Introduction
Adenoid cystic carcinoma (ACC) is a malignant tumor
that originates from the secretory glands, accounting for only 1%
of head and neck malignancies. It most commonly arises in the
salivary glands, where it constitutes ~30% of all salivary gland
malignancies worldwide (1). Known
risk factors remain to be elucidated, although prior radiation
exposure and certain genetic alterations (such as MYB-nuclear
factor I B) fusion and NOTCH1 mutations) have been associated with
ACC (2,3). ACC exhibits distinct biological
behaviors: Slow growth but high propensity for perineural invasion,
local recurrence and distant metastasis (4). The primary treatment for localized ACC
is surgical resection with postoperative radiotherapy (4). However, metastatic ACC has a poor
prognosis due to limited systemic options. Palliative chemotherapy
regimens (such as cisplatin-based or anthracycline-containing
combinations) and molecular targeted agents (lenvatinib) are
commonly used; however, objective response rates remain modest,
typically ranging between 10 and 30%, underscoring the need for
more effective therapeutic strategies (5,6).
In the present study, a case report of maxillary ACC
with distant metastases to the pleura and brain is reported. The
patient underwent multimodal treatment, including surgery,
radiotherapy, chemotherapy and targeted therapy.
Case report
This case report was prepared in accordance with the
CARE guidelines for case reports (7). A 52-year-old male farmer of Han
Chinese ethnicity presented to Nanchong Central Hospital (Nanchong,
China) in January 2020 with a painless, slowly enlarging left
maxillary mass. Family history was negative for malignancies. As
the primary wage-earner for their household, the main concern of
the patient was whether functional impairment would affect their
work capacity.
Physical examination demonstrated a lack of mucosal
lesions, such as ulcers, on the palate, buccal mucosa or gingiva.
No cervical lymphadenopathy was noted. A panoramic dental X-ray
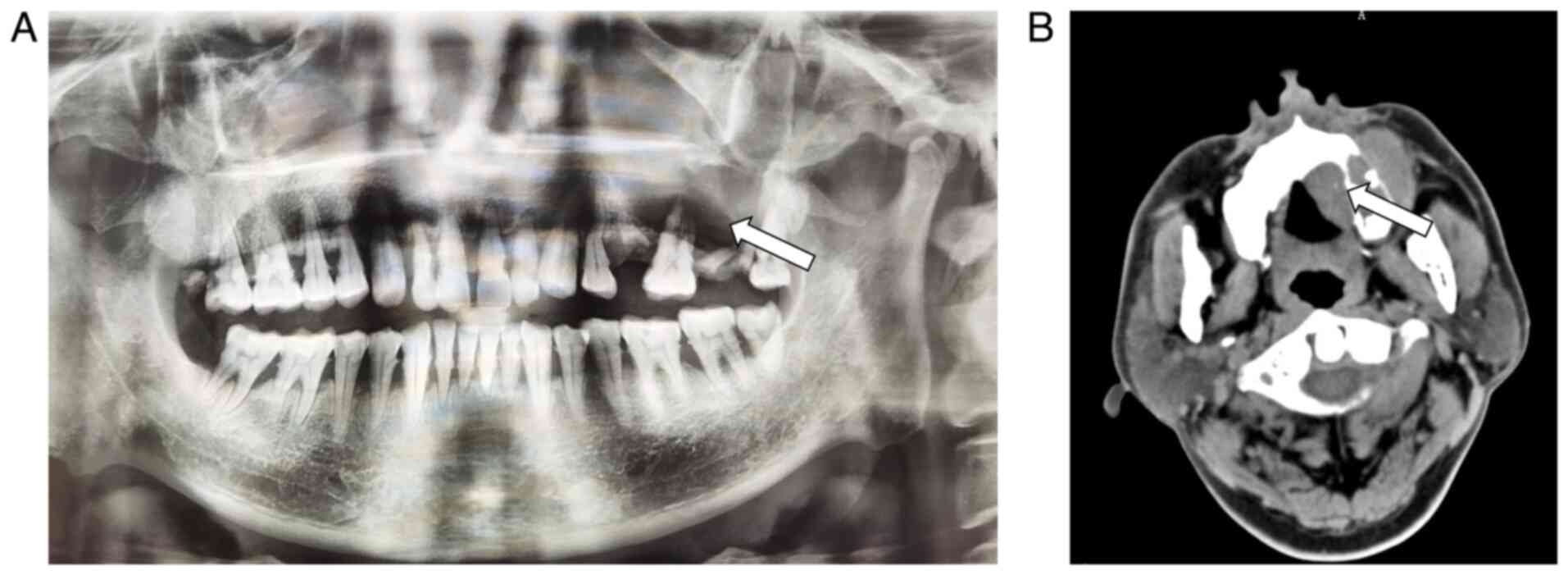
(Fig. 1A) and maxillofacial CT scan
(Fig. 1B) were performed, and
initially left maxillary ameloblastoma was suggested. The panoramic
radiograph (Fig. 1A) indicated
expansile osteolysis indistinguishable from ameloblastoma. The
lesion primarily involved the left maxillary alveolar process and
body, forming a soft tissue mass measuring ~4.7×4.2 cm with
expansile growth on CT imaging (Fig.
1B). The patient underwent total resection of the left maxilla,
partial resection of the right maxilla, implantation of a
bioabsorbable membrane, formation of a fascial flap and exploration
of the deep cervicofacial mass. Intraoperatively, the tumor was
demonstrated to have invaded the anterior and posterior lateral
walls of the maxillary sinus, completely filling the sinus.
