Introduction
Glioma, originating from brain glial cells, is the
most common malignant primary brain tumor in adults, accounting for
4% of primary central nervous system tumors and 81% of malignant
brain tumors (1–3). According to the World Health
Organization (WHO) classification of central nervous system tumors
(4), gliomas are named and
classified based on a combination of molecular characteristics-such
as mutations in the IDH1/2 genes, 1p/19q codeletion, TERT promoter
mutation, and homozygous deletion of CDKN2A/2B-along with
histological phenotypes, which together provide precise guidance
for glioma treatment and improve prognosis.
Currently, histopathological examination of tumor
specimens obtained through surgical resection or stereotactic
biopsy is considered the gold standard for the classification and
grading of gliomas (3). Medical
imaging techniques, such as computed tomography (CT) (5) and magnetic resonance imaging (MRI)
(6), are important for cancer
staging and follow-up after glioma treatment (7). However, these methods are often
expensive and not widely accessible, typically only being performed
on patiFents who already exhibit adverse symptoms (8).
By contrast, blood indicators have been well
established for cancer screening and early diagnosis, monitoring
treatment response and predicting recurrence (9–11).
Inflammation and immunity are important aspects of the hallmarks of
cancer, playing a notable role in tumor development and progression
(12). In previous years, several
studies have shown that some inflammation-related biomarkers
derived from preoperative peripheral blood routine tests such as
the neutrophil-to-lymphocyte ratio (NLR) (13,14),
lymphocyte-to-monocyte ratio (15),
platelet-to-lymphocyte ratio (PLR) (16) and their combinations are able to
refine patient stratification for treatment and predict survival
outcomes in various cancers, including gastric cancer, colorectal
cancer, hepatocellular carcinoma, ovarian cancer and non-small cell
lung cancer (17–19). However, preoperative peripheral
blood routine tests typically include >20 biomarkers, and the
combined effects of all these indicators on the diagnosis and
prognosis of gliomas have not yet been systematically studied under
the new WHO 2021 guidelines (4).
Based on the role of systemic inflammation in tumor
progression, the present study hypothesized that preoperative blood
indicators could predict glioma diagnosis and prognosis. The
present study aimed to systematically evaluate 22 routine blood
indicators for their diagnostic and prognostic value in glioma
under the WHO 2021 classification.
Patients and methods
Patients and healthy controls
(HCs)
The preoperative peripheral blood routine tests were
conducted and retrospectively analyzed. All patients were confirmed
to have glioma by the pathology department of the First Affiliated
Hospital of Nanjing Medical University (Nanjing, China), and were
classified according to the 2021 WHO classification of central
nervous system tumors (4). The
inclusion criteria were as follows: i) Patients had no previous
antitumor treatments, including surgery, radiotherapy or
chemotherapy; and ii) they underwent routine blood tests and
biochemical examinations within 1 week prior to surgery. The
exclusion criteria were: i) Patients with a history of other
malignant tumors; ii) patients with acute or chronic inflammatory
diseases; iii) individuals with diabetes, heart disease,
hypertension, thyroid dysfunction or autoimmune diseases; iv)
patients who received steroid treatment within the last 6 months;
and v) patients with incomplete case and follow-up data.
Data collection
The present study included clinical data from 571
patients with glioma who visited The First Affiliated Hospital of
Nanjing Medical University between December 2014 and September
2021, along with 1,899 HCs from the hospital's Health Examination
Center during the same period (Table
I). Retrospective analysis compared a total of 22 indicators
between the HC group and glioma group, including: Hemoglobin
concentration (HGB); mean corpuscular hemoglobin concentration
(MCHC); hematocrit (HCT); monocyte count (MONO#); white blood cell
count (WBC); monocyte percentage (MONO%); red blood cell count
(RBC); red cell distribution width-coefficient of variation
(RDW-CV); lymphocyte percentage (LYMPH%); lymphocyte count
(LYMPH#); mean corpuscular volume (MCV); mean corpuscular
hemoglobin (MCH); mean platelet volume (MPV); basophil percentage
(BASO%); basophil count (BASO#); eosinophil count (EOS#);
eosinophil percentage (EOS%); platelet count (PLT); platelet
distribution width (PDW); plateletcrit (PCT); neutrophil percentage
(NEUT%); and neutrophil count (NEUT#). Hematological indicators
were measured using 3 ml of fasting venous blood collected in the
morning that was subsequently mixed in a sodium citrate
anticoagulant tube and analyzed with a Sysmex AC-500 (Sysmex
Corporation) fully automated immunoassay analyzer. All data were
obtained from the last test conducted within the week prior to
surgery. Enrolled patients were followed up via outpatient visits
or phone calls. Follow-up occurred every 3 months for the first 2
years post-surgery, every 6 months from the third to the fifth
year, and then twice annually thereafter until death or loss to
follow-up. The survival endpoint for the present study was
tumor-related death or the date of the last follow-up, with the
last follow-up date being May 1, 2023. Overall survival (OS) was
defined as the time from the first day after surgery until death or
the date of the last follow-up.
 | Table I.Clinical characteristics of glioma
patients by World Health Organization grade. |
Table I.
