Introduction
The retroperitoneal space is located in the
posterior abdomen, between the parietal peritoneum and the
transversalis fascia, and is divided into the pre-renal space,
pararenal posterior space, perirenal space and midline great vessel
area (1). Retroperitoneal tumors
originate from the retroperitoneal space, rather than from the
organs within it. They account for 0.1–0.2% of all tumors, with
malignant tumors being more common (2). The primary types of retroperitoneal
tumors include lymphoproliferative, soft tissue and extragonadal
germ cell tumors (3). Primary
retroperitoneal SCC is extremely rare with an unclear incidence
rate and a higher prevalence in female compared with men (4). The etiology of retroperitoneal SCC
remains unknown. Previous cases have mostly been associated with
human papillomavirus (HPV) infection or hysterectomy (3,5–10).
Surgical treatment is the optimal therapeutic approach for
retroperitoneal tumors. However, owing to the complex anatomical
location of the retroperitoneum, tumors often metastasize to
surrounding vessels and tissues. Moreover, there are differences in
treatment approaches between pathological types, such as
retroperitoneal squamous cell carcinoma and other retroperitoneal
tumors. Therefore, accurate preoperative tumor classification is
crucial for formulating effective surgical plans (11). Imaging studies, including CT and
MRI, are the preferred diagnostic modality for retroperitoneal
tumors. However, existing literature has predominantly focused on
therapeutic aspects, with relatively limited exploration of imaging
characteristics. Thus, the present article reports a case of
primary retroperitoneal SCC and provides an in-depth analysis of
its imaging features, aiming to provide valuable references and
guidance for clinical diagnosis and treatment.
Case report
Clinical presentation and
examination
A 49-year-old female patient was admitted to The
Fifth Affiliated Hospital of Zunyi Medical University (Zhuhai,
China) in July 2023 after presenting to the outpatient department
with an abdominal mass. An ultrasound examination revealed two
abnormal solid masses in the left adnexal region. The mass had been
detected 2 years earlier but was not given attention and the
patient did not seek medical consultation. The patient had a good
past health status, with an obstetric history of three pregnancies
and two deliveries. The patient had no history of tumors, no family
history of diseases, and no surgical history. The external
genitalia were normally developed, with a mature and parous
appearance. The vagina was patent, with a small amount of white
discharge visible inside. The cervix was smooth and of normal size,
with a closed cervical os. The uterus was reduced in size, soft in
consistency and without tenderness. No cervical motion tenderness
was observed. A solid mass, ~10.0×7.0 cm in size, could be palpated
in the left adnexal region. The mass was closely associated with
the surrounding tissues, with indistinct margins and poor mobility;
no tenderness was noted. No obvious abnormalities were palpable in
the right adnexal region. The rectal and anal examination was
normal, as was the colonoscopy.
Laboratory tests
The levels of tumor markers carcinoembryonic antigen
(CEA) and cancer antigen 125 (CA125) were 14.3 ng/ml (normal range,
≤5 ng/ml) and 65.8 U/ml (normal range, <35 U/ml), respectively.
Other routine blood tests, including liver and kidney function and
electrolyte levels, were normal.
