Introduction
The fifth edition of the World Health Organization
(WHO) classification of breast cancer introduced a new form of the
disease called uncommon and salivary gland tumor (1). The majority of these breast and
salivary gland tumors have a good prognosis and limited
invasiveness, and are typically negative for immunohistochemistry
markers including estrogen receptor (ER), progesterone receptor
(PR) and human epidermal growth factor receptor 2 (HER2) (2,3). They
are typically indolent tumors with an excellent long-term
prognosis, even when triple negative. They have notably low rates
of lymph node metastasis and distant spread. By contrast,
conventional triple negative breast cancer (TNBC) is biologically
aggressive, and is associated with a higher risk of early
recurrence and distant metastasis, and a markedly worse overall
prognosis. Thus, it is crucial to differentiate it from other
notably dangerous TNBC types. The prognosis of mucoepidermoid
carcinoma (MEC) is related to the grading of Elston-Ellis scoring
system, which is classified as low, medium and high grade according
to the proportion of tumor cystic components, nerve invasion,
necrosis and the number of mitoses per 10 high power fields
(4).
MEC accounts for 0.2–0.3% of the incidence rate of
breast cancer (1). It was first
reported in 1979 (5). Up to 2023,
the number of breast MEC cases reported in the global literature
was <70. Therefore, the present study aimed to improve the
current understanding of this tumor through a breast MEC and
literature review; understand its clinical features, typical
pathological morphology, immunohistochemical and molecular
characteristics and differential diagnosis; and to provide clinical
guidance for the diagnosis, treatment and prognosis of this
tumor.
Case presentation
In August 2024, a 43 year old female patient found a
nodule in the upper outer quadrant of her right breast and was
admitted to Wuhu Hospital affiliated with East China Normal
University and Wuhu Second People's Hospital (Wuhu, China). There
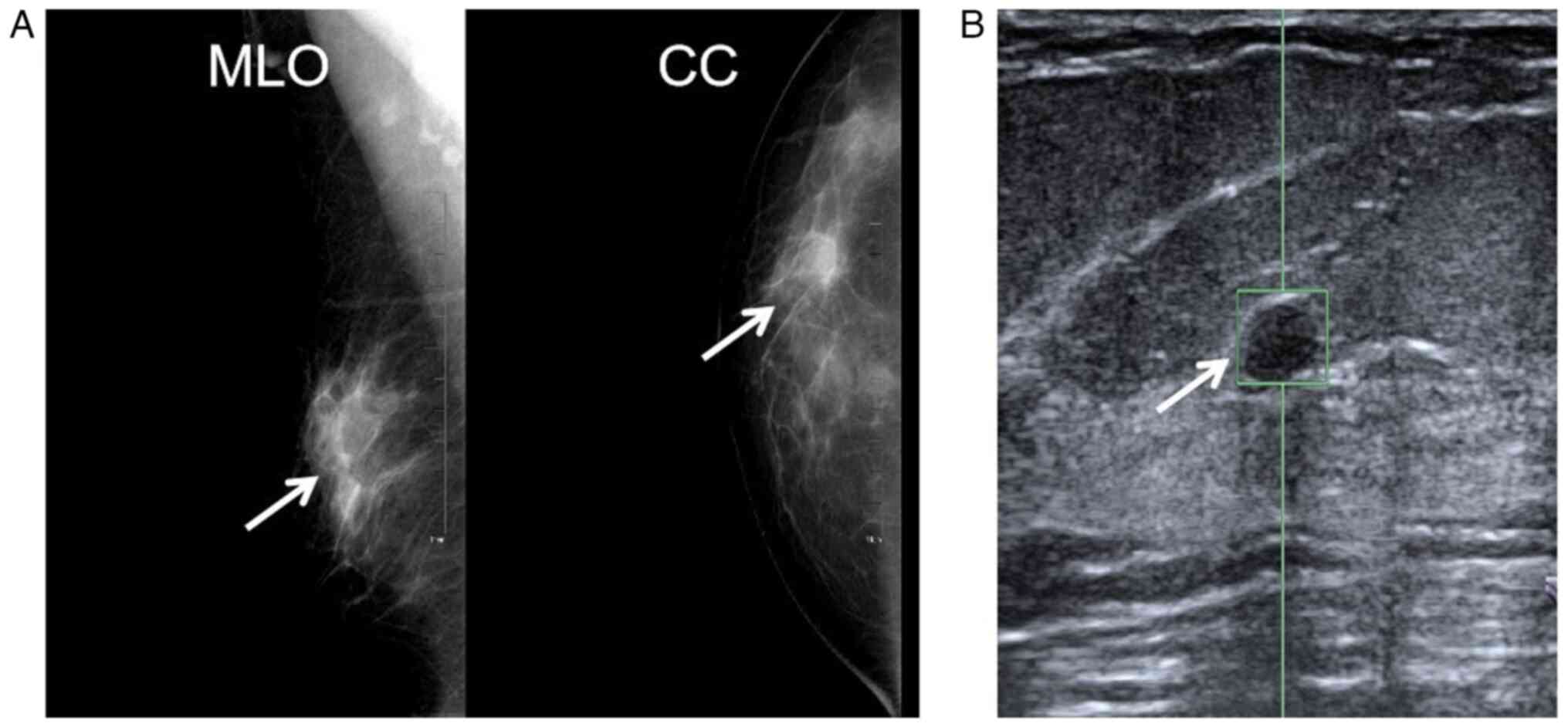
was no abnormal skin or nipple discharge. The mammogram showed an