Postoperative pathological diagnosis confirmed left maxillary ACC
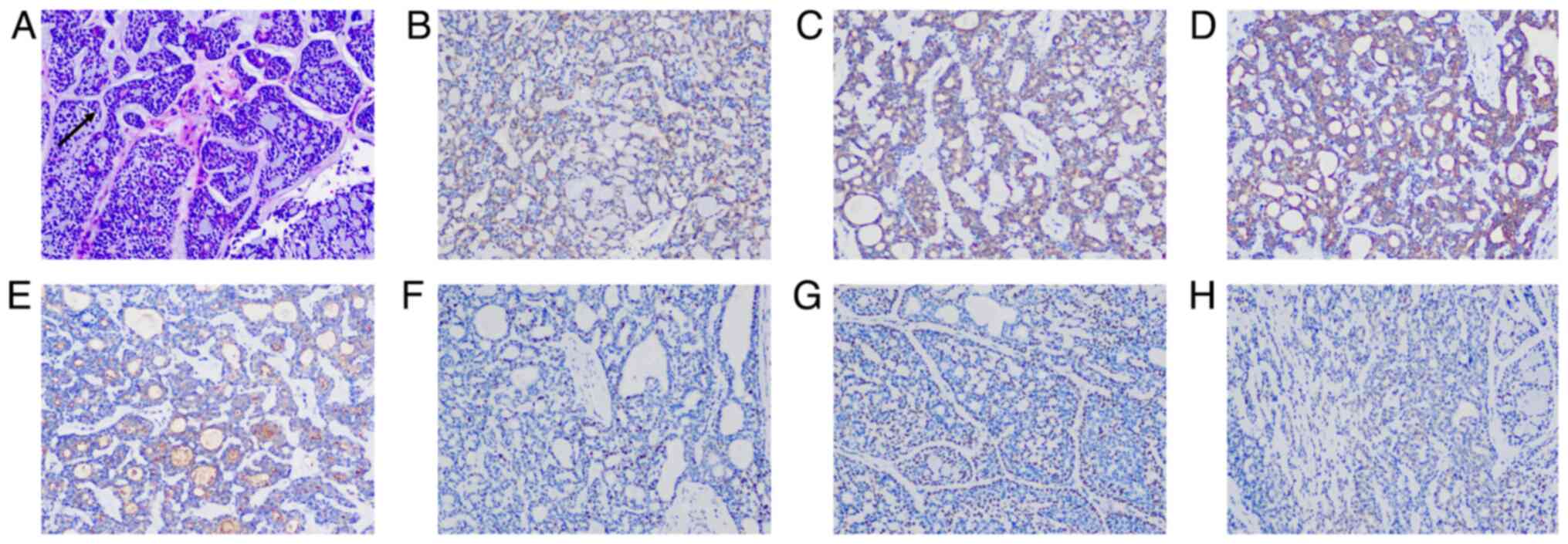
with negative margins and perineural invasion. Tumor cells were
arranged in nests of varying sizes and exhibited a cribriform
pattern with cystic spaces containing basophilic mucoid material. A
layer of mucin-secreting myoepithelial cells surrounded the cystic
spaces (Fig. 2A).
Immunohistochemical analysis demonstrated the following results:
CD117(+), epithelial membrane antigen (EMA)(+), cytokeratin
(CK)8/18(+), P63(+), actin(+), S-100(+), Ki-67 (20% +) (Fig. 2B-H).
Tissue samples were fixed in 4% neutral buffered
formalin at room temperature for 24–48 h, embedded in paraffin, and
sectioned at a thickness of 4 µm. Hematoxylin and eosin staining
was performed at room temperature: sections were stained with
hematoxylin for 5–7 min, followed by eosin for 1–2 min.
For immunohistochemical analysis, 4-µm-thick
paraffin-embedded sections were deparaffinized in xylene and
rehydrated through a graded ethanol series. Heat-induced antigen
retrieval was performed using citrate buffer (pH 6.0) at 95–100°C
for 20 min. Endogenous peroxidase activity was quenched by
incubation with 3% hydrogen peroxide for 15 min at room
temperature. Non-specific binding was blocked with 5% normal
mouse/rabbit serum (Fuzhou Maixin Biotech, China) for 30 min at
room temperature. Sections were then incubated with primary
antibodies (1:100-1:200) overnight at 4°C. The primary antibodies
used were as follows: CD117 (cat# Kit-0029), EMA (clone: E29; cat#
Kit-0011), CK8/18 (clone: MX004+MX035; cat# MAB-1002), P63 (clone:
MX013; cat# MAB-0694), actin (clone: MX083; cat# MAB-0871), S-100
(clone: MXR034; cat# RMA-1075), Ki-67 (clone: MXR002; cat#
RMA-0731), CK (clone: MX005; cat# MAB-0671), vimentin (clone:
MX034; cat# MAB-0735), calponin (clone: MX023; cat# MAB-0712),
TTF-1 (clone: MX011; cat# MAB-0599), and PD-L1 (clone: MXR006; cat#
RMA-0894); all from Fuzhou Maixin Biotech, China. After washing,
sections were incubated with an HRP-conjugated anti-mouse/rabbit
IgG secondary antibody (ready-to-use; cat# KIT-5005, Fuzhou Maixin
Biotech, China) for 1 h at room temperature. Signal detection was
performed using DAB (cat# TT-0805, Fuzhou Maixin Biotech, China).
Sections were counterstained with hematoxylin for 1–2 min at room
temperature. All slides were examined under a light microscope
(Olympus BX43; Olympus Corporation, Japan).
Based on the postoperative pathology, the patient
was diagnosed with ACC, stage T4aN0M0 (IVA) according to the 8th
edition of the AJCC Cancer Staging Manual (8), and underwent adjuvant radiotherapy at
the General Hospital of Western Theater Command of the Chinese
People's Liberation Army (Chengdu, China) in February 2020.
Radiotherapy was delivered as intensity-modulated radiation at 72.6
Gy in 33 fractions. Grade 1 mucositis, graded according to the
Common Terminology Criteria for Adverse Events (CTCAE) version 5.0
(9), occurred but was resolved with
supportive care. Fig. 3A and B
present the entire treatment process of the patient as well as the
timeline.
