Introduction
Non-small cell lung cancer (NSCLC) is the most
common type of lung cancer, and treatment strategies vary depending
on the stage of disease and patient performance status. For
patients with locally advanced NSCLC who have a good performance
status, concurrent chemoradiotherapy (CCRT) is the standard
treatment, combining systemic chemotherapy with thoracic
radiotherapy to enhance local control and improve survival outcomes
(1,2). Following definitive CCRT,
consolidation with durvalumab, an anti-PD-L1 antibody, has been
shown in the phase III PACIFIC trial to significantly improve
progression-free and overall survival, and is now widely regarded
as the standard post-CCRT treatment (3,4).
However, a substantial proportion of patients relapse even after
CCRT and durvalumab consolidation therapy, and no consensus exists
on how to manage recurrence in this setting. The present case
report describes a patient with stage IIIB lung adenocarcinoma who
experienced recurrence during durvalumab consolidation and was
subsequently treated with a combination of durvalumab, tremelimumab
and chemotherapy.
Case report
A 52-year-old man with a 20-pack-year smoking
history presented to Toranomon Hospital (Tokyo, Japan) in April
2023 with an elevated carcinoembryonic antigen level of 17.8 ng/ml
(day 0). Chest computed tomography revealed a 23 mm irregular mass
in the left upper lobe hilum, with lymphadenopathy in the left
hilar and mediastinal regions (Fig.
1A-D). 18F-fluorodeoxyglucose (18F-FDG)
positron emission tomography/computed tomography (PET/CT) revealed
FDG uptake in the left upper lobe mass, left hilar lymph nodes, and
bilateral paratracheal lymph nodes. Relevant 18F-FDG
PET/CT images are presented in Fig.
1E-G. No findings suggestive of distant metastases were
observed elsewhere (Fig. 1H). On
day 7, bronchoscopy with transbronchial biopsy of the left
B3a bronchus and endobronchial ultrasound-guided
transbronchial needle aspiration of the 4R lymph node confirmed the
presence of an adenocarcinoma (Fig.
1I). Immunohistochemical analysis was negative for cytokeratin
5/6 and p40, positive for thyroid transcription factor 1 and napsin
A, and focally positive for hepatocyte nuclear factor 4 alpha.
Staining of programmed death-ligand 1 (PD-L1) using the 22C3 assay
revealed a tumor proportion score of less than 1% (Fig. 1J-M). Oncomine Dx testing was
negative for B-Raf proto-oncogene V600E, epidermal growth factor
receptor, and Kirsten rat sarcoma viral oncogene homolog G12C
mutations. Mutations in other driver gene could not be detected
because of insufficient sample quantity. Based on these findings,
stage IIIB primary lung adenocarcinoma (cT1cN3M0) was diagnosed
according to the 8th edition of the American Joint Committee on
Cancer staging system. On day 30, after 2 cycles of chemotherapy
(cisplatin (CDDP) 80 mg/m2, vinorelbine (VNR) 20
mg/m2) and 60 Gy radiation therapy, a reduction in the
size of the primary tumor and the left hilar lymph nodes was
observed, resulting in a partial response (PR) (Fig. 2C). This was followed by durvalumab
(10 mg/kg) consolidation therapy. Radiotherapy was delivered as
intensity-modulated radiation therapy (IMRT) using 6 MV photons, at
a total dose of 60 Gy in 30 fractions over 6 weeks. In concurrent
chemoradiotherapy (CCRT) for stage III non-small cell lung cancer
(NSCLC), carboplatin plus paclitaxel (CBDCA plus PTX) is considered
one of the standard treatment options (5,6). and
CDDP plus etoposide or CDDP plus pemetrexed (PEM) regimens have
also been comparatively evaluated. In contrast, CDDP plus VNR has
been well studied in Japanese patients, with sufficient data
supporting its efficacy and safety (5,7–9). It is
also covered by insurance and widely used in clinical practice in
Japan. Therefore, in this case, CDDP plus VNR were selected.
