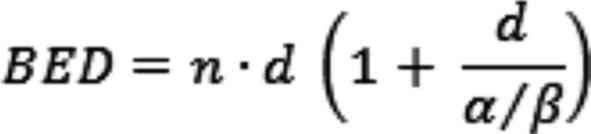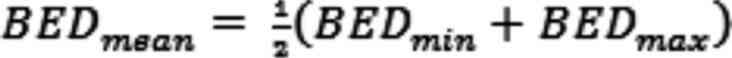Introduction
According to recent global cancer statistics, lung
cancer is the second most frequently diagnosed malignancy,
accounting for ~2.3 million new cases annually (11.7%). Despite
this, it remains the leading cause of cancer-related mortality,
responsible for an estimated 1.8 million deaths worldwide (18%)
(1). Tobacco smoking is well
established as the primary risk factor for lung cancer (2). However, additional contributors
include genetic susceptibility, environmental pollutants,
occupational exposures, socioeconomic status and biological sex
(3).
Although lung cancer continues to be associated with
a poor prognosis, the 5-year relative survival rate for all types
has improved significantly over the past four decades, increasing
from 10.7 to 19.8% (4). This
improvement is largely attributed to advances in diagnostic and
therapeutic strategies, including earlier detection, molecular
profiling, refined staging methods and enhanced treatment
approaches, such as surgery, radiotherapy, targeted therapies and
immunotherapy (5,6).
For cases deemed medically inoperable or for
patients those who decline surgery, stereotactic ablative
radiotherapy (SABR) has emerged as a highly effective alternative
(7,8). SABR is a non-invasive, image-guided
radiotherapy technique that delivers high-dose radiation with
sub-millimeter precision, allowing for maximal tumor control while
minimizing damage to surrounding healthy tissues (9). Clinical studies have demonstrated
excellent local control rates with SABR, reaching up to 98% at 3
years, and a favorable toxicity profile (10,11).
For inoperable peripherally located stage I lung
cancer, stereotactic body radiation therapy (SBRT) can result in
superior local control rates without increasing major toxicities
compared with standard radiotherapy (12). However, the two randomized phase 3
trials on patients with operable stage I NSCLC (STARS and ROSEL)
have closed early due to slow patient accrual. Chang et al
(13) assessed the overall survival
(OS) of patients treated with SBRT vs. surgery by pooling data from
the STARS trial [NCT00840749] and the ROSEL trial [NCT00687986].
The estimated 3-year OS was 95% [95% confidence interval (CI),
85–100%] in the SBRT group compared with 79% (95% CI, 64–97%) in
the surgery group; therefore, it was concluded that SBRT could be a
suitable option for operable patients with stage I NSCLC (13). However, due to the small sample size
and short-follow-up duration this analysis has notable limitations.
A subsequent revised STARS trial, which provided a larger sample
size of SBRT patients, along with a protocol-specified
propensity-matched prospectively registered cohort of patients who
underwent video-assisted thoracoscopic surgical lobectomy with
mediastinal lymph node dissection (VATS L-MLND) (14). Long-term survival outcomes after
SABR were found to be non-inferior to those achieved with VATS
L-MLND in operable patients with stage IA NSCLC (14). Despite these promising results,
lobectomy with mediastinal lymph node dissection remains the
standard of care for operable patients with operable early-stage
NSCLC (15).
According to the Californian Cancer Center registry
data, long-term survival analysis of untreated patients with stage
I NSCLC suggests that a large proportion patients die of lung
cancer specific reasons; the 5-year OS of untreated patients with
stage I NSCLC was 6% overall (16).
There are numerous factors affecting treatment outcomes after SBRT
in patients with NSCLC. Baine et al (17) investigated the role of histology on
post-SBRT treatment outcomes such as local, regional and distant
control; the multi-institutional analysis showed higher local,
regional and distant recurrence rates and worse OS in NSCLC
patients with squamous cell cancer after SBRT. The biologically
effective dose (BED) affects the efficacy and the OS of patients
with NSCLC treated with SBRT. Medium or medium to high BEDs have
been associated with improved OS compared with high or low BEDs
(18).
The assessment of frequency and severity of
comorbidities also serves a critical role in terms of treatment
outcomes after SBRT. In lung cancer, one of the most frequently
used comorbidity scores is the Charlson comorbidity index (CCI)
(19,20). The CCI includes 19 different
diseases, each of which is weighted with scores ranging from 1 to 6
points according to the risk of long-term mortality (21).
For treatment planning, position emission tomography
(PET) computed tomography (CT) scans with a fludeoxyglucose-18
tracer (FDG) can predict stage I and stage II disease, as well as
the Tumor-Node-Metastasis (TNM) staging (22,23)
(T- and N-status), among patients with NSCLC more effectively
compared with dedicated PET alone (24). Furthermore, FDG-PET-CT may to be a
suitable diagnostic tool for predicting the therapeutic outcomes of
patients with early-stage NSCLC treated with SBRT more accurately
(25).
The specific aim of the present study was to provide
data on the long-term (10-year) survival rates of patients with
NSCLC treated with SBRT in a real-world setting. Specifically, the
present study aimed to analyze clinical and treatment-related
prognostic factors including age, performance status, comorbidities
(CCI), BEDmean and gross tumor volume (GTV), as well as their
associations with OS. Using Cox regression models and Kaplan-Meier
survival analysis, the present study aimed to identify patient- and
tumor-related factors influencing long-term outcomes, evaluate the
prognostic impact of BEDmean and GTV and explore whether older
patients experience comparable or superior outcomes compared with
that of younger patients. The present analyses were conducted to
support clinical decision-making and individual risk stratification
in patients with early-stage NSCLC treated with SBRT outside of
randomized controlled trials.
Patients and methods
Data and material
Patient recruitment was conducted retrospectively
using the databases of the Department of Radiation Oncology at the
University Hospital Halle [Halle (Saale), Germany]. Patient data
were anonymized and retrieved from the hospital information system
ORBIS (version 03.20.02.01; Moody's Analytics, Inc.). Information
regarding diagnostic imaging and radiation treatment were obtained
from Centricity PACS (Cytiva) and from Elekta Mosaiq (version 2.84;
Elekta Instrument AB).
All patients (n=99) treated with SBRT between
January 2009 and December 2013, were considered. After the initial
screening process, further analysis focused on patients with NSCLC
with M0-status according to the TNM staging system. Prior to any
data collection, the present study was approved by the ethics
committee of the Medical Faculty of Martin Luther University
Halle-Wittenberg (ethics approval number 2025-006). A total of 64%
of patients were male and 36% were female. The median age was 72
years, with a range of 44 to 91 years. Inclusion criteria comprised
age >18 years, receipt of at least one SBRT treatment and
curative treatment intent. The last date of follow-up was October
11, 2024.
Patient characteristics were categorized in multiple
classifications for analysis. Sex was classified as male or female,
while performance status was assessed using the Karnofsky
performance status (KPS). The KPS is a widely used method of
assessing the functional status of patients with cancer. This scale
allows clinicians to quantify a patient's functional impairment,
facilitating comparisons of therapeutic effectiveness and
prognostic assessments (26). Lower
KPS scores correlate with decreased survival rates across various
serious illnesses, including cancer, chronic obstructive pulmonary
disease (COPD), congestive heart failure and advanced neurological
disorders, such as stroke or dementia (27). In the present study, KPS scores were
dichotomized into >70% (indicating superior physical condition)
and ≤70% (indicating poorer condition). T-status was grouped into
T1, T2, T3, T4 and unknown stage based on the TNM staging system;
nodal involvement (N-status) was categorized as N0 (no nodal
involvement), N1, N2 or unknown. The Union for International Cancer
Control (UICC) stage was recorded as stage I, II, III or unknown
stage according to the 7th edition of the UICC classification
(23).
Histological grading was documented as either known
or unknown, while tumor histology was categorized into
adenocarcinoma, large cell lung carcinoma, squamous cell carcinoma,
not otherwise specified (NOS) or unknown histology. PET-based
treatment was recorded as performed or not performed. Radiation
dose was reported as single dose (such as 7, 8 and 12.5 Gy) and
number of fractions delivered. Furthermore, treatment regimens and
prescription isodoses were recorded.
COPD is a common comorbidity in patients with lung
cancer and is classified into four grades based on the severity of
airflow limitation, as defined by the Global Initiative for Chronic
Obstructive Lung Disease (28).
These grades are determined using spirometry, specifically
assessing the post-bronchodilator forced expiratory volume (FEV) in
a 1-sec duration (FEV1), as a percentage of the
predicted value. Grade I (mild) is characterized by an
FEV1 ≥80% of the predicted value. Symptoms may include
chronic cough and sputum production, but airflow limitation is
mild. Grade II (moderate) corresponds to an FEV1 between
50–79% of the predicted value. Patients typically experience
shortness of breath on exertion, along with cough and sputum
production. Grade III (severe) includes an FEV1 between
30–49% of the predicted value, often accompanied by significant
shortness of breath, reduced exercise capacity, fatigue and
frequent exacerbations. Grade IV (very severe) is defined as a
FEV1 <30% of the predicted value or <50% with
chronic respiratory failure. Grade IV diseases is associated with
severe airflow limitation, a significant decline in quality of life
and life-threatening exacerbations (29).
For analysis, COPD status of each patient was
categorized into COPD unspecified, COPD I, COPD II, COPD II–III,
COPD III, COPD IV or unknown status. Overall comorbidities were
evaluated using the CCI and the age adjusted CCI. The CCI is a
widely used tool to estimate the prognosis and 10-year survival of
patients based on their comorbidities. Table I provides an overview of CCI
estimation based on different comorbidities and their weight in the
score (21).
 | Table I.Charlson comorbidity index
calculation chart. |
Table I.
