Introduction
Oral squamous cell carcinoma (OSCC) is the most
common malignancy of the oral cavity and accounts for approximately
90% of all oral cancers (1–3). It represents a major subgroup of head
and neck squamous cell carcinomas (HNSCCs), which are among the
sixth most common cancers worldwide, with over 500,000 new cases
annually (2). Despite advances in
surgery, chemotherapy, and radiotherapy, the prognosis of patients
with recurrent or metastatic OSCC (R/M-OSCC) remains poor.
Since the 1980s, platinum-based chemotherapy
regimens have served as the standard treatment for advanced head
and neck cancers. The EXTREME regimen, which combines
5-fluorouracil, cisplatin, and cetuximab, became a standard of care
in 2012 (4). More recently, immune
checkpoint inhibitors (ICIs), such as nivolumab and pembrolizumab,
targeting the programmed death-1 (PD-1)/programmed death-ligand 1
(PD-L1) pathway, have expanded the therapeutic landscape and shown
promising efficacy in R/M-HNSCC, including OSCC (5,6). These
agents are now widely used in clinical practice in Japan and
globally. However, ICIs are not universally effective, and their
clinical benefit is limited to a subset of patients (7). Therefore, there is a pressing need to
identify reliable biomarkers to predict which patients are most
likely to respond to ICI therapy. While previous studies have
proposed PD-L1 expression, tumor mutational burden, and
tumor-infiltrating lymphocytes (TILs) as candidate predictive
biomarkers, none have demonstrated sufficient sensitivity or
specificity for routine clinical use (8–10).
In recent years, inflammation- and nutrition-based
biomarkers have emerged as potential prognostic indicators in
various malignancies, including head and neck cancers (11–25).
These include the neutrophil-to-lymphocyte ratio (NLR) (13–16,23,25–30),
platelet-to-lymphocyte ratio (PLR) (14–18,23,24),
lymphocyte-to-monocyte ratio (LMR) (25), C-reactive protein-to-albumin ratio
(CAR) (12,20), prognostic nutritional index (PNI)
(31,32), and modified Glasgow Prognostic Score
(mGPS) (22,24). These markers are simple to calculate
using routinely available laboratory data and reflect the complex
interplay between systemic inflammation, nutritional status, and
cancer progression. Although several reports have explored the
prognostic significance of these markers in HNSCC, limited data
exist regarding their role in predicting the response and outcomes
of ICI treatment specifically in patients with OSCC. Moreover, it
remains unclear whether the timing of biomarker evaluation, before
or after ICI initiation, affects their prognostic utility.
Therefore, the aim of this retrospective study was
to evaluate the clinical relevance of inflammation- and
nutrition-based biomarkers as predictors of treatment response and
prognosis in patients with R/M-OSCC receiving ICI therapy. We also
investigated whether biomarker levels assessed after ICI
administration offer superior prognostic value compared to those
assessed prior to treatment.
Materials and methods
Study design and population
We conducted a retrospective analysis of the
clinical data of 45 patients with R/M-OSCC treated with ICIs
between October 2017 and December 2024 at Hiroshima University
Hospital (Hiroshima, Japan). Eligible patients were aged ≥18 years,
had histologically confirmed OSCC arising exclusively from the oral
cavity (excluding oropharyngeal subsites, such as the soft palate,
base of the tongue, and tonsillar region; this definition aligns
with previous clinical studies that focus solely on cancers of the
oral cavity), received at least one cycle of ICI therapy, and had
adequate clinical data including laboratory results and follow-up
information. Patients were excluded if they had concurrent
malignancies, active autoimmune diseases, or insufficient medical
records. The observation period was defined as the interval from
the initiation of ICI therapy to the date of death, last follow-up,
or the data cutoff (February 1, 2025), whichever came first.
Data collection and definitions
We assessed the following parameters: Age, sex,
Eastern Cooperative Oncology Group Performance Status (ECOG PS),
primary tumor site, disease stage, presence of target lesions,
treatment line, history of surgery, history of radiotherapy,
combined positive score (CPS), chemotherapy regimen and dose,
initial treatment, presence or absence of radiotherapy, observation
period, number of treatment cycles, IBPSs before and after
treatment, overall survival (OS), one-year and two-year survival
rates, progression-free survival (PFS), objective response rate
(ORR), disease control rate (DCR), best overall response, presence
or absence of immune-related adverse events (irAEs), and details of
irAEs. All patients were staged according to the eighth edition of
the International Union Against Cancer (UICC) TNM staging system
(33). In this study, OSCC was
defined as squamous cell carcinoma that arises exclusively from the
oral cavity, excluding oropharyngeal subsites, such as the soft
palate, base of the tongue, and tonsillar region.
The IBPSs were calculated as follows: the NLR was
determined by dividing the absolute neutrophil count
(/mm3) by the absolute lymphocyte count
(/mm3); LMR was calculated by dividing the absolute
lymphocyte count (/mm3) by the absolute monocyte count
(/mm3); PLR was determined by dividing the absolute
platelet count (/mm3) by the absolute lymphocyte count
(/mm3); CAR was calculated by dividing the C-reactive
protein (CRP) concentration (mg/dl) by the serum albumin
concentration (g/dL); and PNI was calculated as 10× serum albumin
concentration (g/dl) + 0.005× total lymphocyte count
(/mm3). These values were obtained from the blood test
results obtained within 1 week prior to the first day of ICI
administration and 4–6 weeks after the initiation of ICI
therapy.
In our department, the treatment strategy for
R/M-OSCC is guided by platinum sensitivity. For platinum-resistant
cases, nivolumab was administered intravenously at a dose of 3
mg/kg (between 2017 and September 2018) and at 240 mg every two
weeks (from October 2018 to February 2025). For platinum-sensitive
cases, pembrolizumab was used either as monotherapy or in
combination therapy. In platinum-sensitive patients with adequate
tolerance for combination therapy involving platinum agents and
5-fluorouracil (5-FU), treatment selection was based on the CPS.
Specifically, PD-L1 expression was evaluated using
immunohistochemistry with the Dako 22C3 pharmDx assay on
formalin-fixed, paraffin-embedded tumor specimens. The CPS was
calculated by dividing the number of PD-L1-positive cells
(including tumor cells, lymphocytes, and macrophages) by the total
number of viable tumor cells, then multiplying by 100. A CPS of ≥1
was considered positive, in accordance with established diagnostic
criteria. All CPS evaluations were conducted by board-certified
oral pathologists with expertise in head and neck malignancies.
Pembrolizumab monotherapy was administered if CPS was ≥20%, while
pembrolizumab was combined with cisplatin (80 mg/m2) and
5-FU (800 mg/m2) for CPS values of 1–19 and <1%,
respectively. However, treatment decisions were made
comprehensively, considering factors such as age, comorbidities,
tumor growth rate, and overall performance status. In cases where
patients were unable to tolerate platinum-based agents or 5-FU,
pembrolizumab monotherapy or the EXTREME regimen was considered as
alternative treatment options. Following the discontinuation of
ICIs, subsequent chemotherapy was administered at the physician's
discretion.
Treatment efficacy was assessed using the Response
Evaluation Criteria in Solid Tumors version 1.1 (34). Following the administration of ICIs,
imaging studies, including computed tomography, magnetic resonance
imaging, and F-fluorodeoxyglucose positron emission tomography,
were performed every two to three months or as clinically
indicated. The DCR was calculated as the sum of the complete
response (CR), partial response (PR), and stable disease (SD)
rates. OS was defined as the time from the first day of ICI
administration to the date of death or the date of the final
analysis (February 1, 2025), whichever was earlier. PFS was defined
as the time from the first day of ICI administration to the date of
disease progression or death.
Statistical analysis
To determine the cutoff values of biomarkers before
and after treatment, receiver operating characteristic (ROC) curves
were constructed for disease control (CR + PR + SD). OS and PFS
were compared using the log-rank test and Kaplan-Meier survival
curves. P-values were calculated using the log-rank test.
Univariate and multivariate analyses were conducted to identify
prognostic factors, with the multivariate analysis performed using
the Cox proportional hazards model to determine predictors of OS
and PFS. P<0.05 was considered to indicate a statistically
significant difference. P-values were determined using chi-square
tests to assess the association between categorical variables. In
cases with small sample sizes or expected cell counts, Fisher's
exact test was employed to ensure accurate statistical inference.
All statistical analyses were performed using SPSS Statistics
(version 30.0; IBM Corp., Armonk, NY, USA).
Ethics statements
This study was conducted in accordance with the
principles of The Declaration of Helsinki and was approved by the
Ethics Committee of Hiroshima University (approval no. E2024-0196).
The requirement for informed consent was waived by the Ethics
Committee owing to the retrospective design of the study.