Clinical characteristics of glioma
patients by World Health Organization grade.
| Group | Total cases, n | Age, mean ±
standard deviation | Gender, n (%) | Cases with
prognostic information, n (%) | Survival/death, n
(%) |
|---|
| Glioma | 571 | 54.19±13.77 | Male 332
(58.14) | 397 (69.53) | 185/212 |
|
|
|
| Female 239
(41.86) |
| (46.60/53.40) |
| Astrocytoma | 92 | 43.02±12.42 | Male 51
(55.43) | 67 (72.8) | 45/22 |
|
|
|
| Female 41
(44.57) |
| (67.16/32.84) |
|
Oligodendroglioma | 77 | 46.86±10.12 | Male 44
(57.14) | 63 (81.8) | 60/3 |
|
|
|
| Female 33
(42.86) |
| (95.24/4.76) |
| Glioblastoma | 402 | 58.16±12.87 | Male 267
(66.41) | 267 (66.4) | 79/188 |
|
|
|
| Female 155
(33.59) |
| (29.59/70.41) |
| Healthy
controls | 1899 | 47.54±13.97 | Male 1034
(54.45) | - | - |
|
|
|
| Female 865
(45.55) |
|
|
Statistical analysis
Categorical variables were presented as case
numbers, while continuous variables were expressed as the mean ±
standard deviation. One-way ANOVA tests were performed to compare
continuous variables across multiple groups, followed by Tukey's
post-hoc test for pairwise comparisons. To address
multicollinearity, the correlations among 22 preoperative
peripheral blood routine indicators were analyzed using Spearman
correlation analysis, and biomarkers with a correlation coefficient
|r|<0.35 were retained to reduce redundancy. The optimal
thresholds for preoperative blood indicators were determined using
receiver operating characteristic (ROC) curves and binary logistic
regression was applied. Survival analysis was performed using the
Kaplan-Meier (K-M) method and differences between groups were
assessed with log-rank tests. Biomarkers with multicollinearity
were excluded based on variance inflation factor (VIF) calculations
and the remaining biomarkers were included in the Cox proportional
hazards model. The proportional hazards assumption was verified by
plotting Schoenfeld residuals. The model's performance was
evaluated using the concordance index (C-index) and calibration
curves for 3-year survival rates. All statistical analyses were
conducted using R software (version 4.2.2; R Foundation for
Statistical Computing). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics of
patients
The HC group comprised 1,034 males (54.45%) and 865
females (45.55%), with an age range of 18 to 84 years and a median
age of 48 years. The glioma group included 332 males (58.14%) and
239 females (41.86%), with an age range of 19 to 84 years and a
median age of 56 years (Table I).
Age was higher in the glioma group compared with the HC group (mean
± SD: 54.19±13.77 vs. 47.54±13.97 years, P=0.002). The sex
distribution did not differ between groups (male, 58.14 vs. 54.45%;
χ2=3.21, P=0.073). The distribution of patient data was
as follows: i) Patients were separated into the HC group (n=1,899)
and the glioma group (n=571); and ii) within the glioma group,
patients were divided into three molecular subtypes: Astrocytoma
(n=92), oligodendroglioma (n=77) and glioblastoma (n=402).
Peripheral blood routine data (comprising 22 indicators) from 1,899
HCs and 571 patients with glioma were analyzed using one-way ANOVA,
with results presented as the mean ± standard deviation (Table SI).
Correlation analysis of preoperative
peripheral blood routine indicators
Due to the presence of multiple interrelated
preoperative peripheral blood routine indicators, such as the NEUT%
and NEUT#, EOS% and EOS#, retaining all indicators would affect the
accuracy of subsequent analyses. Therefore, the present study first
performed a Spearman correlation analysis of the 22 biomarkers and
visualized the results in a heatmap (Fig. 1A). A total of 10 indicators with low
correlation coefficients (|r|<0.35) (Fig. 1B) were retained for further study.
The present study assessed the distribution differences of the same
biomarkers between HCs and different glioma molecular subtypes
(astrocytoma, oligodendroglioma and glioblastoma) (Fig. 1C-L, Table SI). HCs displayed significantly
higher levels in HCT, RDW-CV, MCH, BASO#, EOS# and PDW compared
with all glioma subtypes (P<0.0001). Conversely, NEUT%
demonstrated a significant increase in value with the progression
of glioma grades when compared with the HC group, and patients
diagnosed with glioblastoma showed the highest levels compared with
lower-grade gliomas and HCs (P<0.0001) (Fig. 1K). Despite significant differences
between HCs and oligodendroglioma (P=0.0364) and between
oligodendroglioma and glioblastoma (P=0.0181), no statistically
significant difference was observed in MONO% with the progression
of glioma grades (Fig. 1E).
