Introduction
Brainstem gliomas are malignant primary tumors
originating from glial cells within the brainstem and account for
10–20% of all pediatric central nervous system (CNS) tumors
(1). Among these, nearly 80% are
classified as diffuse midline gliomas [World Health Organization
(WHO) grade IV] (2), which are
marked by highly aggressive biological behavior and an
exceptionally poor prognosis, with a 2-year survival rate of
<10% (3–5). The remaining 20% are predominantly
focal low-grade gliomas (WHO grades I–II), which tend to grow more
slowly and are associated with more favorable outcomes, with
reported 5-year survival rates ranging between 60 and 80% (6). The majority of brainstem gliomas
(70–80%) occur in individuals <18 years of age, with an overall
5-year survival rate of ~30% in this pediatric population (7).
Radiotherapy remains the cornerstone of treatment
for brainstem gliomas, particularly in high-grade tumors. In
pediatric patients over the age of 3 years, clinical improvement is
often observed within 1 to 2 weeks of radiotherapy (8). This modality enables temporary local
tumor control in 50–70% of focal brainstem lesions and has been
shown to extend median progression-free survival (PFS) time from
2.4 to 6 months, as well as median overall survival (OS) time from
3.4 to 9.8 months (9–13). Despite its benefits, radiotherapy is
frequently regarded as a double-edged sword due to the potential
for serious long-term side effects, including growth delays,
neurocognitive impairment, hearing loss, visual deficits and a
general decline in quality of life (14,15).
As a result, radiation oncologists are actively investigating
refined treatment strategies that maximize therapeutic benefit
while reducing collateral damage, especially with the emergence of
advanced techniques such as volumetric modulated arc therapy
(VMAT). VMAT has been shown to surpass both three-dimensional
conformal radiotherapy and intensity-modulated radiotherapy by
enhancing target dose conformity, better sparing of normal tissues,
reducing monitor units and shortening overall treatment time,
improving both therapeutic outcomes and patient experience
(10,16,17).
While VMAT has demonstrated promising potential in
pediatric glioma treatment, most existing studies have focused on
coplanar techniques (18). However,
the dosimetric benefits of non-coplanar VMAT (NC-VMAT),
particularly in the context of pediatric brain radiotherapy, remain
underexplored, with limited evidence supporting the practical
application of the technique. The key innovation of this study lies
in being the first, to the best of our knowledge, to evaluate and
validate the dosimetric advantages of NC-VMAT in the context of
pediatric brainstem glioma. This study addresses a significant gap
in the radiotherapy literature, where previous investigations have
primarily focused on adult populations or non-brainstem pediatric
tumors. By focusing on this anatomically and clinically sensitive
region, the study provides novel insights into potential avenues
for dose optimization and tissue sparing in pediatric CNS
radiotherapy.
Materials and methods
Patients
The present study included 10 pediatric patients
(aged <18 years) who were diagnosed with brainstem glioma and
underwent postoperative VMAT at the Department of Radiotherapy,
Fujian Children's Hospital (Fuzhou, China) between August 2022 and
September 2024. The inclusion criteria were as follows: i)
Histopathological confirmation of glioma; ii) availability of
complete medical records; and iii) documented clinical
administration of VMAT. All patients received standard coplanar
VMAT as part of their routine treatment protocol. The study cohort
consisted of 4 male and 6 female patients, with a median age of 5.5
years (range, 4–12 years).
For this study, NC-VMAT plans were retrospectively
created using the same computed tomography (CT) datasets as the
clinically implemented VMAT plans, allowing for direct dosimetric
comparison. It is important to emphasize that NC-VMAT was not
delivered to patients in clinical practice. Patient follow-up was
conducted through a combination of regular outpatient visits,
telephone interviews and, where applicable, death certificate
verification. All follow-up data were reviewed and validated by
attending clinical physicians to ensure accuracy and
completeness.
Patient positioning and imaging
CT imaging was performed with patients positioned in
a supine manner on an integrated immobilization system (Huayuxin
HYX-UTS-CM; Jinan Huayu New Casting and Forging Materials Co.,
Ltd.) using a customized thermoplastic mask and headrest to ensure
a stable and reproducible posture. Contrast-enhanced scans were
acquired using a 16-slice, large-aperture CT simulator (Discovery
RT590; GE Healthcare) following intravenous administration of
iodinated contrast. Axial images were obtained at a slice thickness
of 2.5 mm for precise anatomical delineation. The acquired images
were then imported into the Eclipse treatment planning system (TPS)
(version 15.5; Varian; Siemens Healthineers), where radiation
oncologists delineated the target volumes and organs at risk (OARs)
to develop the treatment plans. All radiotherapy treatments were
delivered using a Varian TrueBeam linear accelerator (Varian;
Siemens Healthineers).
Target volume delineation
To improve the accuracy of target and normal tissue
delineation, both preoperative and postoperative contrast-enhanced
T1-weighted and fluid attenuated inversion recovery magnetic
resonance imaging (MRI) sequences were imported into the TPS for
all patients. These MRI datasets were automatically aligned with
the planning CT images using rigid registration within the TPS.