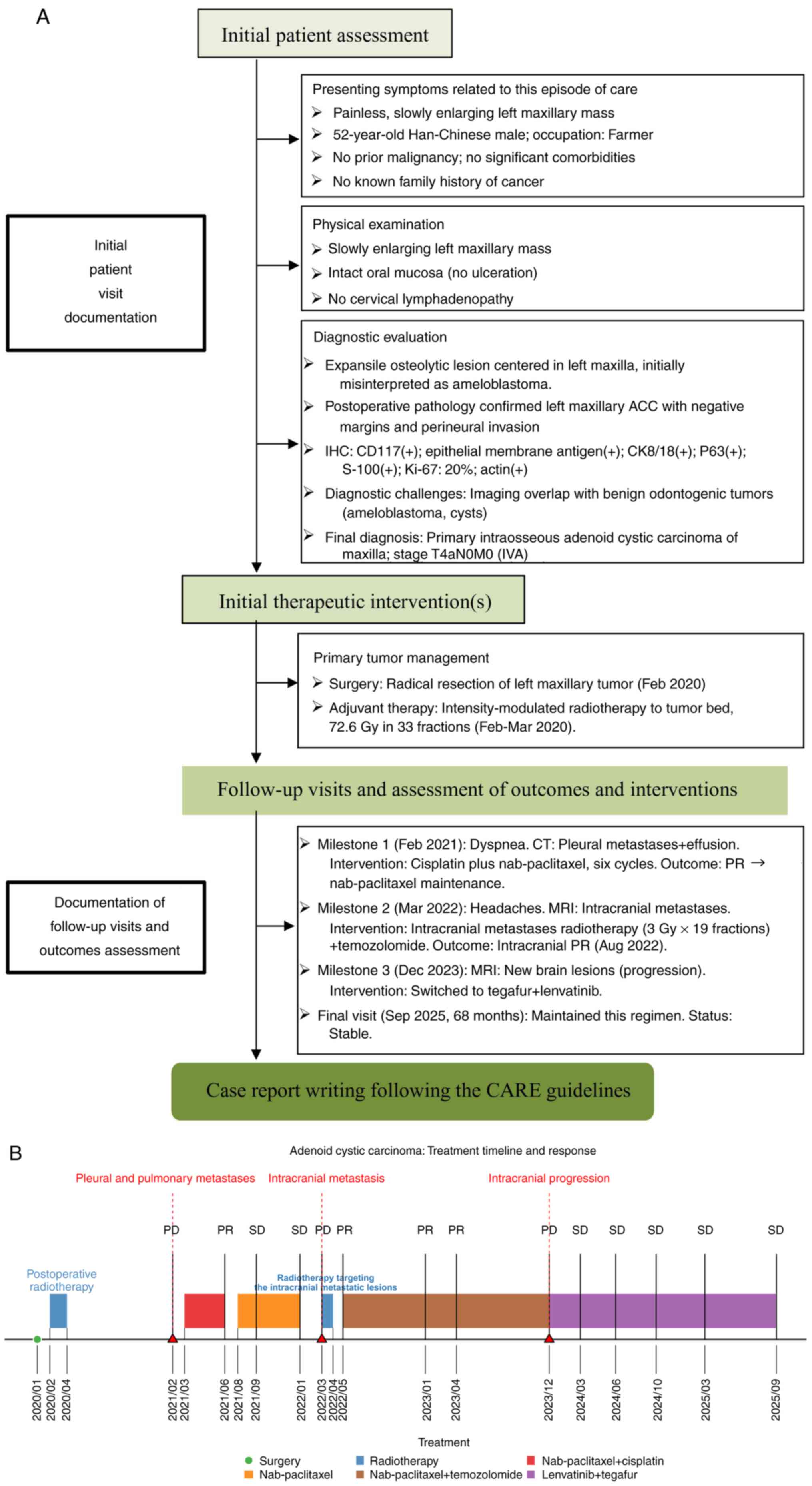 | Figure 3.Treatment strategy and clinical
course. (A) Outline of a CARE-guided workflow for comprehensive
case reporting, encompassing initial assessment, therapeutic
interventions and longitudinal follow-up of this rare malignancy.
(B) Timeline illustrating the sequential adaptation of multimodal
therapy for pleural and intracranial metastases, achieving
sustained disease control and 68-month overall survival. ACC,
adenoid cystic carcinoma; IHC, immunohistochemistry; CK,
cytokeratin; MRI, magnetic resonance imaging; PR, partial response;
PD, progressive disease; SD, stable disease. |
In February 2021, the patient developed dyspnea and
orthopnea. A chest CT scan at an external hospital revealed
multiple enhancing nodules on the right pleura with massive pleural
effusion. The patient underwent closed thoracostomy drainage at the
General Hospital of Western Theater Command, yielding hemorrhagic
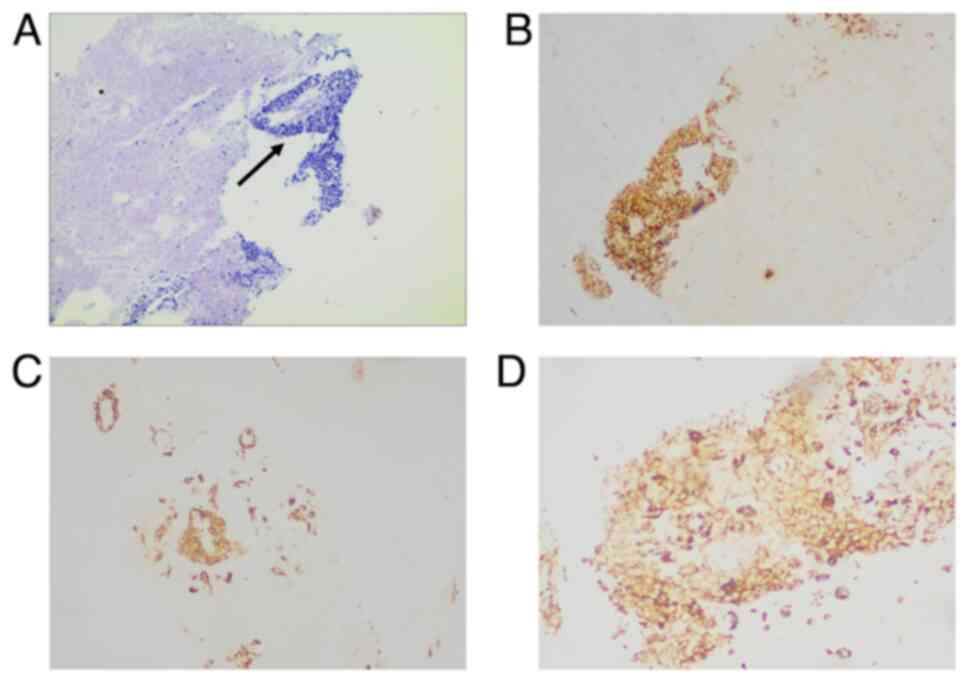
pleural fluid. Pleural biopsy pathology was suggestive of
metastatic carcinoma (Fig. 4). The
immunohistochemical results of the biopsy specimen were as follows:
Carcinoma cells positive for CK, Ki-67 (10%+), smooth muscle actin
and vimentin (Fig. 4); and negative
for calponin, P63, S-100, thyroid transcription factor 1 (TTF-1)
and programmed death-ligand 1 (Fig.