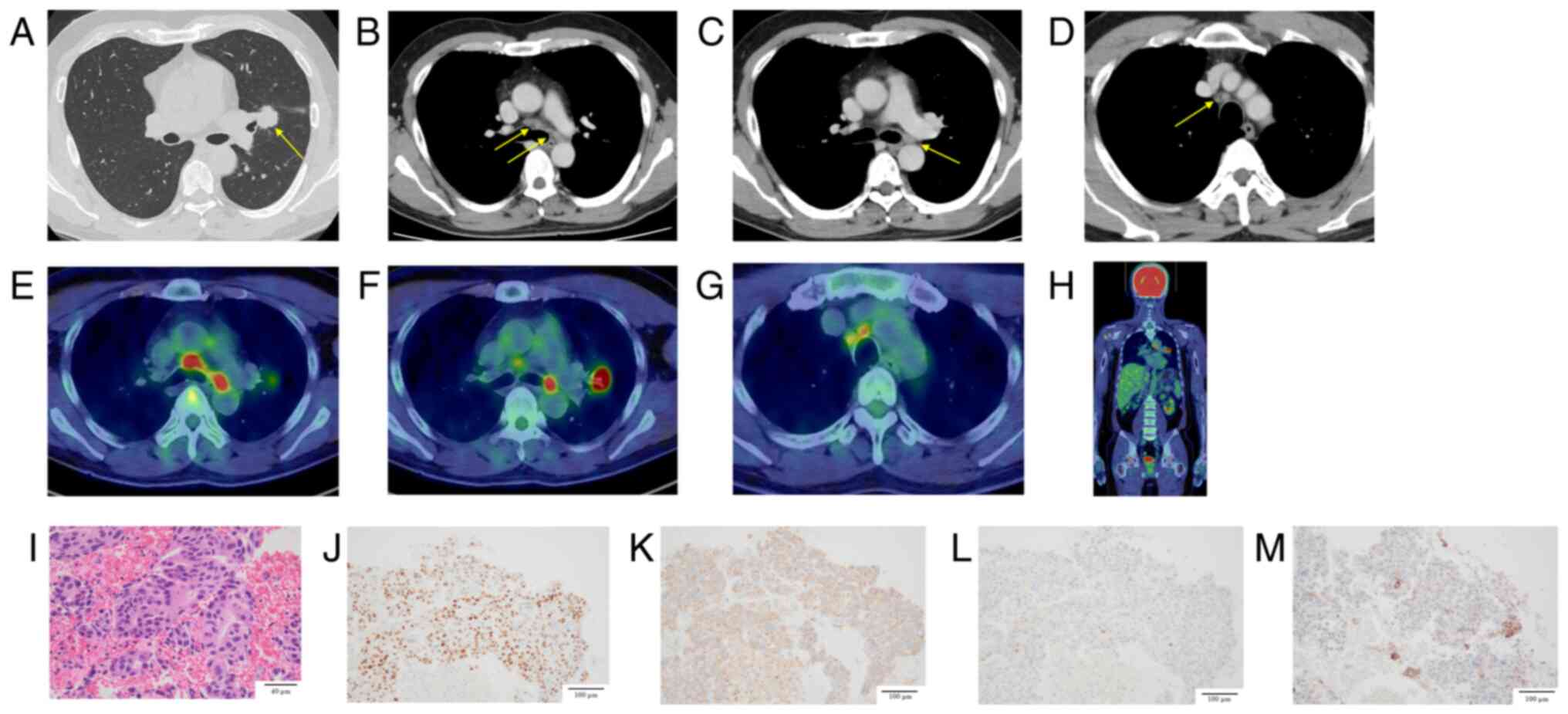 | Figure 1.(A) Chest CT revealed a 23-mm
irregular nodular lesion with spiculated margins in the left upper
lobe hilum, suspected to be the primary tumor. (B) Enlarged right
lower paratracheal and left lower paratracheal lymph nodes. (C)
Enlarged left hilar lymph node. (D) Enlarged right upper
paratracheal lymph node. (E) 18F-FDG positron emission
tomography/CT showed high FDG uptake in the right lower
paratracheal lymph node (SUVmax, 7.71) and the left lower
paratracheal lymph node (SUVmax, 7.83). (F) High FDG uptake in the
irregular left upper lobe mass near the hilum, suspected to be the
primary tumor (SUVmax, 16.73), and in the left hilar lymph node
(SUVmax, 9.39). (G) High FDG uptake in the right upper paratracheal
lymph node (SUVmax, 5.44). (H) No abnormal FDG uptake suggestive of
other distant metastases was observed. (I) Pathological findings
revealed atypical cells with hyperchromatic nuclei proliferating
while exhibiting a poorly formed glandular structure, indicative of
adenocarcinoma. (J) Dual immunostaining: TTF-1 (nucleus) positive,
CK5/6 (cytoplasm) negative. (K) Dual immunostaining: Napsin A
(cytoplasm) positive, p40 (nucleus) negative. (L) Hepatocyte
nuclear factor 4 alpha was negative. (M) Programmed death-ligand 1
immunostaining revealed a tumor proportion score of less than 1%.
