Introduction
As the malignancy with the third highest incidence
rate and the second highest rate of cancer-related deaths globally,
colorectal cancer (CRC) accounts for 1.2 million new cases and
~600,000 fatalities annually (1,2).
Epidemiological modeling predicts a notable increase in disease
burden, with 2.5 million new CRC diagnoses forecast globally by
2035 (3). The epidemiological
landscape of China is particularly concerning, as the country
exhibits the world's highest absolute CRC burden due to its
population size. Recent surveillance data from the National Cancer
Center suggests that the incidence of CRC has increased to the
extent that the disease is now the second most commonly occurring
malignancy in China (4). Despite
advancements in early diagnosis and treatment methods, the
long-term survival rate and prognostic outcomes of patients with
CRC still face challenges (5).
Accurate prognosis assessment is crucial for formulating
individualized treatment plans, predicting survival duration and
optimizing patient management.
In recent years, numerous studies have shown that
inflammatory response, nutritional status and immune function play
pivotal roles in cancer initiation, progression and outcomes
(6–8). Therefore, identifying biomarkers that
can comprehensively reflect a patient's inflammatory, nutritional
and immune status holds significant importance for improving the
prognostic assessment of patients with CRC. The preoperative
hematological parameters of patients with cancer can reflect their
inflammatory, immune and nutritional statuses. Thus, multiple
inflammation indices derived from hematological examinations have
been demonstrated to be closely associated with cancer prognosis.
Key validated prognostic indicators include the
neutrophil-to-lymphocyte ratio (NLR) (9), the platelet-to-lymphocyte ratio
(10), the lymphocyte-to-monocyte
ratio (11), the
fibrinogen-to-albumin ratio (12),
the derived NLR (dNLR) (13), the
mean corpuscular volume-to-lymphocyte ratio (14), the systemic inflammation response
index (15), the systemic
immune-inflammation index (16),
the prognostic nutritional index (PNI) (17), the cumulative inflammation index
(18), the prognostic inflammatory
and nutritional index (19), the
hemoglobin, albumin, lymphocyte and platelet score (20) and pan-immune-inflammation values
(21). Recently, the C-reactive
protein (CRP)-albumin-lymphocyte (CALLY) index, an emerging
immune-nutrition scoring system, has garnered increasing attention
from researchers (22). The CALLY
index, integrating CRP, albumin and lymphocyte levels, provides a
comprehensive assessment of a patient's inflammatory, nutritional
and immune status.
Multiple studies have confirmed that the CALLY index
serves as an independent prognostic factor in patients with gastric
cancer and that it can predict prognosis (23–25).
Conversely, relatively limited evidence exists in the literature
regarding the association between the CALLY index and prognosis in
patients with CRC. Given this context, the present study was
designed to evaluate the prognostic value of the CALLY index in
patients with stage I–III CRC. Through retrospective analysis of
clinicopathological data and preoperative hematological parameters
of patients who underwent radical resection, the study sought to
determine whether the CALLY index could serve as an independent
predictor for both recurrence-free survival (RFS) and overall
survival (OS) rates, thereby potentially offering a novel biomarker
for prognostic assessment in CRC.
Patients and methods
Patients
The present retrospective study analyzed
clinicopathological data and preoperative laboratory hematological
parameters (measured within 1 week before surgery) from patients
with stage I–III CRC who underwent radical resection (R0) at
Jingdezhen First People's Hospital (Jingdezhen, China) between
January 2012 and March 2020. All consecutive patients meeting the
eligibility criteria during this period were initially screened.
The inclusion criteria were as follows: i) Histologically confirmed
primary CRC; ii) no neoadjuvant therapy; and iii) R0 resection with
curative intent. Exclusion criteria eliminated patients with: i)
Synchronous/metachronous malignancies; ii) hematological disorders;
iii) preoperative infection/immunodeficiency; iv) incomplete data;
v) non-radical resection; and vi) receival of neoadjuvant therapy.
All data were extracted from the hospital's maintained
database.
Treatment and follow-up
The disease staging was determined using the eighth
edition of the American Joint Committee on Cancer
Tumor-Node-Metastasis (TNM) classification (26). Comorbidities was defined as
pre-existing comorbidities, including cardiovascular diseases,
pulmonary diseases, diabetes mellitus, chronic kidney disease and
chronic liver disease. Postoperative anastomotic leakage
specifically referred to anastomotic leakage occurring within 30
days after surgery. Postoperative adjuvant therapy primarily
comprised chemotherapy, radiotherapy and other treatment modalities
based on these core therapeutic approaches. Patients with stage II
or III CRC received postoperative adjuvant therapy when deemed
clinically appropriate based on their overall health status.
Postoperative surveillance included contrast-enhanced computed
tomography scans performed at minimum every 6 months and blood
tests conducted every 3 months. Patients were followed up regularly
through outpatient visits or telephone interviews every 3 months
beginning on postoperative day 1 until the study endpoint, defined
as either patient death or March 31, 2025, whichever occurred
first. For outcome assessment, RFS time was calculated as the time
from surgery to CRC recurrence, last follow-up or death, while OS
time was defined as the time from surgery to death from any cause
or last follow-up for surviving patients.
Determination of inflammatory
markers
All calculations for inflammatory markers are
presented in Table I, with the
CALLY index calculated as follows: Albumin (g/dl) × lymphocyte
count (n/µl)/[CRP (mg/dl) ×104] (22).
 | Table I.Inflammatory marker names, formulae
and optimal cut-off values. |
Table I.
Inflammatory marker names, formulae
and optimal cut-off values.
| Marker | Calculation
formula | Optimal cutoff
value |
|---|
| C-reactive
protein-albumin-lymphocyte index | Albumin (g/dl) ×
lymphocyte count (/µl)/[CRP (mg/dl) ×104] | 6.790 |
| Derived
neutrophil-to-lymphocyte ratio | Neutrophils/(white
blood cells-neutrophils) | 2.320 |
| Fibrinogen-to-albumin
ratio | Fibrinogen
(g/l)/albumin (g/l) ×100 | 0.095 |
| Hemoglobin, albumin,
lymphocyte and platelet scores | Hemoglobin (g/l) ×
albumin (g/l) × lymphocytes/platelets | 35.130 |
| Cumulative
inflammatory index | (Corpuscular volume ×
width of erythrocyte distribution × neutrophils)/(lymphocytes
×1,000) | 7.275 |
|
Lymphocyte-to-monocyte ratio |
Lymphocyte/monocyte | 2.890 |
| Ratio between the
mean corpuscular volume and lymphocytes | Corpuscular
volume/lymphocytes | 72.310 |
|
Neutrophil-to-lymphocyte ratio |
Neutrophil/lymphocytes | 3.225 |
| Prognostic immune and
nutritional index | [Albumin (g/dl)
×0.9]-[monocytes (mm3) × 0.0007] | 3.255 |
|
Pan-immune-inflammatory values | Neutrophils ×
monocytes × platelets/lymphocytes | 391.72 |
|
Platelet-to-lymphocyte ratio |
Platelets/lymphocytes | 135.980 |
| Prognostic
nutritional index | Albumin (g/l) + 5 ×
lymphocytes (109/l) | 45.825 |
| Systemic
immune-inflammation index | Platelets ×
neutrophils/lymphocytes | 532.985 |
| Systemic
inflammatory response index | Neutrophils ×
monocytes/lymphocytes | 2.390 |
Statistical analysis
All statistical analyses were performed using R
software (version 4.3.3; R Foundation for Statistical Computing).
