Introduction
Adrenal cysts are rare lesions, with an incidence of
0.06–0.18% in autopsies (1),
accounting for 1–2% of all adrenal incidentalomas (2,3). These
cysts are commonly benign and non-functional, with the majority of
cases being incidentally identified during imaging examinations
performed for unrelated reasons (2–4).
Adrenal pseudocysts, the most common subtype, likely evolve from
prior intraglandular hemorrhage triggered by anticoagulation,
trauma, coagulopathy, pregnancy or underlying neoplasia, whereas
endothelial cysts are thought to derive from vascular or lymphatic
malformations and parasitic cysts arise almost exclusively in
endemic regions after exposure to parasitic infections (such as
Echinococcus) (3,5). Only 10% of all patients with adrenal
cyst present with symptoms (3,5). The
majority of adrenal cysts are asymptomatic and slow-growing, with a
slight predominance in women and a common age of diagnosis between
40 and 60 years (3). Current
evidence supports conservative management for small, asymptomatic,
non-functional lesions. However, surgical intervention (such as
laparoscopic adrenalectomy or selective cystectomy) is recommended
for growing, symptomatic or hormonally active cysts after
multidisciplinary review (3,6–8).
Adrenal cysts can occasionally be indistinguishable from benign
renal cysts located at the upper pole of the kidney and the cystic
transformation of renal cancer, thus increasing the risk of
misdiagnosis. The present study reports the case of a patient with
an adrenal cyst, which was initially misdiagnosed as cystic renal
cell carcinoma (RCC), and outlines the diagnostic and treatment
approaches applied at the People's Hospital of Tuanfeng (Huanggang,
China). Furthermore, the clinical, imaging and pathological
characteristics of adrenal cysts reported in the literature were
reviewed, thus highlighting the diagnostic challenges in
differentiating adrenal cysts from renal cysts and cystic RCC. In
the present study, comprehensive literature searches in both the
PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Web of Science
(https://www.webofscience.com) databases
were performed. The search identified only 1 reported case of
adrenal cyst misdiagnosed as RCC (9), and no documented cases of adrenal
cysts being misdiagnosed as cystic RCC to date. The detailed search
strategy is provided in Table
SI.
Case report
The present study reports the case of a 41-year-old
male patient with left flank pain for >3 days. The patient had
been diagnosed with a left renal cyst and left renal calculi via
ultrasound at another hospital 2 years ago. Upon admission to the
People's Hospital of Tuanfeng in December 2023, a plain and