Charlson comorbidity index
calculation chart.
| Comorbidity | Weight | Criteria |
|---|
| Myocardial
infarction | 1 | History of
myocardial infarction or coronary artery disease |
| Congestive heart
failure | 1 | History of heart
failure |
| Peripheral vascular
disease | 1 | Claudication,
peripheral artery disease or previous vascular surgery |
| Cerebrovascular
disease | 1 | Stroke, transient
ischemic attack or history of cerebral hemorrhage |
| Dementia | 1 | Clinical diagnosis
of dementia |
| Chronic pulmonary
disease | 1 | Chronic obstructive
pulmonary disease or asthma |
| Connective tissue
disease | 1 | Rheumatoid
arthritis or systemic lupus erythematosus |
| Peptic ulcer
disease | 1 | History of gastric
or duodenal ulcer |
| Mild liver
disease | 1 | Chronic liver
disease without liver failure (for example, cirrhosis without
ascites) |
| Diabetes without
complications | 1 | Diabetes mellitus
without end-organ damage |
| Diabetes with
complications | 2 | Diabetes with
end-organ damage, such as retinopathy or nephropathy |
| Hemiplegia or
paraplegia | 2 | Paralysis due to
stroke, spinal cord injury or other causes |
| Moderate or severe
renal disease | 2 | Chronic kidney
disease with creatinine >3 mg/dL or dialysis-dependent |
| Cancer
(non-metastatic, active treatment) | 2 | Any solid tumor
without metastasis, currently treated |
| Leukemia | 2 | Chronic or acute
leukemia |
| Lymphoma | 2 | Non-Hodgkin's or
Hodgkin's lymphoma |
| Moderate or severe
liver disease | 3 | Cirrhosis with
complications (ascites, encephalopathy) |
| Metastatic solid
tumor | 6 | Any solid tumor
with metastasis |
| HIV/AIDS | 6 | HIV infection with
AIDS or opportunistic infections |
The age-adjusted CCI incorporates the age of the
patient to provide a more refined assessment of prognosis. In this
approach, one additional point is added to the total CCI score for
each decade of life starting from 50 years of age. For example,
patients aged 50 to 59 years receive one additional point, those
aged 60 to 69 years receive two points, those aged 70 to 79 years
receive three points and patients aged ≥80 years receive four
points. This adjustment reflects the increasing risk of mortality
associated with aging, independent of the presence or severity of
comorbid conditions (21,30). By accounting for age, the
age-adjusted CCI provides a more accurate prediction of outcomes,
particularly in older populations where age serves a significant
role in overall health and survival (30). In the present study, CCI was used to
evaluate the burden of comorbidities in the patient population. The
CCI was analyzed in two forms: The original version (without age
adjustment) and the age-adjusted version. The CCI without
age-adjustment was categorized into 3 groups: Low comorbidity
(<4), moderate comorbidity (4–5) and
high comorbidity (>5). The age-adjusted CCI was further
stratified into 4 groups: Very low (<3), low (3–5),
moderate (6–8) and high (>8).
Stereotactic radiation treatment
Treatment plans were carried out in accordance with
the national recommendations in place at the time of data
collection. The equipment consisted of a ‘Light Speed RT’ (GE
Healthcare) for scanning the patient geometry, the ‘Oncentra Master
Plan’ treatment planning system (Elekta Instrument AB) and two
linear accelerators (‘MXE’ and ‘Primus’; Siemens Healthineers),
each equipped with a collimator offering 1-cm leaf width in the
center part (measured at isocenter distance) and a portal imaging
system for position verification.
Patients were immobilized by means of a vacuum
mattress on a stereotactic board to which a stereotactic frame
(acrylic box) was attached (Fig.
1). A radiopaque coordinate system embedded in the acrylic
glass was used for patient alignment under the treatment machine.
Several CT series were collected with a slice thickness of 2.5 mm
using different scan techniques to visualize the tumor's movement
during a breathing cycle. Target volume was defined as this
movement space enlarged by 5 mm in all directions to account for
overall geometrical uncertainties of the treatment chain. Dose was
calculated using the ‘collapsed cone algorithm’ on the ‘slow’ CT
series. This series was scanned with a gantry rotation time of 4
sec and therefore displayed an averaged anatomy during ~1 breathing
cycle.
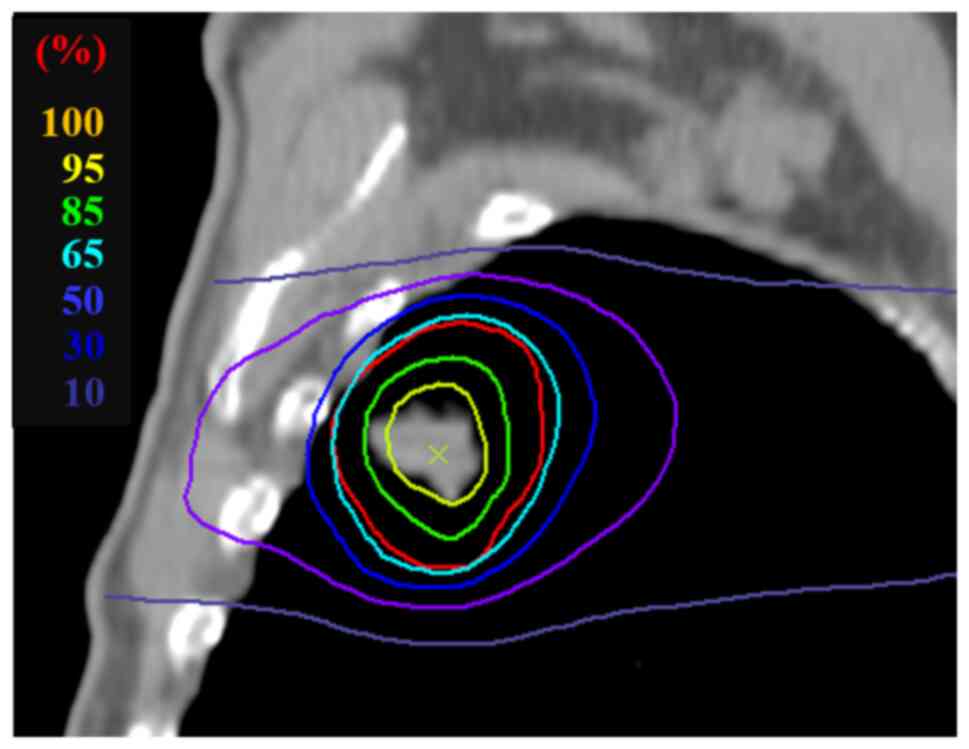
Dose was prescribed in 1 to 6 fractions onto the 65%
isodose enclosing the target volume, with the dose rising steeply
to a maximum of 100% in the center of the target (Fig. 2). All treatment plans consisted of
6–13 fixed beams of 6 MV acceleration potential and were created
using 3D-conformal technique with the beam isocenter in the center
of the tumor. Prior to each irradiation session, a control CT
examination (slow series) of the movement space of the tumor was
taken in which the center coordinates of the tumor, as documented
in the treatment plan, were localized relative to the origin of the
stereotactic frame. The beam isocenter of the treatment device was
then set to that location and orthogonal MV images were taken.
Finally, these images were compared with the respective digitally
reconstructed radiographs from the treatment planning system to
verify correct patient position. Patients were irradiated daily,
unless if three fractions were applied. Those patients were
irradiated every other day.
Calculation of biologically equivalent
dose
The varying effectiveness of the different dose
concepts can be partially explained by the use of a biologically
equivalent dose BED according to the linear quadratic model
(31):
Where n, d and α⁄β are the number of treatment
sessions, their dose and the characteristic parameter of the linear
quadratic model, which is inversely proportional to the repair
capacity of the tissue under consideration, respectively (1). As per previous research for the
stereotactic irradiation of lung tumors (32) the arithmetic mean was calculated
from the prescription BEDmin and the isocenter
BEDmax with α/β=10 Gy:
In the present study, the BED is given in units of
Gy10 in order to visualize the numerical value of α⁄β
used in the calculation. GTV was defined based on the radiotherapy
planning CT acquired prior to the start of SBRT.
Statistical analyses
Proportional hazard Cox regression models were used
to assess the association of cancer-related parameters with OS and
computed hazard ratios (HR) with 95% confidence intervals (CI). In
the univariate analysis, each clinical factor was evaluated
individually. All significant predictors, as well as CCI and KPS,
from the univariate models were subsequently considered in a
multivariate analysis.
To identify the best-fitting model, a stepwise
variable selection approach was applied (both forward inclusion and
backward elimination) based on the Akaike Information Criterion
(33). The final multivariable
model was derived from this stepwise selection procedure. The
primary endpoint of the analysis was OS, defined as the time
interval between the end of radiotherapy and either death or the
last known follow-up as documented in the local citizen
registration.
Survival curves were generated using the
Kaplan-Meier method to analyze cumulative patient survival.
Differences between groups were assessed using the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference. Cut-off values for both BEDmean and GTV were defined as
the thresholds that provided the best separation in OS, as
determined by the log-rank test. To assess the robustness of the
findings in the presence of missing data, a sensitivity analysis
using five imputations was performed. All statistical analyses were
conducted using RStudio (version 2024.04.2+764; Posit Software,
PBC).
Results
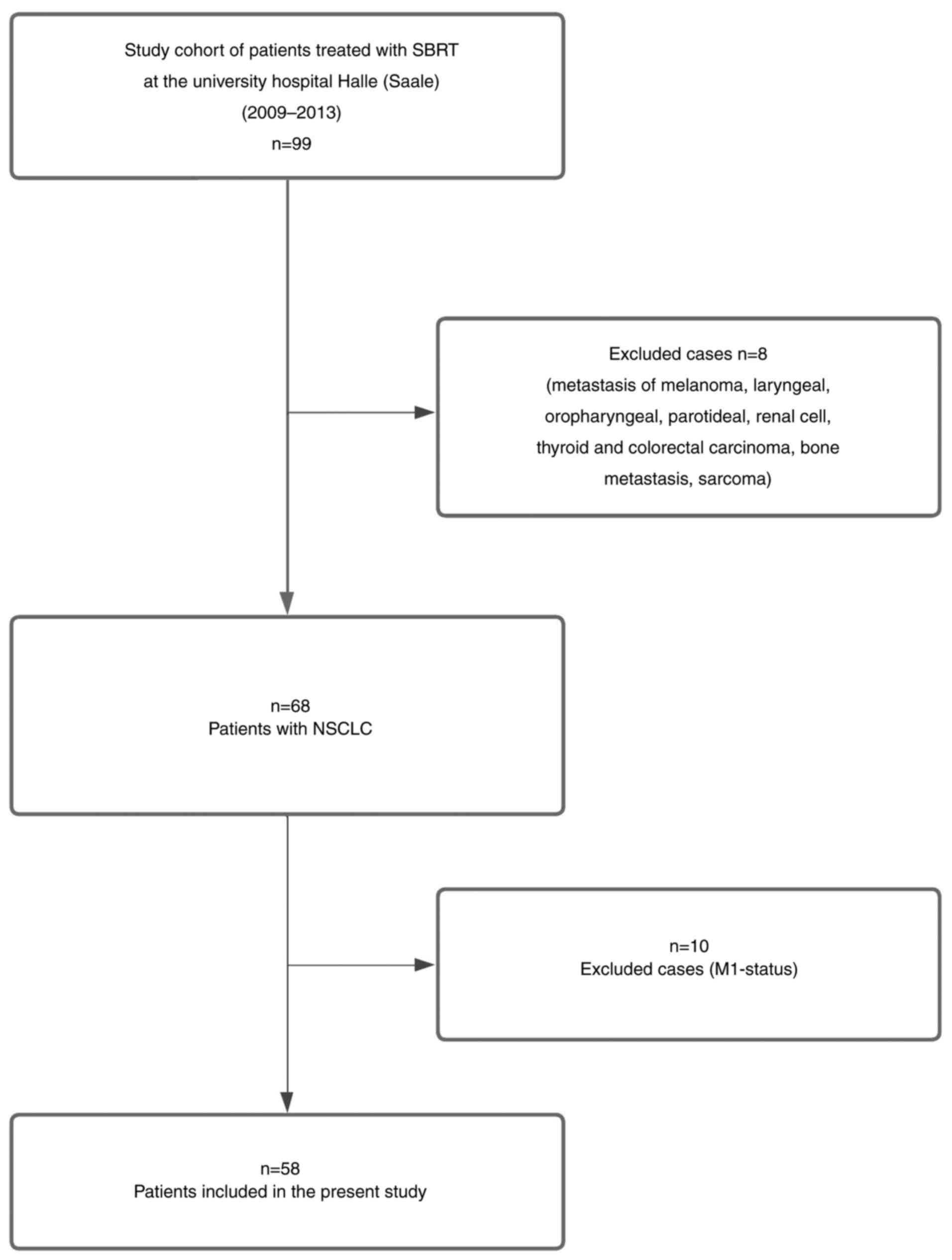
Case selection
The present retrospective study cohort was initially
comprised of 99 patients who were treated with SBRT at the
radiation oncology department of the University Hospital Halle
(Saale) between 2009 and 2013. However, 31 patients that were
treated for lung or bone metastasis caused by various primary
tumors (such as melanoma, laryngeal, oropharyngeal, parotid, renal
cell, thyroid, colorectal carcinoma or sarcoma) were excluded. The
remaining 68 patients were diagnosed with lung cancer.
