Introduction
Gallbladder cancer (GBC) is regarded as the most
common biliary malignancy, accounting for 80–95% of all biliary
malignancies worldwide (1). The
average overall survival of patients with GBC is 6 months, and the
5-year survival rate is 5% (2).
Radical resection remains the only curative approach for GBC, with
postoperative radiotherapy and chemotherapy routinely given.
However, there still exists >1/3 chance of metastasis and
recurrence after surgery (3,4). Once
GBC recurs, whether it is local recurrence or distant metastasis,
the 5-year overall survival rate of patients remains notably low at
just 15–20% (5).
For advanced patients with multiple distant
metastases, surgical resection does not meet the needs of the
patients. Patients with poor general condition are also unable to
tolerate chemotherapy (6,7). Minimally invasive interventional
therapy is characterized by minimal trauma and accurate
positioning, which is suitable for these patients. In several
cases, studies have focused on the local efficacy of interventional
therapy, but have ignored the systemic immune response caused by
interventional therapy (8,9). Only sporadic reports have shown the
efficacy of thermal ablation in achieving distant or systemic
responses (10–13). Microwave ablation (MWA) can kill
tumor cells in situ, activate the immune system of the body
and exert an immune attack on distant metastases, which is
consistent with the concept of the tumor in situ vaccine
(14). However, it is reported that
the immune response induced by it is relatively weak, which is
affected by the immunosuppressive microenvironment, and the effect
is not sustainable (15).
Radioactive particles can change the tumor microenvironment (TME)
from an immunosuppressive state to an immune activation state
(16,17).
The present study reports a case of a female patient
with advanced GBC whose disease course extended >5 years from
the initial diagnosis in order to validate the potential of
local-only therapy for metastatic GBC and investigate the immune
dynamics enabling durable remission. During the disease course
time, new metastatic tumors occurred several times, and the patient
underwent sequential treatment, including five rounds of ultrasound
(US)-guided microwave ablation (MWA) and one occurrence of
radioactive iodine-125 (125I) seed implantation
(RSI).
Case report
Case presentation
In 2018, a 60-year-old woman presented with
recurrent epigastric pain for 6 months. US was performed at
Zhenjiang Hospital of Chinese Traditional and Western Medicine
(Zhenjiang, China) and showed the gallbladder was almost filled
with an heterogeneous isoechoic mass, meeting the criteria for
high-risk polyp resection. Therefore, although the nature of the
mass was not clear, early intervention was needed. The patient
underwent laparoscopic cholecystectomy, with final pathology
showing poorly differentiated adenocarcinoma of the gallbladder,
with a size of 1.5×0.8×0.6 cm invading the whole layer, and a small
