Introduction
Cancer is a critical health issue characterized by
high rates of morbidity and mortality. Cancer is a major societal,
public-health and economic challenge in the 21st century,
accounting for ~1 in 6 deaths (16.8%) and nearly 1 in 4 deaths from
non-communicable diseases (NCD; 22.8%) worldwide. Cancer
contributes to 30.3% of premature NCD deaths among those aged 30–69
years and ranks among the three leading causes of death in this age
group in 177 of 183 countries (1).
Beyond being a key barrier to increasing life expectancy, cancer
incurs substantial societal and macroeconomic costs that vary by
cancer type, geography and sex (2).
Underscoring the disproportionate burden on women, an estimated one
million children became maternal orphans in 2020 due to a maternal
cancer death, nearly half attributable to breast or cervical cancer
(3). The extracellular matrix
(ECM), as a key component of the tumor stroma, notably influences
cancer progression by serving as both a physical scaffold and a
regulator of cell and tissue functions. Beyond merely transmitting
signals, the ECM also generates biochemical and biophysical cues
that activate cellular responses (4). The interaction between tumor cells and
the ECM is dynamic and reciprocal, leading to continuous reshaping
of the surrounding malignant tissue. Research indicates that tumors
can exploit ECM remodeling to establish a microenvironment
conducive to tumorigenesis and metastasis (5). In turn, cellular behaviors such as
adhesion, migration, angiogenesis and malignant transformation are
influenced by changes in the ECM (6,7).
Within this intricate relationship between the ECM and tumor cells,
collagens serve a pivotal role. As the primary component of the
ECM, collagens constitute ~30% of its structure, with 28 distinct
collagen types identified. These various collagens contribute to
the formation of the basal membrane and interstitial matrix,
creating tissue-specific ECM compositions (8). Alterations in collagen within the
tumor microenvironment generate biomechanical signals that are
detected by both tumor and stromal cells, initiating a series of
biological processes. A previous study has highlighted the abnormal
behaviors of collagens in cancer progression, including
degradation, remodeling, fragmentation, linearization and
fasciculation (9), demonstrating
the role of collagens in precancerous lesions and cancer
development. Understanding the mechanisms by which collagens
influence different stages of cancer progression has enhanced their
diagnostic and prognostic value while opening new avenues for
therapeutic target identification (10).
Several antioxidants, anti-inflammatory and
antimicrobial compounds targeting the synthesis and function of
reactive oxygen species (ROS) and inflammatory processes are
already available as anticancer agents. These compounds offer
notable advantages over traditional chemotherapeutic drugs by
precisely targeting cancerous processes while minimizing harm to
healthy cells (11). The Purple
Collagen complex (PCC) incorporates several bioactive compounds
known for their diverse health benefits, including inulin which is
a prebiotic fiber that promotes gut health by stimulating the
growth of beneficial intestinal bacteria (12). Another compound is the elderberry,
which is derived from Sambucus nigra, rich in flavonoids,
particularly anthocyanins, which exhibit antioxidant properties.
These compounds support immune function and have been traditionally
used to alleviate cold and flu symptoms (13). Another plant-based compound is the
black cumin extract sourced from Nigella sativa, which is
known for its antioxidant, anti-inflammatory and antimicrobial
effects, attributed to its active component, thymoquinone (14). Magnesium citrate and malate found in
PCC are highly bioavailable. Magnesium citrate is commonly used to
treat constipation and improve bone health, while magnesium malate
has been suggested to benefit individuals with fibromyalgia
(15). The eggshell membrane
contains naturally occurring glycosaminoglycans and proteins that
support joint health by reducing pain and stiffness associated with
joint disorders (16). Another
compound found in the complex is the bromelain, an enzyme extracted
from pineapples, bromelain possesses anti-inflammatory and
proteolytic properties, aiding in digestion and potentially
reducing inflammation (17). The
final ingredient of PCC is the liposomal vitamin C which is a
potent antioxidant that supports immune function and skin health.
Encapsulating vitamin C in liposomes enhances its bioavailability,
ensuring efficient delivery and absorption (18). Collectively, these components
contribute to the multifaceted health-promoting properties of
PCC.
Due to the established links between the bioactive
compounds and cancer dynamics, the present study aimed to examine
in vitro effects of Purple Collagen, a complex containing
bioactive double hydrolyzed collagens type I, II, III, V and X
collagen peptides and several antioxidant and anti-inflammatory
agents (inulin, elderberry extract, magnesium citrate and malate,
eggshell membrane, bromelain, black cumin extract and liposomal
vitamin C), on cell viability, migration, oxidative stress,
antioxidant status and apoptotic markers of two cancer types HCT116
colon carcinoma and MIA PaCa-2 pancreas carcinoma cells. By
evaluating the mechanisms through which PCC influences these
processes, the present study aimed to uncover its potential as a
therapeutic agent in these cancer types.
Materials and methods
Cell culture
The study protocol was approved by Scientific
Research Ethical Committee of Biruni University in May 2024 with an
approval number of 2024-BIAEK/01-41. HCT116 colon carcinoma cells
were obtained from ATCC (cat. no. CCL-247) and MIA PaCa-2cells
obtained from ATCC (cat. no. CRL-1420). The present study includes
the MIA PaCa-2 pancreatic carcinoma cell line and the HCT116 cell
line, both of which are of human origin.
All cell lines were cultured in DMEM (Nutriculture;
EcoTech Biotechnology Inc.) supplemented with 10% fetal bovine
serum (FBS) and 1% penicillin-streptomycin antibiotics (Diagnovum)
at 37°C in a humidified incubator containing 5% CO2
(Kabin Incubator; Esco Lifesciences Group). The cells were
maintained in these conditions throughout the experiments.
Purple Collagen, the commercial test compound used
in the present study, was supplied by the company Kiperin
Pharmaceutical Food Industry and Trade Limited Company.
Kiperin® PCC contains 10 g collagen (bioactive double
hydrolyzed collagens containing type I, II, III, V and X collagen
peptides derived from grass-fed, pasture-raised calves), 3 g of
inulin, 500 mg of elderberry (Sambucus) extract, 180 mg
magnesium citrate (providing 29 mg magnesium), 180 mg magnesium
malate (providing 27 mg magnesium), 100 mg eggshell membrane, 90 mg
bromelain (2,400 gelatin-digesting units), 90 mg black cumin
extract, 90 mg liposomal vitamin C and 40 mg 100% natural blueberry
flavor. The dosage was determined in accordance with the Turkish
Ministry of Agriculture and Forestry's regulatory standards, which
specify that dietary supplements containing hydrolyzed collagen
should not provide >10 g per day (19). A concentration was used similar to a
previous double hydrolyzed collagen study, which positively
affected healthy cells and suppressed cancer cells (20), and the dosage was determined
accordingly. The selected doses, 0.5, 1 and 1.5 µg/ml, were
normalized to the in vitro cell number (6×104
cells/well) to approximate physiologically attainable levels while
minimizing off-target cytotoxic responses. Due to the structural
and functional similarities between hydrolyzed collagen and purple
collogen, the same dosage range in the experiments was applied.
