Introduction
Castleman disease is a relatively rare non-clonal
lymphoproliferative disease characterized by the histopathological
findings of the lymph nodes, as reported by Castleman et al
(1). Clinically, it is classified
into unicentric Castleman disease (UCD), in which the affected
lymph nodes are confined to a single region, and multicentric
Castleman disease (MCD), characterized by generalized
lymphadenopathy (2–5). The clinical symptoms of CD include a
variety of systemic symptoms and abnormalities upon testing. CD is
characterized by histological findings such as lymphoid follicle
hyperplasia and vascular invasion into lymphoid follicles, and is
definitively diagnosed based on histopathological findings. The
most common site of Castleman disease is the mediastinum,
accounting for approximately 70% of all cases; however, 15% of such
cases occur in the head and neck (6). Therefore, it is necessary to keep in
mind the concept of Castleman disease when cervical lymphadenopathy
is observed. However, it is extremely rare for Castleman disease to
occur in association with head and neck cancer cases, including
oral cancer, in which case it becomes difficult to distinguish
between the metastatic lymph nodes from head and neck cancer and
Castleman disease. Furthermore, the treatment for the two diseases
is completely different, so proper diagnosis is essential.
We experienced a case of MCD that was incidentally
detected during an evaluation of tongue cancer, and report this
case with a review of the pertinent literature.
Case report
A 73-year-old male was referred to our department by
our Internal Medicine Department in May 2022 under a suspicion of
tongue cancer on the left side. The patient became aware of pain in
the same region one month prior to his initial visit. His medical
history included chronic kidney disease, hypothyroidism, chronic
stasis dermatitis, and anemia. At his initial visit, a 25×15 mm
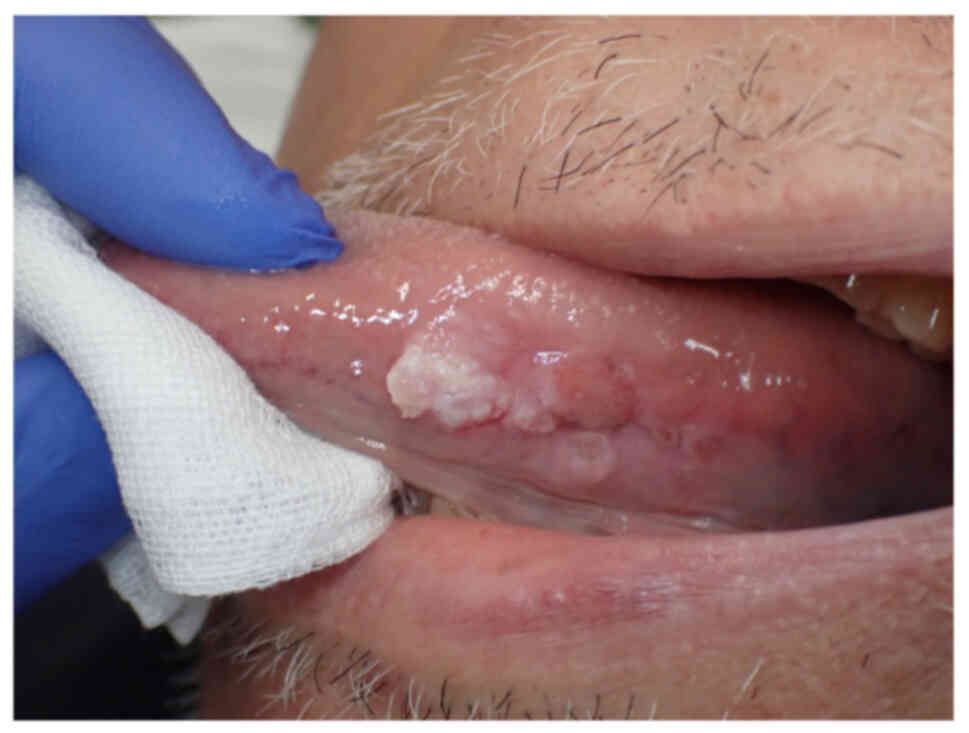
exophytic tumor with a slightly ambiguous border was observed on
the left side of his tongue; however, no paresthesia of the lingual
nerve was observed (Fig. 1). In
addition, no obvious swelling or tenderness of the cervical lymph
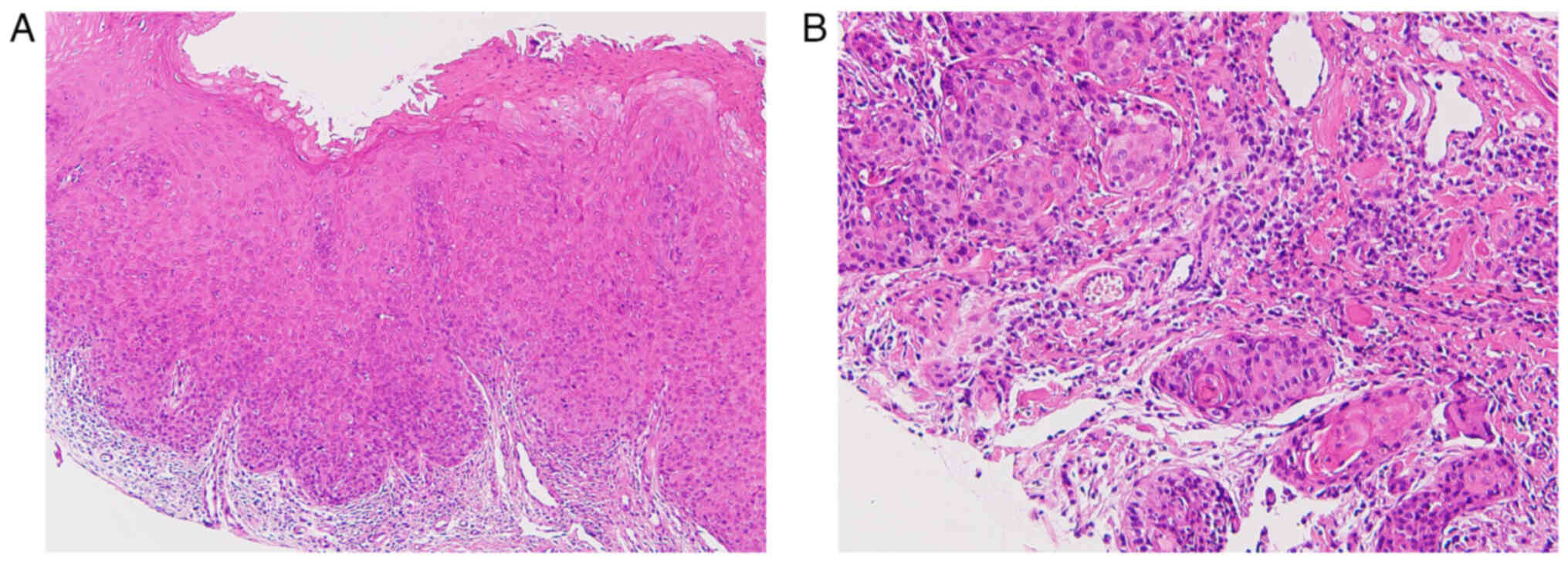
nodes was palpable. A biopsy revealed well-differentiated squamous
cell carcinoma (Fig. 2). A
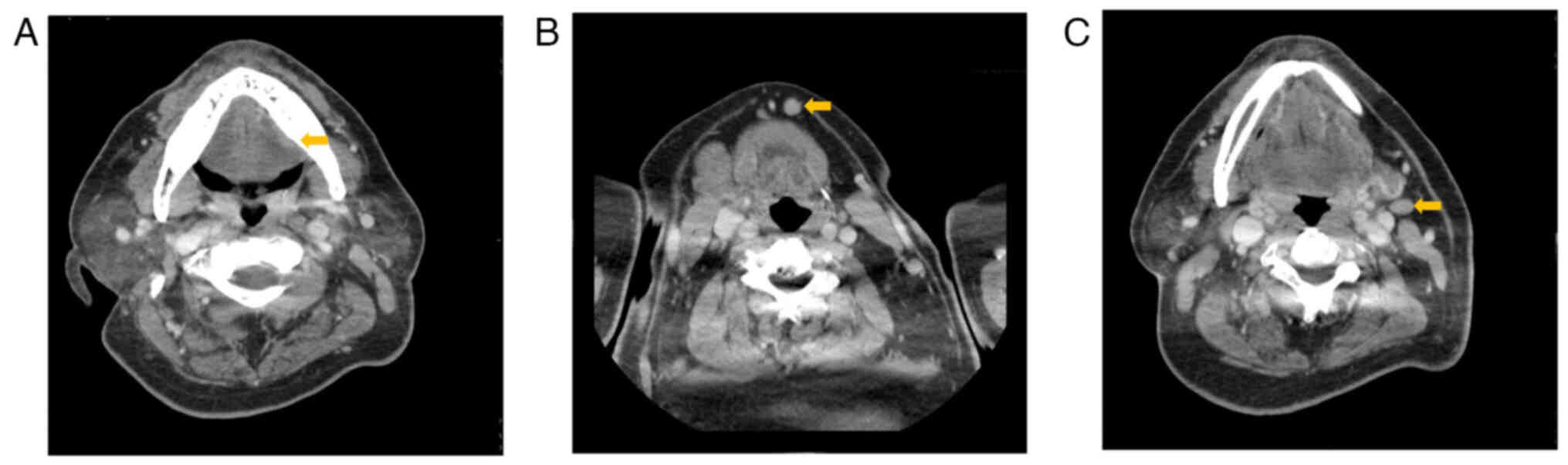
neoplastic lesion measuring 25×15×4 mm in size was observed on the
left side of his tongue upon Computed tomography (CT) and Magnetic
resonance imaging (MRI), with mild enlargement observed in the
submental lymph nodes and left submandibular lymph nodes; however,
no lung lesions were observed (Fig.