Radiation oncologists manually reviewed and, if necessary, adjusted
the image registration to ensure anatomical accuracy. The gross
tumor volume (GTV) of the tumor bed was defined as the surgical
resection cavity along with all regions of contrast enhancement
observed on the preoperative T1-weighted MRI scans. The clinical
target volume (CTV) was generated by expanding the GTV by 2 cm in
all directions, followed by manual modification by the physician to
conform to anatomical boundaries. A uniform 0.3-cm margin was then
added to the CTV to create the planning target volume (PTV).
Surrounding OARs were also carefully delineated with regard to the
target area.
Treatment planning
All treatment plans were developed by the same team
of radiation physicists using calibrated 6 MV X-rays within the
TPS. For each patient, two distinct plans were retrospectively
generated using the same CT dataset. In the control group (VMAT), a
standard coplanar technique was employed, consisting of two full
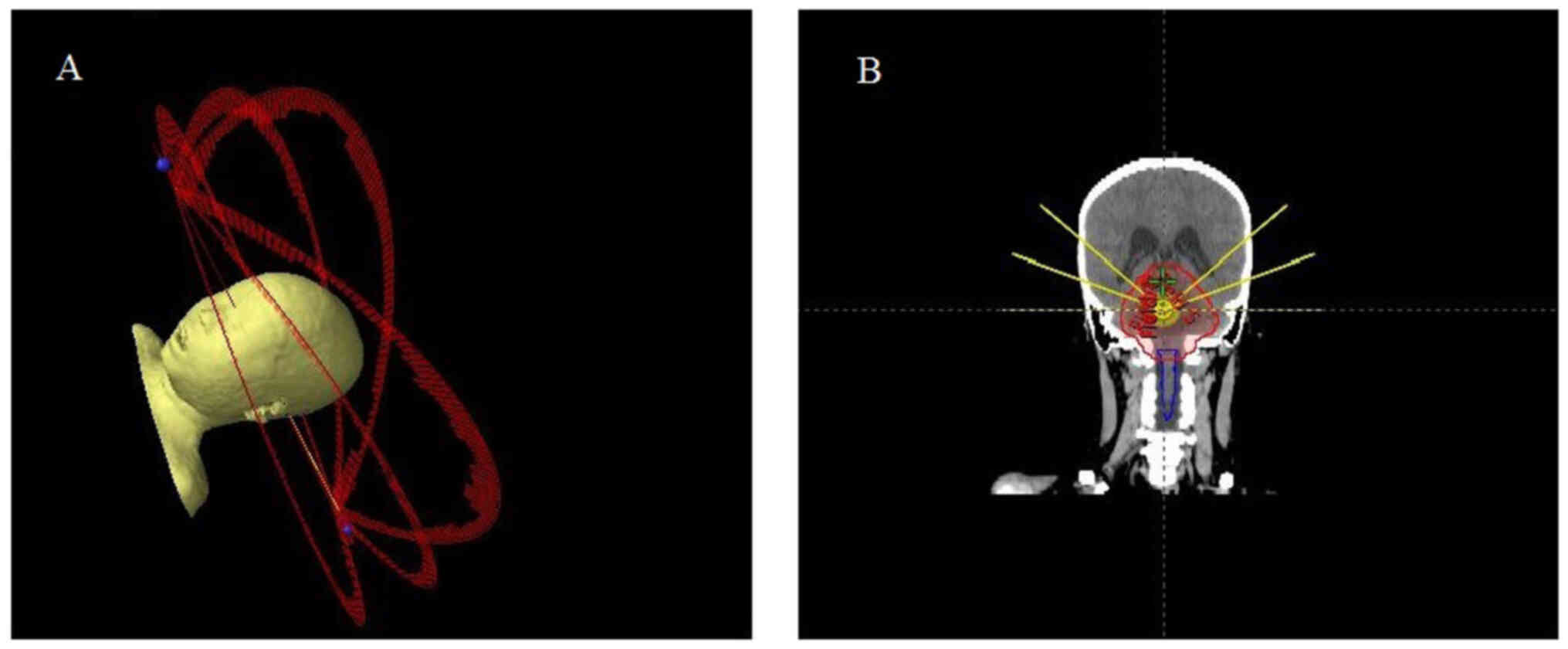
360° arcs delivered with the treatment couch fixed at 0°. In the
experimental group (NC-VMAT), the plan began with a single 360° arc
at a couch angle of 0°, followed by four non-coplanar 180°
half-arcs delivered at couch angles of 20°, 40°, 320° and 340°,
respectively (schematic diagrams are shown in Fig. 1). Both planning approaches were
optimized using the analytical anisotropic algorithm (19). To ensure a fair comparison focused
on the dosimetric merits of each technique, identical optimization
objectives and priority settings were applied across both plans.
The prescription dose for all patients was uniformly set at a PTV
of 50.4 Gy, delivered in 28 fractions of 180 cGy each [the dosing
regimen was selected following National Comprehensive Cancer
Network guidelines (20)], ensuring
that 95% of the target volume received 100% of the prescribed dose.
