Introduction
Esophageal squamous cell carcinoma (ESCC) has a poor
prognosis, ranking 11th in overall mortality, 7th among men and
17th among women, worldwide (1).
Treatment options include surgery, endoscopic submucosal
dissection, chemotherapy, chemoradiotherapy (CRT), and heavy ion
therapy.
Neoadjuvant chemotherapy with cisplatin and
5-fluorouracil (CF) plus docetaxel (DCF) had a better prognosis
than neoadjuvant CRT in patients with locally advanced, resectable
ESCC (2). One of the reasons for
the worse prognosis of neoadjuvant CRT might be the side effects
due to CRT, despite CRT having a high pathological complete
response (pCR) rate and few local recurrences (2). Therefore, methods to predict the CRT
treatment response prior to treatment in ESCC need to be developed,
would be clinically useful, and could be effective in planning
personalized treatments for ESCC patients.
18F-fluorodeoxyglucose (FDG) uptake is
elevated in malignant tumor cells. Therefore, FDG-positron emission
tomography/computed tomography (FDG-PET/CT), which can visualize
FDG accumulation, has been used to identify malignant tumors. In
clinical practice, FDG-PET/CT is used to assess the presence and
location of distant metastases. The most commonly used FDG-PET/CT
parameter is the maximum standardized uptake value (SUVmax), which
represents tissue metabolism. Moreover, SUVmax has been used to
determine treatment response. Previous studies reported that
post-treatment PET parameters can predict patients who will respond
to treatment. Murakami et al (3) reported that comparing post-treatment
PET parameters with pre-treatment PET parameters contributes to
confirming the prognosis of patients with ESCC treated with
neoadjuvant chemotherapy. Swisher et al (4) reported that post-treatment SUVmax was
an independent predictor of survival in patients with ESCC treated
with CRT. To the best of our knowledge, pretreatment PET parameters
have not been able to predict the prognosis or treatment response
of patients with ESCC.
Recently, whole-body continuous imaging using flow
motion has become possible allowing for the measurement of FDG
dynamics. To the best of our knowledge, there are no reports on the
use of dynamic whole-body PET/CT (DW-PET/CT) for esophageal cancer
imaging. In this study, we enrolled ESCC patients who underwent CRT
and investigated the relationship between pretreatment
DW-PET/CT-derived parameters, such as the Patlak slope (PS) and
Patlak intercept (PI), and treatment response.
Highly proliferative cells exhibit greater
radiosensitivity (5). Additionally,
in malignant tumors, cells proliferate rapidly, metabolic activity
is heightened, and glycolysis is upregulated (6,7). Under
such conditions, the PS, which reflects the metabolic rate, is
expected to be elevated. Since the PS reflects active cell
division, we hypothesized that a higher PS may indicate patients
more likely to have favorable response to CRT. In addition, the PI
is considered a parameter reflecting perfusion (8). During CRT resistance, reduced blood
perfusion within the tumor leads to hypoxia, low pH, and decreased
drug delivery, all of which contribute to treatment resistance
(9,10). Thus, we hypothesize that a high PI
would indicate stable perfusion and potentially reduce treatment
resistance and improve CRT efficacy.
Materials and methods
Patient population
This retrospective study included patients with
pathologically proven ESCC who underwent pretreatment DW-PET/CT
followed by CRT between August 2021 and May 2024. We identified
fifty-nine ESCC patients who received CRT after DW-PET/CT at our
institute during the study period (Table I). Three patients were excluded
because their tumors were too small to identify on PET/CT images.
Finally, 56 patients were eligible for this study. The subjects
included 44 men and 12 women with a mean age of 73.0 years (range
28–90 years). The clinical stage was determined by gastrointestinal
endoscopy, barium esophagography, chest and abdominal computed
tomography (CT) scans, magnetic resonance imaging (MRI), and
18F-FDG-PET, based on the 12th Edition of the Japanese
Classification of Esophageal Cancer (4,11).
Clinicopathological data including age, sex, clinical TNM stage,
and pathological findings were collected from electronic medical
records. This study was approved by the Institutional Review Board
at Chiba University Hospital (IRB reference number: HK202302-10;
Chiba, Japan). Written informed consent was obtained for all
examinations and treatments, including DW-PET/CT. However, with
regard to consent for participation in this study, the opt-out
approach was used because of the retrospective design. The study
details were made publicly available on websites or at locations
accessible to potential participants, and all participants were
given the freedom to opt out of the study.
 | Table I.Characteristics of patients treated
with chemoradiotherapy (n=56). |
Table I.
Characteristics of patients treated
with chemoradiotherapy (n=56).
| Characteristic | Value |
|---|
| Median age, years
(range) | 73 (28–90) |
| Sex,
Male/Female | 44/12 |
| Tumor location,
Ce/Ut/Mt/Lt | 12/9/18/17 |
| Clinical T status,
T1/T2/T3/T4 | 6/4/12/34 |
| Clinical N status,
N-/N+ | 13/43 |
| Clinical M status,
M0/M1 | 46/10 |
| Clinical stage,
I/II/III/IV | 5/6/11/34 |
| Surgery, +/- | 13/43 |
| SCC histology | 56 |
| Median
neutrophil/lymphocyte ratio (range) | 3.1 (1.3–11.3) |
| Median prognostic
nutritional index (range) | 48.1
(33.6–57.9) |
| CEA,
≤5.0/>5.0 | 44/12 |
| SCC-associated
antigen, ≤1.5/>1.5 | 26/30 |
| CYFRA,
≤3.5/>3.5/not available | 50/5/1 |
| Serum p53
antibodies, ≤10/>10/not available | 46/8/2 |
| Chemotherapy,
CF/FOLFOX | 52/4 |
| Therapeutic
response, Responder/Non- | 38/18 |
| Responder |
|
| Median tumor
length, mm (range) | 50.5 (15–110) |
| Median tumor
contraction rate, % (range) | 33.33 (−14-70) |
Treatment plan and response
evaluation
CRT consisted of two cycles of CF [800
mg/m2 5-fluorouracil (5-FU) in a continuous infusion for
5 days and 80 mg/m2 cisplatin on day 1] or two cycles of
FOLFOX (400 mg/m2 5-FU, 85 mg/m2 oxaliplatin,
and 200 mg/m2 levofolinate on day 1, followed by 1,600
mg/m2 5-FU in a continuous infusion for 2 days). All
patients received 2 Gy × 15–30 fractions of radiation therapy.