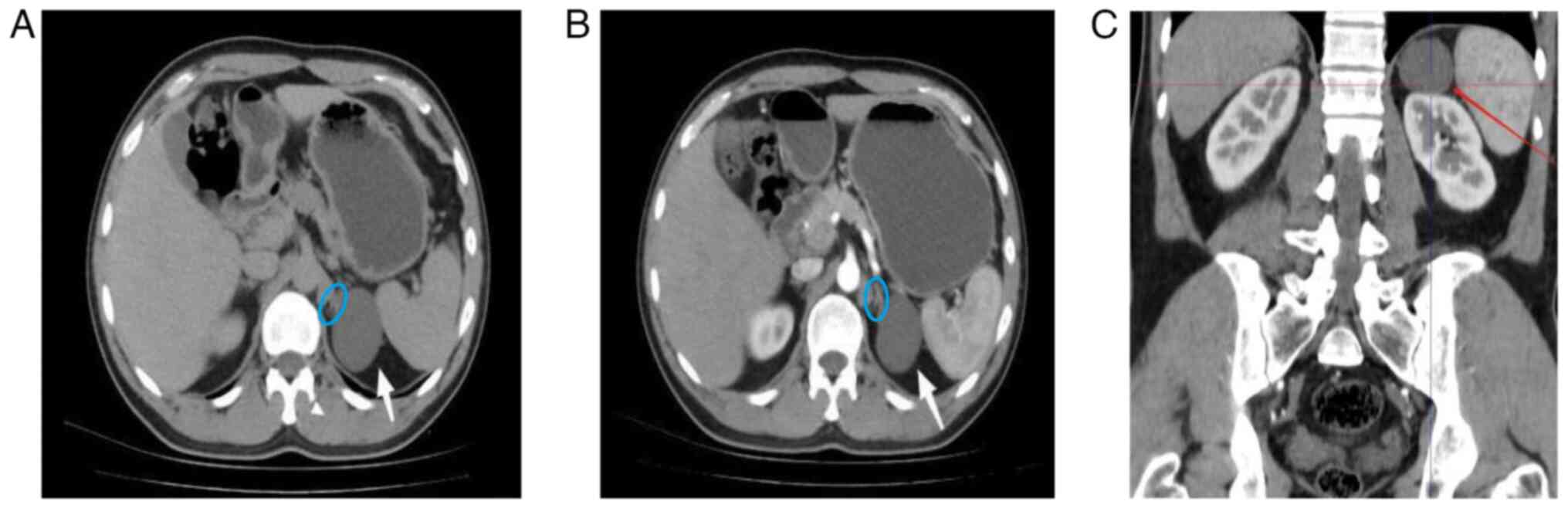
contrast-enhanced CT scan of both kidneys was performed, revealing
a simple renal cyst, ~5 cm in diameter, located at the upper pole
of the left kidney. The cyst was closely adherent to the left
adrenal gland and was classified as a Bosniak classification
(10) Grade I (Fig. 1). No other notable abnormalities
were recorded in the remaining examinations (including complete
blood count, urinalysis, comprehensive metabolic panel and
coagulation tests). The patient was anxious about the cyst and
experienced left flank pain. After excluding surgical
contraindications, posterior retroperitoneoscopic left renal cyst
decortication under general anesthesia was performed (11). During surgery, despite careful
dissection, it was not possible to determine whether the cyst
originated from the kidney or the adrenal gland.
The surgically resected cyst tissue specimens were
fixed in 10% neutral buffered formalin at room temperature within
30 min after resection for ~20 h. After complete sampling,
dehydration (gradient ethanol) was performed, followed by paraffin
embedding to create wax blocks. Sections were cut at a thickness of
3 μm. Permeabilization was performed with 5 mM EDTA retrieval
solution, pH 9.0, at 100°C for 20 min. Endogenous peroxidase
activity was quenched by incubation with 3% hydrogen peroxide for 5
min at room temperature. All primary antibodies were incubated at
room temperature. The primary antibody information is summarized in
Table SII. The secondary
antibodies were conjugated to an HRP-polymer complex, which binds
to the primary antibodies to form a detectable signal. All
secondary antibodies used in the present study were the undiluted
ready-to-use Bond Polymer Refine Detection kit (cat no. DS9800;
Leica Biosystems) or the EnVision FLEX+, Mouse, High pH (Link) kit
(cat. no. K8002; Agilent Technologies, Inc.) and were incubated
with the samples for 8 (Leica) or 20 (Agilent) min at room
temperature. For chromogenic detection, 3,3′-diaminobenzidine (DAB)
was prepared by mixing DAB substrate with DAB buffer at a 1:20
ratio, which was incubated with the sample for 10 min at room
temperature. For counterstaining, the sections were washed with
purified water, followed by the addition of 100 μl hematoxylin
staining solution for 3–5 min. The sections were then washed with
purified water for bluing. The microscope used for the specimens
was an optical microscope (Olympus CX31; magnification, 40–400×).
Neutral balsam was used as the mounting medium. Digital slide
scanning and analysis were carried out using the
PRECICE® 610 Digital Slide Scanning and Analysis System
(Youyun Intelligent Technology Co., Ltd.).