Cells were detached with 0.25% trypsin-EDTA, neutralized with
medium containing 10% FBS, pelleted at 300 × g for 5 min at 37°C,
and resuspended in PBS. The suspension was mixed 1:1 with 0.4%
trypan blue, incubated for 2–3 min at room temperature, and then 10
µl was loaded onto a Thoma hemocytometer. Cells were counted under
a light microscope (10X objective) in the four large corner
squares, including cells touching the top and left borders. Counts
from both chambers were averaged. Viable (unstained) and non-viable
(blue) cells were recorded separately; cell density was calculated
as cells/ml=(mean cells per square) × dilution factor (2) × 104, and %
viability=viable/(viable + non-viable) ×100. The resulting density
was used to adjust seeding for subsequent assays.
The effective dose of Purple Collagen was determined
as 1 µg/ml concentration among three doses tested (0.5, 1 and 1.5
µg/ml) by cell counts measured for three times using the Mindray
BC-6800 (Shenzhen Mindray Bio-Medical Electronics Co., Ltd.) cell
counter. Each cell line was divided into two experimental groups:
Control cells (untreated) and collagen-treated cancer cells (1
µg/ml concentration of Purple Collagen). Cells were incubated with
the respective treatments for 48 h at 37°C.
Wound healing assay
To evaluate cell migration, a wound healing assay
was performed in DMEM supplemented with 10% FBS, consistent with
previous studies utilizing HCT116 and MIA PaCa-2 cell lines
(21–23). At least 90% confluent monolayers of
cells were scratched with a sterile pipette tip, creating a uniform
gap. Images of wound closure of HCT116 and MIA PaCa-2 cells were
taken at 0, 6, 15, 30 and 43 h using an inverted light microscope
(Inverted ICX41 SOPTOP; China) at the same marked position. Wound
healing was quantified by measuring the percentage of wound closure
over time (24). Wound closure area
was measured using ImageJ (1.54g; National Institutes of Health)
software.
Measurement of oxidative stress levels
and antioxidant capacity
Cell lysates were prepared from HCT116 and MIA
PaCa-2 cell lines following standard protocols (25,26)
and stored at −80°C until analysis. All reagents and samples were
equilibrated to room temperature before use.
The total oxidant status (TOS) and total antioxidant
status (TAS) levels in two cell lines were assessed using
commercial assay kits provided by Rel Assay Diagnostics. Both
assays were performed according to the manufacturer's protocol,
utilizing a spectrophotometer (Epoch Microplate Spectrophotometer;
BioTek; Agilent Technologies, Inc.) to quantify the absorbance
changes associated with the oxidative and antioxidative properties
of the samples.
To measure TOS, the assay relied on the oxidation of
ferrous ions to ferric ions by oxidant molecules in the sample. The
ferric ions formed a colored complex with a chromogen in an acidic
medium, and the color intensity was proportional to the total
oxidant molecules present. For the assay, 45 µl cell lysate,
standard solution or distilled water (used as a blank) was mixed
with 300 µl buffer solution (pH 1.75). The absorbance of the
reaction mixture was first read at 530 nm after 30 sec.
Subsequently, 15 µl ferrous ion solution was added to the mixture,
and after incubation for 5 min at 37°C or 10 min at room
temperature, the absorbance was read again. The TOS was calculated
based on the change in absorbance and expressed as µmol
H2O2 equivalents (Eq)/l.
For TAS measurements, antioxidants in the sample
reduced dark blue-green ABTS radical cations to their colorless
form. The assay used 18 µl cell lysate, standard solution or
distilled water (blank), which was mixed with 300 µl acetate buffer
(pH 5.8). The initial absorbance of the reaction mixture was
measured at 660 nm after 30 sec. Subsequently, 45 µl ABTS
prochromogen solution was added, and after incubation for 5 min at
37°C or 10 min at room temperature, the final absorbance was
recorded. The antioxidant capacity was quantified based on the
change in absorbance and expressed as mmol Trolox Eq/l.
The TOS and TAS levels were calculated and analyzed
statistically, with results expressed as mean ± standard deviation
(SD).
Reverse transcription
(RT)-quantitative (q)PCR for apoptotic and cell cycle regulatory
markers expression
Total RNA was extracted from cells, and the
concentration was assessed using a Qubit fluorometer. Subsequently,
500 ng RNA was reverse transcribed into complementary DNA (cDNA)
using the OneScript® Plus cDNA Synthesis Kit (Applied
Biological Materials Inc.) according to the manufacturer's
instructions. The RT reaction was carried out with Moloney-Murine
Leukemia Virus reverse transcriptase. The commercial kit used in
the present study was applied according to the manufacturer's
instructions. Internal quality control steps, including positive
and negative control samples, were performed to validate the
reliability and specificity of the assay. These controls were
included in every experimental run. For experimental validation,
PCR amplification was performed, followed by agarose gel
electrophoresis. The observation of a single band of the expected
size confirmed that each primer pair was specific and
effective.
RT-qPCR was performed using the BlasTaq™ 2X qPCR
MasterMix (Applied Biological Materials Inc.) following the
supplier's protocol. Each reaction contained 2 µg of cDNA template,
1 µM each primer, and the appropriate volume of MasterMix, adjusted
to a final volume of 25 µl with nuclease-free water. The primers
used were as follows: BAX, forward
5′-GCCCTTTTGCTTCAGGGTTTCA-3′ and reverse
5′-CTGTCCAGTTCGTCCCCGAT-3′; BCL2 forward
5′-GTGGATGACTGAGTACCT-3′ and reverse 5′-CCAGGAGAAATCAAACAGAG-3′;
TP53 forward 5′-AATCTCCGCAAGAAAGGGGAG-3′ and reverse
5′-TTGGGCAGTGCTCGCTTAG-3′; cyclin-dependent kinase inhibitor 1
(p21) forward 5′-GACTGTGATGCGCTAATGGC-3′ and reverse
5′-CCGTGGGAAGGTAGAGCTTG-3′; GAPDH (housekeeping gene)
forward 5′-CCACCCATGGCAAATTCC-3′ and reverse
5′-TGGGATTTCCATTGATGACAAG-3′.