amount of atypical glands at the margin of the bile neck (Fig. 1A). According to tumor, lymph node
and metastasis (TNM) staging, it was T3NxMO and belonged to stage
IIa, with immunohistochemistry analysis of tissue samples showing
cytokeratin (CK; +), CK18 (+; Fig.
1B), Ki-67 (+, 30%) and α-fetoprotein (AFP; -) (Fig. S1A).
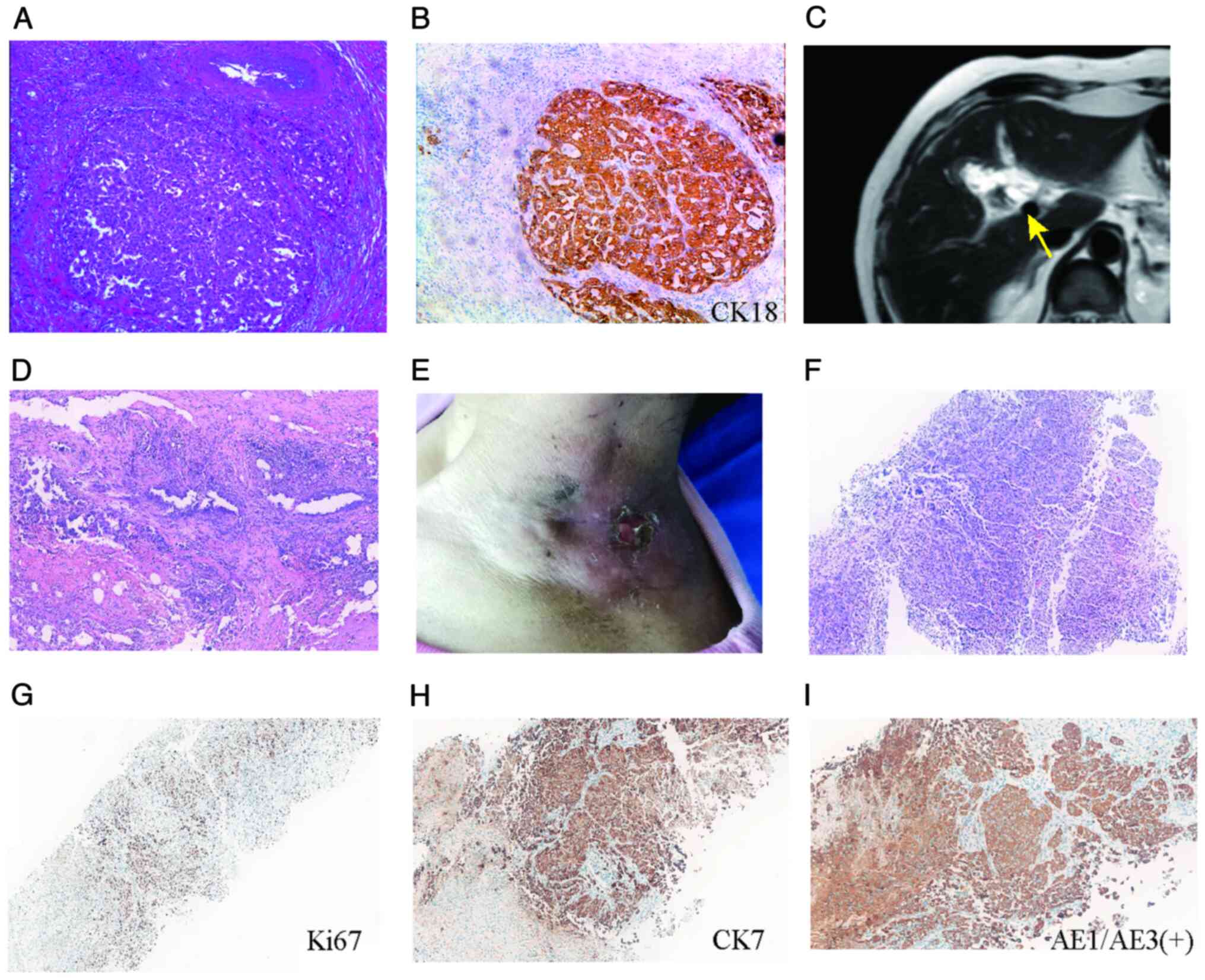 | Figure 1.Diagnostic and pathological
progression of gallbladder cancer. (A) Laparoscopic cholecystectomy
specimen showing poorly differentiated adenocarcinoma (H&E).
(B) IHC of primary tumor with positive CK18 confirming epithelial
origin. Magnification, ×400×. (C) Preoperative MRI showing
irregular gallbladder wall thickening (arrow) with adjacent hepatic
infiltration, suggesting residual malignancy. (D) Radical resection
specimen of residual poorly differentiated adenocarcinoma in
gallbladder bed (H&E, 200× magnification). (E) Clinical
presentation showing left neck mass (8×8 cm) with surface
ulceration prior to treatment. (F) Core-needle biopsy of neck mass
demonstrated malignant epithelial cells (H&E), consistent with
metastatic carcinoma. IHC of neck metastasis showing (G) Ki-67 (+,
75%) indicative of a high proliferative index, (H) CK7 (+),
indicating biliary differentiation and (I) AE1/AE3 (+) indicating
pan-cytokeratin confirmation of epithelial lineage. Magnification,
×100. IHC, immunohistochemistry; CK, cytokeratin; AE1/AE3,
cytokeratin AE1/AE3; H&E, hematoxylin and eosin. |
For definitive management, the patient was referred
to Affiliated Drum Tower Hospital, Medical School of Nanjing
University (Nanjing, China). Preoperative MRI demonstrated
irregular gallbladder wall thickening with adjacent hepatic
infiltration, suggesting residual malignancy (Fig. 1C). Radical cholecystectomy was
performed, with pathology confirming residual poorly differentiated
adenocarcinoma in the gallbladder bed (Figs. 1D and S1B). After surgery, the patient declined
chemoradiotherapy.
In September 2020, the patient presented to the
Department of Medical Ultrasound (Affiliated Hospital of Jiangsu
University) with the chief complaint of a large mass on their left
neck for 3 months. A physical examination revealed an 8×8 cm mass
in the left neck, with skin ulcers visible on its surface (Fig. 1E).
To make a definite diagnosis, a core-needle biopsy
of the left neck mass was performed. The pathological result
indicated malignant epithelial cells (Fig. 1F), possibly originating from the
gallbladder, demonstrated using immunohistochemistry analysis of
cytokeratin AE1/AE3 (+), vimentin (−), transcription termination
factor 1 (TTF-1; -), thyroglobulin (TG; -), CK5/6 (−), CK7 (+),
CK20 (+), caudal type homeobox 2 (CDX-2; -), Ki-67 (+, 75%)
(Figs. 1G-I and S1C). The Ki-67 proliferative index was
quantitatively assessed by a pathologist by visually estimating the
percentage of positive tumor nuclei in representative high-power
fields, according to standard diagnostic protocols.