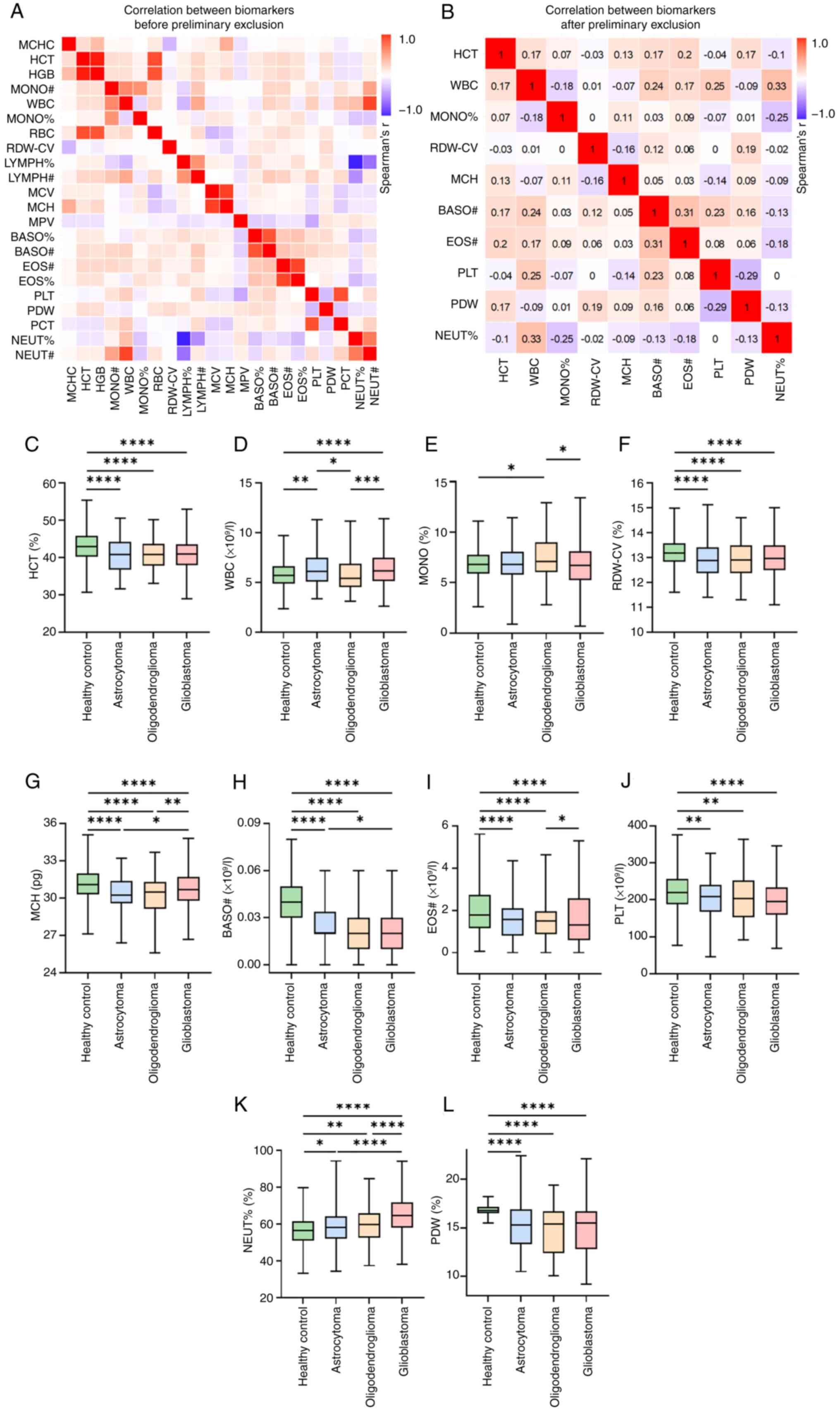 | Figure 1.Correlations between preoperative
peripheral blood routine indicators. (A) Spearman correlation
coefficients for all 22 biomarkers from preoperative peripheral
blood routine tests. (B) Spearman correlation coefficients among 10
biomarkers after preliminary exclusion. A correlation coefficient
of |r|<0.35 was used as the screening criterion. (C)
Distribution of HCT across healthy controls, astrocytoma,
oligodendroglioma and glioblastoma. Distribution of (D) WBC, (E)
MONO, (F) RDW-CV, (G) MCH, (H) BASO#, (I) EOS#, (J) PLT, (K) NEUT%
and (L) PDW across the four groups. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. MCHC, mean corpuscular
hemoglobin concentration; HCT, hematocrit; HGB, hemoglobin; MONO#,
monocyte count; WBC, white blood cell count; MONO%, monocyte
percentage; RBC, red blood cell count; RDW-CV, red cell
distribution width-coefficient of variation; LYMPH%, lymphocyte
percentage; LYMPH#, lymphocyte count; MCV, mean corpuscular volume;
MCH, mean corpuscular hemoglobin; MPV, mean platelet volume; BASO%,
basophil percentage; BASO#, basophil count; EOS#, eosinophil count;
EOS%, eosinophil percentage; PLT, platelet count; PDW, platelet
distribution width; PCT, plateletcrit; NEUT%, neutrophil
percentage; NEUT#, neutrophil count. |
Binary logistic regression model for
differentiating glioma molecular subtypes using preoperative
peripheral blood routine indicators
To construct the binary logistic regression model,
all of the biomarkers were first transformed into binary
classification data based on their optimal cut-off values. Data
greater than or equal to the optimal threshold were recorded as
high-level, while the remaining data were classified as low-level
(Table SII). Subsequently, a
binary logistic regression model was used to test the
classification performance of the remaining 10 biomarkers between
the HC group and glioma group. The results indicated that, except
for EOS#, all other biomarkers showed significant associations with
the classification outcomes (Fig.
2A). Among them, the odds ratios (OR) for WBC, MONO% and NEUT%
were 2.20 (95% CI: 1.73–2.80; P<0.001), 1.71 (95% CI: 1.37–2.14;
P<0.001) and 3.26 (95% CI: 2.60–4.09; P<0.001), respectively,
which means that as the levels of these biomarkers increased, the
risk of developing glioma also increased significantly. By
contrast, a decrease in the levels of the remaining six biomarkers
indicated that the likelihood of developing glioma in healthy
individuals significantly increased. This suggested that lower
levels of these biomarkers were negatively associated with health.
The ROC curve analysis for the classification model showed an area
under the curve (AUC) of 0.9293 (P<0.0001), indicating excellent
classification performance in differentiating the HC group from the
glioma group (Fig. 2B).
