Introduction
Pancreatic ductal adenocarcinoma (PDAC) is among the
most lethal malignancies, characterized by an extremely poor
prognosis (1,2). In recent years, neoadjuvant
chemotherapy has become the standard treatment for borderline
resectable PDAC (3–5). Moreover, even in patients initially
diagnosed with unresectable PDAC, surgical resection following a
favorable response to chemotherapy has been shown to improve
survival outcomes (6).
Additionally, several studies suggest that neoadjuvant chemotherapy
may also be beneficial for patients with resectable PDAC (7,8). These
findings demonstrate the important role of chemotherapy in the
multidisciplinary management of PDAC.
Several chemotherapeutic regimens are currently
available for the treatment of PDAC. However, their efficacies vary
among patients. In some cases, tumor progression has been observed
despite treatment, resulting in loss of resectability (9). Therefore, the development of
personalized treatment strategies based on tumor characteristics is
becoming an important consideration for optimizing the selection of
chemotherapy for PDAC.
In a previous study, we found that a higher tumor
blood flow (TBF) prior to treatment was associated with a better
response to neoadjuvant chemotherapy (10). However, that study was limited by
its small sample size and lack of stratification according to the
chemotherapy regimen. Furthermore, PDAC generally possesses an
intense stromal pathology surrounding cancer cells, which is called
desmoplastic stroma (2,11). Desmoplastic stroma leads to high
intra-tumoral tissue pressure, blood vessel collapse, and decreased
tumor perfusion (12). Chemotherapy
may affect desmoplastic stroma by reducing the number of cancer
cells. Furthermore, the previous report described the experience of
tumor molecular and cellular alterations in PDAC (13). Therefore, it is important to
evaluate not only pre-treatment TBF but also post-treatment TBF.
Although one study examined changes in TBF after chemotherapy
(14), a comparison between pre-
and post-treatment TBF according to chemotherapy regimen has not
been explored.
The aim of this study was to evaluate the
association between pre-treatment TBF and histopathological
therapeutic response stratified by chemotherapy regimen and to
assess changes in TBF before and after chemotherapy. In addition,
intra-tumoral vessels, which may affect the TBF, were evaluated
using immunostaining.
Materials and methods
Patient selection
We conducted a retrospective review of the
electronic medical records at Shiga University of Medical Science
Hospital between January 2011 and December 2023 to identify
patients who underwent pancreatectomy following preoperative
chemotherapy or chemoradiotherapy for PDAC. The medical records of
the patients were accessed for the purpose of this study in April
2024. A total of 58 consecutive patients who underwent
contrast-enhanced computed tomography (CECT) before the initiation
of preoperative therapy were included. Clinical and pathological
data were collected for the analysis.
This study was approved by the Ethics Committee of
Shiga University of Medical Science (approval number R2017-171) and
was conducted in accordance with the principles outlined in the
Declaration of Helsinki. Informed consent was obtained from all the
patients or their legal representatives on an opt-out basis.
CECT examinations protocol and image
analysis
Three-phase CECT examinations were performed using a
64-detector row computed tomography (CT) scanner (Aquilion CX
Edition; Canon Medical Systems Corporation, Tochigi, Japan). An
intravenous bolus of 100 ml of nonionic iodinated contrast material
(Iopamiron®, Bayer Schering Pharma, Berlin, Germany) was
administered at a dose of ≥600 mgI/kg body weight and an injection
rate of 3 ml/s through the median cubital vein. Scans were acquired
with a slice thickness of 5 mm according to institutional
protocols. Imaging was performed at 35 s (arterial phase), 70 s
(portal venous phase), and 130 s (late phase) after contrast
injection.
CT images were independently reviewed by two
experienced surgeons, each with >10 years of expertise in
pancreatic imaging, who were blinded to all clinical and
pathological data. Tumor and aortic attenuation values were
measured during each phase (non-enhanced, arterial, portal venous,
and late) using a picture archiving and communication system
(ShadeQuest/ViewR-DG; Yokogawa Medical Solutions Corporation,
Tokyo, Japan). In cases of discrepancies, a consensus was reached
through a joint review.
Tumor attenuation values (TAV) and aortic
attenuation values (AAV) were determined following a previously
described methodology (10,15). Regions of interest were drawn to
encompass as much of the tumor and aorta as possible, while
avoiding their margins. For tumor measurements, cystic or vascular
regions were excluded. The average of three measurements per phase
was recorded. In all cases, the peak aortic enhancement occurred at
35 s.