Imaging studies
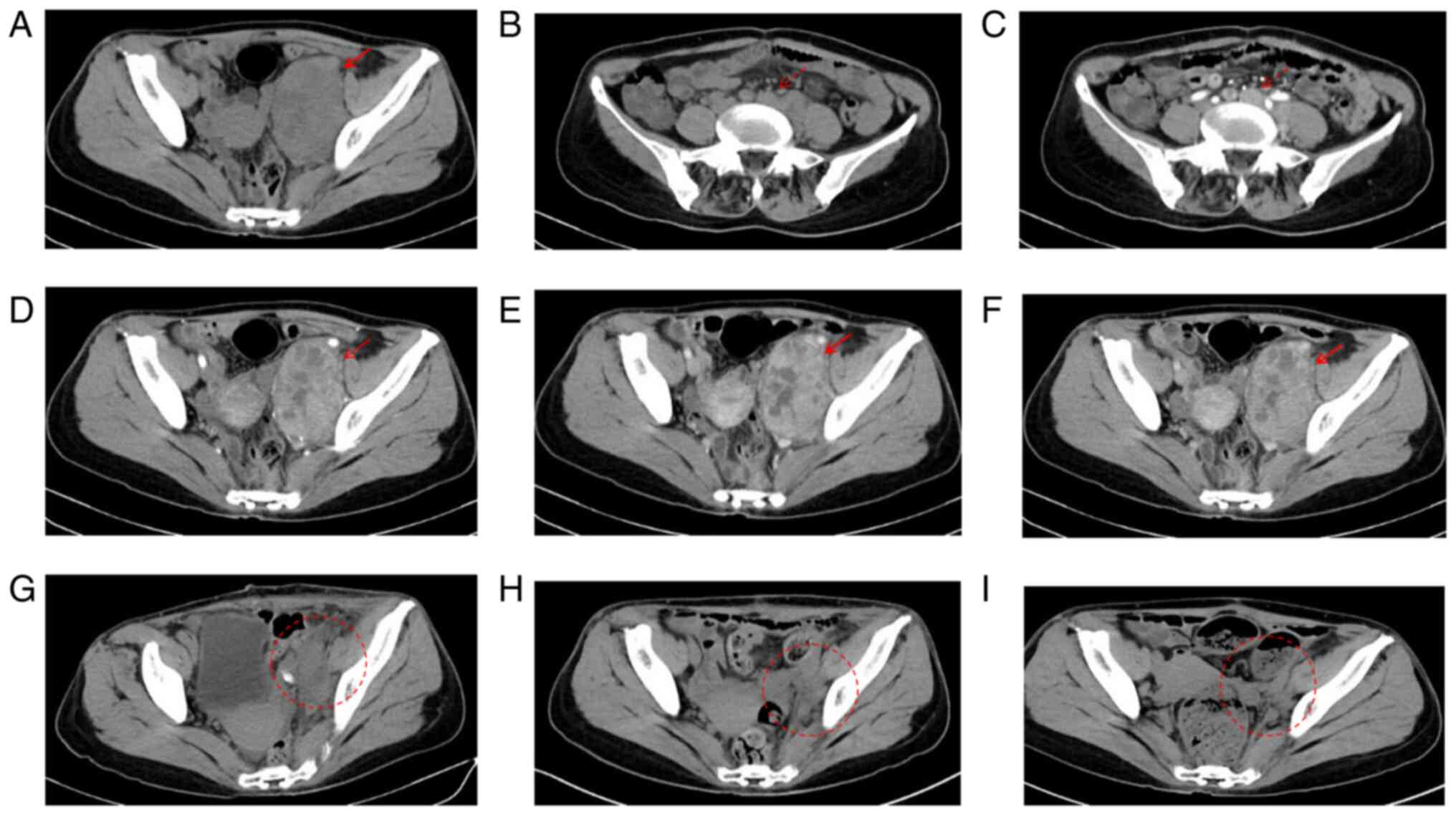
Contrast-enhanced spiral computed tomography (CT;
Fig. 1) revealed an irregular soft
tissue mass (81×59×105 mm) in the left pelvic retroperitoneum
(Fig. 1A), with inhomogeneous
density and moderate heterogeneous enhancement (Fig. 1D-F). Multiple enlarged lymph nodes
were observed around the abdominal aorta and left common iliac
artery (Fig. 1B), with the largest
measuring ~27×30 mm. These lymph nodes also exhibited heterogeneous
moderate enhancement on contrast-enhanced scanning (Fig. 1C). The bowel loops appeared normal
in morphology, with no evidence of mass lesions. No definite signs
of tumor recurrence were observed immediately following surgery, at
1 month postoperatively, and at 2 months postoperatively (Fig. 1G-I).
Pelvic magnetic resonance imaging (MRI; Fig. 2) revealed a mixed signal mass
(107×67×78 mm) in the left pelvic retroperitoneum, primarily
iso-intense on T1-weighted imaging (WI) (Fig. 2A, E) and iso-hyperintense on T2WI
(Fig. 2B), with restricted
diffusion (Fig. 2C-D) and marked
heterogeneous enhancement (Fig.
2F-I) in solid parts. An enlarged lymph node was visible above
the lesion (Fig. 2I), measuring
~20×12 mm, with restricted diffusion and mild to moderate
enhancement. CT and MRI suggested a left pelvic retroperitoneal
mass, likely a malignant schwannoma or leiomyosarcoma, with
multiple lymph node metastases.
