Introduction
The global burden of colorectal cancer (CRC) is
substantial, with incidence rates rising, particularly in
developing countries, where healthcare systems often struggle to
provide adequate cancer care (1).
CRC is the third most common malignant tumor worldwide and the
second leading cause of cancer-related death following lung cancer
(2). The International Agency for
Research on Cancer estimates that the number of worldwide novel CRC
cases and associated deaths will be ~3.2 and 1.6 million,
respectively, in 2040 (3). The
disease presents substantial challenges to public health, not only
due to the physical and psychological burdens experienced by
patients, but also due to the notable healthcare costs involved in
its management (4).
Current CRC treatments, including surgery,
chemotherapy and radiation therapy, are often constrained by
serious side effects, such as adverse effects and high recurrence
rates, and inconsistent efficacy across patients. These limitations
underscore the urgent need for innovative adjunctive therapeutic
strategies that can improve treatment outcomes while minimizing
adverse effects (5,6). Postoperative infection, a common
complication following surgery, profoundly impacts both surgical
outcomes and patient prognosis; therefore, it remains a key focus
for prevention and control during the perioperative period
(7).
Recently, the use of probiotics as a supportive CRC
treatment has gained attention due to their ability to modulate the
gut microbiota and enhance immune responses (8). Probiotics are live microorganisms that
provide health benefits to the host, particularly through their
regulatory effects on the gut microbiome and immune system
(9). Certain probiotic strains,
such as Lactobacillus rhamnosus GG, Bifidobacterium longum
and Lactobacillus casei, may help prevent CRC by inhibiting
tumor growth and metastasis, reducing inflammation and improving
the overall gut environment (10).
Furthermore, probiotics have been found to support gut health and
alleviate the adverse effects commonly associated with conventional
cancer therapies, such as chemotherapy-induced gastrointestinal
toxicity (11). The beneficial
effects of probiotics may be mediated through mechanisms such as
modulation of the intestinal microbiota, strengthening of the
epithelial barrier function and regulation of systemic inflammatory
responses (12). These attributes
suggest that probiotics may not only improve the quality of life of
patients with CRC but also contribute to enhanced treatment
outcomes.
Despite a promising theoretical foundation, the
clinical application of probiotics in CRC management remains
inconsistent. A systematic review of the existing literature
revealed mixed findings A systematic review of the existing
literature revealed mixed findings regarding the effects of
probiotics on patients with CRC (13). Some studies reported beneficial
effects, suggesting that probiotics may modify the gut microbiota,
reduce inflammatory cytokines and secrete anticancer metabolites
(14). Probiotics have also been
revealed to modulate T-lymphocyte and dendritic cell activities
(15). Furthermore, specific lactic
acid-producing bacteria, valued for their immunomodulatory
properties, have demonstrated efficacy for instance, combinations
such as Lactobacillus salivarius and Lactobacillus
fermentum with Lactobacillus acidophilus have been
associated with reduced CRC cell proliferation (16). Other studies demonstrated no notable
impact on disease progression or survival outcomes (17,18).
This variability may stem from differences in study design,
probiotic strain dosage regimens and patient populations,
contributing to the heterogeneity of the results. Moreover, the
absence of robust, high-quality randomized controlled trials (RCTs)
limits the ability to draw definitive conclusions regarding the
efficacy and safety of probiotics in this context (19). To address these gaps, the present
meta-analysis and trial sequential analysis (TSA) were employed to
systematically assess the available evidence on the use of
probiotics as an adjunct to standard CRC therapies. Meta-analysis
enables the synthesis of data across multiple studies, which
provides a more comprehensive evaluation of treatment effects. In
parallel, TSA can be used to estimate the required sample size to
confirm the reliability of the findings, which reduces the risk of
type I errors (20). The primary
aim of the present study was to clarify the role of probiotics in
enhancing the efficacy and safety of CRC treatment. By offering a
thorough, evidence-based assessment, the present study aims to
inform clinical practice and support the potential integration of
probiotics into CRC management. Future research should focus on
identifying the most effective probiotic strains and elucidating
the underlying mechanisms through which these microorganisms
influence tumor biology and patient outcomes (4).
Materials and methods
Study registration
The present study adhered to the Preferred Reporting
Items for Systematic Reviews and Meta-Analyses guidelines and was
registered with the International Prospective Register of
Systematic Reviews under the identifier CRD42024510734 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024510734).
Literature search strategy
A systematic review was conducted by searching
multiple databases, including PubMed (https://pubmed.ncbi.nlm.nih.gov), Embase (https://www.embase.com), Cochrane Central Register of
Controlled Trials (https://www.cochranelibrary.com/central), Allied and
Complementary Medicine Database (https://www.proquest.com/amed), the American
Association for Cancer Research abstract database (https://www.aacr.org/) and China Biology Medicine disc
(http://www.sinomed.ac.cn). The search
covered all relevant articles published from the inception of each
database until December 26, 2023, without language restrictions.
The search strategy employed a combination of Medical Subject
Headings terms (https://meshb.nlm.nih.gov) and free-text keywords,
including disease-related search terms such as ‘CRC’, ‘colorectal
tumor’ and ‘colorectal neoplasm’. Intervention terms were comprised
of ‘probiotics’, ‘Lactobacilli’, ‘Bifidobacteria’,
‘Lactococci’, ‘yeast’, ‘Enterococci’,
‘Bifidobacterium’, ‘Lactobacillus’ and
‘Saccharomyces’. To identify relevant study designs, filters
such as ‘RCT’, and ‘placebo-controlled’ were applied. Boolean
operators (AND/OR) and proximity operators (NEAR/3) were used to
enhance search sensitivity. The full search strategy, adapted to
the syntax requirements of each database, is provided in Data S1.
Manual searches were additionally conducted to
ensure literature saturation. These included screening the
reference lists of the included studies and relevant review
articles, as well as reviewing journals subscribed to by the
People's Hospital of Putuo District library. Printed copies of
conference proceedings from the National Conference on Clinical
Nutrition (2021–2023), obtained during conference attendance were
also reviewed.
Where applicable, the corresponding authors of
potentially eligible studies were contacted via email to request
the missing data. Any unresolved missing data were addressed using
sensitivity analyses following the Cochrane Handbook for Systematic
Reviews of Interventions (version 6.3; section 16.1.2; http://training.cochrane.org/handbook/archive/v6.3/chapter-16).
Study inclusion and exclusion
criteria
Publicly accessible RCTs that investigated the
efficacy of probiotic-assisted treatment in patients with CRC who
underwent open, laparoscopic or robotic CRC surgery were included
in this study. Sex and ethnicity were not restricted in the present
study. In the RCTs, the treatment group was treated with any dose
and type of probiotic preparation including Lactobacillus,
Bifidobacterium, Saccharomyces, and multi-strain combinations,
and the control group was treated with a placebo. The efficacy and
safety of the primary outcome measures, including the incidence of
postoperative infections and mean number of hospitalization days,
were explored. Studies in which the patients had advanced CRC who
could not undergo surgery, received other organ resections or had a
combination of other malignant tumors or gastrointestinal diseases
were excluded. Studies that could not extract the primary outcome
data and used probiotics combined with other adjunctive therapies,
in addition to studies on the pharmacokinetics of probiotics
without long-term follow-up outcome data were also excluded.
Literature screening and data
extraction
Two researchers conducted a thorough review of the
literature, extracted the data and cross-checked their findings. In
the event of discrepancies, they engaged in discussions to resolve
them or sought assistance from a third party for adjudication. Data
extraction was performed using a customized form, which encompassed
essential information, such as the authors of the included studies,
publication dates, characteristics of the study subjects, details
of interventions, elements of bias assessment and outcome measures
of interest. During data extraction, the probiotic genus (or
species/strain) used in each intervention arm was recorded for
every included study. The subgroup analyses (Lactobacillus,
Bifidobacterium, Saccharomyces and multi-strain combinations)
were then defined based on the predominant probiotic genus or
formulation type that consistently emerged from this extracted data
across the included studies.
Quality assessment
The risk of bias in the studies included in the
analysis was independently assessed by two investigators and
subsequently verified. The assessment of bias in the included
studies was conducted using the RCT risk of bias assessment tool
recommended by the Cochrane Handbook (21). This evaluation considered multiple
factors, such as the randomization method, allocation concealment,
blinding procedures for patients and investigators, blinding of
outcome assessors, completeness of outcome data, selective
reporting of study results and identification of other potential
sources of bias such as industry sponsorship bias and
geographic/ethnic heterogeneity.