The diagnosis of metastatic GBC was solidified by
systematically excluding other primary sites. The
immunohistochemical profile, notably CK7/CK20 positivity indicating
biliary origin, coupled with negative markers for lung (TTF-1),
thyroid (TG), colorectal (CDX-2) and squamous carcinomas (CK5/6),
provided definitive cellular evidence against alternative
primaries. Immunohistochemical staining was performed on 4-μm-thick
formalin-fixed paraffin-embedded sections using standard diagnostic
protocols. Antigen retrieval involved citrate buffer incubation,
followed by primary antibody incubation (Jiangsu Lanou Biological
Material Co., Ltd.), polymer-HRP detection with DAB chromogen and
hematoxylin counterstain. This molecular characterization was
reinforced by the patient's established history of GBC resection 2
years prior. Regional imaging using physical examination and
targeted ultrasonography of the neck/axilla revealed no evidence of
new primary lesions. Although comprehensive whole-body PET/CT was
not performed due to the palliative treatment focus, the
convergence of pathological, clinical and localized radiological
findings confirmed metastatic GBC as the sole etiology.
Treatment
After consulting with multidisciplinary experts at
the Affiliated Hospital of Jiangsu University, the application of
US-guided MWA for neck mass was considered as a palliative therapy
for alleviating the patient's condition. Written informed consent
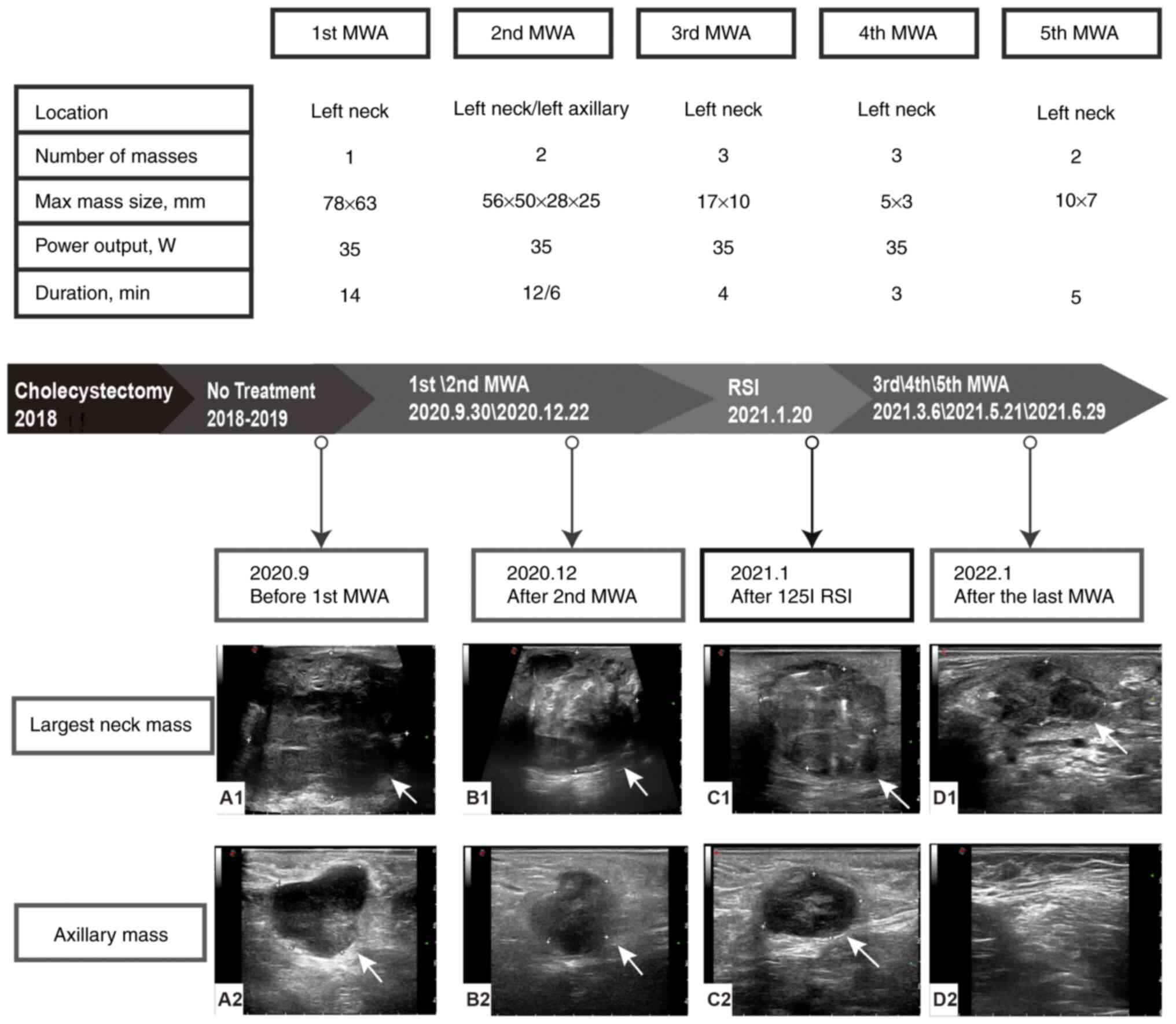
was obtained from the patient before MWA. Fig. 2 shows the specific treatment
timelines and details regarding the patient's metastases. Arrows
indicate the location of the mass vs. the ablation foci.