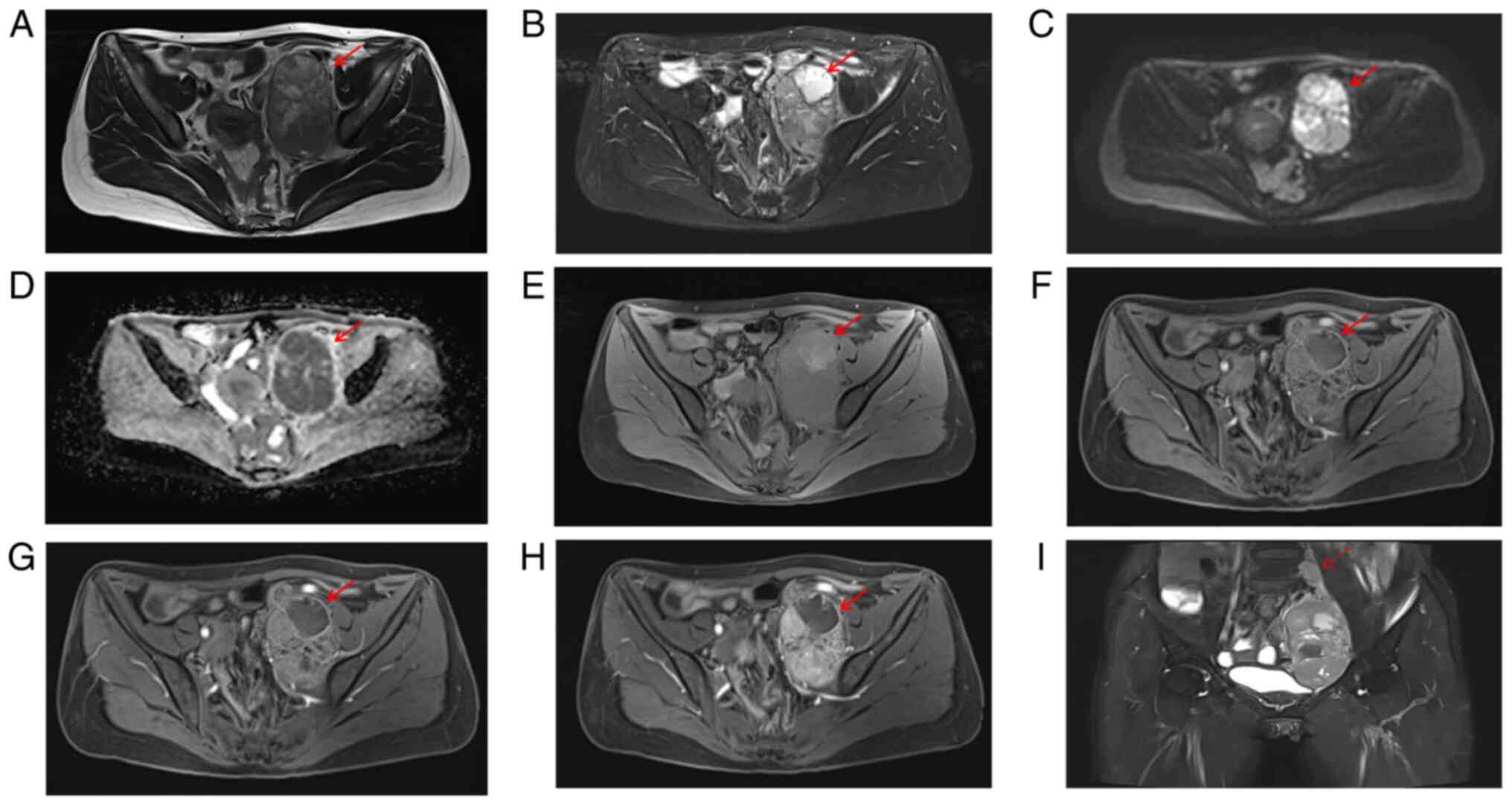 | Figure 2.Magnetic resonance imaging features of
the lesion. (A) Axial T1-weighted imaging (T1WI) demonstrates
heterogeneous signal intensity, with small patchy hyperintense foci
observed within the lesion, suggesting possible intratumoral
hemorrhage. (B) Axial T2-weighted imaging with fat-suppression
shows heterogeneous signal intensity, with small patchy
hyperintense areas noted, indicating possible cystic degeneration
or necrosis within the tumor. (C) Diffusion-weighted imaging (DWI)
reveals hyperintense signal within the lesion. (D) The apparent
diffusion coefficient (ADC) map shows corresponding hypointensity.
