Introduction
Recent advances in immune checkpoint inhibitors
(ICIs) targeting the programmed cell death protein 1
(PD-1)/programmed cell death ligand 1 (PD-L1) pathway have
significantly improved treatment outcomes in non-small cell lung
cancer (NSCLC), with improvements in overall survival (OS) and
progression-free survival (PFS) (1–6). ICIs
can yield favorable clinical outcomes even in patients with NSCLC
and bone metastases (BoMs) (7–11), a
condition traditionally associated with poor prognosis (12–14).
However, not all patients respond favorably, highlighting a need
for continued research to enhance the therapeutic efficacy of
ICIs.
In our clinical experience, we have observed several
cases of NSCLC with BoMs where bone-targeted radiotherapy (RT)
combined with ICIs achieved lung tumor shrinkage and prolonged
survival. This phenomenon, where tumors shrink at sites distant
from the irradiated area, is known as the abscopal effect (15). RT promotes an antitumor immune
response by inducing antigen release and immunogenic cell death,
enhancing maturation and antigen presentation by antigen-presenting
cells, mobilizing T cells, and sensitizing tumor cells to
immune-mediated cell death, which may underlie the abscopal effect
(15,16). Although the abscopal effect is rare
and its mechanisms are unclear (17), ICIs may enhance immune responses
induced by RT, suggesting a potential synergistic effect that could
improve ICI efficacy (16–20). Notably, increased infiltration and
enhanced cytotoxic function of CD8+ T cells within the
tumor immune microenvironment have been reported to play a crucial
role in augmenting the abscopal effect (21,22).
However, the relationship between RT for BoMs and the abscopal
effect in ICI-treated NSCLC has not been elucidated. The study
aimed to determine whether RT for BoMs induces an abscopal effect
in ICI-treated NSCLC and the clinical benefits of this effect.
Patients and methods
Study design and patient
population
This retrospective study included patients with
advanced NSCLC diagnosed with BoMs before receiving ICI treatment
between January 2016 and March 2024 at Kanazawa University
Hospital. The ICIs used in this study were PD-1 inhibitors
(nivolumab and pembrolizumab) and PD-L1 inhibitors (atezolizumab
and durvalumab). For patients with negative driver-gene mutations
and a PD-L1 tumor proportion score (TPS) >50%, first-line
treatment was PD-1/PD-L1 inhibitor monotherapy or combination
therapy with platinum-based agents. In other cases, ICIs were
employed as second-line or later therapy following failure of
conventional chemotherapy or molecular-targeted treatments. To
accurately evaluate the effects of RT on BoMs, patients who had
received RT for lung lesions or brain metastases, as well as those
who had been treated with bone-modifying agents for BoMs, were
excluded. Additionally, patients with a performance status of 3 or
higher and those who received combination therapy with PD-1/PD-L1
inhibitors and cytotoxic T-lymphocyte-associated protein-4
inhibitors were excluded. This study was approved by the Medical
Ethics Committee of Kanazawa University (approval number: 3339-1)
and was conducted in accordance with relevant laws and
institutional guidelines as well as with the tenets enunciated in
the Declaration of Helsinki. Written informed consent was waived
due to the retrospective design. Instead, consent was obtained via
an opt-out method approved by the ethics committee, with study
information provided publicly to allow patients to decline
participation.
Data analysis
The medical records used in this study were
collected from the Kanazawa University Hospital database. Data
included patient characteristics such as age, sex, histological
type, Eastern Cooperative Oncology Group performance status, PD-L1
TPS, number of BoM, visceral metastasis, and ICI and RT history,
including timing and site of BoM. All data were reviewed
independently by at least two investigators to ensure accuracy and
consistency. Patients who received RT to treat multiple bone
lesions were excluded to evaluate the therapeutic effect of
radiation on a single metastatic bone lesion. Participants were
divided into two groups: irradiated (RT-BoM) and non-irradiated
BoMs (non-RT-BoM), and clinical outcomes were evaluated by
assessing responses in lung lesions, OS, PFS, and the incidence of
immune-related adverse events (irAEs, grade ≥3 based on Common
Terminology Criteria for Adverse Events version 5.0). To evaluate
local control of BoMs with and without irradiation, spinal
paralysis and pathological fractures during ICI treatment were
assessed. Lung lesion response was assessed based on Response
Evaluation Criteria in Solid Tumors version 1.1, with size changes
measured via computed tomography between ICI initiation and the
final follow-up. This radiological evaluation was performed by at
least two physicians, including radiologists, to ensure accuracy
and reliability.