Re-evaluation of the primary tumor was performed by CT, endoscopy,
and gastrography 3 to 4 weeks after the completion of CRT.
Endoscopic ultrasound and PET were not always used for response
evaluation. The treatment response was evaluated according to the
Response Evaluation Criteria in Solid Tumors (11). Complete response (CR) was defined as
the disappearance of the target lesion and as the disappearance of
endoscopically suggestive neoplastic lesions and the absence of
histological evidence of cancer on biopsy. Partial response (PR)
was a reduction in the target lesion by 30% or more, progressive
disease (PD) was an increase in the target lesion by 20% or more,
and stable disease (SD) was defined as no reduction equivalent to
PR and no increase equivalent to PD. We calculated the response of
the target lesion according to the thickness of the tumor. We
targeted the main lesion because we wanted to examine the effects
of CRT on the main lesion. In this study, patients who showed a
response, CR or PR, were categorized as clinical responders. The
remaining patients with either SD or PD were categorized as
clinical non-responders. We also calculated the contraction rate of
the main tumor during CRT. The equation for this contraction rate
is as follows: Tumor contraction rate (%)=(Tumor thickness before
treatment-Tumor thickness after treatment)/Tumor thickness before
treatment ×100.
DW-PET/CT protocol
The study participants were scanned on a Siemens
Biograph mCT Flow (Siemens Medical Solutions) with a 221-cm axial
field of view. The crista was a lutetium oxyorthosilicate (LSO),
the gantry opening diameter was 78 mm, the bismuth germanate
detectors were set for 300 sec, the LSO for 40 ns, and the
semiconductor for 214 ps. Patients were asked not to eat for 6 h
before imaging, and their blood glucose levels were measured
immediately before scanning. The blood sugar levels of all patients
were below 150 mg/dl at the time of the PET study. FDG was
administered with an auto-dispensing injector (UG-05; Universal
GIKEN). First, the static whole-body images were acquired 60 min
after the injection of FDG (3.7 MBq/kg [max 370 MBq]). DW-PET/CT
image series were acquired every 5 min starting 60 min after the
injection of FDG (65, 70, 75, and 80 min) using
continuous-bed-motion technique (Flow motion technique) (12,13).
DW-PET/CT analysis
DW-PET/CT analysis was performed using the syngo.via
software. Using this software, kinetic analysis of FDG was
performed according to the compartment model. PS and PI were
calculated as DW-PET/CT parameters based on four whole-body PET/CT
image series acquired at every 5 min starting 60 min (65, 70, 75,
and 80 min) after the injection of FDG, and DW-PET/CT images of the
PS and PI were generated. PS was presumed to represent the absolute
metabolic rate of FDG, while PI represented the percentage of blood
concentration and represents perfusion within the tissue (14–16). A
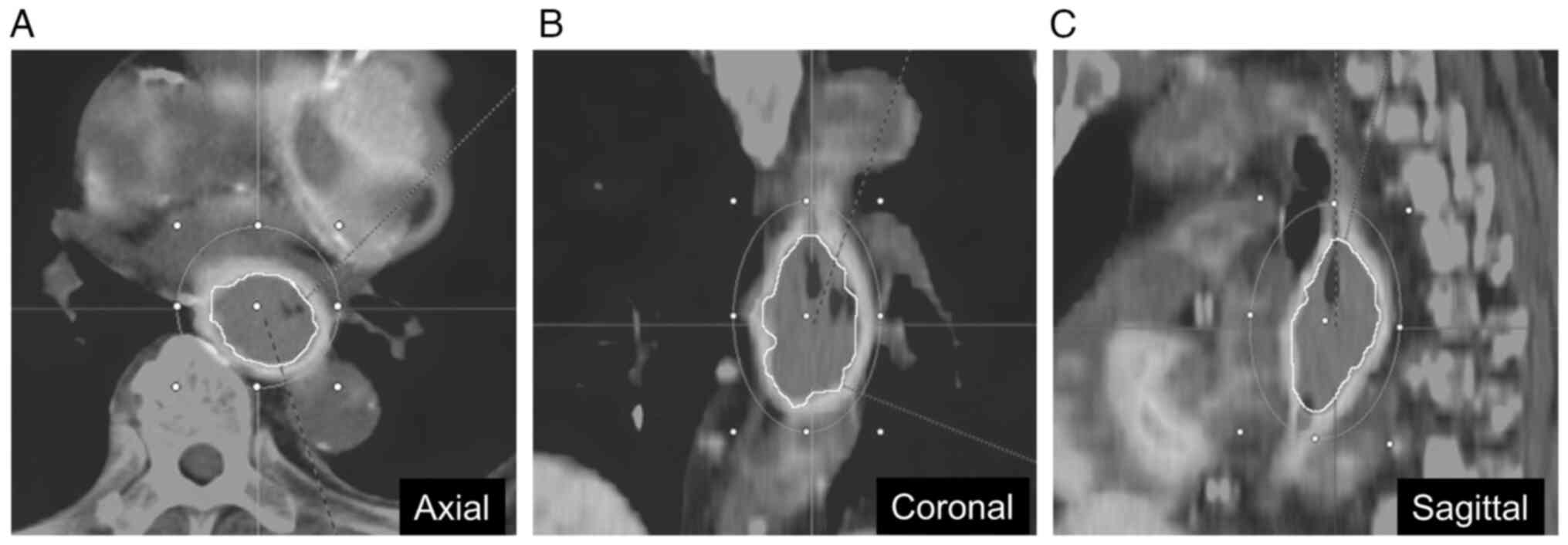
spherical volume of interest (VOI) was placed over the primary
tumor with the highest glucose uptake on the trans-axial PET images
acquired at 60 min after the injection of FDG, and the tumor lesion
was automatically delineated by using threshold of 40% of SUVmax
within the VOI (Fig. 1) (13,17).