All plans were thoroughly reviewed and approved by senior radiation
physicists and chief radiation oncologists to ensure consistency
and clinical acceptability.
Dosimetric evaluation
The dosimetric characteristics of the target dose
distribution for the two treatment groups were evaluated using
dose-volume histogram (DVH) analysis. Key parameters included the
conformity index (CI) and homogeneity index (HI), calculated as
follows: i) CI=TV95%/PTVtotal, where TV95% represents
the volume receiving at least 95% of the prescribed dose, and
PTVtotal denotes the total planning target volume. A CI
value of 1 indicates ideal conformity. ii)
HI=(D2-D98)/D50, where
D2, D50 and D98 correspond to the
doses received by 2, 50 and 98% of the target volume, respectively.
An HI value approaching 0 reflects optimal dose uniformity.
In addition to these indices, comparisons were made
between the two groups for the maximum dose delivered to critical
structures, including the optic nerves, optic chiasm, lenses and
cochleae, as well as the mean dose (Dmean) delivered to
the temporal lobes (TLs) and surrounding normal brain tissue. The
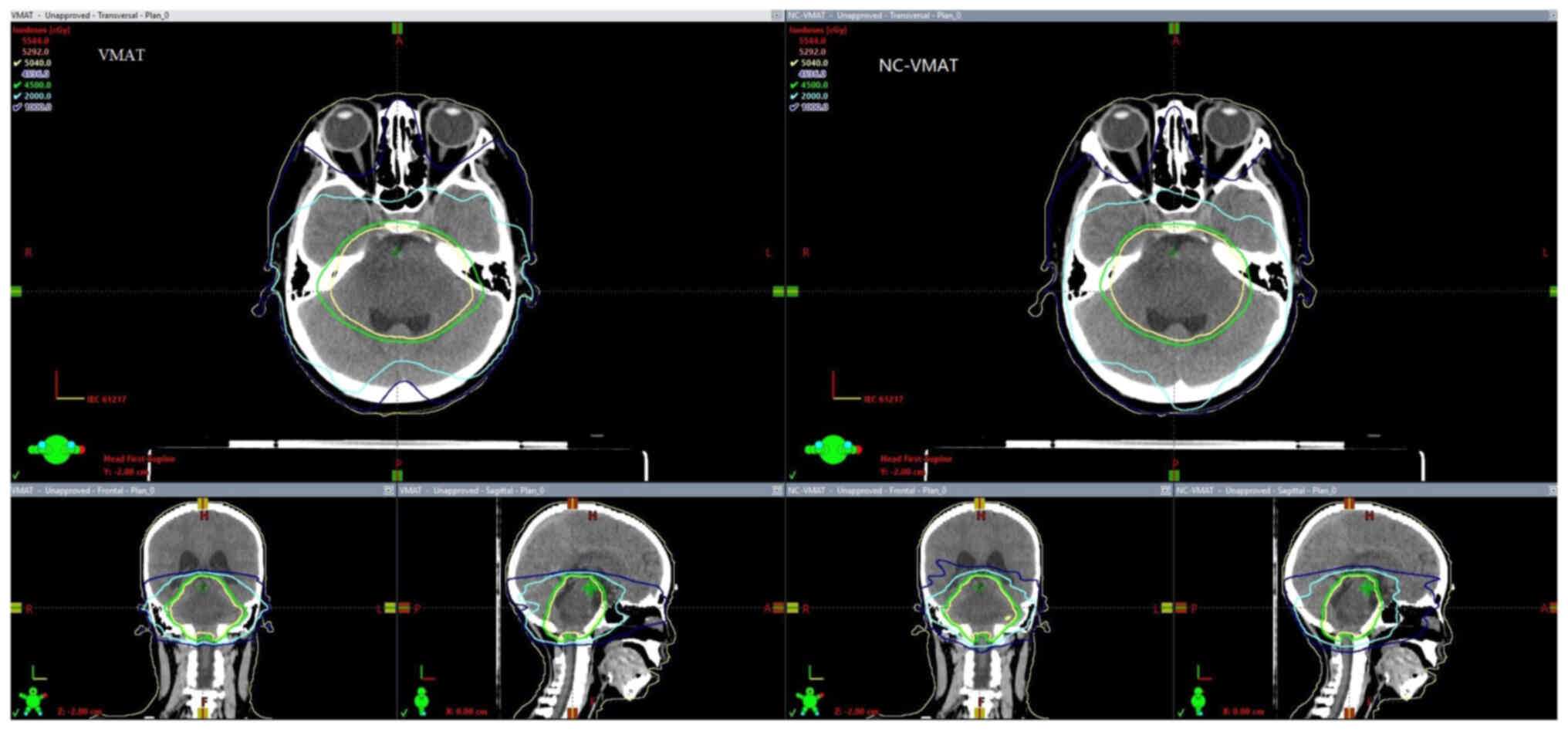
final dose level diagram for the plans in these two groups is shown
in Fig. 2.