Amplification and detection were performed on a
Bio-Rad CFX96™ Real-Time PCR System (Bio-Rad Laboratories, Inc.)
under the following cycling conditions: Initial denaturation at
95°C for 3 min, followed by 40 cycles of denaturation at 95°C for
15 sec, annealing at 60°C for 15 sec and extension at 72°C for 15
sec. Fluorescence data were collected at the end of each extension
step. Relative gene expression levels were calculated using the
2−ΔΔCq method, normalizing the gene expressions to
GAPDH as the internal control (27).
Statistical analysis
All statistical analyses were performed using
GraphPad Instat Software 8.0.1 (Dotmatics). The distribution of the
data was assessed for normality using the Kolmogorov-Smirnov test.
For data that followed a normal distribution, comparisons between
groups were performed using an unpaired t-test. For non-normally
distributed data, a Mann-Whitney U test was applied. P<0.05 was
considered to indicate a statistically significant difference. To
ensure the objectivity and integrity of the findings, all
statistical analyses were performed by an independent researcher
not affiliated with Kiperin Pharmaceutical.
Results
Cell viability and proliferation
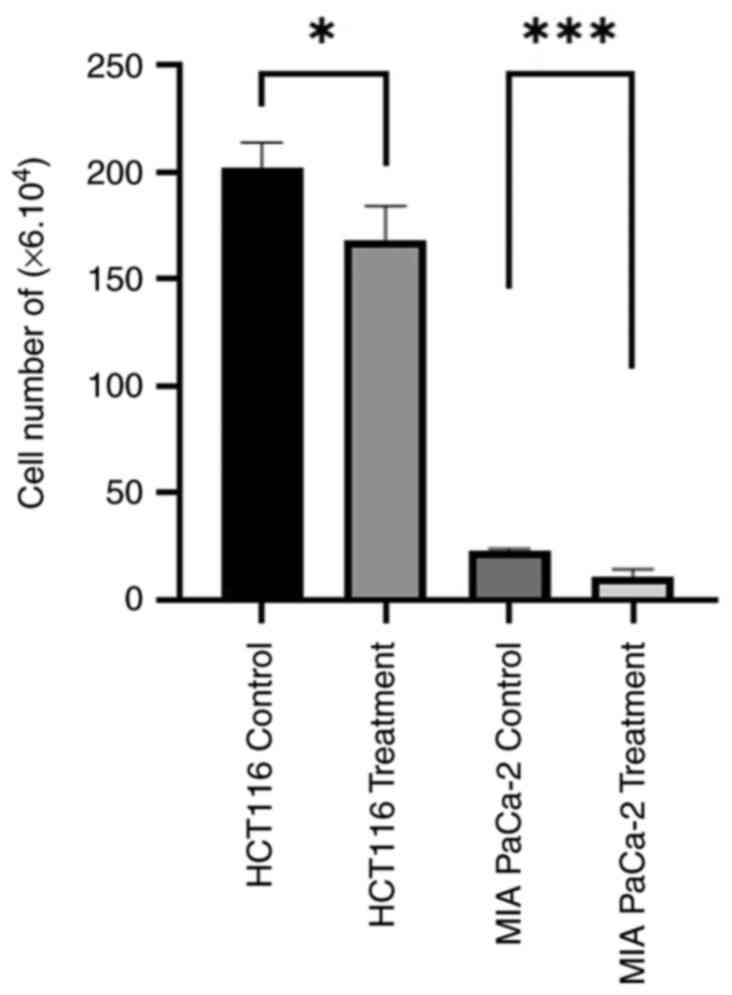
The cell numbers of HCT116 and MIA PaCa-2 cell lines
were assessed in the control conditions and after treatment with 1
µg/ml dose of PCC (Fig. 1). The
results indicate a significant decrease in the number of HCT116
cells treated with the PCC compared with the control group
(P=0.0141). Similarly, a significant reduction in the number of MIA
PaCa-2 cells was observed following treatment with the PCC compared