Statistical analysis
Statistical analysis was performed utilizing RevMan
software (version 5.4; The Cochrane Collaboration), with the mean
difference (MD) employed as the effect size for continuous
variables and the risk ratio (RR) for dichotomous variables, each
presented with 95% CIs. Heterogeneity among the study outcomes was
assessed using the χ2 test (α=0.1) in conjunction with
the I2 statistic to measure the extent of
heterogeneity.
Meta-analysis was performed with a random-effects
model. In instances of significant statistical heterogeneity within
the findings of the incorporated studies, the origin of
heterogeneity was explored through subgroup analysis.
Publication bias was evaluated using funnel plots.
Additionally, TSA was performed using TSA software (version 0.9;
Copenhagen Clinical Trials Centre in Denmark) (22). TSA, a form of cumulative
meta-analysis, addresses the issue of random errors (both
false-negative and false-positive outcomes) that can occur in
conventional meta-analysis during repeated updates. Furthermore,
TSA helps determine the sample size required to achieve a reliable
conclusion. TSA analysis excluded zero-event studies (studies with
no infection events in both groups). The cumulative Z-curve
constituted the core graphical output of TSA. This curve plotted
the cumulative Z-scores (standard normal deviates) derived from
sequentially incorporating trial data as they accumulate over time,
thereby tracking the evolving strength of evidence for or against
the intervention effect. TSA employed this curve in conjunction
with pre-specified monitoring boundaries to assess potential early
stopping points. Benefit (efficacy) boundaries are defined such
that if the cumulative Z-curve crosses above this boundary, it
provides statistically significant evidence favoring the
intervention effect, allowing for an early conclusion of benefit.
Conversely, crossing a predefined futility boundary suggests a lack
of clinically meaningful effect. The region between these
boundaries represents a zone of insufficient evidence, indicating
that more data are required. A crucial parameter is the Required
Information Size (RIS), which estimates the total number of
participants needed to detect (or reject) a predefined target
intervention effect (such as, a 15% relative risk reduction) with a
prespecified power (typically 80–90%) while controlling the Type I
error rate (typically 5%). The RIS determines the maximum extent of
the X-axis (representing cumulative information size) on the TSA
graph. Decision rules based on the cumulative Z-curve position
relative to the boundaries are: i) If Z > Upper boundary, firm
evidence of benefit is concluded; ii) if Z < Lower boundary,
firm evidence of futility (or harm) is concluded; iii) if Z remains
between the boundaries, evidence is deemed inconclusive,
necessitating further trials.
Results
Literature search results
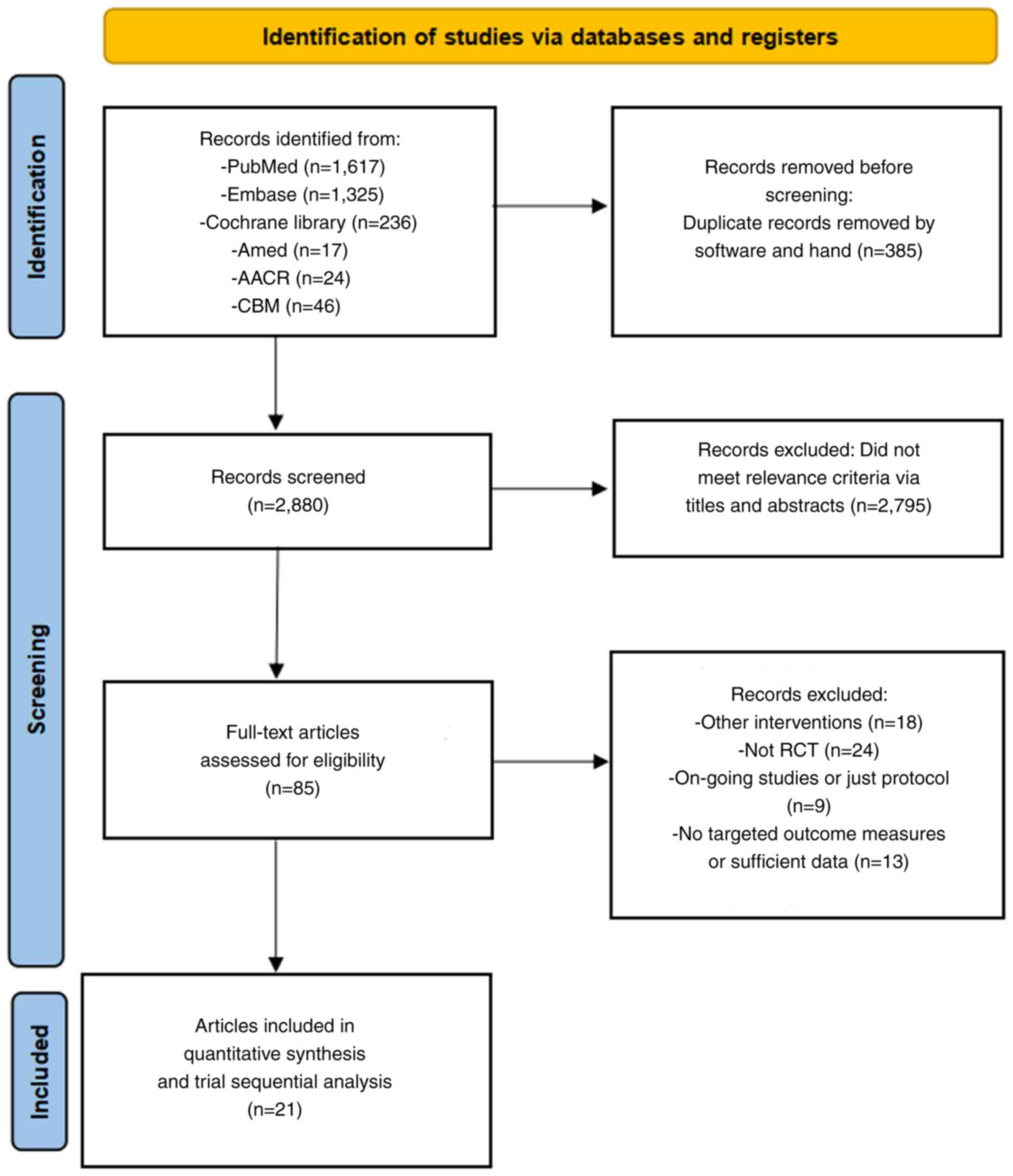
The search strategy initially retrieved 3,265
documents. After removing duplicates and conducting a preliminary
assessment of titles and abstracts, 2,880 documents remained.
Scrutiny of the full texts further narrowed down the total to 64
documents for rescreening, which ultimately identified 21 studies
suitable for quantitative meta-analysis and TSA (23–43).
These studies included 1,602 patients with CRC who had undergone
surgery or were scheduled to undergo surgery. The literature
screening process and outcomes are displayed in Fig. 1.
Basic characteristics of included
studies
Table I presents the
fundamental characteristics of the included studies. The detailed
information includes the country of origin, age range of the
participants, sample size, specific intervention measures and
duration of administration.
 | Table I.Basic characteristics of included
studies. |
Table I.