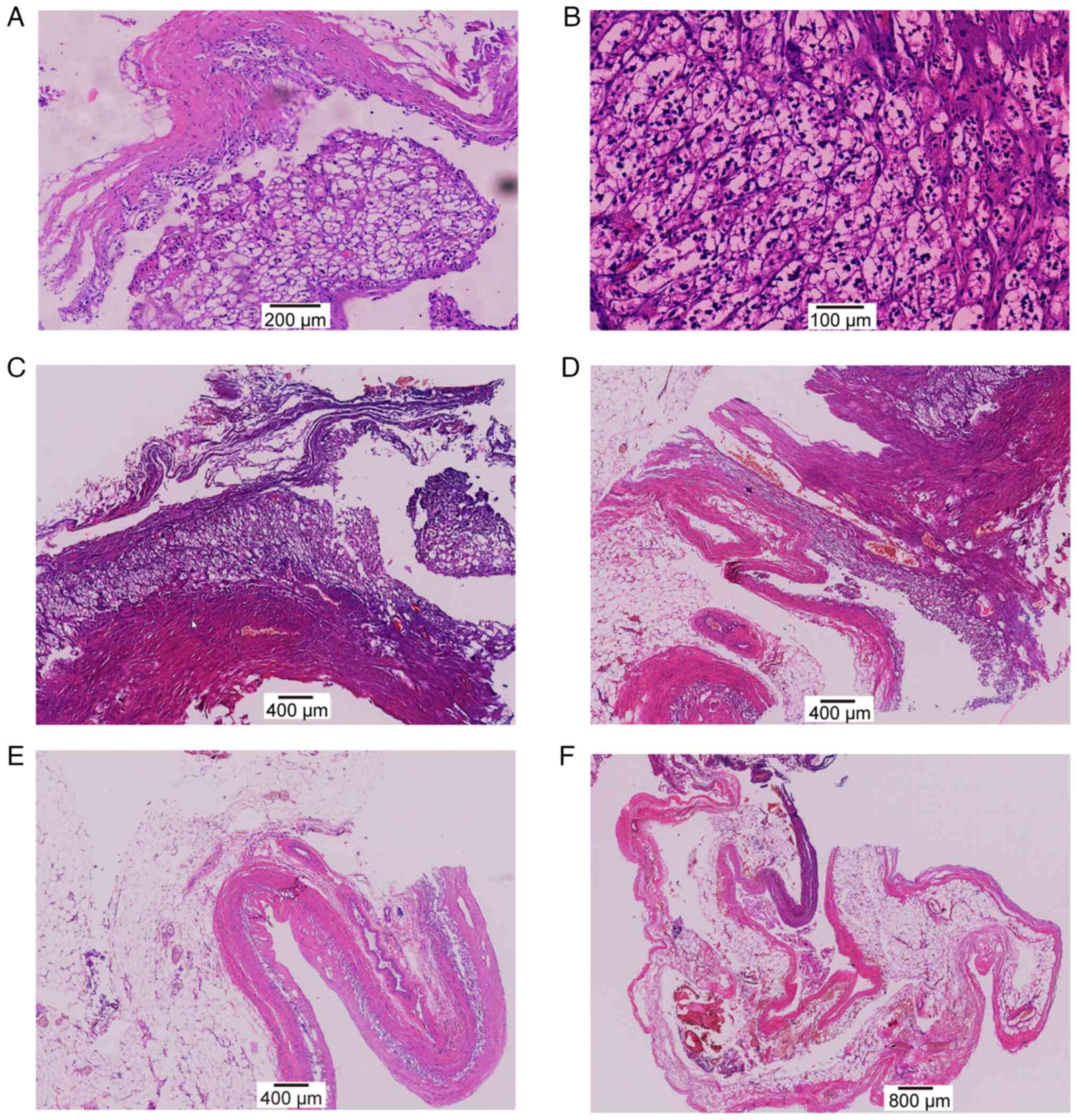
Histopathological examination revealed irregularly
shaped clear cells lining the cyst wall (Fig. 2), which suggested cystic involvement
with clear cell RCC. Immunohistochemical (IHC) staining indicated
the following profile: Cytokeratin 7 (−), α-methylacyl-CoA racemase
(−), cluster of differentiation (CD)117 (−), epithelial membrane
antigen (−), carbonic anhydrase IX (−), CD68 (−), Ki-67 (<1%),
CD10 (+) and vimentin (+) (Fig. 3A and
B). This combination, particularly CD10 and vimentin positivity
with low proliferation, closely resembles the typical
immunophenotype of low-grade clear cell RCC. Based on the initial
diagnosis of clear cell RCC, radical nephrectomy was scheduled.