In this automatically defined tumor area, the SUVmax and mean PS
and PI values were calculated. This analysis was performed by a
single observer (M.I. with 3 years of experience in PET and CT
interpretation) supervised by an experienced gastroenterologist
(Y.K. with 10 years of experience in PET and CT
interpretation).
Statistical analysis
All statistical analyses were performed using the
JMP Pro software (version 17.0; SAS Institute, Inc.). Differences
were considered statistically significant at P<0.05. The
Mann-Whitney U test was applied for comparison of DW-PET/CT
parameters between responder and non-responder groups. Correlations
between DW-PET/CT parameters and tumor contraction rate during CRT
was analyzed using Spearman's rank correlation coefficients. Area
under the receiver operating characteristic curve (AUC) analyses
were performed to assess the predictive value of DW-PET/CT
parameters for predicting treatment responders. Logistic regression
analysis was also performed to improve the AUC.
In addition to evaluating the predictive accuracy of
the PS and PI using AUC analyses, we evaluated the characteristics
of misclassified cases. Categorical variables were analyzed using
Fisher's exact test; continuous variables were assessed using the
Mann-Whitney U test.
Results
Relationship between DW-PET/CT-derived
parameters and treatment response
We compared DW-PET/CT parameters between treatment
responders and non-responders. Tumors of responders showed
significantly higher SUVmax, PS, and PI than non-responders
(P=0.04, P=0.0012, and P=0.0018, respectively; Table II). Next, we investigated the
relationships between the PS, PI, and clinical parameters. A weak
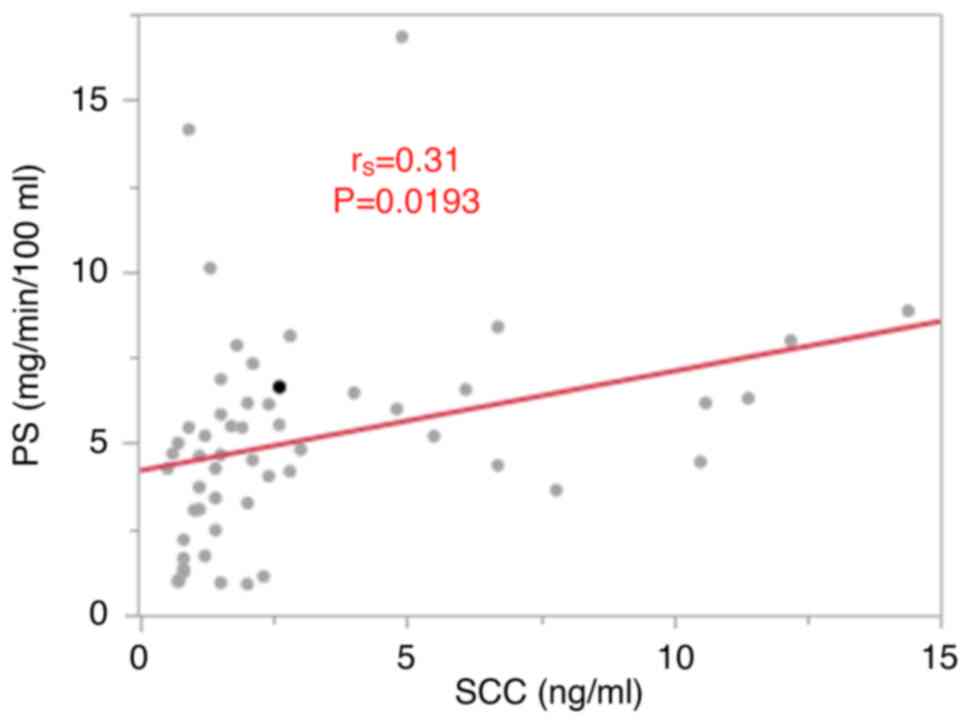
correlation was observed between squamous cell carcinoma-associated
antigen levels and PS (rs=0.31, P=0.0193; Fig. 2). No correlations were found between
SUVmax or PI and clinical parameters. Additionally, the PS did not
correlate with the neutrophil-lymphocyte ratio, prognostic
nutritional index, carcinoembryonic antigen levels, cytokeratin
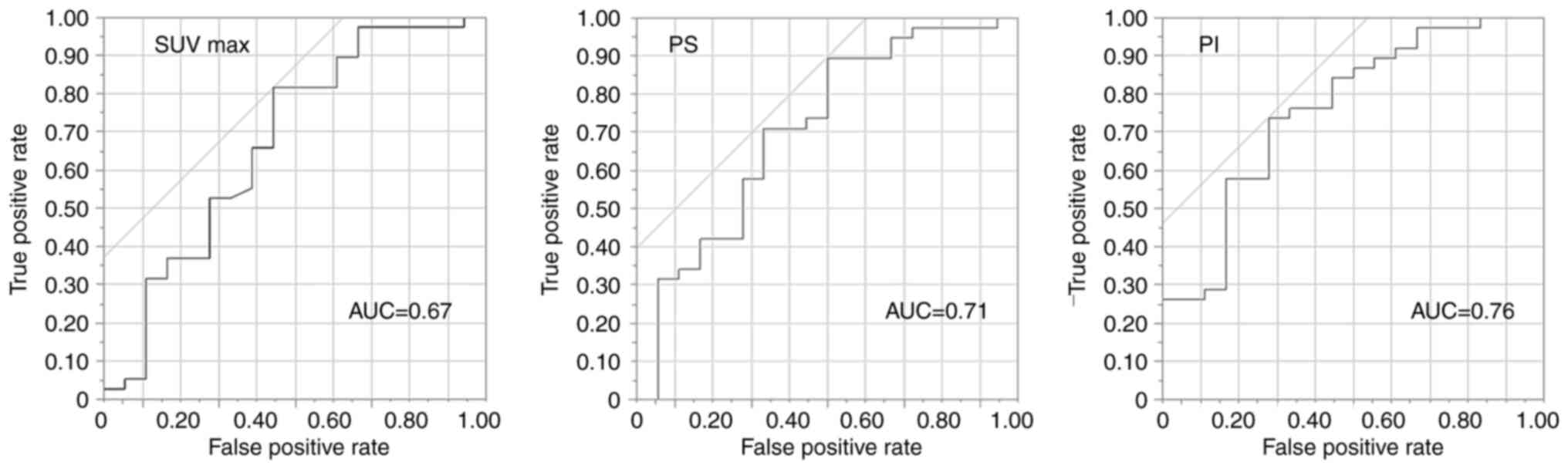
fragment levels, or serum p53 antibodies. The PS and PI showed good
performances in predicting treatment response with AUCs of 0.71 and
0.76, whereas the SUVmax showed a poor performance with an AUC of