Results
Patient characteristics and treatment
outcomes
A total of 45 patients were enrolled in the study
(Table SI). The age of the
patients ranged from 35 to 84 years, with a median of 67 years. The
cohort consisted of 29 (64%) males and 16 (36%) females. Regarding
ECOG PS, 34 patients had a PS of 0, six had a PS of 1, one had a PS
of 2, and four had a PS of 3. The primary tumor site was
predominantly the tongue, observed in 24 (53%) patients. Other
primary sites included the maxillary gingiva in six (13%) patients,
mandibular gingiva in five (11%) patients, and buccal mucosa and
floor of the mouth in four (9%) patients each. At the initial
diagnosis, four patients were classified as stage I, 13 as stage
II, three as stage III, and 24 as stage IV. Nivolumab was
administered to 23 (51%) patients, while pembrolizumab was used in
22 (49%) patients. Surgical resection was performed as the initial
treatment in 42 (93%) patients, and radiotherapy was administered
to 39 (87%) patients. Of the 42 patients who underwent surgery, 41
had neck dissections, and one had surgery on the primary tumor. In
the one case, the patient discontinued outpatient follow-up after
primary tumor resection. Upon re-presentation, disease progression
was observed, and curative surgery was considered unfeasible due to
local recurrence and cervical lymph node metastasis. Therefore,
treatment with an ICI was initiated. Regarding target lesions,
distant metastases were present in 21 (47%) patients, whereas 12
(27%) patients had local residual disease. CPS expression of ≥ 20%
was observed in 29 (64%) patients. Regarding ICI administration,
monotherapy was used in 33 (73%) patients, while 12 (27%) patients
received combination therapy with chemotherapy.
The number of ICI administrations ranged from one to
68, with a median of five (Table
SII). Treatment efficacy was evaluated according to the
Response Evaluation Criteria in Solid Tumors criteria. CR was
observed in four (9%) patients, PR in six (13%) patients, SD in
five (11%) patients, and progressive disease in 30 (67%) patients.
The ORR, calculated as the sum of CR and PR, was 22%, while the
DCR, defined as the sum of CR, PR, and SD, was 33%. irAEs occurred
in 13 (29%) patients. The most common irAE was thyroid dysfunction,
observed in four (9%) patients, followed by interstitial pneumonia
in three (7%) patients and colitis in two (4%) patients. Other
reported adverse events included dermatologic disorders,
interstitial nephritis, hypopituitarism, neurological impairment,
atrial fibrillation, and acute kidney failure.
Survival analysis for OS and PFS after
ICI administration
Figure 1 presents the results of OS analysis. In the
overall cohort of ICI-treated patients, the 1-year survival rate
was 40%, 2-year survival rate was 22%, and median OS was 8.1 months
[95% confidence interval (CI): 5.2–14.2] (Fig. 1A). For patients receiving ICI
monotherapy, the median OS was 7.5 months (95% CI: 016.2), whereas
for those receiving ICI in combination with chemotherapy, the
median OS was 9.7 months (95% CI: 7.1–12.2), with a log-rank test
P-value of 0.788 (Fig. 1B).
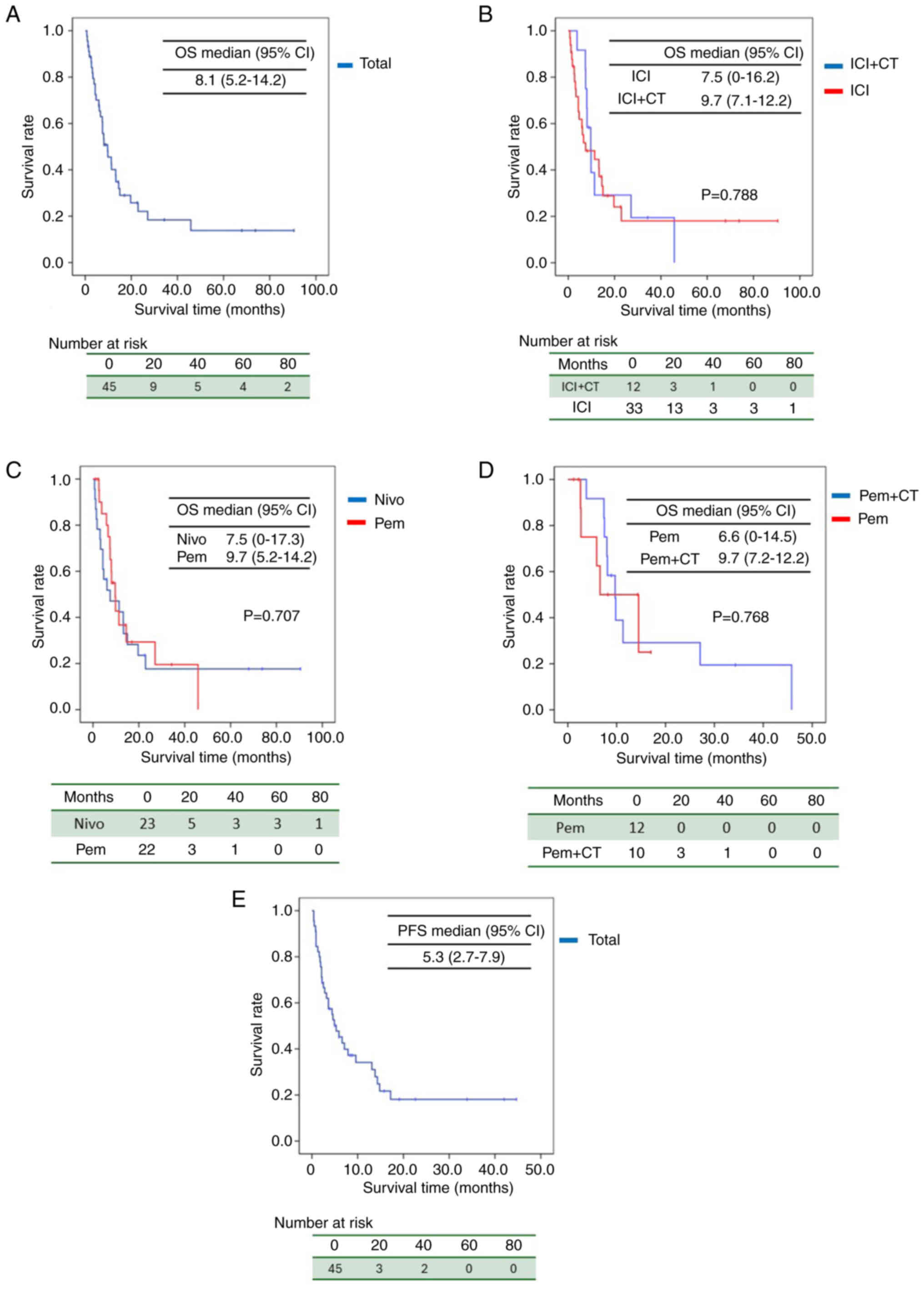 | Figure 1.Kaplan-Meier curves for OS. (A) OS of
the ICI-treated cohort, (B) OS of patients receiving ICI
monotherapy or in combination with chemotherapy, (C) OS of patients
treated with nivolumab or pembrolizumab, (D) OS of patients treated
with pembrolizumab or in combination with chemotherapy, and (E) PFS
of the ICI-treated cohort. ICI, immune checkpoint inhibitor; OS,
overall survival; PFS, progression-free survival; CI, confidence
interval; CT, chemotherapy; Nivo, nivolumab; Pem,
pembrolizumab. |
For patients treated with nivolumab, the median OS
was 7.5 months (95% CI: 0–17.3), whereas for those treated with
pembrolizumab, the median OS was 9.7 months (95% CI: 5.2–14.2),
with a P-value of 0.707 (Fig. 1C).
Among patients treated with pembrolizumab, those receiving
chemotherapy alone had a median OS of 6.6 months (95% CI: 0–14.5),
whereas those receiving pembrolizumab in combination with
chemotherapy had a median OS of 9.7 months (95% CI: 7.2–12.2)
(Fig. 1D). Similar to the findings
of the KEYNOTE-048 trial, a trend toward prolonged OS was observed
with the addition of chemotherapy. The one-year and two-year PFS
rates for all patients were 34 and 6.7%, respectively, with a
median PFS of 5.3 months (Fig.
1E).