Arrows indicate the primary tumor in (A), the right and left lower
paratracheal nodes in (B), the left hilar node in (C), and the
right upper paratracheal node in (D). 18F-FGD,
18F-fluorodeoxyglucose; CT, computed tomography; SUVmax,
standardized uptake value. |
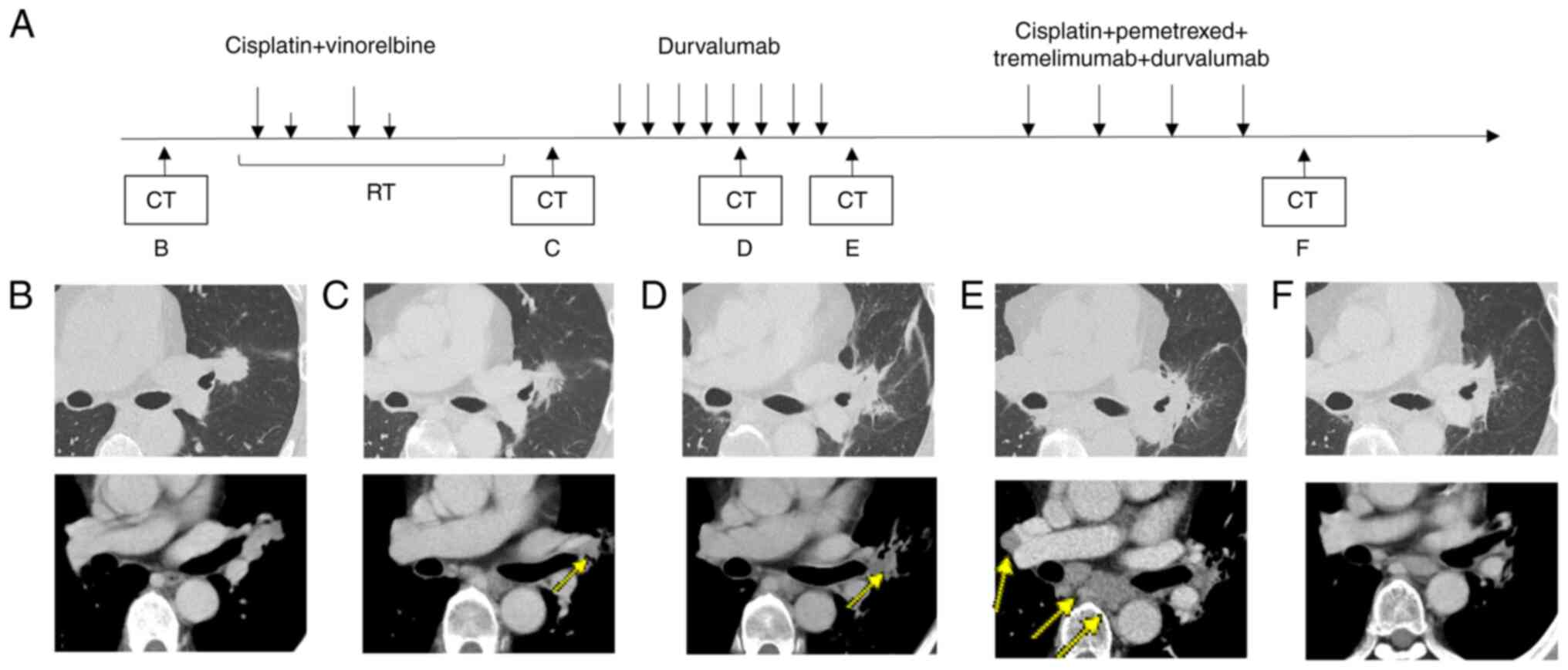 | Figure 2.(A) Imaging and therapeutic response
before and during treatment. (B) Prior to treatment, an irregular
mass was observed near the hilum of the left upper lobe,
accompanied by enlargement of the mediastinal and left hilar lymph
nodes. (C) Chest CT after concurrent chemoradiotherapy, showing a
partial response; the arrow indicates the primary lesion. (D) CT
after 5 cycles of durvalumab, showing a partial response; the arrow
indicates the primary lesion. (E) CT after 8 cycles of durvalumab,
showing progressive disease; the arrows indicate metastatic lesions
in the right hilar and mediastinal lymph nodes. (F) CT after 4
cycles of cisplatin plus pemetrexed plus tremelimumab plus
durvalumab, showing stable disease. CCRT, concurrent
chemoradiotherapy; CT, computed tomography; PD, progressive
disease; PR, partial response; SD, stable disease. |
During the initial concurrent chemoradiotherapy, no
significant adverse events were observed. On day 92, maintenance
therapy with durvalumab (10 mg/kg, every 2 weeks) was started.