with the control group (P=0.0004). These findings suggest that
treatment with PCC has inhibitory effects on the proliferation of
colon cancer and pancreas carcinoma cells.
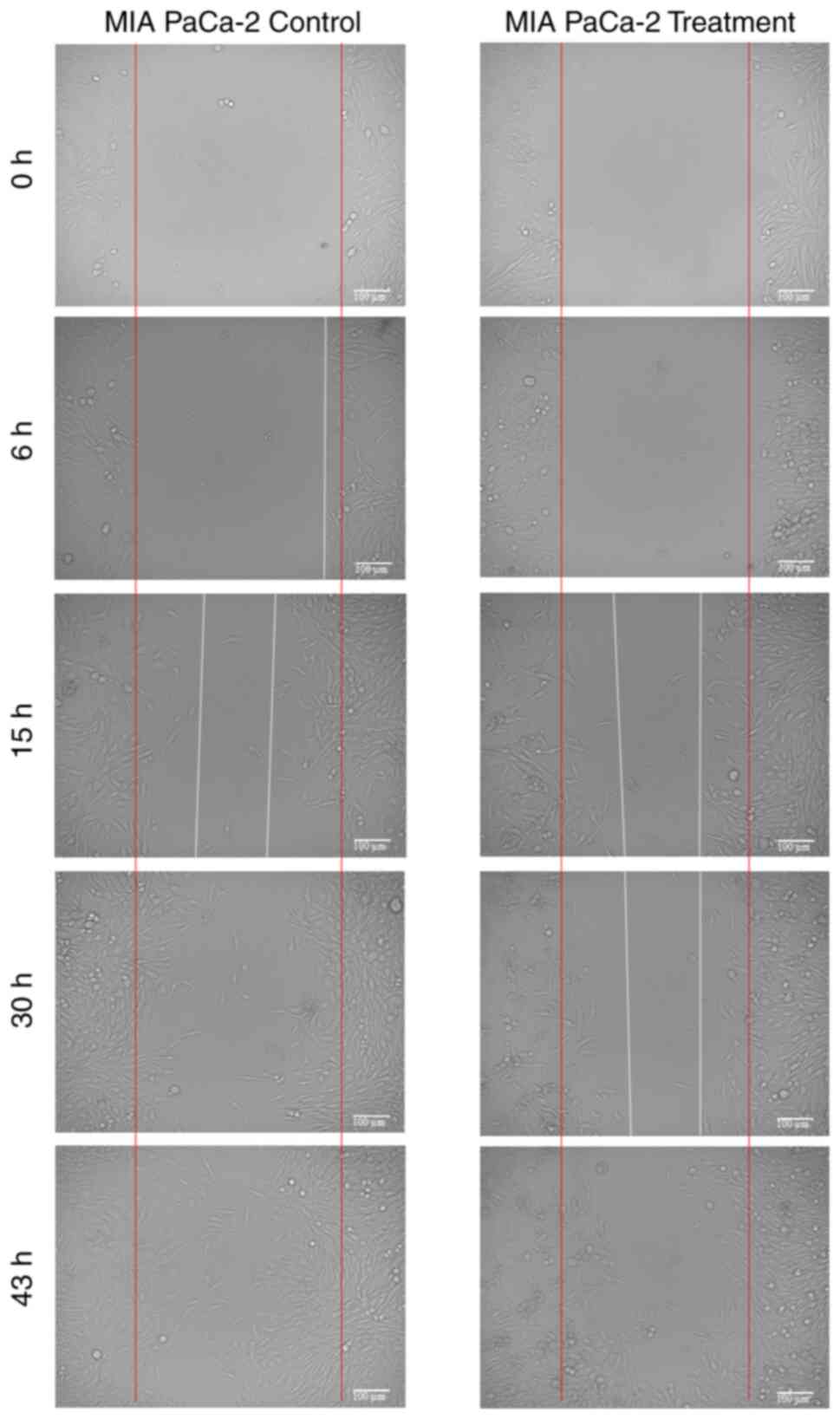
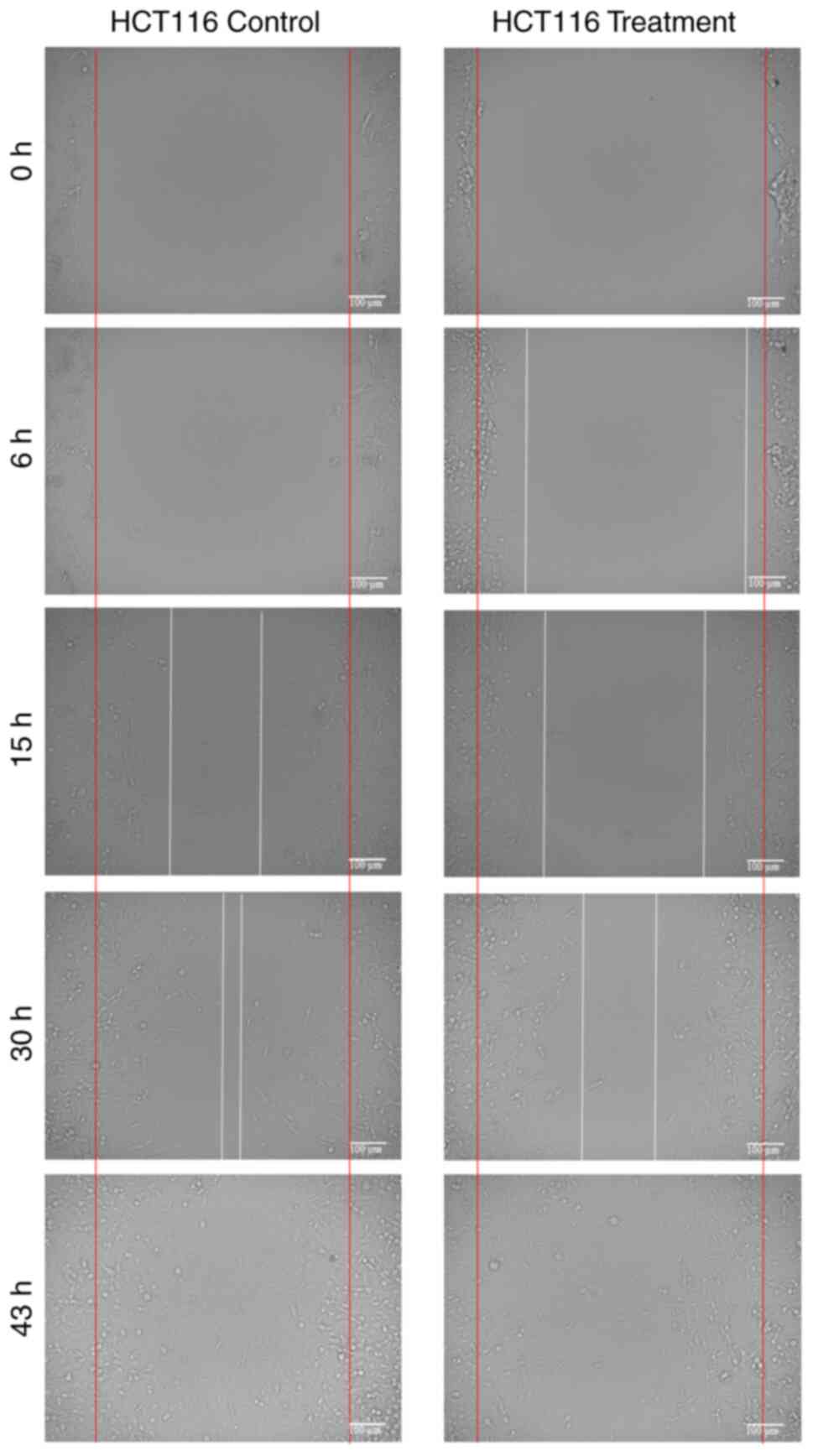
Cell migration
The migration rates of HCT116 and MIA PaCa-2 cells
over different incubation periods are presented in Table I, and representative microscopic
images are shown in Figs. 2 and
3. In both cell lines, treatment
with 1 µg/ml PCC reduced migration compared with the controls,
although the extent and timing of inhibition varied between the two
cell lines.
 | Table I.Migration rate of the wound healing
assay in control or PCC-treated cells. |
Table I.
Migration rate of the wound healing
assay in control or PCC-treated cells.
| Incubation | Migration rate of
MIA PaCa-2 cells (%) | Migration rate of
HCT116 cells (%) |
|---|
| 6 h |
|
|
|
Control | 7.8 | 1.6 |
| 1 µg/ml
PCC | 5.0 | 12.8 |
|
P-value | 0.006 | <0.00014 |
| 15 h |
|
|
|
Control | 46.1 | 43.3 |
| 1 µg/ml
PCC | 43.8 | 30.4 |
|
P-value | 0.549 | <0.0003 |
| 30 h |
|
|
|
Control | 74.0 | 63.8 |
| 1 µg/ml
PCC | 42.3 | 61.2 |
|
P-value | 0.226 | 0.003 |
| 43 h |
|
|
|
Control | 91.7 | 100 |
| 1 µg/ml
PCC | 72.1 | 85.9 |
|
P-value | 0.002 | >0.999 |
For MIA PaCa-2 cells, migration was significantly
reduced in the PCC group at 6 h (5.0 vs. 7.8% P=0.006) and at 43 h
(72.1 vs. 91.7%; P=0.002). However, at 15 and 30 h, the reductions
(43.8 vs. 46.1% and 42.3 vs. 74.0%, respectively) did not reach
statistical significance (P=0.549 and P=0.226, respectively).