S1). Due to the extreme fading and the limited quantity of
tissue obtained from the pleural biopsy, immunohistochemical
staining images for Ki-67 and P63 are not presented.
Although the pleural biopsy showed poorly
differentiated carcinoma (Fig. 4A),
differing from the cribriform pattern of the primary ACC (Fig. 2A), both lesions exhibited an
infiltrative growth pattern with focal stromal collagenization. The
immunohistochemical discrepancy (P63/S-100 negative in pleura vs.
positive in primary ACC) likely reflects tumor dedifferentiation at
the metastatic site. Alternative diagnoses, including primary
pleural mesothelioma and metastatic lung adenocarcinoma, were
considered but excluded: Mesothelioma was ruled out by negative
calponin and absence of mesothelial morphology, and lung
adenocarcinoma was unlikely due to TTF-1 negativity. The
chronological progression from primary ACC to pleural metastasis,
coupled with the absence of other primary tumors, further supports
the diagnosis of metastatic ACC.
Due to the pleural metastasis, every 21 days the
patient received six cycles of cisplatin (75 mg/m2 IV,
days 1–3) combined with nab-paclitaxel (260 mg/m2 IV,
day 1) regimen chemotherapy, achieving partial response. Grade 2
neutropenia (according to CTCAE version 5.0) occurred and was
managed with granulocyte colony-stimulating factor (G-CSF; 5 µg/kg
subcutaneously for 3–5 days until neutrophil recovery). Grade 1
peripheral neuropathy resolved post-treatment. Maintenance
nab-paclitaxel (260 mg/m2 every 21 days) continued for 8
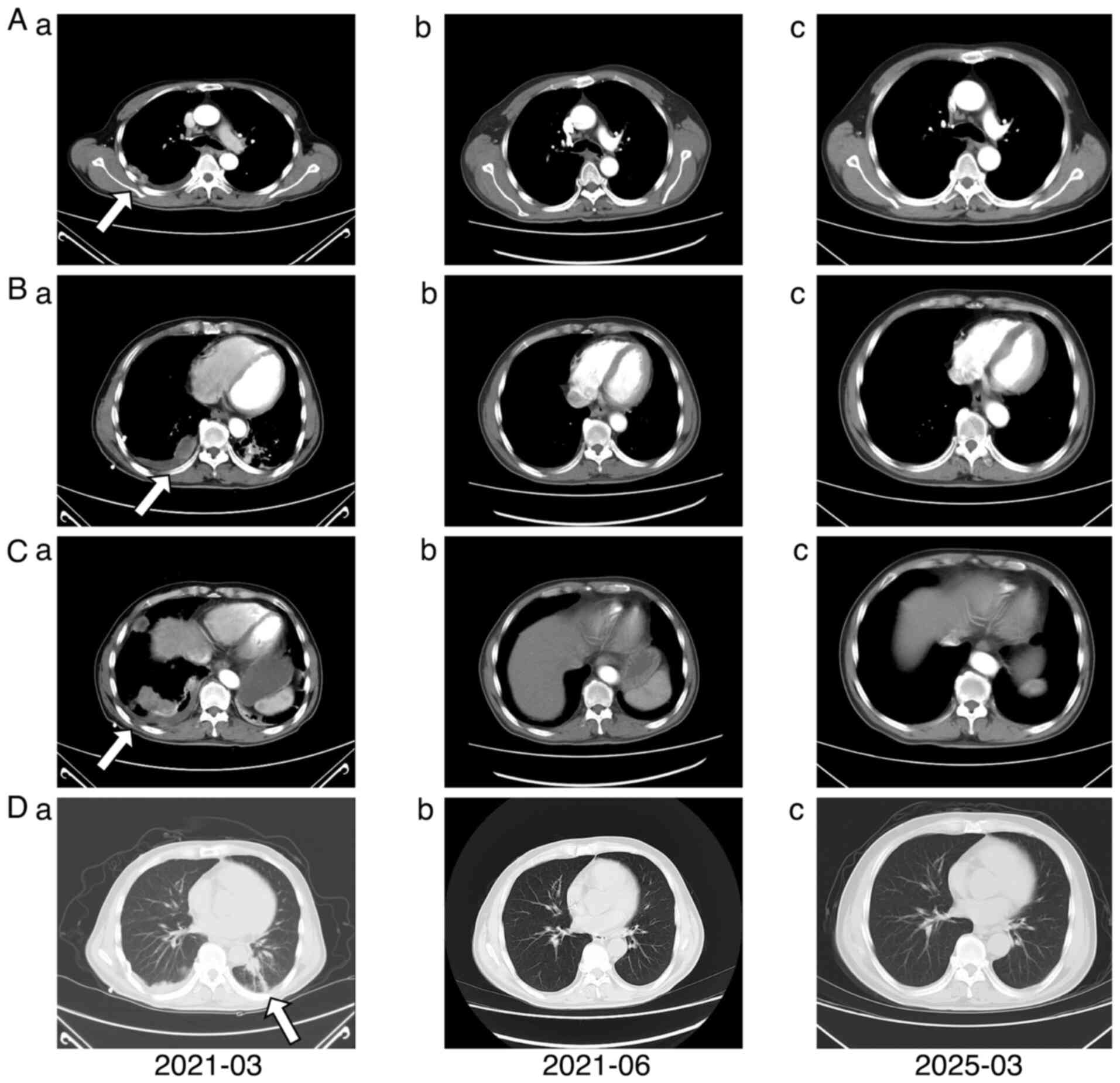
months until intracranial progression. The thoracic lesion remained
stable (Fig. 5).