Plan verification and treatment
duration
Plan verification for both treatment groups was
conducted using the PTW OCTAVIUS 4D dosimetric validation system in
conjunction with Verisoft 7.1 analysis software (PTW Freiburg
GmbH). The γ passing rate was evaluated following AAPM Task Group
218 guidelines (21), using
criteria of 3% dose difference and 2 mm distance-to-agreement, with
a minimum dose threshold of 10% and an acceptance criterion of γ
passing rate ≥95%. Treatment time was defined as the duration from
beam-on to beam-off.
Statistical analysis
Statistical analyses were performed using IBM SPSS
Statistics version 24.0 (IBM Corp.). Given the paired design of the
study, where both treatment techniques were applied to the same
patient cohort, continuous variables were first tested for
normality using the Shapiro-Wilk test. Data following a normal
distribution are presented as the mean ± standard deviation, and
comparisons between techniques were conducted using paired-samples
t-tests. For data that did not meet normality assumptions, values
are presented as the median and interquartile range (IQR), with
comparisons made using the Wilcoxon signed-rank test. A two-sided
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
The baseline characterestics of the 10 pediatric
patients included in this study are summarized in Table I.
 | Table I.Baseline characteristics of the study
participants. |
Table I.
Baseline characteristics of the study
participants.
| SN | Age, years | Sex | PTVtotal
(cc) | Histology | WHO grade |
|---|
| 1 | 5 | Female | 121.10 | Ependymoma | II |
| 2 | 6 | Female | 132.70 | Diffuse midline
glioma | IV |
| 3 | 4 | Male | 125.20 | Anaplastic
astrocytoma | III |
| 4 | 7 | Female | 154.60 | Diffuse midline
glioma | IV |
| 5 | 5 | Female | 176.20 | Diffuse midline
glioma | IV |
| 6 | 4 | Female | 171.40 | Ependymoma | II |
| 7 | 7 | Female | 218.40 | Diffuse midline
glioma | IV |
| 8 | 12 | Male | 179.00 | Ependymoma | II |
| 9 | 4 | Male | 165.40 | Glioblastoma | IV |
| 10 | 12 | Male | 155.70 | Glioblastoma | IV |
Dosimetric comparisons of the PTVs revealed that the
NC-VMAT technique outperformed conventional VMAT in terms of
Dmean, CI and HI, all of which showed statistically
significant improvements (all P≤0.001). However, VMAT demonstrated
a significantly shorter treatment time compared with NC-VMAT
(P<0.001). The γ pass rates for the target volume were
comparable between the two techniques, with no statistically
significant differences observed (P>0.05). Detailed results are
presented in Table II.
 | Table II.Dosimetric parameter comparisons for
the planning target volume in the VMAT and NC-VMAT patient
cohorts. |
Table II.
Dosimetric parameter comparisons for
the planning target volume in the VMAT and NC-VMAT patient
cohorts.