0.67 (Fig. 3). For the PS, when the
cut-off point was set at 3.26 (calculated by the Youden Index
method), the sensitivity was 89.5%, specificity was 50.0%, and
accuracy was 76.8% for the prediction of patients who showed good
response to CRT. For the PI, when the cut-off point was set at
71.37 (calculated by Youden Index method), the sensitivity was
73.7%, specificity was 72.2%, and accuracy was 73.2% for the
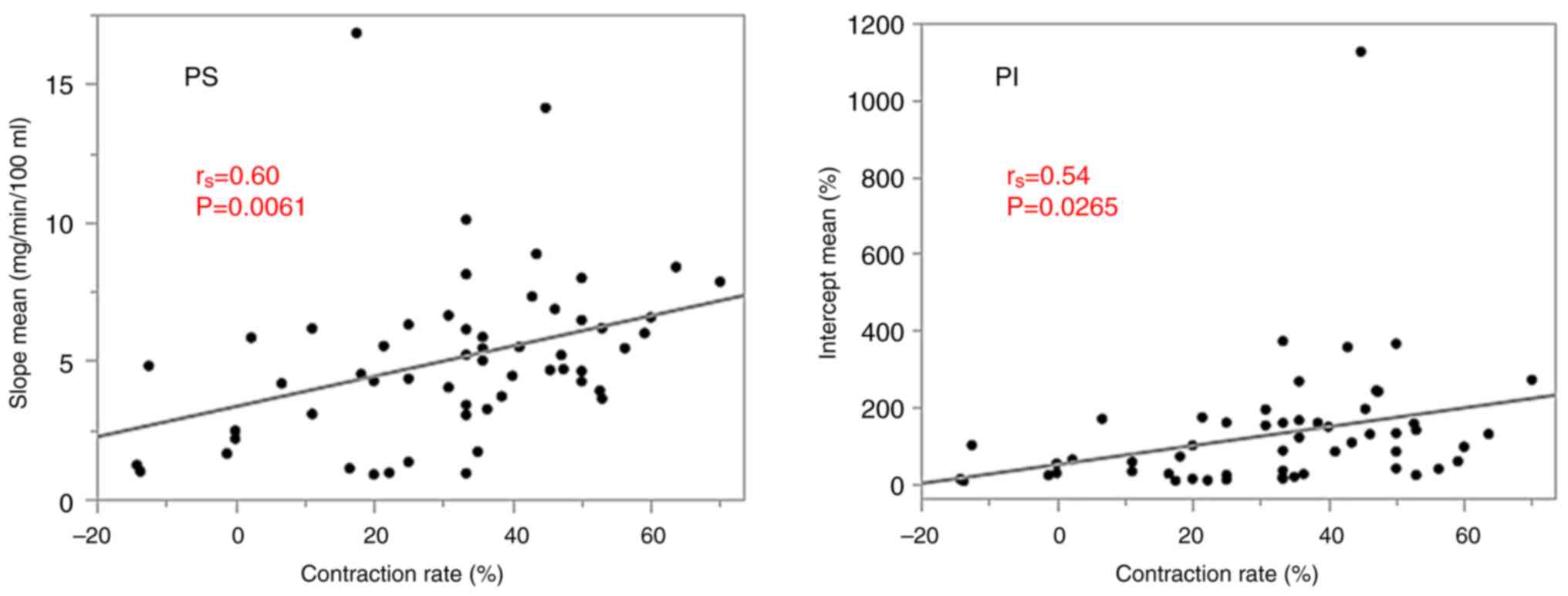
prediction of patients who showed good response to CRT. The tumor
contraction rate during CRT showed significant positive
correlations with the PS and PI (rs=0.60, P=0.0061 and
rs=0.54, P=0.0265, respectively; Fig. 4).
 | Table II.Relationship between therapeutic
responders and SUVmax, Patlak slope and Patlak intercept in
patients with esophageal cancer treated with chemoradiotherapy. |
Table II.
Relationship between therapeutic
responders and SUVmax, Patlak slope and Patlak intercept in
patients with esophageal cancer treated with chemoradiotherapy.
| Measured
parameter | Responder
(n=38) | Non-responder
(n=18) | P-value |
|---|
| SUVmax | 17.57±8.22 | 12.86±8.38 | 0.04 |
| Patlak slope,
mg/min/100 ml | 5.55±2.51 | 4.10±3.72 | 0.0012 |
| Patlak intercept,
% | 161.42±188.30 | 57.96±58.03 | 0.0018 |
To evaluate the predictive accuracy of the PS and PI
using AUC analyses, we evaluated the characteristics of
misclassified cases. No significant differences in clinical or
imaging features were observed between the PS true-positive and
false-positive groups. However, when comparing the PS true-positive
and false-negative groups, the false-negative group was
characterized by less advanced T and N classifications and overall
stage (P<0.0001, P=0.0237, and P=0.0002, respectively), as well
as lower squamous cell carcinoma-associated antigen levels
(P=0.0360). Similarly, no significant differences were found
between the PI true-positive and false-positive groups. In
contrast, the PI false-negative group showed significantly less
advanced T classification and overall stage compared to the
true-positive group (P=0.0221 and P=0.0479, respectively). These
findings suggest that cases with less advanced T classification and
overall disease stage are more likely to result in false-negative
predictions for both PS and PI.