TBF was calculated using the maximum slope method
[10, 14]:
The previous study demonstrated that TBF ≥0.36
s−¹ provides prediction of the destruction of over 50%
of tumor cells in PDAC (10).
Hence, the patients were classified into two groups according to
the pre-treatment TBF: the high TBF group (TBF ≥0.36
s−¹) and the low TBF group (TBF <0.36
s−¹).
Assessment of therapeutic pathological
response
The therapeutic pathological response was graded
according to the Japanese classification of pancreatic carcinoma by
the Japan Pancreas Society: Eight edition (16). The evaluations were performed by two
specialized pancreatic pathologists who were blinded to the
clinical outcomes. Hematoxylin and eosin-stained sections from the
largest cross-section of the tumor were assessed.
Assessment of intra-tumoral vessels by
CD31 immunostaining
The tissue specimens were cut into 4 µm slices from
10% formalin-fixed and paraffin-embedded blocks. The specimens were
then deparaffinized and rehydrated, followed by antigen retrieval
by heating the slides in distilled water at 98°C for 45 min with an
antigen retrieval solution (Immunosaver®, Nisshin EM,
Tokyo, Japan). The slides were then treated in 3%
H2O2 in methanol for 10 min at 25°C to block
endogenous peroxidase activity. To prevent nonspecific protein
binding, the slides were treated with Blocking One Hist (Nacalai
Tesque) and incubated overnight with a monoclonal antibody against
CD31 (1:50, MA5-16337, Invitrogen, CA, USA). Subsequently, they
were incubated with horseradish peroxidase-labeled
polymer-conjugated secondary antibody [Simple Stain MAX PO (MULTI);
Nichirei Bioscience] at 25°C for 30 min. The antigen was visualized
by diaminobenzidine staining (#415172; Nichirei Bioscience)
chromogen for 2–5 min. Finally, the slides were counterstained with
hematoxylin, dehydrated, and mounted. Negative controls were
prepared by excluding the primary antibodies.
The tumor sections were examined under a microscope.
Image analysis was performed using the ImageJ software (National
Institutes of Health, Bethesda, MD, USA). The CD31-positive area
ratio was determined by evaluating five randomly selected fields,
excluding the adenocarcinoma ductal structures. For vessel
quantification, five high-power fields (200× magnification) were
selected and patent (open-lumen) vessels were visually counted.
Clinical data collection and
statistical analysis
The patient characteristics were compared between
the low and high TBF groups. Categorical variables are expressed as
numbers and percentages (%), whereas continuous variables are
expressed as medians with interquartile ranges. Fisher's exact test
(for categorical variables) and the Mann-Whitney U test (for
continuous variables) were used to evaluate significant differences
between the two groups. The Jonckheere-Terpstra trend test was used
to examine the trends. The Wilcoxon signed rank test was used to
evaluate significant difference between pre-treatment and
post-treatment TBF in each regimen. Spearman's rank correlation
analysis was performed to evaluate the relationship between the
intra-tumoral vessels and the post-treatment TBF. In two-tailed
tests, P<0.05 was considered statistically significant. All
statistical analyses were performed using EZR (Saitama Medical
Center, Jichi Medical University, Saitama, Japan), a graphical user
interface for R software (The R Foundation for Statistical
Computing, version 2.13.0, Vienna, Austria) (17) and Bell Curve for Excel software
version 3.23 (Social Survey Research Information Co., Ltd., Tokyo,
Japan).
Results
Comparison of clinical features
between high and low TBF groups
A total of 58 patients were enrolled in the study.
Of these, 35 (60.3%) were categorized into the low TBF group and 23
(39.7%) into the high TBF group. The treatment regimens included
gemcitabine plus S-1 (GS, n=23), gemcitabine plus nab-paclitaxel
(GnP, n=18), and modified FOLFIRINOX (mFOLFIRINOX, n=17). The
clinical characteristics of each group are summarized in Table I. There were no significant
differences between the groups in pre-treatment tumor size or tumor
marker levels, including carcinoembryonic antigen, carbohydrate
antigen 19-9 (CA19-9), and Duke pancreatic monoclonal antigen type
2 (DUPAN-2). Additionally, no significant differences were observed
in pre-treatment duration, treatment regimen, or resectability
classification. Furthermore, the pre-treatment duration showed no
significant differences between low and high TBF groups according
to the treatment regimen (P=0.708 in GS, P=0.138 in GnP, and
P=0.768 in mFOLFIRINOX, respectively). The patients who received
chemoradiotherapy did not show significant differences between low
and high TBF groups (8.6% vs. 8.7%, P=1.000). However,
post-treatment tumor size (P=0.046) and DUPAN-2 (P=0.044) were
significantly lower in the high TBF group. Moreover,
pancreaticoduodenectomy was performed significantly more frequently
in the high TBF group (P=0.004). Although no significant difference
was found in the RECIST classification between groups, the
prevalence of a therapeutic pathological response of Grade II or
higher was significantly greater in the high TBF group (P=0.027).
