Introduction
Due to the continuous advancement of modern medical
technology, the survival of patients with cancer has been markedly
prolonged. However, this progress has also led to a corresponding
rise in the risk of developing other primary malignancies (1–3).
Multiple primary cancers (MPC) denote the diagnosis of ≥2 primary
malignant tumors in a patient, either simultaneously or
sequentially (4). The etiology of
MPC is complex and may be associated with numerous factors,
including patient genetics, immune dysregulation, exposure to
carcinogens, environmental factors, treatment modalities and
increased disease surveillance (5–10). In
clinical practice, the diagnosis and treatment of MPC present
numerous challenges (4,11). Its symptoms and imaging features
closely mimic those of metastatic cancer, often leading to
misdiagnosis or delayed diagnosis, which may delay the optimal
timing for treatment. Furthermore, the treatment of MPC lacks
standardized guidelines, and clinical decision-making requires a
comprehensive consideration of several factors, including the
physical condition of the patient, type of tumor pathology, tumor
staging and prior treatment history, further complicating clinical
decision-making (4,5,12).
Nasopharyngeal carcinoma (NPC) is a prevalent
malignancy localized to the head and neck region. It is
characterized by a distinct geographical distribution, with notably
higher incidence rates in Southeast Asia and Southern China
compared with other regions worldwide (13,14).
Despite advancements in diagnostic and therapeutic technologies,
NPC remains a marked public health burden due to its high incidence
and mortality rates (15). Notably,
the incidence of MPC among patients with NPC is relatively high
(1.9–8.7%) (16–19), which may be attributed to the unique
biological behavior of NPC and its treatment modalities
(radiotherapy and/or chemotherapy), both of which may potentially
induce second primary cancers (17,20).
When patients with NPC develop MPC, clinical management and
prognosis become even more complex. Therefore, a deeper
understanding of the clinical characteristics and survival outcomes
of patients with NPC-related MPC (NPC-MPC) is crucial for
optimizing treatment strategies and improving prognosis.
Although previous studies have explored the
incidence and outcomes of MPC among specific groups of cancer
survivors (5,18,21,22),
certain reports have indicated that patients with metachronous MPC
(mMPC) have an improved prognosis compared with patients with
synchronous MPC (sMPC) (4,23–26).
The number of studies specifically focused on NPC-MPC cases remains
limited and it is unclear whether the previously reported survival
advantage of mMPC compared with sMPC is also applicable to patients
with NPC in association with other primary tumors. Additionally,
there is no consensus on the clinical characteristics, prognosis
and prognostic factors of this complex and unique patient
population. Therefore, identifying prognostic factors in patients
with NPC-MPC is of critical clinical importance. These factors will
not only facilitate accurate patient assessment and personalized
treatment planning but also provide evidence-based guidance for
clinicians to optimize therapeutic strategies. For example,
identifying high-risk patient groups may help guide early detection
and intervention, with the potential for improved patient
prognosis.
Given the current knowledge gaps and clinical needs
in the field of NPC-MPC, the present retrospective study aimed to
analyze the clinical data of 306 patients with NPC-MPC, with a
focus on comparing the clinical characteristics, treatment
responses and survival outcomes between patients with sMPC and
mMPC. Survival and multivariate regression analysis were employed
to identify key prognostic factors, with the aim to provide a
theoretical foundation for the clinical management of NPC-MPC
cases, optimize treatment strategies and ultimately improve patient
prognosis and quality of life.
Materials and methods
Clinical subjects
The clinical data of patients with NPC
[International Classification of Diseases, Tenth Revision code C11
(27)] who were admitted to the
First Affiliated Hospital of Guangxi Medical University (Nanning,
China) from January 2012 to December 2023 were retrospectively
collected by accessing patient medical records whilst following the
appropriate research criteria and guidelines. The clinical data of
patients who had been initially diagnosed with NPC-MPC were then
extracted.
The diagnosis of MPC was based on the criteria
established by Warren and Gates in 1932 (28). The inclusion criteria were as
follows (29): i) Each tumor must
be pathologically confirmed as malignant; ii) each tumor must
possess distinct pathological characteristics; iii) tumors should
be located in different organs or, if in the same organ, be
non-contiguous; and iv) one of the tumors must be pathologically
diagnosed as NPC. Furthermore, the exclusion criteria were as
follows: i) No pathological evidence; ii) recurrence or metastasis
of the primary cancer; and iii) incomplete clinical data. Based on
the time interval between the onset of the two primary tumors,
patients were further categorized into two groups: i) sMPC group,
defined as those occurring simultaneously or within 6 months of
each other; and ii) mMPC group, defined as those with a diagnostic
interval of >6 months (30).
Based on the cancer onset sequence, the NPC first (NCF) and the
other cancer first (OCF) groups were defined. In NCF MPCs, NPC was
the first-occurring tumor, and in OCF MPCs, other malignancies
occurred before NPC. For sMPCs, classification relied on the
earliest documented evidence (pathological confirmation,
radiological suspicion or symptom onset). For cases with identical
diagnostic timestamps (truly simultaneous presentations),
precedence was assigned to the malignancy demonstrating a more
advanced tumor-node-metastasis (TNM) stage. Persisting diagnostic
uncertainties were resolved by symptom chronology analysis or
multidisciplinary tumor board consensus.
The present retrospective study was reviewed and
approved by the Medical Ethics Committee of the First Affiliated
Hospital of Guangxi Medical University. As it was a retrospective
study, the Ethics Committee waived the requirement for informed
patient consent.
Clinical data collection
The inclusion and exclusion criteria were applied to
the patient cohort and 35 sMPC and 271 mMPC cases were selected for
retrospective analysis. The clinical characteristics of patients
assessed included age, sex, marital status, smoking and alcohol
consumption status, family history of cancer, histological subtypes
of cancer, TNM stage, treatment details and survival time. Marital
status was classified as either married or unmarried. Based on the
World Health Organization (WHO) classification, the histological
subtypes of NPC included keratinizing squamous cell carcinoma (WHO
I subtype), differentiated non-keratinizing squamous cell carcinoma
(WHO II subtype) and undifferentiated non-keratinizing squamous
cell carcinoma (WHO III subtype) (31). Smoking status was classified as
never smoker, current or ex-smoker or heavy smoker (≥20 pack-years)
(32). Alcohol consumption status
was categorized into never drinker, current or ex-drinker and heavy
drinker (≥30 g/day) (33). A total
of two experienced pathologists independently reviewed all
pathology slides to confirm the diagnosis. Discrepancies were
resolved by achieving consensus. To ensure quality control,
diagnostic reports were cross validated with institutional cancer
registry data.
Treatment
The clinical staging of NPC was determined according
to the 2017 Eighth Edition of the American Joint Committee on
Cancer staging system (34), where
the TNM stage was defined as early-stage (stage I–II) or
advanced-stage (stage III–IV). In accordance with the guidelines of
the National Comprehensive Cancer Network for head and neck cancer
(35), a standardized treatment
plan was established according to the TNM stage of the patient.