After 5 cycles of durvalumab maintenance therapy, the follow-up
chest CT revealed mild radiation pneumonitis around the lesions,
which did not require treatment. In addition, a reduction in the
size of the primary tumor and left hilar lymph node metastases was
observed (Fig. 2D), and the
therapeutic response was assessed as partial response (PR).
However, after 8 cycles of durvalumab, on day 196, the patient's
carcinoembryonic antigen levels increased to 27.8 ng/ml.
18F-FDG PET/CT revealed additional FDG uptake in the
right hilar, mediastinal, and bilateral supraclavicular lymph
nodes, indicating disease progression (Fig. 2E). Therefore, durvalumab was
discontinued. On day 210, tissue specimens were obtained via repeat
bronchoscopy. However, the sample collected during the procedure
was insufficient for comprehensive analysis; thus, next-generation
sequencing could not be performed.
On day 217, the treatment regimen was adjusted to
include durvalumab (1,500 mg), tremelimumab (75 mg, every 3 weeks),
CDDP (75 mg/m2, every 3 weeks), plus PEM (500
mg/m2, every 3 weeks). Although disease progress
occurred, only 2 cycles of CDDP plus VNR were administered during
the concurrent chemoradiotherapy, and there is no evidence that the
effect of CDDP was insufficient. In the POSEIDON trial, either CDDP
or CBDCA could be selected. We chose CDDP plus PEM, which has more
extensive data compared to CBDCA plus PEM (10). Following the initial combination
phase, the patient transitioned to maintenance therapy with
durvalumab (1,500 mg every 4 weeks) plus PEM (500 mg/m2
every 4 weeks), following the dosing protocol established in the
phase II and III POSEIDON trial, which led to its approval by the
U.S. Food and Drug Administration and subsequent approval in Japan
(6). Thyroid function monitoring
resulted in the continuation of thyroid hormone replacement therapy
because of hypothyroidism as an immune-related adverse event.
During the first cycle of this combination therapy, the patient
experienced Grade 1 appetite loss, which was resolved without
intervention. Subsequently, during the maintenance phase with
durvalumab plus PEM, Grade 1 hepatotoxicity was observed. This was
managed with ursodeoxycholic acid and did not require treatment
interruption. After 4 cycles of combination therapy, the primary
tumor and right hilar lymph node metastases showed less than 30%
reduction in size, consistent with stable disease (Fig. 2F).
Maintenance therapy was administered for 5 cycles.
On day 477, a 6-mm brain metastasis developed in the left
cerebellum (Fig. 3B), and on day
509, 18F-FDG PET/CT revealed multiple bone metastases
and supraclavicular lymph node metastases (Fig. 3B). Therefore, disease progression
(PD) was determined, and maintenance therapy with durvalumab plus
PEM was discontinued. Radiotherapy to the cervical spine (C2) was
administered at a dose of 7 Gy per fraction for 5 fractions, and
Gamma Knife radiosurgery was performed at NTT Medical Center Tokyo
for brain metastasis in the left cerebellum.