| Parameter | VMAT | NC-VMAT | t | P-value |
|---|
| Dmean,
cGy | 5271.90±20.91 | 5220.40±18.98 | 5.848 | <0.001 |
| HI | 0.849±0.004 | 0.066±0.003 | 13.481 | <0.001 |
| CI | 1.095±0.010 | 1.045±0.003 | 4.701 | 0.001 |
| Treatment time,
min | 3.06±0.54 | 5.90±0.10 | −32.111 | <0.001 |
| γ pass rate, % | 99.10±0.30 | 98.80±0.20 | 0.896 | 0.394 |
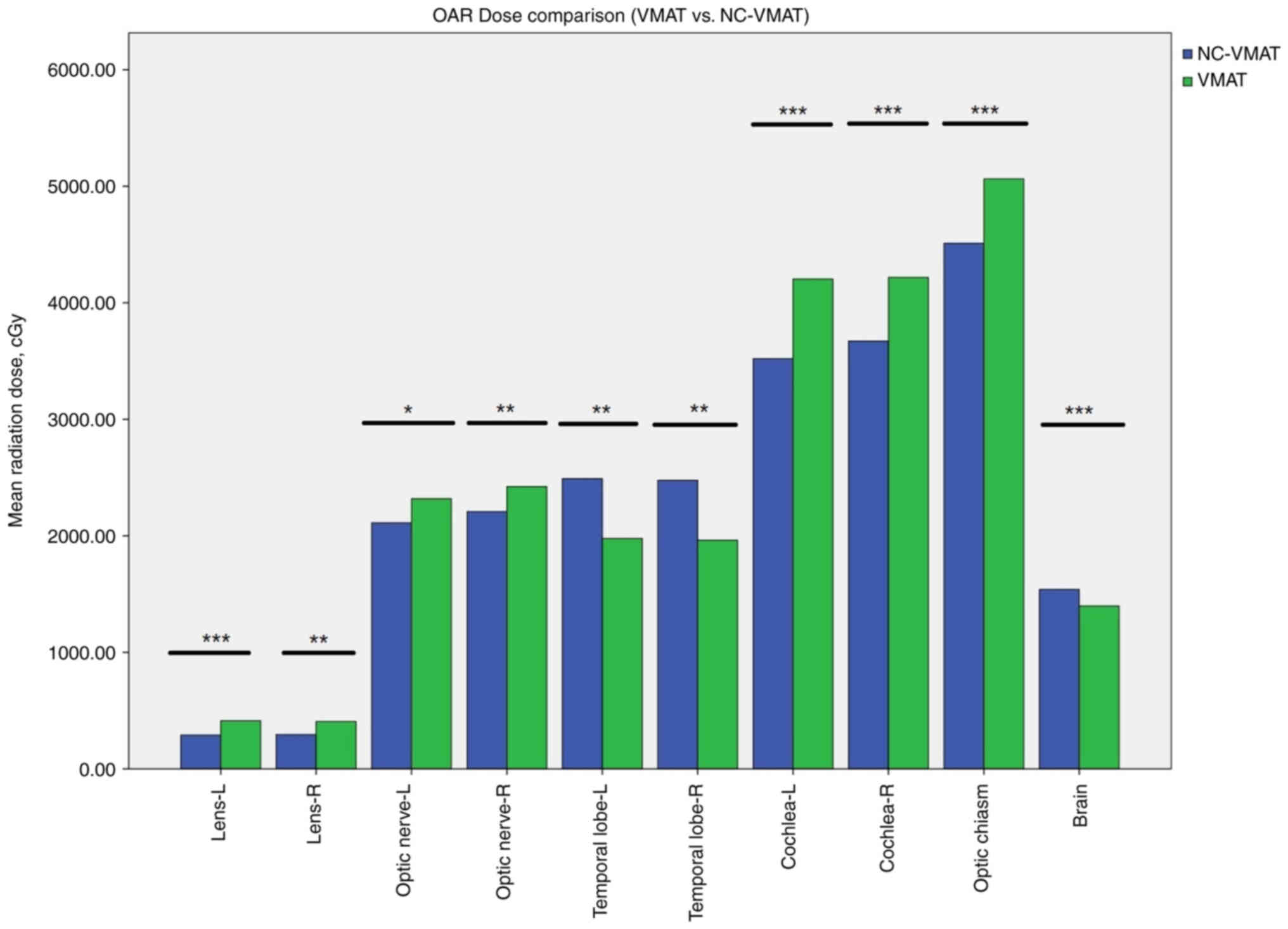
Regarding radiation dose to OARs surrounding the
target volume, NC-VMAT significantly reduced exposure to the
lenses, optic nerves, cochlea, and optic chiasm compared to VMAT
(all P<0.05). VMAT resulted in substantially lower radiation
doses to the TLs and normal brain tissue relative to NC-VMAT
(P<0.05). Corresponding DVH parameters and statistical analyses
are provided in Table III and
Fig. 3 for comprehensive
comparison.
 | Table III.Comparative analysis of OAR dose
exposure between two planning groups. |
Table III.
Comparative analysis of OAR dose
exposure between two planning groups.
|
| Mean radiation
dose, cGy |
|
|
|---|
|
|
|
|
|
|---|
| OAR | VMAT | NC-VMAT | t | P-value |
|---|
| Lens-L | 413.20±40.65 | 289.50±30.00 | −6.147 | <0.001 |
| Lens-R | 407.30±32.05 | 294.00±27.74 | −4.487 | 0.002 |
| Optic nerve-L |
2,318.50±312.59 |
2,111.10±293.98 | −3.274 | 0.010 |
| Optic nerve-R |
2,422.30±336.65 |
2,208.20±286.93 | −3.661 | 0.005 |
| Temporal
lobe-L |
1,977.70±155.11 | 2,491.20±88.48 | 4.480 | 0.002 |
| Temporal
lobe-R |
1,961.50±144.14 | 2,477.00±86.29 | 4.688 | 0.001 |
| Optic chiasm | 5,063.70±40.71 | 4,511.10±62.58 | −17.969 | <0.001 |
| Cochlea-L |
4,203.70±221.98 |
3,520.20±197.45 | −9.052 | <0.001 |
| Cochlea-R |
4,218.00±154.47 |
3,671.90±164.12 | −7.969 | <0.001 |
| Brain | 1,398.50±10.28 | 1,540.30±17.79 | 8.242 | <0.001 |
Discussion
The results of the present retrospective dosimetric
analysis demonstrated that NC-VMAT radiotherapy provides superior
target CI and HI compared with coplanar VMAT, aligning with
findings from previous studies (22–24).
These outcomes reinforce the potential of NC-VMAT to increase tumor
dose coverage and reduce dose inhomogeneity, underscoring its
dosimetric advantages over conventional VMAT techniques. NC-VMAT
allows for flexible adjustment of beam angles through coordinated
gantry and couch rotation, facilitating more precise dose
modulation and enabling multidirectional beam delivery to the
target volume. This approach enhances high-dose conformity to the
tumor while effectively sparing surrounding healthy tissues,
consistent with prior research (25,26).
Despite the increased computational and mechanical demands
associated with NC-VMAT planning and delivery, the comparable γ
passing rates observed between the two techniques in the present
study confirm that the TrueBeam linear accelerator can reliably and
accurately implement NC-VMAT plans.