Basic characteristics of included
studies.
|
|
| Sample size, n |
|
|
|
|
|---|
|
|
|
| Age (range),
years |
|
|
|
|---|
| First author,
year | Country | T | C | Interventions | Duration of
administration | (Refs.) |
|---|
| Xia et al,
2010 | China | 30 | 30 | 67.3 (37–82) | Lactobacillus
casei, | >5 days
preoperatively | (38) |
|
|
|
|
|
| Lactobacillus
acidophilus |
|
|
| Zhang et al,
2010 | China | 30 | 30 | T, 66.7 (41–83); |
Bifidobacterium | For 5 days
preoperatively | (42) |
|
|
|
|
| C, 63.0 (39–81) |
|
|
|
| Liu et al,
2011 | China | 50 | 50 | T, 65.3±11.0; | Lactobacillus
plantarum, | For 6 days
preoperatively | (28) |
|
|
|
|
| C, 65.7±9.9 | Lactobacillus
acidophilus | and 10 days
postoperatively |
|
|
|
|
|
|
| and
Bifidobacterium longum |
|
|
| Zhang et al,
2012 | China | 30 | 30 | T, 67.5 (45–87); | Bifidobacterium
longum, | For 3–5 days
preoperatively | (43) |
|
|
|
|
| C, 61.5 (46–82) | Lactobacillus
acidophilus |
|
|
|
|
|
|
|
| and Enterococcus
faecalis |
|
|
| Liu et al,
2013 | China | 75 | 75 | T,
62.28±12.41; | Lactobacillus
plantarum, | For 6 days
preoperatively | (29) |
|
|
|
|
| C, 66.06±11.02 | Lactobacillus
acidophilus | and 10 days
postoperatively |
|
|
|
|
|
|
| and
Bifidobacterium longum |
|
|
| Pellino et
al, 2013 | Italy | 10 | 8 | T, 71.5±2.1; | Streptococcus
thermophilus, | For 1 day to 4
weeks after | (34) |
|
|
|
|
| C, 72.9±1.6 | Bifidobacterium
longum, | discontinuing
antibiotics |
|
|
|
|
|
|
| Bifidobacterium
breve, |
postoperatively |
|
|
|
|
|
|
| Bifidobacterium
infantis, |
|
|
|
|
|
|
|
| Lactobacillus
acidophilus, |
|
|
|
|
|
|
|
| Lactobacillus
plantarum, |
|
|
|
|
|
|
|
| Lactobacillus
paracasei, |
|
|
|
|
|
|
|
| Lactobacillus
delbrueckii |
|
|
|
|
|
|
|
| subsp.
bulgaricus |
|
|
| Mangell et
al, | Sweden | 32 | 32 | T, 74 (70–80); | Lactobacillus
plantarum | For 8 days
preoperatively | (31) |
| 2012 |
|
|
| C, 70 (64–79) |
| and 5 days
postoperatively |
|
| Chen et al,
2014 | China | 35 | 35 | T, 60.3±17.2; | Clostridium
typhimurium | For 5 days | (24) |
|
|
|
|
| C, 59.8±18.7 | and
Bifidobacterium infantis | preoperatively and
7 days |
|
|
|
|
|
|
|
|
postoperatively |
|
| Sadahiro et
al, | Japan | 100 | 95 | T, 67±9; |
Bifidobacteria | For 7 days
preoperatively | (36) |
| 2014 |
|
|
| C, 66±12 |
| and 5–10 days
postoperatively |
|
| Consoli et
al, | Brazil | 15 | 18 | T, 51 (28–76); | Saccharomyces
boulardii | For at least 7
days | (25) |
| 2016 |
|
|
| C, 59 (17–83) |
| preoperatively |
|
| Liu et al,
2015 | China | 66 | 68 | T,
65.62±18.18; | Lactobacillus
plantarum, | For 6 days
preoperatively | (30) |
|
|
|
|
| C, 60.16±16.20 | Lactobacillus
acidophilus | and 10 days
postoperatively |
|
|
|
|
|
|
| and
Bifidobacterium longum |
|
|
| Mizuta et
al, 2016 | Japan | 31 | 29 | T, 68.9±10.4; | Bifidobacterium
longum | For 7–14 days
preoperatively | (32) |
|
|
|
|
| C, 71.2±9.5 |
| and 14 days
postoperatively |
|
| Yang et al,
2016 | China | 30 | 30 | T, 63.90±
2.25; | Bifidobacterium
longum, | For 5 days
preoperatively | (40) |
|
|
|
|
| C, 62.17±11.06 | Lactobacillus
acidophilus | to 7 days
postoperatively |
|
|
|
|
|
|
| and Enterococcus
faecalis |
|
|
| Kotzampassi et
al, | Greece | 84 | 80 | T, 65.9±11.5; | Lactobacillus
acidophilus, | From the day of
surgery to | (27) |
| 2015 |
|
|
| C, 66.4±11.9 | Lactobacillus
plantarum, | 14 days
postoperatively |
|
|
|
|
|
|
| Bifidobacterium
lactis and |
|
|
|
|
|
|
|
| Saccharomyces
boulardii |
|
|
| Tan et al,
2016 | Malaysia | 20 | 20 | T, 64.3±14.5; | Lactobacillus
acidophilus, | For 8 days
preoperatively | (37) |
|
|
|
|
| C, 68±11.9 | Lactobacillus
casei, |
|
|
|
|
|
|
|
| Lactobacillus
lactis, |
|
|
|
|
|
|
|
| Bifidobacterium
bifidum, |
|
|
|
|
|
|
|
| Bifidobacterium
longum |
|
|
|
|
|
|
|
| and
Bifidobacterium infantis |
|
|
| Bajramagic et
al, | Bosnia and | 39 | 39 | NA | Lactobacillus
acidophilus, | For 3 days | (23) |
| 2019 | Herzegovina |
|
|
| Lactobacillus
casei, | preoperatively
to |
|
|
|
|
|
|
| Lactobacillus
plantarum, | 30 days |
|
|
|
|
|
|
| Lactobacillus
rhamnosus, |
postoperatively |
|
|
|
|
|
|
| Bifidobacterium
lactis, |
|
|
|
|
|
|
|
| Bifidobacterium
bifidum, |
|
|
|
|
|
|
|
| Bifidobacterium
breve, |
|
|
|
|
|
|
|
| Streptococcus
thermophiles |
|
|
| Xu et al,
2019 | China | 30 | 30 | T,
61.03±15.28; | Bifidobacterium
longum, | For 7
consecutive | (39) |
|
|
|
|
| C, 62.35±13.71 | Lactobacillus
acidophilus | days |
|
|
|
|
|
|
| and Enterococcus
faecalis | preoperatively |
|
| Zaharuddin et
al, | Malaysia | 27 | 25 | T, 67.33±9.44; | Lactobacillus
acidophilus, | For 4 weeks | (41) |
| 2019 |
|
|
| C, 66.5±8.57 | Lactobacillus
lactis, | postoperatively
to |
|
|
|
|
|
|
| Lactobacillus
casei subsp, | 6 months |
|
|
|
|
|
|
| Bifidobacterium
longum, |
|
|
|
|
|
|
|
| Bifidobacterium
bifidum |
|
|
|
|
|
|
|
| and
Bifidobacterium infantis |
|
|
| Park et al,
2020 | Korea | 29 | 31 | T, 60.1±10.37; | Bifidobacterium
animalis | For 7 days | (33) |
|
|
|
|
| C, 61.03±7.02 | subsp. Lactis,
Lactobacillus | preoperatively
to |
|
|
|
|
|
|
| casei, and
Lactobacillus | 21 days |
|
|
|
|
|
|
|
plantarum |
postoperatively |
|
|
Rodríguez-Padilla | Spain | 34 | 35 | T, 65 (45–81); | Lactobacillus
acidophilus, | For 20 days | (35) |
| et al,
2021 |
|
|
| C, 68 (41–80) | Lactobacillus
plantarum, | preoperatively |
|
|
|
|
|
|
| Lactobacillus
paracasei, | every second
day |
|
|
|
|
|
|
| Lactobacillus
delbrueckii |
|
|
|
|
|
|
|
| subsp.