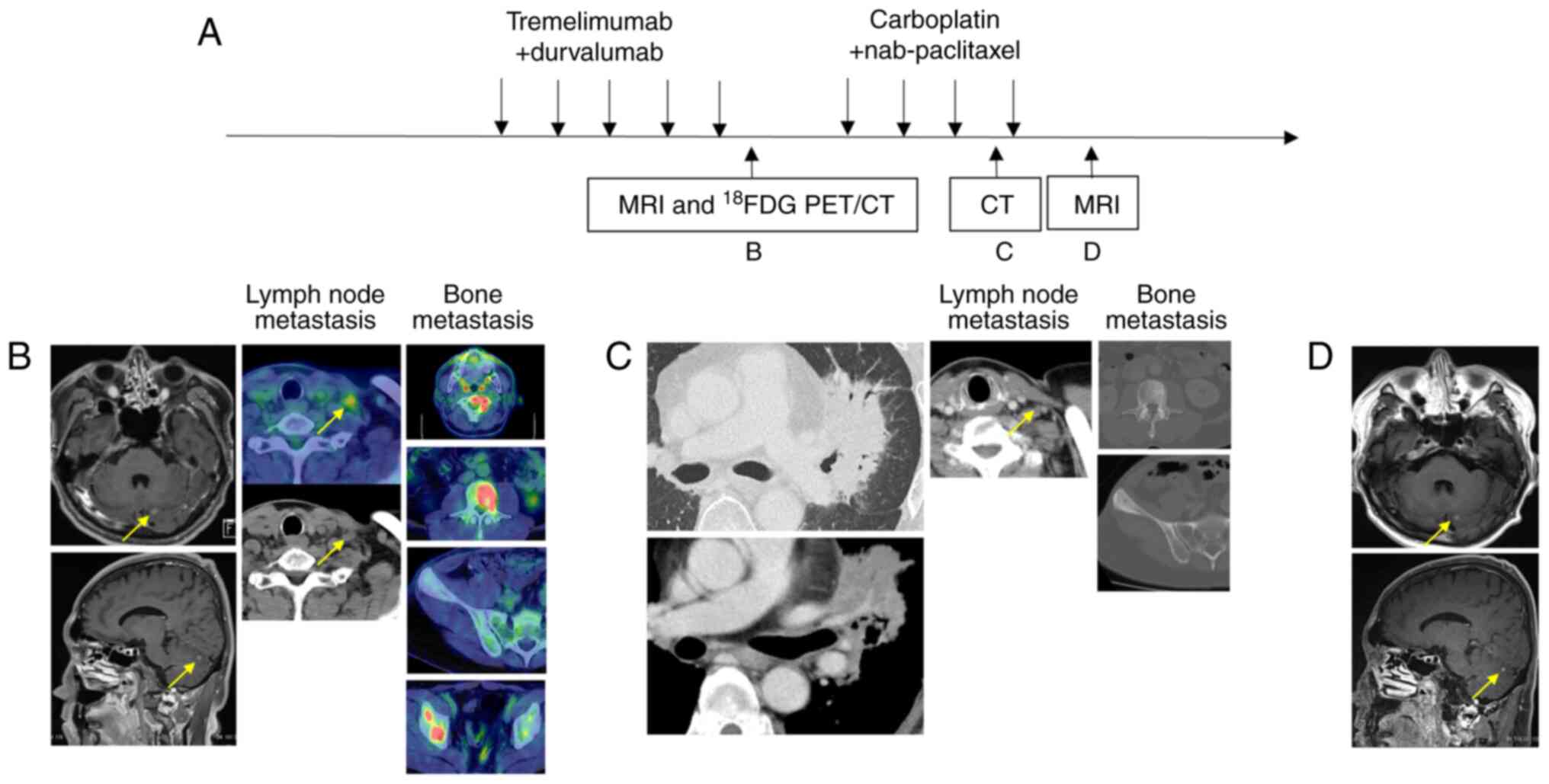 | Figure 3.(A) Imaging and therapeutic response
before and during treatment. (B) MRI and 18F-FDG PET/CT
after 5 cycles of maintenance therapy with durvalumab + pemetrexed,
showing progressive disease; the arrows indicate cerebellar
metastasis and supraclavicular lymph node metastasis, respectively.
(C) CT after 3 cycles of CBDCA plus nab-PTX, showing stable
disease; the arrow indicates supraclavicular lymph node metastasis.
(D) MRI after 3 cycles of CBDCA plus nab-PTX, showing stable
disease; the arrows indicate cerebellar metastasis.