The following R packages were employed: pROC (version 1.18.5) for
receiver operating characteristic (ROC) curve analysis, with the
area under the curve (AUC) calculated to determine the optimal
cutoff value using the Youden index; survival (version 3.6.4) and
survminer (version 0.4.9) for survival analyses; and rstatix
(version 0.7.2) for statistical testing. Based on the optimal
cutoff, the patients were stratified into high-CALLY and low-CALLY
groups. Continuous variables with normal distribution are expressed
as mean ± standard deviation (SD) and compared using independent
samples t-tests, while non-normally distributed continuous
variables are presented as median (Q1-Q3) and
analyzed using Wilcoxon rank-sum tests. Categorical variables are
reported as n (%) and were compared using χ2 tests or
Fisher's exact tests, as appropriate. All tests were two-sided,
with P<0.05 considered to indicate a statistically significant
difference. Univariate and multivariate Cox proportional hazards
regression models were constructed using the survival package to
estimate hazard ratios (HRs) with 95% confidence intervals (CIs)
for RFS and OS. Variables with values of P<0.05 upon univariate
analysis were included in the multivariate model. Survival
probabilities were estimated using Kaplan-Meier curves, with
between-group differences assessed by log-rank tests.
Ethical approval
The present study was conducted in accordance with
the Declaration of Helsinki and approved by the Research Ethics
Committee at Jingdezhen First People's Hospital (approval no.
jdzyykt202514). The requirement for informed consent was waived due
to the retrospective nature of the study and data
anonymization.
Results
Patient characteristics
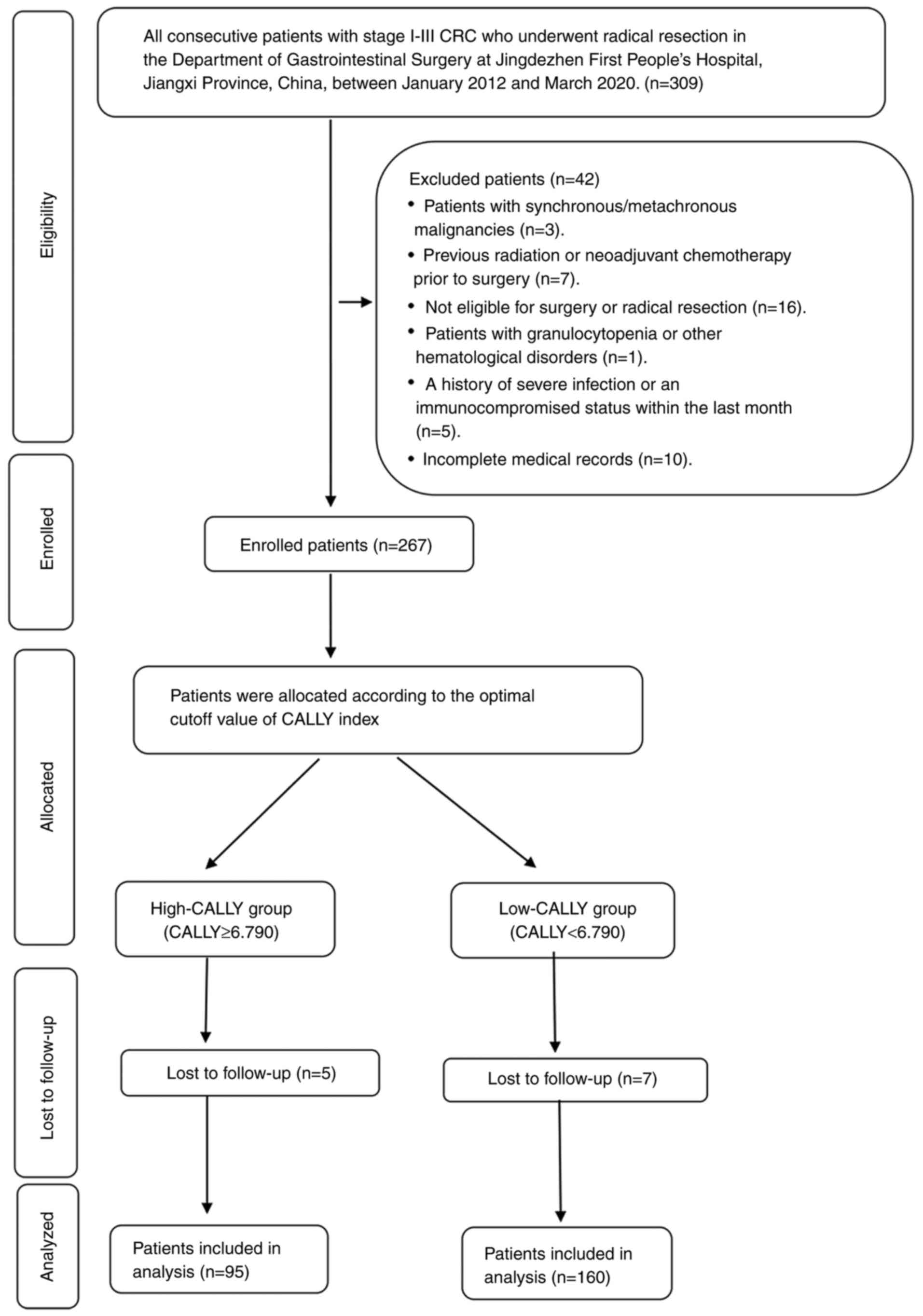
The present study consecutively screened 309
patients with stage I–III CRC undergoing radical resection
(Fig. 1). After applying exclusion
criteria (n=42) and accounting for loss to follow-up (n=12), the
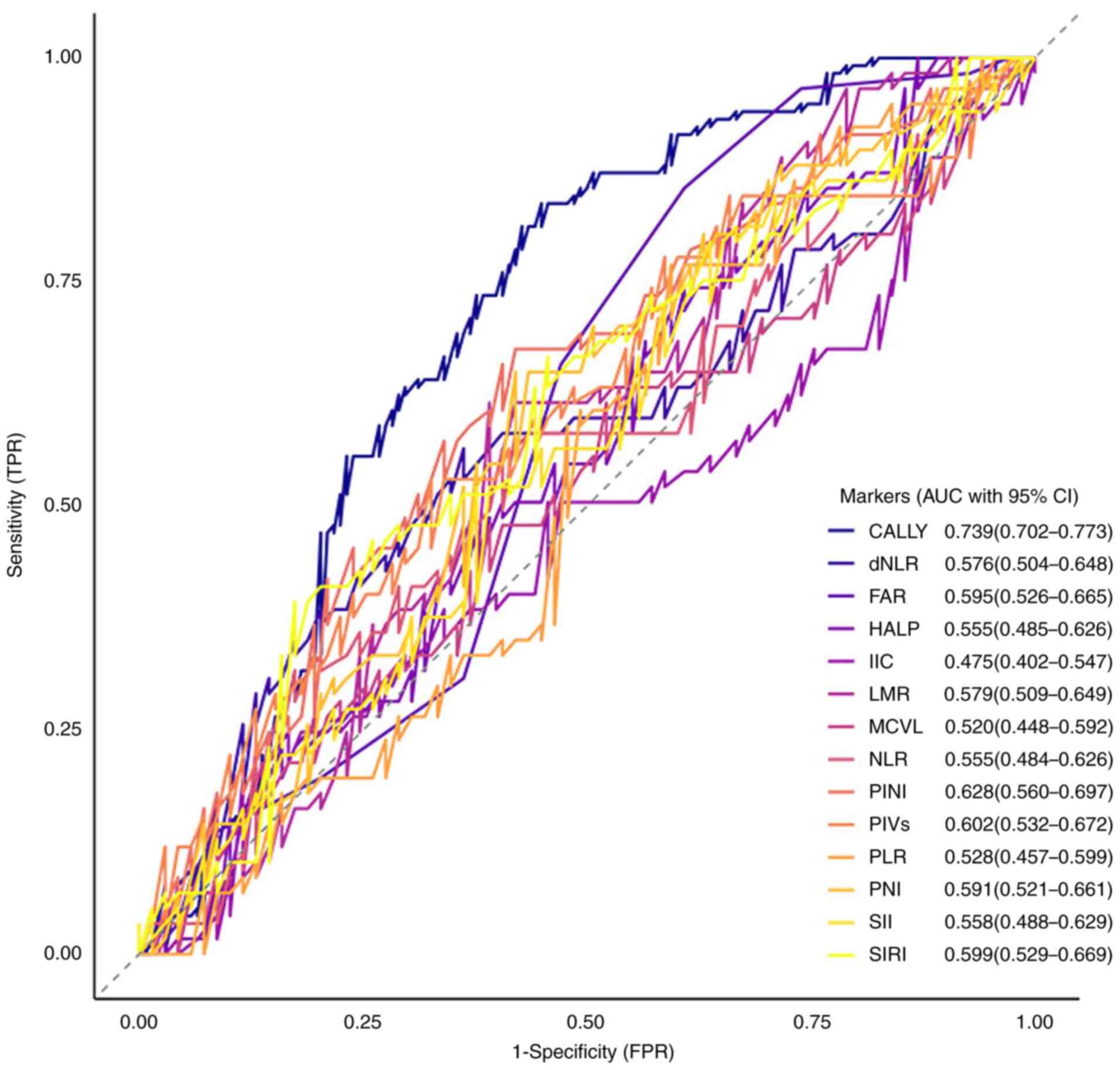
final cohort comprised 255 patients. ROC curve analysis evaluated