bulgaricus., |
|
|
|
|
|
|
|
| Bifidobacterium
breve, |
|
|
|
|
|
|
|
| Bifidobacterium
longum, |
|
|
|
|
|
|
|
| Bifidobacterium
infantis, |
|
|
|
|
|
|
|
| Streptococcus
thermophilus |
|
|
| Huang et al,
2023 | China | 50 | 50 | T,
57.7±11.9; | Bifidobacterium
infants, | From the third | (26) |
|
|
|
|
| C,
62.1±10.5 | Lactobacillus
acidophilus, | postoperative day
to |
|
|
|
|
|
|
| Enterococcus
faecalis, | the end of the
first |
|
|
|
|
|
|
| Bifidobacterium
cereus | 6 weeks |
|
Meta-analysis results
Incidence of postoperative infection
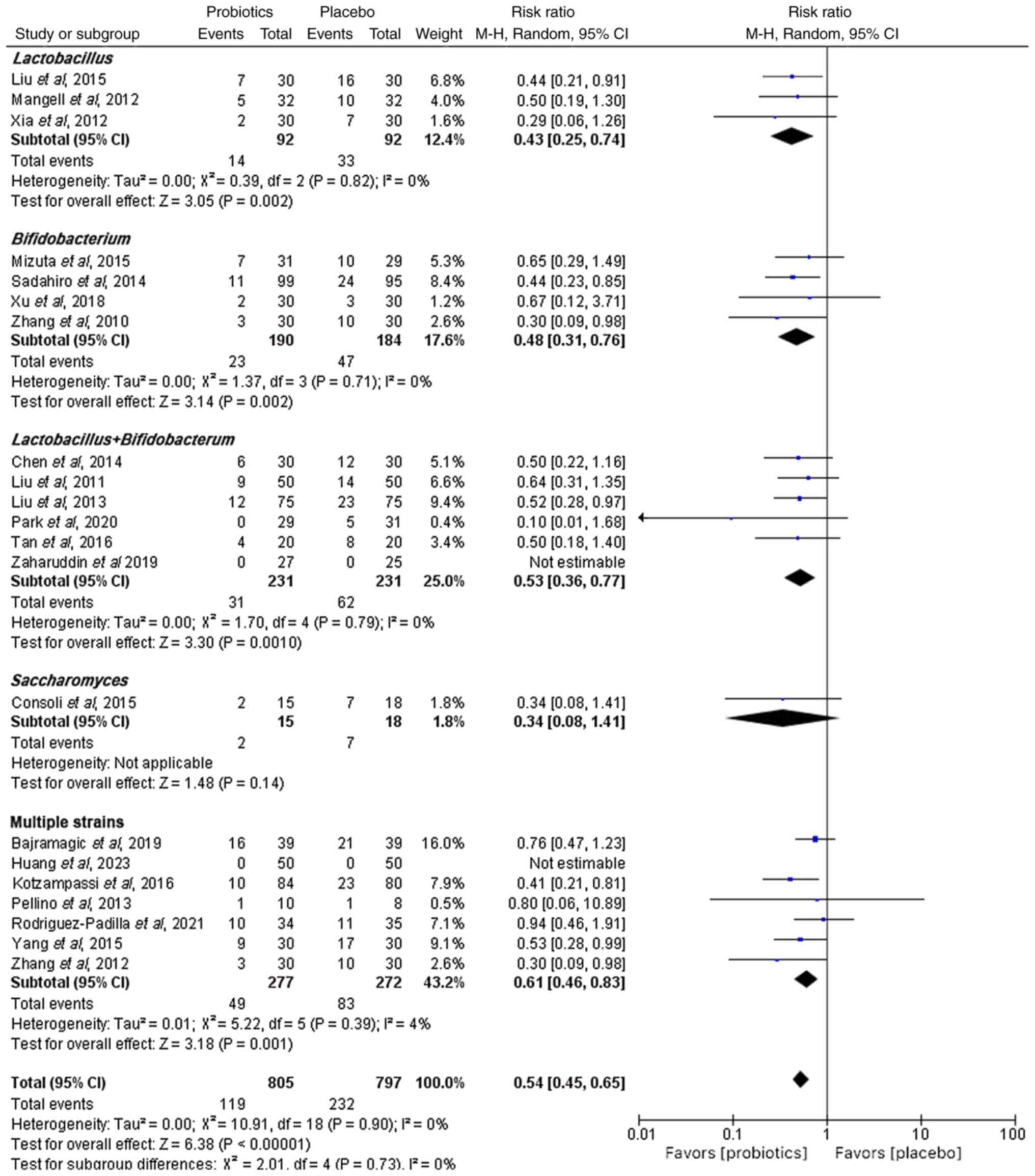
The incidence of postoperative infection was
examined in 21 studies that investigated the efficacy and safety of
probiotics as adjuvant therapy for CRC. A random-effects
meta-analysis demonstrated a significantly lower incidence of
postoperative infection in the probiotic group compared with that
in the placebo group (RR, 0.54; 95% CI, 0.45–0.65; P<0.05)
(Fig. 2). Numerically, the
infection rate was 14.8% (119/805) in probiotic groups vs. 29.1%
(232/797) in controls. Given the various genera of probiotics used
in the included studies, a subgroup analysis based on probiotic
species was conducted, which revealed that using multiple strains
(RR, 0.61; 95% CI, 0.46–0.83; P<0.05), Lactobacillus
species (RR, 0.43; 95% CI, 0.25–0.74; P<0.05),
Bifidobacterium species (RR, 0.48; 95% CI, 0.31–0.76;
P<0.05) and a combination of Lactobacillus and
Bifidobacterium species (RR, 0.53; 95% CI, 0.36–0.77;
P<0.05) significantly reduced the incidence of postoperative
infections compared with the placebo group. However, no significant
difference in the incidence of postoperative infections of the
single strains of Saccharomyces (RR, 0.34; 95% CI,
0.08–1.41; P>0.05) was found compared with that in placebo
group.
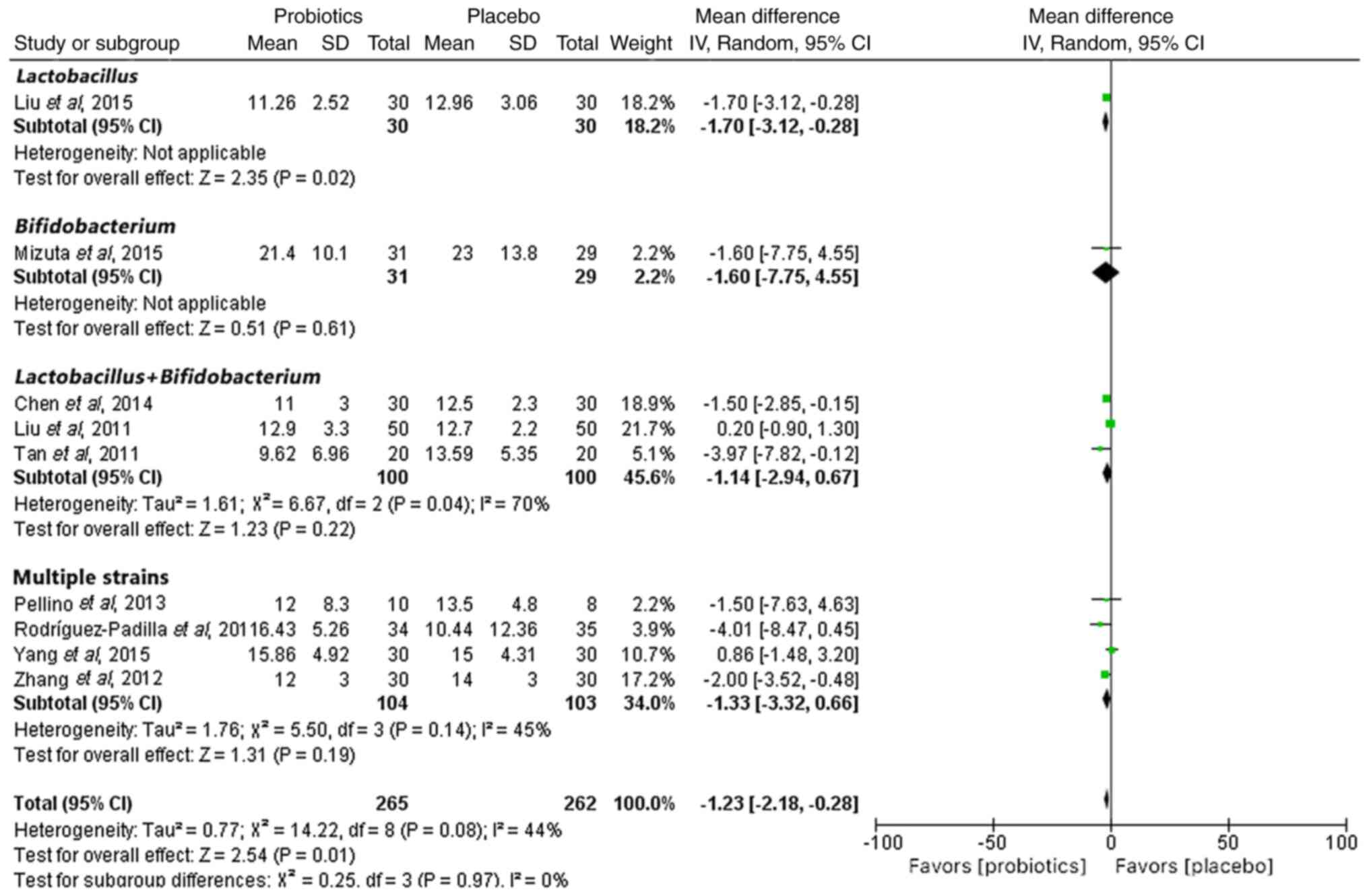
Mean length of hospitalization
The length of hospitalization was reported in nine
of the RCTs. A random-effects meta-analysis indicated that
probiotics significantly reduced the length of hospital stay
compared with that in the placebo group (MD, −1.23; 95% CI, −2.18
to −0.28; P<0.05) (Fig. 3).