The overall survival was significantly better in the high TBF group
than that in the low TBF group (not reached vs. 27 months,
P=0.043). Furthermore, the progression-free survival was better in
the high TBF group than that in low TBF group (55 months vs. 10
months, P=0.002).
 | Table I.Clinical features in the high and low
pre-treatment TBF groups. |
Table I.
Clinical features in the high and low
pre-treatment TBF groups.
| Factors | Low TBF group
(n=35) | High TBF group
(n=23) | P-value |
|---|
| Sex, male | 25 (71.4) | 11 (47.8) | 0.098 |
| Age, years | 65.0 (62.5,
71.5) | 69.0 (61.0,
72.0) | 0.556 |
| Resectability |
|
| 0.286 |
|
Resectable | 14 (40.0) | 8 (34.8) |
|
|
Borderline-resectable | 10 (28.6) | 11 (47.8) |
|
|
Unresectable | 11 (31.4) | 4 (17.4) |
|
| Pre-treatment CEA,
ng/dl | 4.0 (2.5, 5.5) | 4.0 (3.2, 7.2) | 0.643 |
| Pre-treatment CA19-9,
U/l | 114 (33, 215) | 114 (33, 215) | 0.279 |
| Pre-treatment
DUPAN-2, U/l | 270 (74, 1,400) | 145 (37, 1,020) | 0.698 |
| Pre-treatment tumor
size, mm | 32.4 (28.0,
44.0) | 26.2 (23.5,
37.3) | 0.057 |