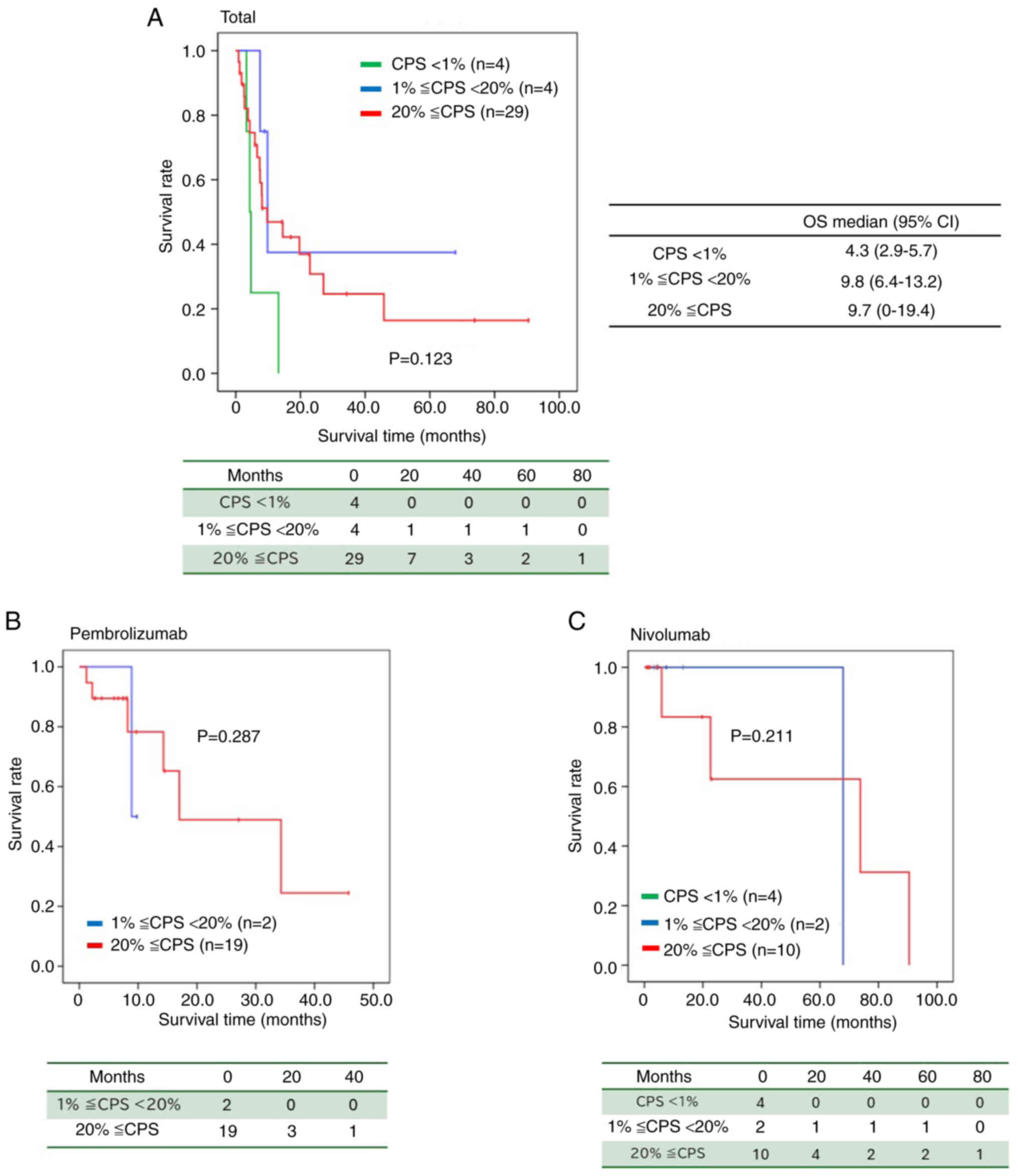
Figure 2 illustrates the OS according to CPS values.
In patients with CPS <1, the median OS was 4.3 months (95% CI:
2.9–5.7), whereas for those with CPS between 1 and <20, the
median OS was 9.8 months (95% CI: 6.4–13.2). For patients with CPS
≥20, the median OS was 9.7 months (95% CI: 0–19.4), with a P-value
of 0.123 (Fig. 2A).
Furthermore, in patients treated with pembrolizumab,
those with CPS between 1 and <20 had a median OS of 8.9 months,
whereas those with CPS ≥20 had a median OS of 17.0 months (95% CI:
0–34.6) (P=0.287) (Fig. 2B).
Compared with patients with CPS <1, those with CPS ≥1 tended to
have a prolonged median OS (Fig.
2A-C).
Cutoff values for pre- and
post-treatment markers according to ROC curve
ROC curves were constructed to assess the
discriminatory ability of biomarkers before and after treatment,
and the area under the curve (AUC) was calculated (Fig. S1). The AUC values for pre-treatment
markers were as follows: 0.53802 for pre-NLR, 0.4735 for pre-LMR,
0.59217 for pre-PNI, 0.50691 for pre-PLR, 0.39516 for pre-CAR,
0.55876 for pre-eosinophils, and 0.58641 for pre-lymphocytes. In
contrast, the AUC values for post-treatment markers were 0.76959
for post-NLR, 0.68779 for post-LMR, 0.69585 for post-PNI, 0.64747
for post-PLR, 0.73618 for post-CAR, 0.51382 for post-eosinophils,
and 0.69935 for post-lymphocytes. Among all markers evaluated
before and after treatment, post-treatment NLR demonstrated the
highest AUC of 0.76959, with a cutoff value of 7.17 (Table SIII).
Univariate and multivariate analyses
results of prognostic factors for OS and PFS
We evaluated the impact of various factors on OS,
including age, sex, ECOG PS, type of ICI, presence or absence of
irAEs, ICI combination therapy, history of radiotherapy, body mass
index (BMI) based on cutoff values calculated from the ROC curve,
CPS value, DCR, and primary site (tongue vs. other), as shown in
Table I. In univariate analysis, a
higher ECOG PS was associated with poorer OS [hazard ratio
(HR)=2.50; 95% CI=1.17–5.33; P=0.0185], and patients with PD as the
efficacy of ICI had poorer OS (HR=4.30; 95% CI=1.79–10.33;
P=0.0011). Conversely, in multivariate analysis, male sex was
associated with poorer OS (HR=3.37; 95% CI=1.11–10.19; P=0.0316),
and patients with PD as the efficacy of ICI demonstrated poorer OS
(HR=6.96; 95% CI=1.80–27.03; P=0.0050).
 | Table I.Univariate and multivariate analysis
of prognosis factors for OS. |
Table I.
Univariate and multivariate analysis
of prognosis factors for OS.
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Variable | Number | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
<65 | 20 | Ref. |
|
|
|
|
|
|
≥65 | 25 | 1.06 | 0.53–2.14 | 0.864 |
|
|
|
| Sex |
|
|
|
|
|
|
|
|
Male | 29 | 1.46 | 0.70–3.04 | 0.298 | 3.37 | 1.11–10.19 | 0.0316a |
|
Female | 16 | Ref. |
|
|
|
|
|
| ECOG PS |
|
|
|
|
|
|
|
| 0 | 34 | Ref. |
|
|
|
|
|
| ≥1 | 11 | 2.50 | 1.17–5.33 | 0.0185a |
|
|
|
| ICI |
|
|
|
|
|
|
|
|
Nivolumab | 23 | 1.14 | 0.57–2.28 | 0.707 |
|
|
|
|
Pembrolizumab | 22 | Ref. |
|
|
|
|
|
| irAE |
|
|
|
|
|
|
|
|
Positive | 13 | Ref. |
|
|
|
|
|
|
Negative | 32 | 1.18 | 0.47–2.96 | 0.736 |
|
|
|
| ICI
combination |
|
|
|
|
|
|
|
|
Monotherapy | 33 | Ref. |
|
|
|
|
|
| With
chemotherapy | 12 | 1.09 | 0.41–2.86 | 0.865 |
|
|
|
| Radiation |
|
|
|
|
|
|
|
|
Previously | 39 | Ref. |
|
|
|
|
|
| No | 6 | 2.09 | 0.82–5.28 | 0.146 |
|
|
|
| BMI,
kg/m2 |
|
|
|
|
|
|
|
|
<18.37 | 20 | 1.57 | 0.69–3.14 | 0.200 |
|
|
|
|
≥18.37 | 25 | Ref. |
|
|
|
|
|
| CPS |
|
|
|
|
|
|
|
|
<20 | 8 | 1.18 | 0.47–2.96 | 0.736 |
|
|
|
|
≥20 | 29 | Ref. |
|
|
|
|
|
| DCR |
|
|
|
|
|
|
|
|
CR/PR/SD | 15 | Ref. |
|
|
|
|
|
| PD | 40 | 4.30 | 1.79–10.33 | 0.0011a | 6.96 | 1.80–27.03 | 0.0050a |
| Primary site |
|
|
|
|
|
|
|
|
Tongue | 24 | Ref. |
|
|
|
|
|
|
Otherwise | 21 | 1.34 | 0.67–2.67 | 0.403 |
|
|
|
In the univariate analysis of the same variables for
PFS, ECOG PS, a history of radiotherapy, and DCR showed significant
differences. Specifically, higher ECOG PS was associated with
poorer PFS (HR=4.64; 95% CI=2.01–10.72; P=0.0003), while patients
without prior radiotherapy exhibited poorer PFS (HR=2.57; 95%
CI=1.03–6.44; P=0.043). Additionally, patients with PD as a
response to ICI were associated with poor PFS (HR=5.54; 95%
CI=2.15–14.27; P=0.0004). In the multivariate analysis, male sex
was associated with poorer PFS (HR=3.86; 95% CI=1.32–11.31;
P=0.0138). Furthermore, patients treated with nivolumab
demonstrated poorer PFS (HR=11.75; 95% CI=2.68–51.49; P=0.0011),
and those with PD as a response to ICI were also associated with
poor PFS (HR=9.15; 95% CI=2.38–35.14; P=0.0013). Lastly, patients
with primary tumors located in sites other than the tongue
demonstrated significantly shorter PFS than those with tongue
cancers (HR=4.87; 95% CI=1.51–15.72; P=0.0081) (Table SIV).