For HCT116 cells, PCC treatment resulted in
consistently significant reductions in migration at early and
intermediate time points. At 6 h, migration was markedly higher in
treated cells (12.8 vs. 1.6%; P=0.00014), compared with the control
cells. At 15 and 30 h, PCC-treated cells showed significantly lower
migration compared with the controls (30.4 vs. 43.3%; P=0.0003;
61.2 vs. 63.8%; P=0.003). At 43 h, the control cells had achieved
complete closure (100%), while treated cells reached 85.9%;
however, this difference was not statistically significant
(P>0.999).
Oxidative and antioxidant status
The TOS and TAS levels in HCT116 and MIA PaCa-2
cells under control conditions and after treatment with 1 µg/ml PCC
are presented in Table II. For
HCT116 cells, TOS levels did not differ significantly between the
control (0.302±0.181 µmol H2O2 Eq/l) and
PCC-treated groups (1.253±2.401 µmol H2O2
Eq/l; P=0.7619). However, TAS levels demonstrated a significant
reduction in the PCC-treated group (0.122±0.102 mmol Trolox Eq/l)
compared with the control (2.414±2.39 mmol Trolox Eq/l;
P=0.0095).
 | Table II.TOS and TAS levels in HCT116 and MIA
PaCa-2 cells treated with 1 µg/ml PCC. |
Table II.
TOS and TAS levels in HCT116 and MIA
PaCa-2 cells treated with 1 µg/ml PCC.
| A, HCT116
cells |
|---|
|
|---|
| Group | TOS (µmol
H2O2 Eq/l) | TAS (mmol Trolox
Eq/l) |
|---|
| Control | 0.302±0.181 | 2.414±2.390 |
| 1 µg/ml PCC | 1.253±2.401 | 0.122±0.102 |
|
P-value | 0.762 | 0.01 |
|
| B, MIA PaCa-2
cells |
|
| Group | TOS (µmol
H2O2 Eq/l) | TAS (mmol Trolox
Eq/l) |
|
| Control | 5.090±0.560 | 2.300±0.004 |
| 1 µg/ml PCC | 8.720±2.450 | 0.007±0.00014 |
|
P-value | 0.292 | 0.600 |
For MIA PaCa-2 cells, no significant differences
were observed in either TOS levels (control, 5.09±0.56 µmol
H2O2 Eq/l; PCC-treated, 8.72±2.45 µmol
H2O2 Eq/l; P=0.292) or TAS levels (control,
2.300±0.004 mmol Trolox Eq/l; PCC-treated, 0.007±0.000 mmol Trolox
Eq/l; P=0.686). These results indicate that 1 µg/ml PCC has a
cell-specific effect on oxidative and antioxidant balance; however,
there was a significant decrease in TAS levels increase in TOS
levels in HCT116 and MIA PaCa-2 cell lines.
Expression levels of apoptotic and
cell cycle regulatory markers
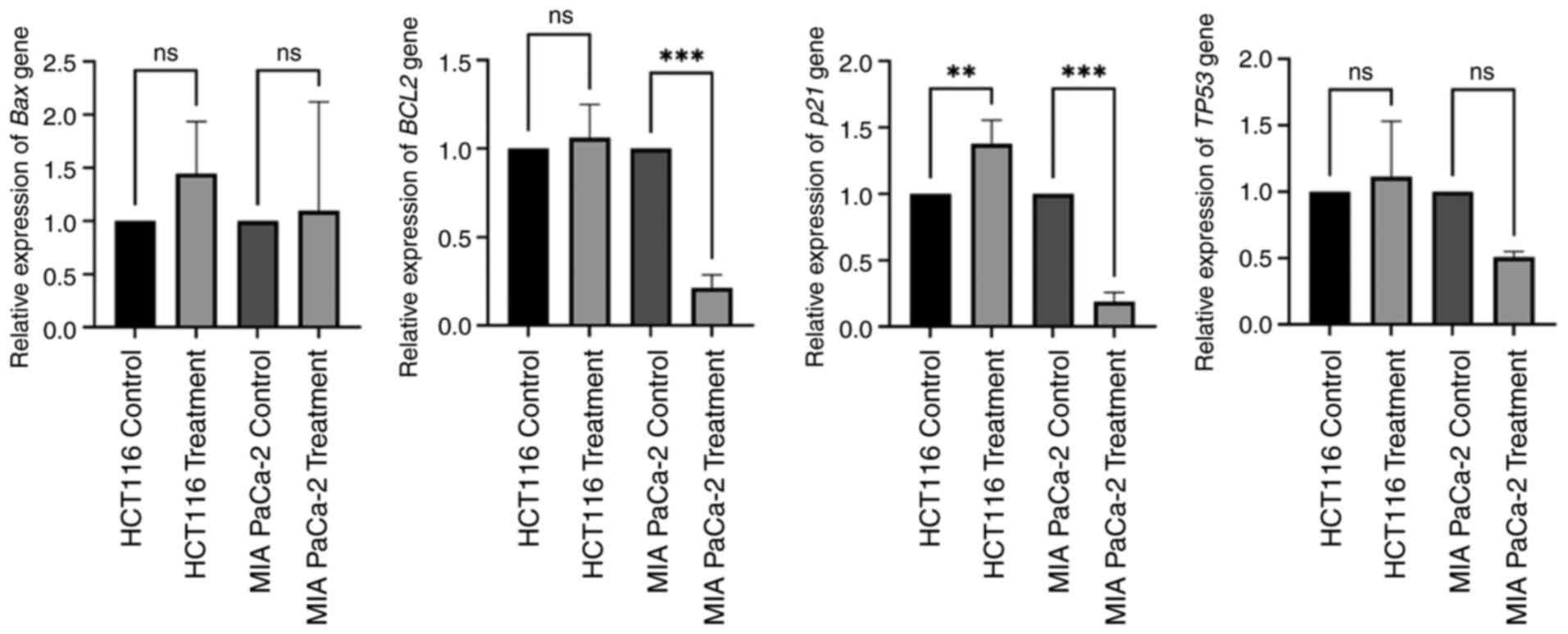
The expression levels of BAX, BCL2, TP53 and
p21 in HCT116 and MIA PaCa-2 cells under control conditions
and after treatment with 1 µg/ml PCC are presented in Fig. 4. In both cell lines, BAX
expression was not significantly altered with PCC treatment
compared with the control. BCL2 expression was significantly
decreased in MIA PaCa-2 cells (P=0.00029), but not in HCT116 cells.