the prognostic performance of 14 inflammatory markers for clinical
outcomes in stage I–III CRC. The CALLY index demonstrated superior
discriminative ability, with an AUC of 0.739 (95% CI, 0.702–0.773),
significantly outperforming all other biomarkers (Fig. 2). Stratified by the CALLY cutoff of
6.790 (Table I), the cohort
comprised 160 low-CALLY (<6.790) and 95 high-CALLY (≥6.790)
patients (Table II). The low-CALLY
group was associated with more aggressive tumor biology,
characterized by higher rates of poorly differentiated histology
(26.25 vs. 6.32%; P<0.001), advanced T4 stage (71.25 vs. 49.47%;
P<0.001), nodal metastasis (N1-2: 74.38 vs. 16.84%; P<0.001),
and stage III disease (73.12 vs. 16.84%; P<0.001). Clinically,
these patients more frequently required adjuvant therapy (75.62 vs.
45.26%; P<0.001), alongside significantly worse RFS (38.33±19.03
vs. 52.93±15.57 months; P<0.001) and OS (44.01±16.22 vs.
54.26±13.46 months; P<0.001) times that were visually
substantiated by pronounced separation in Kaplan-Meier curves
(Figs. 3 and 4). Notably, elevated CA125 (23.08 vs.
10.14%; P=0.005) and CA19-9 (24.79 vs. 14.49%; P=0.038) were more
prevalent in the high-CALLY group despite its less advanced
pathology. No significant intergroup differences existed in terms
of age, sex, tumor location, surgical approach, blood loss,
comorbidities, anastomotic leakage and tumour size P>0.05),
while the median (Q1-Q3) CALLY values
robustly distinguished the cohorts [low-CALLY, 2.62 (1.62–4.64) vs.
high-CALLY, 12.97 (9.88–17.16); P<0.001]. Collectively, low
CALLY status is associated with advanced disease, intensified
treatment needs and inferior survival, graphically validated by
stratified survival analyses, positioning it as a significant
prognostic integrator of inflammatory-nutritional imbalance in
CRC.
 | Table II.Clinicopathological characteristics
of colorectal cancer patients by CALLY score group. |
Table II.
Clinicopathological characteristics
of colorectal cancer patients by CALLY score group.