| Pre-treatment
regimen |
|
| >0.999 |
| GS | 14 (40.0) | 9 (39.1) |
|
|
GnP | 11 (31.4) | 7 (30.4) |
|
|
mFOLFIRINOX | 10 (28.6) | 7 (30.4) |
|
| Pre-treatment
period, months | 3.0 (2.0, 5.0) | 3.0 (2.0, 4.0) | 0.453 |
| Chemoradiotherapy,
yes | 3 (8.6) | 2 (8.7) | >0.999 |
| Post-treatment CEA,
ng/dl | 4.7 (2.4, 5.7) | 3.6 (2.9, 6.4) | 0.905 |
| Post-treatment
CA19-9, U/l | 41 (23, 77) | 33 (18, 59) | 0.279 |
| Post-treatment
DUPAN-2, U/l | 98 (25, 338) | 25 (25, 92) | 0.044a |
| Post-treatment
tumor size, mm | 26.2 (19.0,
33.5) | 22.1 (17.5,
27.5) | 0.046a |
| RECIST
classification |
|
| 0.427 |
|
Progressive disease | 1 (2.9) | 1 (4.3) |
|
| Stable
disease | 23 (65.7) | 11 (47.8) |
|
| Partial
response | 11 (31.4) | 11 (47.8) |
|
| Surgical procedure,
PD | 18 (51.4) | 21 (91.3) | 0.004a |
| Pathological
response, at least grade II | 8 (22.9) | 12 (52.2) | 0.027a |
Association between TBF and
therapeutic pathological response in each regimen
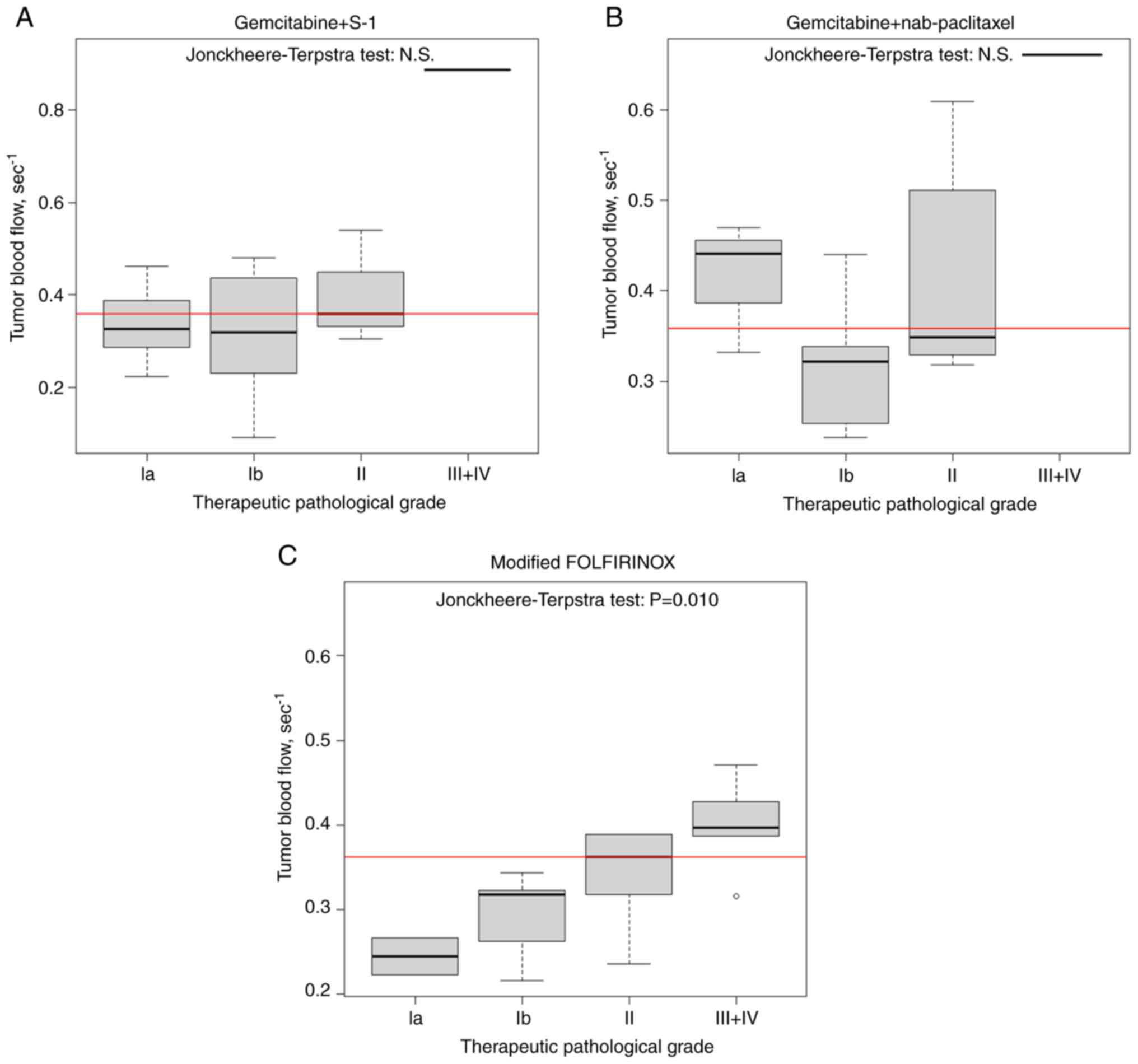
Fig. 1 illustrates
the association between TBF and therapeutic pathological responses.