Univariate and multivariate analyses
results of pre- and post-treatment factors for OS and PFS
Univariate analysis was performed to evaluate the
association between pre-treatment factors, including age, sex, ECOG
PS, CPS value, ICI combination, prior radiotherapy, BMI, and IBPS
markers, encompassing both pre-treatment and post-treatment values
for NLR, LMR, PNI, PLR, CAR, eosinophils, lymphocytes, and mGPS,
and post-treatment factors, including the presence of irAE and IBPS
markers, with OS and PFS as endpoints (Tables II and SV). Regarding OS, only a higher ECOG PS
was associated with poor OS (HR=2.50; 95% CI=1.17–5.33; P=0.0185),
and no significant differences were observed in pre-IBPS markers in
the univariate analysis. Multivariate analysis indicated that
pre-IBPS markers were not associated with prognosis; only sex and
BMI were significantly linked to poor OS (Table II). However, 4–6 weeks after ICI
administration, significant differences were found in NLR, PNI,
CAR, and mGPS in the univariate analysis. Specifically, higher NLR
(HR=2.15; 95% CI=1.07–4.33; P=0.034), lower PNI (HR=4.02; 95%
CI=1.98–8.18; P=0.0002), higher CAR (HR=2.98; 95% CI=1.46–6.06;
P=0.0025), and a mGPS of 1 (HR=2.26; 95% CI=1.02–5.05; P=0.0341)
were associated with poorer OS. Additionally, higher NLR (HR=4.17;
95% CI=1.12–15.57; P=0.0337) and lower PNI (HR=3.92; 95%
CI=1.44–10.64; P=0.0073) were associated with poorer OS in
multivariate analysis.
 | Table II.Univariate and multivariate analysis
of pre- and post-treatment factors for OS. |
Table II.
Univariate and multivariate analysis
of pre- and post-treatment factors for OS.
| A, Pre-treatment
factors |
|---|
|
|---|
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Variable | Number | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
<65 | 20 | Ref. |
|
|
|
|
|
|
≥65 | 25 | 1.06 | 0.53–2.14 | 0.864 |
|
|
|
| Sex |
|
|
|
|
|
|
|
|
Male | 29 | 1.46 | 0.70–3.04 | 0.298 | 4.11 | 1.50–11.24 | 0.0059a |
|
Female | 16 | Ref. |
|
|
|
|
|
| ECOG PS |
|
|
|
|
|
|
|
| 0 | 34 | Ref. |
|
|
|
|
|
| ≥1 | 11 | 2.50 | 1.17–5.33 | 0.0185a |
|
|
|
| CPS |
|
|
|
|
|
|
|
|
<20 | 8 | 1.18 | 0.47–2.96 | 0.736 |
|
|
|
|
≥20 | 29 | Ref. |
|
|
|
|
|
| ICI
combination |
|
|
|
|
|
|
|
|
Mono | 33 | Ref. |
|
|
|
|
|
| With
chemotherapy | 12 | 1.09 | 0.41–2.86 | 0.865 |
|
|
|
| Radiation |
|
|
|
|
|
|
|
|
Previously | 39 | Ref. |
|
|
|
|
|
| No | 6 | 2.09 | 0.82–5.28 | 0.146 |
|
|
|
| BMI,
kg/m2 |
|
|
|
|
|
|
|
|
<18.37 | 20 | 1.57 | 0.69–3.14 | 0.200 | 3.33 | 1.22–9.08 | 0.0188a |
|
≥18.37 | 25 | Ref. |
|
|
|
|
|
| Pre-NLR |
|
|
|
|
|
|
|
|
<4.7 | 16 | Ref. |
|
|
|
|
|
|
≥4.7 | 29 | 1.42 | 0.69–2.90 | 0.338 |
|
|
|
| Pre-LMR |
|
|
|
|
|
|
|
|
<1.29 | 32 | 1.73 | 0.88–3.40 | 0.123 |
|
|
|
|
≥1.29 | 13 | Ref. |
|
|
|
|
|
| Pre-PNI |
|
|
|
|
|
|
|
|
<43.75 | 30 | 1.31 | 0.69–2.48 | 0.397 |
|
|
|
|
≥43.75 | 15 | Ref. |
|
|
|
|
|
| Pre-PLR |
|
|
|
|
|
|
|
|
<453.5 | 31 | Ref. |
|
|
|
|
|
|
≥453.5 | 14 | 1.18 | 0.62–2.29 | 0.630 |
|
|
|
| Pre-CAR |
|
|
|
|
|
|
|
|
<6.35 | 43 | Ref. |
|
|
|
|
|
|
≥6.35 | 2 | 4.33 | 0.99–18.99 | 0.100 |
|
|
|
| Pre-eosinophil |
|
|
|
|
|
|
|
|
<120 | 18 | Ref. |
|
|
|
|
|
|
≥120 | 27 | 1.21 | 0.65–2.22 | 0.550 |
|
|
|
|
Pre-lymphocytes |
|
|
|
|
|
|
|
|
<670 | 29 | 1.09 | 0.52–2.31 | 0.822 |
|
|
|
|
≥670 | 16 | Ref. |
|
|
|
|
|
|
| B,
Post-treatment factors |
|
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
|
Variable | Number | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| irAE |
|
|
|
|
|
|
|
|
Positive | 13 | Ref. |
|
|
|
|
|
|
Negative | 32 | 1.18 | 0.47–2.96 | 0.736 |
|
|
|
| Post-NLR |
|
|
|
|
|
|
|
|
<7.17 | 22 | Ref. |
|
|
|
|
|
|
≥7.17 | 23 | 2.15 | 1.07–4.33 | 0.034a | 4.17 | 1.12–15.57 | 0.0337 |
| Post-LMR |
|
|
|
|
|
|
|
|
<1.23 | 15 | 1.40 | 0.66–2.97 | 0.386 |
|
|
|
|
≥1.23 | 30 | Ref. |
|
|
|
|
|
| Post-PNI |
|
|
|
|
|
|
|
|
<36.6 | 16 | 4.02 | 1.98–8.18 | 0.0002a | 3.92 | 1.44–10.64 | 0.0073 |
|
≥36.6 | 29 | Ref. |
|
|
|
|
|
| Post-PLR |
|
|
|
|
|
|
|
|
<221.1 | 13 | Ref. |
|
|
|
|
|
|
≥221.1 | 32 | 1.34 | 0.62–2.91 | 0.442 |
|
|
|
| Post-CAR |
|
|
|
|
|
|
|
|
<0.44 | 22 | Ref. |
|
|
|
|
|
|
≥0.44 | 23 | 2.98 | 1.46–6.06 | 0.0025a |
|
|
|
|
Post-eosinophil |
|
|
|
|
|
|
|
|
<260 | 32 | 1.00 | 0.46–2.20 | 0.991 |
|
|
|
|
≥260 | 13 | Ref. |
|
|
|
|
|
|
Post-lymphocytes |
|
|
|
|
|
|
|
|
<770 | 21 | 1.26 | 0.62–2.56 | 0.529 |
|
|
|
|
≥770 | 25 | Ref. |
|
|
|
|
|
| Post-mGPS |
|
|
|
|
|
|
|
| 0 | 13 | Ref. |
|
|
|
|
|
| 1 | 33 | 2.26 | 1.02–5.05 | 0.0341a |
|
|
|
For PFS, no significant differences were observed
prior to ICI administration. Moreover, in univariate analysis,
pre-IBPS markers showed no association with prognosis, though
significant differences were noted in ECOG PS and history of
radiotherapy. In multivariate analysis, pre-IBPS markers were not
associated with prognosis; only sex was significantly correlated
with poorer PFS. However, four to six weeks after ICI
administration, significant differences were observed in NLR, PNI,
and CAR. Higher NLR (HR=2.21; 95% CI=1.14–4.29; P=0.014), lower PNI
(HR=3.25; 95% CI=1.61–6.55; P=0.0015), and higher CAR (HR=2.93; 95%
CI=1.44–5.96; P=0.0029) were associated with poorer PFS. Moreover,
significant differences were observed in NLR, LMR, and PNI in
multivariate analysis. Higher NLR (HR=3.80; 95% CI=1.11–13.02;
P=0.00339), lower PNI (HR=2.96; 95% CI=1.08–8.06; P=0.0342), and
higher LMR (HR=4.02; 95% CI=1.14–14.18; P=0.0304) were associated
with poorer PFS (Table SV).
Univariate analysis results for
prognostic factors for the DCR
A univariate analysis was conducted to evaluate
factors influencing the DCR (Table
SVI). Significant differences were observed in ECOG PS
(P=0.0018), CPS (P=0.007), BMI (P=0.032), and history of
radiotherapy (P=0.027). The relationship between each IBPS item
immediately prior to ICI administration and DCR was analyzed, but
no significant differences were observed for any of the items
(Table SVI). In contrast, when the
relationship between post-treatment IBPS items and DCR was analyzed
four to six weeks after ICI administration, significant differences
were found in post-NLR (P=0.0006), post-LMR (P=0.0065), post-PNI
(P=0.0358), post-PLR (P=0.0396), post-CAR (P=0.0062), and
post-lymphocytes (P=0.0321).