|
|
| CALLY |
|
|---|
|
|
|
|
|
|---|
| Variables | Total (n=255) | Low (n=160) | High (n=95) | P-value |
|---|
| Age, years | 62.58±12.49 | 62.34±12.87 | 62.98±11.90 | 0.695 |
| Sex |
|
|
| 0.927 |
|
Male | 144 (56.47) | 90 (56.25) | 54 (56.84) |
|
|
Female | 111 (43.53) | 70 (43.75) | 41 (43.16) |
|
| Tumor location |
|
|
| 0.808 |
|
Colon | 149 (58.43) | 95 (59.38) | 54 (56.84) |
|
|
Rectum | 106 (41.57) | 65 (40.63) | 41 (43.16) |
|
| Surgical
approach |
|
|
| 0.662 |
|
Open | 173 (67.84) | 114 (71.25) | 59 (62.11) |
|
|
Laparoscopic | 82 (32.16) | 46 (28.75) | 36 (37.89) |
|
| Tumor size ≥3.5
cm |
|
|
| 0.077 |
| No | 95 (37.25) | 53 (33.13) | 42 (44.21) |
|
|
Yes | 160 (62.75) | 107 (66.88) | 53 (55.79) |
|
| Blood loss,
mla | 50.00 | 50.00 | 50.00 | 0.093 |
|
| (50.00–100.00) | (50.00–100.00) | (50.00–100.00) |
|
| Comorbidities |
|
|
| 0.062 |
| No | 201 (78.82) | 132 (82.50) | 69 (72.63) |
|
|
Yes | 54 (21.18) | 28 (17.50) | 26 (27.37) |
|
| Anastomotic
leakage |
|
|
| 0.437 |
| No | 240 (94.12) | 152 (95.00) | 88 (92.63) |
|
|
Yes | 15 (5.88) | 8 (5.00) | 7 (7.37) |
|
| Pathological
pattern |
|
|
| <0.001 |
|
Well | 18 (7.06) | 10 (6.25) | 8 (8.42) |
|
|
Moderate | 189 (74.12) | 108 (67.50) | 81 (85.26) |
|
|
Poor | 48 (18.82) | 42 (26.25) | 6 (6.32) |
|
| T stage |
|
|
| <0.001 |
| I | 2 (0.78) | 0 (0.00) | 2 (2.11) |
|
| II | 57 (22.35) | 21 (13.13) | 36 (37.89) |
|
|
III | 35 (13.73) | 25 (15.6) | 10 (10.53) |
|
| IV | 161 (63.14) | 114 (71.25) | 47 (49.47) |
|
| N stage |
|
|
| <0.001 |
| 0 | 120 (47.06) | 41 (25.63) | 79 (83.16) |
|
| I | 103 (40.39) | 92 (57.50) | 11 (11.58) |
|
| II | 32 (12.55) | 27 (16.88) | 5 (5.26) |
|
| TNM stage |
|
|
| <0.001 |
| I | 42 (16.47) | 4 (2.50) | 38 (40.00) |
|
| II | 80 (31.37) | 39 (24.38) | 41 (43.16) |
|
|
III | 133 (52.16) | 117 (73.13) | 16 (16.84) |
|
| P-adjuvant
therapy |
|
|
| <0.001 |
| No | 91 (35.69) | 39 (24.38) | 52 (54.74) |
|
|
Yes | 164 (64.31) | 121 (75.63) | 43 (45.26) |
|
| RFS, months | 43.76±19.14 | 38.33±19.03 | 52.93±15.57 | <0.001 |
| OS, months | 47.83±16.01 | 44.01±16.22 | 54.26±13.46 | <0.001 |
| CEA ≥5 ng/ml |
|
|
| 0.031 |
| No | 166 (65.10) | 120 (75.00) | 46 (48.42) |
|
|
Yes | 89 (34.90) | 40 (25.00) | 49 (51.58) |
|
| CA19-9 ≥30
U/ml |
|
|
| 0.038 |
| 0 | 206 (80.78) | 140 (87.50) | 66 (69.47) |
|
| 1 | 49 (19.22) | 20 (12.50) | 29 (30.53) |
|
| CA125 ≥25 U/ml |
|
|
| 0.005 |
| No | 214 (83.92) | 146 (91.25) | 68 (71.58) |
|
|
Yes | 41 (16.08) | 14 (8.75) | 27 (28.42) |
|
| CALLYa | 4.93 | 2.62 | 12.97 | <0.001 |
|
| (2.16–10.53) | (1.62–4.64) | (9.88–17.16) |
|
Prognostic significance of CALLY in
CRC survival
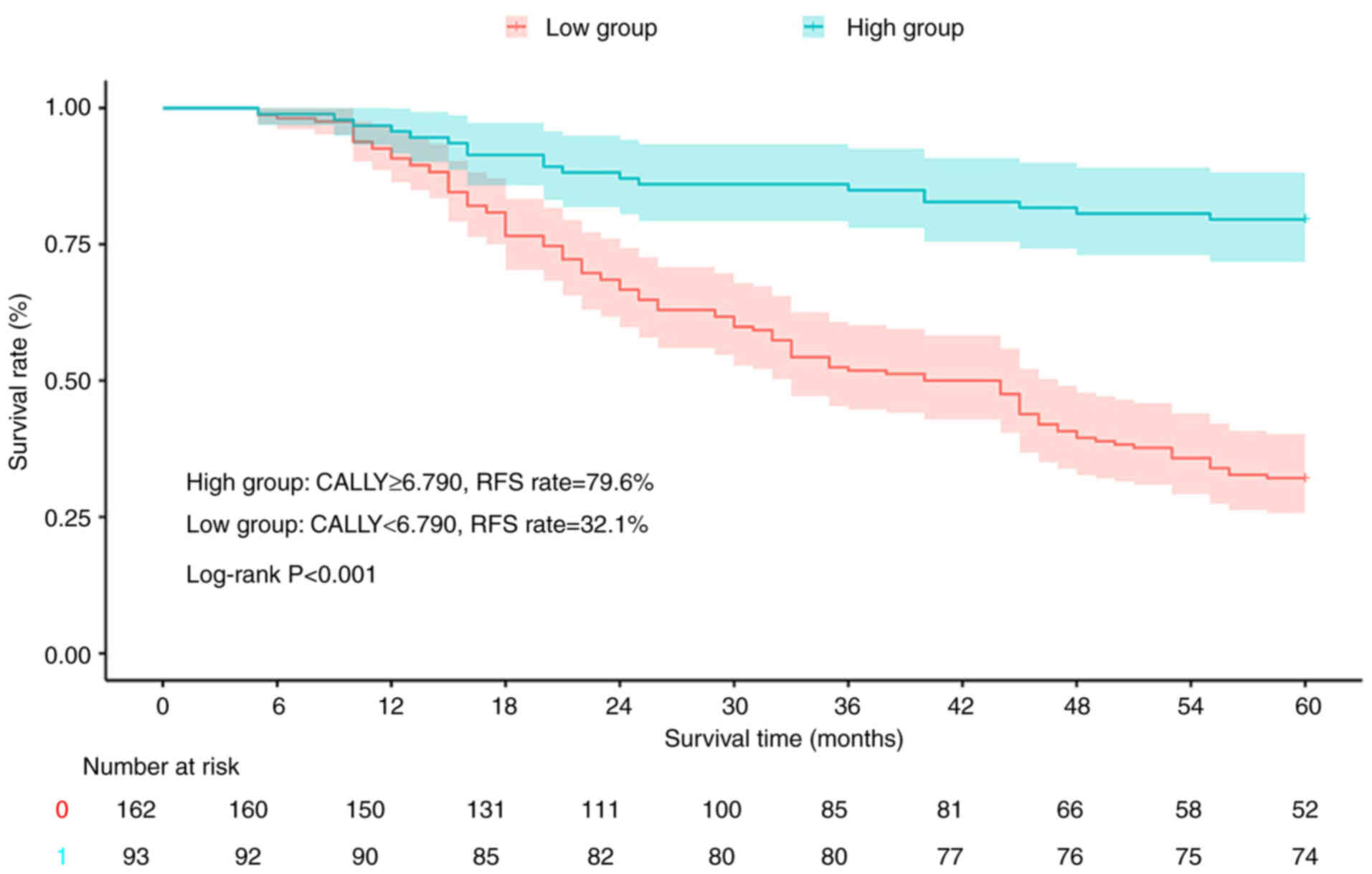
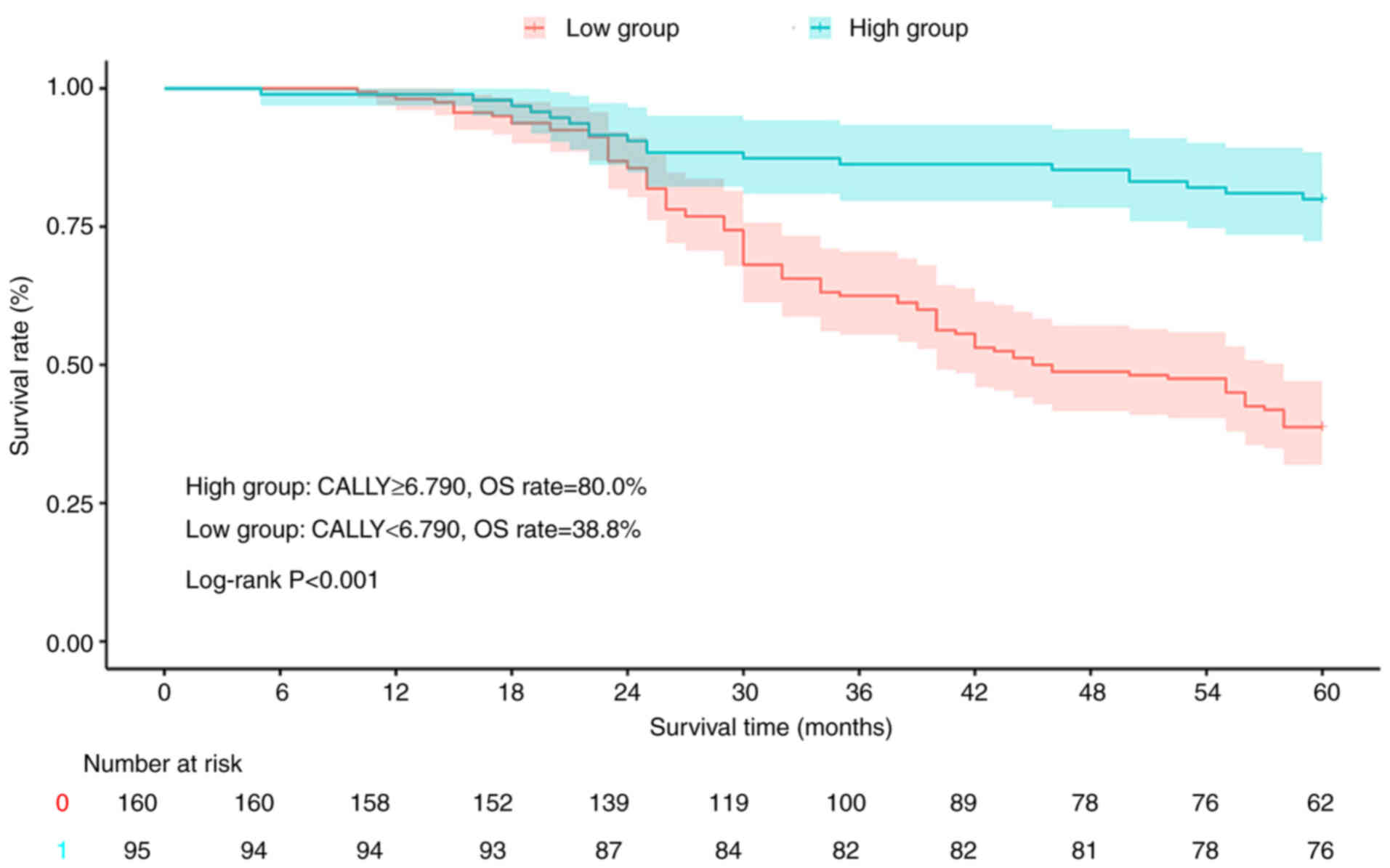