Statistical analysis
OS and PFS from ICI treatment initiation were
assessed using the Kaplan-Meier curve analysis. All clinical data
were used as variables, with Fisher's exact and log-rank tests to
compare between the two groups. In addition, logistic regression
and the COX proportional hazards models were used in multivariate
analysis to assess correlations between clinical data and outcomes
and identify independent predictive factors. Cases with missing
data for key variables or primary outcomes were excluded from the
analysis. However, PD-L1 TPS was not assessed in some patients;
these cases were included in the analysis as a separate unknown
category. A P-value <0.05 was considered significant, and EZR
software (Saitama Medical Center, Jichi Medical University,
Saitama, Japan) was used for all statistical analyses.
Results
Clinical characteristics
In total, 108 patients with NSCLC having BoMs were
enrolled, and their clinical characteristics are summarized in
Table I. This study included 80 men
and 28 women, with a mean age of 66.6±8.6 years. The median
follow-up time from initiation of ICI treatment was 21.8 (2–101)
months. The RT-BoM and non-RT-BoM groups included 33 and 75
patients, respectively, with no significant difference observed for
clinical characteristics (Table I).
In the RT-BoM group, the dose/fraction was 2-8 Gy/1-15 fx (total
dose 8-39 Gy) and the most frequently irradiated site was the
spine. Additionally, 66.7% of cases received radiation before
initiating ICI (Table I).
 | Table I.Clinical characteristics of
participants. |
Table I.
Clinical characteristics of
participants.
| Clinical
characteristic | RT-BoM, n
(n=33) | Non-RT-BoM, n
(n=75) | P-value |
|---|
| Sex |
|
| 0.63 |
|
Male | 26 | 54 |
|
|
Female | 7 | 21 |
|
| Age, years |
|
| 0.52 |
|
≥70 | 21 | 42 |
|
|
<70 | 12 | 33 |
|
| Histology |
|
| 0.81 |
|
Adenocarcinoma | 25 | 59 |
|
|
Non-adenocarcinoma | 8 | 16 |
|
| PD-L1 TPS, % |
|
| 0.98 |
|
<50 | 15 | 34 |
|
|
≥50 | 7 | 17 |
|
|
Unknown | 11 | 24 |
|
| PS |
|
| 0.51 |
| ≤1 | 28 | 68 |
|
| 2 | 5 | 7 |
|
| ICIs |
|
| 0.98 |
| PD-1
inhibitor | 22 | 51 |
|
| PD-L1
inhibitor | 11 | 24 |
|
| Treatment line of
ICIs |
|
| 0.19 |
|
1st | 18 | 51 |
|
|
≥2nd | 15 | 24 |
|
| Number of BoM |
|
| 0.81 |
|
Monostotic | 9 | 18 |
|
|
Multiple | 24 | 57 |
|
| Visceral
metastasis |
|
| 0.62 |
|
Yes | 27 | 57 |
|
| No | 6 | 18 |
|
| Radiation to BoM
timing |
|
|
|
| Pre-ICI
treatment | 22 |
|
|
|
Post-ICI treatment | 11 |
|
|
| Site |
|
|
|
|
Spine | 20 |
|
|
|
Pelvis | 11 |
|
|
| Long
bones of the extremities | 2 |
|
|
Clinical outcomes between RT-BoM vs.