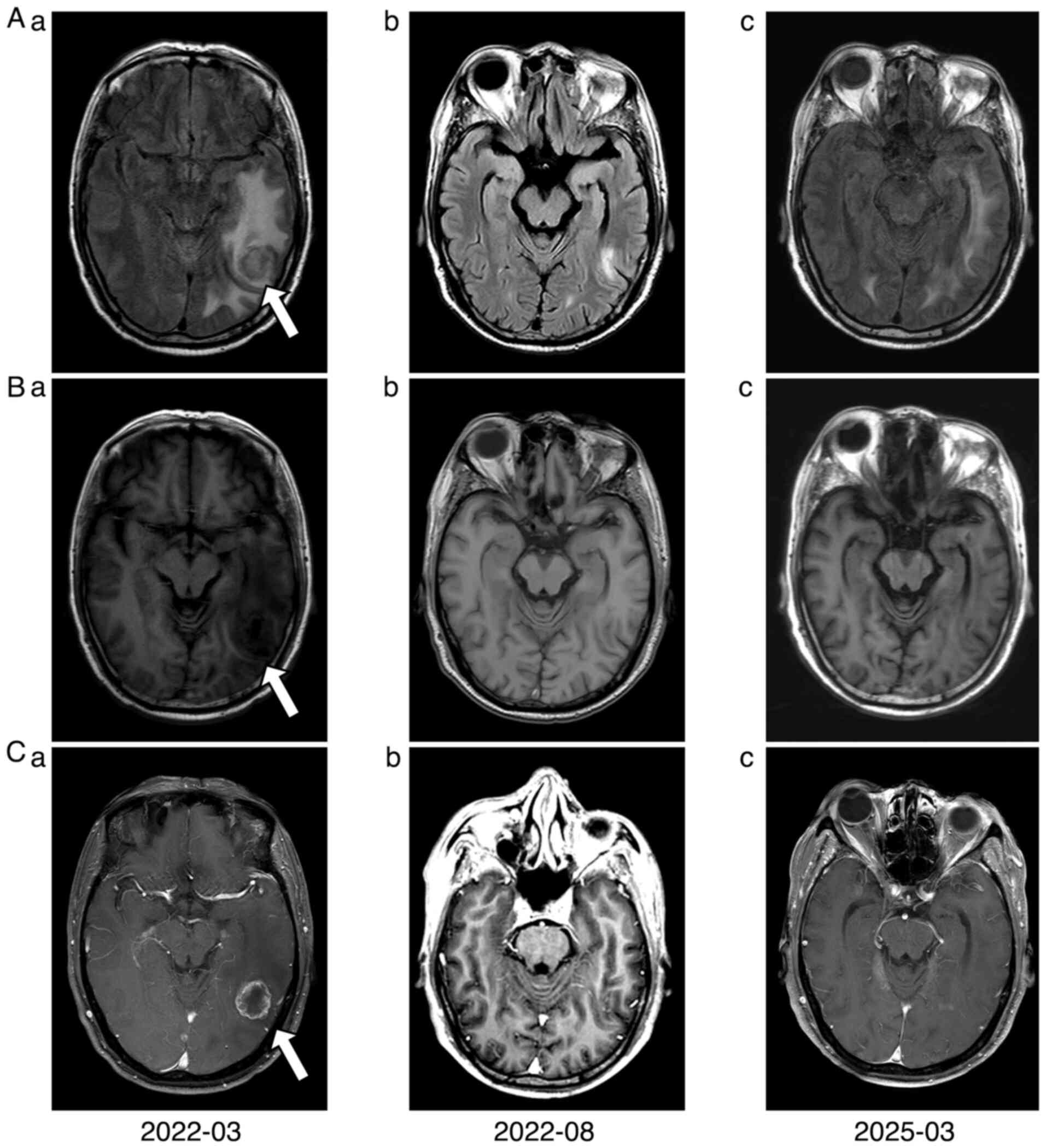
In March 2022, the patient underwent cranial
magnetic resonance imaging (MRI) for headaches, revealing possible
brain metastases. Radiotherapy targeting the intracranial
metastatic lesions commenced within one week following the MRI (57
Gy in 19 fractions). Considering the poor central nervous system
penetration of previous chemotherapeutic agents, temozolomide (75
mg/m2 orally, once daily) was added to the
nab-paclitaxel regimen and continued until disease progression. No
grade ≥3 adverse events (according to CTCAE version 5.0) occurred;
only grade 1 nausea was reported. Follow-up imaging in August 2022
indicated partial response of the intracranial lesions (Fig. 6).