In September 2020, the patient underwent US-guided
MWA treatment of the left neck masses. Preoperative US showed that
the size of the left neck mass was 78×63×57 mm, with a clear
boundary and slightly irregular edge. The left common carotid
artery and internal jugular vein were notably compressed (Fig. 2A). An ECO-100A1 microwave
therapeutic system (Nanjing ECO Medical Technology Co., Ltd.)
consisting of a microwave generator, a hollow water-cooled shaft
antenna (16 gauge) and a flexible coaxial cable was employed. The
whole ablation process was performed under the guidance of US. A
total of 10 ml 2% lidocaine was injected at the puncture site for
local infiltration anesthesia, followed by 20 ml saline to isolate
and protect the surrounding tissue. The needle pin was inserted
into the lesion through previously determined path, and ablation
was performed using a continuous method, with a power output of 35
W. The ablation continued until the transient hyperechoic zone
covered the entire mass. The whole procedure lasted 14 min and 8
sec. The procedure was smooth, and the patient had no obvious
abnormal symptoms.
In December 2020, the patient underwent the second
MWA treatment for both left neck and left axillary masses.
Preoperative US showed that the size of the left axillary mass was
28×25 mm, and the left neck mass had enlarged to 56×50 mm. The
intraoperative power output was 35 W. The ablation time for the
left axillary mass was 5 min and 47 sec, and for the left neck
mass, it was 12 min and 30 sec. The levels of postoperative tumor
indicators, measured using electrochemiluminescence immunoassay
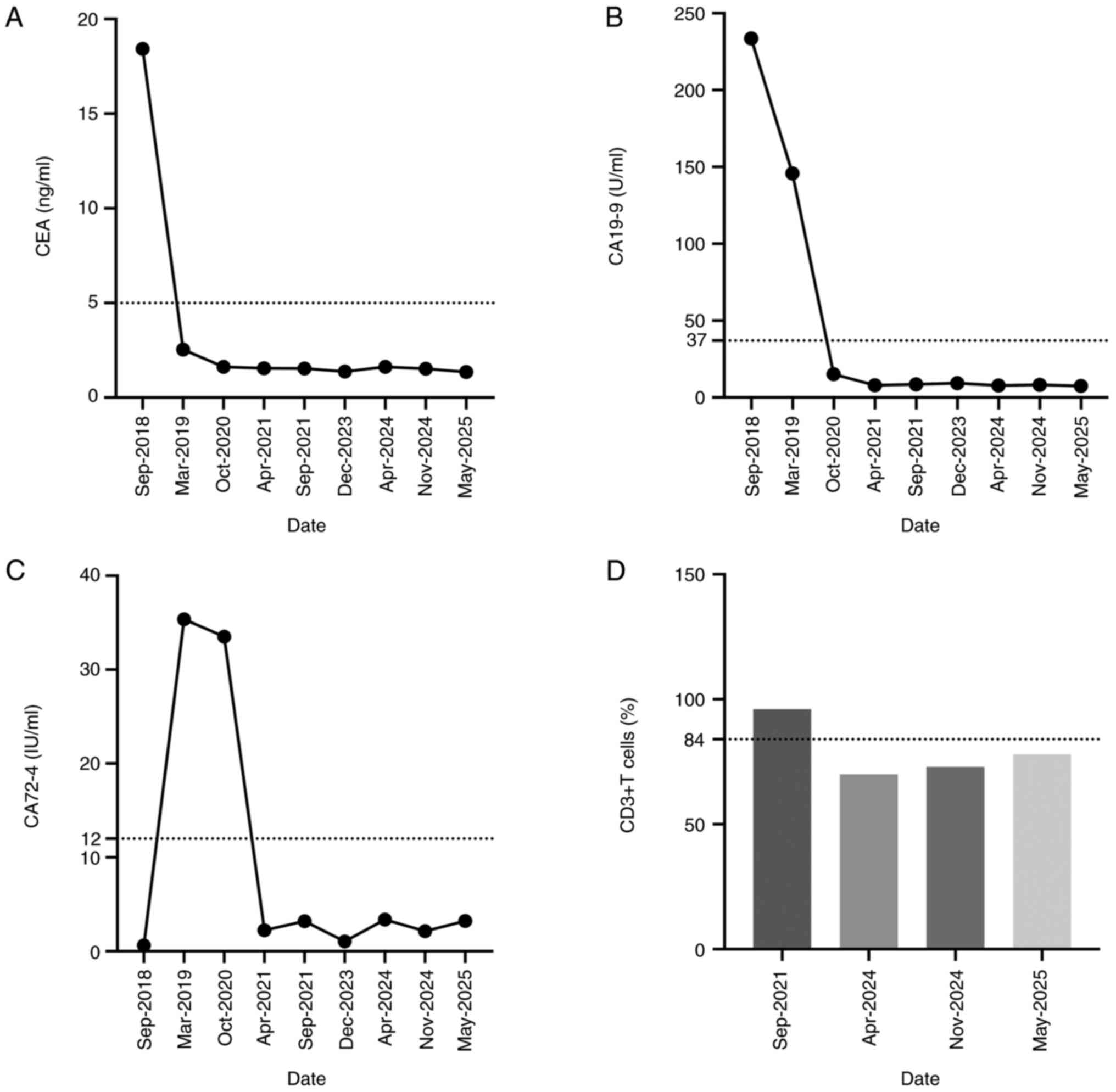
were: Carcinoembryonic antigen (CEA), 1.62 ng/ml; carbohydrate
antigen CA19-9, 15.11 U/ml; and CA72-4, 33.51 IU/ml (Fig. 3A-C).