To improve the AUCs for PS and PI, logistic
regression analysis was performed; however, the AUCs showed no
improvement. Logistic regression was conducted using the following
explanatory variables: PS≥3.26 vs. PS<3.26 and PI≥71.37 vs.
PI<71.37. Individually, neither PS (OR=3.38, 95% CI: 0.63–21.00,
P=0.16) nor PI (OR=3.73, 95% CI: 0.74–18.83, P=0.11) were
significant indicators of the responder status. However, a combined
model incorporating both PS and PI significantly distinguished
responders from non-responders (log-likelihood ratio=6.37,
χ2=12.75, degree of freedom=2, P=0.0017,
R2=0.18, AIC=64.04). The ROC analysis yielded an AUC of
0.76. When PS and PI were treated as continuous variables, the
overall model test yielded the following parameters: log-likelihood
ratio=5.74, χ2=11.48, degree of freedom=2, P=0.0032,
R2=0.16, and AIC=65.31. The corresponding ROC curve
analysis showed an AUC of 0.75.
Discussion
Patients with ESCC have a poor prognosis and are
treated using a multidisciplinary approach that combines various
therapies. The JCOG1109 trial (2)
showed that postoperative DCF therapy has a better prognosis than
CF or CRT in terms of overall survival. In contrast, the pCR rates
for preoperative DCF therapy and CRT with CF therapy were 18.6 and
36.7%, respectively. DCF also carries a higher risk of adverse
events such as myelosuppression and neutropenia, with 42–90% of
patients developing grade 3 or higher neutropenia and 10–39%
developing febrile neutropenia (18–20).
These data indicate the presence of a population for which CRT is
suitable. Determining which cases are suitable for CRT can be
useful for treatment decision-making. The results of our study
indicate that, in ESCC patients undergoing CRT, those with higher
measured PS and PI had better responses. Similarly, the tumor
contraction rate correlated with a higher PS. The PS measured using
FDG-PET may be promising imaging biomarker for treatment selection
in patients with ESCC.
Various methods have been proposed to predict CRT
efficacy, including analyses of genes, RNA, proteins, metabolites,
the tumor microenvironment, and the microbiome. Among these,
imaging techniques offer the distinct advantage of being
non-invasive. In this study, we focused on imaging biomarkers
obtained during baseline clinical assessments.
Several studies have reported on the use of various
imaging modalities, such as CT, MRI, and PET, in assessing the
response of esophageal cancer to CRT. During CT perfusion imaging,
blood flow has been shown to correlate with treatment response and
overall survival (21,22). Other CT-derived parameters, such as
tumor thickness (23) and radiomic
features like LLLEnergy (24), have
also been reported to be associated with treatment response and
overall survival. Among MRI-derived parameters, the apparent
diffusion coefficient has been reported to correlate with survival
outcomes (25,26). Ktrans has been associated with pCR
(27,28), and has also been shown to be useful
for predicting response (29).
Additionally, histogram analysis of apparent diffusion coefficient
values has been reported to correlate with both pCR and
recurrence-free survival (30).
The advantages of PET imaging include not needing
contrast agents, its applicability in patients with metallic
implants, and a lower susceptibility to motion artifacts caused by
cardiac activity. van der Aa et al (31) reported that the SUVmax, which has
been widely used in clinical practice, is not predictive of pCR. In
our study, however, we demonstrated the potential utility of
alternative PET parameters, PS and PI, in predicting treatment
response.
Several studies have examined dynamic PET parameters
in cancers other than esophageal cancer. Many of these studies
suggest that such parameters are useful in distinguishing between
benign and malignant lesions including in lung cancer, liver
cancer, nasopharyngeal tumors, bone lesions, post-operative
gastroesophageal cancers, and colorectal lesions (13,32–36).
Additionally, dynamic PET has been used to predict hormone receptor
status in breast cancer (37) and
to differentiate subtypes of pheochromocytoma and paraganglioma
(38).
Differences in metabolic behavior have also been
reported among different cancer types within the same tissue. For
example, Kaneko et al (39)
showed that MRI-FDG imaging could differentiate between
intrahepatic cholangiocarcinoma, hepatocellular carcinoma, and
liver metastases. Similarly, Lv et al (40) demonstrated that ΔKi is useful for
distinguishing between primary and synchronous multiple lung
cancers.
Several studies have investigated the relationship
between cancer and treatment response using dynamic PET parameters.
Wang et al (41) reported
that the Palak Ki is associated with the response to immunotherapy
in non-small cell lung cancer. In addition, de Geus-Oei et
al (42) demonstrated that
MRI-FDG correlates with overall survival and progression-free
survival in colorectal cancer, while Dimitrakopoulou-Strauss et
al (43) found that the influx
rate was useful for predicting response to neoadjuvant chemotherapy
in soft-tissue sarcoma. In concert, Hofheinz et al (44) reported an association between the
Kslope and progression-free survival in non-small cell lung cancer.
Dimitrakopoulou-Strauss et al (45) have also examined prognosis in
non-small cell lung cancer and shown that k3 is related to
progression-free survival in multiple myeloma. To the best of our
knowledge, no studies have reported on these parameters in
esophageal cancer.