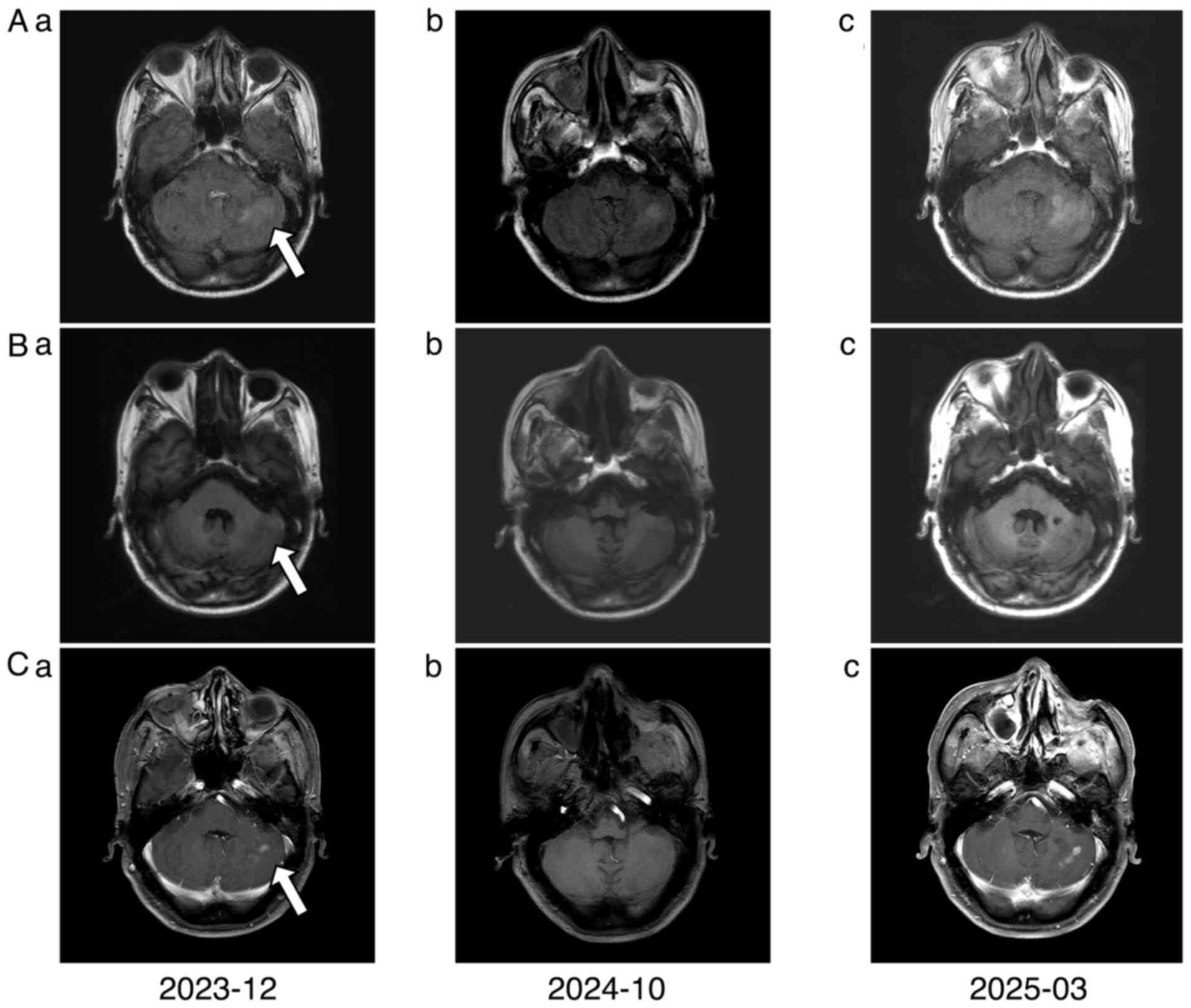
The patient continued regular surveillance. In
December 2023, follow-up cranial MRI revealed new intracranial
lesions, indicating resistance to second-line chemotherapy and
intracranial progression. Due to decreased treatment compliance
associated with prolonged disease, therapy was switched to tegafur
(40 mg/m2 orally twice daily, days 1–14) combined with
lenvatinib (8 mg orally daily), an anti-angiogenic targeted agent.
Disease evaluations in October 2024 and March 2025 confirmed stable
disease (Fig. 7).
The patient maintained this regimen with ongoing
surveillance. The last follow-up was conducted in September 2025,
at which time the patient was alive. In terms of patient
perspective, the 5-year survival milestone allowed the patient to
witness their daughter's high school graduation and university
enrollment moments. Overall, the treatment provided to the patient
highlighted that advanced cancer is not necessarily incurable when
innovative therapies are persistently pursued.
Discussion
ACC is a rare, slow-growing and often asymptomatic
malignancy. Intraosseous ACC (IACC) accounts for only <0.4% of
all ACCs, with cases in the maxilla being even rarer (10). The pathogenesis of IACC remains to
be elucidated; however, ACC typically arises from the secretory
glands, suggesting that primary intraosseous ACC may originate from
ectopic salivary tissues during mandibular embryogenesis (11). Although typically indolent, ACC
exhibits perineural invasion, allowing the spread along nerves,
leading to recurrence and metastasis. Hematogenous spread to the
lungs and bones is most common (12), while pleural and brain metastases
are rare and have not, to the best of our knowledge, been
previously reported in maxillary ACC.
The observed immunohistochemical discordance,
specifically the loss of myoepithelial markers P63 and S-100 in the
metastatic pleural lesion, is a particularly noteworthy finding.
This phenotypic alteration aligns with high-grade transformation or
dedifferentiation, a recognized pattern of tumor progression in
advanced ACC that often leads to loss of lineage-specific markers
at metastatic sites (13,14). Such alterations reinforce the
necessity of integrating clinical context and histomorphology with
biomarker interpretation to avoid diagnostic ambiguity.