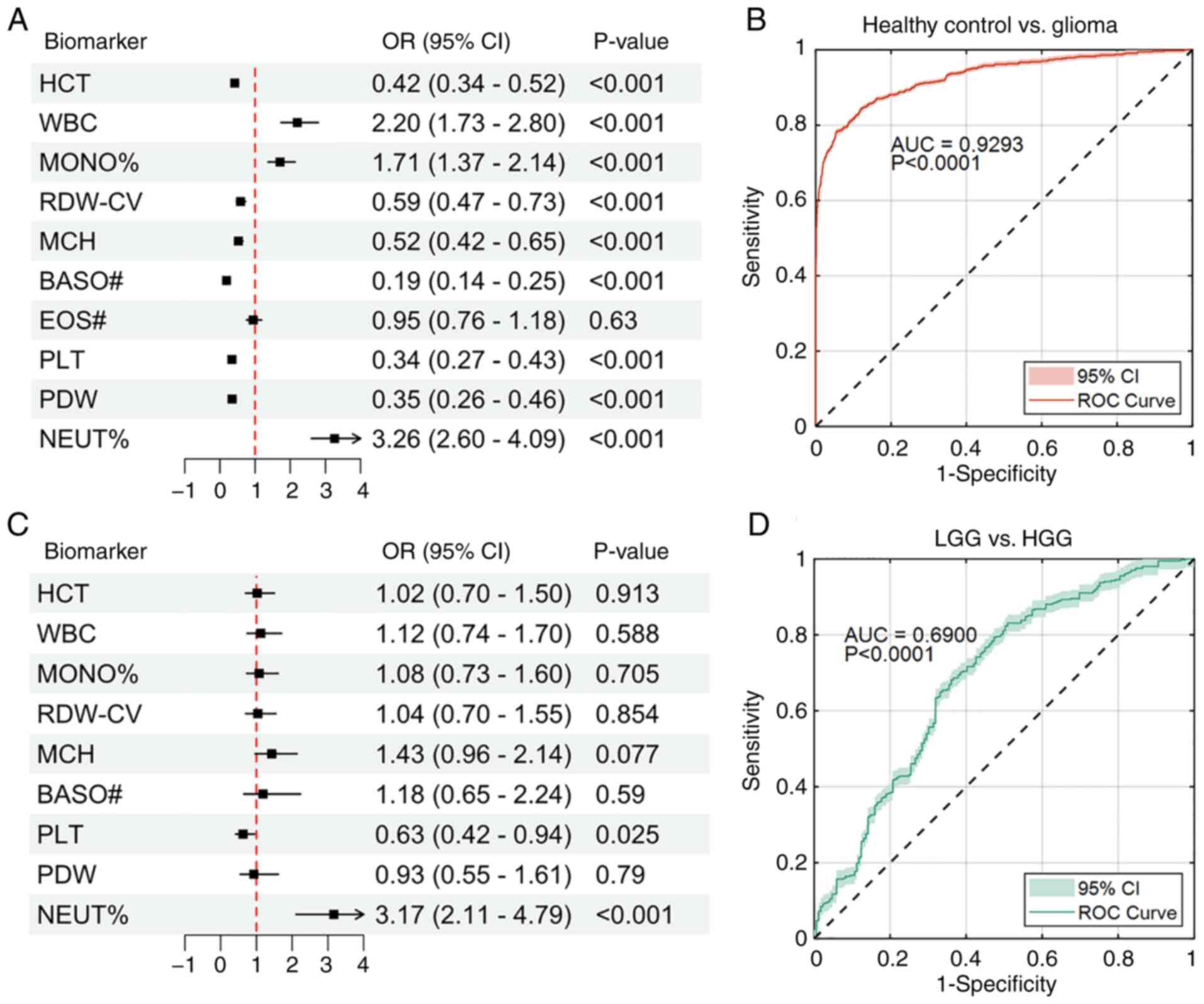 | Figure 2.Results of binary logistic regression
between different groups with preoperative blood routine
indicators. (A) Forest plot of binary logistic regression comparing
the effects of biomarkers in classification of healthy control
group vs. glioma group. (B) ROC curve analysis for the
classification model in A. (C) Forest plot of binary logistic
regression comparing the effects of biomarkers in the
classification of LGG vs. HGG. (D) ROC curve analysis for the
classification model in C. ROC, receiver operating characteristic;
LGG, low-grade glioma; HGG, high-grade glioma; OR, odds ratio; CI,
confidence interval; AUC, area under curve; HCT, hematocrit; WBC,
white blood cell count; MONO%, monocyte percentage; RDW-CV, red
cell distribution width-coefficient of variation; MCH, mean
corpuscular hemoglobin; BASO#, basophil count; PLT, platelet count;
PDW, platelet distribution width; NEUT%, neutrophil percentage. |
A similar model was also constructed for the
classification of the low-grade glioma (LGG) and high-grade glioma
(HGG) groups using the nine biomarkers that showed significant
effects in the aforementioned binary logistic regression model.
Astrocytoma and oligodendroglioma were classified as LGGs, while
glioblastoma was classified as a HGG. The results showed that the
PLT (OR, 0.63; P=0.025) and NEUT% (OR, 3.17; P<0.001) were
identified as key biomarkers for distinguishing between LGG and
HGG. A decrease in PLT was associated with a significantly higher
risk of developing HGG in patients with glioma, while an increase
in NEUT% indicated a significantly elevated risk of HGG
progression. However, the remaining biomarkers did not show any
significant differences (P>0.05) (Fig. 2C). The ROC analysis yielded an AUC
of 0.6900 (P<0.0001), suggesting some discriminative potential;
however, the sub-0.70 AUC indicates limited performance and should
not be taken as evidence of a strong diagnostic marker (Fig. 2D).
Association of preoperative blood
routine indicators with survival outcomes in patients with
glioma
To evaluate whether preoperative blood routine
indicators can predict survival (OS) in patients with glioma,
patients with complete prognostic information were selected in the
present study based on the WHO 2021 glioma grading guidelines
(4). The group included 67 patients
with astrocytoma, 63 patients with oligodendroglioma and 267
patients with glioblastoma. Survival analysis was first performed
using the K-M method, with log-rank tests assessing intergroup
differences. K-M survival curves were plotted for all 22
preoperative blood routine indicators and 6 biomarkers were
statistically significant, as shown in Fig. 3 (all P<0.05). The results showed
that these 6 biomarkers significantly affected the OS of the
patients with glioma. Since LYMPH% and LYMPH#, NEUT% and NEUT# were
highly clinically correlated (Fig.