oval-shaped isodense shadow in the upper outer quadrant of the
right breast, measuring ~1.7 cm in diameter. Automated breast
volume scanning showed a hypoechoic mass at the 9 o'clock position
of the right breast (~30 mm from the nipple and 15 mm from the skin
surface), which measured ~20×11 mm. The mass exhibited well-defined
borders, an irregular shape with shallow lobulation at the margins
and heterogeneous internal echogenicity (Fig. 1), suggestive of a benign tumor.
Tissues from breast rotational resection were sent
for standard pathological analysis. A pile of medium-textured,
gray-white, gray-yellow rotary-cut tissue measuring 1.5×1.0×1.0 cm
was found in the gross area. Microscopically, the tumor showed
cystic-solid growth, and papillary processes could be observed in
the capsule. The tumor was composed of mild intermediate,
epidermoid, mucous and eosinophilic fine cells. Cribriform gland
cavities and microcapsules of varying sizes were formed, which
contained mucus or eosinophilic secretions (Fig. 2A-C). Lymphoid tissue hyperplasia
could be observed in the breast tissue around the tumor (Fig. 2D). Mitoses were infrequent. The
present case is classified as low-grade according to the
Elston-Ellis grading system (5).
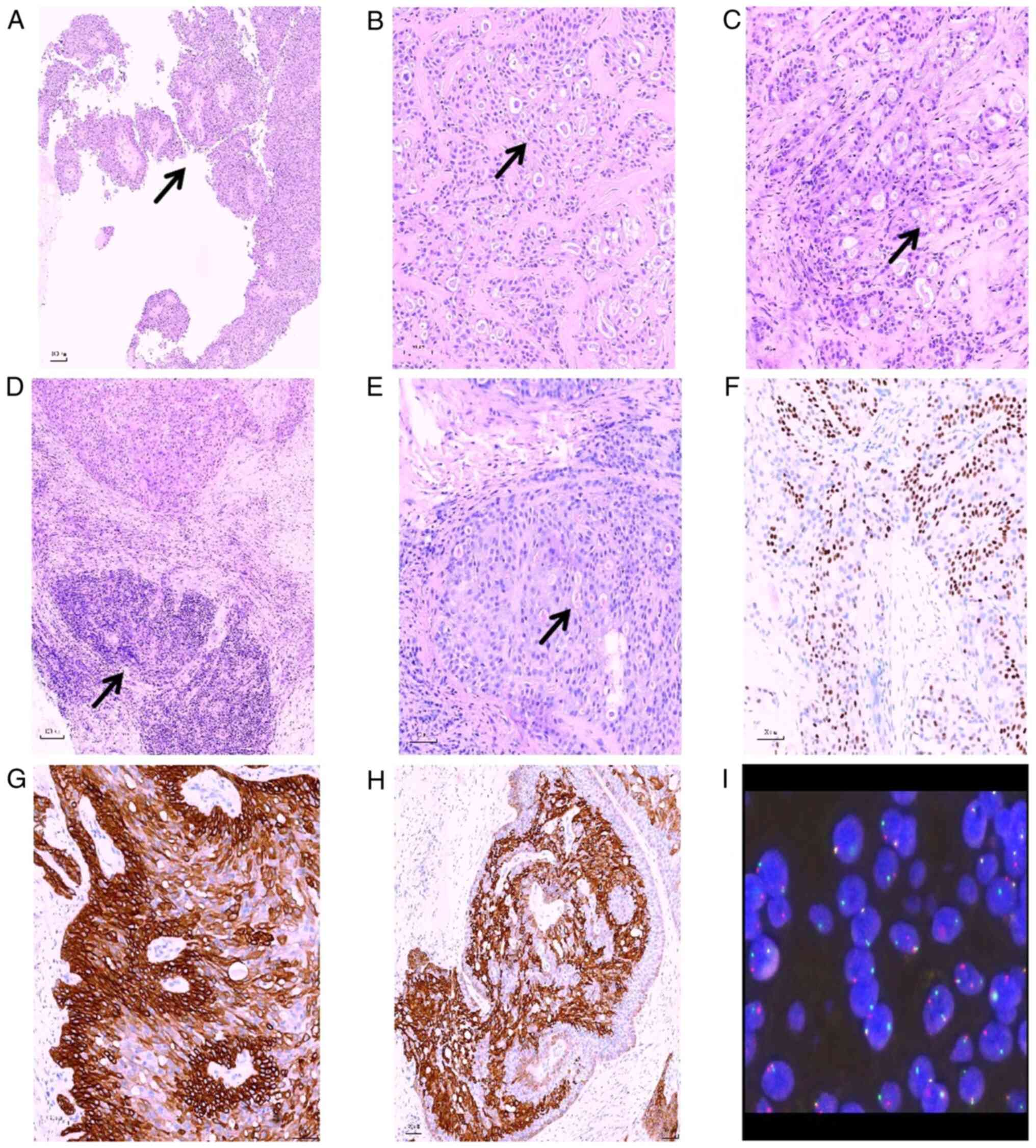 | Figure 2.Pathological characteristics of breast
mucoepidermoid carcinoma. (A) The tumor was cystic, and the tumor
cells protruded into the cystic cavity to form a papillary
structure (arrow; H&E staining; magnification, ×100). (B) The
tumor cells were composed of alternating polygonal and mucoid
cells, and exhibited markedly small and visible nuclei. Nucleoli
was observed in polygonal cells, as well as eosinophilic cytoplasm
(arrow; H&E staining; magnification, ×200). (C) Ethmoid
glandular cavity or microcapsule structure could be observed
between the tumor cells, and there were eosinophilic secretions,
which may appear in the same cavity as blue-stained mucus (arrow;
H&E staining; magnification ×200). (D) Lymphoid tissue
hyperplasia could be observed in the breast tissue around the tumor
(arrow; H&E staining; magnification ×100). (E) Mucicarmine
staining showed positive staining of mucus both inside and outside