Kaplan-Meier curves of overall
survival according to each IBPS
Using the cutoff values determined from the ROC
curve for each IBPS marker before and after treatment, patients
were divided into two groups: High and Low. Then, Kaplan-Meier
survival curves were generated with OS as the endpoint. Significant
differences in OS were observed for the following markers: pre-NLR
(P=0.004), pre-LMR (P<0.001), pre-PLR (P=0.008), pre-CAR
(P=0.001), pre-lymphocytes (P=0.022), post-NLR (P=0.042), post-LMR
(P=0.036), and post-lymphocytes (P=0.007) (Fig. 3).
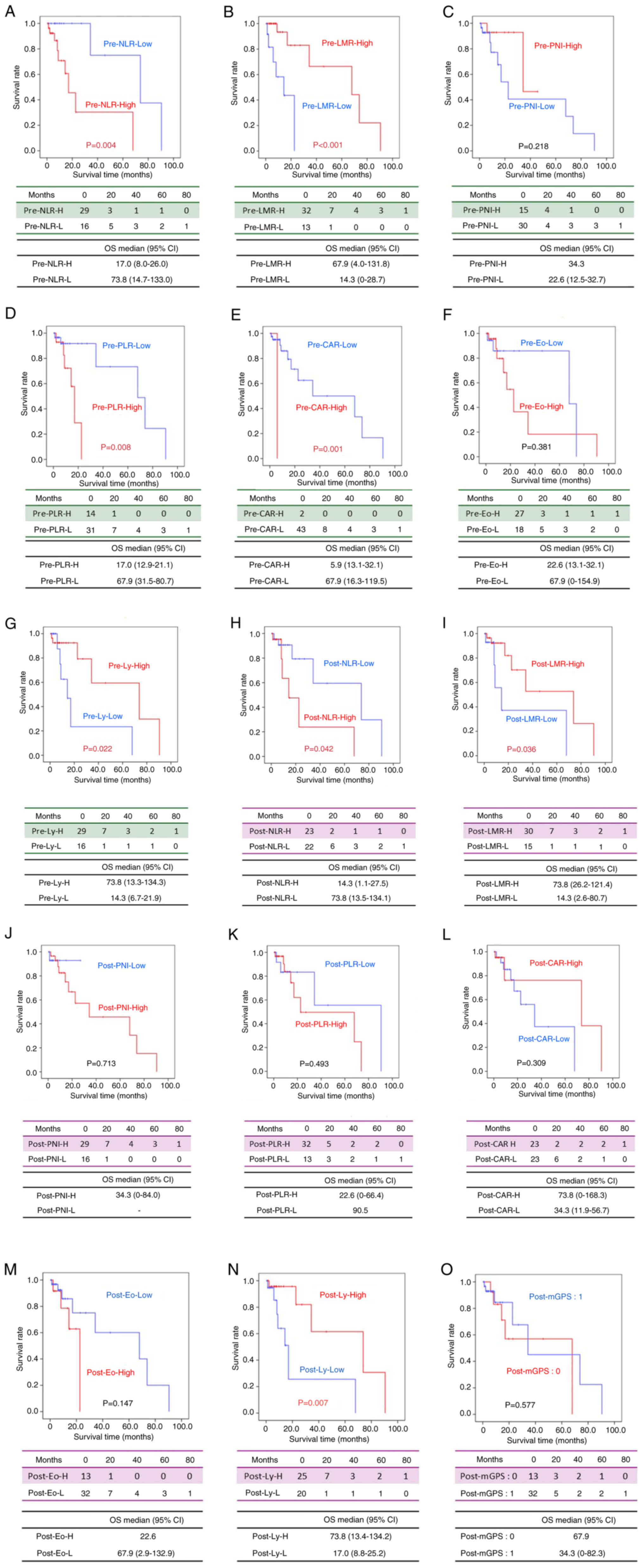 | Figure 3.Kaplan-Meier curves of OS according
to each inflammation-based prognostic score. OS according to the
(A) pre-NLR (A), (B) pre-LMR, (C) pre-PNI, (D) pre-PLR, (E)
pre-CAR, (F) pre-Eo, (G) pre-Ly, (H) post-NLR, (I) post-LMR, (J)
post-PNI, (K) post-PLR, (L) post-CAR, (M) post-Eo, (N) post-Ly and
(O) post-mGPS. CAR, C-reactive protein-to-albumin ratio; Eo,
eosinophils; LMR, lymphocyte-to-monocyte ratio; Ly, lymphocytes;
mGPS, modified Glasgow Prognostic Score; NLR,
neutrophil-to-lymphocyte ratio; OS, overall survival; PLR,
platelet-to-lymphocyte ratio; PNI, prognostic nutritional
index. |
In the Pre-NLR group, a significant difference in OS
was observed between the Low group (NLR <7.17) and the High
group (NLR ≥7.17) (P=0.042) (Fig.
3A). Similarly, in the Pre-LMR group, the Low group (LMR
<1.29) and the High group (LMR ≥1.29) showed a significant
difference (P<0.001) (Fig. 3B).
However, no significant difference was found in the Pre-PNI group
between the Low group (PNI <43.75) and the High group (PNI
≥43.75) (P=0.218) (Fig. 3C). In the
Pre-PLR group, OS differed significantly between the Low group (PLR
<453.5) and the High group (PLR ≥453.5) (P=0.008) (Fig. 3D). In the Pre-CAR group, a
significant difference was found between the Low group (CAR
<6.35) and the High group (CAR ≥6.35) (P=0.001) (Fig. 3E). Conversely, in the Pre-eosinophil
group, no significant difference in OS was observed between the Low
group (eosinophils <120/µl) and the High group (eosinophils
≥120/µl) (P=0.381) (Fig. 3F). A
significant difference was observed in the Pre-lymphocytes group
between the Low group (lymphocytes <670/µl) and the High group
(lymphocytes ≥670/µl) (P=0.022) (Fig.
3G).
Regarding post-treatment IBPS markers, in the
Post-NLR group, a significant difference in OS was found between
the Low group (NLR <4.7) and the High group (NLR ≥4.7) (P=0.042)
(Fig. 3H). In the Post-LMR group,
the Low group (LMR <1.23) and the High group (LMR ≥1.23) also
showed a significant difference (P=0.036) (Fig. 3I). No significant difference was
found in the Post-PNI group between the Low group (PNI <36.6)
and the High group (PNI ≥36.6) (P=0.713) (Fig. 3J). Similarly, no significant
difference was observed in the Post-PLR group (Low: PLR <221.1,
High: PLR ≥221.1, P=0.493) (Fig.
3K), Post-CAR group (Low: CAR <0.44, High: CAR ≥0.44,
P=0.309) (Fig. 3L), and the
Post-eosinophil group (Low: eosinophils <260/µl, High:
eosinophils ≥260/µl, P=0.147) (Fig.
3M). However, in the Post-lymphocytes group, a significant
difference was observed between the Low group (lymphocytes
<770/µl) and the High group (lymphocytes ≥770/µl) (P=0.007)
(Fig. 3N). No significant
difference was found between the Post-mGPS=0 group and the
Post-mGPS=1 group (P=0.577) (Fig.
3O).
Discussion
In this study, pre-treatment IBPS values did not
show significant associations with OS or PFS. However, post-ICI
treatment values for NLR, PNI, CAR, and mGPS were significantly
correlated with OS. Similarly, post-treatment values for NLR, PNI,
and CAR were significantly associated with PFS. While no
significant association was observed between pre-treatment IBPS
values and the DCR, post-treatment values of NLR, LMR, PNI, PLR,
and CAR were significantly correlated with DCR. Survival analysis
using Kaplan–Meier curves, with cutoff values derived from ROC
analysis, revealed that high NLR, low LMR, high PLR, high CAR, and
low lymphocyte counts prior to ICI administration were associated
with shorter OS. Furthermore, high NLR, low LMR, and low lymphocyte
counts following ICI administration were significantly associated
with shorter OS.
NLR is a marker of systemic inflammation and has
been reported as a prognostic factor in various cancers, including
R/M-OSCC (26–30). In our study, post-ICI NLR
demonstrated the highest predictive accuracy (AUC=0.7696), with
patients exhibiting NLR ≥7.17 having significantly shorter OS than
those with NLR <7.17. An elevated NLR reflects an increase in
neutrophils and a decrease in lymphocytes, suggesting
tumor-associated inflammation and immune suppression (35). Several studies have reported that an
increase in TILs is associated with a favorable prognosis (29,30).
The mechanism underlying the association between elevated NLR and
poor prognosis may be attributed to the inflammatory response, in
which increased neutrophil production promotes tumor proliferation,
invasion, and angiogenesis by inducing the secretion of chemokines
and cytokines, further enhancing tumor-associated inflammation and
suppressing anti-tumor immunity.