Stratification based on the CALLY index revealed
significant survival disparities among the patients with CRC.
Kaplan-Meier analysis showed that the high-CALLY group (≥6.790) had
superior RFS (79.6 vs. 32.1%; log-rank P<0.001; Fig. 3) and OS (80.0 vs. 38.8%; log-rank
P<0.001; Fig. 4) rates compared
with the low-CALLY group (<6.790).
COX regression analysis of 5-year RFS
rate in patients with CRC
Univariate and multivariate Cox regression analyses
established CALLY as an independent predictor of RFS in CRC
(Table III). In the univariate
analysis, high CALLY exhibited a strong association with improved
RFS (HR, 0.22; 95% CI, 0.14–0.35; P<0.001), outperforming
conventional biomarkers including CA19-9 (HR, 1.57; 95% CI,
1.04–2.38; P=0.032), CA125 (HR, 2.05; 95% CI, 1.36–3.07;
P<0.001) and CEA (HR, 1.45; 95% CI, 1.01–2.06; P=0.041). After
adjusting for clinicopathological confounders in multivariate
analysis, CALLY retained independent significance (HR, 0.56; 95%
CI, 0.35–0.90; P=0.016), while among the other factors, only nodal
metastasis (N1-2 vs. N0: HR, 6.71; 95% CI, 4.17–10.81; P<0.001),
poor differentiation (moderate/poor vs. well: HR, 2.79; 95% CI,
1.88–4.16; P<0.001), advanced TNM stage (I vs. II–III: HR 2.59,
1.03–6.51; P=0.043) and elevated CA19-9 (>30 vs. ≤30 U/ml: HR,
1.69; 95% CI, 1.06–2.71; P=0.028) remained significant. Notably,
tumor size (P=0.114), blood loss (P=0.100), CEA (0.213), CA125
(0.755), advanced T stage (P=0.056) and adjuvant therapy (P=0.082)
lost statistical significance after adjustment.
 | Table III.Univariate and multivariate analysis
for recurrence-free survival (RFS) in patients with colorectal
cancer patients. |
Table III.
Univariate and multivariate analysis
for recurrence-free survival (RFS) in patients with colorectal
cancer patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (female vs.
male) | 0.85
(0.60–1.20) | 0.352 | - | - |
| Age (≥60 vs. <60
years) | 0.80
(0.56–1.14) | 0.211 | - | - |
| Tumor location
(rectum vs. colon) | 0.90
(0.63–1.28) | 0.564 | - | - |
| Surgical approach
(laparoscopic vs. open) | 0.20
(0.03–1.42) | 0.107 | - | - |
| Tumor size (≥3.5
vs. <3.5 cm) | 1.78
(1.22–2.61) | 0.003 | 1.51
(0.90–2.54) | 0.114 |
| Blood loss (≥100
vs. <100 ml) | 2.53
(1.56–4.08) | <0.001 | 1.83
(0.89–3.76) | 0.100 |
| Predisease (yes vs.
no) | 0.72
(0.46–1.13) | 0.156 | - | - |
| Anastomotic leakage
(yes vs. no) | 0.58
(0.24–1.42) | 0.236 | - | - |
| Pathological
pattern (moderate and poor vs. well) | 3.76
(2.58–5.48) | <0.001 | 2.79
(1.88–4.16) | <0.001 |
| T stage (I and II
vs. III and IV) | 3.29
(1.89–5.73) | <0.001 | 1.75
(0.98–3.12) | 0.056 |
| N stage (0 vs. I
and II) | 7.88
(4.95–12.54) | <0.001 | 6.71
(4.17–10.81) | <0.001 |
| TNM stage (I vs.