the tumor cells (arrow; H&E staining; magnification ×200).
Immunohistochemical staining showed that the tumor cells exhibited
a regional positive staining pattern for (F) p63, (G) CK5/6 and (H)
CK7 (magnification, ×200). (I) The mastermind like transcriptional
coactivator 2 gene showed a disrupted rearrangement, according to
the results of fluorescence in situ hybridization detection.
H&E, hematoxylin and eosin; CK, cytokeratin. |
Mucicarmine staining showed positive staining of
mucus inside and outside tumor cells (Fig. 2E). Tissues were fixed in 4% neutral
buffer formaldehyde solution at room temperature for 24 h, then
dehydrated and embedded in paraffin. Following sectioning at a
thickness of 4 µm, paraffin-embedded tissue sections were dried in
an incubator at 60°C for 30 min to enhance adhesion to the slides.
The sections were then deparaffinized in xylene and rehydrated
through a graded ethanol series. To quench endogenous peroxidase
activity, the tissues were treated with 3% hydrogen for 20 min at
room temperature. Immunohistochemical staining was performed on a
Benchmark XT automated immunohistochemistry stainer (Roche Tissue
Diagnostics) according to the manufacturer's kit (OptiView DAB IHC
Detection Kit; cat. no. 760-700; Roche Diagnostics). This detection
kit is designed specifically for the Ventana Bench Mark series
fully automatic immunohistochemical staining instrument. All steps
(including blocking, primary antibody incubation, secondary
antibody/linker incubation, HRP polymer incubation, washing, and
DAB color development) are automatically completed by the
instrument according to the preset program.
Sections from known positive tissues were used as
positive controls, and PBS instead of primary antibody was used as
a negative control. The following primary antibodies were used: ER
(cat. no. IR078; Clone: SP1; Guangzhou LBP Medicine Science &
Technology Co., Ltd.), PR (cat. no. IM361; Clone:16; Guangzhou LBP
Medicine Science & Technology Co., Ltd.), cytokeratin5/6
(CK5/6; cat. no. IM060; Clone: LBP1-CK5/6;Guangzhou LBP Medicine
Science & Technology Co., Ltd.); cytokeratin7 (CK7; cat. no.