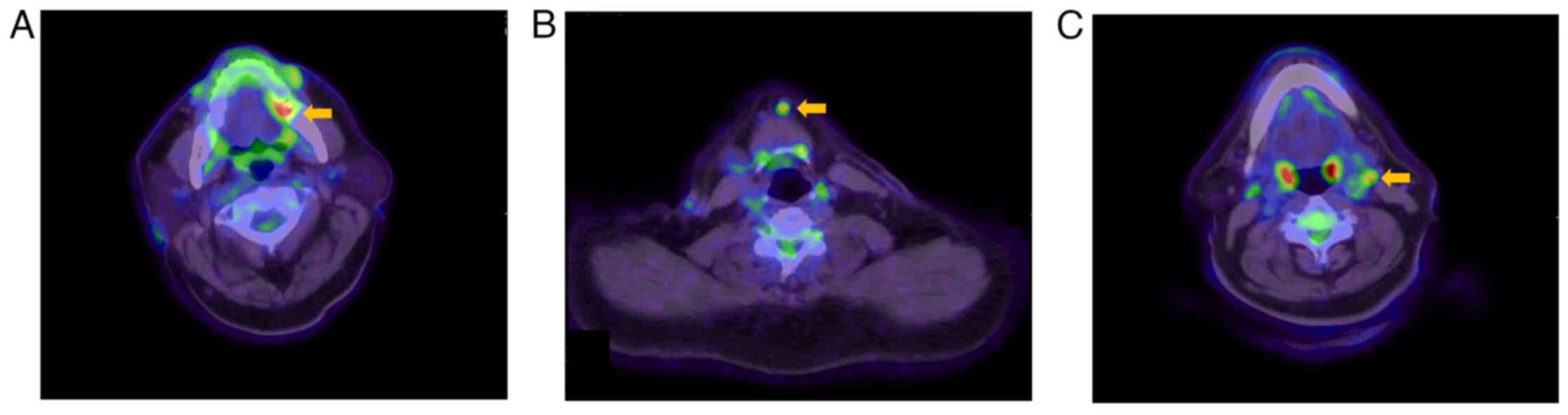
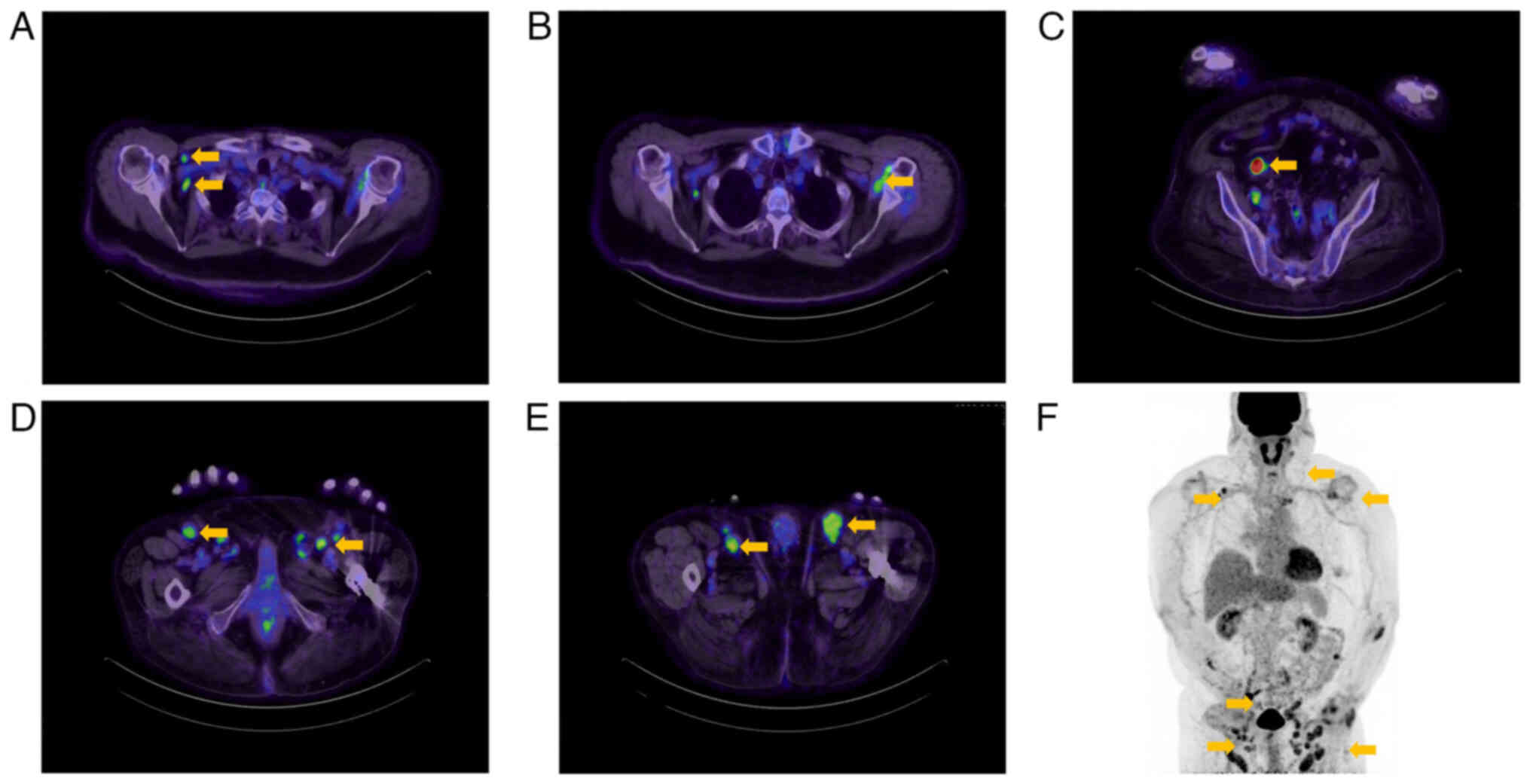
3). F-fluorodeoxyglucose positron emission tomography/computed
tomography (FDG-PET/CT) revealed a strong accumulation of SUVmax
6.9 on the left side of his tongue. An abnormal FDG uptake was also
observed in the submental lymph node (SUVmax 5.2) and the left
submandibular lymph node (SUVmax 4.8) (Fig. 4). In addition, multiple intense
accumulations (SUVmax 1.7–6.5) were detected in the lymph nodes of
both axillae, around the abdominal aorta, in the pelvic cavity, and
in both inguinal regions (Fig. 5).