Furthermore, the non-specific clinical
manifestations of ACC, such as a slow-growing mass accompanied by
pain and swelling, often mimic odontogenic lesions (such as
odontogenic cysts, ameloblastoma and apical periodontitis), leading
to misdiagnosis. The erroneous initial diagnosis of ameloblastoma
underscores the diagnostic complexity of maxillary ACC. In the
present case, panoramic radiography demonstrated expansile
osteolytic changes with cortical thinning, which are features
indistinguishable from ameloblastoma. This diagnostic pitfall
reinforces that histopathological verification remains mandatory
for expansile maxillary lesions, regardless of benign-appearing
imaging characteristics (15).
Therefore, histopathological examination remains the diagnostic
gold standard.
In the present case report, the initial panoramic
radiograph indicated expansile changes consistent with imaging
features of ameloblastoma; however, subsequent tissue biopsy
confirmed ACC. The lesion primarily involved the left maxillary
alveolar process and body, forming a soft tissue mass measuring
~4.7×4.2 cm with expansile growth. This pattern suggests a tumor
epicenter within the maxillary body. In contrast to these
observations, tumors originating in the maxillary sinus with
secondary maxillary invasion typically demonstrate eccentric growth
centered on the sinus cavity (16).
Based on these findings, a diagnosis of primary ACC arising within
the maxilla was proposed.
The standard treatment for ACC is radical surgery
combined with postoperative radiotherapy, with a 5-year survival
rate of ~75%. However, due to high recurrence and metastasis rates,
10 and 15-year survival rates drop to 50–60 and 30–35%,
respectively (4). For recurrent or
metastatic ACC, palliative chemotherapy is the mainstay, although
outcomes are limited. This underscores the value of documenting
novel strategies in challenging cases, as emphasized by Wáng
(17) regarding the role of case
reports in driving therapeutic innovation. The present case, with
its unprecedented metastatic pattern and sequential multimodal
approach, contributes to this body of knowledge. The sequential
strategy used for the present patient aligns with the framework by
Zupancic et al (18). This
approach extended overall survival to 68 months from initial
diagnosis (January 2020) to last follow-up (September 2025).
Research on the cisplatin + nab-paclitaxel (TP)
regimen in metastatic ACC is controversial (19), however, both drugs are recommended
by the National Comprehensive Cancer Network guidelines (20). The majority of the studies support
combination therapy over monotherapy, although data on maintenance
chemotherapy are limited and inconclusive (5). The patient in the present study
achieved sustained stabilization of pleural metastases following
six cycles of TP chemotherapy supplemented with nab-paclitaxel
maintenance therapy. This favorable outcome suggests that adding
nab-paclitaxel maintenance following six cycles of TP chemotherapy
may represent an effective treatment modality for pleural
metastasis.
The patient developed brain metastasis despite
stable extracranial disease, likely due to cisplatin and paclitaxel
poorly penetrating the blood-brain barrier, allowing tumor cells to
spread intracranially via perineural invasion. Notably, the patient
presented with pleural metastasis first, which may have a higher
risk for brain involvement, an area which requires further
research.
Due to the propensity of ACC for perineural invasion
and the ability of temozolomide to cross the blood-brain barrier,
broad-spectrum antitumor activity demonstrates significant efficacy
against the majority of intracranial malignancies (for example,
gliomas) (21). With the consent of
the patient, temozolomide (75 mg/m2 orally, once daily)
was administered orally daily. This regimen achieved favorable
therapeutic outcomes without severe adverse reactions.
Lenvatinib, a multitargeted tyrosine kinase
inhibitor, is recommended for the treatment of advanced ACC by
multiple guidelines (20,22). Furthermore, studies have
demonstrated the efficacy of tegafur in ACC treatment (23,24).
Due to the current patient's intracranial disease progression,
preserved performance status, high tumor burden and the anticipated
limited efficacy of monotherapy, combined therapy with tegafur and
lenvatinib was initiated under close monitoring for adverse events.
This regimen resulted in stabilization of both intracranial and
extracranial metastases for >1 year. These findings suggest that
tegafur plus lenvatinib represents a viable treatment option for
patients with multi-metastatic ACC experiencing localized
progression following previous treatment with chemotherapy.
Overall, to the best of our knowledge, the present
study reports the first documented case of maxillary ACC with
sequential metastases to the pleura and intracranial sites.
Following six cycles of TP regimen chemotherapy, maintenance
therapy with nab-paclitaxel resulted in sustained significant
control of pleural metastases. In addition, to the best of our
knowledge, the present study represents the first application of
temozolomide combined with nab-paclitaxel following brain
metastasis radiotherapy, yielding a favorable intracranial
response. Furthermore, the tegafur-lenvatinib combination proved to
be a viable regimen following second-line chemotherapy resistance
in this patient. Whether this multimodal approach can be extended
to other metastatic maxillary ACC cases warrants further
investigation.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Ting Yu
(Department of Pathology, The General Hospital of Western Theater
Command, Chengdu, China) for diagnostic support.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BH and JS were responsible for clinical management
of the patient, analyzed and interpreted clinical data, constructed
figures, and drafting the manuscript. QYY was responsible for
diagnostic analysis, performed imaging, advising on treatment, and
manuscript revision. LZ was responsible for conceptualization,
supervision, critical revision and final approval. BH and JS
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient for treatment.