The tumor suppressor marker p21 showed significant
upregulation in HCT116 cells (0.00221) after PCC treatment, but
downregulation in MIA PaCa-2 cells (P=0.00025). TP53
expression did not differ with PCC treatment in both cell lines
compared with the control. These results suggest that 1 µg/ml PCC
caused an apoptotic and cell cycle regulatory effect, affecting
BCL2 and p21 expression levels in different cell
lines. It was observed that pro-apoptotic pathways were activated
in both the HCT116 and MIA PaCa-2 cell lines. This highlights the
potential role of the PCC in modulating apoptosis and cell cycle
regulation.
Discussion
The findings of the present experimental study
demonstrate that the PCC exhibits significant cell-type-specific
effects on cancer cell proliferation, migration, oxidative stress
and the expression of apoptotic and cell cycle regulatory markers.
The observed reduction in cell viability and proliferation in
HCT116 and MIA PaCa-2 cells suggests that PCC treatment selectively
inhibits the growth of colon cancer and pancreas carcinoma cells.
Furthermore, the differential modulation of oxidative and
antioxidant status, with a notable reduction in TAS levels in
HCT116 cells, highlights the potential of PCC to influence cellular
redox balance. The significant reduction in migration observed in
both cell lines, particularly at intermediate time points, further
underscores the potential role of PCC in inhibiting cancer cell
motility and metastatic capacity.
Colorectal cancer is the second leading cause of
cancer-associated mortalities globally (28). While approximately half of patients
with colorectal cancer initially respond positively to conventional
therapies (29), long-term survival
remains poor due to the high likelihood of metastasis following
multimodal treatment (30).
Therefore, alternative therapies are needed. The treatment of
HCT116 colorectal cancer cells with PCC, which includes bioactive
double-hydrolyzed collagens (types I, II, III, V and X) and various
bioactive components (inulin, elderberry extract, magnesium citrate
and malate, eggshell membrane, bromelain, black cumin extract,
liposomal vitamin C), resulted in a significant reduction in cell
proliferation and migration. This inhibitory effect may be
associated with downregulation of anti-apoptotic markers
BCL2 and p21. These findings align with existing
literature indicating that certain bioactive compounds can modulate
apoptotic pathways in colorectal cancer cells (31). For instance, a previous study has
demonstrated that targeting specific molecular pathways can enhance
the expression of pro-apoptotic signals, thereby promoting cancer
cell death (32). Additionally, the
observed modulation of oxidative and antioxidant status, evidenced
by changes in TOS and TAS levels, suggests that components of the
PCC may influence the redox balance within HCT116 cells. This is
consistent with research indicating that alterations in oxidative
stress can impact cancer cell behavior (33,34).
Collectively, these results suggest that PCC exerts its anticancer
effects through the modulation of apoptosis, oxidative stress and
cell migration pathways in HCT116 colorectal cancer cells.
Sato and Seiki (35)
also suggest that hydrolyzed collagen fragments may trigger
intracellular stress responses, potentially explaining the
upregulation of tumor suppressor proteins such as TP53 and
p21 in the present study. According to Madri and Furthmayr
(36), the interaction of collagen
with cellular signaling pathways may affect apoptosis and cell
cycle regulation. Bioactive peptides in the PCC may modulate these
pathways, leading to the observed changes in apoptotic and cell
cycle markers. Blueberry extract, a dietary phytochemical, has been
reported to have a chemo-preventive activity in triple negative
breast cancer cell lines in vitro and in vivo
(37,38). Blueberry reduced cell proliferation
in HCC38, HCC1937 and MDA-MB-231 cells and reduced the metastatic
potential of MDA-MB-231 cells through modulation of the
PI3K/AKT/NFkB pathway. Bilberry treatment decreased MMP-9
activity and urokinase-type plasminogen activator secretion, and
increased tissue inhibitor of MMP-1 and plasminogen
activator inhibitor-1 secretion in MDA-MB-231 cells (38,39).
Minker et al (40) reported
that the proanthocyanidins extracted from 11 berry species
including blueberry may be preventive against human colorectal
cancer cell lines by inducing apoptosis. In line with the
literature, the PCC, which also contains blueberry extract, may
modulate the metastatic features of the cell lines HCT116 and MIA
PaCa-2 with different incubation durations.
Notably, the inhibitory effect on migration,
observed in both HCT116 and MIA PaCa-2 cells at intermediate time
points, aligns with prior studies suggesting that hydrolyzed
collagen fragments can interfere with ECM integrity, integrin
signaling, and MMP activity, and the phytochemicals with anticancer
features. These findings emphasize the complexity of PCC which has
potential role against the cancer progression and highlight the
potential therapeutic value of bioactive compounds in complex, in
selectively modulating cancer cell behavior. Future studies should
explore the mechanistic underpinnings of these effects, with a
focus on optimizing collagen formulations and dosages to enhance
their anticancer efficacy across diverse tumor types.
The findings showed a significant decrease in
BCL2 gene expression in MIA PaCa-2 cells as well as
modulation of p21 expression in both cell types treated with
PCC. These changes indicate that although the collagen complex
regulates p21 gene expression, which serves an important
role in the repair mechanism and the process leading to apoptosis,
it indicates its pro-apoptotic and cell cycle regulatory effect by
decreasing BCL2 gene expression. Mammoto et al
(41) and Payne and Huang (42) describe how specific collagen
interactions can modulate intracellular signaling pathways,
including those regulating apoptosis. Hydrolyzed collagen fragments
in the PCC can disrupt essential survival pathways by interfering
with ECM-integrin signaling, resulting in increased pro-apoptotic
markers. Furthermore, Yin et al (43) identified collagen genes associated
with epithelial-mesenchymal transition and immune infiltration,
highlighting their role in pancreatic carcinoma progression. The
observed decrease in BCL2 expression may indicate that the
PCC modulates stress response pathways associated with these
processes, pushing pancreatic carcinoma cells towards apoptosis
rather than proliferation.
The present study has several limitations that
should be considered. The in vitro design does not fully
replicate the complexity of the tumor microenvironment, including
interactions with immune cells and vascular structures.