Blood test results indicated low values of hemoglobin content (Hb)
at 10.4 g/dl and albumin (Alb) at 3 1g/dl, a slightly elevated
C-reactive protein (CRP) at 1.79 mg/dl, and a high γ-globulin
fraction in the protein fractionation pattern at 38.4%. Although
lymphoproliferative disorder was suspected, metastasis from tongue
cancer to the cervical lymph nodes could not be ruled out. For this
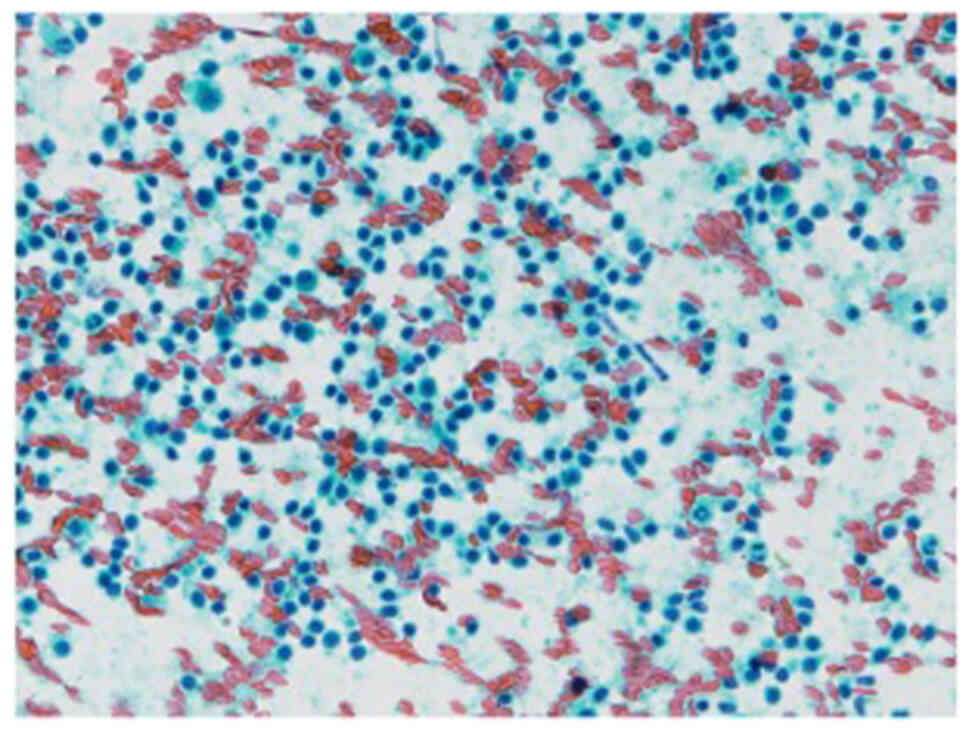
reason, we performed fine needle aspiration (FNA) on the submental
lymph nodes and left submandibular lymph nodes, while observing no
atypical epithelial cells suggesting metastasis (Fig. 6). In addition, we consulted our