III and III) | 12.04
(3.83–37.87) | <0.001 | 2.59
(1.03–6.51) | 0.043 |
| P-adjuvant therapy
(yes vs. no) | 3.77
(2.40–5.92) | <0.001 | 1.59
(0.94–2.69) | 0.082 |
| CEA (>5 vs. ≤5
ng/ml) | 1.45
(1.01–2.06) | 0.041 | 0.76
(0.49–1.17) | 0.213 |
| CA125 (>25 vs.
≤25 U/ml) | 2.05
(1.36–3.07) | <0.001 | 0.92
(0.54–1.56) | 0.755 |
| CA19-9 (>30 vs.
≤30 U/ml) | 1.57
(1.04–2.38) | 0.032 | 1.69
(1.06–2.71) | 0.028 |
| CALLY (high vs.
low) | 0.22
(0.14–0.35) | <0.001 | 0.56
(0.35–0.90) | 0.016 |
COX regression analysis of 5-year OS
rate in patients with CRC
Univariate and multivariate Cox regression analyses
established CALLY as an independent predictor of OS in CRC
(Table IV). Upon univariate
analysis, high CALLY exhibited a profound protective effect on OS,
with an HR of 0.26 (95% CI, 0.16–0.43; P<0.001), outperforming
all other variables including nodal metastasis (HR, 7.95; 95% CI,
4.79–13.18; P<0.001), poor differentiation (HR, 3.69; 95% CI,
2.50–5.45; P<0.001) and elevated CA19-9 (HR, 1.74; 95% CI,
1.14–2.65; P=0.010). Following multivariate adjustment for
clinicopathological confounders, CALLY retained robust independent
significance (HR, 0.47; 95% CI, 0.27–0.82; P=0.008), while nodal
metastasis (HR, 5.41; 95% CI, 2.93–9.98; P<0.001), poor
differentiation (HR, 2.42; 95% CI, 1.54–3.81; P<0.001) and
elevated CA19-9 (HR, 1.61; 95% CI, 1.02–2.57; P=0.043) remained
significant, alongside advanced TNM stage (HR, 1.74; 95% CI,
1.03–2.95; P=0.040). Conventional biomarkers (CEA and CA125),
anatomical factors (T stage), and treatment variables (adjuvant
therapy) lost statistical significance (P>0.05) after
adjustment, along with tumor size (P=0.121) and intraoperative
blood loss (P=0.154), which were significant in the univariate
analysis.
 | Table IV.Univariate and multivariate analysis
for overall survival in patients with colorectal cancer. |
Table IV.
Univariate and multivariate analysis
for overall survival in patients with colorectal cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (female vs.
male) | 0.77
(0.53–1.11) | 0.158 | - | - |
| Age (≥60 vs. <60
years) | 0.81
(0.56–1.17) | 0.263 | - | - |
| Tumor location
(rectum vs. colon) | 0.86
(0.59–1.25) | 0.440 | - | - |
| Surgical approach
(laparoscopic vs. open) | 0.20
(0.03–1.42) | 0.107 | - | - |
| Tumor size (≥3.5
vs. <3.5 cm) | 2.28
(1.50–3.47) | <0.001 | 1.57
(0.89–2.76) | 0.121 |
| Blood loss (≥100
vs. <100 ml) | 2.94
(1.81–4.78) | <0.001 | 2.01
(0.77–5.25) | 0.154 |
| Predisease (yes vs.
no) | 0.75
(0.47–1.20) | 0.230 | - | - |
| Anastomotic leakage
(yes vs. no) | 0.66
(0.27–1.63) | 0.372 | - | - |
| Pathological
pattern (moderate and poor vs. well) | 3.69
(2.50–5.45) | <0.001 | 2.42
(1.54–3.81) | <0.001 |
| T stage (I and II
vs. III and IV) | 4.65
(2.35–9.18) | <0.001 | 1.44
(0.63–3.30) | 0.394 |
| N stage (0 vs. I
and II) | 7.95
(4.79–13.18) | <0.001 | 5.41
(2.93–9.98) | <0.001 |
| TNM stage (I vs.
III and III) | 15.56
(3.84–63.01) | <0.001 | 1.74
(1.03–2.95) | 0.040 |
| P-adjuvant therapy
(yes vs. no) | 4.27
(2.58–7.06) | <0.001 | 1.64
(0.95–2.85) | 0.077 |
| CEA (>5 vs. ≤5
ng/ml) | 1.61
(1.11–2.32) | 0.011 | 0.90
(0.58–1.38) | 0.622 |
| CA125 (>25 vs.
≤25 U/ml) | 1.82
(1.19–2.81) | 0.006 | 1.01
(0.60–1.71) | 0.971 |
| CA19-9 (>30 vs.
≤30 U/ml) | 1.74
(1.14–2.65) | 0.010 | 1.61
(1.02–2.57) | 0.043 |
| CALLY (high vs.
low) | 0.26
(0.16–0.43) | <0.001 | 0.47
(0.27–0.82) | 0.008 |
Discussion
The CALLY index derives its prognostic power from
quantifying a pathophysiological triad that orchestrates CRC
progression through tumor microenvironment (TME)-specific
mechanisms. Elevated CRP levels reflect activation of
interleukin-6/Janus kinase/signal transducer and activator of
transcription 3 signaling, which expands myeloid-derived suppressor
cells (MDSCs) that spatially exclude cytotoxic T lymphocytes from
tumor nests, a hallmark of ‘immune-excluded’ CRC subtypes (27,28).
Concurrently, hypoalbuminemia disrupts gut barrier integrity,
permitting translocation of procarcinogenic microbiota (for
example, Fusobacterium nucleatum) that activate transforming
growth factor-β signaling (29,30).
This further amplifies MDSC-mediated immunosuppression by inducing
regulatory T cell differentiation and programmed death-ligand 1
(PD-L1) upregulation on tumor-associated macrophages (31). Lymphopenia completes this vicious
cycle by depleting CD103+ tissue-resident memory T cells
critical for controlling microsatellite-stable CRC (32). Collectively, these processes
establish an immunosuppressive TME favoring metastasis.
In gastric cancer research, Hashimoto et al
(33) demonstrated that the
high-CALLY group (cut-off value: 3.28) exhibited a significantly
higher proportion of early-stage cases (stage I: 71.5%; P=0.019)
and lower venous invasion rates compared with the low-CALLY group.
These findings align closely with the present study, where the
high-CALLY group (≥6.790) displayed superior tumor biological
characteristics, including significantly reduced rates of poor
differentiation (6.32 vs. 26.25%), T4 invasion (49.47 vs. 71.25%),
lymph node metastasis (N1-2 stage: 16.84 vs. 74.38%) and stage III
disease (16.84 vs. 73.12%) (all P<0.001). Paradoxically, despite
the favorable prognosis, the high-CALLY group showed elevated
levels of CA125 (23.08 vs. 10.14%) and CA19-9 (24.79 vs. 14.49%).