IM061 Clone: LBP1-CK7; Guangzhou LBP Medicine Science &
Technology Co., Ltd), transformation-related protein63 (p63; cat.
no. IM311; Clone:LBP1-P63; Guangzhou LBP Medicine Science &
Technology Co., Ltd.), GATA binding protein 3 (GATA3; cat. no.
IM277; Clone:LBP1-GATA3; Guangzhou LBP Medicine Science &
Technology Co., Ltd.), SOX-10 (cat. no. IR349; Clone:LBP2-SOX10;
Guangzhou LBP Medicine Science & Technology Co., Ltd.), Ki-67
(cat. no. IM098; Clone:LBP1-Ki67; Guangzhou LBP Medicine Science
& Technology Co., Ltd.), TRPS-1 (cat. no. IR474; Clone:ZR382;
Guangzhou LBP Medicine Science & Technology Co., Ltd.), Syn
(cat. no. IM136; Clone:LBP1-Syn; Guangzhou LBP Medicine Science
& Technology Co., Ltd.), S100 (cat. no. IM135; Clone:LBP1-S100;
Guangzhou LBP Medicine Science & Technology Co., Ltd.). The
stained sections were examined and imaged under a DM-2000 light
microscope (Leica Biosystems).
Immunohistochemical results showed that cytokeratin
(CK) 5/6 and p63 were positive in polygonal cells (Fig. 2F and G) cells; CK7 was positive in
mucoid cells (Fig. 2H); GATA
binding protein 3 (GATA3) and transcriptional repressor GATA
binding 1 (TRPS1) were positive (Fig.
S1A and B); S100 and SOX-10 were focal positive (Fig. S1C and D); SYN was negative
(Fig. S1E); ER was 20% positive
(Fig. S1F); PR was negative
(Fig. S1G); HER2 was 1+ (>10%
of invasive tumor cells showed faint, incomplete membrane staining;
Fig. S1H); and the Ki-67
proliferation index was ~10% (Fig.
S1I).
Formalin-fixed, paraffin-embedded tissue sections
were analyzed for mastermind like transcriptional coactivator 2
(MAML2) gene rearrangements using the ZytoLight SPEC MAML2 Dual
Color Break Apart Probe (ZYTOVISION GmbH; cat. no. Z-2014-200),
which consists of a SpectrumGreen-labeled probe targeting the 5′
region and a SpectrumOrange-labeled probe targeting the 3′ region
of the MAML2 gene. All aspects of probe design, including
nucleotide sequence, fragment length, enzymatic labeling and DNase
treatment for template removal, are optimized by the manufacturer
and constitute proprietary intellectual property. Sections of 4–5
µm thickness were mounted on charged slides, baked at 60°C for 60
min, deparaffinized in xylene, and dehydrated through a graded
ethanol series. Heat-induced pretreatment was performed in 1×
saline-sodium citrate buffer (SSC; pH 6.3) at 80°C for 30 min,
followed by enzymatic digestion with 0.5 mg/ml pepsin in 0.01 N HCl
at 37°C for 15–30 min. Tissues were then post-fixed in 10% neutral
buffered formalin and dehydrated through a series of ethanol
solutions of increasing concentration. Hybridization was carried
out by applying 10 µl of the probe mixture onto the sample,
followed by a critical co-denaturation step at 82°C for 5 min. This
elevated temperature provides the energy required to disrupt the
hydrogen bonds in both the double-stranded DNA of the tissue of the
patient and the applied probe, denaturing them into single strands
and making the target genomic sequences accessible. Subsequently,
hybridization was allowed to proceed at 45°C for 16–20 h in a
humidified chamber.
Notably, post-hybridization stringency washing is a
critical step that must be carefully optimized according to the
manufacturer's instructions; commonly recommended conditions were
carried out, including washing in pre-warmed 2× SSC with 0.3% NP-40
at 73°C for 5 min, followed by a rinse in 2× SSC/0.05% Tween 20 at
room temperature. Slides were air-dried in the dark and
counterstained with DAPI. Analysis was performed on an
epifluorescence microscope using single interference filter sets
for orange and green. For each interference filter, monochromatic
images were acquired and merged using CytoVision (Leica
Microsystems). Analyses were performed in a minimum of 50 nuclei
harboring at least one copy of each signal. A specimen was
considered positive for MAML2 rearrangement when a minimum of 20%
of analyzed cells displayed split 3′MAML2 and 5′MAML2 signals or
single signals under a Leica DM6000 B motorized fluorescence
microscope. Fluorescence in situ hybridization detection
revealed a broken rearrangement of the MAML2 gene in the tumor
cells (Fig. 2I).