According to Radiation Therapy Oncology Group,
American Society for Radiation Oncology and Pediatric Normal Tissue
Effects in the Clinic guidelines, the risk of radiation-induced
toxicity in pediatric patients significantly increases when the
lens is exposed to doses >3 Gy, when the optic nerves or optic
chiasm receive doses >45 Gy or when the cochlea is subjected to
a Dmean >35 Gy (27,28).
In the present study, dosimetric comparisons of OARs revealed that
NC-VMAT achieved significantly better dose sparing of the lens,
optic nerves, optic chiasm and cochlea than conventional VMAT
(P<0.05), which was consistent with earlier findings (29,30).
These results provide further support for the efficacy of NC-VMAT
in reducing treatment-associated toxicities during radiotherapy for
pediatric brainstem gliomas.
However, the present analysis also revealed that the
Dmean delivered to the TLs and normal brain tissue was
significantly higher in the NC-VMAT group compared with that in the
VMAT group. This finding, which contrasts with a prior report
(26), likely stems from the
inherent design of NC-VMAT, which involves multiple beam paths that
traverse these regions, increasing exposure to low-dose radiation.
These observations underscore the need for careful clinical
judgment when applying NC-VMAT, especially in pediatric patients
with low-grade brainstem gliomas and longer projected survival
times. As shown by Pokhrel et al (31) and Bertholet et al (32), fine-tuning key parameters of
NC-VMAT, such as gantry angles, dose rate and beam delivery time,
can help optimize dose distributions to minimize radiation exposure
to critical structures. With the clinical adoption of NC-VMAT,
radiation oncologists are afforded greater flexibility to
individualize treatment parameters, improving the protection of
surrounding OARs and reducing the risk of long-term
complications.
Although NC-VMAT technology offers significant
advantages, its clinical implementation presents several
challenges. As demonstrated in the present study, treatment time
for NC-VMAT was nearly double that of conventional VMAT, which may
pose difficulties during radiotherapy sessions for young children.
Prolonged treatment durations can reduce patient compliance,
particularly in pediatric patients who may struggle to remain
still, potentially compromising irradiation accuracy. Moreover,
children requiring sedation may need increased doses of anesthetic
agents. Previous studies by Guilcher et al (33) and Docking and Knijnik (34) have highlighted the risks associated
with repeated sedation, noting that cumulative exposure to
anesthetic agents in pediatric populations may elevate the risk of
long-term adverse effects.
Further limitations to the widespread adoption of
NC-VMAT include its higher equipment and operational costs, the
complexity of its treatment planning process and the need for
highly skilled technical personnel (35). These practical constraints
underscore the importance of developing strategies to optimize the
balance between treatment quality, efficiency and feasibility in
pediatric settings.
Future studies are warranted to evaluate the
clinical application of NC-VMAT further. These should include
long-term follow-up to assess dose distribution, tumor control
efficacy, patient survival outcomes, and treatment-related
complications across various tumor types and patient subgroups.
Whether the dosimetric benefits of NC-VMAT ultimately translate
into meaningful clinical improvements remains an open question, and
addressing this gap will be essential for informing evidence-based
clinical guidelines.
The present study has several limitations. Firstly,
as a single-center investigation with a relatively small sample
size (n=10), the statistical power to detect subtle clinical
differences may be limited. Although strict inclusion and exclusion
criteria were applied to minimize potential confounding factors,
larger multicenter studies with expanded cohorts are necessary to
improve the generalizability and robustness of the findings.
Furthermore, the current research lacks a comprehensive assessment
of long-term neurocognitive outcomes in brain regions exposed to
low-dose irradiation. Given the heightened radiosensitivity of the
developing pediatric nervous system, the potential for delayed
adverse effects from low-dose exposure warrants further
investigation through extended follow-up.
Furthermore, none of the patients received NC-VMAT
treatment in clinical practice. The NC-VMAT plans were generated
retrospectively using the same CT images and planning data as the
clinically delivered VMAT plans. These experimental plans were used
solely for dosimetric comparison within the study framework.
In conclusion, NC-VMAT demonstrates significant
advantages in the radiotherapeutic management of pediatric
brainstem gliomas, particularly in terms of improved target dose
conformity and enhanced protection of critical organs. Despite
these benefits, its clinical implementation presents certain
challenges, including longer treatment durations and increased
radiation exposure to the TLs and surrounding brain tissue.
However, the dosimetric strengths of NC-VMAT suggest that it holds
potential for optimizing radiotherapy outcomes and improving the
overall treatment experience for children with brainstem
gliomas.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science Foundation of
Fujian Province (grant nos. 2023J011305 and 2023J011299).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZL, JJ, YS, BG, KS, YL and JY contributed to the
study conception and design. ZL and YS were mainly responsible for
study conception. BG and ZL were responsible for methodology.
Formal analysis and investigation was performed by YL and KS.