Dermatology Department and performed a lymph node biopsy from the
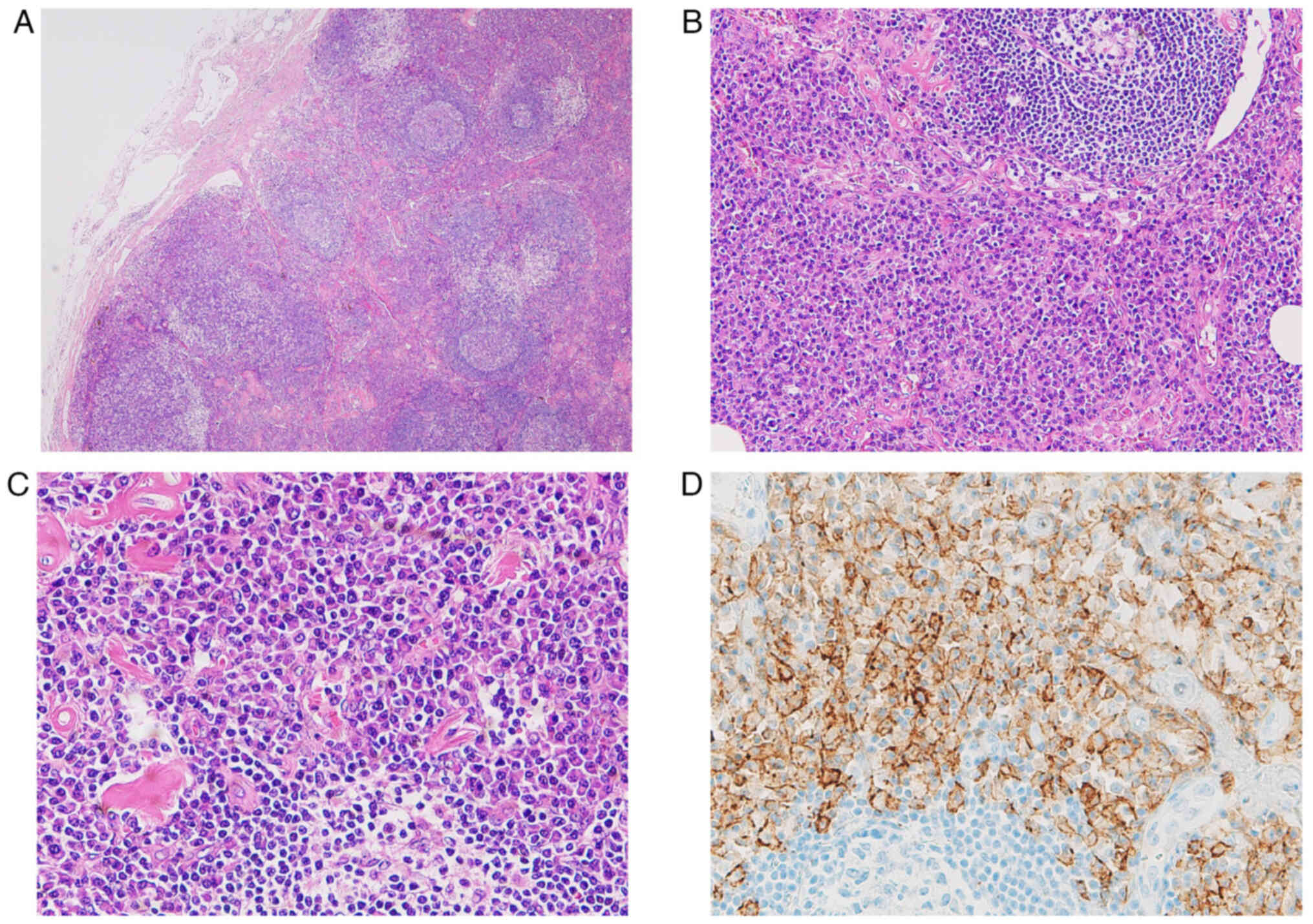
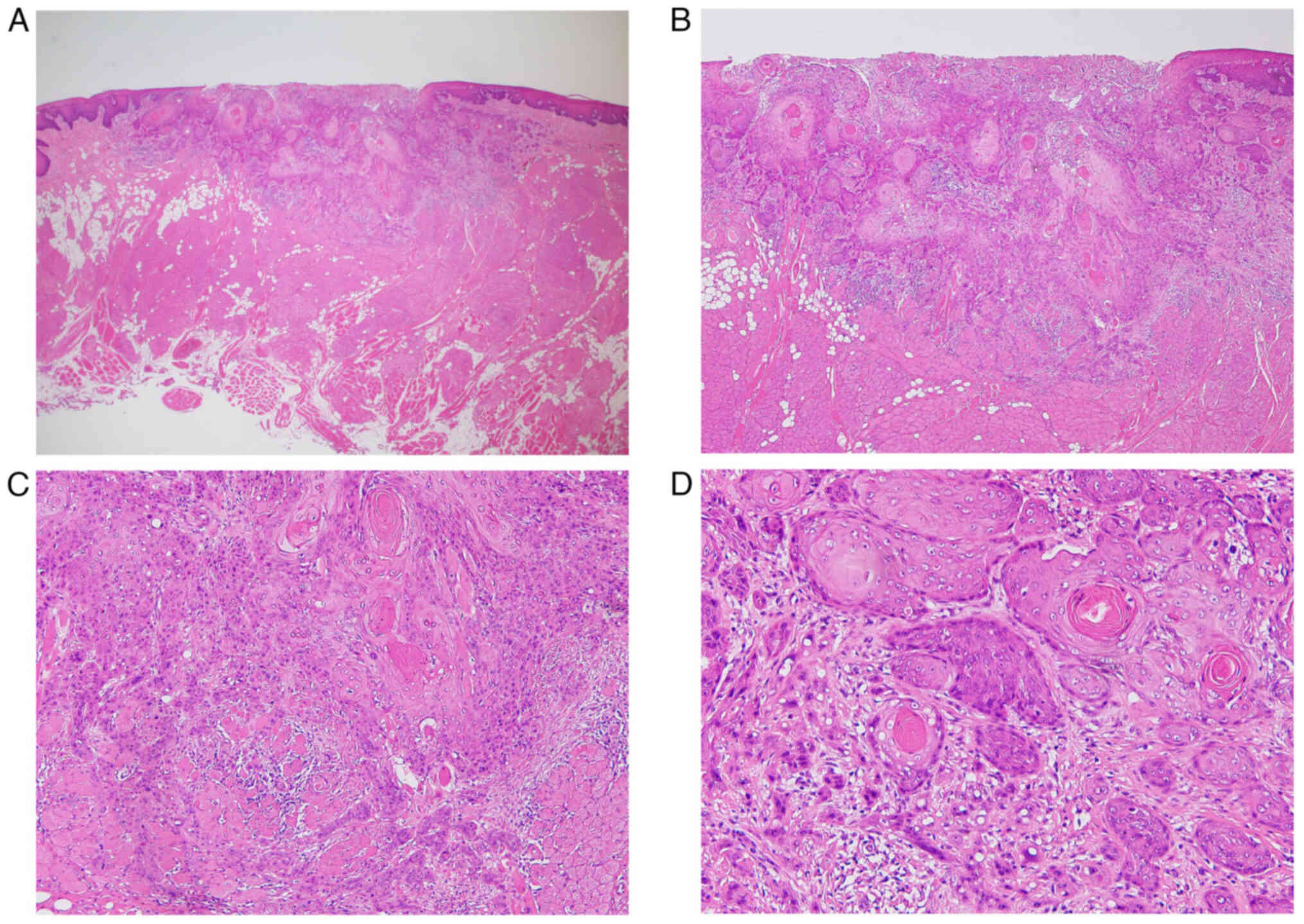
left inguinal region. Histologically, we observed follicle
formation with an embryonic center and significant plasma cell
proliferation around the follicle. When we investigated
kappa/lambda chains by means of clonality studies, the plasma cells
were not biased by kappa/lambda chains and immunohistochemistry did
not reveal any findings that could be considered to be tumor
proliferation. Since there was also no amyloid deposition, the
patient was diagnosed with a plasma cell variant of Castleman
disease (Fig. 7).