Patient consent for publication
The patient provided written informed consent for
publication of de-identified clinical details and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Din MAU and Shaikh H: Adenoid cystic
cancer. StatPearls. StatPearls Publishing; Treasure Island, FL:
2025
|
|
2
|
Persson M, Andrén Y, Mark J, Horlings HM,
Persson F and Stenman G: Recurrent fusion of MYB and NFIB
transcription factor genes in carcinomas of the breast and head and
neck. Proc Natl Acad Sci USA. 106:18740–18744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrarotto R, Mitani Y, Diao L, Guijarro
I, Wang J, Zweidler-McKay P, Bell D, William WN Jr, Glisson BS,
Wick MJ, et al: Activating NOTCH1 mutations define a distinct
subgroup of patients with adenoid cystic carcinoma who have poor
prognosis, propensity to bone and liver metastasis, and potential
responsiveness to notch1 inhibitors. J Clin Oncol. 35:352–360.
2017. View Article : Google Scholar
|
|
4
|
Coca-Pelaz A, Rodrigo JP, Bradley PJ,
Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A,
Haigentz M Jr, Takes RP, et al: Adenoid cystic carcinoma of the
head and neck-an update. Oral Oncol. 51:652–661. 2015. View Article : Google Scholar
|
|
5
|
Onaga R, Enokida T, Ito K, Ueda Y, Okano
S, Fujisawa T, Wada A, Sato M, Tanaka H, Takeshita N, et al:
Combination chemotherapy with taxane and platinum in patients with
salivary gland carcinoma: A retrospective study of docetaxel plus
cisplatin and paclitaxel plus carboplatin. Front Oncol.
13:11851982023. View Article : Google Scholar
|
|
6
|
Tchekmedyian V, Sherman EJ, Dunn L, Tran
C, Baxi S, Katabi N, Antonescu CR, Ostrovnaya I, Haque SS, Pfister
DG and Ho AL: Phase II study of lenvatinib in patients with
progressive, recurrent or metastatic adenoid cystic carcinoma. J
Clin Oncol. 37:1529–1537. 2019. View Article : Google Scholar
|
|
7
|
Gagnier JJ, Kienle G, Altman DG, Moher D,
Sox H and Riley D; CARE Group, : The CARE guidelines:
Consensus-based clinical case reporting guideline development. J
Med Case Rep. 7:2232013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017.PubMed/NCBI
|
|
9
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE)
Version 5.0. https://www.ctc.ucl.ac.uk/TrialDocuments/Uploaded/Common%20Terminology%20Criteria%20for%20Adverse%20Events%20(CTCAE)%20v5.0_14092023_0.pdfSeptember
13–2025
|
|
10
|
Vinuth D, Agarwal P, Dhirawani RB and Dube
G: Atypical case of primary intraosseous adenoid cystic carcinoma
of mandible. J Oral Maxillofac Pathol. 17:436–439. 2013. View Article : Google Scholar
|
|
11
|
Sasaki E, Yamagata K, Hagiwara T, Takasaki
R, Fukuzawa S, Uchida F, Ishibashi-Kanno N and Bukawa H: A case of
primary intraosseous adenoid cystic carcinoma of the mandible. Case
Rep Dent. 2023:24220862023.
|
|
12
|
Savithri V, Suresh R, Janardhanan M,
Aravind T and Mohan M: Primary intraosseous adenoid cystic
carcinoma with widespread skeletal metastases showing features of
high-grade transformation. Head Neck Pathol. 15:715–722. 2021.
View Article : Google Scholar
|
|
13
|
Nagao T: ‘Dedifferentiation’ and
high-grade transformation in salivary gland carcinomas. Head Neck
Pathol. 7 (Suppl 1):S37–S47. 2013. View Article : Google Scholar
|
|
14
|
Seethala RR, Hunt JL, Baloch ZW, Livolsi
VA and Leon Barnes E: Adenoid cystic carcinoma with high-grade
transformation: A report of 11 cases and a review of the
literature. Am J Surg Pathol. 31:1683–1694. 2007. View Article : Google Scholar
|
|
15
|
MacDonald DS: Maxillofacial fibro-osseous
lesions. Clin Radiol. 70:25–36. 2015. View Article : Google Scholar
|
|
16
|
Vidović Juras D, Škrinjar I, Manojlović S,
Blivajs I, Franćeski D, Manojlović L and Vučićević Boras V: Case of
unrecognised of maxillary adenoid cystic carcinoma. Acta Stomatol
Croat. 53:82–85. 2019. View Article : Google Scholar
|
|
17
|
Wáng YXJ: Advance modern medicine with
clinical case reports. Quant Imaging Med Surg. 4:439–443. 2014.
|
|
18
|
Zupancic M, Näsman A, Friesland S and
Dalianis T: Adenoid cystic carcinoma, clinical presentation,
current treatment and approaches towards novel therapies.
Anticancer Res. 44:1325–1334. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee RH, Wai KC, Chan JW, Ha PK and Kang H:
Approaches to the management of metastatic adenoid cystic
carcinoma. Cancers (Basel). 14:56982022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu S, Li Y and Liu F: Standardized
treatment of oral cancer under the guidance of clinical practice
guidelines of national comprehensive cancer network. Hua Xi Kou
Qiang Yi Xue Za Zhi. 42:566–571. 2024.(In English, Chinese).
|
|
21
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C,
Bogdahn U, et al: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Herpen C, Vander Poorten V, Skalova A,
Terhaard C, Maroldi R, van Engen A, Baujat B, Locati LD, Jensen AD,
Smeele L, et al: Salivary gland cancer: ESMO-European reference
network on rare adult solid cancers (EURACAN) clinical practice
guideline for diagnosis, treatment and follow-up. ESMO Open.
7:1006022022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hiraga S, Miyoshi Y and Kishimoto S:
Successful treatment of multiple pulmonary metastases of adenoid
cystic carcinoma of the external auditory meatus with TS-1. Gan To
Kagaku Ryoho. 31:1115–1117. 2004.(In Japanese). PubMed/NCBI
|
|
24
|
Matsumoto M, Nomiyama T, Nakae J and
Nishikawa H: Combination chemotherapy for a senile patient with
adenoid cystic carcinoma of the esophagus: A case report. Jpn J
Clin Oncol. 23:258–262. 1993.
|