Several studies have used CT, MRI, and conventional
FDG-PET images as biomarkers for prognosis prediction and treatment
selection in patients with ESCC (46,47). A
common limitation of these studies was the objectivity of the
region of interests generated when the parameters were obtained
from the images. To ensure objectivity, many studies have taken
measurements at the site of the maximum tumor diameter when
obtaining regions of interest from images, and in some cases,
artificial intelligence has been employed. However, the methods
evaluated only one image slice of the tumor and could not evaluate
the entire tumor. The method employed in this study
semi-automatically set the VOI to surround the entire tumor,
allowing for evaluation of the whole tumor and ensuring
objectivity. We believe that these results are superior to those of
previous studies. Compared to CT and MRI, FDG-PET has drawbacks
such as patient exposure to radiation and examination costs;
however, if the results of these studies can be introduced into
clinical practice, the test will be a useful tool for making
treatment decisions and predicting prognoses.
This study included patients with cT0-1 ESCC. These
patients are generally candidates for surgery or endoscopic
treatment; however, CRT is often performed for elderly patients,
patients with poor activities of daily living, and patients with
circumferential lesions. Therefore, we decided to include such
cases in our analysis.
Our study had several limitations. First, the
biological investigations were not performed to confirm the
parameters obtained from DW-PET/CT. Second, our study was based on
single-center retrospective data and had a relatively small sample
size. Thus, prospective multicenter investigations with larger
populations are required to strengthen the statistical power of our
results. Third, we drew the VOI semi-automatically, which is
therefore objective. Fourth, this study included several types of
treatment, which could be biased. Finally, we did not assess the
histological tumor grade in this study.
In conclusion, our findings suggest that parameters
obtained from DW-PET/CT, such as the PS and PI, can be useful
predictors for treatment response in ESCC patients treated with
CRT.
Acknowledgements
Part of this study was presented at the AACR Annual
Meeting, 23–25 April, 2025 in Chicago, IL, USA. Poster No. 2543,
Section 1, Board 22.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MI, YK, KH, GO, ToT, AH, RM, MU, TaT, YM, AN, TS,
NS, TI and HM contributed to the study conception and design.
Material preparation, data collection and analysis were performed
by MI and KH. The first draft of the manuscript was written by MI,
and YK, KH, GO, ToT, AH, RM, MU, TaT, YM, AN, TS, NS, TI and HM
commented on previous versions of the manuscript. MI and YK confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All procedures followed were in accordance with the
ethical standards of the responsible committee on human
experimentation (institutional and national) and with the Helsinki
Declaration of 1964 and later versions. Written informed consent
was obtained from all participants before examinations and
treatments, including DW-PET/CT. However, with regard to consent
for participation in this study, the opt-out approach was used due
to the retrospective design of the study. The study details were
made publicly available on websites or at locations accessible to
potential participants; all participants were given the freedom to
opt out of the study. This retrospective study was approved by the
Clinical Research Center (Institutional Review Board) of Chiba
University Hospital (IRB reference number: HK202302-10).
Patient consent for publication
Consent for publication of the study was obtained
via the opt-out approach.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
AUC
|
area under the receiver operating
characteristic curve
|
|
CF
|
cisplatin and 5-fluorouracil
|
|
CR
|
complete response
|
|
CRT
|
chemoradiotherapy
|
|
CT
|
computed tomography
|
|
DCF
|
cisplatin and 5-fluorouracil plus
docetaxel
|
|
DW-PET/CT
|
dynamic whole-body positron emission
tomography-computed tomography
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
FDG
|
18F-fluorodeoxyglucose
|
|
FDG-PET/CT
|
18F-fluorodeoxyglucose
positron emission tomography-computed tomography
|
|
FOLFOX
|
5-fluorouracil, oxaliplatin and
levofolinate
|
|
LSO
|
lutetium oxyorthosilicate
|
|
MRI
|
magnetic resonance imaging
|
|
pCR
|
pathological complete response
|
|
PD
|
progressive disease
|
|
PET/CT
|
positron emission tomography-computed
tomography
|
|
PI
|
Patlak intercept
|
|
PR
|
partial response
|
|
PS
|
Patlak slope
|
|
SD
|
stable disease
|
|
SUVmax
|
maximum standardized uptake value
|
|
VOI
|
volume of interest
|
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48.
2023.PubMed/NCBI
|
|
2
|
Kato K, Machida R, Ito Y, Daiko H, Ozawa
S, Ogata T, Hara H, Kojima T, Abe T, Bamba T, et al: Doublet
chemotherapy, triplet chemotherapy, or doublet chemotherapy
combined with radiotherapy as neoadjuvant treatment for locally
advanced oesophageal cancer (JCOG1109 NExT): A randomised,
controlled, open-label, phase 3 trial. Lancet. 404:55–66. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murakami K, Yoshida N, Taniyama Y,
Takahashi K, Toyozumi T, Uno T, Kamei T, Baba H and Matsubara H:
Maximum standardized uptake value change rate before and after
neoadjuvant chemotherapy can predict early recurrence in patients
with locally advanced esophageal cancer: A multi-institutional
cohort study of 220 patients in Japan. Esophagus. 19:205–213. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swisher SG, Maish M, Erasmus JJ, Correa
AM, Ajani JA, Bresalier R, Komaki R, Macapinlac H, Munden RF,
Putnam JB, et al: Utility of PET, CT, and EUS to identify
pathologic responders in esophageal cancer. Ann Thorac Surg.
78:1152–1160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bergonie J and Tribondeau L:
Interpretation of some results of radiotherapy and an attempt at
determining a logical technique of treatment. Radiat Res.
11:587–588. 1959. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vaupel P and Multhoff G: The warburg
effect: Historical dogma versus current rationale. Adv Exp Med
Biol. 1269:169–177. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dimitrakopoulou-Strauss A, Hohenberger P,
Pan L, Kasper B, Roumia S and Strauss LG: Dynamic PET with FDG in
patients with unresectable aggressive fibromatosis:
Regression-based parametric images and correlation to the FDG
kinetics based on a 2-tissue compartment model. Clin Nucl Med.