(E) Axial T1-weighted imaging with fat-suppression displays
heterogeneous signal intensity, with small patchy hyperintense foci
visible within the tumor. (F) In the arterial phase, the tumor
exhibits marked but heterogeneous enhancement. (G) In the venous
phase, the tumor continues to show marked heterogeneous
enhancement, which is more pronounced compared to the arterial
phase. (H) In the delayed phase, the tumor demonstrates persistent
heterogeneous enhancement, with small patchy hypointense areas
indicating non-enhancing regions. (I) Coronal T2-weighted imaging
with fat suppression reveals regional lymph nodes, which show
heterogeneous enhancement on contrast-enhanced imaging. The
adjacent bladder and uterus are compressed and displaced to the
right. Solid arrows indicate the tumor area; dashed arrows indicate
enlarged lymph nodes. DWI, diffusion-weighted imaging; ADC,
apparent diffusion coefficient. |
Surgery and pathology
The patient underwent resection of the
retroperitoneal lesion and regional lymphadenectomy.
Intraoperatively, the tumor was found in the left pelvic
retroperitoneum, adhering to the left pelvic wall and iliopsoas
muscle (12×10 cm), compressing the left adnexa, sigmoid colon and
rectum. The left internal and external iliac vessels, fallopian
tube, femoral nerve and obturator nerve were infiltrated.
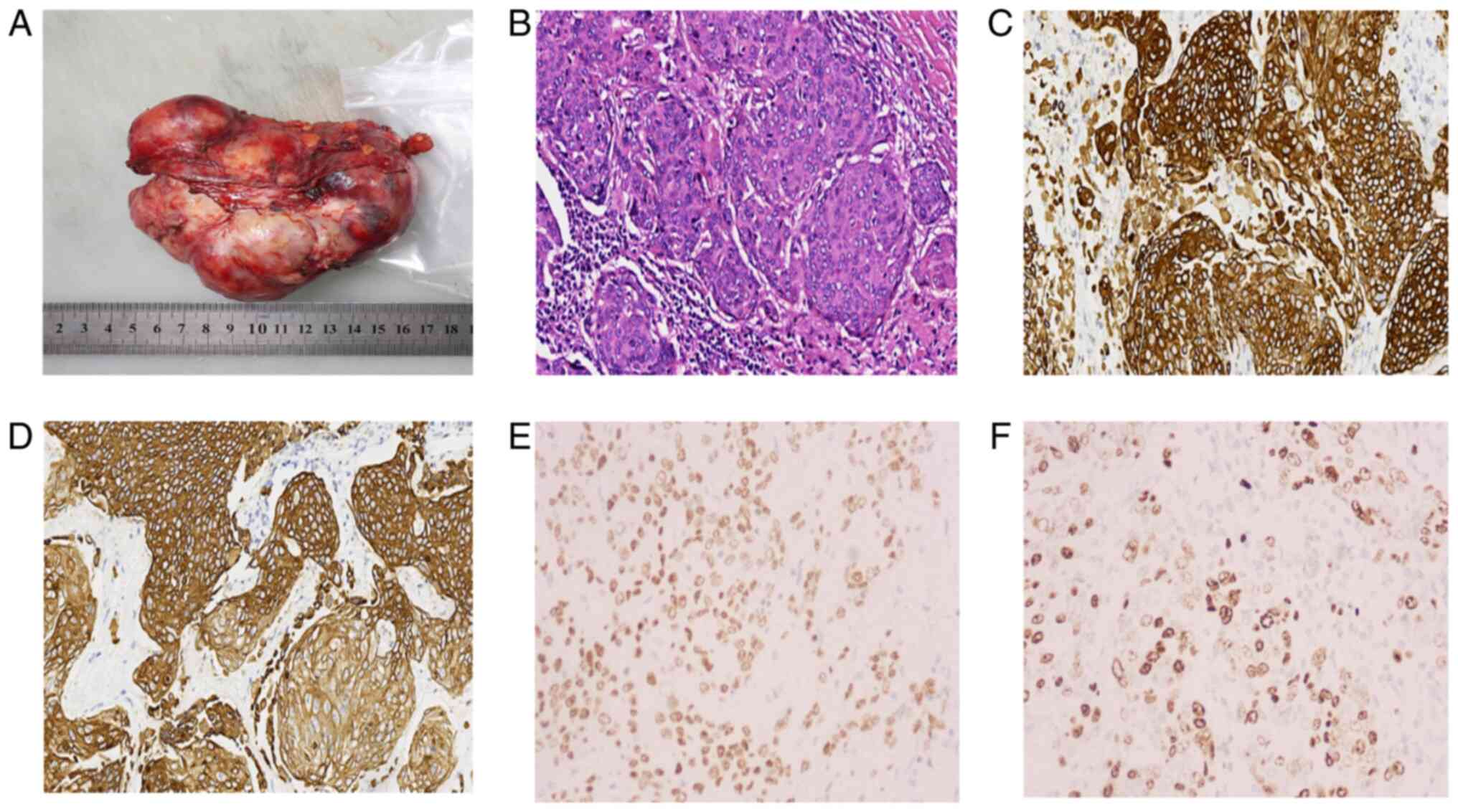
Gross pathological examination
A single grayish-white mass measuring 11.5×9×5 cm
with a multinodular appearance was observed (Fig. 3A). The cut surface was grayish-white
to pale yellow with multiple nodules. The cystic spaces contained
purulent fluid and pale yellow necrotic foci. The texture was
finely textured, resembling fish flesh. Postoperative pathology
confirmed poorly differentiated retroperitoneal SCC (Fig 3B). Following surgical resection,
tissue specimens were fixed in 10% neutral buffered formalin at
room temperature for 24 h, subjected to routine dehydration and
paraffin embedding, and sectioned at 4 µm thickness. After mounting
on Leica Bond Plus slides, all immunohistochemical staining
procedures were conducted on the Leica BOND-MAX fully automated
system. Heat-induced epitope retrieval was performed at 100°C for
20 min using the instrument's proprietary EDTA-based alkaline
retrieval solution (BOND Epitope Retrieval Solution 2, AR9640;
Leica Biosystems). Subsequently, Leica BOND™ Primary Antibody
Diluent (DS9800; Leica Biosystems) was used as a blocking reagent;
this ready-to-use reagent was incubated at room temperature