non-RT-BoM groups
The overall response rate of lung lesions in the
RT-BoM group was 42.4%, which was significantly better than that in
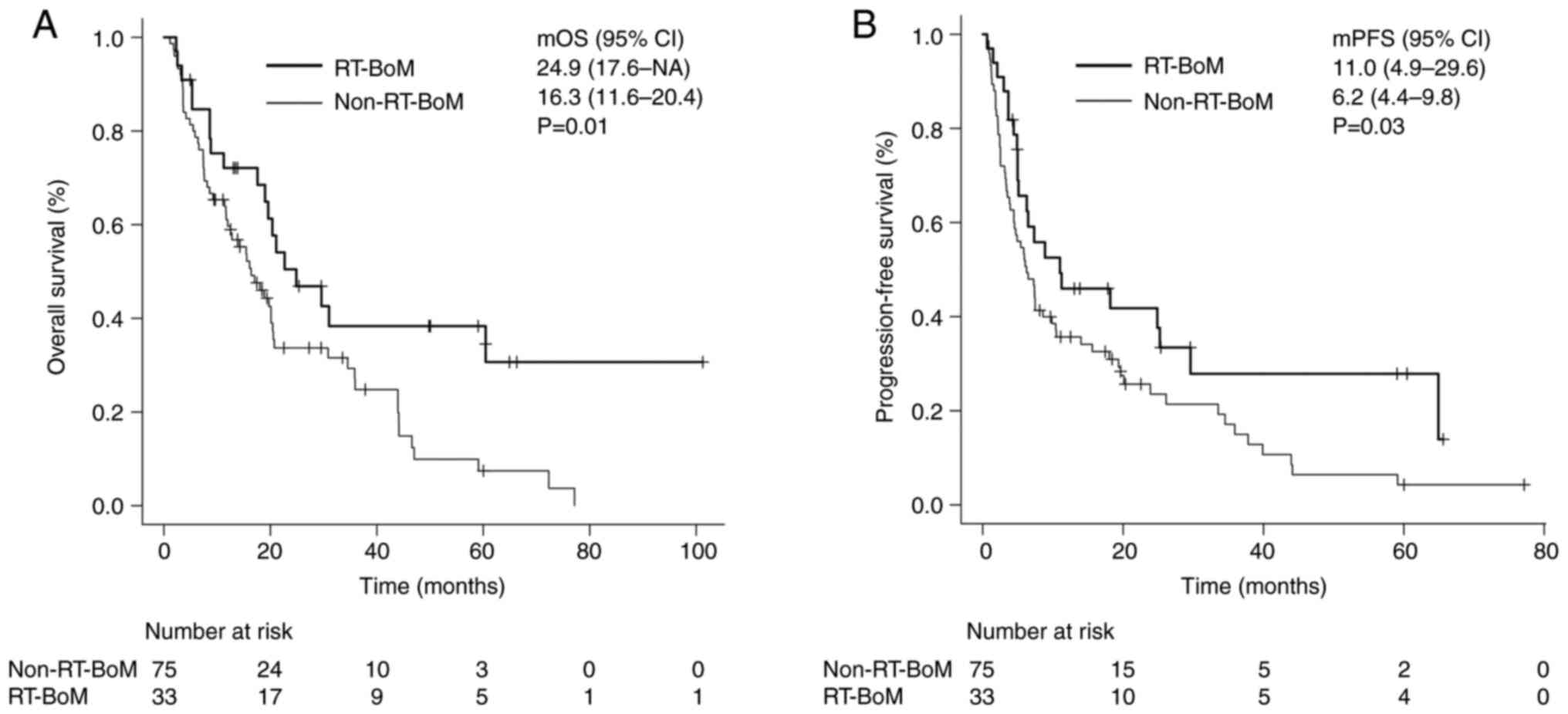
the non-RT-BoM group (21.3%, P=0.03). The median OS and PFS in the
RT-BoM group were 24.9 (17.6-NA) and 11.0 (4.9-29.6) months,
respectively, significantly longer than those in the non-RT-BoM
group [16.3 (11.6-20.4) months, P=0.01; 6.2 (4.4-9.8) months,
P=0.03] (Fig. 1A and B). The
incidence of irAEs in the RT-BoM group was 21.2%, similar to that
in the non-RT-BoM group (21.3%), with no significant difference
(P=0.99). No skeletal-related events (SREs) were observed after
radiation in the RT-BoM group; however, two cases of pathological
fracture were observed in the non-RT-BoM group during ICI treatment
(both cases required surgery for vertebral pathological fractures
and paralysis).
Furthermore, subgroup analyses were performed for
the RT-BoM group. The group that received radiation before ICI
initiation (n=22) had a significantly better lung lesion response
rate than the group that received radiation after ICI initiation
(n=11) (54.5 vs. 9.1%, P=0.02). Although no significant difference
was observed in OS [24.9 (18.9-NA) vs. 21.0 (3.3-NA)months, P=0.58]
or PFS [18.2 (5.1-NA) vs. 6.4 (1.4-64.9) months, P=0.45] between
the two groups, a trend toward prolonged OS and PFS was noted in
the group that received radiation before initiating ICIs.
Irradiation sites of BoM were analyzed in the spine (n=22) and
pelvis (n=11), where case numbers were high. No significant
differences were observed between lung lesion response (22.7 vs.
36.3%, P=0.61), OS [24.8 (8.6-NA) vs. 22.6 (8.6-NA) months,
P=0.44), and PFS [8.9 (3.6-NA) vs. 18.1 (4.8-NA) months, P=0.27].
However, due to the limited number of cases in each group, these
findings should be interpreted with caution.