Endpoints and follow-up
All enrolled patients were followed up using
outpatient reviews, inpatient examinations and telephone calls
until the death of the patient or until March 2024. Overall
survival (OS) was calculated from the time of diagnosis of the
first cancer to the death of the patient or last follow-up.
Patients lost to follow-up were treated as censored cases, with
their survival time calculated up to the date of the last confirmed
contact (such as their final hospital visit or telephone contact),
meaning that their observation was ended at that point without an
event being recorded. All surviving patients without adverse events
were censored at the study end date (March 2024).
Statistical analysis
The χ2 test was used for categorical
variables when all expected cell counts were >5 or when ≤20% of
cells had expected counts of ≤5; otherwise, Fisher's exact test was
applied for 2×2 tables, and the Fisher-Freeman-Halton exact test
(Monte Carlo method, 100,000 replicates, two-sided) was used for
larger contingency tables. An unpaired (independent samples) t-test
was used for continuous variables. OS was estimated using the
Kaplan-Meier method, and survival outcomes between the two groups
were compared via the log-rank test. To explore the factors
influencing prognosis, multivariate Cox regression analysis was
performed. The proportional hazards assumption was assessed using
Schoenfeld residuals, and all covariates satisfied this assumption.
Multicollinearity among covariates was evaluated using the variance
inflation factor (VIF), where all VIF values were observed to be
below the commonly accepted threshold of 5, indicating no
significant collinearity. SPSS (version 27.0; IBM Corp.) and R
Statistical Software (version 4.2.2; http://www.r-project.org) were used for all
statistical analyses. Two-sided P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics of patients
with sMPC and mMPC
A total of 306 patients diagnosed with MPC-involving
NPC were included in the present study (Table I). Among these, 35 patients (11.4%)
had sMPC, whilst 271 patients (88.6%) had mMPC. The proportion of
patients with other cancer as the first primary cancer (OCF group)
was significantly higher in the sMPC group (37.1%) compared with in
the mMPC group (11.8%), with the difference between groups being
statistically significant (P<0.001). Conversely, the proportion
of NPC as the first primary cancer (NCF) was notably higher in the
mMPC group (88.2%) than in the sMPC group (62.9%). The mean age at
diagnosis of MPC was significantly higher in the sMPC group
(53.3±12.4 years) compared with in the mMPC group (46.2±11.9 years;
P=0.001). No significant differences were observed between groups
in terms of sex distribution (P=0.194), family history of cancer
(P=0.100) or marital status (P=0.704). However, smoking history
differed significantly, with a higher proportion of heavy smokers
in the sMPC group (11.4%) compared with in the mMPC group (3.9%;
P=0.042).
 | Table I.Clinical characteristics of 306
patients with multiple primary cancers. |
Table I.
Clinical characteristics of 306
patients with multiple primary cancers.
| Variable | Total (n=306) | sMPC group
(n=35) | mMPC group
(n=271) | P-value | Test statistic |
|---|
| Order of
occurrence |
|
|
|
<0.001a | - |
|
OCF | 45 (14.7) | 13 (37.1) | 32 (11.8) |
|
|
|
NCF | 261 (85.3) | 22 (62.9) | 239 (88.2) |
|
|
| Sex |
|
|
| 0.194 | 1.686 |
|
Male | 216 (70.6) | 28 (80.0) | 188 (69.4) |
|
|
|
Female | 90 (29.4) | 7 (20.0) | 83 (30.6) |
|
|
| Age at MPC
diagnosis, years | 47.0±12.2 | 53.3±12.4 | 46.2±11.9 | 0.001 | 10.822 |
| Family history of
cancer |
|
|
| 0.100a | - |
| No | 267 (87.3) | 27 (77.1) | 240 (88.6) |
|
|
|
Yes | 39 (12.7) | 8 (22.9) | 31 (11.4) |
|
|
| Marital status |
|
|
| 0.704a | - |
|
Married | 291 (95.1) | 34 (97.1) | 257 (94.8) |
|
|
|
Unmarried | 15 (4.9) | 1 (2.9) | 14 (5.2) |
|
|
| Smoking status |
|
|
| 0.042a | - |
| Never
smoker | 204 (66.7) | 19 (54.3) | 185 (68.3) |
|
|
| Current
or ex-smoker | 90 (29.4) | 12 (34.3) | 78 (28.8) |
|
|
| Heavy
smokerb | 12 (3.9) | 4 (11.4) | 8 (3.0) |
|
|
| Alcohol status |
|
|
| 0.061a | - |
| Never
drinker | 231 (75.5) | 21 (60.0) | 210 (77.5) |
|
|
| Current
or ex-drinker | 49 (16.0) | 9 (25.7) | 40 (14.8) |
|
|
| Heavy
drinkerc | 26 (8.5) | 5 (14.3) | 21 (7.7) |
|
|
| TNM stage - first
cancer |
|
|
| 0.002a | - |
|
I/II | 129 (42.2) | 6 (17.1) | 123 (45.4) |
|
|
|
III/IV | 173 (56.5) | 28 (80.0) | 145 (53.5) |
|
|
|
Unknown | 4 (1.3) | 1 (2.9) | 3 (1.1) |
|
|
| TNM stage - second
cancer |
|
|
| 0.587a | - |
|
I/II | 120 (39.2) | 14 (40.0) | 106 (39.1) |
|
|
|
III/IV | 181 (59.2) | 20 (57.1) | 161 (59.4) |
|
|
|
Unknown | 5 (1.6) | 1 (2.9) | 4 (1.5) |
|
|
| Histological
type |
|
|
| 0.492a | - |
| I | 7 (2.3) | 0 (0.0) | 7 (2.6) |
|
|
| II | 29 (9.5) | 5 (14.3) | 24 (8.9) |
|
|
|
III | 270 (88.2) | 30 (85.7) | 240 (88.6) |
|
|
| Surgery - first
cancer |
|
|
| 0.003a | - |
| No | 270 (88.2) | 25 (71.4) | 245 (90.4) |
|
|
|
Yes | 36 (11.8) | 10 (28.6) | 26 (9.6) |
|
|
| Radiotherapy -
first cancer |
|
|
|
<0.001a | - |
| No | 39 (12.7) | 14 (40.0) | 25 (9.2) |
|
|
|
Yes | 267 (87.3) | 21 (60.0) | 246 (90.8) |
|
|
| Chemotherapy -
first cancer |
|
|
| 0.819 | 0.052 |
| No | 117 (38.2) | 14 (40.0) | 103 (38.0) |
|
|
|
Yes | 189 (61.8) | 21 (60.0) | 168 (62.0) |
|
|
| Surgery - second
cancer |
|
|
| <0.001 | 11.308 |
| No | 129 (42.2) | 24 (68.6) | 105 (38.7) |
|
|
|
Yes | 177 (57.8) | 11 (31.4) | 166 (61.3) |
|
|
| Radiotherapy -
second cancer |
|
|
| 0.002 | 9.993 |
| No | 232 (75.8) | 19 (54.3) | 213 (78.6) |
|
|
|
Yes | 74 (24.2) | 16 (45.7) | 58 (21.4) |
|
|
| Chemotherapy -
second cancer |
|
|
| 0.116 | 2.473 |
| No | 186 (60.8) | 17 (48.6) | 169 (62.4) |
|
|
|
Yes | 120 (39.2) | 18 (51.4) | 102 (37.6) |
|
|
| Survival state |
|
|
| 0.047 | 3.942 |
|
Survived | 119 (38.9) | 19 (54.3) | 100 (36.9) |
|
|
|
Dead | 187 (61.1) | 16 (45.7) | 171 (63.1) |
|
|
| Median survival
time of first cancer, months | 155.2
(137–172.5) | 41.3
(37.7–44.9) | 160.3
(139.0–181.6) | <0.001 | 77.794 |
| Median survival
time of second cancer, months | 32.0
(26.3–37.7) | 40.8
(29.0–52.7) | 30.5
(24.2–36.8) | 0.302 | 1.065 |
Patients in the sMPC group had a significantly
higher proportion of advanced-stage first cancers (III/IV) compared
with in the mMPC group (80.0 vs. 53.5%; P=0.002). No significant
differences were observed in the TNM stage of the second primary
cancer (P=0.587). Most patients had histological type III cancer
(88.2%), with no significant difference between the sMPC and mMPC
groups (P=0.492) (Table I).