At 2 weeks after the rotational mastectomy, the
patient underwent right breast-conserving surgery, right axillary
sentinel lymph node biopsy and right breast fascial glandular flap
plasty. Postoperative pathology showed no metastatic carcinoma in
the right sentinel lymph nodes, no cancer infiltration at the
surgical margins of the breast-conserving procedure and a small
quantity of residual cancer tissue in the original surgical cavity.
Postoperative radiotherapy was administered, including whole-breast
radiotherapy (50 Gy/25 fractions) and tumor bed boost (10 Gy/5
fractions). After 8 months of monthly telephone and outpatient
follow-up the patient was alive, without any signs of
recurrence.
Discussion
The salivary glands are where MEC is most commonly
identified, followed by the tonsils, thyroid, pleura, lung, thymus,
esophagus and other areas (6–8).
However, it rarely happens in the breast. The breast gland MEC is
classified as salivary gland tumor according to the 2019 edition
WHO breast tumor classification (1). Its histological morphology is similar
to that of salivary gland MEC, which is composed of mucous,
intermediate (basal-like) and epidermis-like (squamous) cells.
Transparent cells and eosinophils can also be observed.
Pathological grading of mammary MEC is critically dependent on
histological features adapted from salivary gland systems,
emphasizing architecture, cytological atypia, mitoses and necrosis.
Low-grade tumors are indolent, while high-grade tumors behave
aggressively (9).
The majority of breast MEC cases are characterized
by negative expression of ER, PR and HER2. However, unlike other
TNBCs, they exhibit relatively good prognosis (10). Thus, distinguishing it from other
notably dangerous TNBC types is crucial. The clinical indications,
pathological morphology, molecular immunohistochemical
characteristics, pathological differential diagnosis, clinical
treatment and prognosis of breast MEC are as follows: Regarding
clinical features, up to 2023, the number of breast MEC cases
reported in the global literature was <70. The majority of cases
were case reports or small-scale case series studies. Case reports
were mainly from USA, Europe, Japan, China and other areas where
medical research is more developed. In previous years, a small
number of cases have been reported in China; however, the total
number of cases remains small. The age range of the patients is
wide, but the majority of patients are middle-aged and elderly
women (27–86 years old) (11). Most
patients exhibit minor pain or no discernible symptoms. Similar to
other breast cancer types, the common symptoms of MEC are breast
masses, and certain cases are accompanied by nipple discharge or
pain. The tumor can be cystic or solid, with a size of 0.5–11.0 cm
(mean, 3.5 cm). In previous studies, ultrasonography examination
revealed that the mass showed cystic and solid changes with clear
boundaries (9,11,12).
In regard to typical pathomorphological features,
MEC tumors show a cystic-solid growth pattern, marked vitreous
degeneration of the cyst wall, lymphocyte infiltration and
follicular structure in the stroma around the cyst wall. Solid or
papillary growth of the tumor can be observed in the capsule,
infiltrating into the surrounding cyst wall tissue, while the tumor
cells have a glandular cavity-like structure and secrete mucus in
part of the cavity. The tumor cells contain mucus-secreting cells,
clear cytoplasm and intermediate cells. Cystic or polycystic
structures and solid nests are typical structural patterns of
breast MEC. In low-grade MEC, the tumor cells in the cyst wall form
a papillary or polyp-like structure protruding into the cyst
cavity. Ethmoid glandular cavities and microcapsules of different
sizes can be formed between tumor cells, which contain mucus or
eosinophilic secretions. The ethmoid gland lumen or microcapsule
structure is considered a valuable morphological change in breast
MEC. Peritumor-associated lymphoid tissue hyperplasia is an
important diagnostic marker for breast MEC, which has been fully
recognized in salivary gland MEC. High-grade MEC of the breast has
shown the same cellular composition, but more solid components,
mainly epidermoid cells and intermediate cells, fine cell atypia,
increased mitosis, necrosis and nerve invasion. However, the
histological changes of high-grade breast MEC have been suggested
to be non-specific, and the diagnostic criteria remain unclear
(13). Previously reported
high-grade breast MEC cases may be a group of heterogeneous
tumors.