1A), it was decided to include LYMPH#, MONO%, PCT and NEUT% in
the multivariate Cox regression analysis. Previously, the VIF was
calculated for these indicators and plotted in Table II, which showed that all Variance
Inflation Factor (VIF) values were low (MONO%=1.34, LYMPH#=1.88,
PCT=1.09, NEUT%=1.16), indicating that multicollinearity was not a
concern in the present model.
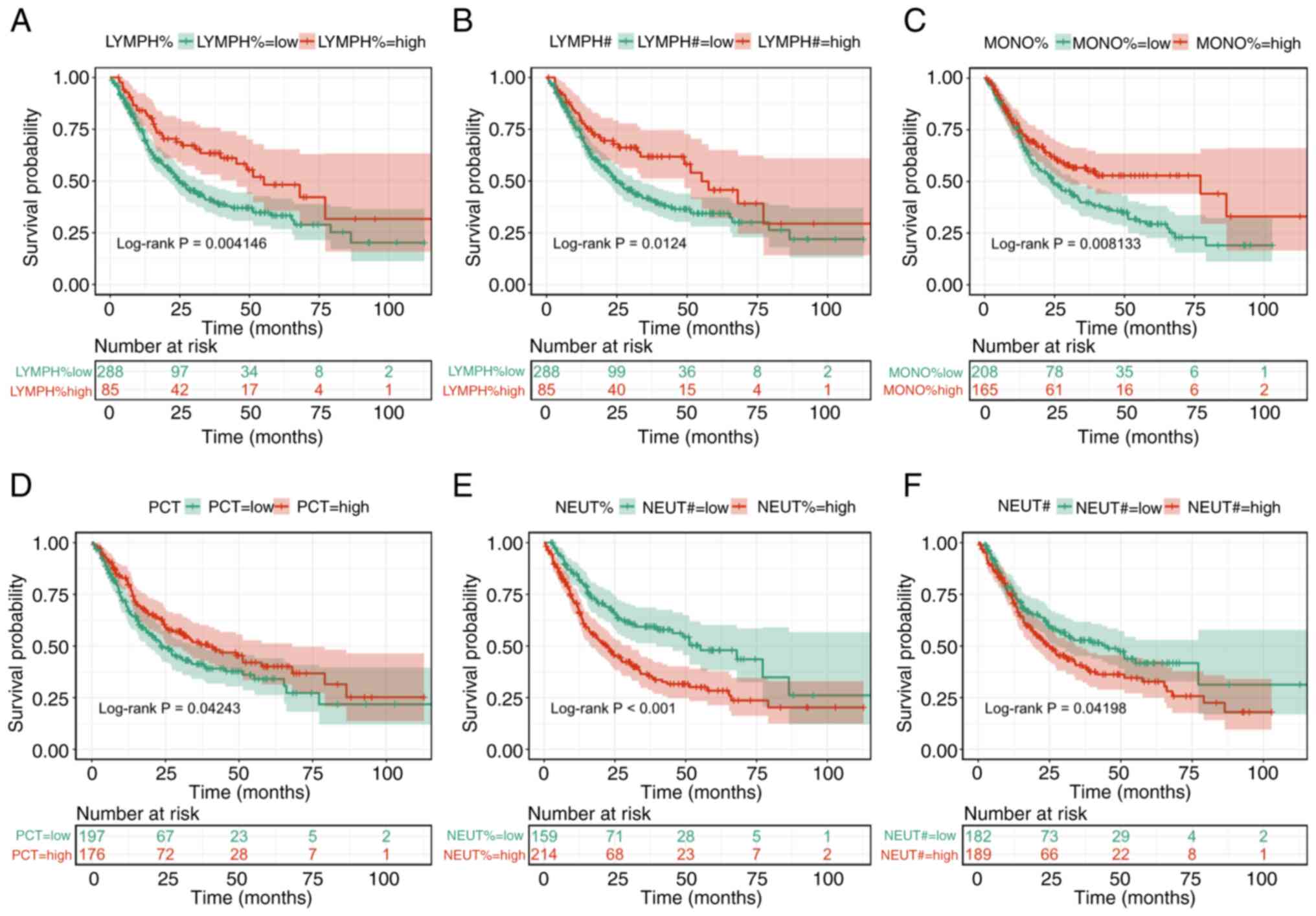 | Figure 3.Kaplan-Meier survival curves for
different biomarkers in the glioma group. Survival curves were
stratified by baseline (A) LYMPH%, (B) LYMPH#, (C) MONO%, (D) PCT,
(E) NEUT% and (F) NEUT# levels. The curves compared overall
survival probabilities between low- and high-level groups defined
by biomarker thresholds. LYMPH%, lymphocyte percentage; LYMPH#,
lymphocyte count; MONO%, monocyte percentage; PCT, plateletcrit;
NEUT%, neutrophil percentage; NEUT#, neutrophil count. |
 | Table II.Results of VIF between different
biomarkers and multivariable Cox regression analysis. |
Table II.