Age at occurrence of MPCs in patients
with NPC
Patients with sMPC exhibited distinct age patterns
at the time of cancer occurrence. The age at first cancer diagnosis
was significantly higher in the sMPC group (53.3±12.3 years)
compared with in the mMPC group (45.5±11.5 years; P<0.001).
Conversely, the age at second cancer diagnosis did not differ
significantly between the groups (53.3±12.4 vs. 54.5±10.6 years;
P=0.814) (Table II).
 | Table II.Age at occurrence of multiple primary
cancers involving nasopharyngeal carcinoma. |
Table II.
Age at occurrence of multiple primary
cancers involving nasopharyngeal carcinoma.
|
|
|
| NCF group
(n=261) | OCF group
(n=45) |
|---|
|
|
|
|
|
|
|---|
| Variable | sMPC group
(n=35) | mMPC group
(n=271) | sMPC | mMPC | sMPC | mMPC |
|---|
| Age at first cancer
diagnosis, years | 53.3±12.3 |
45.5±11.5a | 52.9±15.3 | 48.6±10.4 | 53.5±10.6 |
45.1±11.6b |
| Age at second
cancer diagnosis, years | 53.3±12.4 | 54.5±10.6 | 53.0±15.4 | 54.3±11.3 | 53.5±10.6 | 54.5±10.5 |
When stratified by NCF and OCF groups, the age at
first cancer diagnosis did not significantly differ within the NCF
group (52.9±15.3 years for sMPC group vs. 48.6±10.4 years for mMPC
group; P>0.05). However, in the OCF group, patients in the mMPC
group were significantly younger at first cancer diagnosis
(45.1±11.6 years) compared with those in the sMPC group (53.5±10.6
years; P=0.001). No significant differences were observed in the
age at second cancer diagnosis within either the NCF or OCF groups
(Table II).
Distribution of affected sites in
MPCs
The affected site distribution was based on the
anatomical locations involved in the first and second primary
cancers. Although both sites were reviewed for each patient, the
nasopharynx was excluded from this summary as all patients had NPC
as the index cancer. Statistical analysis revealed no significant
differences between patients with sMPC and mMPC (P=0.940; Table III) with reference to all major
affected sites of cancer (excluding the nasopharynx). The head and
neck region was the most frequently involved site (29.0%), followed
by the digestive (27.4%) and respiratory (24.4%) systems, as
detailed in Table III.
 | Table III.Distribution of affected sites in
synchronous and metachronous multiple primary cancers. |
Table III.
Distribution of affected sites in
synchronous and metachronous multiple primary cancers.
| Affected site | Total (n=303) | sMPC group
(n=36) | mMPC group
(n=267) | P-value |
|---|
| Respiratory
system | 74 (24.4) | 10 (27.8) | 64 (24.0) | 0.940 |