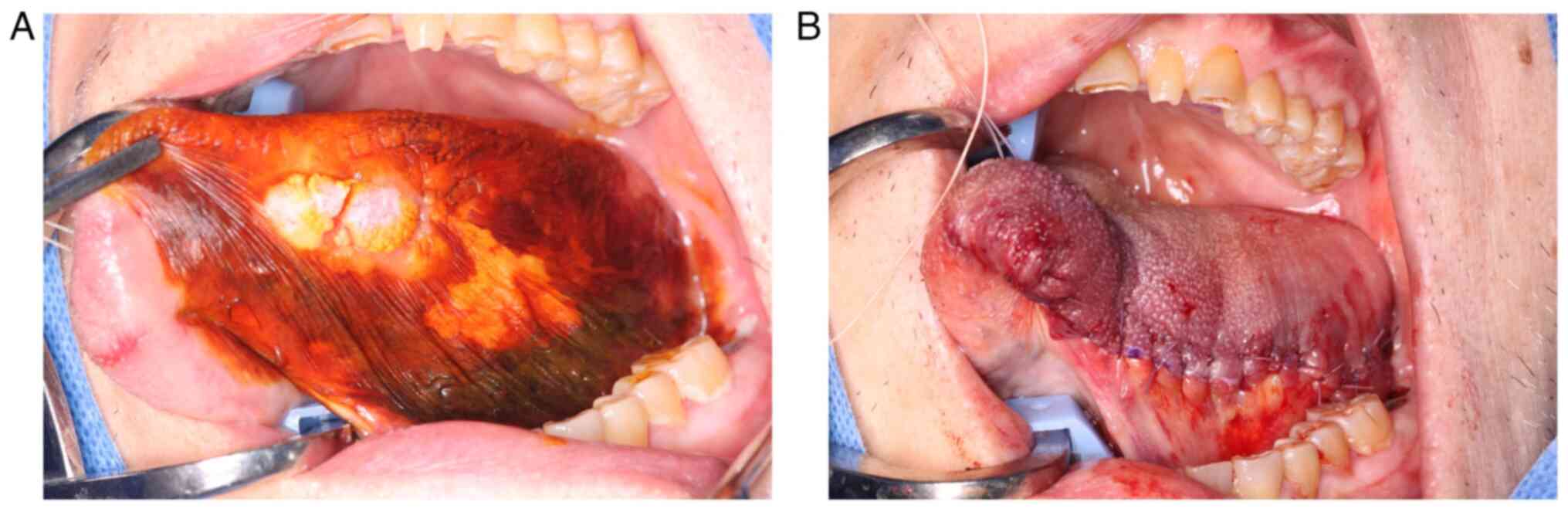
Based on the above, in August 2022, a left partial
glossectomy was performed under general anesthesia with a diagnosis
of left lingual squamous cell carcinoma (cT2N0M0, stage II) along
with MCD. Following the resection, the wound was sutured with 4-0
absorbent sutures (Fig. 8).
Histopathologically, the lesion showed a sheet-like proliferation
of atypical squamous cells with a tendency to keratinize, thus
forming large and small nests and infiltrating into the superficial
muscle layer. Moreover, low-grade dysplasia was observed in the
white mucosa around the tumor (Fig.
9). The depth of invasion was 2 mm and venous infiltration was
observed; however, no lymphatic vessels or nerve infiltration was
observed. Based on the above, the lesion was diagnosed as squamous
cell carcinoma (SCC) of the tongue (pT2N0M0, grade 1, stage II).
Our hematology department thereafter was responsible for the
treatment for Castleman disease. Because the symptoms were mild and
asymptomatic, with the 10-year survival rate expected to be 80%
even without treatment, our course of action was to monitor his
progress without using any immunosuppressive drugs and tocilizumab.
It was decided that if symptoms such as fatigue and slight fever
appeared, then the administration of 0.3 mg/kg/day of prednisolone
will be initiated, and if these symptoms were to either worsen or
if organ symptoms are observed, then the administration of
tocilizumab would be considered while increasing the dose of
prednisolone to 1 mg/kg/day. Postoperatively, the patient has been
undergoing monthly follow-up visits and CT scans every three months
to monitor the status of his tongue and the lymph node lesions.
Currently, there has been no evidence of any recurrent metastasis
of the tongue cancer even 30 months following surgery, and with no
progression of Castleman disease observed.
Discussion
Castleman disease is a non-neoplastic
lymphoproliferative disorder of unknown origin and it is a
relatively rare disease that was reported by Castleman et al
(1) in 1956. Subsequently, it was
histopathologically classified into three types by Keller et
al (7): hyaline vascular type
(HV type); plasma cell type (PC type); and mixed type (M type). HV
type is histologically characterized by the proliferation of
lymphoid follicles and the proliferation and vitrification of blood
vessels between follicles. It has few clinical symptoms and it
rarely demonstrates abnormal values upon blood testing. On the
other hand, PC type is characterized by the diffuse, sheet-like
infiltration of plasma cells into the interfollicular tissue and
expansion of the follicular spaces, which often causes a variety of
clinical symptoms such as generalized lymphadenopathy, anemia, and
abnormal blood tests. Although there are no specific
immunohistochemical markers for diagnosis, plasma cells may
occasionally be identified using CD138 immunostaining. Moreover,
depending on the distribution of the lesions, it is also
distinguished from UCD, which occurs only in a single lymph node,
and MCD, which spreads to multiple regions. In principle, UCD
corresponds to HV type, and MCD corresponds to PC type and M type.