Results of VIF between different
biomarkers and multivariable Cox regression analysis.
| Biomarker | VIF | β | Sx̄ | Wald
χ2 | HR | 95% CI | P-value |
|---|
| MONO% | 1.341141 | 0.017 | 0.039 | 0.186 | 1.017 | 0.943–1.097 | 0.666 |
| LYMPH# | 1.878677 | 0.119 | 0.175 | 0.462 | 1.126 | 0.798–1.588 | 0.496 |
| PCT | 1.091518 | −2.198 | 1.398 | 2.469 | 0.111 | 0.007–1.253 | 0.116 |
| NEUT% | 1.163272 | 0.035 | 0.009 | 12.776 | 1.035 | 1.016–1.055 | <0.001 |
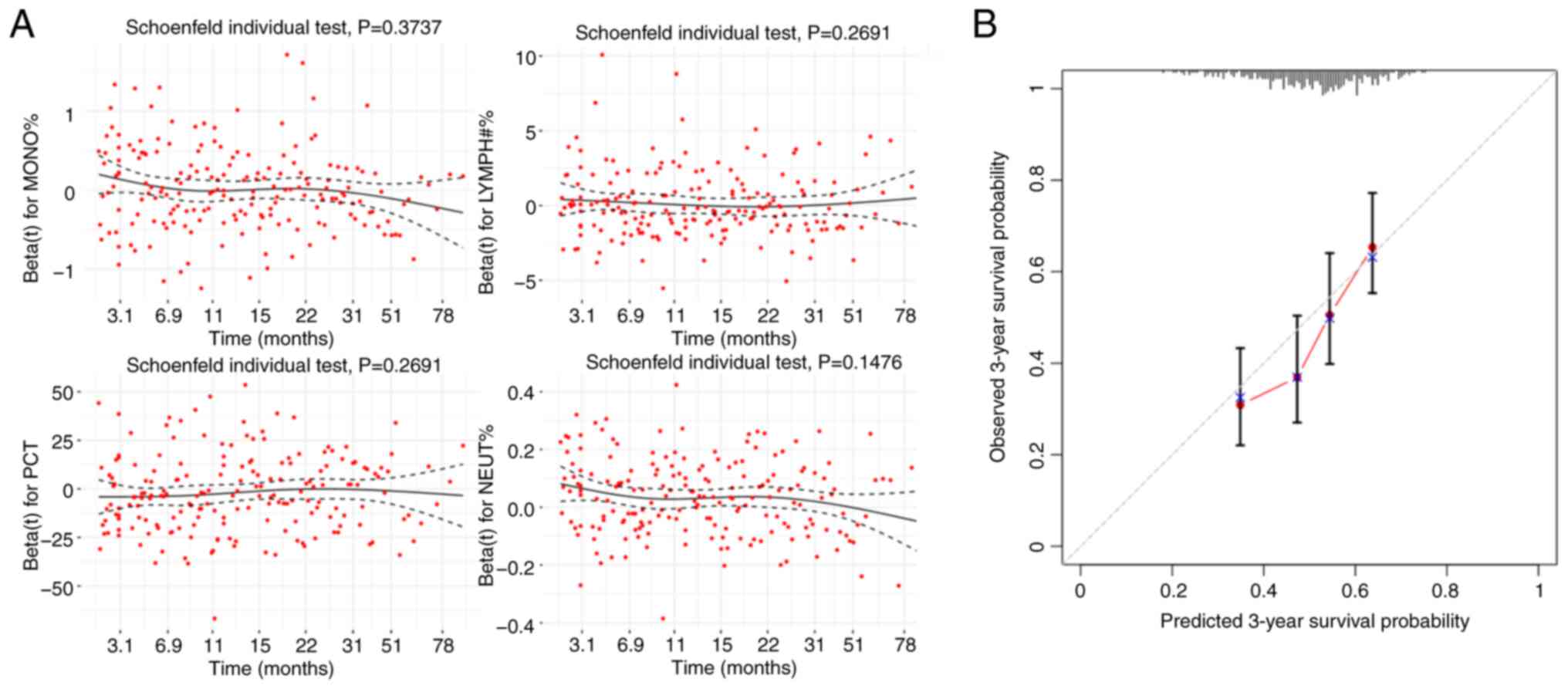
Subsequently, a multivariable Cox regression
analysis was performed, and residual plots were generated using the
Schoenfeld residuals method (Fig.
4A). These plots indicated that the residual distributions of
the remaining 4 indicators were uniform, suggesting independence
from time and their suitability for inclusion in the multivariable
Cox regression analysis (Table
II). PCT showed a protective trend but did not reach
statistical significance (P=0.116). NEUT% was the only independent
prognostic factor with statistical significance (P<0.001).
Although the associated hazard ratio of 1.035 indicated a
relatively small effect size, NEUT% elevation was consistently and
significantly associated with poor prognosis.
The model showed good overall fit (likelihood ratio
χ2=22.45, df=4, P=<0.0001), acceptable discrimination
(C-index=0.62, SD=0.02) (data not shown) and good 3-year
calibration (Fig. 4B). The
predicted probabilities, indicated by the red curve in Fig. 4B, align well with the actual
observed outcomes, depicted by the gray dashed line, indicating
that the model has relatively high predictive accuracy.
Discussion
The early diagnosis of glioma is important for
patient treatment and recovery. It can be achieved via imaging
modalities such as CT, MRI and positron emission tomography-CT
(5,6), but routine peripheral blood
examination has the advantage of simplicity and can cover a wider
population. Existing studies have shown that multiple hematological
indicators are associated with the prediction and prognosis of
gliomas. Blood inflammatory markers, such as NLR (13,14)
and PLR (16), have been identified
as useful for the early detection and staging of gliomas. This
study assessed preoperative peripheral blood routine indicators and
explored their correlations with the integrated diagnosis
(incorporating both histology and molecular subtypes), tumor grade,
and prognosis in gliomas.