according to the instrument's preset protocol. Then, the following
ready-to-use primary antibodies from Anbiping (China) were applied
directly and incubated at room temperature for 30 min:
Pan-Cytokeratin (clone AE1/AE3; Catalog No. IM067-4), CK5/6 (clone
D5/16B4; Catalog No. IM060-4), p40 (clone BC28; Catalog No.
IM257-4), and Ki-67 (clone MIB-1; Catalog No. IM098-4). Detection
was performed using the Leica BOND™ Polymer Refine Detection System
(HRP-based system; catalog No. DS9800, Leica Biosystems) at 25°C
for 8 min. Finally, chromogenic development was performed with the
Leica BOND DAB Refine kit (DS9800; Leica Biosystems), and all
stained sections were examined and imaged using an Olympus BX53
light microscope. The quantification of Ki-67-positive cells was
performed using the Mshot Microscope Digital Measurement Analysis
System (Guangzhou Micro-shot Technology Co., Ltd.). At least five
representative fields of view were randomly selected from each
sample and a 40× objective lens (total magnification 400×) was
used. A total of two pathologists blinded to the grouping,
independently counted the number of positive nuclei among all tumor
cells. The final results were expressed as the percentage of
positive cells (Ki-67 index). The final immunohistochemical results
(Fig. 3C-F) showed Pan-Cytokeratin
(+), CK5/6 (+), p40 (+) and Ki-67 (+, 70%).
The patient received adjuvant chemotherapy (167
mg/m2 paclitaxel + 0.3 g/m2 carboplatin)
every 4 weeks for six cycles. The patient was followed up via
telephone every 2 months for a total of 12 months. During the
follow-up period, the patient was in good condition without any
signs of recurrence.
Discussion
SCC is a malignant tumor that often occurs in areas
covered by squamous epithelium, such as the skin, esophagus, cervix
and vagina (12). However,
retroperitoneal SCC is extremely rare, with only nine articles and
16 cases reported globally, and its etiology remains unclear. SCC
may be associated with metaplasia as a result of chronic irritation
during the embryonic dormancy period (13). SCC has also been linked to
endometriosis (7) and HPV infection
(14). Currently, studies on
retroperitoneal SCC are lacking, and primarily rely on individual
case reports that focus on treatment options, with little
description or summary of its imaging features. Therefore, the
present article reports a case of primary retroperitoneal SCC and
analyzes its imaging manifestations to provide a reference for
future diagnosis and treatment.