However, when analyzing the genera of probiotics used, there was no
significant reduction in the number of days of hospitalization for
any genus except for those using Lactobacillus species (MD,
−1.70; 95% CI, −3.12 to −0.28; P<0.05).
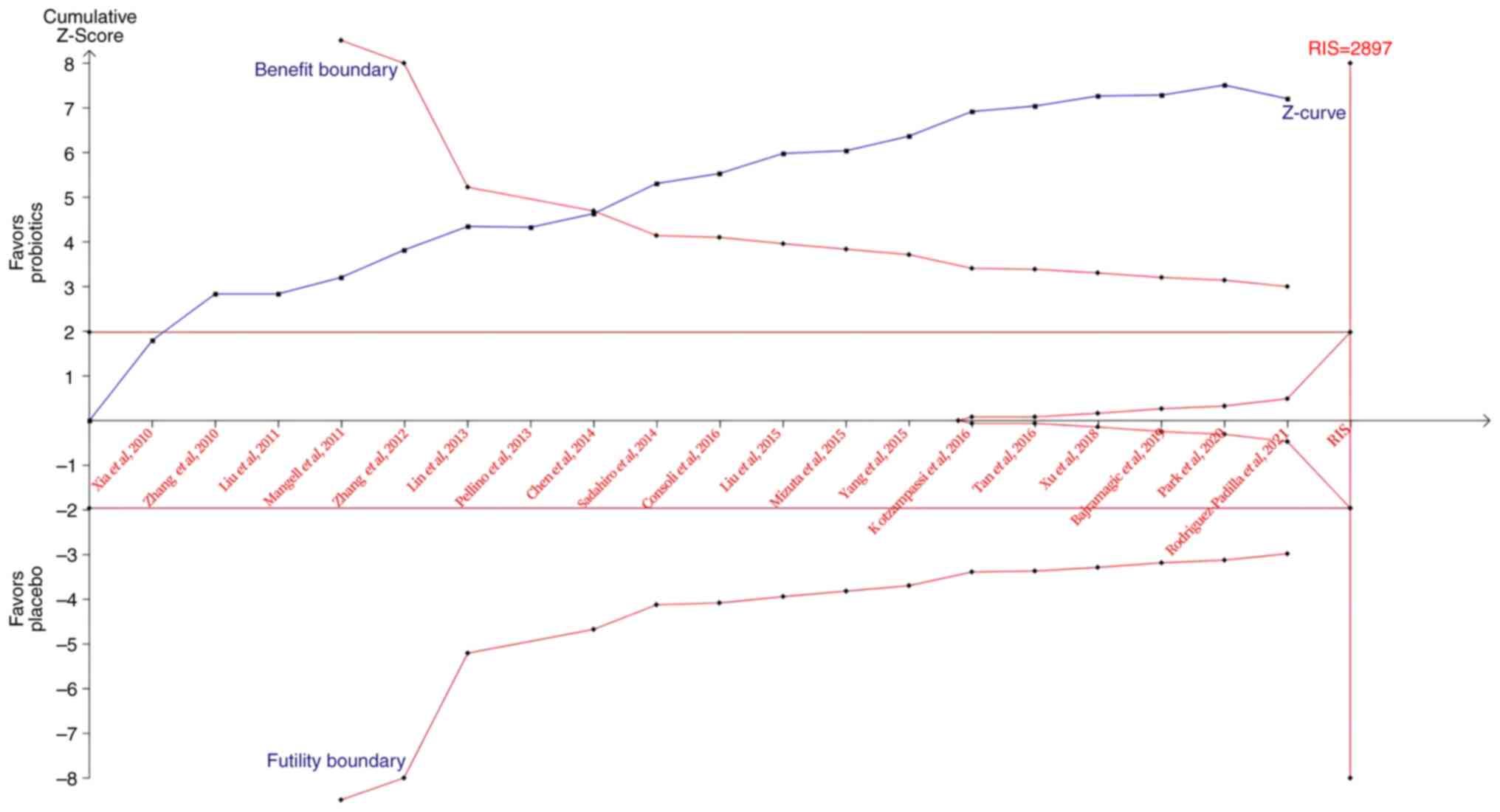
TSA of probiotics in adjuvant therapy
for CRC
TSA results indicated that the cumulative Z-curve
crossed both the traditional and trial sequential monitoring
boundaries for benefit (Fig. 4),
which supports the fact that probiotics are effective in the
prevention of postoperative infections. Although the RIS (2,897
participants) was not reached, the results suggested no need for
further RCTs to confirm this finding.
Risk of bias and publication bias
assessment
The results of the methodological quality
assessment, which illustrate the risk of bias in the included
studies, are presented in Fig. S1.
The funnel plot, constructed using data on postoperative infection
rates, revealed an asymmetrical distribution of studies around the
funnel, which indicated the potential presence of publication bias.
Publication bias analysis is depicted in Fig. S2.
Discussion
In the realm of cancer research, there is a growing
focus on the interplay between diet, gut microbiota and CRC
(44). The human microbiota, which
consists of ~100 trillion microorganisms, forms an intricate
ecosystem that comprises bacteria, viruses, eukaryotes and archaea.
This ecosystem serves a key role in facilitating nutrient
absorption, bolstering host immunity against infections, fortifying
the intestinal immune system and regulating host metabolism
(45). Notably, the composition of
the microbiome differs between healthy individuals and those
diagnosed with CRC. The bacteria implicated in CRC to date include
Fusobacterium nucleatum, Enterococcus faecalis, Streptococcus
gallolyticus, enterotoxigenic Bacteroides fragilis,
Escherichia coli, Peptostreptococcus spp. and
Ruminococcus spp. Conversely, species such as
Lactobacillus spp., Bifidobacterium spp.,
Faecalibacterium spp., Roseburia spp.,
Clostridium spp., Granulicatella spp.,
Streptococcus thermophilus and other members of the
Lachnospiraceae family are diminished in patients with CRC
(46). Based on existing research,
persistent dysbiosis in gut microbiota diversity hinders the
efficacy of chemotherapy and promotes CRC development and
progression. Therefore, the regulation of gut microbiota has
emerged as a novel strategy for managing CRC progression (47,48).
Probiotics, recognized as potential agents that
influence the composition and functionality of human gut
microbiota, can provide therapeutic advantages in patients with CRC
when administered in appropriate dosages (49). Current clinical evidence indicates
that effective dosing typically ranges between 109 and
1011 colony-forming units per day, contingent upon
specific probiotic strains, disease status and therapeutic
objectives. Rigorous dose-finding studies remain necessary to
establish standardized protocols for CRC management (50–52).
The advantageous impacts of probiotics, which act in a species- or
strain-specific manner, encompass the preservation of a healthy
microbiome, reversal of dysbiosis, prevention of pathogenic
infections (such as, rotavirus-induced diarrhea and Clostridioides
difficile infections) and mucosal adhesion of pathogens, and
stabilization and enhancement of intestinal barrier function
(53–56). Probiotic bacteria may confer these
advantages through the production of anti-carcinogenic,
anti-inflammatory, anti-mutagenic and other biologically notable
compounds, such as short-chain fatty acids, which are frequently
associated with gastrointestinal disorders, including CRC (57). Another potential mechanism of
anticancer activity is the enhancement or restoration of natural
killer (NK) cell activity. NK cells serve a key role in regulating
immunity against both cancer and infections, with higher NK cell
activity being associated with a reduced risk of cancer. The
ability of probiotics to boost NK cell activity appears to be
connected to their production of IL-12 (58).