Furthermore, only a single dose of PCC was tested, and a broader
dose-response analysis was not performed. The short-term nature of
the experiments does not account for potential long-term effects or
resistance mechanisms. While significant changes in apoptotic and
cell cycle markers were observed, the specific molecular mechanisms
underlying these effects were not explored in detail. Future
studies are warranted to confirm the observed gene expression
changes at the protein and functional levels. Additionally, in
vivo experiments were not performed, which limits the
translational relevance of the results. The study also did not
isolate the contributions of individual bioactive components within
the collagen complex or assess detailed oxidative stress markers
beyond TOS and TAS levels. As the formulation contains multiple
bioactive ingredients, it remains unclear whether the observed
biological effects are due to individual components or synergistic
interactions. Future studies are warranted to investigate the
specific contributions and potential synergistic mechanisms of each
component A more comprehensive migration analysis and consideration
of cell line variability would further strengthen the findings.
Addressing these limitations in future research will provide more
robust and clinically relevant insights.
In conclusion, the findings of the present study
underscore the cell-type-specific effects of PCC on cancer cell
proliferation, migration, oxidative stress and apoptosis. While the
complex demonstrated significant inhibitory effects on the
viability and migration of HCT116 colorectal cancer cells, its
effects on MIA PaCa-2 cells varied, reflecting the distinct
biological characteristics of these cancer types. The pro-apoptotic
and cell cycle-regulatory effects, marked by upregulation of
BCL2 and p21, suggest that PCC may modulate key
molecular pathways to promote apoptosis in certain cancers.
Oxidative and antioxidant modulation, particularly TOS decrease and
TAS increase in cancer cells, highlights the role of collagen in
influencing redox balance.
Acknowledgements
Not applicable.
Funding
Purple Collagen complex is a product developed by Kiperin
Pharmaceutical Food Industry and Trade Limited Company at Biruni
University Technopark with support from the Ministry of Industry
and Technology of the Republic of Türkiye (project support no.
93867). The consumables and reagents used in the study were
provided by Kiperin Pharmaceutical Food Industry and Trade Limited
Company.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LKB served as the project manager, supervised the
study, contributed to the data analysis and interpretation, and
participated in the manuscript writing and editing. CY performed
the statistical analysis, optimized the methodology and provided
critical revisions to the manuscript. NH contributed to data
interpretation, literature review and manuscript editing. LKB and
CY confirm the authenticity of all the raw data. All authors
participated in an independent and impartial evaluation of the
study. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Scientific
Researches Ethical Committee of Biruni University in May 2024 with
an approval number of 2024-BIAEK/01-41. All procedures were
performed in accordance with ethical research guidelines.
Patient consent for publication
Not applicable.
Competing interests
LKB serves as a scientific consultant for Kiperin
Pharmaceutical Food Industry and Trade Limited Company (Istanbul,
Turkey). The authors used Kiperin collagen in the study from
Kiperin Pharmaceutical Food Industry and Trade Limited Company,
although the study was conducted independently, and the company had
no role in the study design, data collection, data analysis,
interpretation of results or manuscript preparation. The other
authors declare that they have no competing interests.
References
|
1
|
Bray F, Laversanne M, Weiderpass E and
Soerjomataram I: The ever-increasing importance of cancer as a
leading cause of premature death worldwide. Cancer. 127:3029–3030.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen S, Cao Z, Prettner K, Kuhn M, Yang J,
Jiao L, Wang Z, Li W, Geldsetzer P, Bärnighausen T, et al:
Estimates and projections of the global economic cost of 29 cancers
in 204 countries and territories from 2020 to 2050. JAMA Oncol.
9:465–472. 2023. View Article : Google Scholar
|
|
3
|
Guida F, Kidman R, Ferlay J, Schüz J,
Soerjomataram I, Kithaka B, Ginsburg O, Vega RB, Galukande M,
Parham G, et al: Global and regional estimates of orphans
attributed to maternal cancer mortality in 2020. Nat Med.
28:2563–2572. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paszek MJ, Zahir N, Johnson KR, Lakins JN,
Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M,
Boettiger D, et al: Tensional homeostasis and the malignant
phenotype. Cancer Cell. 8:241–254. 2005. View Article : Google Scholar
|
|
5
|
Desai N, Sahel D, Kubal B, Postwala H,
Shah Y, Chavda VP, Fernandes C, Khatri DK and Vora LK: Role of the
extracellular matrix in cancer: Insights into tumor progression and
therapy. Adv Ther. 8:24003702025. View Article : Google Scholar
|
|
6
|
Theocharis AD, Skandalis SS, Gialeli C and
Karamanos NK: Extracellular matrix structure. Adv Drug Deliv Rev.
97:4–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winkler J, Abisoye-Ogunniyan A, Metcalf KJ
and Werb Z: Concepts of extracellular matrix remodelling in tumour
progression and metastasis. Nat Commun. 11:51202020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frantz C, Stewart KM and Weaver VM: The
extracellular matrix at a glance. J Cell Sci. 123:4195–4200. 2010.
View Article : Google Scholar
|
|
9
|
Zaffar M and Pradhan A: Assessment of
anisotropy of collagen structures through spatial frequencies of
Mueller matrix images for cervical pre-cancer detection. Appl Opt.
59:1237–1248. 2020. View Article : Google Scholar
|
|
10
|
Song K, Yu Z, Zu X, Li G, Hu Z and Xue Y:
Collagen remodeling along cancer progression providing a novel
opportunity for cancer diagnosis and treatment. Int J Mol Sci.
23:105092022. View Article : Google Scholar
|
|
11
|
Kaur R, Bhardwaj A and Gupta S: Cancer
treatment therapies: Traditional to modern approaches to combat
cancers. Mol Biol Rep. 50:9663–9676. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hiel S, Bindels LB, Pachikian BD, Kalala
G, Broers V, Zamariola G, Chang BPI, Kambashi B, Rodriguez J, Cani
PD, et al: Effects of a diet based on inulin-rich vegetables on gut
health and nutritional behavior in healthy humans. Am J Clin Nutr.
109:1683–1695. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Domínguez R, Zhang L, Rocchetti G, Lucini
L, Pateiro M, Munekata PES and Lorenzo JM: Elderberry (Sambucus
nigra L.) as potential source of antioxidants: Characterization,
optimization of extraction parameters and bioactive properties.
Food Chem. 330:1272662020. View Article : Google Scholar
|
|
14
|
Srinivasan K: Cumin (Cuminum cyminum) and
black cumin (Nigella sativa) seeds: Traditional uses, chemical
constituents, and nutraceutical effects. Food Qual Saf. 2:1–6.
2018. View Article : Google Scholar
|
|
15
|
Morel V, Pickering ME, Goubayon J, Djobo
M, Macian N and Pickering G: Magnesium for pain treatment in 2021?