However, during preoperative planning, discrepancies between the
radiological and clinical findings raised concerns regarding the
pathological diagnosis, which prompted a multidisciplinary team
(MDT) consultation. The MDT recommended expert pathological
consultation and additional IHC staining analysis was carried out,
which revealed positivity for inhibin-α, melan-A, synaptophysin and
vimentin (Fig. 3C-F). The
established scoring criteria for immunohistochemical staining
intensity are provided in Table
SIII. Based on the aforementioned findings, the final diagnosis
was revised to adrenal cyst, thus avoiding the need for radical
nephrectomy. After a 1-year follow-up (December 2024), no
recurrence of the adrenal cyst was observed in the patient.
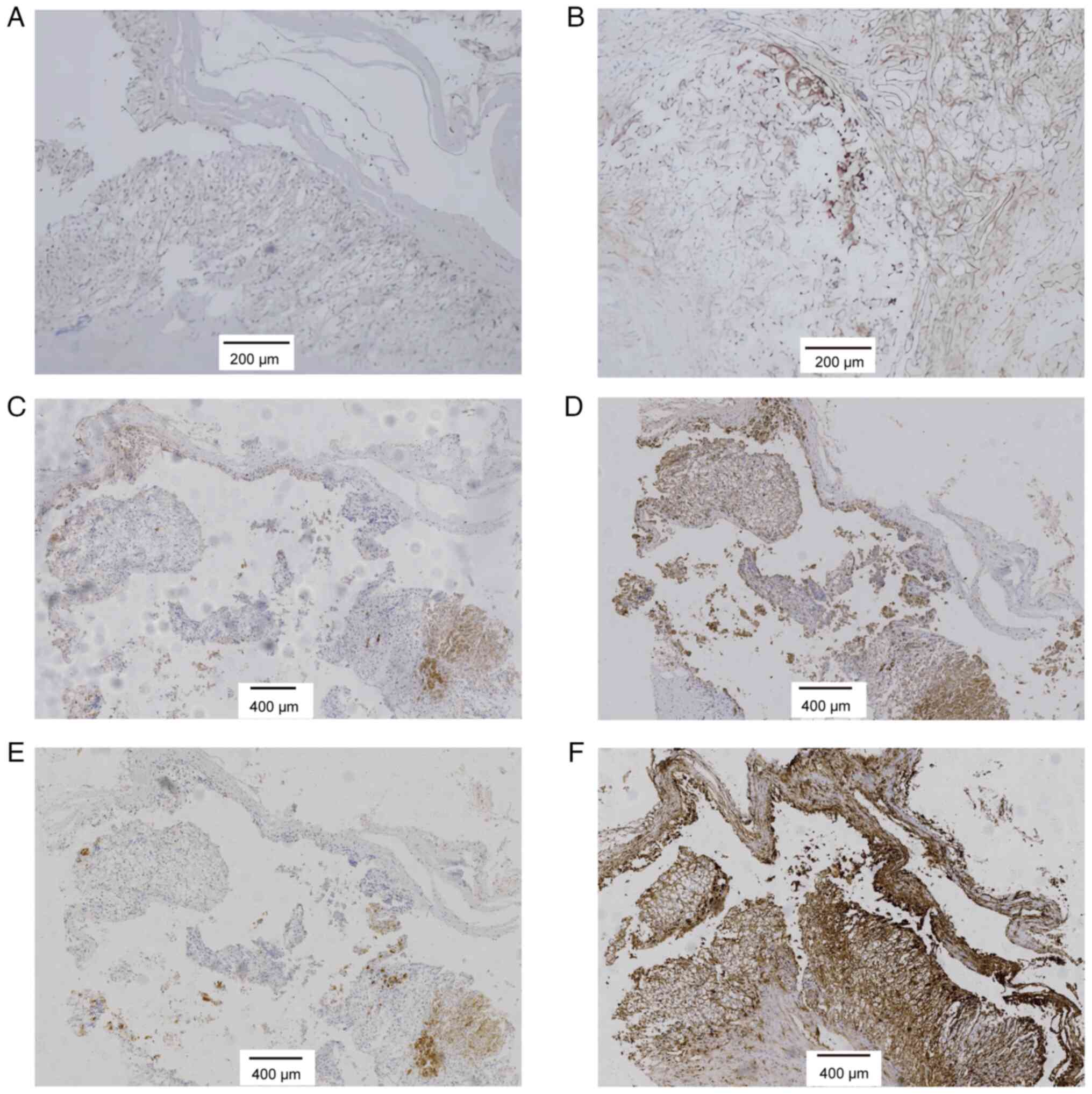 | Figure 3.Immunohistochemical staining of the
adrenal cyst. (A) Cluster of differentiation 10-positive
(magnification, ×100), (B) vimentin-positive (magnification, ×100),
(C) inhibin-α-positive (magnification, ×40), (D) melan-A-positive
(magnification, ×40), (E) synaptophysin-positive (magnification,
×40), and (F) vimentin-positive (magnification, ×40) staining are
shown. The panels (A and B) represent the first immunohistochemical
stains, while (C-F) show the additional immunohistochemical
analysis. |
Discussion
Benign adrenal cysts typically appear as
well-demarcated, commonly rounded masses with thin walls and
homogeneous internal structures. On imaging, they present with low
attenuation on CT, low signal on T1-weighted MRI images and with
high signal on T2-weighted sequences (12,13).
Due to the anatomical location of the lesion, the imaging
manifestations of adrenal cysts are complex and variable, which
makes their differentiation from renal cysts and abdominal
lymphangiomas occasionally difficult (3,5,14).
Renal or pancreatic cysts can also invade the adrenal gland.