Regarding immunohistochemical and molecular
characteristics, epidermoid cells and intermediate cells express
high molecular weight CKs (such as CK5/6) and p63, while the
majority of mucoid cells express low-molecular weight keratins
(such as CK7). Breast MEC usually presents a typical
triple-negative immunophenotype (14). However, previous studies have shown
that it can express hormone receptors in different degrees. Most of
the reported cases of breast MEC have low ER expression (15,16).
The positive rate of ER in the present case was 20%. Previous
research has shown that the prognosis of patients with low levels
of hormone receptor expression was good (17). This is likely due to unique tumor
origin and molecular characteristics.Breast MEC arises from
salivary gland-like ductal cells (similar to salivary gland
tumors), not hormone-sensitive breast luminal cells (18). Its molecular drivers are unrelated
to estrogen signaling. Because tumor growth is not fueled by
estrogen signaling, it lacks the aggressive proliferative and
metastatic drive associated with hormone-dependent pathways. This
intrinsic ‘indolence’ is a key reason for its improved prognosis
(19,20). Low ER/PR positivity reflects passive
expression, not functional dependence. The tumor does not depend on
these signals for survival or growth (21). Low ER/PR expression typically occurs
in low-grade MEC, which inherently has positive outcomes regardless
of ER/PR status.
Breast MEC exhibits a lower incidence of MAML2
rearrangements (~38% of reported cases) compared with salivary
gland MEC (55–88%). The dominant fusion is CREB-regulated
transcription coactivator 1 (CRTC1)-MAML2 from t(11;19) (q21;p13),
with rare CRTC3-MAML2 t(11;15) (q21;q26) fusion.
CRTC1/3:MAML2-positive breast MECs are associated with lower
histological grade and improved survival, similar to salivary MEC
(22,23). However, fusion-negative cases may
show aggressive behavior due to genomic instability. To date, there
have been 21 molecular analysis reports of breast MEC, including 7
cases carrying CRTC1-MAML2 and 1 case carrying CRTC3-MAML2
translocation (24). Breast MECs
lack TP53 mutations, which are found in high-grade forms of TNBCs,
and the MAML2 or EWS RNA binding protein 1 rearrangements
pathognomonic of salivary MECs. Breast MEC is molecularly distinct
from both salivary gland MEC and conventional TNBCs. While
CRTC1/3-MAML2 fusions define a subset with favorable prognosis,
dominant PI3K/AKT/mTOR dysregulation and EGFR/amphiregulin
activation offer actionable targets. Larger cohort studies using
RNA in situ hybridization and targeted Next-Generation
Sequencing are needed to validate these alterations and explore
fusion-independent oncogenic mechanisms (25). The molecular genetic characteristics
of tumors require further research.
With regard to differential diagnosis, intraductal
papilloma with common epithelial hyperplasia/squamous metaplasia
includes fibrous cystic wall, solid sieve-like hyperplasia of tumor
cells protruding from the cystic cavity, papillary structures with
fibrous vascular axis, relatively mild cells and
immunohistochemical expression of CK5/6. Careful identification of
different cellular components and morphological features, such as
mucus secretion, in MEC can aid in identification (1). Solid papillary carcinoma presents as
cystic papillary hyperplasia, with cells of the same size and
visible mucus inside, as well as invasive growth. However, there is
a lack of thick-walled cystic cavity and peripheral lymphocyte
infiltration. In a previous study, immunohistochemical staining
showed strong ER/PR positivity, CK5/6 and p63 negativity and SYN
positivity (1). Secretory carcinoma
presents as cystic, solid or papillary structures, and secretions
inside the microvesicles can be observed both inside and outside
the tumor cells. Furthermore, no thick infiltration of lymphocytes
is detected in the cyst wall or its surroundings. The
immunohistochemical results show that S100, SOX-10 and CD117 are
positive, whereas p63 and CK5/6 are negative. The combination of
the ETS variant transcription factor 6-neurotrophic tyrosine
receptor kinase 3 gene is the most relevant molecular
characteristic (15). Squamous
metaplasia is usually focal, with clear cellular atypia, keratosis
(keratinized beads) and lack of intracellular and extracellular
mucus (1). Clear cell sweat gland
adenoma is more common in the dermis, subcutaneous tissue, nipple
and areola. The histological features of the tumor are mostly
cystic and papillary hyperplasia, with clear cell morphology mixed
with transparent squamous cells. Small glandular structures are
scattered in the transparent cells, arranged by cuboidal
eosinophils, but there is no lymphocyte infiltration around the
cyst wall, mucous epithelium, intracellular or extracellular mucus.
There are multiple overlaps in histological features and molecular
genetic changes between low-grade MEC and breast clear cell sweat
gland adenoma (26).