State of the art. Nutrients. 13:13972021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruff KJ, DeVore DP, Leu MD and Robinson
MA: Eggshell membrane: A possible new natural therapeutic for joint
and connective tissue disorders: Results from two open-label human
clinical studies. Clin Interv Aging. 4:235–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kansakar U, Trimarco V, Manzi MV, Cervi E,
Mone P and Santulli G: Exploring the therapeutic potential of
bromelain: Applications, benefits, and mechanisms. Nutrients.
16:20602024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maurya VK, Shakya A, McClements DJ,
Srinivasan R, Bashir K, Ramesh T, Lee J and Sathiyamoorthi E:
Vitamin C fortification: Need and recent trends in encapsulation
technologies. Front Nutr. 10:12292432023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Republic of Türkiye Ministry of
Agriculture and Forestry, . List of restricted substances for food
supplements. 2025.Available from:. https://www.tarimorman.gov.tr/GKGM/Belgeler/DB_Gida_Isletmeleri/Takviye_Edici_Gidalar_Kisitli_Maddeler_Listesi.pdf
|
|
20
|
Batur LK, Yavas C and Hekim N: Kiperin
double-hydrolyzed collagen as a potential anti-tumor agent: Effects
on HCT116 colon carcinoma cells and oxidative stress modulation.
Curr Issues Mol Biol. 47:3642025. View Article : Google Scholar
|
|
21
|
Huang YP, Yeh CA, Ma YS, Chen PY, Lai KC,
Lien JC and Hsieh WT: PW06 suppresses cancer cell metastasis in
human pancreatic carcinoma MIA PaCa-2 cells via the inhibitions of
p-Akt/mTOR/NF-κB and MMP2/MMP9 signaling pathways in vitro. Environ
Toxicol. 39:2768–2781. 2024. View Article : Google Scholar
|
|
22
|
Abassi H, Ayed-Boussema I, Shirley S, Abid
S, Bacha H and Micheau O: The mycotoxin zearalenone enhances cell
proliferation, colony formation and promotes cell migration in the
human colon carcinoma cell line HCT116. Toxicol Lett. 254:1–7.
2016. View Article : Google Scholar
|
|
23
|
Liu S, Yang W, Li Y and Sun C: Fetal
bovine serum, an important factor affecting the reproducibility of
cell experiments. Sci Rep. 13:19422023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jonkman JEN, Cathcart JA, Xu F, Bartolini
ME, Amon JE, Stevens KM and Colarusso P: An introduction to the
wound healing assay using live-cell microscopy. Cell Adh Migr.
8:440–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Batur LK, Yavas C, Merdan YE and Aygan A:
Kiperin postbiotic supplement-enhanced bacterial supernatants
promote fibroblast function: Implications for regenerative
medicine. Biomedicines. 13:14302025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sevindik M, Akgul H, Bal C, Altuntas D,
Korkmaz AI and Dogan M: Oxidative stress and heavy metal levels of
Pholiota limonella mushroom collected from different regions. Curr
Chem Biol. 12:169–172. 2018. View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI
|
|
29
|
Singh A, Sweeney MF, Yu M, Burger A,
Greninger P, Benes C, Haber DA and Settleman J: TAK1 inhibition
promotes apoptosis in KRAS-dependent colon cancers. Cell.
148:639–650. 2012. View Article : Google Scholar
|
|
30
|
Dienstmann R, Salazar R and Tabernero J:
Personalizing colon cancer adjuvant therapy: Selecting optimal
treatments for individual patients. J Clin Oncol. 33:1787–1796.
2015. View Article : Google Scholar
|
|
31
|
Wu X, Cai J, Zuo Z and Li J: Collagen
facilitates the colorectal cancer stemness and metastasis through
an integrin/PI3K/AKT/Snail signaling pathway. Biomed Pharmacother.
114:1087082019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Turner BTX, Kacem MB, Liu A, Hsieh CER,
Hegde SM and Curran MA: 787 Targeting DNA damage response modulates
antiphagocytic signals to convert radiotherapy into a systemic
immunotherapy to treat metastatic colorectal cancer. J Immuno
Therapy Cancer. 12:2024.
|
|
33
|
Skowronki K, Andrews J, Rodenhiser DI and
Coomber BL: Genome-wide analysis in human colorectal cancer cells
reveals ischemia-mediated expression of motility genes via DNA
hypomethylation. PLoS One. 9:e1032432014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hamidian K, Saberian MR, Miri A, Sharifi F
and Sarani M: Doped and un-doped cerium oxide nanoparticles:
Biosynthesis, characterization, and cytotoxic study. Ceram Int.
47:13895–13902. 2021. View Article : Google Scholar
|
|
35
|
Sato H and Seiki M: Regulatory mechanism
of 92 kDa type IV collagenase gene expression which is associated
with invasiveness of tumor cells. Oncogene. 8:395–405.
1993.PubMed/NCBI
|
|
36
|
Madri JA and Furthmayr H: Collagen
polymorphism in the lung: An immunochemical study of pulmonary
fibrosis. Hum Pathol. 11:353–366. 1980. View Article : Google Scholar
|
|
37
|
Reboredo-Rodríguez P: Potential roles of
berries in the prevention of breast cancer progression. J Berry
Res. 8:307–323. 2018. View Article : Google Scholar
|
|
38
|
Adams LS, Kanaya N, Phung S, Liu Z and
Chen S: Whole blueberry powder modulates the growth and metastasis
of MDA-MB-231 triple-negative breast tumors in nude mice. J Nutr.
141:1805–1812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fang M, Yuan J, Peng C and Li Y: Collagen
as a double-edged sword in tumor progression. Tumour Biol.
35:2871–2882. 2014. View Article : Google Scholar
|
|
40
|
Minker C, Duban L, Karas D, Järvinen P,
Lobstein A and Muller CD: Impact of procyanidins from different
berries on caspase 8 activation in colon cancer. Oxid Med Cell
Longev. 2015:1541642015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mammoto T, Jiang A, Jiang E, Panigrahy D,
Kieran MW and Mammoto A: Role of collagen matrix in tumor
angiogenesis and glioblastoma multiforme progression. Am J Pathol.
183:1293–1305. 2013. View Article : Google Scholar
|
|
42
|
Payne LS and Huang PH: The pathobiology of
collagens in glioma. Mol Cancer Res. 11:1129–1140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yin W, Zhu H, Tan J, Xin Z, Zhou Q, Cao Y,
Wu Z, Wang L, Zhao M, Jiang X, et al: Identification of collagen
genes related to immune infiltration and epithelial-mesenchymal
transition in glioma. Cancer Cell Int. 21:2762021. View Article : Google Scholar
|