CAR is a marker that reflects systemic inflammation
and nutritional status. In patients with a high CAR, elevated
inflammatory cytokines stimulate CRP production while inhibiting
albumin synthesis and accelerating its degradation, signaling the
progression of cachexia (36–38).
In head and neck squamous cell carcinoma, high interleukin-6 (IL-6)
expression has been reported, and IL-6 signaling promotes
immunosuppression and tumor progression (39,40).
Local and systemic increases in IL-6 correlate with elevated CRP
concentrations across various cancers (41,42).
In our study, a high CAR was associated with a poor prognosis,
highlighting its potential as an important prognostic marker for
patients with squamous cell carcinoma undergoing chemoradiotherapy
or cytotoxic chemotherapy.
Similarly, mGPS, calculated based on serum albumin
and CRP levels (43), has been
recognized as a prognostic factor for patients with recurrent or
metastatic head and neck cancer (44–48).
Consistent with these findings, our study also demonstrated a
significant association between post-ICI mGPS values and OS.
PLR, another hematological inflammatory marker, is
calculated from platelet and lymphocyte counts. Platelets, similar
to neutrophils, play a key role in inflammation and are often
elevated in patients with chronic inflammation associated with
solid tumors (49,50). Elevated platelet counts have been
linked to cancer progression, making PLR a promising predictive
marker for tumor progression (31,32).
Several studies have suggested that preoperative PLR values are
valuable for prognosis prediction in gastrointestinal cancers, and
more recent research has highlighted its potential for predicting
the efficacy of systemic chemotherapy (14).
PNI, calculated using albumin levels and lymphocyte
counts, reflects nutritional and immunological statuses (51). Previous studies have demonstrated
that pre-ICI PNI is associated with the efficacy of ICIs in
advanced or recurrent non-small cell lung cancer (52). Given the strong relationship between
immune function and nutritional status, low PNI values are
typically associated with poor prognosis. Our study further
corroborates this, demonstrating that low PNI values are associated
with reduced ICI efficacy and worse prognosis.
Notably, in our study, the AUC of nearly all
hematological markers improved after treatment compared with
pre-treatment values. This suggests that post-treatment values may
serve as more reliable prognostic indicators in patients receiving
ICI therapy. Matsuo et al reported that pre-treatment IBPSs
were insufficient for predicting prognosis in patients with
R/M-head and neck squamous cell carcinoma, while post-nivolumab
GPS, NLR, CAR, and eosinophil counts were significantly associated
with survival outcomes (48).
Similarly, Tachinami et al emphasized the importance of
post-treatment IBPS, including NLR, CAR, and PLR, in predicting
prognosis in R/M-OSCC (53). Our
findings are consistent with those of these studies, suggesting
that post-treatment data could be instrumental in predicting
treatment response, enabling the early identification of patients
with poor responses to ICI therapy and allowing for timely
transition to alternative treatment strategies. In this study, the
AUC values of biomarkers such as NLR, PNI, and CAR were below 0.8.
While an AUC above 0.8 is considered to indicate high predictive
accuracy, biomarkers with AUC values between 0.6 and 0.8 can still
be clinically useful (54,55). Especially in patients with R/M-OSCC
undergoing ICI therapy, the tumor immune microenvironment is
complex, and it is challenging to predict prognosis with a single
biomarker alone. In our analysis, even though the AUC values were
moderate, NLR and PNI were found to be independent prognostic
factors in multivariable analysis. Therefore, these markers may
still have clinical value in supporting treatment decisions.
Further improvements in predictive performance may be achieved by
developing composite models that integrate multiple clinical and
biological factors. Recent studies have identified several factors
influencing the efficacy of ICIs, including irAEs, BMI, history of
radiotherapy, ECOG PS, and CPS for PD-L1 expression (38,48,56–60).
In our study, sex and BMI were significant predictors of OS,
whereas sex, ICI type, presence of irAEs, and BMI were significant
predictors of PFS. Additionally, ECOG PS, CPS values, BMI, and
radiotherapy history were significantly associated with DCR.
In addition to hematological biomarkers such as
IBPS, various other factors have been proposed as potential
predictors of response to ICIs. Given the accessibility of
peripheral blood samples, numerous studies have analyzed
circulating cytokine levels, including tumor necrosis factor-alpha,
interferon-γ (IFN-γ), IL-6, IL-8, and transforming growth factor-β
(TGF-β) (61–63). Elevated IFN-γ levels have been
associated with improved ICI efficacy and toxicity, while higher
baseline levels of IL-8, IL-6, and TGF-β have been identified as
negative predictive markers. However, the extent to which these
circulating factors accurately reflect the tumor microenvironment
remains debatable. Additionally, studies have indicated that a high
neoantigen burden correlates with better treatment outcomes
(64), whereas genetic mutations
(e.g., epidermal growth factor receptor, KRAS, PTEN, TP53)
and dysregulation of the Wnt signaling pathway may impact ICI
response (65–69). Moreover, the use of corticosteroids,
antibiotics, or proton pump inhibitors influences ICI efficacy,
potentially through alterations in gut microbiota composition
(70–72). Future research should aim to
integrate these molecular, clinical, and therapeutic factors to
optimize patient selection and enhance treatment strategies for ICI
therapy.
One major limitation of this study is the small
sample size, particularly after stratification into subgroups. This
limitation is inherent to the retrospective design and the rarity
of patients with R/M-OSCC treated with ICIs at a single
institution. Although the limited cohort size reduced statistical
power, we were still able to identify consistent trends in the
performance of prognostic and predictive biomarkers, especially for
NLR and PNI. These results aligned with those of previous reports
(31,32,48,53)
and suggest that inflammation- and nutrition-based biomarkers may
provide meaningful clinical insights, even in smaller populations.
In addition, potential selection biases related to treatment
history, clinical stage, and histological type were not fully
controlled. Thus, further validation in prospective, multi-center
studies with larger cohorts is essential to confirm our findings
and develop robust predictive models. This study primarily aimed to
identify pre-treatment biomarkers predictive of ICI efficacy in
R/M-OSCC. While we also evaluated post-treatment biomarkers, their
prognostic value seemed limited, likely due to their reflection of
early treatment dynamics rather than baseline tumor biology.
Nevertheless, post-treatment biomarkers assessed 4–6 weeks after
ICI initiation showed potential for early prediction of treatment
efficacy, which may help identify non-responders and guide timely
treatment modifications. As our study did not include a control
group receiving alternative treatments such as chemotherapy or
targeted agents, the generalizability of our findings across
different treatment modalities remains uncertain. Future
prospective, multi-arm studies are warranted to evaluate the
prognostic consistency of these biomarkers and to determine whether
post-treatment markers retain predictive value independent of
treatment type.
In conclusion, our study demonstrated that post-ICI
values of NLR, PNI, CAR, and mGPS were significantly associated
with survival outcomes in patients with R/M-OSCC. While
pre-treatment IBPS values did not demonstrate significant
associations with DCR, post-ICI values of NLR, LMR, PNI, PLR, and
CAR exhibited significant correlations. These findings suggest that
hematological biomarkers, including IBPS, may serve as valuable
predictors of ICI treatment efficacy. Early prediction of ICI
response and timely adjustments to treatment may lead to improved
prognosis and the early identification of patients who could
benefit from sequential treatment.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was partially supported by a Grant-in-Aid for
Scientific Research from the Ministry of Education, Science,
Sports, and Culture of Japan (grant no. 23K09398).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SaY and NI designed the study. SaY and AH organized
the data collection and analyzed the data. SaY, NI, FO, TN, MH, SO,
KK, TA and SoY participated in the treatment of patients with
R/M-OSCC, and contributed to data analysis and interpretation. SaY
and SoY were the main contributors to manuscript writing. SaY, NI
and SoY confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
principles of The Declaration of Helsinki and was approved by the
Ethics Committee of Hiroshima University (approval no. E2024-0196).