CT and MRI are the primary imaging modalities for
retroperitoneal tumors. Distinguishing whether a lesion is
retroperitoneal or organ-derived is crucial for accurate diagnosis
and treatment. In the present case, imaging demonstrated that the
adjacent uterus was displaced to the right, the tumor encircled the
left external iliac artery and its pedicle extended outward behind
the ilium, suggesting a retroperitoneal origin. CT indicated that
the tumor had an inhomogeneous density with mixed high and low
densities, which may be caused by abnormal vascular structures
prone to hemorrhage, resulting in high densities, and tumor
size-related uneven blood supply, vascular invasion and necrosis,
leading to low densities. Enhanced scanning revealed heterogeneous
marked enhancement, likely due to angiogenesis-promoting factors
such as VEGF, but with unenhanced or weakly enhanced areas
resulting from rapid tumor growth, hypoxia, necrosis or vascular
compression/occlusion.
MRI, with its superior soft tissue resolution,
complements CT in assessing such tumors. Using T1WI, small
high-signal areas suggested possible hemorrhage, whereas mixed
signals on T2WI and fat-suppressed T2WI indicated possible cystic
change and necrosis, corroborating the CT findings.
Diffusion-weighted imaging revealed high signals, and apparent
diffusion coefficient maps showed low signals, indicating
restricted diffusion. This restricted diffusion may be attributed
to high cell density, which reduces the extracellular space, and
tumor stromal fibrosis, forming a meshwork that hinders water
diffusion. The irregular shape of the tumor, caused by inconsistent
growth rates in different directions, further suggested malignancy.
Retroperitoneal SCC predominantly manifests as cystic lesions
(3,8), differing from the present case. This
variability highlights the non-specific imaging features of
retroperitoneal SCC, likely due to the limited number of reported
cases. Nonetheless, the present case provides a valuable reference
for clinical practice, highlighting the importance of considering
this rare tumor in the diagnosis of retroperitoneal tumors, given
its distinct treatment and prognosis.
Retroperitoneal SCC should be differentiated from
retroperitoneal schwannoma and leiomyosarcoma. retroperitoneal
schwannoma typically has a complete capsule, often exhibits soft
tissue density, is prone to cystic changes with possible
calcification and demonstrates progressive delayed enhancement on
imaging (15). On a T2WI sequence
of MRI, it presents with slightly high central signal and markedly
high marginal signal, or multiple ring-shaped low signals in a
high-signal background, which is characteristic of retroperitoneal
schwannoma. By contrast, retroperitoneal leiomyosarcoma typically
appears as an inhomogeneous mass, is prone to necrosis and cystic
change, is richly vascularized and shows uneven and marked
enhancement on imaging. Moreover, it tends to invade crucial
structures, such as the inferior vena cava and renal veins
(16).
The diagnosis of retroperitoneal SCC is challenging
due to its low incidence and non-specific clinical manifestations.
A comprehensive judgment integrating multiple sources of
information is required. In addition to CT and MRI, PET/CT can be
used to detect primary lesions and metastases, providing a
reference for subsequent surgical and treatment plans. Percutaneous
CT-guided biopsy is a crucial method for differentiating tumors. In
the present case, this examination was not performed because the
patient refused, and the relevant ethical implications were
considered.
Most patients with retroperitoneal SCC lack typical
clinical symptoms in the early stages. Symptoms such as abdominal
masses, perineal pain, constipation, urinary retention and lower
limb thrombosis may appear as the tumor grows and compresses
surrounding tissues or organs. Some cases also present with
symptoms of distant metastasis (14). In the present case, despite the
large size of the tumor, the patient only presented with a palpable
mass in the lower abdomen and did not experience other symptoms
such as lower gastrointestinal discomfort, which may be attributed
to the relatively spacious retroperitoneal space. Additionally,
both the whole-abdomen spiral CT scan and colonoscopy revealed no
notable abnormalities, suggesting that the patient's intestines
were likely not invaded. Therefore, lower gastrointestinal
symptoms, such as hematochezia and changes in bowel habits, were
absent.
The majority of reported patients are female, and
most are HPV-positive or have a history of gynecological surgery.