It is well established that postoperative
complications following CRC surgeries notably impact treatment
efficacy, recurrence rates and overall survival in patients.
Minimizing postoperative infection is key for the improvement of
surgical and long-term oncological outcomes. Numerous meta-analyses
have indicated the potential advantages of probiotics in mitigating
postoperative complications among patients with CRC. Persson et
al (59) focused on diarrhea as
the primary outcome, reporting an odds ratio (OR) of 0.42 (95% CI,
0.31–0.55; P<0.05). An et al (60) examined perioperative mortality,
yielding an RR of 0.17 (95% CI, 0.02–1.38; P<0.05), as well as
overall postoperative complications, yielding an RR of 0.45 (95%
CI, 0.27–0.76; P<0.05). Araújo et al (61) investigated various complications,
including ileus, which is impairment of intestinal motility (OR,
0.13; 95% CI, 0.02–0.78; P<0.05), diarrhea (OR, 0.32; 95% CI,
0.15–0.69; P<0.05), abdominal collection (OR, 0.35; 95% CI,
0.13–0.92; P<0.05), sepsis (OR, 0.41; 95% CI, 0.22–0.80;
P<0.05), pneumonia (OR, 0.39; 95% CI, 0.19–0.83; P<0.05) and
surgical site infection (OR, 0.53; 95% CI, 0.36–0.78; P<0.05).
Chen et al (62) assessed
total postoperative infections (OR, 0.31; 95% CI, 0.15–0.64;
P<0.05) and surgical site infections (OR, 0.62; 95% CI,
0.39–0.99; P<0.05). There was no notable rise in adverse events
associated with probiotic use (63).
In the present study, the effectiveness and safety
of probiotics was examined in the prevention of postoperative
infections by incorporating unique methodological features, notably
the use of RRs instead of ORs to reduce the potential
overestimation of adverse effects associated with OR. The present
study involved a more extensive and detailed analysis, including
subgroup and TSA analyses, to evaluate evidence credibility. These
results indicated that probiotics may notably and positively
influence the reduction of postoperative infectious complications.
Postoperative infection represents a multifaceted challenge that
was intentionally not explored in depth within the present study.
Nevertheless, postoperative infection was identified as a primary
outcome due to its substantial clinical importance, as these
infections contribute to increased mortality rates and elevate both
direct and indirect healthcare expenditures. The placebo group
exhibited a postoperative infection incidence of 29.1% (232/797),
whereas the probiotic group demonstrated ~60% lower odds of
infection in patients with CRC. As a result, this outcome may
indirectly underscore the potential advantages of probiotics to
clinicians. In future research, we plan to utilize Mendelian
randomization to investigate the association between gut microbiota
and CRC prognosis. Additionally, the potential advantages of
probiotics in decreasing the mean length of hospital stay for
patients with CRC was investigated. The probiotic group experienced
a decrease in the mean number of hospitalization days by 1 day,
which led to potential cost savings in healthcare. However, it is
well established that length of hospital stay is influenced by
various confounding factors, including patient comorbidities (for
example, diabetes or coronary artery disease), psychological
conditions (for example, anxiety or depression) and the choice of
surgical approach. To mitigate the effects of these confounders, a
random-effects model was utilized. Furthermore, subgroup analyses
based on surgical techniques were performed and aimed to
standardize hospitalization duration by employing hospitalization
costs. However, this cost-based adjustment was not achievable due
to the lack of available data. Future research can include
cost-related outcomes to assess the economic benefits of probiotics
from a health economics perspective.
Furthermore, subgroup analysis indicated that the
effectiveness of various probiotic types in the prevention of
postoperative infections was evident in the combination of multiple
strains, Lactobacillus species, Bifidobacterium
species, and the combination of Lactobacillus and
Bifidobacterium species, but not in single strains of
Saccharomyces. Additionally, smaller studies with shorter
durations tended to exhibit exaggerated effect sizes compared with
larger and longer studies. Accordingly, caution should be exercised
when interpreting the results of small, short-duration studies.
The variability in probiotic types, duration of
administration, study design and outcomes presents a challenge in
conducting TSA. While this variability was considered in the
present analysis, the assumptions regarding the results of future
large trials were based on the expectation of consistency with
existing trial findings. This assumption appears to be justified
for probiotics, given the coherence observed among the results of
large RCTs.
Despite not reaching the RIS of 2,897, the
cumulative Z-curve crossed the boundaries, which allowed a
conclusion to be reached without the need for further trials. To
the best of our knowledge, the present study represents the first
application of TSA to assess the effect of probiotics on the
incidence of postoperative infections, which thereby enhances the
validity of these findings. However, the clinical application of
probiotics in CRC management poses several challenges. Variability
in study designs, probiotic formulations and patient populations
complicates the interpretation and comparison of the results.
Moreover, the optimal strains, dosages and duration of probiotic
therapy have not yet been defined. The limitations of the present
study are largely attributed to the heterogeneity of the included
trials. Several studies featured relatively small sample sizes,
which may have compromised the robustness and generalizability of
their findings. Additionally, the lack of long-term follow-up data
limits the ability to evaluate the sustained effects of probiotics
on CRC outcomes. Variations in patient demographics and clinical
settings further introduce potential statistical biases, which make
it more difficult to draw definitive conclusions from the
aggregated data.
In the past decade, research has increasingly
demonstrated the complex interplay between gut microbiota and
immune responses in maintaining physiological balance and
influencing the development, progression and treatment outcomes of
various diseases, including cancer (64–67).
Leveraging the gut mycobiome for diagnostic, prognostic and
therapeutic purposes in cancer and other disorders (such as
inflammatory bowel disease, metabolic diseases and autoimmune
conditions) demonstrates its potential in precision medicine.
Future research should prioritize large multicentre trials with
standardized protocols to strengthen the evidence for the use of
probiotics as adjunctive therapy in CRC management.
Future therapeutic strategies targeting the
microbiome or drawing inspiration from it should be designed to
modulate gut bacteria synergistically to enhance the effectiveness
of probiotics in clinical settings (68). Consequently, probiotics have the
potential to serve as a preventive measure for CRC and to enhance
treatment, thereby improving clinical outcomes for patients with
CRC in the future. The future of probiotic research in CRC should
focus on developing personalized treatment strategies and
conducting in-depth mechanistic studies. Tailoring probiotic
interventions to individual patient profiles and uncovering the
specific mechanisms through which probiotics exert their effects
will be key to realizing their full potential as adjunctive
therapies. Continued investigation in these areas will not only
enhance the current understanding of probiotics but also pave the
way for more effective targeted treatment strategies to potentially
improve outcomes for patients with CRC in the future.
In conclusion, probiotics demonstrate potential as
an effective strategy for the prevention of postoperative
infectious complications. The current meta-analysis with TSA
suggested that probiotics are efficacious in the prevention of
postoperative infections in patients undergoing colorectal
resection. Therefore, integrating probiotics into routine
perioperative care for CRC surgery may offer notable benefits for
patients in the future. However, further large-scale RCTs are
required to investigate the optimal composition, timing, duration
and dosing schedule of probiotics, particularly in patients with
immunodeficiency and dysbiosis who may be advised against probiotic
use.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Bureau of Science and
Technology of Zhoushan, Zhejiang province (grant no. 2022C31035)
and the Zhejiang Pharmaceutical Association (grant no.
2021ZYYJC04).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YZ, JH and WS conceptualized and designed the study,
conducted the statistical analysis and edited the manuscript. YZ,
JH, QX, FZ, YY and HY conducted data collection and wrote the
manuscript. FZ and WS are responsible for the interpretation of the
research results and confirm the authenticity of all the raw data.
FZ reviewed and gave final approval of the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of People's Hospital of Putuo District (Zhoushan, China;
grant no. 2024014KYLW).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dang HX, Krasnick BA, White BS, Grossman
JG, Strand MS, Zhang J, Cabanski CR, Miller CA, Fulton RS,
Goedegebuure SP, et al: The clonal evolution of metastatic
colorectal cancer. Sci Adv. 6:eaay96912020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD 2021 Diseases, Injuries Collaborators,
. Global incidence, prevalence, years lived with disability (YLDs),
disability-adjusted life-years (DALYs), and healthy life expectancy
(HALE) for 371 diseases and injuries in 204 countries and
territories and 811 subnational locations, 1990–2021: A systematic
analysis for the Global Burden of Disease Study 2021. Lancet.