This apparent contradiction may be explained by enhanced
immunoediting mechanisms, such as intact lymphocyte function,
mediated through antibody-dependent cellular cytotoxicity, which
efficiently eliminates antigen-expressing tumor cells, leading to
the release of tumor-associated antigens (e.g., CA125/CA19-9) into
the bloodstream (34,35). Single-cell sequencing further
reveals that specific T-cell subsets (for example, tissue-resident
memory T cells) may modulate biomarker release dynamics by
regulating immune checkpoint molecules such as PD-L1 (36). Future studies should explore the
combined prognostic value of serial CALLY measurements and tumor
markers.
The prognostic role of the CALLY index in advanced
CRC has been established. Furukawa et al (37) demonstrated its superiority in
metastatic settings, showing a 2.8-fold increased mortality risk in
patients with colorectal liver metastasis (95% CI, 1.6–4.9;
P<0.001) compared with conventional NLR/PNI biomarkers. The
present study extends these findings to stage I–III CRC through
three key analytical approaches. First, in a head-to-head
comparison of 14 prognostic markers, the CALLY index achieved the
highest discriminative power (AUC, 0.739). Second, multivariable
Cox models confirmed its independence from TNM stage and age (OS:
HR, 2.15; 95% CI, 1.16–3.98; P=0.015; and RFS: HR, 2.34; 95% CI,
1.32–4.15; P=0.003). Most notably, Kaplan-Meier analysis revealed
significant survival disparities, with low-CALLY patients
exhibiting 23.8 and 31.6% absolute reductions in 5-year OS (58.3
vs. 82.1%; P=0.008) and RFS (45.2 vs. 76.8%; P<0.001),
respectively. This tripartite validation, spanning ROC performance,
regression stability and survival curve divergence, solidifies the
CALLY index as a pan-stage prognostic tool.
The present study has several limitations that
warrant consideration. First, the single-center retrospective
design and relatively limited sample size may introduce selection
bias, highlighting the need for future multicenter studies with
larger cohorts to validate the findings. Second, the proposed CALLY
cutoff value was derived from a single institutional dataset,
necessitating external validation through collaborative multicenter
research to confirm its generalizability across diverse
populations. Specifically, the lack of an independent external
cohort for validating the optimal cutoff (6.790) and prognostic
performance limits the immediate clinical translatability of the
findings. Future studies should prioritize multi-institutional
collaboration to establish population-adjusted thresholds. Third,
the exclusive reliance on single-timepoint preoperative
measurements precluded assessment of dynamic changes in CALLY
values, suggesting that prospective studies incorporating serial
measurements would provide more comprehensive insights into its
clinical utility. Fourth, the absence of key prognostic confounders
in the analysis, including molecular subtypes (RAS/BRAF mutation
status), microsatellite instability status, perioperative
nutritional support and postoperative complications, may have
influenced survival outcomes independent of the CALLY index.
Finally, the analysis did not incorporate circulating tumor DNA or
other molecular residual disease markers, which could potentially
miss early micrometastatic signals.
The present study establishes the CALLY index as a
simple yet effective prognostic marker for patients with stage
I–III CRC after radical resection. Key findings demonstrate its
ability to: i) Independently predict RFS and OS rates; ii) stratify
patients by tumor aggressiveness; and iii) complement traditional
TNM staging. As a composite of routine blood parameters, this
multifaceted marker, integrating inflammatory, nutritional and
immune indicators, enhances clinical relevance while offering
cost-effectiveness and immediate clinical implementability.
Although further validation is warranted, the CALLY index may guide
personalized management by identifying high-risk patients requiring
intensified surveillance, ultimately optimizing CRC therapeutic
decisions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JL and SZ were responsible for the study
conceptualization and methodology. JL and XH were responsible for
visualization, validation, investigation and data curation. JL
provided resources and wrote the original draft manuscript. SZ
helped to review and edit the manuscript, and supervised the study.
JL, XH and SZ were responsible for project administration. JL and
SZ confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and was approved by the Research Ethics
Committee at The Jingdezhen First People's Hospital (Jingdezhen,
China; approval no. jdzyykt202514). The requirement for informed
consent was waived due to the retrospective nature of the study and
data anonymization.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rezazadeh M, Agah S, Kamyabi A, Akbari A,
Ghamkhari Pisheh R, Eshraghi A, Babakhani A, Ahmadi A, Paseban M,
Heidari P, et al: Effect of diabetes mellitus type 2 and
sulfonylurea on colorectal cancer development: A Case-control
study. BMC Gastroenterol. 24:3822024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mozooni Z, Golestani N, Bahadorizadeh L,
Yarmohammadi R, Jabalameli M and Amiri BS: The role of
interferon-gamma and its receptors in gastrointestinal cancers.
Pathol Res Pract. 248:1546362023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Han Z, Li X, Huang A, Shi J and Gu
J: Epidemiology and risk factors of colorectal cancer in China.
Chin J Cancer Res. 32:729–741. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benson AB III, Venook AP, Cederquist L,
Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC,
Fichera A, et al: Colon cancer, version 1.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burzinskis E, Janulaityte I, Jievaltas M,
Skaudickas D, Burzinskiene G, Dainius E, Naudziunas A and
Vitkauskiene A: Inflammatory markers in prostate cancer: Potential
roles in risk stratification and immune profiling. J Immunotoxicol.
22:24977762025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan J, Wu Z, Liu Y, Wang W, Qin W, Pan G,
Xiong Y, Ma J, Zhao J, Zhou H, et al: Transcriptional profiling
reveals H.pylori-associated genes induced inflammatory cell
infiltration and chemoresistance in gastric cancer. Front Immunol.
16:15925582025. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan S, Zhu L, Chen X and Lin Q: Huanglian
Jiedu Tang regulates the inflammatory microenvironment to alleviate
the progression of breast cancer by inhibiting the RhoA/ROCK
pathway. Tissue Cell. 95:1028502025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JY, Jung EJ, Kim JM, Lee HS, Kwag SJ,
Park JH, Park T, Jeong SH, Jeong CY and Ju YT: Dynamic changes of
neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio
predicts breast cancer prognosis. BMC Cancer. 20:12062020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang XZ, Chen WJ, Zhang X, Wu CC, Zhang
CY, Sun SS and Wu J: An Elevated Platelet-to-Lymphocyte ratio
predicts poor prognosis and clinicopathological characteristics in
patients with colorectal cancer: A Meta-analysis. Dis Markers.
2017:10531252017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nøst TH, Alcala K, Urbarova I, Byrne KS,
Guida F, Sandanger TM and Johansson M: Systemic inflammation
markers and cancer incidence in the UK Biobank. Eur J Epidemiol.