Predictive factors of clinical
outcomes
Univariate analysis identified predictors of lung
lesion response, revealing significant differences in sex [odds
ratio and 95% confidence interval: 0.23 (0.06-0.85), P=0.02] and
radiation for BoM (2.72 (1.12-6.58), P=0.01]. Multivariate analysis
incorporating these factors revealed that radiation for BoM was an
independent predictor [3.69 (1.20-11.40), P=0.02] (Table II). Univariate analysis for OS
predictors showed significant differences in visceral metastasis
[hazard ratio and 95% confidence interval: 2.72 (1.12-6.58),
P=0.02] and radiation for BoM [2.21 (1.22-3.97), P<0.01].
Multivariate analysis confirmed radiation for BoM as an independent
predictor of OS [2.22 (1.23-4.01), P<0.01] (Table III). For PFS, univariate analysis
showed significant differences in sex [1.68 (1.05-2.70), P=0.03],
treatment line of ICIs [1.73 (1.12-2.67), P=0.01], and radiation
for BoM [0.60 (0.36-0.97), P=0.04]. Multivariate analysis
incorporating these factors revealed that treatment line of ICIs
(1.84 [1.16-2.91], P=0.01) and radiation for BoM (0.56 [0.33-0.93],
P=0.02) were independent predictors of PFS (Table IV).
 | Table II.Univariate and multivariate analyses
of lung lesion response. |
Table II.
Univariate and multivariate analyses
of lung lesion response.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | Comparison | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Sex | Male vs.
female | 0.23
(0.06-0.85) | 0.02 | 0.48
(0.11-2.17) | 0.34 |
| Age | ≥70 vs. <70
years | 1.11
(0.46-2.58) | 0.82 |
|
|
| Histology | ADC vs.
non-ADC | 2.29
(0.88-5.94) | 0.09 |
|
|
| PD-L1 TPS | <50 vs. ≥50 | 1.25
(0.43-3.58) | 0.67 |
|
|
| PS | ≤1 vs. 2 | 2.03
(0.59-6.97) | 0.26 |
|
|
| ICIs | PD-1 inhibitor vs.
PD-L1 inhibitor | 0.42
(0.15-1.15) | 0.09 |
|
|
| Treatment line of
ICIs | 1st vs. ≥2nd | 0.43
(0.16-1.14) | 0.09 |
|
|
| Number of BoMs | Monostotic vs.
multiple | 1.65
(0.59-4.59) | 0.33 |
|
|
| Visceral
metastasis | Yes vs. no | 2.50
(0.77-8.02) | 0.12 |
|
|
| Radiation to
BoM | Yes vs. no | 2.72
(1.12-6.58) | 0.02 | 3.69
(1.20-11.40) | 0.02 |
 | Table III.Univariate and multivariate analyses
of overall survival. |
Table III.
Univariate and multivariate analyses
of overall survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | Comparison | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex | Male vs.
female | 1.33
(0.82-2.17) | 0.23 |
|
|
| Age | ≥70 vs. <70
years | 1.42
(0.91-2.23) | 0.11 |
|
|
| Histology | ADC vs.
non-ADC | 1.01
(0.57-1.74) | 0.99 |
|
|
| PD-L1 TPS | <50 vs. ≥50 | 1.01
(0.53-1.86) | 0.98 |
|
|
| PS | ≤1 vs. 2 | 1.17
(0.61-2.29) | 0.63 |
|
|
| ICIs | PD-1 inhibitor vs.
PD-L1 inhibitor | 1.31
(0.81-2.11) | 0.28 |
|
|
| Treatment line of
ICIs | 1st vs. ≥2nd | 1.56
(0.99-2.46) | 0.06 |
|
|
| Number of BoMs | Monostotic vs.
multiple | 1.54
(0.90-2.61) | 0.11 |
|
|
| Visceral
metastasis | Yes vs. no | 2.72
(1.12-6.58) | 0.02 | 1.28
(0.80-2.04) | 0.31 |
| Radiation to
BoM | Yes vs. no | 2.21
(1.22-3.97) | <0.01 | 2.22
(1.23-4.01) | <0.01 |
 | Table IV.Univariate and multivariate analyses
of progression-free survival. |
Table IV.
Univariate and multivariate analyses
of progression-free survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | Comparison | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex | Male vs.
female | 1.68
(1.05-2.70) | 0.03 | 1.51
(0.93-2.45) | 0.08 |
| Age | ≥70 vs. <70
years | 0.91
(0.59-1.41) | 0.69 |
|
|
| Histology | ADC vs.
non-ADC | 0.87
(0.52-1.47) | 0.62 |
|
|
| PD-L1 TPS | <50 vs. ≥50 | 0.85
(0.47-1.51) | 0.58 |
|
|
| PS | ≤1 vs. 2 | 1.45
(0.76-2.74) | 0.25 |
|
|
| ICIs | PD-1 inhibitor vs.