Multiplanar CT/MRI scans serve a key role in determining the origin
of the lesion. For example, renal cysts typically arise within the
contour of the kidney, while pancreatic pseudocysts are associated
with peripancreatic inflammation and a history of pancreatitis
(3,13). In the present case report, the left
adrenal cyst was closely adherent to the upper pole of the left
kidney, thus compressing the kidney. However, the origin of the
cyst could not be distinguished by preoperative CT. Therefore, a
cyst decortication was performed. Furthermore, the intraoperative
findings of close adhesion to the left adrenal gland suggested that
the cystic mass arose from the adrenal gland. The aforementioned
observation highlighted the importance of re-evaluating the
preoperative diagnosis and considering a broader intraoperative
dissection to verify the origin of the mass. Based on the present
case, the following measures are recommended for similar cases in
the future: i) High-resolution MRI should be performed to further
localize the cyst and determine whether it originates from the
kidney, adrenal gland or another organ; ii) preoperative MDT
discussions should be also conducted to establish the most
appropriate treatment plan, in accordance with the current European
Society of Endocrinology clinical practice guidelines (7); and iii) a larger area should be
intraoperatively dissected to accurately assess the origin of the
cyst.
Histologically, adrenal cysts are categorized into
pseudocysts and endothelial, epithelial and parasitic cysts. Among
them, pseudocysts are the most common surgical cases, accounting
for ~80% of all cases, and often result from encapsulated adrenal
hemorrhage (3,15). Endothelial cysts, the most frequent
type in autopsy series (~45%) (4),
are lined by a monolayer of endothelial cells, while epithelial
cysts have a thin capsule lined by flat-to-cuboidal cells (3,16).