In the present study, significant differences were
found between the healthy group and the glioma group in the
following 7 indicators: HCT, RDW-CV, MCH, BASO#, EOS#, PDW and
NEUT%. Many of these indicators are known to be influenced by the
systemic inflammatory and nutritional status of patients. In this
context, a study by Han et al (20) reported that low preoperative levels
of serum albumin, a well-established marker of malnutrition and
inflammation (21), are associated
with shorter survival in patients with glioblastoma. Low albumin
levels typically reflect malnutrition and inflammatory status in
patients (21); patients with
glioma often experience significant changes in their nutritional
status, especially as the tumor progresses and treatment advances,
leading to a higher prevalence of malnutrition and weight loss. The
present study found a significant difference in MCH between the
healthy and glioma groups, consistent with prior research on
anemia-related hematologic alterations in cancer, including glioma
(21–24). Here, MCH is cited as a marker of
anemia, linking this finding to the preceding discussion of
malnutrition and inflammation (low albumin) (21). HCT, RDW-CV and MCH are highly
correlated hematologic markers of anemia, which are associated with
the development of a variety of tumors, such as prostate cancer
(22–24). In addition to anemia- and
nutrition-related markers, tumor-associated inflammation and immune
responses can affect peripheral blood counts; therefore,
inflammation/immune activity may manifest as changes in indicators
such as BASO# and NEUT%. A study by Zheng et al (25) first reported elevated preoperative
NLR and derived NLR in patients with glioma. Subsequent studies by
Li et al (26) and
Stepanenko et al (27)
further supported that systemic inflammatory responses,
particularly neutrophil-dominated immunity, play an important role
in glioma progression. A study presented by Liang et al
(28) reported that increased
neutrophil infiltration in tumors is significantly associated with
glioma grade. In the present study, it was found that the
distribution levels of the neutrophil percentage gradually
increased across the groups of healthy individuals, astrocytoma,
oligodendroglioma and glioblastoma. This suggested that for healthy
individuals, NEUT% was a risk factor for developing glioma, while
for those with glioma, it was a risk factor for developing
glioblastoma.
Inflammatory responses may play an important role in
the progression of gliomas (29,30),
particularly the elevated levels of neutrophils in preoperative
peripheral blood, which may reflect chronic inflammation in the
tumor microenvironment that is closely associated with tumor growth
and invasion. The NEUT% not only served as an independent
prognostic factor for patients with glioma but may also be an
effective indicator for early identification of high-risk patients
with glioma. The present study may provide an important clinical
reference and future studies could integrate other indicators for a
more comprehensive prognostic assessment. In summary, NEUT% holds
significant potential in the diagnosis, molecular subtyping and
prognostic evaluation of gliomas, warranting further investigation
into its mechanisms in glioma and clinical applications.
Despite the promising findings of the present study,
several limitations should be acknowledged: i) Age differed between
the glioma and HC groups, but age was not incorporated into the
analyses. Because age is associated with both disease risk and
outcomes, the lack of age adjustment may introduce residual
confounding and could partly account for some observed
associations. Future work should include age (and other baseline
covariates) as adjustment variables, perform stratified analyses or
apply matching/weighting approaches to mitigate this bias. ii)
Sample size disparity-while the overall number of patients was
substantial (n=571), subgroup analyses for rare molecular subtypes,
such as oligodendroglioma (n=88) and glioblastoma (n=402), may have
lacked statistical power; iii) model performance-the prognostic
model exhibited moderate discriminative ability (C-index, 0.62),
indicating a need for integrating complementary biomarkers, such as
genetic or imaging markers, to enhance clinical utility.
In conclusion, the present study demonstrated that
the combination of certain preoperative peripheral blood routine
indicators has significant predictive value for glioma diagnosis.
NEUT% exhibited significant predictive power for glioma diagnosis
and warrants close attention. Although NEUT% showed a limited
impact in the prediction of the OS of patients with glioma, it was
significantly associated with poor outcomes in patients with
glioma. The NEUT% level in preoperative peripheral blood tests
holds clinical significance for both the diagnosis and prognosis of
glioma and should be carefully monitored in clinical practice.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by the National Natural Science
Foundation of China (grant no. 82272651) and the Jiangsu Province
Capability Improvement Project through Science, Technology and
Education (grant no. ZDXK202225).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YYo and YW conceived and designed the study. QY, JN
and JZ acquired the clinical data. YYu and ZS assisted with patient
enrollment and clinical data interpretation. QY, JN, JZ, XF and XC
analyzed and interpreted the data. XF and XC performed statistical
analyses, developed analysis pipelines, and ensured data
reproducibility. YYu developed the data visualization pipeline. YYu
and QY drafted the manuscript. YYu, ZS, YW, XF and XC critically
revised the manuscript for important intellectual content. ZS
provided critical expertise on the 2021 WHO classification of
gliomas. YYo and YW supervised the study. YYo, YYu and YW confirm
the authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was completed with ethics
committee approval from The First Affiliated Hospital of Nanjing
Medical University (approval no. 2024-SR-1144).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCT
|
hematocrit
|
|
RDW-CV
|
red cell distribution
width-coefficient of variation
|
|
LYMPH#
|
lymphocyte count
|
|
MCH
|
mean corpuscular hemoglobin
|
|
BASO#
|
basophil count
|
|
EOS#
|
eosinophil count
|
|
PLT
|
platelet count
|
|
PDW
|
platelet distribution width
|
|
PCT
|
plateletcrit
|
|
NEUT%
|
neutrophil percentage
|
|
NEUT#
|
neutrophil count
|
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. New Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grossman SA, Ye X, Piantadosi S, Desideri
S, Nabors LB, Rosenfeld M and Fisher J; NABTT CNS Consortium, :
Survival of patients with newly diagnosed glioblastoma treated with
radiation and temozolomide in research studies in the United
States. Clin Cancer Res. 16:2443–2449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang K, Wu Z, Zhang H, Zhang N, Wu W, Wang
Z, Dai Z, Zhang X, Zhang L, Peng Y, et al: Glioma targeted therapy:
Insight into future of molecular approaches. Mol Cancer. 21:392022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
WHO Classification of Tumours Editorial
Board, . World Health Organization Classification of Tumours of the
Central Nervous System. 5th Edition. International Agency for
Research on Cancer; Lyon: 2021
|
|
5
|
Mettler FA Jr, Thomadsen BR, Bhargavan M,
Gilley DB, Gray JE, Lipoti JA, McCrohan J, Yoshizumi TT and Mahesh
M: Medical radiation exposure in the US in 2006: Preliminary
results. Health Phys. 95:502–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Le Bihan D, Breton E, Lallemand D, Grenier
P, Cabanis E and Laval-Jeantet M: MR imaging of intravoxel
incoherent motions: Application to diffusion and perfusion in
neurologic disorders. Radiology. 161:401–407. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Upadhyay N and Waldman A: Conventional MRI
evaluation of gliomas. Brit J Radiol. 84((Spec Iss 2)): S107–S111.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu S, Guan J and Liu Y: Identification of
microRNAs as novel biomarkers for glioma detection: A meta-analysis
based on 11 articles. J Neurol Sci. 348:181–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loke SY and Lee ASG: The future of
blood-based biomarkers for the early detection of breast cancer.