PD-L1 inhibitor | 1.31
(0.82-2.11) | 0.24 |
|
|
| Treatment line of
ICIs | 1st vs. ≥2nd | 1.73
(1.12-2.67) | 0.01 | 1.84
(1.16-2.91) | 0.01 |
| Number of BoMs | Monostotic vs.
multiple | 1.56
(0.93-2.61) | 0.08 |
|
|
| Visceral
metastasis | Yes vs. no | 1.43
(0.83-2.44) | 0.18 |
|
|
| Radiation to
BoM | Yes vs. no | 2.21
(1.22-3.97) | <0.01 | 2.22
(1.23-4.01) | <0.01 |
Discussion
Our study found that combined therapy with ICIs and
RT for BoM in advanced NSCLC may induce a favorable response in
lung lesions, considered an abscopal effect, and prolong prognosis,
in addition to providing good local control of BoMs.
Recent, large-scale studies on immunotherapy
combining ICIs and RT (23–32) have demonstrated that ICI treatment
enhances the immune response induced by RT, whereas RT enhances the
therapeutic effect of ICIs, confirming the existence of the
abscopal effect (18–20). Several systematic reviews and
meta-analyses have reported the occurrence of the abscopal effect
at distant sites, alongside prolonged OS and PFS with the
combination of ICIs and RT (20,33–36).
However, the abscopal effect remains rare, and its underlying
mechanism is not yet fully understood (17). Additionally, the relationship
between irradiation of BoMs and abscopal effect in NSCLC has rarely
been studied.
BoM is a poor prognostic factor in lung cancer
(12–14), and SREs, such as severe pain,
pathological fracture, and spinal cord compression, significantly
reduces daily activity and quality of life (37,38).
Therefore, early management of BoMs is crucial to prevent SREs.
Generally, RT and bone-modifying agents are commonly used in
combination to avoid interfering with systemic therapy (39–41).
This study focused on RT for BoMs and explored its potential to
improve ICI treatment efficacy. This favorable treatment effect,
which can be considered an abscopal effect, may improve clinical
outcomes for patients with NSCLC presenting BoMs, who typically
have a poor prognosis.
Our study found that irradiation of BoMs improved
the response rate of lung lesions and prolonged prognosis,
consistent with reports of the abscopal effect induced by RT of
lung or brain lesions (20,33–36),
as well as recent findings by Facilissimo et al (42) demonstrating similar benefits of RT
to BoMs in NSCLC patients receiving ICIs. In addition, no spinal
paralysis and pathological fractures occurred at the irradiated
site, and good local control was achieved. The incidence of grade 3
or higher irAEs, evaluated as a safety measure, was similar between
the RT-BoM and non-RT-BoM groups and consistent with previous
studies (36,43,44).
Subgroup analysis for investigating optimal RT strategy suggested
that RT prior to ICI treatment may result in better clinical
outcomes. Our results aligned with the suggestion that the optimal
timing for RT is either concomitant with or prior to ICI
administration (19,45). Based on these results, irradiated
BoMs before initiating ICIs may provide good local control,
pulmonary response, and prolonged prognosis, which can be
considered an abscopal effect, for advanced NSCLC. Our analysis
found no significant differences in clinical outcomes among
different irradiated BoM sites, suggesting that therapeutic
benefits may apply broadly. However, due to limited sample size and
site heterogeneity, further studies are needed to explore
site-specific effects and optimize treatment. While our results are
promising, the retrospective nature and heterogeneity in RT
protocols preclude definitive clinical recommendations. Further
prospective studies are needed to determine the optimal timing,
dosage, fractionation, and the role of irradiated sites to
establish standardized treatment protocols for clinical
practice.
This study had several limitations. First, it was a
retrospective analysis conducted at a single center without
randomization or a control group, which may have introduced
selection bias and limited generalizability. Second, the RT-BoM
group included a small number of patients (n=33), and RT regimens
varied in dose, fractionation, and treatment site, limiting the
evaluation of specific RT parameters. Third, although a possible
abscopal effect was suggested, its underlying mechanisms remain
unclear. Notably, we did not assess changes in the tumor immune
microenvironment or PD-L1 expression, which limits interpretation
of the immunological response. Further prospective, multi-center
studies, including randomized controlled trials with standardized
RT protocols and immune profiling, are necessary to validate and
expand upon these findings.