403:2133–2161. 2024.PubMed/NCBI
|
|
3
|
Morgan E, Arnold M, Gini A, Lorenzoni V,
Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N and Bray F:
Global burden of colorectal cancer in 2020 and 2040: Incidence and
mortality estimates from GLOBOCAN. Gut. 72:338–344. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saffar KN, Larypoor M and Torbati MB:
Analyzing of colorectal cancerrelated genes and microRNAs
expression profiles in response to probiotics Lactobacillus
acidophilus and Saccharomyces cerevisiae in colon cancer cell
lines. Mol Biol Rep. 51:1222024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Türk S, Yılmaz A, Safari Baesmat A, Malkan
UY, Ucar G and Türk C: The probiotic-induced disregulation of
immune-related genes in colon cells and relation with colorectal
cancer. Cell Mol Biol (Noisy-le-grand). 69:37–45. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023.PubMed/NCBI
|
|
7
|
Matsuda A, Maruyama H, Akagi S, Inoue T,
Uemura K, Kobayashi M, Shiomi H, Watanabe M, Arai H, Kojima Y, et
al: Do postoperative infectious complications really affect
long-term survival in colorectal cancer surgery? A multicenter
retrospective cohort study. Ann Gastroenterol Surg. 7:110–120.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo J, Zhou B, Niu Y, Liu L and Yang L:
Engineered probiotics introduced to improve intestinal microecology
for the treatment of chronic diseases: present state and
perspectives. J Diabetes Metab Disord. 22:1029–1038. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SH and Lim YJ: The role of microbiome
in colorectal carcinogenesis and its clinical potential as a target
for cancer treatment. Intest Res. 20:31–42. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Pan L, Wang F, Long F, Yang B and
Tang D: Interventional effects of oral microecological agents on
perioperative indicators of colorectal cancer: A meta-analysis.
Front Oncol. 13:12291772023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khorashadizadeh S, Abbasifar S, Yousefi M,
Fayedeh F and Moodi Ghalibaf A: The role of microbiome and
probiotics in chemo-radiotherapy-induced diarrhea: A narrative
review of the current evidence. Cancer Rep (Hoboken).
7:e700292024.PubMed/NCBI
|
|
12
|
He Z, Xie H, Xu H, Wu J, Zeng W, He Q,
Jobin C, Jin S and Lan P: Chemotherapy-induced microbiota
exacerbates the toxicity of chemotherapy through the suppression of
interleukin-10 from macrophages. Gut Microbes. 16:23195112024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Imanbayev N, Iztleuov Y, Bekmukhambetov Y,
Abdelazim IA, Donayeva A, Amanzholkyzy A, Aigul Z, Aigerim I and
Aslan Y: Colorectal cancer and microbiota: Systematic review. Prz
Gastroenterol. 16:380–396. 2024.PubMed/NCBI
|
|
14
|
Sanders ME, Merenstein DJ, Reid G, Gibson
GR and Rastall RA: Probiotics and prebiotics in intestinal health
and disease: from biology to the clinic. Nat Rev Gastroenterol
Hepatol. 16:605–616. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Azcárate-Peril MA, Sikes M and
Bruno-Bárcena JM: The intestinal microbiota, gastrointestinal
environment and colorectal cancer: A putative role for probiotics
in prevention of colorectal cancer? Am J Physiol Gastrointest Liver
Physiol. 301:G401–G424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paolillo R, Romano Carratelli C,
Sorrentino S, Mazzola N and Rizzo A: Immunomodulatory effects of
Lactobacillus plantarum on human colon cancer cells. Int
Immunopharmacol. 9:1265–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan L, Zhang S, Li H, Yang F, Mushtaq N,
Ullah S, Shi Y, An C and Xu J: The influence of gut microbiota
dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal
cancer. Biomed Pharmacother. 108:184–193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar A, Pramanik J, Batta K, Bamal P,
Gaur M, Rustagi S, Prajapati BG and Bhattacharya S: Impact of
metallic nanoparticles on gut microbiota modulation in colorectal
cancer: A review. Cancer Innov. 3:e1502024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao J, Liao Y, Wei C, Ma Y, Wang F, Chen
Y, Zhao B, Ji H, Wang D and Tang D: Potential ability of probiotics
in the prevention and treatment of colorectal cancer. Clin Med
Insights Oncol. 17:117955492311882252023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu J, Ma X, Wang X, Zhu G, Wang H, Zhang Y
and Li J: Efficacy and safety of compound kushen injection for
advanced colorectal cancer: A systematic review and meta-analysis
of randomized clinical trials with trial sequential analysis.
Integr Cancer Ther. 23:153473542412584582024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Higgins JP, Savovic J and Page MJ: Chapter
8: Assessing risk of bias in a randomized trial. Cochrane Handbook
for Systematic Reviews of Interventions version 6.2. Higgins JP,
Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al: Cochrane;
2021, Available from:. training.cochrane.org/handbook
|
|
22
|
Weng H, Li S and Zeng XT: Application of
the trial sequential analysis soft ware for meta-analysis. Chin J
Evid-Based Med. 16:604–611. 2016.(In Chinese).
|
|
23
|
Bajramagic S, Hodzic E, Mulabdic A, Holjan
S, Smajlovic SV and Rovcanin A: Usage of probiotics and its
clinical significance at surgically treated patients sufferig from
colorectal carcinoma. Med Arch. 73:316–320. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen H, Xia Y, Shi C, Liang Y, Yang Y and
Qin H: Effects of perioperative probiotics administration on
patients with colorectal cancer. Chin J Clin Nutr. 22:82014.(In
Chinese).
|
|
25
|
Consoli ML, da Silva RS, Nicoli JR,
Bruña-Romero O, da Silva RG, de Vasconcelos Generoso S and Correia
MI: Randomized clinical trial: Impact of oral administration of
saccharomyces boulardii on gene expression of intestinal cytokines
in patients undergoing colon resection. JPEN J Parenter Enteral
Nutr. 40:1114–1121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang F, Li S, Chen W, Han Y, Yao Y, Yang
L, Li Q, Xiao Q, Wei J, Liu Z, et al: Postoperative Probiotics
administration attenuates gastrointestinal complications and gut
microbiota dysbiosis caused by chemotherapy in colorectal cancer
patients. Nutrients. 15:3562023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kotzampassi K, Stavrou G, Damoraki G,
Georgitsi M, Basdanis G, Tsaousi G and Giamarellos-Bourboulis EJ: A
Four-Probiotics regimen reduces postoperative complications after
colorectal surgery: A randomized, double-blind, placebo-controlled
study. World J Surg. 39:2776–2783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang
J, Jiang Y, Zhang H, Yang Z, Wang Y and Zheng Q: Randomised
clinical trial: The effects of perioperative probiotic treatment on
barrier function and post-operative infectious complications in
colorectal cancer surgery - a double-blind study. Aliment Pharmacol
Ther. 33:50–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu ZH, Huang MJ, Zhang XW, Wang L, Huang
NQ, Peng H, Lan P, Peng JS, Yang Z, Xia Y, et al: The effects of
perioperative probiotic treatment on serum zonulin concentration
and subsequent postoperative infectious complications after
colorectal cancer surgery: A double-center and double-blind
randomized clinical trial. Am J Clin Nutr. 97:117–126. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Li C, Huang M, Tong C, Zhang X,
Wang L, Peng H, Lan P, Zhang P, Huang N, et al: Positive regulatory
effects of perioperative probiotic treatment on postoperative liver
complications after colorectal liver metastases surgery: A
double-center and double-blind randomized clinical trial. BMC
Gastroenterol. 15:342015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mangell P, Thorlacius H, Syk I, Ahrné S,
Molin G, Olsson C and Jeppsson B: Lactobacillus plantarum 299v does
not reduce enteric bacteria or bacterial translocation in patients
undergoing colon resection. Dig Dis Sci. 57:1915–1924. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mizuta M, Endo I, Yamamoto S, Inokawa H,
Kubo M, Udaka T, Sogabe O, Maeda H, Shirakawa K, Okazaki E, et al:
Perioperative supplementation with bifidobacteria improves
postoperative nutritional recovery, inflammatory response, and
fecal microbiota in patients undergoing colorectal surgery: A
prospective, randomized clinical trial. Biosci Microbiota Food
Health. 35:77–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park IJ, Lee JH, Kye BH, Oh HK, Cho YB,
Kim YT, Kim JY, Sung NY, Kang SB, Seo JM, et al: Effects of
probiotics on the symptoms and surgical ouTComes after anterior
REsection of colon cancer (POSTCARE): A randomized, double-blind,
placebo-controlled trial. J Clin Med. 9:21812020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pellino G, Sciaudone G, Candilio G,
Camerlingo A, Marcellinaro R, De Fatico S, Rocco F, Canonico S,
Riegler G and Selvaggi F: Early postoperative administration of
probiotics versus placebo in elderly patients undergoing elective
colorectal surgery: A double-blind randomized controlled trial. BMC
Surg. 13 (Suppl 2):S572013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rodríguez-Padilla Á, Morales-Martín G,
Pérez-Quintero R, Gómez-Salgado J, Balongo-García R and Ruiz-Frutos
C: Postoperative ileus after stimulation with probiotics before
ileostomy closure. Nutrients. 13:6262021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sadahiro S, Suzuki T, Tanaka A, Okada K,
Kamata H, Ozaki T and Koga Y: Comparison between oral antibiotics
and probiotics as bowel preparation for elective colon cancer
surgery to prevent infection: Prospective randomized trial.