Original draft preparation was performed by ZL and YS. JJ and BG
reviewed and edited the manuscript. Funding was acquired by ZL and
BG. YS and JY supervised the study. ZL and BG confirm the
authenticity of all the raw data. All authors commented on previous
versions of the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was conducted in strict adherence to the
Ethical Review Measures for Biomedical Research Involving Human
Subjects, the Declaration of Helsinki (as revised in 2013) and the
International Ethical Guidelines for Health-related Research
Involving Humans. All research activities involving human
participants were approved by the Ethics Committee of Fujian
Children's Hospital (Fuzhou, China; approval no. 2024ETKLRK10007).
The ethics committee approved the waiver of parental consent due to
the retrospective nature of the study and the anonymized nature of
the data, and no identifiable MRI was included in the
manuscript.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hassan H, Pinches A, Picton SV and
Phillips RS: Survival rates and prognostic predictors of high grade
brain stem gliomas in childhood: A systematic review and
meta-analysis. J Neurooncol. 135:13–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Green AL and Kieran MW: Pediatric
brainstem gliomas: New understanding leads to potential new
treatments for two very different tumors. Curr Oncol Rep.
17:4362015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Upadhyaya SA, Koschmann C, Muraszko K,
Venneti S, Garton HJ, Hamstra DA, Maher CO, Betz BL, Brown NA, Wahl
D, et al: Brainstem low-grade gliomas in children-excellent
outcomes with multimodality therapy. J Child Neurol. 32:194–203.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ostrom QT, Price M, Neff C, Cioffi G,
Waite KA, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2016–2020. Neuro Oncol. 25 (Suppl
4):iv1–iv99. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pasqualetti F, Lombardi G, Gadducci G,
Giannini N, Montemurro N, Feletti A, Zeppieri M, Somma T, Caffo M,
Bertolotti C and Ius T: Brain stem glioma recurrence: Exploring the
therapeutic frontiers. J Pers Med. 14:8992024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grimm SA and Chamberlain MC: Brainstem
Glioma: A review. Curr Neurol Neurosci Rep. 13:3462013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Janssens GO, Gidding CE, Van Lindert EJ,
Oldenburger FR, Erasmus CE, Schouten-Meeteren AY and Kaanders JH:
The role of hypofractionation radiotherapy for diffuse intrinsic
brainstem glioma in children: A pilot study. Int J Radiat Oncol
Biol Phys. 73:722–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laghari AA, Baig MZ, Bari E, Darbar A,
Mushtaq N, Hani Abdullah UE and Khan DA: Pediatric brainstem
gliomas: An institutional experience. Asian J Neurosurg.
14:1144–1150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Briere TM, McAleer MF, Levy LB and Yang
JN: Sparing of normal tissues with volumetric arc radiation therapy
for glioblastoma: Single institution clinical experience. Radiat
Oncol. 12:792017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schulz-Ertner D, Debus J, Lohr F, Frank C,
Höss A and Wannenmacher M: Fractionated stereotactic conformal
radiation therapy of brain stem gliomas: Outcome and prognostic
factors. Radiother Oncol. 57:215–223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan L, Soliman H, Sahgal A, Perry J, Xu W
and Tsao MN: External beam radiation dose escalation for high grade
glioma. Cochrane Database Syst Rev. 5:CD0114752020.PubMed/NCBI
|
|
13
|
Hatim G, Chekrine T, Houjami M, Boughafour
M, Bouchbika Z, Benchakroun N, Jouhadi H, Tawfiq N, Benider A and
Sahraoui S: Pediatric brainstem glioma. J Neurosci Neurol Disord.
6:001–004. 2022. View Article : Google Scholar
|
|
14
|
Kortmann RD, Timmermann B, Taylor RE,
Scarzello G, Plasswilm L, Paulsen F, Jeremic B, Gnekow AK,
Dieckmann K, Kay S and Bamberg M: Current and future strategies in
radiotherapy of childhood low-grade glioma of the brain: Part II:
Treatment-related late toxicity. Strahlenther Onkol. 179:585–597.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Merchant TE, Conklin HM, Wu S, Lustig RH
and Xiong X: Late effects of conformal radiation therapy for
pediatric patients with low-grade glioma: Prospective evaluation of
cognitive, endocrine, and hearing deficits. J Clin Oncol.
27:3691–3697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shaffer R, Nichol AM, Vollans E, Fong M,
Nakano S, Moiseenko V, Schmuland M, Ma R, McKenzie M and Otto K: A
comparison of volumetric modulated arc therapy and conventional
intensity-modulated radiotherapy for frontal and temporal
high-grade gliomas. Int J Radiat Oncol Biol Phys. 76:1177–1184.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sheu T, Briere TM, Olanrewaju AM and
McAleer MF: Intensity modulated radiation therapy versus volumetric
arc radiation therapy in the treatment of glioblastoma-does
clinical benefit follow dosimetric advantage? Adv Radiat Oncol.