In conclusion, in ICI treatment of NSCLC with BoMs,
irradiation of BoMs was associated with improved lung lesion
response and prolonged prognosis, suggesting a possible abscopal
effect. In addition to local control of BoMs, systemic clinical
benefits were observed. However, due to the limitations of this
study, including its retrospective single-center design, small
sample size, and heterogeneity in RT protocols, these findings
should be interpreted with caution. Further prospective,
multi-center studies and mechanistic investigations are warranted
to confirm and better understand the observed effects and to
develop an optimized immunoradiotherapeutic strategy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available due to privacy or ethical restrictions but may
be requested from the corresponding author.
Authors' contributions
YA, KH, MO, IM, SY and SD conceptualized the study.
YA, KH, SM, YT, MO, IM and SY developed the methodology. YA
performed the formal analysis. YA, KH, SM, YT, MO, IM and SY
conducted the investigation and data acquisition. KH, SY and SD
confirm the authenticity of all the raw data. YA wrote the original
draft. KH, MO, IM, SY and SD performed review and editing. KH and
SD supervised the study. All authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Kanazawa University (no. 3339-1; Kanazawa, Japan). The
requirement for written informed consent was waived due to the
retrospective design. Instead, consent was obtained via an opt-out
method approved by the ethics committee, with study information
provided publicly to allow patients to decline participation.
Patient consent for publication
Written informed consent for publication was not
required for the present study because all data were fully
anonymized and contained no identifiable patient information.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BoM
|
bone metastasis
|
|
ICI
|
immune checkpoint inhibitor
|
|
irAE
|
immune-related adverse event
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
PD-1
|
programmed cell death protein 1
|
|
PD-L1
|
programmed cell death ligand 1
|
|
PFS
|
progression-free survival
|
|
RT
|
radiotherapy
|
|
SRE
|
skeletal-related event
|
|
TPS
|
tumor proportion score
|
References
|
1
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-Small-Cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-Small-Cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, Von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G, Srimuninnimit V, Laktionov KK, Bondarenko
I, et al: Pembrolizumab versus chemotherapy for previously
untreated, PD-L1-expressing, locally advanced or metastatic
non-small-cell lung cancer (KEYNOTE-042): A randomised, Open-label,
controlled, phase 3 trial. Lancet. 393:1819–1830. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asano Y, Yamamoto N, Demura S, Hayashi K,
Takeuchi A, Kato S, Miwa S, Igarashi K, Higuchi T, Yonezawa H, et
al: The therapeutic effect and clinical outcome of immune
checkpoint inhibitors on bone metastasis in advanced non-small-cell
lung cancer. Front Oncol. 12:8716752022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asano Y, Yamamoto N, Demura S, Hayashi K,
Takeuchi A, Kato S, Miwa S, Igarashi K, Higuchi T, Taniguchi Y, et
al: Combination therapy with immune checkpoint inhibitors and
denosumab improves clinical outcomes in non-small cell lung cancer
with bone metastases. Lung Cancer. 193:1078582024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asano Y, Yamamoto N and Demura S, Takeuchi
A, Kato S, Miwa S, Okuda M, Matsumoto I, Yano S and Demura S: Serum
inflammatory dynamics as novel biomarkers for immune checkpoint
inhibitors in non-small-cell lung cancer with bone metastases.
Anticancer Res. 44:4493–4503. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asano Y, Yamamoto N, Hayashi K, Hayashi K,
Takeuchi A, Kato S, Miwa S, Igarashi K, Higuchi T, Taniguchi Y, et
al: Novel predictors of immune checkpoint inhibitor response and
prognosis in advanced non-small-cell lung cancer with bone
metastasis. Cancer Med. 12:12425–12437. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asano Y, Hayashi K, Takeuchi A, Kato S,
Miwa S, Taniguchi Y, Okuda M, Matsumoto I, Yano S and Demura S:
Combining dynamics of serum inflammatory and nutritional indicators
as novel biomarkers in immune checkpoint inhibitor treatment of
non-small-cell lung cancer with bone metastases. Int
Immunopharmacol. 136:1122762024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawachi H, Tamiya M, Tamiya A, Ishii S,
Hirano K, Matsumoto H, Fukuda Y, Yokoyama T, Kominami R, Fujimoto
D, et al: Association between metastatic sites and first-line
pembrolizumab treatment outcome for advanced non-small cell lung
cancer with high PD-L1 expression: A retrospective multicenter
cohort study. Invest New Drugs. 38:211–218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Landi L, D'Incà F, Gelibter A, Chiari R,
Grossi F, Delmonte A, Passaro A, Signorelli D, Gelsomino F, Galetta
D, et al: Bone metastases and immunotherapy in patients with
advanced non-small-cell lung cancer. J Immunother Cancer.