18F-FDG PET, 18F-fluorodeoxyglucose positron
emission tomography; CBDCA, carboplatin; CT, computed tomography;
nab-PTX, nab-paclitaxel; PD, progressive disease; SD, stable
disease. |
From day 561, the patient received combination
therapy with CBDCA (AUC 6, every 3 weeks) plus nab-PTX (100
mg/m2 on days 1, 8, and 15 of each cycle). After 3
cycles of CBDCA plus nab-PTX, CT showed expansion of sclerosis in
the bone metastases of the L3 vertebral body and the right iliac
wing, as well as enlargement of the primary lesion and infiltrative
shadow around the right hilar lymph nodes, likely due to a
radiation recall phenomenon (Fig.
3C). These bone and lung findings were considered
treatment-related changes, as the primary and mediastinal lymph
nodes maintained their size reduction, and the left subclavian
lymph node also showed shrinkage (Fig.
3C), indicating that CBDCA plus nab-PTX was effective. Since
the effectiveness of CBDCA plus nab-PTX was observed, a fourth
course was also administered. Brain magnetic Resonance Imaging
(MRI) after 4 cycles of CBDCA plus nab-PTX confirmed a reduction of
the lesion to 4 mm (Fig. 3D). The
purpose of Gamma Knife treatment is local tumor control (11,12).
In this case, the metastatic brain tumor shrank, and local control
was successfully achieved. CBDCA plus nab-PTX treatment is planned
to be continued.
Discussion
This case demonstrates the potential of combining
durvalumab, tremelimumab, and chemotherapy to treat NSCLC
recurrence during durvalumab consolidation therapy after CCRT. A
five-year follow-up study from the PACIFIC trial reported a median
overall survival (OS) of 47.5 months for patients receiving
durvalumab after CCRT compared to 29.1 months in the placebo group
(13). In the PACIFIC trial,
post-durvalumab therapies consisted predominantly of chemotherapy,
often followed by immunotherapy, most commonly with immune
checkpoint inhibitors such as nivolumab or pembrolizumab. The
current approach for treating recurrent NSCLC without driver
mutations involves immunotherapy, with or without chemotherapy.
Therefore, despite the absence after durvalumab and CCRT, the use
of immunotherapy alone or in combination with chemotherapy remains
a viable strategy.
A retrospective analysis of 15 Japanese institutions
examined patients with locally advanced or unresectable NSCLC who
experienced disease progression after CCRT and durvalumab
consolidation therapy. Patients were categorized into 3 groups:
early discontinuation (progression within 6 months of durvalumab
initiation), late discontinuation (progression between 7 and 12
months after durvalumab initiation), and accomplishment
(progression after 12 months of durvalumab initiation) (14). Among the 127 patients analyzed, 50
(39.4%) were in the early discontinuation group. Subsequent
treatments included platinum-based chemotherapy combined with
immune checkpoint inhibitors in 4 patients (8.0%) in the early
discontinuation group. In this group, the median OS of the 4
patients treated with platinum-based chemotherapy and immune
checkpoint inhibitors was 10.3 months; however, none of these
patients received both durvalumab and tremelimumab.
Previous research showed that patients with
metastatic melanoma who progressed after PD-L1 therapy benefited
more from a combination of PD-L1 and cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4) treatments than from
CTLA-4 monotherapy, showing an objectively higher response rate
(31% vs. 13%; P<0.0001) and longer median OS (20.4 vs. 8.8
months; hazard ratio 0.50; P<0.0001) (15). Similarly, in patients with
metastatic NSCLC who experienced disease progression after PD-L1
inhibitor treatment, addition of CTLA-4 antibodies demonstrated
efficacy in 10% of patients in phase 2 trials (16).
The phase III POSEIDON trial evaluated first-line
treatment for advanced NSCLC by comparing a regimen of durvalumab,
tremelimumab, and chemotherapy with chemotherapy alone. This
combination significantly improved the progression-free survival
and OS of patients (6).
Furthermore, addition of durvalumab to chemotherapy significantly
increased progression-free survival. However, as the patients had
not previously received durvalumab, the effectiveness of the
regimen for recurrence during post-CCRT durvalumab therapy remains
unverified. In contrast, the CheckMate 9LA trial evaluated a
dual-checkpoint strategy using nivolumab and ipilimumab with only 2
chemotherapy cycles while continuing CTLA-4 blockade in the
maintenance phase (17). Both
trials supported the combination of anti-CTLA-4 and anti-PD-L1
antibodies with chemotherapy for treatment-naive NSCLC. Our
patient, who was diagnosed with lung adenocarcinoma without
actionable driver mutations (EGFR or ALK) and with negative PD-L1
expression at initial diagnosis, shares key pathological features
with the populations enrolled in both trials. The POSEIDON trial
reported approximately 70% of enrolled patients with nonsquamous
histology and 38% with PD-L1 expression <1%, while the CheckMate
9LA trial included patients with both squamous and nonsquamous
histologies and a broad range of PD-L1 expression levels. Thus,
from a pathological standpoint, our case is consistent with these
study populations.