Subsequently, 10 of these 68 patients were excluded due to
M1-status, resulting in a final cohort of 58 patients with
M0-status that were included in the present analysis (Fig. 3).
Patient characteristics
The baseline characteristics of all patients with
lung cancer are presented in Table
II, stratified by age groups (<75 and ≥75 years). Among
patients aged <75 years (n=28) and ≥75 years (n=30), the
proportion of females was comparable (32 vs. 33%), with the
majority of patients in both groups being male (68 vs. 67%). A
higher KPS (>70%) was observed more frequently among older
patients (53 vs. 29%), whereas lower KPS (≤70%) occurred more often
in the younger group (71 vs. 47%).
 | Table II.Baseline patient characteristics
(n=58)a. |
Table II.
Baseline patient characteristics
(n=58)a.
| Characteristic | Total, n (%) | Patients aged
<75 years, n (%) | Patients aged ≥75
years, n (%) |
|---|
| Sex |
|
|
|
|
Female | 19 (33) | 9 (32) | 10 (33) |
|
Male | 39 (67) | 19 (68) | 20 (67) |
| Age, years |
|
|
|
|
<75 | 28 (48) |
|
|
|
≥75 | 30 (52) |
|
|
|
Karnofsky-performance-status, % |
|
|
|
|
>70 | 24 (41) | 8 (29) | 16 (53) |
|
≤70 | 34 (59) | 20 (71) | 14 (47) |
| T-status |
|
|
|
| 1 | 26 (45) | 12 (43) | 14 (47) |
| 2 | 19 (33) | 7 (25) | 12 (40) |
| 3 | 2 (3) | 1 (4) | 1 (3) |
| 4 | 3 (5) | 3 (11) | 0 (0) |
|
Unknown | 8 (14) | 5 (18) | 3 (10) |
| N-status |
|
|
|
| 0 | 43 (74) | 18 (64) | 25 (83) |
| 1 | 2 (3) | 2 (7) | 0 (0) |
| 2 | 4 (7) | 3 (11) | 1 (3) |
|
Unknown | 9 (16) | 5 (18) | 4 (13) |
| UICC-stage |
|
|
|
| I | 37 (64) | 16 (57) | 21 (70) |
| II | 8 (14) | 4 (14) | 4 (13) |
|
III | 4 (7) | 3 (11) | 1 (3) |
|
Unknown | 9 (16) | 5 (18) | 4 (13) |
| Grading |
|
|
|
|
Known | 16 (28) | 7 (25) | 9 (30) |
|
Unknown | 42 (72) | 21 (75) | 21 (70) |
| PET-based
treatment |
|
|
|
| No | 15 (26) | 9 (32) | 6 (20) |
|
Yes | 41 (71) | 18 (64) | 23 (77) |
|
Unknown | 2 (3) | 1 (4) | 1 (3) |
| Histology |
|
|
|
|
Adenocarcinoma | 16 (28) | 7 (25) | 9 (30) |
| Large
cell lung carcinoma | 1 (2) | 1 (4) | 0 (0) |
| Not
otherwise specified | 3 (5) | 0 (0) | 3 (10) |
|
Squamous cell carcinoma | 23 (40) | 13 (46) | 10 (33) |
|
Unknown | 15 (26) | 7 (25) | 8 (27) |
| COPD |
|
|
|
| COPD
unspecified | 11 (19) | 4 (14) | 7 (23) |
| COPD
I | 1 (2) | 0 (0) | 1 (3) |
| COPD
II | 8 (14) | 6 (21) | 2 (7) |
| COPD
II–III | 1 (2) | 0 (0) | 1 (3) |
| COPD
III | 4 (7) | 1 (4) | 3 (10) |
| COPD
IV | 11 (19) | 10 (36) | 1 (3) |
|
Unknown | 22 (38) | 7 (25) | 15 (50) |
| CCI without age
adjustment |
|
|
|
|
<3 | 1 (2) | 1 (4) | 0 (0) |
|
>8 | 10 (17) | 4 (14) | 6 (20) |
|
3-5 | 11 (19) | 9 (32) | 2 (7) |
|
6-8 | 36 (62) | 14 (50) | 22 (73) |
| CCI
age-adjusted |
|
|
|
|
<3 | 1 (2) | 1 (4) | 0 (0) |
|
3-5 | 11 (19) | 9 (32) | 2 (7) |
|
6-8 | 36 (62) | 14 (50) | 22 (73) |
|
>8 | 10 (17) | 4 (14) | 6 (20) |
| Treatment regimen
(prescription isodose); BEDmean/Gy10 |
|
|
|
| 37.5
Gy/3 fr. (65%); 126.51 Gy10 | 29 (60) | 12 (52) | 17 (68) |
| 40 Gy/4
fr. (65%); 118.11 Gy10 | 1 (2) | 1 (4) | 0 (0) |
| 28 Gy/4
fr. (65%); 68.53 Gy10 | 1 (2) | 1 (4) | 0 (0) |
| 32 Gy/4
fr. (65%); 83.71 Gy10 | 1 (2) | 0 (0) | 1 (4) |
| 36 Gy/4
fr. (65%); 100.24 Gy10 | 1 (2) | 1 (4) | 0 (0) |
| 50 Gy/5
fr. (80%); 120.31 Gy10 | 1 (2) | 1 (4) | 0 (0) |
| 48 Gy/6
fr. (65%); 125.57 Gy10 | 14 (29) | 7 (30) | 7 (28) |
T1 tumors were the most common in both groups (43
vs. 47%), while T2 tumors were slightly more frequent among older
patients (25 vs. 40%). T3 and T4 stages were rare in both groups.
Unknown T-status was more common in the <75 years group (17 vs.
10%). Regarding nodal status, most patients were N0, particularly
in the older group (83 vs. 64%). N1 and N2 involvement was observed
only in younger patients (N1: 7%; N2: 11%), while N-status remained
unknown in 18 and 14% of the <75 and ≥75 groups,
respectively.
UICC stage I was the most frequent stage in both age
groups (57 vs. 70%). Stage II and III cases were similarly
distributed, and unknown stage was slightly more frequent in the
younger group (18 vs. 14%). Histological grading was unknown in the
majority of patients in both age groups, slightly more so in
younger patients (75 vs. 70%).
A PET-based treatment approach was used more often
in older patients (77 vs. 64%). Squamous cell carcinoma was the
most frequent histological subtype (46 vs. 33%), followed by
adenocarcinoma (25 vs. 30%). Large cell lung carcinoma occurred
only in the younger group (4%). Tumors NOS were found exclusively
in older patients (10%).
Regarding comorbidities, COPD IV was substantially
more frequent in younger patients (36 vs. 3%), while unspecified
COPD was more frequent in the older group (23 vs. 14%). The
proportion of patients with unknown COPD status was higher among
older patients (51 vs. 25%). Age-adjusted CCI values were higher in
the ≥75 years group: 73% had a score of 6–8 and 20% had a score of
>8, compared with 50 and 14% in the <75 years group,
respectively.
The most commonly prescribed treatment regimen was
37.5 Gy in 3 fractions (52 vs. 68%), followed by 48 Gy in 6
fractions (32 vs. 28%). Most patients received a mean biological
effective dose (BEDmean) of 126.51 Gy10 (50%). The
second most frequent BEDmean was 125.57 Gy10,
administered in 24% of patients. All other regimens (e.g., 118.11,
120.31, 100.24, 83.71 and 68.53 Gy10) accounted for
<5% of patients.