| Digestive
system | 83 (27.4) | 10 (27.8) | 73 (27.3) |
|
| Urinary system | 22 (7.3) | 3 (8.3) | 19 (7.1) |
|
| Reproductive
system | 21 (6.9) | 1 (2.8) | 20 (7.5) |
|
| Head and neck | 88 (29.0) | 10 (27.8) | 78 (29.2) |
|
| Others | 15 (5.0) | 2 (5.6) | 13 (4.9) |
|
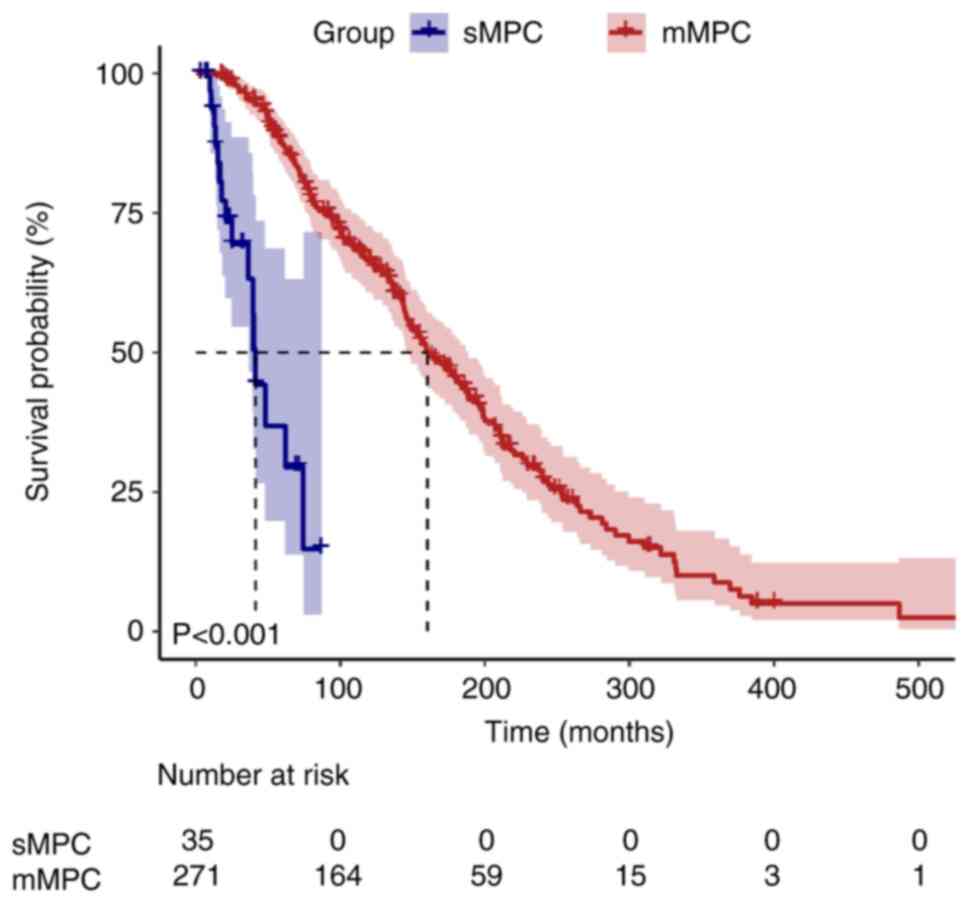
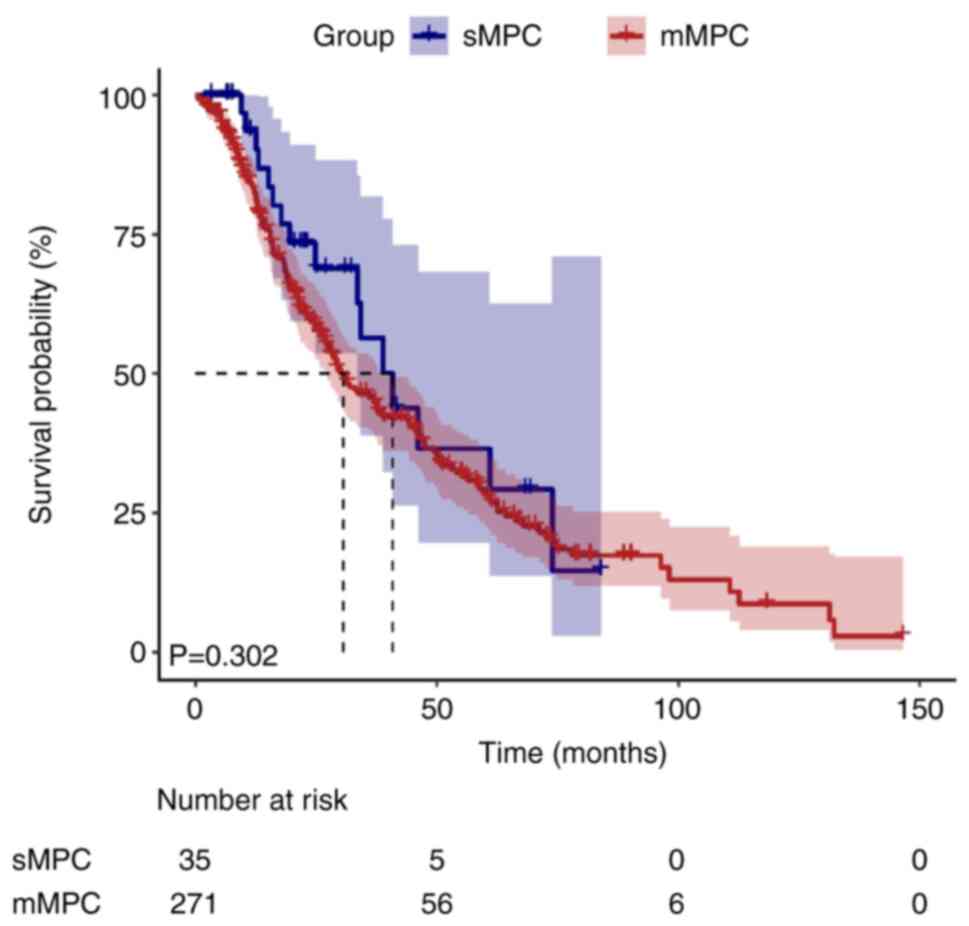
Visualization of survival time
During the median follow-up duration of 155.2 months
[95% confidence interval (CI), 137.9–172.5], 10 patients (3.3%)
were lost to follow-up and censored as per the pre-defined
protocol. Kaplan-Meier survival curves illustrated divergent
survival outcomes between the sMPC and mMPC groups (Figs. 1 and 2). The median survival time for the first
cancer was significantly shorter in the sMPC group (41.3 months;
95% CI, 37.7–44.9) compared with in the mMPC group (160.3 months;
95% CI, 139.0–181.6; log-rank P<0.001). By contrast, the median
survival time for the second cancer did not differ significantly
between the two groups (40.8 vs. 30.5 months; P=0.302).
Survival outcomes and prognostic
factors
Univariate analysis
Univariate analysis (Table IV) identified several factors that
were significantly associated with first cancer survival time.
Notably, an older age at first cancer diagnosis was strongly
associated with shorter survival, with each 1-year increase in age
corresponding to a 5% increase in the risk of death [hazard ratio
(HR), 1.05; 95% CI, 1.03–1.06; P<0.001]. Smoking history also
had a profound effect on survival, with heavy smokers exhibiting
significantly worse survival (HR, 2.71; 95% CI, 1.37–5.38; P=0.004)
compared with current or ex-smokers. Radiotherapy for the first
cancer was significantly associated with improved survival (HR,
0.53; 95% CI, 0.34–0.83; P=0.011), suggesting its potential benefit
for these patients. Conversely, chemotherapy was significantly
associated with reduced survival, with patients receiving
chemotherapy showing significantly worse survival compared with
those who did not receive chemotherapy (HR, 2.01; 95% CI,
1.47–2.75; P<0.001). However, variables such as family history
of cancer and marital status did not show statistically significant
associations with survival (P>0.05).
 | Table IV.Univariate analysis of prognostic
factors in multiple primary cancers. |
Table IV.
Univariate analysis of prognostic
factors in multiple primary cancers.
| Characteristic | HR (95% CI) | P-value |
|---|
| Order of occurrence
(OCF vs. NCF) | 0.64
(0.42–1.01) | 0.054 |
| Sex (Male vs.