Pathologically, adrenal cysts often need to be differentiated from
cystic pheochromocytoma (3). The
key feature of the pathological classification of adrenal cysts is
the structure of the cyst wall. The cyst wall of a pseudocyst is
composed of fibrous tissue containing fibrin hemorrhagic fluid
without an internal cellular structure. Furthermore, endothelial
(vascular) cysts originate from dilated and thrombosed blood
vessels or lymphatics and are composed of a single layer of flat
endothelial cells. Epithelial (mesothelial) cysts have commonly
thin wells, associated with mesothelial remnants and are composed
of flat and cuboidal mesothelial cells arranged side by side.
Lastly, parasitic cysts, most frequently associated with
echinococcosis, can contain parasites within the inner wall
(3,5). These cysts, which are often surrounded
by fibrous calcified cyst walls, can be composed of a single or
multiple cavities, supplemented with a transparent fluid (3,5).
In the present case report, the initial pathological
diagnosis was inconsistent with the preoperative CT scan and
intraoperative findings, and therefore an MDT discussion was
initiated. After the urologists, oncologists, radiologists and
pathologists reviewed the case, it was concluded that the clinical,
radiological and pathological findings were inconsistent. Based on
the aforementioned discrepancy, the pathologists were recommended
to consult with senior reference pathologists. Finally,
pathological assessment combined with IHC verified that the lesion
was an adrenal cyst.
Regarding the reasons for misdiagnosis in the
present case, the following contributing factors were identified.
Firstly, there was insufficient communication between the surgical
and pathological teams. The resected tissue sample was not
thoroughly described in terms of its specific location and was
generally designated as a ‘renal cyst.’ Secondly, adrenal
metastases from clear cell RCC, often cystic and with high lipid
content, can be misdiagnosed on chemical-shift MRI imaging
(17). A fibrous adrenal cyst can
sometimes fuse with the renal capsule, leading to notable
challenges for pathological differentiation from primary renal
cystic lesions (18). In the
present case, the pathologist misinterpreted the lipid cells of the
adrenal tissue as clear cells characteristic of clear cell RCC,
thus leading to a misdiagnosis. Furthermore, the nuclei in this
specimen were poorly defined, thus hindering accurate
differentiation. Thirdly, the IHC antibodies used in the present
case were not selected based on the type of adrenal tumor, thus
resulting in non-specific and inconclusive results. This further
contributed to pathological misdiagnosis. Therefore, to accurately
differentiate cystic lesions from tumors, the following primary
antibodies should be used: Vimentin, pancytokeratin, melan-A,
inhibin-α, synaptophysin, chromogranin A, paired box gene 8 and
carbonic anhydrase IX (19,20).
The outcome of the present case report suggests that
surgeons should not solely rely on the ‘authority’ of pathological
diagnosis. In cases where uncertainty exists, a thorough
differential diagnosis should also be performed to reach a
definitive conclusion. Notably, IHC reactions are indeed highly
susceptible to the physicochemical conditions of the tissue
samples. Engel and Moore (21)
indicated that key variables, such as fixation methods, pH levels
and temperature could markedly affect the accuracy of IHC results.
For example, the choice of the fixative and its pH can greatly
affect antigen preservation, while the temperature during tissue
processing, such as during dehydration and fixation, can also
affect the distribution and intensity of immunostaining.
Additionally, the duration of fixation is also key and therefore
both under- and over-fixation can lead to suboptimal staining
outcomes (21–23). Furthermore, heat-induced epitope
retrieval methods, such as microwave or pressure cooker techniques,
are often employed to reverse formalin-induced cross-linking.
However, excessive heat or improper buffer pH can damage epitopes
(21). Therefore, the
physicochemical milieu of the tissue samples, from fixation to
storage, should be meticulously standardized to ensure accurate and
reliable IHC results.