In patients with a history of gynecological surgery,
retroperitoneal SCC may have resulted from contamination during the
surgical procedure, leading to iatrogenic viral or tumor cell
deposition, or as a result of microscopic lymphatic spread from the
cervix, vaginal vault or anal canal to the retroperitoneal region.
Retroperitoneal SCC is also associated with endometriosis; however,
the exact pathological mechanisms remain unclear (14). Nonetheless, deep-infiltrating
endometriosis is associated with somatic mutations in the
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit a,
KRAS and AT-rich interactive domain-containing protein 1A genes
(17). In HPV-positive cases,
retroperitoneal SCC may be caused by the oncoprotein E6, which
degrades p53 via the ubiquitin-proteasome pathway. This degradation
disrupts DNA repair and apoptosis regulation, as well as E7 binding
and inactivation of the retinoblastoma protein, thereby relieving
its inhibition of the G1/S phase transition in the cell
cycle. This drives abnormal cell proliferation and genomic
instability, ultimately leading to malignant transformation. When
diagnosing retroperitoneal SCC in female patients, differentiating
it from gynecological tumors is crucial. Tumor markers such as
CA125 and CA19-9 can help distinguish ovarian masses from
retroperitoneal tumors, whereas a positive SCC antigen is
indicative of the diagnosis of retroperitoneal SCC (8).
The immunohistochemistry results of the present case
showed positivity for Pan-Cytokeratin, CK5/6, Ki-67 and p40, which
is similar to previous reports where Pan-Cytokeratin, CK5/6, p63,
p40, and p16 were positive in retroperitoneal SCC (3,5,7–10,18).
This finding suggests that Pan-Cytokeratin, CK5/6, Ki-67 and
p40-positivity may be associated with retroperitoneal SCC. However,
this finding is based on a limited number of cases and may not be
representative of a broader population. Therefore, further research
is needed to confirm this association.
The treatment strategy for retroperitoneal SCC is
not well-defined. Surgery is the primary treatment, often followed
by chemotherapy and radiotherapy. The extent of surgical resection
depends on whether the tumor metastasized to or invaded adjacent
organs. Surgical resection ranges from simple tumor removal to
extensive surgery, including resection of nearby organs and
affected segments, as well as regional lymph node dissection. In
chemotherapy, taxanes and platinum-based drugs show some efficacy
in SCC; however, no specific guidelines have been established for
retroperitoneal SCC owing to its rarity. Neoadjuvant chemotherapy
can be used to downstage the tumor for patients who cannot undergo
surgical resection (14). Patients
receiving concurrent chemoradiotherapy have improved clinical
outcomes. In the present case, the patient underwent surgery and
subsequently returned to the hospital regularly for 6 months of
postoperative chemotherapy. As of August 2024, close follow-up has
shown the patient to be stable with no signs of tumor
recurrence.
There are several limitations to the present case.
HPV testing was not performed. Future case studies should include
HPV testing to clarify its role in the pathogenesis of
retroperitoneal SCC. Squamous cell carcinoma antigen was not
tested. Future cases should include this test to aid in accurate
diagnosis. 3. Percutaneous CT-guided core biopsy was not conducted.
Future cases should prioritize percutaneous CT-guided core biopsy
and emphasize educating patients about the safety of the biopsy
procedure to optimize the sequence of treatment.
In conclusion, retroperitoneal SCC is a rare disease
lacking typical clinical and imaging features, making preoperative
diagnosis particularly challenging. Thus, further analysis of the
imaging features in the present case is crucial for future clinical
practice, enabling the development of individualized treatment
plans and ensuring maximum patient benefits.
Acknowledgements
Not applicable.
Funding
The authors thank the National Natural Science Foundation of
China (grant no. 82260341) and the Technology Plan Fund of Guizhou
Provincial [grant no. Qiankehe Foundation-ZK (2022) General 634]
for their financial support of the present study.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FZ performed data acquisition, and drafted, reviewed
and edited the manuscript. YG designed the methodology, validation
and reviewing and editing the manuscript. JZ conceived and designed
the study and was responsible for the acquisition of funding. FZ
and YG confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was granted exemption by the
ethics committee of The Fifth Affiliated Hospital of Zunyi Medical
University. The study was performed in accordance with the 1964
Declaration of Helsinki and subsequent amendments.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the present case report and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mota MMDS, Bezerra ROF and Garcia MRT:
Practical approach to primary retroperitoneal masses in adults.