female) | 0.72
(0.52–0.99) | 0.036 |
| Age at first cancer
diagnosis (continuous variable) | 1.05
(1.03–1.06) | <0.001 |
| Family history of
cancer (Yes vs. no) | 1.05
(0.67–1.66) | 0.832 |
| Age at MPC
diagnosis (continuous variable) | 1.04
(1.02–1.05) | <0.001 |
| Age at second
cancer diagnosis (continuous variable) | 0.99
(0.97–1.00) | 0.050 |
| Marital status
(Unmarried vs. married) | 0.73
(0.30–1.78) | 0.470 |
| Smoking (reference,
Never) |
| 0.005 |
| Current
or ex-smoker | 1.51
(1.08–2.10) |
|
|
Heavya | 2.71
(1.37–5.38) |
|
| Alcohol (reference,
Never) |
| 0.054 |
| Current
or ex-drinker | 1.24
(0.82–1.89) |
|
|
Heavyb | 1.88
(1.14–3.09) |
|
| First cancer
affected site (reference, Nasopharynx) |
| 0.292 |
|
Respiratory system | 2.77
(0.68–11.26) |
|
|
Digestive system | 1.98
(1.07–3.66) |
|
| Urinary
system | 1.22
(0.39–3.83) |
|
|
Reproductive system | 1.27
(0.52–3.1) |
|
|
Others | 1.61
(0.59–4.36) |
|
| Secondary cancer
site (reference, Nasopharynx) |
| 0.455 |
|
Respiratory system | 1.01
(0.63–1.63) |
|
|
Digestive system | 1.01
(0.63–1.60) |
|
| Urinary
system | 0.82
(0.36–1.86) |
|
|
Reproductive system | 0.58
(0.27–1.27) |
|
| Head
and neck system | 0.35
(0.10–1.14) |
|
|
Others | 0.76
(0.49–1.19) |
|
| TNM stage - first
cancer (reference, I/II) |
| <0.001 |
|
III/IV | 4.54
(3.21–6.42) |
|
|
Unknown | 3.58
(1.11–11.56) |
|
| TNM stage - second
cancer (reference, I/II) |
| 0.009 |
|
III/IV | 1.62
(1.18–2.22) |
|
|
Unknown | 1.28
(0.46–3.56) |
|
| Histology type
(reference, I) |
| 0.494 |
| II | 0.72
(0.27–1.92) |
|
|
III | 0.61
(0.25–1.49) |
|
| Surgery - first
cancer (Yes vs. no) | 1.42
(0.88–2.29) | 0.174 |
| Radiotherapy -
first cancer (Yes vs. no) | 0.53
(0.34–0.83) | 0.011 |
| Chemotherapy -
first cancer (Yes vs. no) | 2.01
(1.47–2.75) | <0.001 |
| Surgery - second
cancer (Yes vs. no) | 0.47
(0.35–0.64) | <0.001 |
| Radiotherapy -
second cancer (Yes vs. no) | 1.31
(0.93–1.84) | 0.131 |
| Chemotherapy -
second cancer (Yes vs. no) | 1.55
(1.15–2.10) | 0.005 |
Multivariate analysis
After adjusting for confounding factors,
multivariate survival analysis (Table
V) revealed that mMPC was significantly associated with
improved survival compared with sMPC (adjusted HR, 0.21; 95% CI,
0.11–0.40; P<0.001). Moreover, age at first cancer diagnosis was
identified as an independent predictor of survival (adjusted HR,
1.04; 95% CI, 1.02–1.05; P<0.001). By contrast, advanced TNM
stage (stage III/IV vs. I/II) was a significant independent
predictor of poor survival (adjusted HR, 2.8; 95% CI, 1.84–4.26;
P<0.001). Notably, radiotherapy for the first cancer
demonstrated a protective effect (adjusted HR, 0.55; 95% CI,
0.31–0.98; P=0.042), suggesting that patients who received
radiotherapy may have had improved survival outcomes. Furthermore,
chemotherapy for the first primary cancer revealed a significant
univariate association with a worse survival (HR, 2.01; 95% CI,
1.47–2.75; P<0.001; Table IV);
however, this significance was attenuated in multivariate models
(adjusted HR, 1.12; P=0.549; Table
V). A similar pattern was observed for second primary cancer
chemotherapy, where univariate analysis demonstrated a significant
association (HR, 1.55; 95% CI, 1.15–2.10; P=0.005; Table IV) that was diminished in
multivariate analysis (adjusted HR, 1.24; P=0.200; Table V).
 | Table V.Multivariate analysis of prognostic
factors in multiple primary cancers. |
Table V.
Multivariate analysis of prognostic
factors in multiple primary cancers.
| Variable | Crude HR (95%
CI) | Crude P-value | Adjusted HR (95%
CI) | Adjusted
P-value |
|---|
| MPC type (mMPC vs.
sMPC) | 0.11
(0.06–0.20) | <0.001 | 0.21
(0.11–0.40) | <0.001 |
| Sex (Male vs.
female) | 0.72
(0.52–0.99) | 0.036 | 0.89
(0.62–1.3) | 0.553 |
| Age at first cancer
diagnosis (continuous variable, per year increase) | 1.05
(1.03–1.06) | <0.001 | 1.04
(1.02–1.05) | <0.001 |
| Smoking status
(Current or ex-smoker vs. never smoked) | 1.51
(1.08–2.10) | 0.015 | 0.98
(0.68–1.43) | 0.927 |
| Smoking status
(Heavy smokera vs.
never smoked) | 2.71
(1.37–5.38) | 0.004 | 1.32
(0.64–2.71) | 0.458 |
| TNM stage of first
cancer (III/IV vs. I/II) | 4.54
(3.20–6.42) | <0.001 | 2.8
(1.84–4.26) | <0.001 |
| TNM stage of first
cancer (unknown vs. I/II) | 3.58
(1.11–11.57) | 0.033 | 0.58
(0.16–2.12) | 0.408 |
| TNM stage of second
cancer (III/IV vs. I/II) | 1.62
(1.18–2.22) | 0.003 | 1.81
(1.24–2.63) | 0.002 |
| TNM stage of second
cancer (unknown vs. I/II) | 1.28
(0.46–3.56) | 0.631 | 1.47
(0.50–4.28) | 0.480 |
| Radiotherapy for
first cancer (Yes vs. no) | 0.53
(0.34–0.83) | 0.011 | 0.55
(0.31–0.98) | 0.042 |
| Chemotherapy for
first cancer (Yes vs. no) | 2.01
(1.47–2.75) | <0.001 | 1.12
(0.77–1.63) | 0.549 |
| Surgery for second
cancer (Yes vs. no) | 0.47
(0.35–0.64) | <0.001 | 0.82
(0.57–1.20) | 0.311 |
| Chemotherapy for
second cancer (Yes vs. no) | 1.55
(1.15–2.10) | 0.005 | 1.24
(0.89–1.72) | 0.200 |
Discussion
The current retrospective cohort analysis delineated
distinct clinicopathological patterns and survival trajectories
between the sMPC and mMPC groups of MPCs in patients with NPC. The
following principal findings emerged: i) Patients with sMPC
exhibited unique demographic profiles characterized by older age,
heavier smoking burden and advanced-stage first cancers; and ii)
synchronicity independently predicted survival outcomes, with the
sMPC group demonstrating a 4.8-fold increased mortality risk
compared with the mMPC group. The significant differences in
clinical characteristics observed between the two groups have
important implications for clinical management.
The demographic divergence observed between groups
suggest distinct carcinogenic mechanisms. The older age of the sMPC
cohort at initial diagnosis and its elevated heavy smoking
prevalence aligned with cumulative mutagenic exposure models.