Survival analysis
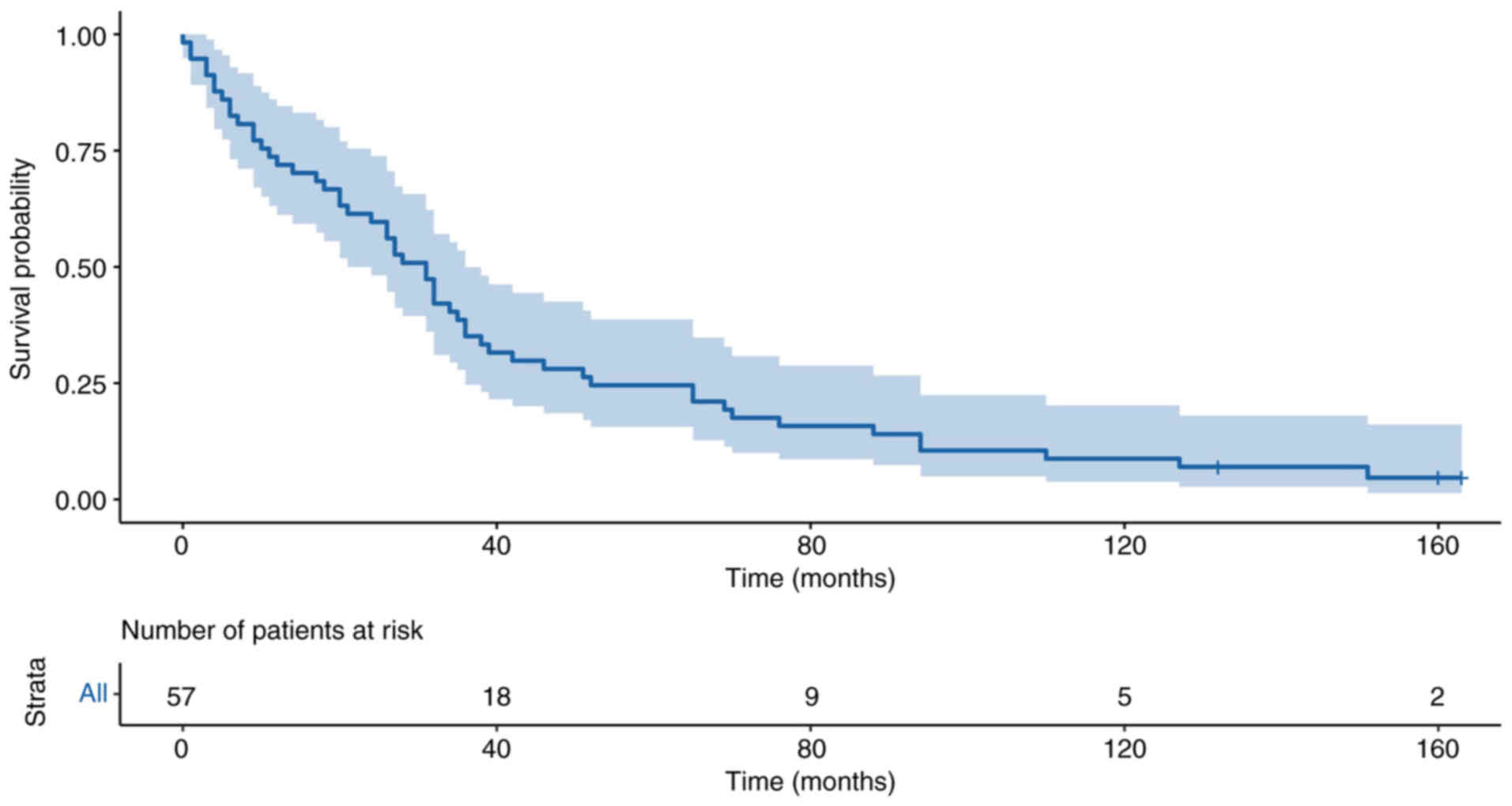
For OS, the 3-, 5- and 10-year OS rate was 35.09,
24.56 and 8.77%, respectively. The overall median survival of all
patients without metastasis was 32 months (95% CI, 10–35 months).
Median survival estimates for the respective patient subgroups are
summarized in Table SI.
The results of the univariate and multivariate Cox
regression analyses are summarized in Table III. Although the CCI and KPS
showed a statistically significant but weak correlation (Pearson's
r=0.29; P=0.029), both variables were retained in the univariate
Cox regression analyses. This decision was based on clinical
reasoning, as comorbid burden and performance status represent
distinct yet complementary aspects of the general health of a
patient and may independently influence survival. Furthermore,
considering their relevance within different age groups, their
inclusion allows for a more nuanced assessment of age-related
heterogeneity in the prognosis of patients with non-metastatic
NSCLC.
 | Table III.Univariate and multivariate
Cox-regression models. |
Table III.
Univariate and multivariate
Cox-regression models.
|
| Univariate Cox
model | Multivariate
stepwise model |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
| 0.025 |
|
| 0.057 |
|
<75 | - | - |
| - | - |
|
|
≥75 | 0.54 | 0.31–0.92 |
| 0.55 | 0.30–1.01 |
|
| Sex |
|
|
|
|
|
|
|
Female | - | - |
|
|
|
|
|
Male | 1.12 | 0.64–1.97 | 0.762 |
|
|
|
| CCI |
|
| 0.654 |
|
|
|
|
<4 | - | - |
|
|
|
|
|
4-5 | 1.36 | 0.73–2.53 |
|
|
|
|
|
>5 | 1.11 | 0.53–2.30 |
|
|
|
|
| Karnofsky
performance status, % |
|
| 0.311 |
|
|
|
|
>70 | - | - |
|
|
|
|
|
≤70 | 1.33 | 0.77–2.30 |
|
|
|
|
|
BEDmean/Gy10 | 0.99 | 0.97–1.00 | 0.257 |
|
|
|
| GTV | 1.01 | 1.00–1.02 | 0.041 | 1.01 | 1.00–1.02 | 0.024 |
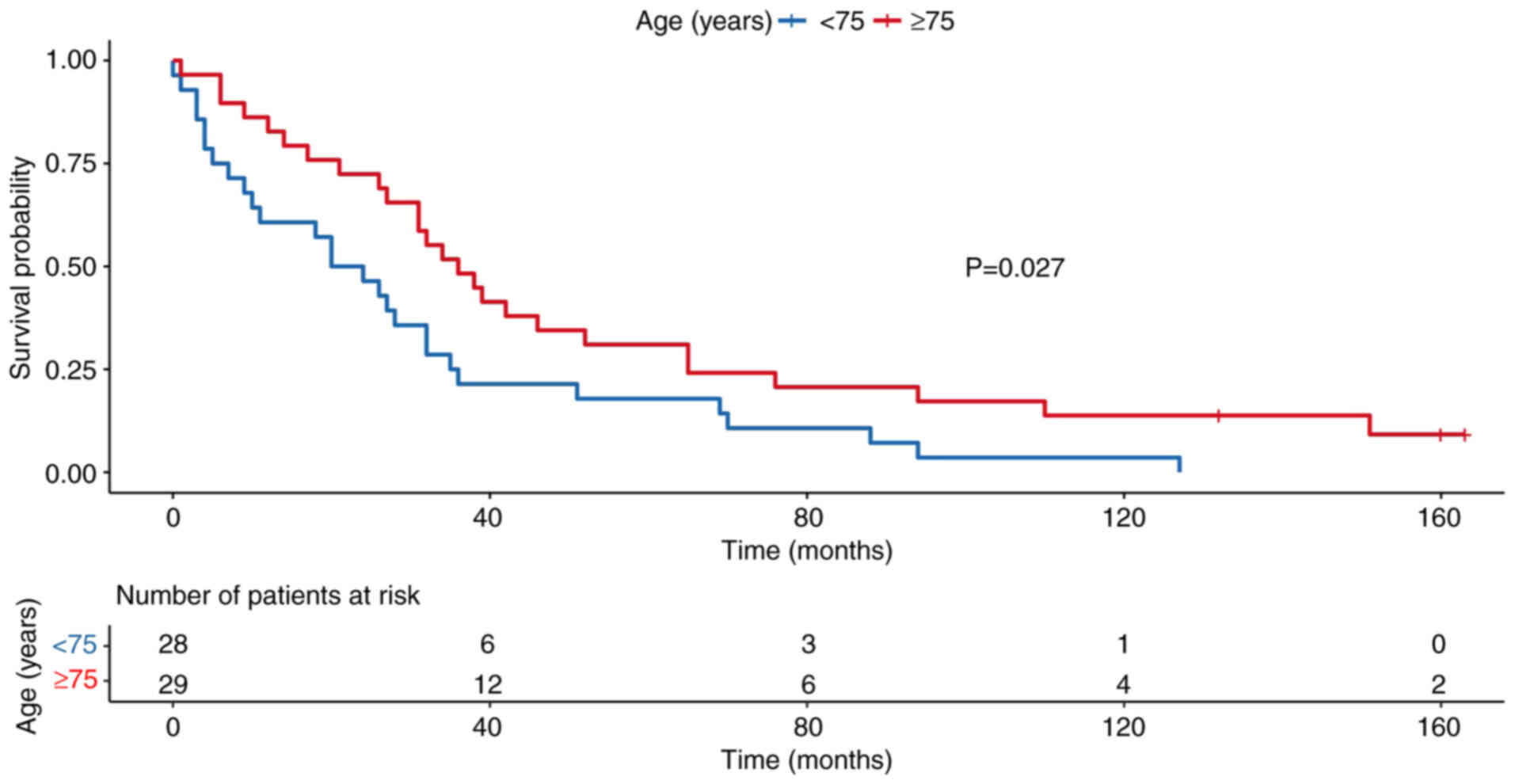
In the univariate analysis, age ≥75 years was
significantly associated with decreased OS (HR, 0.54; 95% CI,
0.31–0.92; P=0.025). However, age ≥75 years was not statistically
significant in the multivariate stepwise model (HR, 0.55; 95% CI,
0.30–1.01; P=0.057), although a similar trend was observed. Sex
(male vs. female) was not significantly associated with OS in the
univariate analysis (HR, 1.12; 95% CI, 0.64–1.97; P=0.7) and was
not selected for the multivariate stepwise model.
The CCI did not show a significant association with
survival in either the univariate analysis or the stepwise model
(P=0.6). When compared with patients with a CCI <4, those with
scores of 4–5 (HR, 1.36; 95% CI, 0.73–2.53) and >5 (HR, 1.11;
95% CI, 0.53–2.30) did not differ significantly in mortality risk.