Eur J Cancer. 92:54–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duffy MJ and O'Byrne K: Tissue and blood
biomarkers in lung cancer: A review. Adv Clin Chem. 86:1–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McMillan DC: Systemic inflammation,
nutritional status and survival in patients with cancer. Curr Opin
Clin Nutr. 12:223–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang ZL, Zhang CB, Liu YQ, Wang Z and
Jiang T: Peripheral blood test provides a practical method for
glioma evaluation and prognosis prediction. CNS Neurosci Ther.
25:876–883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weng W, Chen X, Gong S, Guo L and Zhang X:
Preoperative neutrophil-lymphocyte ratio correlated with glioma
grading and glioblastoma survival. Neurol Res. 40:917–922. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan P, Li JW, Mo LG and Huang QR: A
nomogram combining inflammatory markers and clinical factors
predicts survival in patients with diffuse glioma. Medicine
(Baltimore). 100:e279722021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma G, Jain SK and Sinha VD: Peripheral
inflammatory blood markers in diagnosis of glioma and IDH status. J
Neurosci Rural Pract. 12:88–94. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng Q, He B, Liu X, Yue J, Ying H, Pan Y,
Sun H, Chen J, Wang F, Gao T, et al: Prognostic value of
pre-operative inflammatory response biomarkers in gastric cancer
patients and the construction of a predictive model. J Transl Med.
13:662015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Afari ME and Bhat T: Neutrophil to
lymphocyte ratio (NLR) and cardiovascular diseases: An update.
Expert Rev Cardiovasc Ther. 14:573–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang
J, Wang T, Zhu W and Liu P: Prognostic value of PLR in various
cancers: A meta-analysis. PLoS One. 9:e1011192014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han S, Huang Y, Li Z, Hou H and Wu A: The
prognostic role of preoperative serum albumin levels in
glioblastoma patients. BMC Cancer. 15:1082015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eckart A, Struja T, Kutz A, Baumgartner A,
Baumgartner T, Zurfluh S, Neeser O, Huber A, Stanga Z, Mueller B
and Schuetz P: Relationship of nutritional status, inflammation,
and serum albumin levels during acute illness: A prospective study.
Am J Med. 133:713–722.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ludwig H, Van Belle S, Barrett-Lee P,
Birgegård G, Bokemeyer C, Gascón P, Kosmidis P, Krzakowski M,
Nortier J, Olmi P, et al: The European Cancer Anaemia Survey
(ECAS): A large, multinational, prospective survey defining the
prevalence, incidence, and treatment of anaemia in cancer patients.
Eur J Cancer. 40:2293–2306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Colloca G, Venturino A, Vitucci P and
Gianni W: Management of anaemia in prostate cancer. Cancer Invest.
28:280–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mercadante S, Gebbia V, Marrazzo A and
Filosto S: Anaemia in cancer: Pathophysiology and treatment. Cancer
Treat Rev. 26:303–311. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng SH, Huang JL, Chen M, Wang BL, Ou QS
and Huang SY: Diagnostic value of preoperative inflammatory markers
in patients with glioma: A multicenter cohort study. J Neurosurg.
129:583–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li B, Gao B, Zhu HJ, Luwor RB, Lu J, Zhang
L and Kong B: The prognostic value of preoperative inflammatory
markers for pathological grading of glioma patients. Technol Cancer
Res Treat. 23:153303382412731602024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stepanenko AA, Sosnovtseva AO, Valikhov
MP, Chernysheva AA, Abramova OV, Pavlov KA and Chekhonin VP:
Systemic and local immunosuppression in glioblastoma and its
prognostic significance. Front Immunol. 15:13267532024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang J, Piao Y, Holmes L, Fuller GN,
Henry V, Tiao N and de Groot JF: Neutrophils promote the malignant
glioma phenotype through S100A4. Clin Cancer Res. 20:187–198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Turner MC, Radzikowska U, Ferastraoaru DE,
Pascal M, Wesseling P, McCraw A, Backes C, Bax HJ, Bergmann C,
Bianchini R, et al: AllergoOncology: Biomarkers and refined
classification for research in the allergy and glioma nexus-A joint
EAACI-EANO position paper. Allergy. 79:1419–1439. 2024. View Article : Google Scholar : PubMed/NCBI
|