Notably, 37.1% of patients with sMPC developed OCF malignancies as
first primaries compared with 11.8% in the mMPC group. This is
similar to the results of previous studies (36,37)
and implies potentially shared etiological factors such as aging,
genetic mutations and tobacco-related field cancerization that
simultaneously increase the risk of multiple cancers or exposure to
a unique set of carcinogens that trigger the development of
multiple primary tumors at the same time (7,38–40).
The older age at diagnosis in the sMPC group may be due to the
cumulative exposure to carcinogens over a longer period. This is
consistent with the multistep carcinogenesis process theory, where
genetic mutations and cellular damage accumulates over time, often
due to prolonged exposure to carcinogens, gradually resulting in an
association with cancer development (5,36,41,42).
The head and neck region, and the digestive and
respiratory systems, were the most frequently affected sites in the
secondary malignancies, which is generally consistent with previous
research findings. The presence of multiple synchronous tumors in
the head and neck area and the upper aerodigestive tract has been
well established (16,17,43)
and may be explained by the concept of ‘field cancerization’
(44). This trend may result from
the growing incidence of thyroid, lung and digestive system
malignancies (45–47), and the observation highlights the
need for enhanced surveillance for malignancies of the head and
neck and upper aerodigestive tract to be integrated throughout the
treatment and management process of NPC. Such surveillance can
facilitate the early detection of malignancies and help formulate
personalized treatment strategies for patients with NPC exhibiting
MPC.
Moreover, the survival analysis revealed that
patients with mMPC had an improved prognosis compared with those
with sMPC (median overall survival, 160.3 vs. 41.3 months;
P<0.001). This survival disparity may reflect divergent
biological pathways. In the sMPC cohort, advanced age (53.3±12.4
vs. 46.2±11.9 years; P=0.001) and heavy smoking prevalence (11.4
vs. 3.0%; P=0.042) may indicate a cumulative mutagenic exposure
potentially driving synchronous carcinogenesis through field
cancerization (44). This
mechanism, consistent with previous findings, is associated with
synchronous tumors in related regions such as the head and neck,
lung and colorectal areas, often exhibiting more aggressive
behavior (23–25). Additionally, patients with sMPC have
a significantly higher proportion of advanced-stage first cancers
(80.0 vs. 53.5%; P=0.002), aligning with findings that synchronous
colorectal cancer tends to present with advanced TNM stages and
larger tumor diameters. This higher intrinsic tumor burden
inherently limits therapeutic options and effectiveness (25). Conversely, patients with mMPC
benefit from longer inter-cancer intervals (>6 months): Tissue
repair reduces prior treatment toxicity (for example,
chemotherapy-related risk drops from a univariate HR value of 2.01
to an adjusted HR value of 1.12), which is consistent with the
finding that metachronous colorectal cancer has fewer complications
and improved tolerance due to sufficient inter-cancer intervals
(25). Over time, restored immune
competence and DNA repair capacity in patients with mMPC may
further lower the risk of aggressive tumor progression. Crucially,
regular monitoring enabled by the inter-cancer interval facilitates
early detection of second primary cancers, in line with the
conclusion that metachronous cancers are more likely to be
diagnosed at earlier, curable stages (4). Moreover, a reduced mutual influence
between tumors in mMPC facilitates radical treatments such as
radiotherapy, as observed in the 85% 5-year survival rate with
radiotherapy for metachronous head and neck cancers (23), mirrored by the significant benefit
of radiotherapy observed in the present study (adjusted HR, 0.55;
P=0.042). In summary, sMPC has a poor prognosis that is associated
with field cancerization-driven synchronous progression, a high
tumor burden and treatment limitations. By contrast, mMPC exhibits
survival advantages from inter-cancer interval-driven bodily
repair, early detection and access to radical treatments.
Results of the present multivariate analysis
demonstrated that mMPC is an independent survival predictor, whilst
radiotherapy was revealed to be associated with protective effects
despite the complexity of MPC. This underscores its indispensable
role in NPC management. The identification of independent
prognostic factors, such as mMPC, age at first cancer diagnosis,
TNM stage and radiotherapy for the first cancer provides valuable
guidance for clinicians to stratify patients and formulate
personalized treatment plans. This finding is consistent with
previous studies (16,48). The protective effect of radiotherapy
on patient survival is consistent with its established role in NPC
treatment. Conversely, chemotherapy was observed to be
significantly associated with worse survival in the univariate
analysis; however, this finding lost statistical significance in
the multivariate model. This discrepancy can be attributed to
confounding by indication. Specifically, patients receiving
chemotherapy were more likely to present with advanced-stage
disease, which is itself a strong predictor of poor prognosis. Once
the model was adjusted for TNM stage and other covariates, the
independent effect of chemotherapy diminished, suggesting that
chemotherapy use was a marker of disease severity rather than a
direct cause of worse outcomes.
Clinically, the aforementioned findings advocate for
tailored surveillance strategies. Universal TNM staging remains
critical for all patients given the persistent prognostic impact of
advanced-stage disease. For patients with sMPC who are
characterized by older age, a higher prevalence of advanced-stage
first cancers and heavy smoking, comprehensive baseline assessments
(such as whole-body PET-CT and endoscopy) are essential to avoid
missing synchronous second primary malignancies. Post-treatment
surveillance should be intensified for early detection of
subsequent primaries, alongside mandatory smoking cessation
interventions. For patients with mMPC, long-term surveillance
targeting the head and neck region and upper aerodigestive tract
should commence after NPC diagnosis. Treatment strategies require a
tiered optimization approach: Radiotherapy should be prioritized
for the first cancer given its independent association with patient
survival, whilst chemotherapy decisions warrant a cautious risk
assessment that accounts for age and TNM stage disparities in sMPC
whilst weighing cumulative treatment burdens in mMPC.
Although the present study provides valuable
insights into the survival outcomes of patients with NPC who have
MPC, there are several limitations to consider. The retrospective
nature of the study may have led to selection bias, potentially
affecting the accuracy and reliability of the data. Therefore,
further prospective studies are needed to confirm these findings.
Additionally, the focus of the study on a single cohort of patients
with NPC may limit the generalizability of the results to other
cancer types or populations. Future studies should explore the
molecular mechanisms underlying synchronous and metachronous cancer
development to inform treatment strategies.
In conclusion, the present study highlights the
significant clinical heterogeneity and survival disparities between
patients with NPC who also present with either sMPC or mMPC. Among
patients with mMPC, a younger age, early TNM stage and radiotherapy
for the first cancer are associated with improved survival
outcomes. By identifying key prognostic factors and their
implications for treatment, these findings can inform clinical
practice and guide future research aimed at improving the outcomes
for this complex patient population.
Acknowledgements
Not applicable.
Funding
The present work was supported by the Guangxi Natural Science
Foundation Outstanding Youth Science Fund Project (grant no.