However, typical findings are not always obtained in the
histopathological diagnosis of MCD, so careful judgment is required
for each individual case, while carefully considering the available
clinical information. The clinical symptoms of MCD include general
malaise, fever, anemia, high value of CRP, hypergammaglobulinemia,
and a variety of systemic symptoms and abnormalities upon testing.
A diagnosis is made based on both the clinical symptoms and the
histopathology of the enlarged lymph nodes (2). While clinical symptoms such as general
malaise and fever were not observed in our case, low Hb and Alb
values and a high gamma-globulin fraction were observed upon blood
testing. In addition, due to the fact that a CT scan confirmed the
enlargement of lymph nodes across multiple regions and that the
degree of plasma cell infiltration was histopathologically high, it
was diagnosed as a PC type of MCD.
While the most common site of Castleman disease is
the mediastinum, 15% of such cases occur in the head and neck, and
it is also known to occur in the abdominal cavity and axilla. The
differential diagnosis of Castleman disease occurring in the neck
includes inflammatory cervical lymphadenitis, cervical lymph node
metastasis of malignant tumors, and malignant lymphoma, as well as
sarcoidosis and IgG4-related diseases. If head and neck cancer is
present, then cervical lymph node metastasis should first be
suspected. In our case, multiple abnormal FDG accumulations were
systemically observed in addition to cervical lymph nodes upon
PET/CT, suggesting at least some form of lymphoproliferative
disorder. However, regarding the cervical lymph nodes, because the
possibility of metastasis from tongue cancer could not be ruled
out, FNA was performed. As far as we were able to find,
English-language reported cases of Castleman disease occurring in
conjunction with head and neck cancer are extremely rare, with only
three known cases, including our own case (8,9)
(Table I). The average patient age
was 47.7 years, with two patients in their 30s. There were two
males and one female, with the cancer site being the tongue in all
three cases. Histopathologically, two cases were HV type, and one
case was PC type. Regarding the site of lymphadenopathy, two cases
occurred at a single site (mediastinum, submandibular region) and
one case occurred at multiple sites. As noted earlier, both of the
two non-self cases of UCD corresponded to HV type. Lymphadenopathy
was also observed in the neck in two cases, including our own case.
In one case, under a diagnosis of cT1N3aM0, hemi-glossectomy and
bilateral neck dissection were performed. However, no lymph node
metastasis was observed in the postoperative histopathological
diagnosis, so it may have been possible to treat the disease by
partial resection alone. In our own case, only a partial
glossectomy was performed based on the results of the preoperative
FNA. To differentiate between metastatic lymph nodes from oral
cancer and Castleman disease, cervical ultrasonography, CT, and MRI
are first performed. However, as both conditions present with very
similar imaging findings, a definitive diagnosis requires either
FNA or a biopsy of the lymph node.
 | Table I.Castleman disease occurring in
conjunction with head and neck cancer. |
Table I.
Castleman disease occurring in
conjunction with head and neck cancer.
| Author | Sex | Age | Location of SCC | Location of lymph
node enlargement | Clinical
diagnosis | Diagnosis at
biopsy | Treatment | Histopathological
diagnosis | (Refs.) |
|---|
| Pereira et
al | F | 38 | Tongue | Right posteromedial
mediastinum | Germ cell cell
tumor | Small blue
lesion | Right posterolateral
excision of chest wall and thoracotomy with the right paraspinous
tumor | Hyaline vascular type
Castleman disease | (9) |
| Deshmukh et
al | M | 32 | Tongue | Submandibular
lesion | Cervical lymph node
metastasis | NA | Bilateral modified
neck dissection | Hyaline vascular type
Castleman disease | (8) |
| Present case | M | 73 | Tongue | Submental and left
submandibular, bilateral axillary, abdominal periaortic, pelvic,
and bilateral inguinal lymph nodes | Lymphoproliferative
disorders | Plasma cell type
Castleman disease | Observation | - | - |
MCD is believed to be caused by the sustained
production of interleukin-6 (IL-6) (10). For this reason, treatment is
centered on IL-6 inhibitors, with siltuximab mainly used (11); however, tocilizumab (2), an anti-IL-6 receptor antibody, is the
only approved treatment by the Japanese National Health Insurance
System. In asymptomatic cases with only mild laboratory
abnormalities, as in the present case, careful observation may be
an appropriate management option. In moderate to severe cases, the
concomitant use of steroids is recommended in addition to
tocilizumab (12,13). With proper treatment, the prognosis
is relatively good, with a 5-year overall survival rate of 100% and
a 10-year overall survival rate of 90% or more in Japan (14). That said, obtaining a complete cure
is difficult, with most patients not achieving permanent remission
even via treatment with tocilizumab (15), so further therapeutic drug
development and clinical trials are needed going forward.
While the relationship between Castleman disease and
SCC has not been clarified, the involvement of IL-6 has been
suggested (16). IL-6 is a cytokine
involved in a variety of biological events, including immune
reactions, hematopoiesis, and acute phase reactions, with the
overproduction thereof thought to be involved in the development of
chronic inflammatory diseases and cancers. Riedel et al
(17) reported that they observed
elevated serum IL-6 levels in head and neck SCCs, particularly in
advanced cancers with lymph node metastases. In this case, the
symptoms of Castleman disease were mild and asymptomatic, and since
no active therapeutic intervention was undertaken and only
observation was performed, the IL-6 levels were therefore not
measured. Regarding the monitoring method, if Castleman disease and
oral cancer coexist, as observed in this case, then it is necessary
to confirm both lesions, with imaging examinations conducted once
every 3 to 6 months considered to be appropriate. Although the
course of MCD is generally slow, it is recommended to conduct
systemic examinations such as PET/CT once a year. In addition, in
conjunction with the Hematology Department, we should be prepared
to respond quickly when symptoms appear.