Given the rarity of both adrenal cysts and cystic
RCC, the misdiagnosis of an adrenal cyst as cystic RCC is extremely
rare. The limitations of the present case report were associated
with the experience of the urological surgeon and particularly with
the accuracy of the pathological diagnosis. Furthermore, MRI was
not performed. During preoperative evaluation, the need to
differentiate between a renal cyst and an adrenal cyst was not
considered, therefore the urological CT scan was assumed to be
sufficient for diagnosis. Not performing MRI is also one of the
lessons learned from this case.
In summary, the present case report may provide the
following three implications for urologists: i) This case
highlights the diagnostic pitfalls among renal cysts, adrenal cysts
and cystic RCC, underscoring the need for clinicians to maintain a
broad differential diagnosis and to complete all relevant
preoperative investigations; ii) adrenal cysts and cystic RCC share
similar pathological features, and therefore pathologists should be
vigilant to avoid misdiagnosis. The accurate diagnosis requires
careful consideration of all clinical findings, the use of
appropriate IHC markers and participation in clinical pathological
MDT discussions, to ensure the correct pathological diagnosis; and
iii) urologists should not rely solely on the conclusions drawn by
radiologists and pathologists. When inconsistencies arise during
the treatment process, the timely initiation of MDT discussions
could promote diagnostic accuracy, thus establishing the most
appropriate treatment strategy for patients with adrenal cysts in
the future.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Yu Fang
(Department of Pathology, Zhongnan Hospital of Wuhan University,
Wuhan, China) for assisting with the pathological diagnosis.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YX, XZ, MZ and CC performed the surgical procedure.
YX, XH and XZ conceived and designed the study. YZ performed the
histopathological examination. XH, MZ, YZ and CC wrote the
manuscript. YX and XZ revised the manuscript. YX, MZ, YZ and XZ
confirmed the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present case report was approved by the Ethics
Committee of the People's Hospital of Tuanfeng (Huanggang, China;
approval no. 20240014). Written informed consent was obtained from
the patient.
Patient consent for publication
The patient provided written informed consent for
the publication of the present case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bellantone R, Ferrante A, Raffaelli M,
Boscherini M, Lombardi CP and Crucitti F: Adrenal cystic lesions:
Report of 12 surgically treated cases and review of the literature.
J Endocrinol Invest. 21:109–114. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young WF: Clinical practice. The
incidentally discovered adrenal mass. N Engl J Med. 356:601–610.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calissendorff J, Juhlin CC, Sundin A,
Bancos I and Falhammar H: Adrenal cysts: An emerging condition. Nat
Rev Endocrinol. 19:398–406. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laasri K, El Harras Y, Izi Z, Marrakchi S,
Derqaoui S, Bernoussi Z, El Aoufir O, Laamrani FZ and Jroundi L:
Endothelial cyst of the adrenal gland: A rare case report. SAGE
Open Med Case Rep. 12:2050313X2412615102024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mete O, Erickson LA, Juhlin CC, de Krijger
RR, Sasano H, Volante M and Papotti MG: Overview of the 2022 WHO
Classification of adrenal cortical tumors. Endocr Pathol.
33:155–196. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pogorzelski R, Toutounchi S, Krajewska E,
Ambroziak U, Koperski Ł, Wołoszko T, Celejewski K, Szostek MM,
Jakuczun W and Gałązka Z: Adrenal cysts-optimal laparoscopic
treatment. Wideochir Inne Tech Maloinwazyjne. 13:288–291.
2018.PubMed/NCBI
|
|
7
|
Fassnacht M, Tsagarakis S, Terzolo M,
Tabarin A, Sahdev A, Newell-Price J, Pelsma I, Marina L, Lorenz K,
Bancos I, et al: European society of endocrinology clinical
practice guidelines on the management of adrenal incidentalomas, in
collaboration with the European network for the study of adrenal
tumors. Eur J Endocrinol. 189:G1–G42. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bozic Antic I, Djurisic I and Nikolic S:
Adrenal cysts: To operate or not to operate? J Clin Med.