After 1 week, a follow-up US revealed an ablation
focus measuring 22×18 mm in the left axilla and 56×51 mm in the
left neck, both with clear margins (Fig. 2B). Contrast-enhanced US (CEUS)
demonstrated no enhancement in the early or late phases of
enhancement in the axillary and neck masses. A 1-month follow-up US
revealed the presence of a newly discovered mass, measuring ~33×35
mm in size, located just superior to the left neck ablation
foci.
In January 2021, due to the continuous new
occurrence and substantial volume of the metastasis tumors, MWA
alone was considered insufficient for inactivation. To achieve
further disease control, the patient underwent 125I RSI
for left neck masses. This decision balanced the lesion's critical
location abutting the carotid artery (Fig. 2A), the patient's explicit
chemotherapy refusal and the patient's frailty. RSI is not a
first-line treatment option for metastatic disease, but it has been
shown to be promising for refractory oligometastatic cases in
clinical practice (18).
Preoperative US identified two hypoechoic masses on the left neck,
measuring 54×40 mm and 40×32 mm, with CEUS showing partial necrosis
in the larger mass. A total of 58 125I particles
(physical dimension, 0.6 mm diameter; activity, 0.5 millicurie per
seed) were evenly distributed to the active region of the larger
mass, and 72 particles were evenly distributed to the other
mass.
A 1-month follow-up US revealed a reduction in the
size of the axillary ablation foci. The two neck masses had
decreased in size compared with previous measurements, displaying
clear margins and heterogeneous internal echoes with scattered
strong echogenic particles (Fig.
2C).
After 3 months, tumor indicators levels were as
follows: CEA, 1.55 ng/ml; CA19-9, 7.88 U/ml; and CA72-4, 2.24
IU/ml. All were within the normal range (Fig. 3A-C).
In March, April and June 2021, the patient underwent
the third, fourth and fifth sessions of MWA for neck masses,
respectively, targeting the residual active areas of the left neck
mass and suspicious lymph nodes for repeat ablation.
Outcome and follow-up
Following the last ablation procedure, the patient
returned to the hospital for follow-up at 3, 6, 30, 36, 42 and 47
months. The latest examination results were as follows: Physical
examination revealed smooth skin without redness or swelling and US
indicated the ablation foci had gradually absorbed and disappeared
completely (Fig. 2D). The tumor
markers remained within normal ranges. Flow cytometric analysis of
T cell subsets indicated that CD3+ levels consistently
remained high 2 years postoperatively (Fig. 3D).
Discussion
Due to the large number of metastatic tumors and the
high tendency for recurrence, traditional surgical treatments
struggle to completely eradicate the advanced cancer. This has
become a notable challenge in treatment and a leading cause of
patient mortality, highlighting the refractory nature of metastatic
cancer. Consequently, the focus of treatment and research for
advanced metastatic GBC has shifted to systemic therapies. Among
these, the GemCis chemotherapy protocol, which combines gemcitabine
and cisplatin, has been widely adopted internationally (1). However, a notable number of patients
with advanced cancer choose to refrain from chemotherapy in
clinical practice due to physical and mental distress, economic
pressure and other reasons, instead choosing palliative
interventional therapies which are low-cost. In the present case,
the patient rejected postoperative adjuvant radiotherapy or
chemotherapy, and finally developed distant metastasis. The team
offered only local palliative treatment for the metastatic mass of
the patient, which unexpectedly achieved long-term systemic
remission. Interventional therapy can not only destroy local tumor
tissue but also activate the immune system of the body, which in
turn has a certain immune attack on the distant metastasis, being
consistent with the concept of in situ tumor vaccine. Based
on a comprehensive literature review (PubMed, Web of Science and
EMBASE up to July 2025), no prior case reports or studies have
specifically documented the use of sequential MWA combined with
¹25I-RSI for metastatic GBC.
MWA is a technique that directly utilized thermal
energy to kill tumor cells in situ (19). Previous research has demonstrated
that thermal ablation can induce abscopal effects (9). This is a rare clinical response that
refers to tumor regression or growth at a site distant from
ablation or irradiation site. The abscopal response resulting from
MWA is seldom reported. However, in the present case, the patient
did not receive any systemic therapy prior to metastasis and had a
long postoperative follow-up period of 30 months. Thus, the
immunological mechanism of the abscopal effect warrants discussion.
Natural killer cells, which are effector lymphocytes of the innate
immune system and part of the first line of defense against cancer,
can be activated by MWA to promote the destruction of tumor cells
(20). Subsequently, residual
antigens remain in the tumor site (21). These antigens can release
inflammatory mediators, damage associated molecular patterns, and
immune regulatory factors (such as IL-1, IL-6 and HSP70) (22). Dendritic cells (DCs) phagocytose
antigens, present them on major histocompatibility complex
molecules and display co-stimulatory factors, thereby stimulating T
cells to generate adaptive immune responses and change the TME for
antitumor effects (9). In the
peripheral blood, a previous study reported that B cells are
notable antigen-presenting cells in the MWA-induced systemic
response (23).