We described a case of MCD that was incidentally
detected during an evaluation of tongue cancer and reviewed the
literature on this rare entity. Distinguishing between
lymphadenopathy in Castleman disease and lymph node metastasis in
oral cancer is difficult using just the clinical findings and
images alone. In order to provide appropriate treatment, it is
necessary to carry out a histopathological examination in
cooperation with the relevant departments to make an appropriate
diagnosis and stage classification.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YY conceptualized the case report, performed the
acquisition, analysis and interpretation of data and drafted the
manuscript. YY, SS, MY, MN and KS were involved in the treatment
and follow-up in this case. SA advised on patient treatment,
analyzed patient data, critically revised the manuscript and
provided valuable feedback, provided supervision, and approved the
final manuscript for publication. YY and SA confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
publication, authorizing the use of their imaging, pathological and
clinical data for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Castleman B, Iverson L and Menendez VP:
Localized mediastinal lymphnode hyperplasia resembling thymoma.
Cancer. 9:822–830. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fajgenbaum DC, Uldrick TS, Bagg A, Frank
D, Wu D, Srkalovic G, Simpson D, Liu AY, Menke D, Chandrakasan S,
et al: International, evidence-based consensus diagnostic criteria
for HHV-8-negative/idiopathic multicentric Castleman disease.
Blood. 129:1646–1657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartoli E, Massarelli G, Soggia G and
Tanda F: Multicentric giant lymph node hyperplasia. A hyperimmune
syndrome with a rapidly progressive course. Am J Clin Pathol.
73:423–426. 1980. View Article : Google Scholar
|
|
4
|
Gaba AR, Stein RS, Sweet DL and Variakojis
D: Multicentric giant lymph node hyperplasia. Am J Clin Pathol.
69:86–90. 1978. View Article : Google Scholar
|
|
5
|
Frizzera G, Banks PM, Massarelli G and
Rosai J: A systemic lymphoproliferative disorder with morphologic
features of Castleman's disease. Pathological findings in 15
patients. Am J Surg Pathol. 7:211–231. 1983. View Article : Google Scholar
|
|
6
|
Bonekamp D, Horton KM, Hruban RH and
Fishman EK: Castleman disease: The great mimic. Radiographics.
31:1793–1807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keller AR, Hochholzer L and Castleman B:
Hyaline-vascular and plasma-cell types of giant lymph node
hyperplasia of the mediastinum and other locations. Cancer.
29:670–683. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deshmukh M, Bal M, Deshpande P and
Jambhekar NA: Synchronous squamous cell carcinoma of tongue and
unicentric cervical Castleman's disease clinically mimicking a
stage IV disease: A rare association or coincidence? Head Neck
Pathol. 5:180–183. 2011. View Article : Google Scholar
|
|
9
|
Pereira TC, Landreneau R, Nathan G and
Sturgis CD: Pathologic quiz case. Large posterior mediastinal mass
in a young woman. Pathologic diagnosis: Localized
hyaline-vascular-type Castleman disease (angiofollicular lymphoid
hyperplasia). Arch Pathol Lab Med. 125:964–967. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishimoto N, Kanakura Y, Aozasa K, Johkoh
T, Nakamura M, Nakano S, Nakano N, Ikeda Y, Sasaki T, Nishioka K,
et al: Humanized anti-interleukin-6 receptor antibody treatment of
multicentric Castleman disease. Blood. 106:2627–2632. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Rhee F, Wong RS, Munshi N, Rossi JF,
Ke XY, Fosså A, Simpson D, Capra M, Liu T, Hsieh RK, et al:
Siltuximab for multicentric Castleman's disease: A randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 15:966–974.
2014. View Article : Google Scholar
|
|
12
|
Nishimoto N, Sasai M, Shima Y, Nakagawa M,
Matsumoto T, Shirai T, Kishimoto T and Yoshizaki K: Improvement in
Castleman's disease by humanized anti-interleukin-6 receptor
antibody therapy. Blood. 95:56–61. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dispenzieri A, Armitage JO, Loe MJ, Geyer
SM, Allred J, Camoriano JK, Menke DM, Weisenburger DD, Ristow K,
Dogan A and Habermann TM: The clinical spectrum of Castleman's
disease. Am J Hematol. 87:997–1002. 2012. View Article : Google Scholar
|
|
14
|
Fujimoto S, Sakai T, Kawabata H, Kurose N,
Yamada S, Takai K, Aoki S, Kuroda J, Ide M, Setoguchi K, et al: Is
TAFRO syndrome a subtype of idiopathic multicentric Castleman
disease? Am J Hematol. 94:975–983. 2019. View Article : Google Scholar
|
|
15
|
Carbone A, Borok M, Damania B, Gloghini A,
Polizzotto MN, Jayanthan RK, Fajgenbaum DC and Bower M: Castleman
disease. Nat Rev Dis Primers. 7:842021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsumura N, Shiiki H, Saito N, Uramoto H,
Hanatani M, Nonaka H and Nakamura S: Interleukin-6-producing thymic
squamous cell carcinoma associated with Castleman's disease and
nephrotic syndrome. Intern Med. 41:871–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Riedel F, Zaiss I, Herzog D, Götte K, Naim
R and Hörmann K: Serum levels of interleukin-6 in patients with
primary head and neck squamous cell carcinoma. Anticancer Res.
25:2761–2765. 2005.PubMed/NCBI
|