However, none of these studies included patients
with recurrence after concurrent chemoradiotherapy or durvalumab
consolidation. Our case differs in this regard, as the patient
experienced disease progression during durvalumab consolidation
following CCRT, a clinical context not evaluated in either study.
Although informative, these findings may not be directly applicable
to post-CCRT recurrence. Furthermore, although anti-vascular
endothelial growth factor therapy such as bevacizumab is an
established option for advanced NSCLC, its administration following
stereotactic ablative radiotherapy (SABR) is associated with a
markedly increased risk of pulmonary hemorrhage (18). While SABR was not employed in the
present case, the potential for hemorrhagic complications
associated with prior thoracic irradiation was taken into
consideration, and a treatment regimen incorporating dual immune
checkpoint inhibitors was selected.
In our patient, the combination of immunotherapy and
chemotherapy, including durvalumab, was beneficial for treating
NSCLC recurrence during durvalumab maintenance after CCRT. Given
the absence of driver mutations and negative PD-L1 expression at
the initial diagnosis, this combination was selected to enhance
efficacy by targeting different immune checkpoints with durvalumab
and tremelimumab. Further accumulation of cases is recommended to
strengthen these findings, and additional clinical experience is
essential to validate the effectiveness of this treatment strategy
in a broader patient population.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YM drafted the first version of the manuscript. YM,
TM, HN and MT confirmed the authenticity of all the raw data. HU
and TF contributed to the pathological evaluation. YM, TM, HN, TK,
YT, YN, SH, AM, HU, TF and MT made substantial contributions to the
conception and design of the study, including determination of the
treatment strategy, acquisition of clinical and pathological data,
and analysis and interpretation of data. All authors were involved
in drafting the manuscript or revising it critically for important
intellectual content. Furthermore, all authors agreed to be
accountable for all aspects of the work, ensuring that questions
related to the accuracy or integrity of any part of the study are
appropriately investigated and resolved. Each author participated
sufficiently to take public responsibility for appropriate portions
of the content, and all authors have confidence in the accuracy and
integrity of the contributions of their co-authors. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the case report to be published under anonymity.
Competing interests
SH receives lecture fees from AstraZeneca. The other
authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
CCRT
|
concurrent chemoradiotherapy
|
|
CTLA-4
|
cytotoxic T-lymphocyte-associated
protein 4
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
PD-L1
|
programmed death-ligand 1
|
References
|
1
|
Pritchard RS and Anthony SP: Chemotherapy
plus radiotherapy compared with radiotherapy alone in the treatment
of locally advanced, unresectable, non-small-cell lung cancer. A
mata-anaysis. Ann Intern Med. 125:723–729. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marino P, Preatoni A and Cantoni A:
Randomized trials of radiotherapy alone versus combined
chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell
lung cancer. A meta-analysis. Cancer. 76:593–601. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et
al: Durvalumab after chemoradiotherapy in stage III non-small-cell
lung cancer. N Engl J Med. 377:1919–1929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et
al: Overall survival with durvalumab after chemoradiotherapy in
stage III NSCLC. N Engl J Med. 379:2342–2350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naito Y, Kubota K, Nihei K, Fujii T, Yoh
K, Niho S, Goto K, Ohmatsu H, Saijo N and Nishiwaki Y: Concurrent
chemoradiotherapy with cisplatin and vinorelbine for stage III
Non-small cell lung cancer. J Thorac Oncol. 3:617–622. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson ML, Cho BC, Luft A,
Alatorre-Alexander J, Geater SL, Laktionov K, Kim SW, Ursol G,
Hussein M, Lim FL, et al: Durvalumab with or without tremelimumab
in combination with chemotherapy as first-line therapy for
metastatic non-small-cell lung cancer: The phase III POSEIDON
study. J Clin Oncol. 41:1213–1227. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamamoto N, Nakagawa K, Nishimura Y,
Tsujino K, Satouchi M, Kudo S, Hida T, Kawahara M, Takeda K,
Katakami N, et al: Phase III study comparing second- and
third-generation regimens with concurrent thoracic radiotherapy in
patients with unresectable stage III non-small-cell lung cancer:
West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol.