In the present case, the patient was also treated
with a single RSI due to the repeated progression of the disease.
This treatment approach could create a favorable immune environment
for tumor in situ vaccines. Radioactive 125I
seeds are implanted into tumors to emit large doses of γ rays at
close range to destroy tumor cells without damaging normal tissues
(24,25). RSI is increasingly used in the local
treatment of tumors and has been utilized in the treatment of
various recurrent and refractory tumors (26–30).
RSI can trigger immunogenic tumor cell death. Tumor cells express
molecules that promote phagocytosis of cancer cells by surface DCs,
such as calreticulin, and release molecules that trigger ingested
antigen processing and cross-presentation, such as high mobility
group box 1 protein. This process promotes DC cross-presentation of
tumor antigens, altering the TME and generating antitumor immune
responses (31). A previous study
showed that RT can induce pyroptosis in tumor cells, resulting in
the release of cellular contents from tumor cells, causing an
inflammatory response and activating the immune system (32). RT can also induce abscopal effects.
Siva et al (33) reported 10
cases of abscopal effects after radiotherapy, and their median
duration was 21 months (range, 3–54 months). While RSI demonstrates
notable potential, its physical application faces inherent
constraints in metastatic settings. The technique is primarily
suited for limited, accessible lesions due to technical challenges
in precise seed placement within numerous or sub-centimeter
metastases, with risks of migration and adjacent tissue damage
(25,28). For cervical applications similar to
the present case, radiation damage to adjacent nerves or vessels
remains a concern (27). In the
present case, however, US-guided precision prevented such
complications, underscoring its importance for high-risk
anatomy.
MWA can be utilized as an in situ tumor
vaccine to a certain extent, but due to the relatively weak
response induced by it, it needs to be combined with other
treatment methods. In the present case, initial MWA achieved
partial control, but disease progression necessitated combined RSI.
However, after 125I RSI treatment, the notable effect
was achieved, and the subsequent ablation effect was greatly
enhanced. We hypothesize that the TME was transformed from an
immunosuppressive state to an immune activation state after RSI,
which provided a favorable immune environment for tumor treatment.
While immune checkpoint dynamics (such as programmed cell death
protein 1/programmed death-ligand 1) were not assessed in the
present study, their role in modulating abscopal responses warrants
investigation in future GBC studies (34).
Furthermore, the patient's peripheral blood T cell
subsets demonstrated a continuous increase in CD3+
levels. Notably, the CD3+ T-cell elevation persisted for
>30 months, substantially exceeding the typical duration of
acute inflammatory responses to cellular debris. Its temporal
association with complete clinical remission of all metastatic
lesions (47 months) and sustained normalization of tumor markers
strongly argues against non-specific inflammation (22), instead supporting a systemic
adaptive immune response. These findings align with the paradigm of
adaptive immune memory induced by in situ vaccination
(35,36), whereby localized tumor destruction
(via MWA/RSI) releases tumor-associated antigens, reprograms the
immunosuppressive microenvironment (16,31)
and potentiates sustained immunosurveillance mediated by
antigen-experienced T cells. While definitive proof of
tumor-specific immunity (such as T cell receptor sequencing or
cytotoxic assays) is lacking and remains unavailable due to the
retrospective nature of the present study, three concordant
observations strongly suggest abscopal immunity mediated by in
situ vaccination: i) Sustained CD3+ lymphocytosis;
ii) unprecedented disease control >47 months; and iii) durable
normalization of serum tumor markers. This clinical-immunological
profile provides compelling evidence consistent with long-term
immune memory formation.
The translation importance of the present case is
threefold: i) It challenges the therapeutic nihilism for advanced
GBC by demonstrating that sequential local interventions alone can
achieve durable systemic remission, offering hope for
chemotherapy-ineligible patients; ii) it provides the first
clinical evidence that combining RSI (radiotherapy) with MWA
synergistically overcomes immunosuppression, effectively creating
an in situ vaccine against metastatic GBC; and iii) It
establishes a foundation for minimally invasive strategies to
convert palliative care into curative intent, with 47-month
disease-free survival exceeding historical medians by >300%.
However, as a single-case report, the present study lacks
statistical power to establish causal relationships. The
exceptional response observed may be influenced by unique patient
biology. Comprehensive immune profiling was not available. Future
prospective trials are needed to validate the findings.
The patient's entire disease course lasted 5 years
and 4 months, during which recurrence and metastasis occurred.
Based on the patient's poor condition, a sequential therapy
consisting of multiple local interventions was precisely and
appropriately employed, which ultimately resulted in a favorable
prognosis. In conclusion, while the present single case cannot
confirm efficacy, it provides clinical rationale for exploring
combined ablation-RSI as an immune-activating strategy in
metastatic GBC. Palliative interventional therapy may induce a
systemic effect and can enhance the survival rate of patients with
advanced multiple metastatic cancer. The mechanism of the systemic
antitumor immune response induced by such local treatments merits
further investigation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by National Science Foundation
for Young Scientists of China (grant no. 82302208), Medical
Research Project of Jiangsu Provincial Health Commission (grant no.