4:50–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horbinski C, Nabors LB, Portnow J,
Baehring J, Bhatia A, Bloch O, Brem S, Butowski N, Cannon DM, et
al: NCCN Guidelines® Insights: Central Nervous System
Cancers, Version 2.2022. J Natl Compr Canc Netw. 21:12–20. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van Esch A, Tillikainen L, Pyykkonen J,
Tenhunen M, Helminen H, Siljamäki S, Alakuijala J, Paiusco M, Lori
M and Huyskens DP: Testing of the analytical anisotropic algorithm
for photon dose calculation. Med Phys. 33:4130–4148. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gajjar A, Mahajan A, Abdelbaki M, Anderson
C, Antony R, Bale T, Bindra R, Bowers DC, Cohen K, Cole B, et al:
Pediatric central nervous system cancers, version 2.2023, NCCN
Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw.
20:1339–1362. 2022.PubMed/NCBI
|
|
21
|
Miften M, Olch A, Mihailidis D, Moran J,
Pawlicki T, Molineu A, Li H, Wijesooriya K, Shi J, Xia P, et al:
Tolerance limits and methodologies for IMRT measurement-based
verification QA : Recommendations of AAPM Task Group No. 218. Med
Phys. 45:e53–e83. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hijazi WBM, El-Sayed A, El-Sayed SM and
El-Sayed M: Comparison of coplanar and non-coplanarvmat for brain
cancer by using the dosimetrical and radiobiological indices. Int J
Adv Res. 10:577–596. 2022. View Article : Google Scholar
|
|
23
|
Fitzgerald R, Owen R, Hargrave C, Pryor D,
Barry T, Lehman M, Bernard A, Mai T, Seshadri V and Fielding A: A
comparison of three different VMAT techniques for the delivery of
lung stereotactic ablative radiation therapy. J Med Radiat Sci.
63:23–30. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fitzgerald R, Owen R, Hargrave C, Pryor D,
Lehman M, Bernard A, Mai T, Seshadri V and Fielding A: A comparison
of non-coplanar three-dimensional conformal radiation therapy,
intensity modulated radiation therapy, and volumetric modulated
radiation therapy for the delivery of stereotactic ablative
radiation therapy to peripheral lung cancer. J Med Imaging Radiat
Sci. 48:360–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim ST, An HJ, Kim JI, Yoo JR, Kim HJ and
Park JM: Non-coplanar VMAT plans for lung SABR to reduce dose to
the heart: A planning study. Br J Radiol. 93:201905962020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheung EYW, Lee KHY, Lau WTL, Lau APY and
Wat PY: Non-coplanar VMAT plans for postoperative primary brain
tumour to reduce dose to hippocampus, temporal lobe and cochlea: A
planning study. BJR Open. 3:202100092021.PubMed/NCBI
|
|
27
|
Constine LS, Marks LB, Milano MT, Ronckers
CM, Jackson A, Hudson MM, Marcus KJ, Hodgson DC, Hua CH, Howell RM,
et al: A user's guide and summary of pediatric normal tissue
effects in the clinic (PENTEC): Radiation dose-volume response for
adverse effects after childhood cancer therapy and future
directions. Int J Radiat Oncol Biol Phys. 119:321–337. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee AW, Ng WT, Pan JJ, Chiang CL, Poh SS,
Choi HC, Ahn YC, AlHussain H, Corry J, Grau C, et al: International
guideline on dose prioritization and acceptance criteria in
radiation therapy planning for nasopharyngeal carcinoma. Int J
Radiat Oncol Biol Phys. 105:567–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong FHC, Moleme PA, Ali OA and Mugabe KV:
Clinical implementation of HyperArc. Phys Eng Sci Med. 45:577–587.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xue J, Jin S, Zhang H, Zou K, Sheng J,
Tang J, Zhao W, Yang P, Tang L, Lv X and Lv L: A simplified
non-coplanar volumetric modulated arc therapy for the whole brain
radiotherapy with hippocampus avoidance. Front Oncol.
13:11435642023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pokhrel D, Mallory R, Bush M, St Clair W
and Bernard ME: Feasibility study of stereotactic radiosurgery
treatment of glomus jugulare tumors via HyperArc VMAT. Med Dosim.
47:307–311. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bertholet J, Zhu C, Guyer G, Mueller S,
Volken W, Mackeprang P, Loebner HA, Stampanoni MFM, Aebersold DM,
Fix MK and Manser P: Dosimetrically motivated beam-angle
optimization for non-coplanar arc radiotherapy with and without
dynamic collimator rotation. Med Phys. 51:1326–1339. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guilcher GMT, Rivard L, Huang JT, Wright
NAM, Anderson L, Eissa H, Pelletier W, Ramachandran S, Schechter T,
Shah AJ, et al: Immune function in childhood cancer survivors: A
Children's Oncology Group review. Lancet Child Adolesc Health.
5:284–294. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Docking KM and Knijnik SR: Prospective
longitudinal decline in cognitive-communication skills following
treatment for childhood brain tumor. Brain Inj. 35:1472–1479. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Torizuka D, Uto M and Mizowaki T:
Dosimetric impact of adding non-coplanar arcs for scalp-avoidance
whole-brain irradiation with volumetric-modulated arc radiotherapy
on scalp dose reduction in pediatric patients with
medulloblastomas. J Appl Clin Med Phys. 25:e141892024. View Article : Google Scholar : PubMed/NCBI
|