The KPS (≤70 vs. >70%) was also not significantly associated
with survival (HR, 1.33; 95% CI, 0.77–2.30; P=0.3) and was excluded
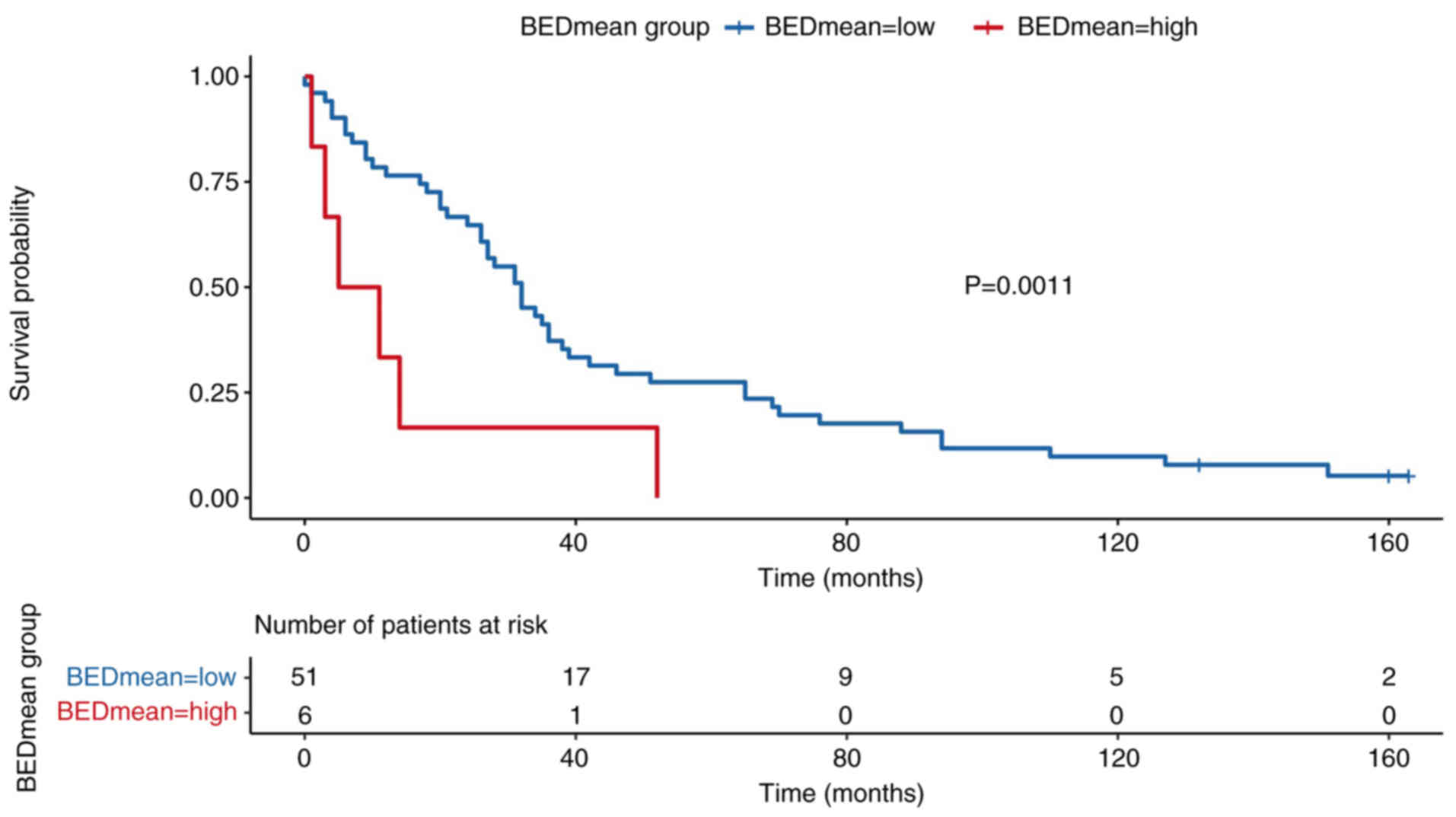
from the multivariate stepwise model. The mean BED
(BEDmean/Gy10) showed no significant association with
survival in the univariate analysis (HR, 0.99; 95% CI, 0.97–1.00;
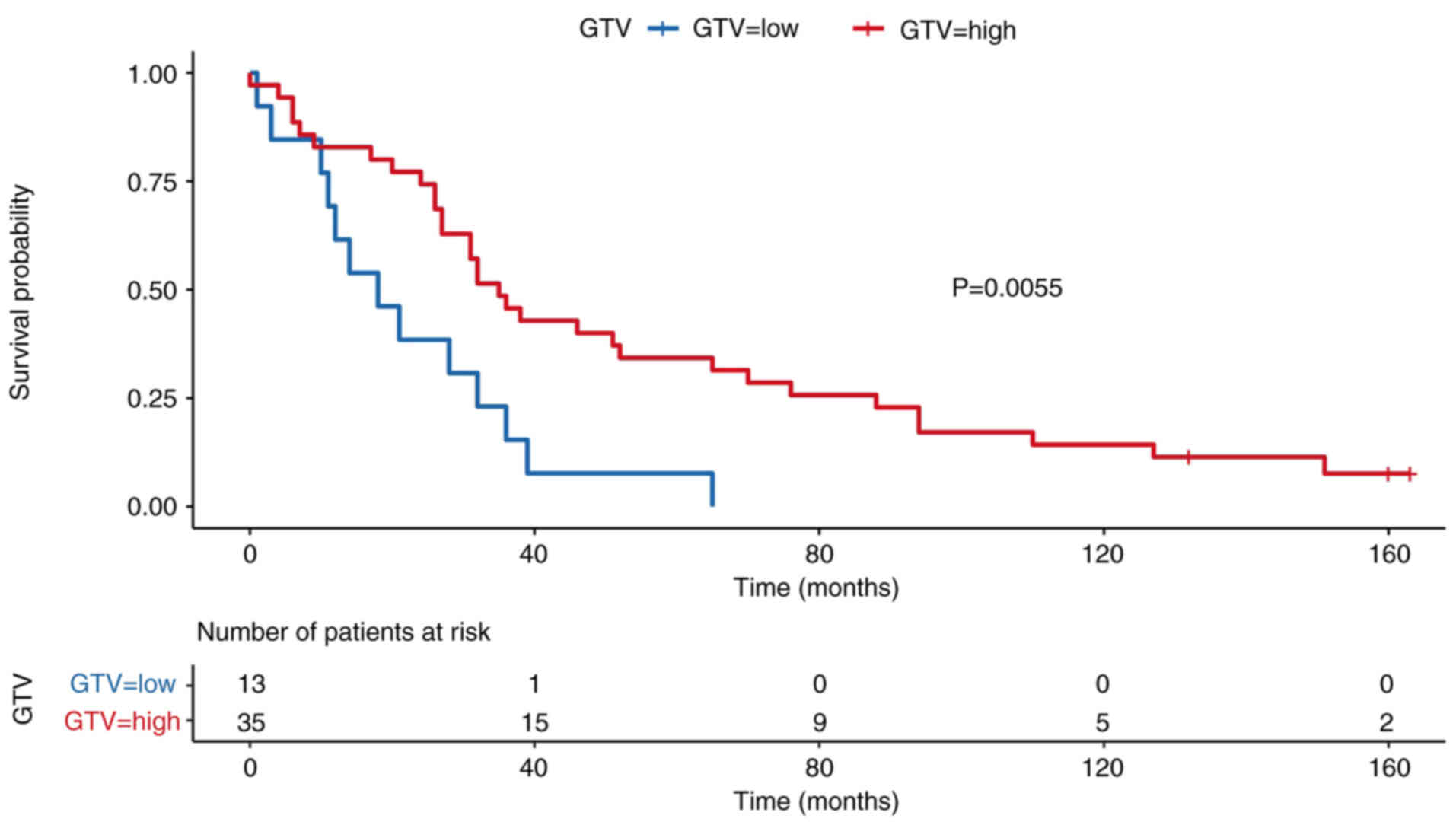
P=0.2) and was not retained in the multivariate model. The GTV was
significantly associated with survival in both the univariate (HR,
1.01; 95% CI, 1.00–1.02; P=0.041) and multivariate (HR, 1.01; 95%
CI, 1.00–1.02; P=0.024) analyses. This indicates that larger tumor
volumes were independently associated with increased mortality
risk.
Kaplan-Meier survival analysis
Kaplan-Meier survival curves were generated to
illustrate OS in the present study cohort and stratified subgroups.
The survival curve for the total cohort up to 10 years (120 months)
is displayed in Fig. 4. OS was
stratified by age group (<75 vs. ≥75 years), which demonstrated
significantly reduced survival in older patients (P=0.023; Fig. 5). Survival rates were also analyzed
stratified according to BEDmean, using a cut-off of 120.31
Gy10 (Fig. 6). Patients
with high BEDmean demonstrated significantly improved survival
rates compared with those with lower doses (P=0.011). Survival was
stratified by GTV, using a cut-off of 25.6 cm3 (Fig. 7). Patients with larger tumor volumes
had significantly poorer survival outcomes (P=0.006).
Sensitivity analysis
To assess the robustness of the Cox regression
results, a sensitivity analysis using multiple imputations was
conducted and compared with the univariate and multivariate
stepwise model based on complete-case analysis. The pooled results
from the imputed datasets were compared with the complete case
analysis. The consistency of effect estimates between both models
supported the validity of the present conclusions. Overall, the
effect estimates were consistent between the imputed and stepwise
models. The variable age ≥75 years remained significantly
associated with improved survival across both approaches
(univariate imputed, HR, 0.50; 95% CI, 0.29–0.88; P=0.016;
multivariate stepwise, HR, 0.49; 95% CI, 0.29–0.85; P=0.012). Sex,
CCI and KPS did not show any significant associations in either
model.
Volume GTV remained significantly associated with
worse survival in both the univariate imputed (HR, 1.01; 95% CI,
1.00–1.02; P=0.007) and multivariate stepwise model (HR, 1.01; 95%
CI, 1.00–1.02, P<0.001). BEDmean also showed improved survival
in the imputed model (HR, 0.99; 95% CI, 0.97–1.00; P=0.14), but
this was not statistically significant. Taken together, the
sensitivity analysis supported the robustness of the aforementioned
findings, particularly regarding age and GTV, and further
validation of the multivariate model despite missing data (Table IV).
 | Table IV.Sensitivity analysis using multiple
imputation. |
Table IV.
Sensitivity analysis using multiple
imputation.
|
| Univariate Cox
model (multiple imputation) | Multivariate
stepwise model |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
<75 | - | - |
| - | - |
|
|
≥75 | 0.50 | 0.29–0.88 | 0.016 | 0.49 | 0.29–0.85 | 0.012 |
| Sex |
|
|
|
|
|
|
|
Female | - | - |
|
|
|
|
|
Male | 1.03 | 0.58–1.83 | >0.924 |
|
|
|
| CCI | 1.05 | 0.87–1.25 | 0.671 |
|
|
|
| Karnofsky
performance status | 1.00 | 0.98–1.01 | 0.542 |
|
|
|
|
BEDmean/Gy10 | 0.99 | 0.97–1.00 | 0.141 |
|
|
|
| GTV | 1.01 | 1.00–1.02 | 0.007 | 1.01 | 1.00–1.02 | <0.001 |
Discussion
The primary aim of the present study was to analyze
data on the long-term (10-year) OS of patients with NSCLC treated
with SBRT in a real-world setting. To the best of our knowledge
there have only been a few analyses that have provided 10-year
follow-up data of patients treated with SBRT (34–37).