H2023141), Social Development Program of Zhenjiang City (grant nos.
SH2023015 and SH2023019) and Medical Education Collaborative
Innovation Fund of Jiangsu University (grant nos. JDY2023005 and
JDY2023012).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WL made substantial contributions to the conception
of the work, analyzed and interpreted the patient data, and drafted
the manuscript. YC, SZ and MA contributed to the acquisition and
analysis of clinical data. HZ and YC contributed to the study
design and methodology. JB and JQ contributed to the data analysis
and interpretation. BC conceived and supervised the study,
critically revised the manuscript for important intellectual
content, and approved the final version to be published. WL and JB
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of any potentially identifiable images
or data included in the present article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roa JC, García P, Kapoor VK, Maithel SK,
Javle M and Koshiol J: Gallbladder cancer. Nat Rev Dis Primers.
8:692022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levy AD, Murakata LA and Rohrmann CA Jr:
Gallbladder carcinoma: Radiologic-pathologic correlation.
Radiographics. 21:295–314. 549–555. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.
|
|
4
|
Margonis GA, Gani F, Buettner S, Amini N,
Sasaki K, Andreatos N, Ethun CG, Poultsides G, Tran T, Idrees K, et
al: Rates and patterns of recurrence after curative intent
resection for gallbladder cancer: A multi-institution analysis from
the US extra-hepatic biliary malignancy consortium. HPB (Oxford).
18:872–878. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jarnagin WR, Ruo L, Little SA, Klimstra D,
D'Angelica M, DeMatteo RP, Wagman R, Blumgart LH and Fong Y:
Patterns of initial disease recurrence after resection of
gallbladder carcinoma and hilar cholangiocarcinoma: Implications
for adjuvant therapeutic strategies. Cancer. 98:1689–1700. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun J, Xie TG, Ma ZY, Wu X and Li BL:
Current status and progress in laparoscopic surgery for gallbladder
carcinoma. World J Gastroenterol. 29:2369–2379. 2023. View Article : Google Scholar
|
|
8
|
Campbell WA IV and Makary MS: Advances in
image-guided ablation therapies for solid tumors. Cancers (Basel).
16:25602024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Slovak R, Ludwig JM, Gettinger SN, Herbst
RS and Kim HS: Immuno-thermal ablations-boosting the anticancer
immune response. J Immunother Cancer. 5:782017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bäcklund M and Freedman J: Microwave
ablation and immune activation in the treatment of recurrent
colorectal lung metastases: A case report. Case Rep Oncol.
10:383–387. 2017. View Article : Google Scholar
|
|
11
|
Xu H, Sun W, Kong Y, Huang Y, Wei Z, Zhang
L, Liang J and Ye X: Immune abscopal effect of microwave ablation
for lung metastases of endometrial carcinoma. J Cancer Res Ther.
16:1718–1721. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao C, Yang M, Pan Y, Xie D, Chen B, Ren
S and Zhou C: Case report: Abscopal effect of microwave ablation in
a patient with advanced squamous NSCLC and resistance to
immunotherapy. Front Immunol. 12:6967492021. View Article : Google Scholar
|
|
13
|
Jiang G, Song C, Xu Y, Wang S, Li H, Lu R,
Wang X, Chen R, Mao W and Zheng M: Recurrent lung adenocarcinoma
benefits from microwave ablation following multidisciplinary
treatments: A case with long-term survival. Front Surg.
9:10382192023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saxena M, van der Burg SH, Melief CJM and
Bhardwaj N: Therapeutic cancer vaccines. Nat Rev Cancer.
21:360–378. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He N and Jiang J: Contribution of immune
cells in synergistic anti-tumor effect of ablation and
immunotherapy. Transl Oncol. 40:1018592024. View Article : Google Scholar
|
|
16
|
Takashima ME, Berg TJ and Morris ZS: The
effects of radiation dose heterogeneity on the tumor
microenvironment and anti-tumor immunity. Semin Radiat Oncol.
34:262–271. 2024. View Article : Google Scholar
|
|
17
|
Zeng P, Shen D, Shu W, Min S, Shu M, Yao
X, Wang Y and Chen R: Identification of a novel peptide targeting
TIGIT to evaluate immunomodulation of 125I seed
brachytherapy in HCC by near-infrared fluorescence. Front Oncol.
13:11432662023. View Article : Google Scholar
|
|
18
|
Wang H, Shi HB, Qiang WG, Wang C, Sun B,
Yuan Y and Hu WW: CT-guided radioactive 125I seed
implantation for abdominal incision metastases of colorectal
cancer: Safety and efficacy in 17 patients. Clin Colorectal Cancer.