13:8462024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gubbiotti MA, LiVolsi V, Montone K and
Baloch Z: A cyst-ematic analysis of the adrenal gland: A
compilation of primary cystic lesions from our institution and
review of the literature. Am J Clin Pathol. 157:531–539. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silverman SG, Pedrosa I, Ellis JH, Hindman
NM, Schieda N, Smith AD, Remer EM, Shinagare AB, Curci NE, Raman
SS, et al: Bosniak classification of cystic renal masses, version
2019: An update proposal and needs assessment. Radiology.
292:475–488. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Richard PO, Violette PD, Bhindi B, Breau
RH, Gratton M, Jewett MAS, Kapoor A, Pouliot F, Leveridge M, So AI,
et al: 2023 Update-Canadian urological association guideline:
Management of cystic renal lesions prior to original publication
(March 2017), this guideline underwent review by the CUA guidelines
committee, CUA members at large, and the CUA executive board. The
2023 updates were approved by the CUA guidelines committee and CUA
executive board. Can Urol Assoc J. 17:162–174. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo YK, Yang ZG, Li Y, Deng YP, Ma ES, Min
PQ and Zhang XC: Uncommon adrenal masses: CT and MRI features with
histopathologic correlation. Eur J Radiol. 62:359–370. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albano D, Agnello F, Midiri F, Pecoraro G,
Bruno A, Alongi P, Toia P, Di Buono G, Agrusa A, Sconfienza LM, et
al: Imaging features of adrenal masses. Insights Imaging. 10:12019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sundin A, Hindié E, Avram AM, Tabarin A,
Pacak K and Taïeb D: A clinical challenge: endocrine and imaging
investigations of adrenal masses. J Nucl Med. 62:26S–33S. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dar SA, Qayyum F, Amir A, Khan MUU, Asif
MA, Ullah AS, Chaudhry MJ, Afzaal H and Mehmood Qadri H:
Pseudocysts of the adrenal gland: A systematic review of existing
scientific literature from 2000 to 2023. Cureus.
16:e705282024.PubMed/NCBI
|
|
16
|
Dogra P, Sundin A, Juhlin CC,
Calissendorff J, Falhammar H and Bancos I: Rare benign adrenal
lesions. Eur J Endocrinol. 188:407–420. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woo S, Cho JY, Kim SY and Kim SH: Adrenal
adenoma and metastasis from clear cell renal cell carcinoma: Can
they be differentiated using standard MR techniques? Acta Radiol.
55:1120–1128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan F, Pietrow P, Wilson LA, Romanas M and
Tawfik OW: Adrenal pseudocyst: A unique case with adrenal renal
fusion, mimicking a cystic renal mass. Ann Diagn Pathol. 8:87–90.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu M, Li J and Xu C: Clear cell renal cell
carcinoma with small intestine invasion mimicking an intestinal
tumor. Rev Esp Enferm Dig. 116:717–718. 2024.PubMed/NCBI
|
|
20
|
Oxley J: Renal immunohistochemistry-with
examples. 2021.Available from:. http://www.jonoxley.com/wp-content/uploads/2021/03/Renal-Immunotable-with-examples-3.pdfJuly
12–2025
|
|
21
|
Engel KB and Moore HM: Effects of
preanalytical variables on the detection of proteins by
immunohistochemistry in formalin-fixed, paraffin-embedded tissue.
Arch Pathol Lab Med. 135:537–543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Magaki S, Hojat SA, Wei B, So A and Yong
WH: An introduction to the performance of immunohistochemistry.
Methods Mol Biol. 1897:289–298. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mebratie DY and Dagnaw GG: Review of
immunohistochemistry techniques: Applications, current status, and
future perspectives. Semin Diagn Pathol. 41:154–160. 2024.
View Article : Google Scholar : PubMed/NCBI
|