In the mFOLFIRINOX group, a higher TBF was significantly associated
with a better therapeutic pathological response, as assessed using
the Jonckheere-Terpstra test (P=0.010). In contrast, TBF was not
associated with therapeutic response in patients receiving GnP
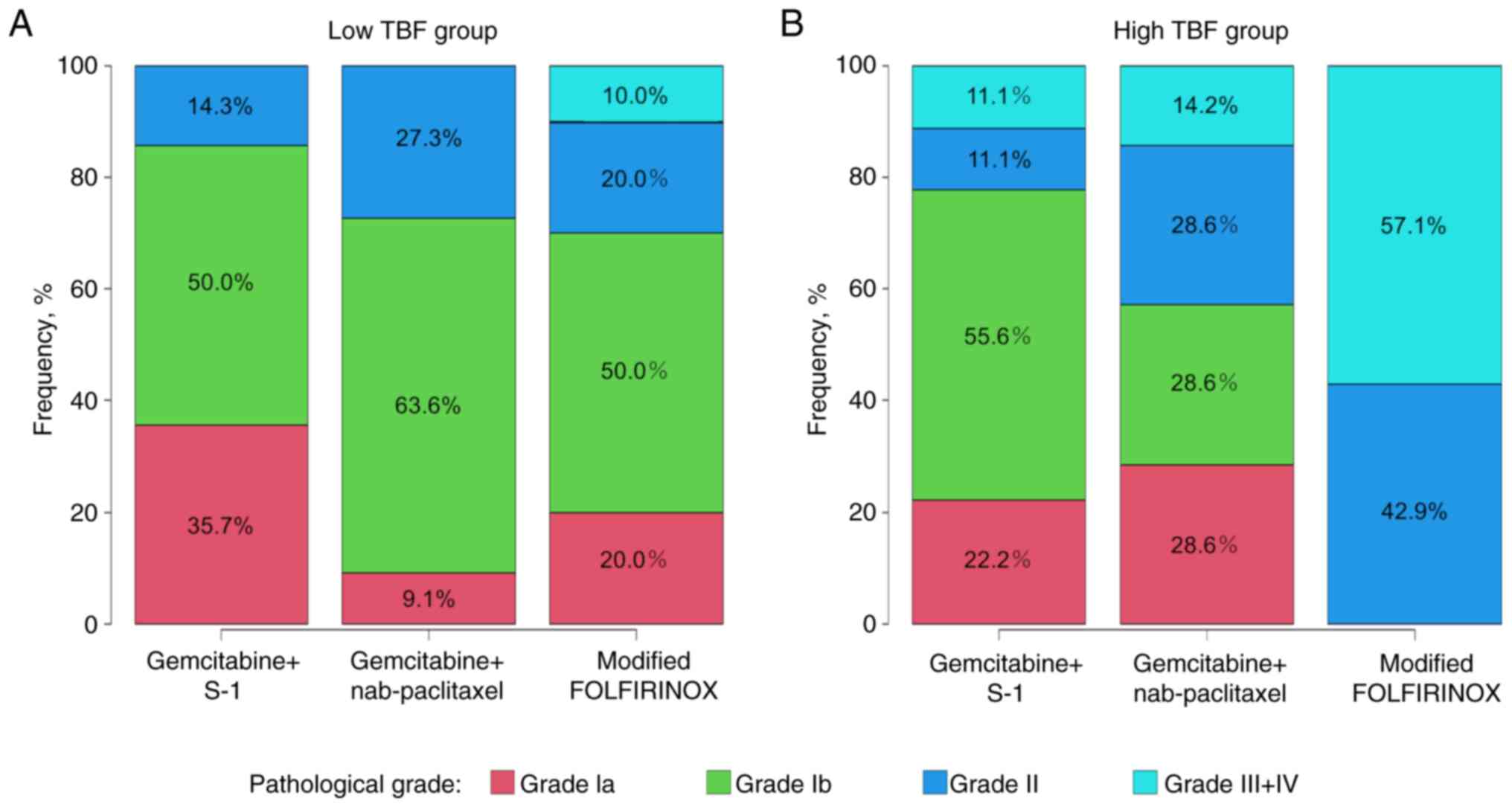
(P=0.490) or GS (P=0.387). Fig. 2
shows the therapeutic pathological response according to regimen in
the low and high TBF groups. In the low TBF group, approximately
90% of patients treated with GnP achieved a therapeutic
pathological response of Grade Ib or higher, compared to 80% of
patients treated with mFOLFIRINOX and approximately 65% of those
treated with GS. These findings suggest that GnP is more effective
than mFOLFIRINOX or GS in patients with a low TBF. Conversely, in
the high TBF group, mFOLFIRINOX demonstrated greater efficacy than
GnP or GS.
Between January 2024 and August 2025, we treated few
new cases; Case 1: Patient with resectable PDAC who received
mFOLFIRINOX for 2 months, followed by radical resection. The TBF
was 0.43, and the therapeutic pathological response was Grade II.
Case 2: Patient with resectable PDAC who received GnP for 2 months,
followed by radical resection. The TBF was 0.24, and the
therapeutic pathological response was Grade II. Case 3: Patient
with resectable PDAC who received GS for 2 months, followed by
radical resection. The TBF was 0.43, and the therapeutic
pathological response was Grade Ia. The medical records of these
patients were accessed in August 2025.