Radiol Bras. 51:391–400. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuqin D, Shi H and Ji Z: Imaging in
diagnosis and treatment of retroperitoneal tumor: application and
expert consensus interpretation. J Surg Concepts Pract. 27:511–516.
2022.
|
|
3
|
Oh HJ, Park EH, Lee YB, Hu J, Lee GJ, Chun
SH, Lee MY, Lee DW, Kim J and Jin JY: HPV-related retroperitoneal
squamous cell carcinoma of unknown primary: A case report. Cancer
Res Treat. 47:954–957. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan H and Lin SD: Case report: HPV related
pelvic retroperitoneal squamous cell cancer of unknown primary
presenting as ovary neoplasm. Int J Surg Case Rep. 125:1105282024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matylevich OP, Kurchankou MA, Kopsсhaj PA
and Schmeler KM: HPV-related metastatic retroperitoneal pelvic
squamous cell carcinoma of unknown primary origin in a patient
previously treated for endometrial cancer. Int J Surg Case Rep.
118:1096242024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yewedalsew SF, Abramowitz BR, Goswami S,
Ahlawat S, Gupta TR and Yu Q: HPV-associated retroperitoneal
squamous cell carcinoma of unknown origin: A case report. Am J
Gastroenterol. 118:S1992–S1993. 2023. View Article : Google Scholar
|
|
7
|
Cucinella G, Sozzi G, Di Donna MC, Unti E,
Mariani A and Chiantera V: Retroperitoneal squamous cell carcinoma
involving the pelvic side wall arising from endometriosis: A case
report. Gynecol Obstet Invest. 87:159–164. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuzaka Y, Yamaguchi K, Moriyoshi K,
Takao Y, Takakura K and Konishi I: Primary retroperitoneal squamous
cell carcinoma: A case report with review of the literature. Int
Cancer Conf J. 8:61–65. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Isbell A and Fields EC: Three cases of
women with HPV-related squamous cell carcinoma of unknown primary
in the pelvis and retroperitoneum: A case series. Gynecol Oncol
Rep. 16:5–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clements A, Euscher E, Lacour R, Merritt
W, Klopp A and Ramondetta L: The presence of human papillomavirus
or p16 in six cases of retroperitoneal carcinoma. Obstet Gynecol.
116:1042–1046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Y, Ta N, Du Z, Qu X, Geng G, Wang S,
Shi T, Feng X and Chen R: Pathological diagnosis of retroperitoneal
mass—an analysis of 1 050 cases in a single center. Acad J Naval
Med Uni. 44:272–277. 2023.(In Chinese).
|
|
12
|
Gliagias V, Wotman M, Herman SW,
Costantino P, Kraus D and Tham T: Investigating the role of octamer
binding transcription Factor-4 (Oct-4) in oral cavity squamous cell
carcinoma: A systematic review and meta-analysis. Am J Otolaryngol.
40:282–288. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schatz JE and Colgan TJ: Squamous
metaplasia of the peritoneum. Arch Pathol Lab Med. 115:397–398.
1991.PubMed/NCBI
|
|
14
|
Pandey A, Kumar D, Gupta P, Khosla D,
Periasamy K and Kapoor R: Primary retroperitoneal squamous cell
carcinoma: A literature review. J Cancer Res Clin Oncol.
149:12507–12512. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang S, Shang L, Zhong Y, Xing X, Xu H and
Li T: Analysis on the image performances of CT and MR on
retroperitoneal schwannomas. China Med Equipment. 20:44–48.
2023.
|
|
16
|
Shu Q, Shi H and Lu C: Case analysis:
Typical imaging manifestations, pathological basis, and
differential diagnosis of retroperitoneal Schwannoma. China J Bases
Clin Gen Surg. 32:300–304. 2025.(In Chinese).
|
|
17
|
Anglesio MS, Papadopoulos N, Ayhan A,
Nazeran TM, Noë M, Horlings HM, Lum A, Jones S, Senz J, Seckin T,
et al: Cancer-associated mutations in endometriosis without cancer.
N Engl J Med. 376:1835–1848. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahdallah R, Morse B, Hakam A and Shahzad
MM: Pelvic squamous cell carcinoma of unknown primary: A case
report and review of the literature. Eur J Gynaecol Oncol.
37:430–433. 2016.PubMed/NCBI
|