37:943–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu J, Panin V, Smith AM, Spottiswoode B,
Shah V and von Gall CA: Design and implementation of automated
clinical whole body parametric PET with continuous bed motion. IEEE
Trans Radiat Plasma Med Sci. 4:696–707. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe M, Kato H, Katayama D, Soeda F,
Matsunaga K, Watabe T, Tatsumi M, Shimosegawa E and Tomiyama N:
Semiquantitative analysis using whole-body dynamic F-18
fluoro-2-deoxy-glucose-positron emission tomography to
differentiate between benign and malignant lesions. Ann Nucl Med.
36:951–963. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patlak CS and Blasberg RG: Graphical
evaluation of blood-to-brain transfer constants from multiple-time
uptake data. Generalizations. J Cereb Blood Flow Metab. 5:584–590.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Osborne DR and Acuff S: Whole-body dynamic
imaging with continuous bed motion PET/CT. Nucl Med Commun.
37:428–431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phelps ME, Huang SC, Hoffman EJ, Selin C,
Sokoloff L and Kuhl DE: Tomographic measurement of local cerebral
glucose metabolic rate in humans with
(F-18)2-fluoro-2-deoxy-D-glucose: Validation of method. Ann Neurol.
6:371–388. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lemarignier C, Di Fiore F, Marre C, Hapdey
S, Modzelewski R, Gouel P, Michel P, Dubray B and Vera P:
Pretreatment metabolic tumour volume is predictive of disease-free
survival and overall survival in patients with oesophageal squamous
cell carcinoma. Eur J Nucl Med Mol Imaging. 41:2008–2016. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamasaki M, Miyata H, Tanaka K, Shiraishi
O, Motoori M, Peng YF, Yasuda T, Yano M, Shiozaki H, Mori M and
Doki Y: Multicenter phase I/II study of docetaxel, cisplatin and
fluorouracil combination chemotherapy in patients with advanced or
recurrent squamous cell carcinoma of the esophagus. Oncology.
80:307–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Umeki Y, Matsuoka H, Fujita M, Goto A,
Serizawa A, Nakamura K, Akimoto S, Nakauchi M, Tanaka T, Shibasaki
S, et al: Docetaxel cisplatin 5-FU(DCF) therapy as a preoperative
chemotherapy to advanced esophageal squamous cell carcinoma: A
single-center retrospective cohort study. Internal Medicine.
62:319–325. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katada C, Sugawara M, Hara H, Fujii H,
Nakajima TE, Ando T, Kojima T, Watanabe A, Sakamoto Y, Ishikawa H,
et al: A management of neutropenia using granulocyte colony
stimulating factor support for chemotherapy consisted of docetaxel,
cisplatin and 5-fluorouracil in patients with oesophageal squamous
cell carcinoma. Jpn J Clin Oncol. 51:199–204. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayano K, Okazumi S, Shuto K, Matsubara H,
Shimada H, Nabeya Y, Kazama T, Yanagawa N and Ochiai T: Perfusion
CT can predict the response to chemoradiation therapy and survival
in esophageal squamous cell carcinoma: Initial clinical results.
Oncol Rep. 18:901–908. 2007.PubMed/NCBI
|
|
22
|
Makari Y, Yasuda T, Doki Y, Miyata H,
Fujiwara Y, Takiguchi S, Matsuyama J, Yamasaki M, Hirao T, Koyama
MK, et al: Correlation between tumor blood flow assessed by
perfusion CT and effect of neoadjuvant therapy in advanced
esophageal cancers. J Surg Oncol. 96:220–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wongwaiyut K, Ruangsin S, Laohawiriyakamol
S, Leelakiatpaiboon S, Sangthawan D, Sunpaweravong P and
Sunpaweravong S: Pretreatment esophageal wall thickness associated
with response to chemoradiotherapy in locally advanced esophageal
cancer. J Gastrointest Cancer. 51:947–951. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kasai A, Miyoshi J, Sato Y, Okamoto K,
Miyamoto H, Kawanaka T, Tonoiso C, Harada M, Goto M, Yoshida T, et
al: A novel CT-based radiomics model for predicting response and
prognosis of chemoradiotherapy in esophageal squamous cell
carcinoma. Sci Rep. 14:20392024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aoyagi T, Shuto K, Okazumi S, Shimada H,
Kazama T and Matsubara H: Apparent diffusion coefficient values
measured by diffusion-weighted imaging predict
chemoradiotherapeutic effect for advanced esophageal cancer. Dig
Surg. 28:252–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Cobelli F, Giganti F, Orsenigo E,
Cellina M, Esposito A, Agostini G, Albarello L, Mazza E, Ambrosi A,
Socci C, et al: Apparent diffusion coefficient modifications in
assessing gastro-oesophageal cancer response to neoadjuvant
treatment: Comparison with tumour regression grade at histology.
Eur Radiol. 23:2165–2174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun NN, Liu C, Ge XL and Wang J: Dynamic
contrast-enhanced MRI for advanced esophageal cancer response
assessment after concurrent chemoradiotherapy. Diagn Interv Radiol.