36:841–848. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Wu Q, Kong Y and Lu C: Mild
cognitive impairment in type 2 diabetes is associated with
fibrinogen-to-albumin ratios. PeerJ. 11:e158262023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu X, Sun T, Zhang X, Wang W, Ji Y and
Jing J: The predicting role of albumin to derived
neutrophil-to-lymphocyte ratio for the prognosis of esophageal
squamous cell carcinoma patients aged 60 years and above. BMC
Cancer. 25:6522025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poenariu IS, Boldeanu L, Ungureanu BS,
Caragea DC, Cristea OM, Pădureanu V, Siloși I, Ungureanu AM, Statie
RC, Ciobanu AE, et al: Interrelation of Hypoxia-inducible factor-1
Alpha (HIF-1 α) and the ratio between the mean corpuscular
Volume/Lymphocytes (MCVL) and the cumulative inflammatory index
(IIC) in ulcerative colitis. Biomedicines. 11:31372023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Gong L, Zhu X, Wang Y and Li C:
Mediation of systemic inflammation response index in the
association of healthy eating Index-2020 in patientis with
periodontitis. Oral Health Prev Dent. 23:225–232. 2025.PubMed/NCBI
|
|
16
|
Wang Y, Lyu J, Jia H, Liang L, Xiao L, Liu
Y, Liu X, Li K, Chen T, Zhang R, et al: Clinical utility of the
systemic immune-inflammation index for predicting survival in
esophageal squamous cell carcinoma after radical radiotherapy.
Future Oncol. 17:2647–2657. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Sun W, Fu S, Wang J, Jin B, Zhang S,
Liu Y, Zhang Q and Wang H: Prognostic value of the preoperative
prognostic nutritional and systemic immunoinflammatory indexes in
patients with colorectal cancer. BMC Cancer. 25:4032025. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Șerban RE, Popescu DM, Boldeanu MV,
Florescu DN, Șerbănescu MS, Șandru V, Panaitescu-Damian A,
Forțofoiu D, Șerban RC, Gherghina FL and Vere CC: The diagnostic
and prognostic role of inflammatory markers, including the new
cumulative inflammatory index (IIC) and mean corpuscular
volume/lymphocyte (MCVL), in colorectal adenocarcinoma. Cancers
(Basel). 17:9902025. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie H, Wei L, Liu M, Liang Y, Yuan G, Gao
S, Wang Q, Lin X, Tang S and Gan J: Prognostic significance of
preoperative prognostic immune and nutritional index in patients
with stage I–III colorectal cancer. BMC Cancer. 22:13162022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong J, Jiang W, Zhang W, Guo T, Yang Y,
Jiang X, Zheng L and Du T: Exploring the J-shaped relationship
between HALP score and mortality in cancer patients: A NHANES
1999–2018 cohort study. Front Oncol. 14:13886102024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li K, Zeng X, Zhang Z, Wang K, Pan Y, Wu
Z, Chen Y and Zhao Z: Pan-immune-inflammatory values predict
survival in patients after radical surgery for non-metastatic
colorectal cancer: A retrospective study. Oncol Lett. 29:1972025.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iida H, Tani M, Komeda K, Nomi T,
Matsushima H, Tanaka S, Ueno M, Nakai T, Maehira H, Mori H, et al:
Superiority of CRP-albumin-lymphocyte index (CALLY index) as a
non-invasive prognostic biomarker after hepatectomy for
hepatocellular carcinoma. HPB (Oxford). 24:101–115. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakashima K, Haruki K, Kamada T, Takahashi
J, Tsunematsu M, Ohdaira H, Furukawa K, Suzuki Y and Ikegami T:
Usefulness of the C-reactive protein (CRP)-Albumin-lymphocyte
(CALLY) index as a prognostic indicator for patients with gastric
cancer. Am Surg. 90:2703–2709. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fukushima N, Masuda T, Tsuboi K, Takahashi
K, Yuda M, Fujisaki M, Ikegami T, Yano F and Eto K: Prognostic
significance of the preoperative C-reactive
protein-albumin-lymphocyte (CALLY) index on outcomes after
gastrectomy for gastric cancer. Surg Today. 54:943–952. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Toda M, Musha H, Suzuki T, Nomura T and
Motoi F: Impact of C-reactive protein-albumin-lymphocyte index as a
prognostic marker for the patients with undergoing gastric cancer
surgery. Front Nutr. 12:15560622025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amin MB, Edge SB and Greene FL: AJCC
Cancer Staging Manual. 8th edition. New York: Springer; 2016
|
|
27
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L and Karin M: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Binnewies M, Roberts EW, Kersten K, Chan
V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI,
Ostrand-Rosenberg S, Hedrick CC, et al: Understanding the tumor
immune microenvironment (TIME) for effective therapy. Nat Med.
24:541–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mima K, Nishihara R, Qian ZR, Cao Y,
Sukawa Y, Nowak JA, Yang J, Dou R, Masugi Y, Song M, et al:
Fusobacterium nucleatum in colorectal carcinoma tissue and
patient prognosis. Gut. 65:1973–1980. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen WD, Zhang X, Zhang YP, Yue CB, Wang
YL, Pan HW, Zhang YL, Liu H and Zhang Y: Fusobacterium
nucleatum is a risk factor for metastatic colorectal cancer.
Curr Med Sci. 42:538–547. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tauriello DVF, Palomo-Ponce S, Stork D,
Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M,
Ibiza S, Cañellas A, Hernando-Momblona X, et al: TGFβ drives immune
evasion in genetically reconstituted colon cancer metastasis.
Nature. 554:538–543. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang A, Wu Y, Cui J, Cai M, Xie Y, Zou J,
Alenzi M, Yang H, Huang P and Huang M: Assessment of
compartment-specific CD103-positive cells for prognosis prediction
of colorectal cancer. Cancer Immunol Immunother. 74:2372025.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashimoto I, Tanabe M, Onuma S, Morita J,
Nagasawa S, Maezawa Y, Kanematsu K, Aoyama T, Yamada T, Yukawa N,
et al: Clinical impact of the C-reactive Protein-albumin-lymphocyte
index in Post-gastrectomy patients with gastric cancer. In Vivo.
38:911–916. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Łuksza M, Sethna ZM, Rojas LA, Lihm J,
Bravi B, Elhanati Y, Soares K, Amisaki M and Dobrin A: Neoantigen
quality predicts immunoediting in survivors of pancreatic cancer.
Nature. 606:389–395. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reina-Campos M, Heeg M, Kennewick K,
Mathews IT, Galletti G, Luna V, Nguyen Q, Huang H, Milner JJ, Hu
KH, et al: Metabolic programs of T cell tissue residency empower
tumour immunity. Nature. 621:179–187. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Furukawa S, Hiraki M, Kimura N, Sakai M,
Ikubo A and Samejima R: The potential of the C-reactive
protein-albumin-lymphocyte (CALLY) index as a prognostic biomarker
in colorectal cancer. Cancer Diagn Progn. 5:370–377. 2025.
View Article : Google Scholar : PubMed/NCBI
|