The working group ‘Extracranial Stereotactic
Radiotherapy’ of the German Society for Radiation Oncology
previously investigated the efficacy and safety of SBRT based on a
retrospective multicentric analysis (38). Data of 582 NSCLC patients treated
with SBRT at 13 institutions between 1998 and 2011 were
retrospectively analyzed. The BED was the most significant factor
associated with freedom from local progression (FFLP) and OS;
3-year FFLP and OS were 92.5 and 62.2%, respectively (38). In the present dataset, the 3-year OS
was lower (35.09%) compared with the of the aforementioned working
group study. However, the analysis by Guckenberger et al
(38) exclusively included patients
with stage I NSCLC. By contrast, the present cohort comprised 64%
patients with stage I NSCLC. Additionally, the baseline KPS in the
SBRT working group cohort was 80%, whereas in the present dataset,
59% of patients with NSCLC had a KPS of ≤70%. Therefore, the
observed difference in 3-year OS may be attributed to the poorer
clinical performance status of the present cohort and the inclusion
of patients with NSCLC with more advanced UICC stages.
In contrast to previously published findings
(39), BEDmean did not remain a
significant predictor of OS in the multivariate analysis (P=0.2),
despite being associated with survival in univariate Cox regression
(HR, 0.99; 95% CI, 0.97–1.00; P=0.2) and showing a significant
difference in Kaplan-Meier analysis (P=0.011). These inconsistent
findings may reflect the complex interplay between dose, tumor
volume and patient frailty (40,41).
However, the current literature specifically addressing the use of
SBRT in elderly patients, particularly with respect to survival
outcomes, is scarce. This gap in the literature highlights the
relevance of the present study and supports the need for further
research in this underrepresented patient subgroup. Furthermore,
the sensitivity analysis using multiple imputations demonstrated
that the BEDmean only had a non-significant trend toward improved
survival (HR, 0.99; 95% CI, 0.97–1.00; P=0.14). This suggested that
while BED remains a key dosimetry parameter, its prognostic impact
on OS may be diminished in heterogeneous, comorbidity-burdened
patient populations such as in the present study. In a Chinese
retrospective multicenter analysis from 2023 analyzing a total of
145 early-stage NSCLC patients treated with SBRT, the 5-year local
recurrence rate was 5.1% and progression-free survival (PFS) rates
at 3- and 5-years were 69.2 and 60.5%, respectively. The
corresponding OS rates were 78.1 and 70.1% (42).
In the present analysis, older age was associated
with a survival benefit compared with younger patients in the
univariate Cox regression (HR, 0.54; 95% CI, 0.31–0.92; P=0.025),
although this association did not remain statistically significant
in the multivariate model (HR, 0.55; 95% CI, 0.30–1.01; P=0.057).
This finding may be explained by notable differences in baseline
characteristics between the two age groups. In the younger cohort
(<75 years), 71% of patients had a KPS of ≤70%, 48% were
diagnosed with COPD stage IV and 64% had an age-adjusted CCI score
of ≥6. By contrast, older patients (≥75 years) were more frequently
characterized by higher performance status and less severe COPD
staging. Given that SBRT is typically offered to patients deemed
medically inoperable, it could be considered that younger patients
in the present cohort were selected for SBRT only when significant
comorbidities or poor functional status precluded surgical
treatment. This selection bias may have contributed to the
unexpected survival disadvantage in the younger subgroup.
Similar to the analysis by Guckenberger et al
(38) real-world outcomes of SBRT
treatment in inoperable patients with stage I NSCLC were reported
from a Japanese cohort (42). In
this retrospective study, 399 patients with a median age of 75
years were analyzed (43). The
overall 3-year survival rate in this cohort was 77%, which was even
higher compared with the 3-year survival rate reported by
Guckenberger et al (38).
Most of these patients had optimal performance status scores (n=237
had an ECOG score of 0) and no pulmonary comorbidities (n=255 had
no emphysema; n=292 had no pulmonary interstitial changes), which
is in contrast to 31% of the patients in the present study who had
COPD grade IV.
Kreinbrink et al (40) investigated the safety and efficacy
of SBRT in patients ≥80 years with early-stage NSCLC, and it
reported that the median survival was 29.1 months. In comparison,
the overall median survival in the present analysis was slightly
higher at 32 months (95% CI, 10–35 months). However, the shorter
survival observed in their cohort could be attributed to a worse
median Eastern Cooperative Oncology Group (ECOG) performance status
of 2 (range, 0–3) and an increased median age of 83 years. By
contrast, only 52% of patients in the present dataset was ≥75
years. Overall, Kreinbrink et al (40) reported high efficacy and low
toxicity rates in a cohort of elderly, inoperable patients with
NSCLC.
A large proportion of patients in the current study
presented with high T-stages (T3-statge, 4.0%; T4-stage, 6%) and N+
status, which may be explained by several factors. In interpreting
the present findings, it is important to acknowledge the potential
variability in T- and N-staging within the cohort, particularly
given the retrospective nature of the study and the heterogeneity
of available documentation. In certain cases, multiple tumor
lesions within the ipsilateral lung may have contributed to a
higher T-stage classification. Additionally, centrally located
tumors could have raised diagnostic uncertainty regarding the
involvement of hilar lymph nodes. In such instances, the
distinction between direct tumor invasion and true nodal metastasis
may have been unclear, potentially leading to an N+ classification
despite the absence of pathological confirmation. These
considerations highlight the inherent limitations of retrospective
staging assessments and underline the need for cautious
interpretation of TN-stage-based survival outcomes in this context.
Such diagnostic challenges, particularly in borderline cases, may
have potentially influenced staging decisions.
Prior studies have shown the superiority of CCI
compared with individual comorbid conditions in the prediction of
survival (19,20,30).
However, to the best of our knowledge, there are only few analyses
available using CCI as predictor for survival in a cohort of
patients treated with SBRT (44–46).
Baker et al (46)
investigated the role of CCI and the cumulative illness rating
scale (CIRS) as prognostic factors for death within 6 months after
SBRT; it was reported that CIRS and tumor diameter were more
accurate predictors in terms of early-mortality in early-stage lung
cancer after SBRT (46). A
subsequent trial performed by Eriguchi et al (47) retrospectively analyzed operable
stage I NSCLC treated with SBRT, staged as cT1-2N0M0. The median
follow-up after SBRT was 40 months and in their multivariate
regression analysis, age and CCI were found to be significantly
associated with OS (47). By
contrast, the present analysis did not find a significant
association between CCI and OS. Patients with a CCI score of 4–5
had a hazard ratio of 1.36 (95% CI, 0.73–2.53) and those with a
score >5 had a hazard ratio of 1.11 (95% CI, 0.53–2.30) compared
to patients with a CCI <4. Neither comparison reached
statistical significance (P=0.6) and these findings were consistent
across both univariate and multivariate models. However, the median
age of the cohort in the study by Eriguchi et al (47) was slightly older at 79 years (range,
55–88 years) compared with that of the present SBRT cohort at 74
years (range, 45–91 years). Furthermore, the median follow-up time
of their study was notably shorter compared with that of the
present analysis (40 vs. 149 months). In addition, different ethnic
backgrounds (Asian vs. European patients) should be considered in
the interpretation of the different outcomes between CCI and
survival.
Ganti et al (48) examined the prognostic role of CCI in
terms of survival based on a large sample size (n=617) of patients
with NSCLC and small cell lung cancer (SCLC). Multivariate
regression models showed no correlation between survival and the
age-adjusted CCI or CCI without age-adjustment. In contrast to the
present findings, another study conducted on 2,221 patients with
lung cancer treated with SBRT showed a significant association
between OS and CCI as well as between lung cancer specific survival
and CCI (49). The patient
characteristics and follow-up time differed when compared with the
present study; the median 5-year OS rate was 34 months for patients
with pathological confirmation of their diagnosis compared with 26
months in the present study.
PET-CT based treatment planning have shown good
clinical outcomes in inoperable adenocarcinoma, with higher PET-CT
SUVmax values before SBRT being associated with increased risk of
failure (50). When using
PET-CT-based radiation planning, the GTV and clinical tumor volume
could be significantly reduced and accuracy improved (51–53).
Additionally, recent studies have established predictive models for
treatment outcomes (PFS and distant metastasis-free survival) after
SBRT based on radiomic features and PET-CT (54–57).
Although PET-CT-based treatment planning has demonstrated clinical
value in previous studies (52,54,58,59),
it was not included in the present multivariate Cox regression
model. Due to the limited overall sample size, the number of
variables that could be entered into the model had to be restricted
to avoid overfitting. As a result, only covariates with
statistically significant associations in the univariate analysis
(P<0.05) were selected for multivariate modeling. PET-CT did not
meet this criterion and was therefore excluded from further
analysis.
PET-CT was already established as a routine imaging
modality during the treatment period and was used in a large
proportion of the present cohort. The contribution of PET-CT to
improved clinical outcomes may have been indirectly captured
through a more accurate GTV definition, which was significantly
associated with OS (52). PET-CT
enables precise delineation of GTV, often resulting in reduced
volumes and improved dose conformity (60). In the present dataset, GTV emerged
as an independent predictor of OS in both univariate and
multivariate analyses. Thus, the prognostic effect of PET-CT-based
planning may have been reflected through its influence on GTV, even
though it was not explicitly included as a covariate in the final
model.
A key limitation of the present retrospective,
single-center analysis lies in the inherent risk of selection bias
and residual confounding due to the non-randomized nature of the
cohort. Although missing data and potential bias was addressed
through multiple imputations and complete-case sensitivity
analyses, these methods rely on assumptions (for example, data
missing at random) that cannot be fully verified. Furthermore, the
absence of randomization limits causal inference and associations
observed in multivariate models should be interpreted as
exploratory rather than confirmatory.
The monocentric design further restricts the
generalizability of the present findings, as treatment protocols,
patient selection and follow-up strategies may differ across
institutions. The relatively small sample size limits statistical
power, particularly for subgroup analyses, and increases the risk
of type II errors. Although GTV and age emerged as significant
prognostic factors in both primary and imputed models, these
findings warrant validation in larger, multicenter cohorts.
Additionally, the retrospective nature of data collection led to
incomplete documentation of certain clinical variables and
inconsistent reporting of toxicity or recurrence, precluding
analysis of endpoints such as local control or PFS. Due to this, OS
remained the only robust endpoint available for analysis. Finally,
the lack of standardized imaging protocols and follow-up intervals
may have introduced variability in outcome assessment. Despite the
application of rigorous statistical methods, these methodological
limitations underscore the need for prospective, multicenter
studies with standardized data collection to improved understand
the long-term outcomes of SBRT in early-stage NSCLC.