24:195–202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lei J, Han Q, Zhu S, Shi D, Dou S, Su Z
and Xu X: Assessment of esophageal carcinoma undergoing concurrent
chemoradiotherapy with quantitative dynamic contrast-enhanced
magnetic resonance imaging. Oncol Lett. 10:3607–3612. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu L, Xie X, Guo Z, Shen W, Qian P, Jiang
N and Fan Y: Dynamic contrast-enhanced magnetic resonance imaging:
A novel approach to assessing treatment in locally advanced
esophageal cancer patients. Niger J Clin Pract. 24:1800–1807. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirata A, Hayano K, Ohira G, Imanishi S,
Hanaoka T, Murakami K, Aoyagi T, Shuto K and Matsubara H:
Volumetric histogram analysis of apparent diffusion coefficient for
predicting pathological complete response and survival in
esophageal cancer patients treated with chemoradiotherapy. Am J
Surg. 219:1024–1029. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van der Aa DC, Gisbertz SS, Anderegg MCJ,
Lagarde SM, Klaassen R, Meijer SL, van Dieren S, Hulshof M, Bergman
J, Bennink RJ, et al: (18)F-FDG-PET/CT to detect pathological
complete response after neoadjuvant treatment in patients with
cancer of the esophagus or gastroesophageal junction: Accuracy and
long-term implications. J Gastrointest Cancer. 55:270–280. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weissinger M, Atmanspacher M, Spengler W,
Seith F, Von Beschwitz S, Dittmann H, Zender L, Smith AM, Casey ME,
Nikolaou K, et al: Diagnostic performance of dynamic whole-body
patlak [(18)F]FDG-PET/CT in patients with indeterminate lung
lesions and lymph nodes. J Clin Med. 12:39422023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Q, Wang RF, Zhang J and Zhou Y:
Differential diagnosis of pulmonary lesions by parametric imaging
in (18)F-FDG PET/CT dynamic multi-bed scanning. J BUON. 18:928–934.
2013.PubMed/NCBI
|
|
34
|
Parodi D, Dighero E, Biddau G, D'Amico F,
Bauckneht M, Marini C, Garbarino S, Campi C, Piana M and Sambuceti
G: Localized FDG loss in lung cancer lesions. EJNMMI Res.
14:1022024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Skawran S, Messerli M, Kotasidis F,
Trinckauf J, Weyermann C, Kudura K, Ferraro DA, Pitteloud J, Treyer
V, Maurer A, et al: Can dynamic whole-body FDG PET imaging
differentiate between malignant and inflammatory lesions? Life
(Basel). 12:13502022.PubMed/NCBI
|
|
36
|
Huang X, Zhuang M, Yang S, Wang Y, Liu Q,
Xu X, Xiao M, Peng Y, Jiang P, Xu W, et al: The valuable role of
dynamic (18)F FDG PET/CT-derived kinetic parameter K(i) in patients
with nasopharyngeal carcinoma prior to radiotherapy: A prospective
study. Radiother Oncol. 179:1094402023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sundaraiya S, T R, Nangia S, Sirohi B and
Patil S: Role of dynamic and parametric whole-body FDG PET/CT
imaging in molecular characterization of primary breast cancer: A
single institution experience. Nucl Med Commun. 43:1015–1025. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van Berkel A, Vriens D, Visser EP, Janssen
MJR, Gotthardt M, Hermus ARMM, Geus-Oei LF and Timmers HJLM:
Metabolic Subtyping of Pheochromocytoma and Paraganglioma by
(18)F-FDG Pharmacokinetics Using Dynamic PET/CT Scanning. J Nucl
Med. 60:745–751. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kaneko K, Nagao M, Yamamoto A, Yano K,
Honda G, Tokushige K and Sakai S: Patlak reconstruction using
dynamic 18 F-FDG PET imaging for evaluation of malignant liver
tumors: A comparison of HCC, ICC, and metastatic liver tumors. Clin
Nucl Med. 49:116–123. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lv W, Yang M, Zhong H, Wang X, Yang S, Bi
L, Xian J, Pei X, He X, Wang Y, et al: Application of dynamic
(18)F-FDG PET/CT for distinguishing intrapulmonary metastases from
synchronous multiple primary lung cancer. Mol Imaging.
2022:80812992022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang D, Qiu B, Liu Q, Xia L, Liu S, Zheng
C, Liu H, Mo Y, Zhang X, Hu Y, et al: Patlak-Ki derived from
ultra-high sensitivity dynamic total body [(18)F]FDG PET/CT
correlates with the response to induction immuno-chemotherapy in
locally advanced non-small cell lung cancer patients. Eur J Nucl
Med Mol Imaging. 50:3400–3413. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
de Geus-Oei LF, van Laarhoven HW, Visser
EP, Hermsen R, van Hoorn BA, Kamm YJ, Krabbe PF, Corstens FH, Punt
CJ and Oyen WJ: Chemotherapy response evaluation with FDG-PET in
patients with colorectal cancer. Ann Oncol. 19:348–352. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dimitrakopoulou-Strauss A, Strauss LG,
Egerer G, Vasamiliette J, Mechtersheimer G, Schmitt T, Lehner B,
Haberkorn U, Stroebel P and Kasper B: Impact of dynamic 18F-FDG PET
on the early prediction of therapy outcome in patients with
high-risk soft-tissue sarcomas after neoadjuvant chemotherapy: A
feasibility study. J Nucl Med. 51:551–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hofheinz F, Hoff JV, Steffen IG, Lougovski
A, Ego K, Amthauer H and Apostolova I: Comparative evaluation of
SUV, tumor-to-blood standard uptake ratio (SUR), and dual time
point measurements for assessment of the metabolic uptake rate in
FDG PET. EJNMMI Res. 6:532016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dimitrakopoulou-Strauss A, Hoffmann M,
Bergner R, Uppenkamp M, Eisenhut M, Pan L, Haberkorn U and Strauss
LG: Prediction of short-term survival in patients with advanced
nonsmall cell lung cancer following chemotherapy based on
2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography: A
feasibility study. Mol Imaging Biol. 9:308–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Okazumi S, Ohira G, Hayano K, Aoyagi T,
Imanishi S and Matsubara H: Novel advances in qualitative
diagnostic imaging for decision making in multidisciplinary
treatment for advanced esophageal cancer. J Clin Med. 13:6322024.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hayano K, Ohira G, Hirata A, Aoyagi T,
Imanishi S, Tochigi T, Hanaoka T, Shuto K and Matsubara H: Imaging
biomarkers for the treatment of esophageal cancer. World J
Gastroenterol. 25:3021–3029. 2019. View Article : Google Scholar : PubMed/NCBI
|