7:3162019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin M, Guan S, Ding X, Zhuang R, Sun Z,
Wang T, Zheng J, Li L, Gao X, Wei H, et al: Construction and
validation of a novel web-based nomogram for patients with lung
cancer with bone metastasis: A real-world analysis based on the
SEER database. Front Oncol. 12:10752172022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Demaria S and Formenti SC: The abscopal
effect 67 years later: From a side story to center stage. Br J
Radiol. 93:202000422020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walle T, Martinez Monge R, Cerwenka A,
Ajona D, Melero I and Lecanda F: Radiation effects on antitumor
immune responses: Current perspectives and challenges. Ther Adv Med
Oncol. 10:17588340177425752018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morita Y, Saijo A, Nokihara H, Mitsuhashi
A, Yoneda H, Otsuka K, Ogino H, Bando Y and Nishioka Y: Radiation
therapy induces an abscopal effect and upregulates programmed
death-ligand 1 expression in a patient with non-small cell lung
cancer. Thorac Cancer. 13:1079–1082. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bang A and Schoenfeld JD: Immunotherapy
and radiotherapy for metastatic cancers. Ann Palliat Med.
8:312–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xing D, Siva S and Hanna GG: The abscopal
effect of stereotactic radiotherapy and immunotherapy: Fool's Gold
or El Dorado? Clin Oncol. 31:432–443. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fiorica F, Tebano U, Gabbani M, Perrone M,
Missiroli S, Berretta M, Giuliani J, Bonetti A, Remo A, Pigozzi E,
et al: Beyond abscopal effect: A meta-analysis of immune checkpoint
inhibitors and radiotherapy in advanced non-small cell lung cancer.
Cancers (Basel). 13:23522021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng Y, Liu X, Li N, Zhao A, Sun Z, Wang
M and Luo J: Radiotherapy combined with immunotherapy could improve
the immune infiltration of melanoma in mice and enhance the
abscopal effect. Radiat Oncol J. 41:129–139. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei J, Montalvo-Ortiz W, Yu L, Krasco A,
Ebstein S, Cortez C, Lowy I, Murphy AJ, Sleeman MA and Skokos D:
Sequence of alphaPD-1 relative to local tumor irradiation
determines the induction of abscopal antitumor immune responses.
Sci Immunol. 6:eabg01172021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park B, Yee C and Lee KM: The effect of
radiation on the immune response to cancers. Int J Mol Sci.
15:927–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mondini M, Levy A, Meziani L, Milliat F
and Deutsch E: Radiotherapy-immunotherapy combinations-Perspectives
and challenges. Mol Oncol. 14:1529–1537. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, De Wit M, et
al: Overall survival with durvalumab after chemoradiotherapy in
stage III NSCLC. N Engl J Med. 379:2342–2350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gray JE, Villegas A, Daniel D, Vicente D,
Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, Cho BC, et al:
Three-year overall survival with durvalumab after chemoradiotherapy
in stage III NSCLC-update from PACIFIC. J Thorac Oncol. 15:288–293.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Theelen WSME, Peulen HMU, Lalezari F, van
der Noort V, De Vries JF, Aerts JG, Dumoulin DW, Bahce I, Niemeijer
AL, De Langen AJ, et al: Effect of pembrolizumab after stereotactic
Body radiotherapy vs. pembrolizumab alone on tumor response in
patients with advanced non-small cell lung cancer: Results of the
PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol.
5:1276–1282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shaverdian N, Lisberg AE, Bornazyan K,
Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P:
Previous radiotherapy and the clinical activity and toxicity of
pembrolizumab in the treatment of Non-small-cell lung cancer: A
secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol.
18:895–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bates JE, Morris CG, Milano MT, Yeung AR
and Hoppe BS: Immunotherapy with hypofractionated radiotherapy in
metastatic non-small cell lung cancer: An analysis of the National
Cancer Database. Radiother Oncol. 138:75–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Foster CC, Sher DJ, Rusthoven CG, Verma V,
Spiotto MT, Weichselbaum RR and Koshy M: Overall survival according
to immunotherapy and radiation treatment for metastatic
non-small-cell lung cancer: A National Cancer Database analysis.