A potential concern in long-term survival analyses
of elderly or comorbid patients is the risk of loss to follow-up or
competing events unrelated to cancer, such as non-cancer-related
deaths. In the present cohort, follow-up was actively conducted via
association with the local citizen registration office, which
allowed for robust and reliable ascertainment of vital status for
all patients. Therefore, no patients were lost to follow-up and OS
could be determined with high confidence. However, due to the
retrospective design and limited availability of cause-of-death
information and cancer-specific survival could not be evaluated. As
such, the reported OS includes both cancer-related and
non-cancer-related deaths. This limitation is particularly relevant
in an elderly cohort with substantial comorbidities, where
non-cancer mortality may compete with oncologic outcomes. While the
CCI was included in the analysis to account for overall comorbidity
burden, it was not significantly associated with survival in the
present models. The influence of competing risks, especially in
older patients or those with severe COPD, should be considered when
interpreting the OS results. Future prospective studies with
detailed documentation of cause of death and competing events are
warranted to separate cancer-specific outcomes from broader
survival metrics in SBRT-treated patients with NSCLC.
In the present multivariate Cox regression analysis,
GTV emerged as an independent predictor of OS, with larger tumor
volumes being significantly associated with worse outcomes. This
finding is consistent with previously published evidence,
reinforcing the prognostic relevance of tumor volume in
SBRT-treated patients with NSCLC. Kessel et al (61) analyzed 219 patients with lung
metastases and confirmed that smaller GTV and PTV were
significantly associated with improved OS using a univariate
analysis. In addition to the favorable oncologic outcomes, low
rates of physician- and patient-reported high-grade toxicity even
after long-term follow-up, were reported. This underlines the
importance of minimizing irradiated volume not only to reduce
toxicity but also to potentially improve survival. The integration
of patient-reported outcomes further emphasized the impact of
respiratory symptoms such as severe dyspnea on patients' quality of
life and highlights the clinical relevance of sparing functional
lung tissue, especially in patients with compromised pulmonary
function (61). These results
underscore the importance of volumetric parameters such as GTV and
PTV in SBRT planning and outcomes. The present results contributed
to this evidence by demonstrating that even in a real-world cohort
with heterogeneous stages and performance statuses, tumor volume
remained a robust and independent prognostic factor for survival.
Future analysis should also focus on the long-term outcomes of
medically operable early-stage patients with lung cancer treated
with surgery compared with SBRT. Due to the lack of prospective,
randomized trials comparing both treatments, advanced statistical
methods such as propensity score matching are needed to evaluate
these treatment outcomes.
The present study provided long-term survival data
for early-stage patients with NSCLC treated with SBRT. Although OS
rates remained limited, patients aged ≥75 years demonstrated better
outcomes, likely reflecting more selective inclusion of older
patients with lower tumor burden and favorable clinical
characteristics. Tumor volume was independently associated with OS,
highlighting its prognostic relevance in this setting. By contrast,
BEDmean and performance status were not significant predictors.
These findings emphasize the importance of tumor characteristics
over chronological age when considering SBRT for early-stage
NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JAM performed the data analysis. SG, CK, CP and DV
contributed to the refinement of the analysis. All authors read and
approved the final manuscript. JAM and DV confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Medical Faculty of Martin Luther University
Halle-Wittenberg [Halle (Saale), Germany; approval no. 2025-006].
As patient data was obtained from the University Hospital Halle, it
should be noted that the University Hospital Halle serves as the
academic teaching hospital and clinical center of the Medical
Faculty of Martin Luther University Halle-Wittenberg.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work the author(s)
used ChatGPT, a language model developed by OpenAI Inc., in order
to improve writing style and check grammar and spelling. After
using this tool, the authors reviewed and edited the content as
needed and took full responsibility for the content of the
publication.
Glossary
Abbreviations
Abbreviations:
|
BED
|
biologically effective dose
|
|
CCI
|
Charlson comorbidity index
|
|
CI
|
confidence interval
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
FDG-PET
|
fluorodeoxyglucose positron emission
tomography
|
|
FEV1
|
forced expiratory volume in 1
second
|
|
HR
|
hazard ratio
|
|
KPS
|
Karnofsky performance status
|
|
SCLC
|
small cell lung cancer
|
|
NSCLC
|
non-SCLC
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
RT
|
radiotherapy
|
|
SBRT
|
stereotactic body radiation
therapy
|
|
UICC
|
Unité International Contre Le
Cancer
|
|
VATS L-MLND
|
video-assisted thoracoscopic surgical
lobectomy with mediastinal lymph node dissection
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Islami F, Torre LA and Jemal A: Global
trends of lung cancer mortality and smoking prevalence. Transl Lung
Cancer Res. 4:327–338. 2015.PubMed/NCBI
|
|
3
|
Koch M, Gräfenstein L, Karnosky J, Schulz
C and Koller M: Psychosocial burden and quality of life of lung
cancer patients: Results of the EORTC QLQ-C30/QLQ-LC29
questionnaire and hornheide screening instrument. Cancer Manag Res.
13:6191–6197. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu T, Yang X, Huang Y, Zhao M, Li M, Ma K,
Yin J, Zhan C and Wang Q: Trends in the incidence, treatment, and
survival of patients with lung cancer in the last four decades.
Cancer Manag Res. 11:943–953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Besse B, Adjei A, Baas P, Meldgaard P,
Nicolson M, Paz-Ares L, Reck M, Smit EF, Syrigos K, Stahel R, et
al: 2nd ESMO consensus conference on lung cancer: Non-small-cell
lung cancer first-line/second and further lines of treatment in
advanced disease. Ann Oncol. 25:1475–1484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan WL, Jain A, Takano A, Newell EW, Iyer
NG, Lim WT, Tan EH, Zhai W, Hillmer AM, Tam WL and Tan DSW: Novel
therapeutic targets on the horizon for lung cancer. Lancet Oncol.
17:e347–e362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palma D, Visser O, Lagerwaard FJ,
Belderbos J, Slotman BJ and Senan S: Impact of introducing
stereotactic lung radiotherapy for elderly patients with stage I
non-small-cell lung cancer: A population-based time-trend analysis.
J Clin Oncol. 28:5153–5159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palma DA and Senan S: Improving outcomes
for high-risk patients with early-stage non-small-cell lung cancer:
Insights from population-based data and the role of stereotactic
ablative radiotherapy. Clin Lung Cancer. 14:1–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Potters L, Kavanagh B, Galvin JM, Hevezi
JM, Janjan NA, Larson DA, Mehta MP, Ryu S, Steinberg M, Timmerman
R, et al: American society for therapeutic radiology and oncology
(ASTRO) and American college of radiology (ACR) practice guideline
for the performance of stereotactic body radiation therapy. Int J
Radiat Oncol Biol Phys. 76:326–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Timmerman R, Paulus R, Galvin J, Michalski
J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone
D, et al: Stereotactic body radiation therapy for inoperable early
stage lung cancer. JAMA. 303:1070–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Senthi S, Haasbeek CJA, Slotman BJ and
Senan S: Outcomes of stereotactic ablative radiotherapy for central
lung tumours: A systematic review. Radiother Oncol. 106:276–282.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ball D, Mai GT, Vinod S, Babington S,
Ruben J, Kron T, Chesson B, Herschtal A, Vanevski M, Rezo A, et al:
Stereotactic ablative radiotherapy versus standard radiotherapy in
stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): A phase 3,
open-label, randomised controlled trial. Lancet Oncol. 20:494–503.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang JY, Senan S, Paul MA, Mehran RJ,
Louie AV, Balter P, Groen HJM, McRae SE, Widder J, Feng L, et al:
Stereotactic ablative radiotherapy versus lobectomy for operable
stage I non-small-cell lung cancer: A pooled analysis of two
randomised trials. Lancet Oncol. 16:630–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang JY, Mehran RJ, Feng L, Verma V, Liao
Z, Welsh JW, Lin SH, O'Reilly MS, Jeter MD, Balter PA, et al:
Stereotactic ablative radiotherapy for operable stage I
non-small-cell lung cancer (revised STARS): Long-term results of a
single-arm, prospective trial with prespecified comparison to
surgery. Lancet Oncol. 22:1448–1457. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altorki N, Wang X, Damman B, Mentlick J,
Landreneau R, Wigle D, Jones DR, Conti M, Ashrafi AS, Liberman M,
et al: Lobectomy, segmentectomy, or wedge resection for peripheral
clinical T1aN0 non-small cell lung cancer: A post hoc analysis of
CALGB 140503 (Alliance). J Thorac Cardiovasc Surg. 167:338–347.e1.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raz DJ, Zell JA, Ou S-HI, Gandara DR,
Anton-Culver H and Jablons DM: Natural history of stage I non-small
cell lung cancer: Implications for early detection. Chest.
132:193–199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baine MJ, Verma V, Schonewolf CA, Lin C
and Simone CB II: Histology significantly affects recurrence and
survival following SBRT for early stage non-small cell lung cancer.
Lung Cancer. 118:20–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Yang F, Li B, Li H, Liu J, Huang
W, Wang D, Yi Y and Wang J: Which is the optimal biologically
effective dose of stereotactic body radiotherapy for Stage I
non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol
Biol Phys. 81:e305–e316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Birim O, Kappetein AP and Bogers AJJC:
Charlson comorbidity index as a predictor of long-term outcome
after surgery for nonsmall cell lung cancer. Eur J Cardiothorac
Surg. 28:759–762. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Birim O, Maat APWM, Kappetein AP, van
Meerbeeck JP, Damhuis RAM and Bogers AJJC: Validation of the
Charlson comorbidity index in patients with operated primary
non-small cell lung cancer. Eur J Cardiothorac Surg. 23:30–34.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th Edition.
Wiley-Blackwell; Hoboken, NJ, USA: pp. 129–136. 2016
|
|
24
|
Cerfolio RJ, Ojha B, Bryant AS, Raghuveer
V, Mountz JM and Bartolucci AA: The accuracy of integrated PET-CT
compared with dedicated PET alone for the staging of patients with
nonsmall cell lung cancer. Ann Thorac Surg. 78:1017–1023. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clarke K, Taremi M, Dahele M, Freeman M,
Fung S, Franks K, Bezjak A, Brade A, Cho J, Hope A and Sun A:
Stereotactic body radiotherapy (SBRT) for non-small cell lung
cancer (NSCLC): Is FDG-PET a predictor of outcome? Radiother Oncol.