The requirement for informed consent was waived by the Ethics
Committee owing to the retrospective design of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
AUC
|
area under the curve
|
|
BMI
|
body mass index
|
|
CAR
|
C-reactive protein-to-albumin
ratio
|
|
CI
|
confidence interval
|
|
CPS
|
combined positive score
|
|
CR
|
complete response
|
|
CRP
|
C-reactive protein
|
|
DCR
|
disease control rate
|
|
ECOG PS
|
Eastern Cooperative Oncology Group
Performance Status
|
|
HR
|
hazard ratio
|
|
IBPS
|
inflammation-based prognostic
score
|
|
ICIs
|
immune checkpoint inhibitors
|
|
IFN-γ
|
interferon-γ
|
|
IL
|
interleukin
|
|
irAEs
|
immune-related adverse events
|
|
LMR
|
lymphocyte-to-monocyte ratio
|
|
mGPS
|
modified Glasgow Prognostic Score
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
ORR
|
objective response rate
|
|
OS
|
overall survival
|
|
OSCC
|
oral squamous cell carcinoma
|
|
PD-L1
|
programmed death-ligand 1
|
|
PFS
|
progression-free survival
|
|
PLR
|
platelet-to-lymphocyte ratio
|
|
PNI
|
prognostic nutritional index
|
|
PR
|
partial response
|
|
R/M-OSCC
|
recurrent or metastatic oral squamous
cell carcinoma
|
|
ROC
|
receiver operating characteristic
|
|
SD
|
stable disease
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
Werning JW: Oral cancer: Diagnosis,
management, and rehabilitation. 1st Edition. Thieme Medical
Publishing Group; New York, USA: pp. 12007
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
3
|
Olson CM, Burda BU, Beil T and Whitlock
EP: Screening for oral cancer: A targeted evidence update for the
U.S. Preventive Services Task Force Rockville (MD): Agency for
Healthcare Research and Quality (US); 2013
|
|
4
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent Squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohen EEW, Soulières D, Le Tourneau C,
Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R,
et al: Pembrolizumab versus standard therapy for recurrent or
metastatic head and neck squamous cell carcinoma (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 393:156–167. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanna GJ, Schoenfeld JD and Ferris RL:
Immunotherapy approaches in head and neck cancer: Current and
emerging strategies. Front Immunol. 13:8409042022.
|
|
8
|
Zhang P, Su DM, Liang M and Fu J:
Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1)
surface expression in breast cancer cells and promote
PD-L1-mediated T cell apoptosis. Mol Immunol. 45:1470–1476. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vogelstein B, Papadopoulos N, Velculescu
VE, Zhou S, Diaz LA and Kinzler KW: Cancer genome landscapes.
Science. 339:1546–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Evaluation of cumulative prognostic
scores based on the systemic inflammatory response in patients with
inoperable Non-small-cell lung cancer. Br J Cancer. 89:1028–1030.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shibutani M, Maeda K, Nagahara H, Iseki Y,
Hirakawa K and Ohira M: The significance of the C-reactive protein
to albumin ratio as a marker for predicting survival and monitoring
chemotherapeutic effectiveness in patients with unresectable
metastatic colorectal cancer. Springerplus. 5:17982016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
An X, Ding PR, Li YH, Wang FH, Shi YX,
Wang ZQ, He YJ, Xu RH and Jiang WQ: Elevated neutrophil to
lymphocyte ratio predicts survival in advanced pancreatic cancer.
Biomarkers. 15:516–522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Li C, Zhao J, Yang L, Liu F, Zheng
H, Wang Z and Xu Y: Neutrophil-to-lymphocyte and
platelet-to-lymphocyte ratios predict chemotherapy outcomes and
prognosis in patients with colorectal cancer and synchronous liver
metastasis. World J Surg Oncol. 14:2892016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, Huang Y, Zhou L, Dai Y and Hu G:
High pretreatment neutrophil-to-lymphocyte ratio as a predictor of
poor survival prognosis in head and neck squamous cell carcinoma:
Systematic review and meta-analysis. Head Neck. 41:1525–1535. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rassouli A, Saliba J, Castano R, Hier M
and Zeitouni AG: Systemic inflammatory markers as independent
prognosticators of head and neck squamous cell carcinoma. Head
Neck. 37:103–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takenaka Y, Oya R, Kitamiura T, Ashida N,
Shimizu K, Takemura K, Yamamoto Y and Uno A: Platelet count and
platelet-lymphocyte ratio as prognostic markers for head and neck
squamous cell carcinoma: Meta-analysis. Head Neck. 40:2714–2723.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bardash Y, Olson C, Herman W, Khaymovich
J, Costantino P and Tham T: Platelet-lymphocyte ratio as a
predictor of prognosis in head and neck cancer: A systematic review
and meta-analysis. Oncol Res Treat. 42:665–677. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishikawa D, Suzuki H, Koide Y, Beppu S,
Kadowaki S, Sone M and Hanai N: Prognostic markers in head and neck
cancer patients treated with nivolumab. Cancers (Basel).
10:4662018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mohammed FF, Poon I, Zhang L, Elliott L,
Hodson ID, Sagar SM and Wright J: Acute-phase response reactants as
objective biomarkers of radiation-induced mucositis in head and
neck cancer. Head Neck. 34:985–993. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kano S, Homma A, Hatakeyama H, Mizumachi
T, Sakashita T, Kakizaki T and Fukuda S: Pretreatment
lymphocyte-to-monocyte ratio as an independent prognostic factor
for head and neck cancer. Head Neck. 39:247–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ikeguchi M: Glasgow prognostic score and
neutrophil-lymphocyte ratio are good prognostic indicators after
radical neck dissection for advanced squamous cell carcinoma in the
hypopharynx. Langenbecks Arch Surg. 401:861–866. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Turri-Zanoni M, Salzano G, Lambertoni A,
Giovannardi M, Karligkiotis A, Battaglia P and Castelnuovo P:
Prognostic value of pretreatment peripheral blood markers in
paranasal sinus cancer: Neutrophil-to-lymphocyte and
platelet-to-lymphocyte ratio. Head Neck. 39:730–736. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mikoshiba T, Ozawa H, Saito S, Ikari Y,
Nakahara N, Ito F, Watanabe Y, Sekimizu M, Imanishi Y and Ogawa K:
Usefulness of hematological inflammatory markers in predicting
severe side-effects from induction chemotherapy in head and neck
cancer patients. Anticancer Res. 39:3059–3065. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yasumatsu R, Wakasaki T, Hashimoto K,
Nakashima K, Manako T, Taura M, Matsuo M and Nakagawa T: Monitoring
the neutrophil-to-lymphocyte ratio may be useful for predicting the
anticancer effect of nivolumab in recurrent or metastatic head and
neck cancer. Head Neck. 41:2610–2618. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferrucci PF, Ascierto PA, Pigozzo J, Del
Vecchio M, Maio M, Antonini Cappellini GC, Guidoboni M, Queirolo P,
Savoia P, Mandalà M, et al: Baseline neutrophils and derived
neutrophil-to-lymphocyte ratio: Prognostic relevance in metastatic
melanoma patients receiving ipilimumab. Ann Oncol. 27:732–738.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kiriu T, Yamamoto M, Nagano T, Hazama D,
Sekiya R, Katsurada M, Tamura D, Tachihara M, Kobayashi K and
Nishimura Y: The time-series behavior of neutrophil-to-lymphocyte
ratio is useful as a predictive marker in non-small cell lung
cancer. PLoS One. 13:e01930182018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sacdalan DB, Lucero JA and Sacdalan DL:
Prognostic utility of baseline neutrophil-to-lymphocyte ratio in
patients receiving immune checkpoint inhibitors: A review and
meta-analysis. Onco Targets Ther. 11:955–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diem S, Schmid S, Krapf M, Flatz L, Born
D, Jochum W, Templeton AJ and Früh M: Neutrophil-to-lymphocyte
ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic
markers in patients with non-small cell lung cancer (NSCLC) treated
with nivolumab. Lung Cancer. 111:176–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bagley SJ, Kothari S, Aggarwal C, Bauml
JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson
JC, et al: Pretreatment neutrophil-to-lymphocyte ratio as a marker
of outcomes in nivolumab-treated patients with advanced
non-small-cell lung cancer. Lung Cancer. 106:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jain S, Harris J and Ware J: Platelets:
Linking hemostasis and cancer. Arterioscler Thromb Vasc Biol.
30:2362–2367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nieswandt B, Hafner M, Echtenacher B and
Männel DN: Lysis of tumor cells by natural killer cells in mice is
impeded by platelets. Cancer Res. 59:1295–1300. 1999.PubMed/NCBI
|
|
33
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. 8th Edition.
Wiley-Blackwell; Hoboken, NJ: 2016
|
|
34
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Granot Z and Jablonska J: Distinct
functions of neutrophil in cancer and its regulation. Mediators
Inflamm. 2015:7010672015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferris RL: Immunology and immunotherapy of
head and neck cancer. J Clin Oncol. 33:3293–3304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pettersen K, Andersen S, Degen S, Tadini
V, Grosjean J, Hatakeyama S, Tesfahun AN, Moestue S, Kim J, Nonstad
U, et al: Cancer cachexia associates with a systemic
autophagy-inducing activity mimicked by cancer cell-derived IL-6
trans-signaling. Sci Rep. 7:20462017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanoue K, Tamura S, Kusaba H, Shinohara Y,
Ito M, Tsuchihashi K, Shirakawa T, Otsuka T, Ohmura H, Isobe T, et
al: Predictive impact of C-reactive protein to albumin ratio for
recurrent or metastatic head and neck squamous cell carcinoma
receiving nivolumab. Sci Rep. 11:27412021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duffy SA, Taylor JM, Terrell JE, Islam M,
Li Y, Fowler KE, Wolf GT and Teknos TN: Interleukin-6 predicts
recurrence and survival among head and neck cancer patients.
Cancer. 113:750–757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Riedel F, Zaiss I, Herzog D, Götte K, Naim
R and Hörmann K: Serum levels of interleukin-6 in patients with
primary head and neck squamous cell carcinoma. Anticancer Res.