Regarding the prognosis and clinical treatment of
breast MEC, at present, there is no standard treatment for breast
MEC, probably because it is rare and the treatment may involve
surgery, radiotherapy and chemotherapy. In the case of low-grade
malignant tumors, the tumor must be removed completely. For
patients with a high malignant degree and strong invasiveness,
radical mastectomy and axillary lymph node dissection should be
performed for a good overall prognosis of MEC; however,
histological grade is an important prognostic factor, and
high-grade tumors have a poor prognosis. The prognosis of breast
MEC is associated with tumor grade and distant metastasis (14,15).
Lymph node metastasis can be found in all grades of breast MEC,
while distant metastasis can only be observed in high-grade cases.
The prognosis of low-grade breast MEC is good, while high-grade MEC
with distant metastasis often leads to mortality.
In conclusion, breast MEC is a rare malignant tumor
prone to misdiagnosis as other lesions. Pathological diagnosis
requires the integration of morphological features,
immunohistochemical profiling, specific staining techniques and
molecular testing. Comprehensive analysis of histological grading
is crucial for clinical management, enabling prognostic evaluation
while emphasizing the importance of differential diagnosis from
other histologically similar tumors. Histological grading provides
critical guidance for clinical management and prognostic
evaluation. Globally reported cases of breast MEC remain scarce.
The rarity of this neoplasm poses considerable challenges to
related research and data aggregation. Future efforts should
prioritize case data accumulation through international
collaboration, as well as multicenter studies to facilitate the
comprehensive investigation of this disease.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MX and ZXX conceived and designed the study. MX
analyzed and summarized the data and wrote the manuscript. CCC and
XYS collected the laboratory examination data and images of the
case. ZXX critically revised the manuscript. MX, CCC, XYS and ZXX
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the principles of the Declaration of Helsinki.
Patient consent for publication
The patient involved in the present study was
subjected to standard clinical practice and provided written
informed consent for the publication of medical data and
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Foschini MP, Geyer FC, Marchio C and
Nishimura R: Mucoepidermoid carcinoma. WHO Classification of
Tumours Editorial Board. WHO Classification of Tumours, Breast
Tumours, International Agency for Research on Cancer; Lyon: pp.
149–50. 2019
|
|
2
|
Cserni G, Quinn CM, Foschini MP, Bianchi
S, Callagy G, Chmielik E, Decker T, Fend F, Kovács A, van Diest PJ,
et al: Triple-negative breast cancer histological subtypes with a
favourable prognosis. Cancers. 13:56942021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cima L, Kaya H, Marchiò C, Nishimura R,
Wen HY, Fabbri VP and Foschini MP: Triple-negative breast
carcinomas of low malignant potential: Review on diagnostic
criteria and differential diagnoses. Virchows Arch. 480:109–126.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cipriani NA, Lusardi JJ, McElherne J,
Pearson AT, Olivas AD, Fitzpatrick C, Lingen MW and Blair EA:
Mucoepidermoid carcinoma: A comparison of histologic grading
systems and relationship to MAML2 rearrangement and prognosis. Am J
Surg Pathol. 43:885–897. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patchefsky AS, Frauenhoffer CM, Krall RA
and Cooper HS: Low-grade mucoepidermoid carcinoma of the breast.
Arch Pathol Lab Med. 103:196–198. 1979.PubMed/NCBI
|
|
6
|
Liu C, Zhao Y, Chu W, Zhang F and Zhang Z:
Mucoepidermoid carcinoma of esophagus combined with squamous
carcinoma of lung: A case report and literature review. J Cancer
Res Ther. 11:6582015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goh GH, Lim CM, Vanacek T, Michal M and
Petersson F: Spindle cell mucoepidermoid carcinoma of the palatine
tonsil with CRTC1-MAML2 fusion transcript: Report of a rare case in
a 17-year-old boy and a review of the literature. Int J Surg
Pathol. 25:705–710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu HJ, Zhou RX, Liu F, Wang JK and Li FY:
You cannot miss it: Pancreatic mucoepidermoid carcinoma: A case
report and literature review. Medicine (Baltimore). 97:e99902018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye RP, Liao YH, Xia T, Kuang HA and Xiao
XL: Breast mucoepidermoid carcinoma: A case report and review of
literature. Int J Clin Exp Pathol. 13:3192–3199. 2020.PubMed/NCBI
|
|
10
|
Burghel GJ, Abu-Dayyeh I, Babouq N,
Wallace A and Abdelnour A: Mutational screen of a panel of tumor
genes in a case report of mucoepidermoid carcinoma of the breast
from Jordan. Breast J. 24:1102–1104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He X, You J, Chen Y, Tang H, Ran J and Guo
D: Mucoepidermoid carcinoma of the breast, 3 cases report and
literature review. Medicine (Baltimore). 102:e337072023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen G, Liu W, Liao X, Yu F and Xie Y:
Imaging findings of the primary mucoepidermoid carcinoma of the
breast. Clin Case Rep. 10:e054492022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Tommaso L, Foschini MP, Ragazzini T,
Magrini E, Fornelli A, Ellis IO and Eusebi V: Mucoepidermoid
carcinoma of the breast. Virchows Arch. 444:13–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan M, Gilmore H and Harbhajanka A:
Mucoepidermoid carcinoma of the breast with MAML2 rearrangement: A
case report and literature review. Int J Surg Pathol. 28:787–792.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bean GR, Krings G, Otis CN, Solomon DA,
García JJ, van Zante A, Camelo-Piragua S, van Ziffle J and Chen YY:
CRTC1-MAML2 fusion in mucoepidermoid carcinoma of the breast.