22:136–142. 2023.PubMed/NCBI
|
|
19
|
Chu KF and Dupuy DE: Thermal ablation of
tumours: Biological mechanisms and advances in therapy. Nat Rev
Cancer. 14:199–208. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu M, Pan H, Che N, Li L, Wang C, Wang Y,
Ma G, Qian M, Liu J, Zheng M, et al: Microwave ablation of primary
breast cancer inhibits metastatic progression in model mice via
activation of natural killer cells. Cell Mol Immunol. 18:2153–2164.
2021. View Article : Google Scholar
|
|
21
|
den Brok MH, Sutmuller RP, van der Voort
R, Bennink EJ, Figdor CG, Ruers TJ and Adema GJ: In situ tumor
ablation creates an antigen source for the generation of antitumor
immunity. Cancer Res. 64:4024–4029. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmad F, Gravante G, Bhardwaj N,
Strickland A, Basit R, West K, Sorge R, Dennison AR and Lloyd DM:
Changes in interleukin-1β and 6 after hepatic microwave tissue
ablation compared with radiofrequency, cryotherapy and surgical
resections. Am J Surg. 200:500–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou W, Yu M, Mao X, Pan H, Tang X, Wang
J, Che N, Xie H, Ling L, Zhao Y, et al: Landscape of the peripheral
immune response induced by local microwave ablation in patients
with breast cancer. Adv Sci (Weinh). 9:e22000332022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang F, Wu J, Hong L, Zhang P and Song J:
Iodine-125 seed inhibits proliferation and promotes apoptosis of
cholangiocarcinoma cells by inducing the ROS/p53 axis. Funct Integr
Genomics. 24:1142024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu W, Du P, Yang C, Jiang F, Xie P, Yang
J, Zhang Z and Ma J: The effect of computed tomography-guided
125I radioactive particle implantation in treating
cancer and its pain. Cancer Biother Radiopharm. 33:176–181.
2018.PubMed/NCBI
|
|
26
|
Sugawara A, Nakashima J, Kunieda E, Nagata
H, Mizuno R, Seki S, Shiraishi Y, Kouta R, Oya M and Shigematsu N:
Incidence of seed migration to the chest, abdomen, and pelvis after
transperineal interstitial prostate brachytherapy with loose (125)I
seeds. Radiat Oncol. 6:1302011. View Article : Google Scholar
|
|
27
|
Zhang G, Wu Z, Yu W, Lyu X, Wu W, Fan Y,
Wang Y, Zheng L, Huang M, Zhang Y, et al: Clinical application and
accuracy assessment of imaging-based surgical navigation guided
125I interstitial brachytherapy in deep head and neck regions. J
Radiat Res. 63:741–748. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ji Z, Ni Y, He C, Huo B, Liu S, Ma Y, Song
Y, Hu M, Zhang K, Wang Z, et al: Clinical outcomes of radioactive
seed brachytherapy and microwave ablation in inoperable stage I
non-small cell lung cancer. Am J Cancer Res. 13:3753–3762.
2023.PubMed/NCBI
|
|
29
|
Chen L, Zhu GY, Jin ZC, Zhong BY, Wang Y,
Lu J, Pan T, Teng GJ and Guo JH: Efficacy and safety of radioactive
125I seed implantation for patients with
oligo-recurrence soft tissue sarcomas. Cardiovasc Intervent Radiol.
45:808–813. 2022. View Article : Google Scholar
|
|
30
|
Zaorsky NG, Davis BJ, Nguyen PL, Showalter
TN, Hoskin PJ, Yoshioka Y, Morton GC and Horwitz EM: The evolution
of brachytherapy for prostate cancer. Nat Rev Urol. 14:415–439.
2017. View Article : Google Scholar
|
|
31
|
Charpentier M, Spada S, Van Nest SJ and
Demaria S: Radiation therapy-induced remodeling of the tumor immune
microenvironment. Semin Cancer Biol. 86:737–747. 2022. View Article : Google Scholar
|
|
32
|
Cao W, Chen G, Wu L, Yu KN, Sun M, Yang M,
Jiang Y, Jiang Y, Xu Y, Peng S and Han W: Ionizing radiation
triggers the antitumor immunity by inducing gasdermin E-mediated
pyroptosis in tumor cells. Int J Radiat Oncol Biol Phys.
115:440–452. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siva S, MacManus MP, Martin RF and Martin
OA: Abscopal effects of radiation therapy: A clinical review for
the radiobiologist. Cancer Lett. 356:82–90. 2015. View Article : Google Scholar
|
|
34
|
Hashimoto K, Nishimura S, Shinyashiki Y,
Ito T, Kakinoki R and Akagi M: Clinicopathological assessment of
PD-1/PD-L1 immune checkpoint expression in desmoid tumors. Eur J
Histochem. 67:36882023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou Y, Xu X, Ding J, Jing X, Wang F, Wang
Y and Wang P: Dynamic changes of T-cell subsets and their relation
with tumor recurrence after microwave ablation in patients with
hepatocellular carcinoma. J Cancer Res Ther. 14:40–45. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Q, Sun Z and Chen L: Memory T cells:
Strategies for optimizing tumor immunotherapy. Protein Cell.
11:549–564. 2020. View Article : Google Scholar
|