Surgery. 155:493–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tan CK, Said S, Rajandram R, Wang Z,
Roslani AC and Chin KF: Pre-surgical administration of microbial
cell preparation in colorectal cancer patients: A randomized
controlled trial. World J Surg. 40:1985–1992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xia Y, Yang Z, Chen HQ and Qin HL: Effect
of bowel preparation with probiotics on intestinal barrier after
surgery for colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi.
13:528–531. 2010.(In Chinese). PubMed/NCBI
|
|
39
|
Xu Q, Xu P, Cen Y and Li W: Effects of
preoperative oral administration of glucose solution combined with
postoperative probiotics on inflammation and intestinal barrier
function in patients after colorectal cancer surgery. Oncol Lett.
18:694–698. 2019.PubMed/NCBI
|
|
40
|
Yang Y, Xia Y, Chen H, Hong L, Feng J,
Yang J, Yang Z, Shi C, Wu W, Gao R, et al: The effect of
perioperative probiotics treatment for colorectal cancer:
short-term outcomes of a randomized controlled trial. Oncotarget.
7:8432–8440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zaharuddin L, Mokhtar NM, Muhammad Nawawi
KN and Raja Ali RA: A randomized double-blind placebo-controlled
trial of probiotics in post-surgical colorectal cancer. BMC
Gastroenterol. 19:1312019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang W, Du P, Chen D, Cui L and Ying C:
Effect of viable bifidobacterium supplementon theimmune statusand
inflanunatory response inpatients undergoing resection for
colorectal cancer. Chin J Gastrointest Surg. 13:40. 2010.
|
|
43
|
Zhang JW, Du P, Gao J, Yang BR, Fang WJ
and Ying CM: Preoperative probiotics decrease postoperative
infectious complications of colorectal cancer. Am J Med Sci.
343:199–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu J, Nong C, Zhao J, Meng L and Song J:
An integrative bioinformatic analysis of microbiome and
transcriptome for predicting the risk of colon adenocarcinoma. Dis
Markers. 2022:79940742022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang H, Lei L, Zhou Y, Ye F and Zhao G:
Dietary flavonoids and the risk of colorectal cancer: An updated
meta-analysis of epidemiological studies. Nutrients. 10:9502018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Al-Nemari R, Al-Senaidy A, Semlali A,
Ismael M, Badjah-Hadj-Ahmed AY and Ben Bacha A: GC-MS profiling and
assessment of antioxidant, antibacterial, and anticancer properties
of extracts of Annona squamosa L. leaves. BMC Complement Med Ther.
20:2962020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wong CC and Yu J: Gut microbiota in
colorectal cancer development and therapy. Nat Rev Clin Oncol.
20:429–452. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun L, Zhu Z, Jia X, Ying X, Wang B, Wang
P, Zhang S and Yu J: The difference of human gut microbiome in
colorectal cancer with and without metastases. Front Oncol.
12:9827442022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang W, An Y, Qin X, Wu X, Wang X, Hou H,
Song X, Liu T, Wang B, Huang X and Cao H: Gut microbiota-derived
metabolites in colorectal cancer: The bad and the challenges. Front
Oncol. 11:7396482021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hill C, Guarner F, Reid G, Gibson GR,
Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S,
et al: Expert consensus document. The international scientific
association for probiotics and prebiotics consensus statement on
the scope and appropriate use of the term probiotic. Nat Rev
Gastroenterol Hepatol. 11:506–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Suez J, Zmora N, Segal E and Elinav E: The
pros, cons, and many unknowns of probiotics. Nat Med. 25:716–729.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sonnenburg JL and Bäckhed F:
Diet-microbiota interactions as moderators of human metabolism.
Nature. 535:56–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Szajewska H and Kołodziej M: Systematic
review with meta-analysis: Saccharomyces boulardii in the
prevention of antibiotic-associated diarrhoea. Aliment Pharmacol
Ther. 42:793–801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Szajewska H, Guarino A, Hojsak I, Indrio
F, Kolacek S, Shamir R, Vandenplas Y and Weizman Z; European
Society for Pediatric Gastroenterology, Hepatology, Nutrition, :
Use of probiotics for management of acute gastroenteritis: A
position paper by the ESPGHAN Working Group for Probiotics and
Prebiotics. J Pediatr Gastroenterol Nutr. 58:531–539. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cani PD and Jordan BF: Gut
microbiota-mediated inflammation in obesity: A link with
gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 15:671–682.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang HC, Cai BH, Suen CS, Lee HY, Hwang
MJ, Liu FT and Kannagi R: BGN/TLR4/NF-B mediates epigenetic
silencing of immunosuppressive siglec ligands in colon cancer
cells. Cells. 9:3972020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu R, Shen Q, Li P and Shang N: Sturgeon
chondroitin sulfate restores the balance of gut microbiota in
colorectal cancer bearing mice. Int J Mol Sci. 23:37232022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Persson JE, Viana P, Persson M, Relvas JH
and Danielski LG: Perioperative or postoperative probiotics reduce
treatment-related complications in adult colorectal cancer patients
undergoing surgery: A systematic review and meta-analysis. J
Gastrointest Cancer. 55:740–748. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
An S, Kim K, Kim MH, Jung JH and Kim Y:
Perioperative probiotics application for preventing postoperative
complications in patients with colorectal cancer: A systematic
review and meta-analysis. Medicina (Kaunas). 58:16442022.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Araújo MM, Montalvão-Sousa TM, Teixeira
PDC, Figueiredo ACMG and Botelho PB: The effect of probiotics on
postsurgical complications in patients with colorectal cancer: A
systematic review and meta-analysis. Nutr Rev. 81:493–510. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen C, Wen T and Zhao Q: Probiotics used
for postoperative infections in patients undergoing colorectal
cancer surgery. Biomed Res Int. 2020:57347182020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen Y, Qi A, Teng D, Li S, Yan Y, Hu S
and Du X: Probiotics and synbiotics for preventing postoperative
infectious complications in colorectal cancer patients: A
systematic review and meta-analysis. Tech Coloproctol. 26:425–436.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jaye K, Li CG and Bhuyan DJ: The complex
interplay of gut microbiota with the five most common cancer types:
From carcinogenesis to therapeutics to prognoses. Crit Rev Oncol
Hematol. 165:1034292021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Iida N, Dzutsev A, Stewart CA, Smith L,
Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S,
et al: Commensal bacteria control cancer response to therapy by
modulating the tumor microenvironment. Science. 342:967–970. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Belkaid Y and Hand TW: Role of the
microbiota in immunity and inflammation. Cell. 157:121–41. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang F, Aschenbrenner D, Yoo JY and Zuo
T: The gut mycobiome in health, disease, and clinical applications
in association with the gut bacterial microbiome assembly. Lancet
Microbe. 3:e969–e983. 2022. View Article : Google Scholar : PubMed/NCBI
|