104:62–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Freeman M, Ennis M and Jerzak KJ:
Karnofsky performance status (KPS) ≤60 is strongly associated with
shorter brain-specific progression-free survival among patients
with metastatic breast cancer with brain metastases. Front Oncol.
12:8674622022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buccheri G, Ferrigno D and Tamburini M:
Karnofsky and ECOG performance status scoring in lung cancer: A
prospective, longitudinal study of 536 patients from a single
institution. Eur J Cancer. 32A:1135–1141. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agustí A, Celli BR, Criner GJ, Halpin D,
Anzueto A, Barnes P, Bourbeau J, Han MK, Martinez FJ, Montes de Oca
M, et al: Global initiative for chronic obstructive lung disease
2023 report: GOLD executive summary. Am J Respir Crit Care Med.
207:819–837. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mirza S, Clay RD, Koslow MA and Scanlon
PD: COPD guidelines: A review of the 2018 GOLD report. Mayo Clin
Proc. 93:1488–1502. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Charlson ME, Carrozzino D, Guidi J and
Patierno C: Charlson comorbidity index: A critical review of
clinimetric properties. Psychother Psychosom. 91:8–35. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fowler JF: The linear-quadratic formula
and progress in fractionated radiotherapy. Br J Radiol. 62:679–694.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klement RJ, Sonke JJ, Allgäuer M,
Andratschke N, Appold S, Belderbos J, Belka C, Blanck O, Dieckmann
K, Eich HT, et al: Correlating dose variables with local tumor
control in stereotactic body radiation therapy for early-stage
non-small cell lung cancer: A modeling study on 1500 individual
treatments. Int J Radiat Oncol Biol Phys. 107:579–586. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Z: Variable selection with stepwise
and best subset approaches. Ann Transl Med. 4:1362016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Henschke CI, Yip R, Sun Q, Li P, Kaufman
A, Samstein R, Connery C, Kohman L, Lee P, Tannous H, et al:
Prospective cohort study to compare long-term lung cancer-specific
and all-cause survival of clinical early stage (T1a-b; ≤20 mm)
NSCLC treated by stereotactic body radiation therapy and surgery. J
Thorac Oncol. 19:476–490. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fan S, Zhang Q, Chen J, Chen G, Zhu J, Li
T, Xiao H, Du S, Zeng Z and He J: Comparison of long-term outcomes
of stereotactic body radiotherapy (SBRT) via Helical tomotherapy
for early-stage lung cancer with or without pathological proof.
Radiat Oncol. 18:492023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Speicher PJ and D'Amico TA: Weighing the
relative importance of short-term versus long-term outcomes when
comparing surgery versus stereotactic body radiation therapy (SBRT)
for early-stage non-small cell lung cancer. J Thorac Dis. 10 (Suppl
17):S2022–S2024. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arnett ALH, Mou B, Owen D, Park SS, Nelson
K, Hallemeier CL, Sio T, Garces YI, Olivier KR and Merrell KW:
Long-term clinical outcomes and safety profile of SBRT for
centrally located NSCLC. Adv Radiat Oncol. 4:422–428. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guckenberger M, Allgäuer M, Appold S,
Dieckmann K, Ernst I, Ganswindt U, Holy R, Nestle U,
Nevinny-Stickel M, Semrau S, et al: Safety and efficacy of
stereotactic body radiotherapy for stage 1 non-small-cell lung
cancer in routine clinical practice: A patterns-of-care and outcome
analysis. J Thorac Oncol. 8:1050–1058. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jimenez-Jimenez E, Marti-Laosa MM,
Nieto-Guerrero JM, Perez ME, Gómez M, Lozano E and Sabater S:
Biologically effective dose (BED) value lower than 120 Gy improve
outcomes in lung SBRT. Clin Transl Oncol. 26:1203–1208. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kreinbrink P, Blumenfeld P, Tolekidis G,
Sen N, Sher D and Marwaha G: Lung stereotactic body radiation
therapy (SBRT) for early-stage non-small cell lung cancer in the
very elderly (≥80 years old): Extremely safe and effective. J
Geriatr Oncol. 8:351–355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pop DD, Hopîrtean C, Coşer F, Dan F, Zah
T, Fekete Z, Chiş A, Tufăscu G, Udrea A and Mihai A: Implementation
of advanced radiotherapy techniques: Stereotactic body radiotherapy
(SBRT) for oligometastatic patients with lung metastasis-a single
institution experience. Med Pharm Rep. 95:410–417. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo Y, Zhu Y, Zhang R, Yang S, Kepka L,
Viani GA, Milano MT, Sio TT, Sun X, Wu H, et al: Five-year
follow-up after stereotactic body radiotherapy for medically
inoperable early-stage non-small cell lung cancer: A multicenter
study. Transl Lung Cancer Res. 12:1293–1302. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Onishi H, Shioyama Y, Matsumoto Y, Matsuo
Y, Miyakawa A, Yamashita H, Matsushita H, Aoki M, Nihei K, Kimura
T, et al: Real-world results of stereotactic body radiotherapy for
399 medically operable patients with stage I histology-proven
non-small cell lung cancer. Cancers (Basel). 15:43822023.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Snider M, Salama JK and Boyer M: Survival
and recurrence rates following SBRT or surgery in medically
operable stage I NSCLC. Lung Cancer. 197:1079622024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wong OY, Yau V, Kang J, Glick D, Lindsay
P, Le LW, Sun A, Bezjak A, Cho BCJ, Hope A and Giuliani M: Survival
impact of cardiac dose following lung stereotactic body
radiotherapy. Clin Lung Cancer. 19:e241–e246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baker S, Sharma A, Peric R, Heemsbergen WD
and Nuyttens JJ: Prediction of early mortality following
stereotactic body radiotherapy for peripheral early-stage lung
cancer. Acta Oncol. 58:237–242. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Eriguchi T, Takeda A, Sanuki N, Tsurugai
Y, Aoki Y, Oku Y, Hara Y, Akiba T and Shigematsu N: Stereotactic
body radiotherapy for operable early-stage non-small cell lung
cancer. Lung Cancer. 109:62–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ganti AK, Siedlik E, Marr AS, Loberiza FR
Jr and Kessinger A: Predictive ability of Charlson comorbidity
index on outcomes from lung cancer. Am J Clin Oncol. 34:593–596.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wilkie JR, Lipson R, Johnson MC, Williams
C, Moghanaki D, Elliott D, Owen D, Atluri N, Jolly S and Chapman
CH: Use and outcomes of SBRT for early stage NSCLC without
pathologic confirmation in the veterans health care administration.
Adv Radiat Oncol. 6:1007072021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Moraes FY, Abreu CECV, Siqueira GSM,
Haddad CK, Degrande FAM, Hopman WM, Neves-Junior WFP, Gadia R and
Carvalho HA: Applying PET-CT for predicting the efficacy of SBRT to
inoperable early-stage lung adenocarcinoma: A Brazilian
case-series. Lancet Reg Health Am. 11:1002412022.PubMed/NCBI
|
|
51
|
Mahasittiwat P, Yuan S, Xie C, Ritter T,
Cao Y, Ten Haken RK and Kong FMS: Metabolic tumor volume on PET
reduced more than gross tumor volume on CT during radiotherapy in
patients with non-small cell lung cancer treated with 3DCRT or
SBRT. J Radiat Oncol. 2:191–202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chirindel A, Adebahr S, Schuster D,
Schimek-Jasch T, Schanne DH, Nemer U, Mix M, Meyer P, Grosu AL,
Brunner T and Nestle U: Impact of 4D-(18)FDG-PET/CT imaging on
target volume delineation in SBRT patients with central versus
peripheral lung tumors. Multi-reader comparative study. Radiother
Oncol. 115:335–341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hoopes DJ, Tann M, Fletcher JW, Forquer
JA, Lin PF, Lo SS, Timmerman RD and McGarry RC: FDG-PET and
stereotactic body radiotherapy (SBRT) for stage I non-small-cell
lung cancer. Lung Cancer. 56:229–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen K, Hou L, Chen M, Li S, Shi Y, Raynor
WY and Yang H: Predicting the efficacy of SBRT for lung cancer with
18F-FDG PET/CT radiogenomics. Life (Basel).
13:8842023.PubMed/NCBI
|
|
55
|
Thor M, Fitzgerald K, Apte A, Oh JH, Iyer
A, Odiase O, Nadeem S, Yorke ED, Chaft J, Wu AJ, et al: Exploring
published and novel pre-treatment CT and PET radiomics to stratify
risk of progression among early-stage non-small cell lung cancer
patients treated with stereotactic radiation. Radiother Oncol.
190:1099832024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yu X, He L, Wang Y, Dong Y, Song Y, Yuan
Z, Yan Z and Wang W: A deep learning approach for automatic tumor
delineation in stereotactic radiotherapy for non-small cell lung
cancer using diagnostic PET-CT and planning CT. Front Oncol.
13:12354612023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu L, Zhang Z, Yi H, Wang J, Li J, Wang X,
Bai H, Ge H, Zheng X, Ni J, et al: A PET/CT radiomics model for
predicting distant metastasis in early-stage non-small cell lung
cancer patients treated with stereotactic body radiotherapy: A
multicentric study. Radiat Oncol. 19:102024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tyran M, Charrier N, Darreon J, Madroszyk
A, Tallet A and Salem N: Early PET-CT after stereotactic
radiotherapy for stage 1 non-small cell lung carcinoma is
predictive of local control. In Vivo. 32:121–124. 2018.PubMed/NCBI
|
|
59
|
Borm KJ, Oechsner M, Schiller K, Peeken
JC, Dapper H, Münch S, Kroll L, Combs SE and Duma MN: Prognostische
Faktoren bei der stereotaktischen Strahlentherapie von
Lungenmetastasen. Strahlenther Onkol. 194:886–893. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Retif P, Verrecchia-Ramos E, Saleh M,
Djibo Sidikou A, Letellier R, Al Salah A, Pfletschinger E, Taesch
F, Ben-Mahmoud S and Michel X: On the use of 4D-PET/CT for the safe
SBRT re-irradiation of central lung recurrence within
radiation-induced fibrosis: A clinical case. J Clin Med.
14:40152025. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kessel KA, Grosser RCE, Kraus KM, Hoffmann
H, Oechsner M and Combs SE: Stereotactic body radiotherapy (SBRT)
in patients with lung metastases-prognostic factors and long-term
survival using patient self-reported outcome (PRO). BMC Cancer.
20:4422020. View Article : Google Scholar : PubMed/NCBI
|