25:2761–2765. 2005.PubMed/NCBI
|
|
41
|
Ravishankaran P and Karunanithi R:
Clinical significance of preoperative serum interleukin-6 and
C-reactive protein level in breast cancer patients. World J Surg
Oncol. 9:182011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McKeown DJ, Brown DJ, Kelly A, Wallace AM
and McMillan DC: The relationship between circulating
concentrations of C-reactive protein, inflammatory cytokines and
cytokine receptors in patients with non-small-cell lung cancer. Br
J Cancer. 91:1993–1995. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Inoue Y, Iwata T, Okugawa Y, Kawamoto A,
Hiro J, Toiyama Y, Tanaka K, Uchida K, Mohri Y, Miki C and Kusunoki
M: Prognostic significance of a systemic inflammatory response in
patients undergoing multimodality therapy for advanced colorectal
cancer. Oncology. 84:100–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsuki T, Okamoto I, Fushimi C, Sawabe M,
Kawakita D, Sato H, Tsukahara K, Kondo T, Okada T, Tada Y, et al:
Hematological predictive markers for recurrent or metastatic
squamous cell carcinomas of the head and neck treated with
nivolumab: A multicenter study of 88 patients. Cancer Med.
9:5015–5024. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ueki Y, Takahashi T, Ota H, Shodo R,
Yamazaki K and Horii A: Predicting the treatment outcome of
nivolumab in recurrent or metastatic head and neck squamous cell
carcinoma: Prognostic value of combined performance status and
modified Glasgow prognostic score. Eur Arch Otorhinolaryngol.
277:2341–2347. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Niwa K, Kawakita D, Nagao T, Takahashi H,
Saotome T, Okazaki M, Yamazaki K, Okamoto I, Hirai H, Saigusa N, et
al: Multicentre, retrospective study of the efficacy and safety of
nivolumab for recurrent and metastatic salivary gland carcinoma.
Sci Rep. 10:169882020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Minohara K, Matoba T, Kawakita D, Takano
G, Oguri K, Murashima A, Nakai K, Iwaki S, Hojo W, Matsumura A, et
al: Novel Prognostic Score for recurrent or metastatic head and
neck cancer patients treated with Nivolumab. Sci Rep. 11:169922021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Matsuo M, Yasumatsu R, Masuda M, Toh S,
Wakasaki T, Hashimoto K, Jiromaru R, Manako T and Nakagawa T:
Inflammation-based prognostic score as a prognostic biomarker in
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma treated with nivolumab therapy. In Vivo. 36:907–917.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wagner DD: New links between inflammation
and thrombosis. Arterioscler Thromb Vasc Biol. 25:1321–1324. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Stone RL, Nick AM, McNeish IA, Balkwill F,
Han HD, Bottsford-Miller J, Rupairmoole R, Armaiz-Pena GN, Pecot
CV, Coward J, et al: Paraneoplastic thrombocytosis in ovarian
cancer. N Engl J Med. 366:610–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang L, Ma W, Qiu Z, Kuang T, Wang K, Hu
B and Wang W: Prognostic nutritional index as a prognostic
biomarker for gastrointestinal cancer patients treated with immune
checkpoint inhibitors. Front Immunol. 14:12199292023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shoji F, Takeoka H, Kozuma Y, Toyokawa G,
Yamazaki K, Ichiki M and Takeo S: Pretreatment prognostic
nutritional index as a novel biomarker in non-small cell lung
cancer patients treated with immune checkpoint inhibitors. Lung
Cancer. 136:45–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tachinami H, Tomihara K, Yamada SI, Ikeda
A, Imaue S, Hirai H, Nakai H, Sonoda T, Kurohara K, Yoshioka Y, et
al: Neutrophil-to-lymphocyte ratio as an early marker of outcomes
in patients with recurrent oral squamous cell carcinoma treated
with nivolumab. Br J Oral Maxillofac Surg. 61:320–326. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou XH, Obuchowski NA and McClish DK:
Statistical methods in diagnostic medicine. 2nd Edition. John Wiley
& Sons; New Jersey: 2011, View Article : Google Scholar
|
|
55
|
Valero C, Lee M, Hoen D, Zehir A, Berger
MF, Seshan VE, Chan TA and Morris LGT: Response rates to anti-PD-1
immunotherapy in microsatellite-stable solid tumors with 10 or more
mutations per megabase. JAMA Oncol. 7:739–743. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Iwasa YI, Kitoh R, Yokota Y, Hori K,
Kasuga M, Kobayashi T, Kanda S and Takumi Y: Post-treatment
neutrophil/lymphocyte ratio is a prognostic factor in head and neck
cancers treated with nivolumab. Cancer Diagn Progn. 4:182–188.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shaverdian N, Lisberg AE, Bornazyan K,
Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P:
Previous radiotherapy and the clinical activity and toxicity of
pembrolizumab in the treatment of non-small-cell lung cancer: A
secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol.
18:895–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Öjlert ÅK, Nebdal D, Lund-Iversen M,
Åstrøm Ellefsen R, Brustugun OT, Gran JM, Halvorsen AR and Helland
Å: Immune checkpoint blockade in the treatment of advanced
non-small cell lung cancer-predictors of response and impact of
previous radiotherapy. Acta Oncol. 60:149–156. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shankar B, Zhang J, Naqash AR, Forde PM,
Feliciano JL, Marrone KA, Ettinger DS, Hann CL, Brahmer JR,
Ricciuti B, et al: Multisystem immune-related adverse events
associated with immune checkpoint inhibitors for treatment of
non-small cell lung cancer. JAMA Oncol. 6:1952–1956. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hsiehchen D, Naqash AR, Espinoza M, Von
Itzstein MS, Cortellini A, Ricciuti B, Owen DH, Laharwal M, Toi Y,
Burke M, et al: Association between immune-related adverse event
timing and treatment outcomes. Oncoimmunology. 11:20171622022.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang M, Zhai X, Li J, Guan J, Xu S, Li Y
and Zhu H: The role of cytokines in predicting the response and
adverse events related to immune checkpoint inhibitors. Front
Immunol. 12:6703912021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sanmamed MF, Perez-Gracia JL, Schalper KA,
Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, Oñate C, Perez G, Alfaro
C, Martín-Algarra S, et al: Changes in serum interleukin-8 (IL-8)
levels reflect and predict response to anti-PD-1 treatment in
melanoma and non-small-cell lung cancer patients. Ann Oncol.
28:1988–1995. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
McGranahan N, Furness AJ, Rosenthal R,
Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak
NJ, Hiley CT, et al: Clonal neoantigens elicit T cell
immunoreactivity and sensitivity to immune checkpoint blockade.
Science. 351:1463–1469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Miao D, Margolis CA, Vokes NI, Liu D,
Taylor-Weiner A, Wankowicz SM, Adeegbe D, Keliher D, Schilling B,
Tracy A, et al: Genomic correlates of response to immune checkpoint
blockade in microsatellite-stable solid tumors. Nat Genet.
50:1271–1281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Giroux Leprieur E, Hélias-Rodzewicz Z,
Takam Kamga P, Costantini A, Julie C, Corjon A, Dumenil C, Dumoulin
J, Giraud V, Labrune S, et al: Sequential ctDNA whole-exome
sequencing in advanced lung adenocarcinoma with initial durable
tumor response on immune checkpoint inhibitor and late progression.
J Immunother Cancer. 8:e0005272020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Guibert N, Jones G, Beeler JF, Plagnol V,
Morris C, Mourlanette J, Delaunay M, Keller L, Rouquette I, Favre
G, et al: Targeted sequencing of plasma cell-free DNA to predict
response to PD1 inhibitors in advanced non-small cell lung cancer.
Lung Cancer. 137:1–6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang W, Li Y, Lyu J, Shi F, Kong Y, Sheng
C, Wang S and Wang Q: An aging-related signature predicts favorable
outcome and immunogenicity in lung adenocarcinoma. Cancer Sci.
113:891–903. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Arbour KC, Mezquita L, Long N, Rizvi H,
Auclin E, Ni A, Martínez-Bernal G, Ferrara R, Lai WV, Hendriks LEL,
et al: Impact of baseline steroids on efficacy of programmed cell
death-1 and programmed death-ligand 1 blockade in patients with
non-small-cell lung cancer. J Clin Oncol. 36:2872–2878. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu C, Guo H, Mao H, Tong J, Yang M and
Yan X: An up-to-date investigation into the correlation between
proton pump inhibitor use and the clinical efficacy of immune
checkpoint inhibitors in advanced solid cancers: A systematic
review and meta-analysis. Front Oncol. 12:7532342022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ochi N, Ichihara E, Takigawa N, Harada D,
Inoue K, Shibayama T, Hosokawa S, Kishino D, Harita S, Oda N, et
al: The effects of antibiotics on the efficacy of immune checkpoint
inhibitors in patients with non-small-cell lung cancer differ based
on PD-L1 expression. Eur J Cancer. 149:73–81. 2021. View Article : Google Scholar : PubMed/NCBI
|