Radiat Oncol. 14:182019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Welsh J, Menon H, Chen D, Verma V, Tang C,
Altan M, Hess K, De Groot P, Nguyen QN, Varghese R, et al:
Pembrolizumab with or without radiation therapy for metastatic
non-small cell lung cancer: A randomized phase I/II trial. J
Immunother Cancer. 8:e0010012020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Theelen WSME, Chen D, Verma V, Hobbs BP,
Peulen HM, Aerts JG, Bahce I, Niemeijer AL, Chang JY, de Groot PM,
et al: Pembrolizumab with or without radiotherapy for metastatic
non-small-cell lung cancer: A pooled analysis of two randomised
trials. Lancet Respir Med. 9:467–475. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Xu T, Chang P, Fu W, Wei J, Xia C,
Wang Q, Li M, Pu X, Huang F, et al: Efficacy and safety of immune
checkpoint inhibitors with or without radiotherapy in metastatic
non-small cell lung cancer: A systematic review and meta-analysis.
Front Pharmacol. 14:10642272023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tomaciello M, Conte M, Montinaro FR,
Sabatini A, Cunicella G, Di Giammarco F, Tini P, Gravina GL,
Cortesi E, Minniti G, et al: Abscopal effect on bone metastases
from solid tumors: A systematic review and retrospective analysis
of challenge within a challenge. Biomedicines. 11:11572023.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rodríguez Plá M, Dualde Beltrán D and
Ferrer Albiach E: Immune checkpoints inhibitors and SRS/SBRT
synergy in metastatic non-small-cell lung cancer and melanoma: A
systematic review. Int J Mol Sci. 22:116212021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geng Y, Zhang Q, Feng S, Li C, Wang L,
Zhao X, Yang Z, Li Z, Luo H, Liu R, et al: Safety and efficacy of
PD-1/PD-L1 inhibitors combined with radiotherapy in patients with
non-small-cell lung cancer: A systematic review and meta-analysis.
Cancer Med. 10:1222–1239. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kan C, Vargas G, Le Pape F and Clézardin
P: Cancer cell colonisation in the bone microenvironment. Int J Mol
Sci. 17:16742016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Santini D, Barni S, Intagliata S, Falcone
A, Ferraù F, Galetta D, Moscetti L, La Verde N, Ibrahim T, Petrelli
F, et al: Natural history of non-small-cell lung cancer with bone
metastases. Sci Rep. 5:186702015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lutz S, Balboni T, Jones J, Lo S, Petit J,
Rich SE, Wong R and Hahn C: Palliative radiation therapy for bone
metastases: Update of an ASTRO Evidence-Based Guideline. Pract
Radiat Oncol. 7:4–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Makita K, Hamamoto Y, Kanzaki H, Kataoka
M, Yamamoto S, Nagasaki K, Ishikawa H, Takata N, Tsuruoka S, Uwatsu
K, et al: Local control of bone metastases treated with external
beam radiotherapy in recent years: A multicenter retrospective
study. Radiat Oncol. 16:2252021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
LeVasseur N, Clemons M, Hutton B, Shorr R
and Jacobs C: Bone-targeted therapy use in patients with bone
metastases from lung cancer: A systematic review of randomized
controlled trials. Cancer Treat Rev. 50:183–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Facilissimo I, Natoli G, Gaspari F,
Comandone T, Bongiovanni D, Gollini P, Provenza C and Comandone A:
The role of bone radiotherapy during immune checkpoint inhibitors
treatment of non-small-cell lung cancer: A single-institution
experience. Ther Adv Med Oncol. 17:175883592513324512025.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jayathilaka B, Mian F, Franchini F,
Au-Yeung G and IJzerman M: Cancer and treatment specific incidence
rates of immune-related adverse events induced by immune checkpoint
inhibitors: A systematic review. Br J Cancer. 132:51–57. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Suazo-Zepeda E, Bokern M, Vinke PC,
Hiltermann TJ, De Bock GH and Sidorenkov G: Risk factors for
adverse events induced by immune checkpoint inhibitors in patients
with non-small-cell lung cancer: A systematic review and
meta-analysis. Cancer Immunol Immunother. 70:3069–3080. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dang TO, Ogunniyi A, Barbee MS and Drilon
A: Pembrolizumab for the treatment of PD-L1 positive advanced or
metastatic Non-small cell lung cancer. Expert Rev Anticancer Ther.
16:13–20. 2016. View Article : Google Scholar : PubMed/NCBI
|