2024JJG140004); National Natural Science Foundation of China (grant
nos. 82272736, 81460460 and 81760542); Key Research and Development
Program of Guangxi Province, China (2018AB61001); Youth Science and
Technology Award of Guangxi Province, China (2023); The Research
Foundation of the Science and Technology Department of Guangxi
Province, China (grant nos. 2023GXNSFDA026009, 2016GXNSFAA380252
and 2014GXNSFBA118114); the Research Foundation of the Health
Department of Guangxi Province, China (grant no. S2018087); Guangxi
Medical University Training Program for Distinguished Young
Scholars (2017); Medical Excellence Award Funded by the Creative
Research Development Grant from the First Affiliated Hospital of
Guangxi Medical University (2016); Guangxi Medical High-level
Talents Training Program (2022); and the Central Government Guide
Local Science and technology Development Projects (grant no.
ZY18057006).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XH, YK, TW, ZM and MK contributed to the study
conception and design. Data management and accuracy verification
were handled by XH, YK, TW and ZM. XH and YK confirm the
authenticity of all the raw data. The statistical analysis,
interpretation of the results and writing of the first draft of the
manuscript were performed by XH. Oversight and critical manuscript
revisions were performed by MK. All authors commented on previous
versions of the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was performed in line with the
principles of the Declaration of Helsinki and was reviewed and
approved by the Medical Ethics Committee of the First Affiliated
Hospital of Guangxi Medical University (Nanning, China; approval
no. 2025-E0109). Due to the retrospective design, the committee
waived the requirement for informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wagle NS, Nogueira L, Devasia TP, Devasia
TP, Mariotto AB, Yabroff KR, Islami F, Jemal A, Alteri R, Ganz PA
and Siegel RL: Cancer treatment and survivorship statistics, 2025.
CA Cancer J Clin. 75:308–340. 2025.PubMed/NCBI
|
|
2
|
Choi E, Hua Y, Su CC, Wu JT, Neal JW,
Leung AN, Backhus LM, Haiman C, Le Marchand L, Liang SY, et al:
Racial and ethnic differences in second primary lung cancer risk
among lung cancer survivors. JNCI Cancer Spectr. 8:pkae0722024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Freire MV, Thissen R, Martin M, Fasquelle
C, Helou L, Durkin K, Artesi M, Lumaka A, Leroi N, Segers K, et al:
Genetic evaluation of patients with multiple primary cancers. Oncol
Lett. 29:42024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanjak P, Suktitipat B, Vorasan N,
Juengwiwattanakitti P, Thiengtrong B, Songjang C, Therasakvichya S,
Laiteerapong S and Chinswangwatanakul V: Risks and cancer
associations of metachronous and synchronous multiple primary
cancers: A 25-year retrospective study. BMC Cancer. 21:10452021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ge S, Wang B, Wang Z, He J and Ma X:
Common multiple primary cancers associated with breast and
gynecologic cancers and their risk factors, pathogenesis, treatment
and prognosis: A review. Front Oncol. 12:8404312022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang BX, Brantley KD, Rosenberg SM,
Kirkner GJ, Collins LC, Ruddy KJ, Tamimi RM, Schapira L, Borges VF,
Warner E, et al: Second primary non-breast cancers in young breast
cancer survivors. Breast Cancer Res Treat. 207:587–597. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trapani JA, Thia KY, Andrews M, Davis ID,
Gedye C, Parente P, Svobodova S, Chia J, Browne K, Campbell IG, et
al: Human perforin mutations and susceptibility to multiple primary
cancers. OncoImmunology. 2:e241852013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aredo JV, Luo SJ, Gardner RM, Sanyal N,
Choi E, Hickey TP, Riley TL, Huang WY, Kurian AW, Leung AN, et al:
Tobacco smoking and risk of second primary lung cancer. J Thorac
Oncol. 16:968–979. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Vries S, Schaapveld M, Janus CPM,
Daniëls LA, Petersen EJ, van der Maazen RWM, Zijlstra JM, Beijert
M, Nijziel MR, Verschueren KMS, et al: Long-term cause-specific
mortality in hodgkin lymphoma patients. J Natl Cancer Inst.
113:760–769. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou B, Zang R, Song P, Zhang M, Bie F,
Bai G, Li Y, Huai Q, Han Y and Gao S: Association between
radiotherapy and risk of second primary malignancies in patients
with resectable lung cancer: A population-based study. J Transl
Med. 21:102023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Division of Cancer Control and Population
Sciences Program Areas, . Long Term Survivorship. Definitions.
National Cancer Institute; 2020
|
|
12
|
Tian M, Shen J, Liu M, Chen XF, Wang TJ
and Sun YS: Prognostic factors and nomogram development for
survival in renal cell carcinoma patients with multiple primary
cancers: A retrospective study. Transl Androl Urol. 14:685–695.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
15
|
Wu T, Miao W, Qukuerhan A, Alimu N, Feng
J, Wang C, Zhang H, Du H and Chen L: Global, regional, and national
burden of nasopharyngeal carcinoma from 1990 to 2021. Laryngoscope.
135:1409–1418. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang LL, Li GH, Li YY, Qi ZY, Lin AH and
Sun Y: Risk assessment of secondary primary malignancies in
nasopharyngeal carcinoma: A big-data intelligence platform-based
analysis of 6,377 long-term survivors from an endemic area treated
with intensity-modulated radiation therapy during 2003–2013. Cancer
Res Treat. 51:982–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chow JCH, Au KH, Mang OWK, Cheung KM and
Ngan RKC: Risk, pattern and survival impact of second primary
tumors in patients with nasopharyngeal carcinoma following
definitive intensity-modulated radiotherapy. Asia Pac J Clin Oncol.
15:48–55. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Svärd F, Alabi RO, Leivo I, Mäkitie AA and
Almangush A: The risk of second primary cancer after nasopharyngeal
cancer: A systematic review. Eur Arch Otorhinolaryngol.
280:4775–4781. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao C, Miao JJ, Hua YJ, Wang L, Han F, Lu
LX, Xiao WW, Wu HJ, Zhu MY, Huang SM, et al: Locoregional control
and mild late toxicity after reducing target volumes and radiation
doses in patients with locoregionally advanced nasopharyngeal
carcinoma treated with induction chemotherapy (IC) followed by
concurrent chemoradiotherapy: 10-year results of a phase 2 study.
Int J Radiat Oncol Biol Phys. 104:836–844. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scélo G, Boffetta P, Corbex M, Chia KS,
Hemminki K, Friis S, Pukkala E, Weiderpass E, McBride ML, Tracey E,
et al: Second primary cancers in patients with nasopharyngeal
carcinoma: A pooled analysis of 13 cancer registries. Cancer Causes
Control. 18:269–278. 2007.PubMed/NCBI
|
|
21
|
Guzzinati S, Buja A, Grotto G, Zorzi M,
Manfredi M, Bovo E, Del Fiore P, Tropea S, Dall'Olmo L, Rossi CR,
et al: Synchronous and metachronous multiple primary cancers in
melanoma survivors: A gender perspective. Front Public Health.