Histopathology. 74:463–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mura MD, Clement C, Foschini MP, Borght
SV, Waumans L, Eyken PV, Hauben E, Keupers M, Weltens C, Smeets A,
et al: High-grade HER2-positive mucoepidermoid carcinoma of the
breast: A case report and review of the literature. J Med Case Rep.
17:5272023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sherwell-Cabello S, Maffuz-Aziz A,
Rios-Luna NP, Pozo-Romero M, López-Jiménez PV and Rodriguez-Cuevas
S: Primary mucoepidermoid carcinoma of the breast. Breast J.
23:753–755. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lakhani SR, Ellis Ian O, Schnitt SJ, Tan
PH and van de Vijver MJ: WHO Classification of Tumours of the
Breast. International Agency for Research on Cancer (IARC);
2012
|
|
19
|
Howitt BE, Vivero M, Nucci MR, Fletcher
CD, Cipriani NA, Ott PA, Sholl LM, Doyle LA, Hornick JL and Parra
AR: The repertoire of genetic alterations in salivary gland-type
mammary carcinomas. Hum Pathol. 56:137–146. 2016.
|
|
20
|
Ross JS, Wang K, Rand JV, Sheehan CE,
Jennings TA, Al-Rohil RN, Otto GA, Curran JJ, Yelensky R, Lipson D,
et al: The distribution of CRTC1-MAML2 fusion gene in salivary
gland and salivary gland-type tumors of the head, neck, and breast:
A multi-institutional study. Modern Pathol. 31:725–731. 2018.
|
|
21
|
Pareja F, Arnaud Da Cruz P, Gularte-Mérida
R, Vahdatinia M, Li A, Geyer FC, da Silva EM, Nanjangud G, Wen HY,
et al: Pleomorphic adenomas and mucoepidermoid carcinomas of the
breast are underpinned by fusion genes. NPJ Breast Cancer.
6:202020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vered M and Wright JM: Update from the 5th
edition of the world health organization classification of head and
neck tumors: Odontogenic and maxillofacial bone tumours. Head Neck
Pathol. 16:63–75. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakayama T, Miyabe S, Okabe M, Sakuma H,
Ijichi K, Hasegawa Y, Nagatsuka H, Shimozato K and Inagaki H:
Clinicopathological significance of the CRTC3-MAML2 fusion
transcript inmucoepidermoid carcinoma. Mod Pathol. 22:1575–1581.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawaguchi K, Ijichi H, Ueo H, Hisamatsu Y,
Omori S, Shigechi T, Kawaguchi K, Yamamoto H, Oda Y, Kubo M, et al:
Breast mucoepidermoid carcinoma with a rare CRTC3-MAML2 fusion. Int
Cancer Conf J. 13:481–487. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Venetis K, Sajjadi E, Ivanova M, Andaloro
S, Pessina S, Zanetti C, Ranghiero A, Citelli G, Rossi C, Lucioni
M, et al: The molecular landscape of breast mucoepidermoid
carcinoma. Cancer Med. 12:10725–10737. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Memon RA, Prieto Granada CN and Wei S:
Clear cell papillary neoplasm of the breast with MAML2 gene
rearrangement: Clear cell hidradenoma or Low-grade mucoepidermoid
carcinoma? Pathol Res Pract. 216:1531402020. View Article : Google Scholar : PubMed/NCBI
|