28:3739–3745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasaki T, Seto T, Yamanaka T, Kunitake N,
Shimizu J, Kodaira T, Nishio M, Kozuka T, Takahashi T, Harada H, et
al: A randomised phase II trial of S-1 plus cisplatin versus
vinorelbine plus cisplatin with concurrent thoracic radiotherapy
for unresectable, locally advanced non-small cell lung cancer:
WJOG5008L. Br J Cancer. 119:675–682. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shukuya T, Takahashi T, Harada H, Akamatsu
H, Sakaguchi C, Imai H, Ono A, Nakamura Y, Tsuya A, Kenmotsu H, et
al: Comparison of vinorelbine plus cisplatin and s-1 plus cisplatin
in concurrent chemoradiotherapeutic regimens for unresectable stage
III Non-small Cell lung cancer. Anticancer Res. 32:675–680.
2012.PubMed/NCBI
|
|
10
|
Scagliotti GV, Parikh P, Von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rogers SJ, Lomax N, Alonso S, Lazeroms T
and Riesterer O: Radiosurgery for five to fifteen brain metastases:
A single centre experience and a review of the literature. Front
Oncol. 12:8665422022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dewan MC, Rattani A, Fieggen G, Arraez MA,
Servadei F, Boop FA, Johnson WD, Warf BC and Park KB: Global
neurosurgery: The current capacity and deficit in the provision of
essential neurosurgical care. Executive summary of the global
neurosurgery initiative at the program in global surgery and social
change. J Neurosurg. 130:1055–1064. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spigel DR, Faivre-Finn C, Gray JE, Vicente
D, Planchard D, Paz-Ares L, Vansteenkiste JF, Garassino MC, Hui R,
Quantin X, et al: Five-year survival outcomes from the PACIFIC
trial: Durvalumab after chemoradiotherapy in stage III
non-small-cell lung cancer. J Clin Oncol. 40:1301–1311. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hasegawa T, Ariyasu R, Tanaka H, Saito R,
Kawashima Y, Horiike A, Sakatani T, Tozuka T, Shiihara J, Saiki M,
et al: Subsequent treatment for locally advanced non-small-cell
lung cancer that progressed after definitive chemoradiotherapy and
consolidation therapy with durvalumab: A multicenter retrospective
analysis (TOPGAN 2021-02). Cancer Chemother Pharmacol. 92:29–37.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pires da Silva I, Ahmed T, Reijers ILM,
Weppler AM, Betof Warner A, Patrinely JR, Serra-Bellver P, Allayous
C, Mangana J, Nguyen K, et al: Ipilimumab alone or ipilimumab plus
anti-PD-1 therapy in patients with metastatic melanoma resistant to
anti-PD-(L)1 monotherapy: A multicentre, retrospective, cohort
study. Lancet Oncol. 22:836–847. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schoenfeld JD, Giobbie-Hurder A,
Ranasinghe S, Kao KZ, Lako A, Tsuji J, Liu Y, Brennick RC, Gentzler
RD, Lee C, et al: Durvalumab plus tremelimumab alone or in
combination with low-dose or hypofractionated radiotherapy in
metastatic non-small-cell lung cancer refractory to previous
PD(L)-1 therapy: An open-label, multicentre, randomised, phase 2
trial. Lancet Oncol. 23:279–291. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker
M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E,
Juan-Vidal O, et al: First-line nivolumab plus ipilimumab combined
with two cycles of chemotherapy in patients with non-small-cell
lung cancer (CheckMate 9LA): An international, randomised,
open-label, phase 3 trial. Lancet Oncol. 22:198–211. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lau B, No HJ, Wu YF, Ko RB, Devine M, Das
M, Neal JW, Wakelee HA, Ramchandran KJ, Wakelee HA, et al:
Pulmonary hemorrhage in patients treated with thoracic stereotactic
ablative radiotherapy and anti-angiogenic agents. Int J Radiat
Oncol Biol Phys. 111:e4232021. View Article : Google Scholar
|

