11:11954582023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Jiang Y, Bai Y and Xu H: Do cancer
survivors have an increased risk of developing subsequent cancer? A
population-based study. J Transl Med. 23:3552025. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bugter O, Van Iwaarden DLP, Dronkers EAC,
de Herdt MJ, Wieringa MH, Verduijn GM, Mureau MAM, Ten Hove I, van
Meerten E, Hardillo JA and Baatenburg de Jong RJ: Survival of
patients with head and neck cancer with metachronous multiple
primary tumors is surprisingly favorable. Head Neck. 41:1648–1655.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang X, Zhang C, Zhou H, Zhang J, Yan W,
Zhong WZ and Chen KN: Multiple pulmonary resections for synchronous
and metachronous lung cancer at two Chinese centers. Ann Thorac
Surg. 109:856–863. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan H, Wen R, Zhou L, Gao X, Lou Z, Hao L,
Meng R, Gong H, Yu G and Zhang W: Clinicopathological features and
prognosis of synchronous and metachronous colorectal cancer: A
retrospective cohort study. Int J Surg. 109:4073–4090. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tamura K, Fujimoto T, Shimizu T, Nagayoshi
K, Mizuuchi Y, Hisano K, Horioka K, Shindo K, Nakata K, Ohuchida K
and Nakamura M: Clinical features, surgical treatment strategy, and
feasibility of minimally invasive surgery for synchronous and
metachronous multiple colorectal cancers: A 14-year single-center
experience. Surg Endosc. 38:7139–7151. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
World Health Organization (WHO), .
International Statistical Classification of Diseases and Related
Health Problems. 10th Revision (ICD-10). WHO; Geneva: 2016,
https://icd.who.int/browse10/2016/en#/C11
|
|
28
|
Warren S and Gates O: Multiple primary
malignant tumors: A survey of the literature and a statistical
study. Am J Cancer. 16:1358–1414. 1932.
|
|
29
|
Working Group Report: International rules
for multiple primary cancers (ICD-0 third edition). Eur J Cancer
Prev. 14:307–308. 2005. View Article : Google Scholar
|
|
30
|
Yang XB, Zhang LH, Xue JN, Wang YC, Yang
X, Zhang N, Liu D, Wang YY, Xun ZY, Li YR, et al: High incidence
combination of multiple primary malignant tumors of the digestive
system. World J Gastroenterol. 28:5982–5992. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo LF, Hong JG, Wang RJ, Chen GP and Wu
SG: Nasopharyngeal carcinoma survival by histology in endemic and
non-endemic areas. Ann Med. 56:24250662024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duncan MS, Freiberg MS, Greevy RA Jr,
Kundu S, Vasan RS and Tindle HA: Association of smoking cessation
with subsequent risk of cardiovascular disease. JAMA. 322:642–650.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho JH, Shin SY, Kim H, Kim M, Byeon K,
Jung M, Kang KW, Lee WS, Kim SW and Lip GYH: Smoking cessation and
incident cardiovascular disease. JAMA Netw Open. 7:e24426392024.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lydiatt WM, Patel SG, O'Sullivan B,
Brandwein MS, Ridge JA, Migliacci JC, Loomis AM and Shah JP: Head
and neck cancers-major changes in the American joint committee on
cancer eighth edition cancer staging manual. CA Cancer J Clin.
67:122–137. 2017.PubMed/NCBI
|
|
35
|
Adelstein D, Gillison ML, Pfister DG,
Spencer S, Adkins D, Brizel DM, Burtness B, Busse PM, Caudell JJ,
Cmelak AJ, et al: NCCN guidelines insights: Head and neck cancers,
version 2.2017. J Natl Compr Canc Netw. 15:761–770. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li F, Zhong WZ, Niu FY, Zhao N, Yang JJ,
Yan HH and Wu YL: Multiple primary malignancies involving lung
cancer. BMC Cancer. 15:6962015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ventura L, Carbognani P, Gnetti L, Rossi
M, Tiseo M, Silini EM, Sverzellati N, Silva M, Succi M, Braggio C,
et al: Multiple primary malignancies involving lung cancer: A
single-center experience. Tumori. 107:196–203. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wynder EL, Mushinski MH and Spivak JC:
Tobacco and alcohol consumption in relation to the development of
multiple primary cancers. Cancer. 40 (4 Suppl):S1872–S1878. 1977.
View Article : Google Scholar
|
|
39
|
Oeffinger KC, Baxi SS, Novetsky Friedman D
and Moskowitz CS: Solid tumor second primary neoplasms: Who is at
risk, what can we do? Semin Oncol. 40:676–689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hung RJ, Baragatti M, Thomas D, McKay J,
Szeszenia-Dabrowska N, Zaridze D, Lissowska J, Rudnai P, Fabianova
E, Mates D, et al: Inherited predisposition of lung cancer: A
hierarchical modeling approach to DNA repair and cell cycle control
pathways. Cancer Epidemiol Biomarkers Prev. 16:2736–2744. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Amer M: Multiple neoplasms, single
primaries, and patient survival. Cancer Manag Res. 6:119–134. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liberale C, Soloperto D, Marchioni A,
Monzani D and Sacchetto L: Updates on larynx cancer: Risk factors
and oncogenesis. Int J Mol Sci. 24:129132023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Goggins WB, Yu IT, Tse LA, Leung SF, Tung
SY and Yu KS: Risk of second primary malignancies following
nasopharyngeal carcinoma in Hong Kong. Cancer Causes Control.
21:1461–1466. 2010.PubMed/NCBI
|
|
44
|
Slaughter DP, Southwick HW and Smejkal W:
Field cancerization in oral stratified squamous epithelium.
Clinical implications of multicentric origin. Cancer. 6:963–968.
1953. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hayat MJ, Howlader N, Reichman ME and
Edwards BK: Cancer statistics, trends, and multiple primary cancer
analyses from the surveillance, epidemiology, and end results
(SEER) program. Oncologist. 12:20–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Watanabe S, Kodama T, Shimosato Y, Arimoto
H, Sugimura T, Suemasu K and Shiraishi M: Multiple primary cancers
in 5,456 autopsy cases in the National cancer center of Japan. J
Natl Cancer Inst. 72:1021–1027. 1984.PubMed/NCBI
|
|
47
|
Boucai L, Zafereo M and Cabanillas ME:
Thyroid cancer: A review. JAMA. 331:425–435. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Wang Z, Chen Y, Lin Q, Chen H, Lin
Y, Lu L, Zheng P and Chen X: Impact of prior cancer on the overall
survival of patients with nasopharyngeal carcinoma. Am J
Otolaryngol. 43:1032352022. View Article : Google Scholar : PubMed/NCBI
|