Change in TBF following treatment in
each regimen
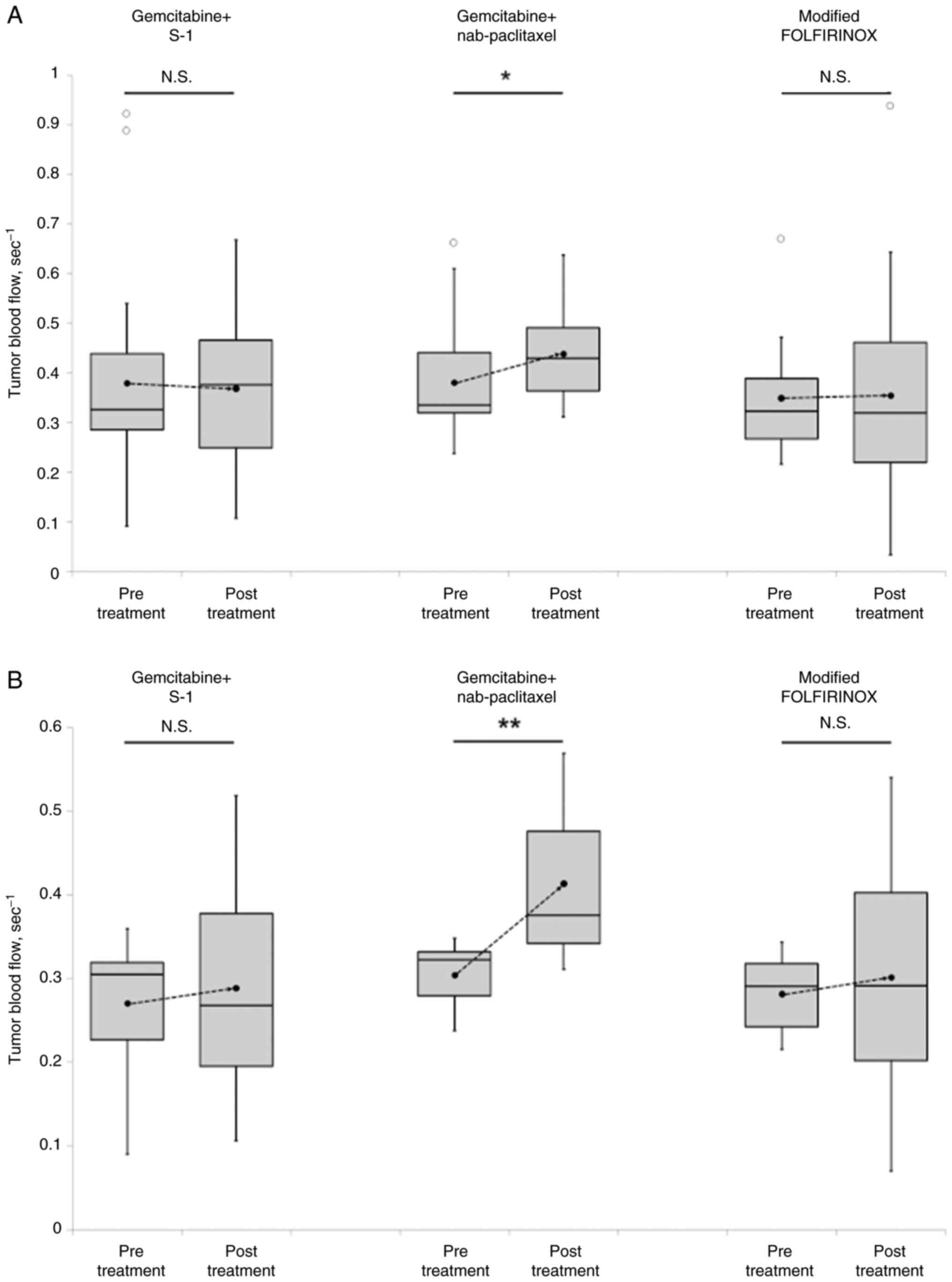
The changes in TBF before and after treatment for
each regimen are shown in Fig. 3.
No significant differences were observed between the pre- and
post-treatment TBF values for mFOLFIRINOX and GS. However, the
post-treatment TBF significantly increased after GnP treatment
(P=0.018). Furthermore, among patients in the low TBF group,
post-treatment TBF significantly increased after GnP treatment
(P=0.004), whereas TBF remained unchanged in patients treated with
mFOLFIRINOX (P=0.445) or GS (P=0.397).
Association between the post-treatment
TBF and the intra-tumoral vessels
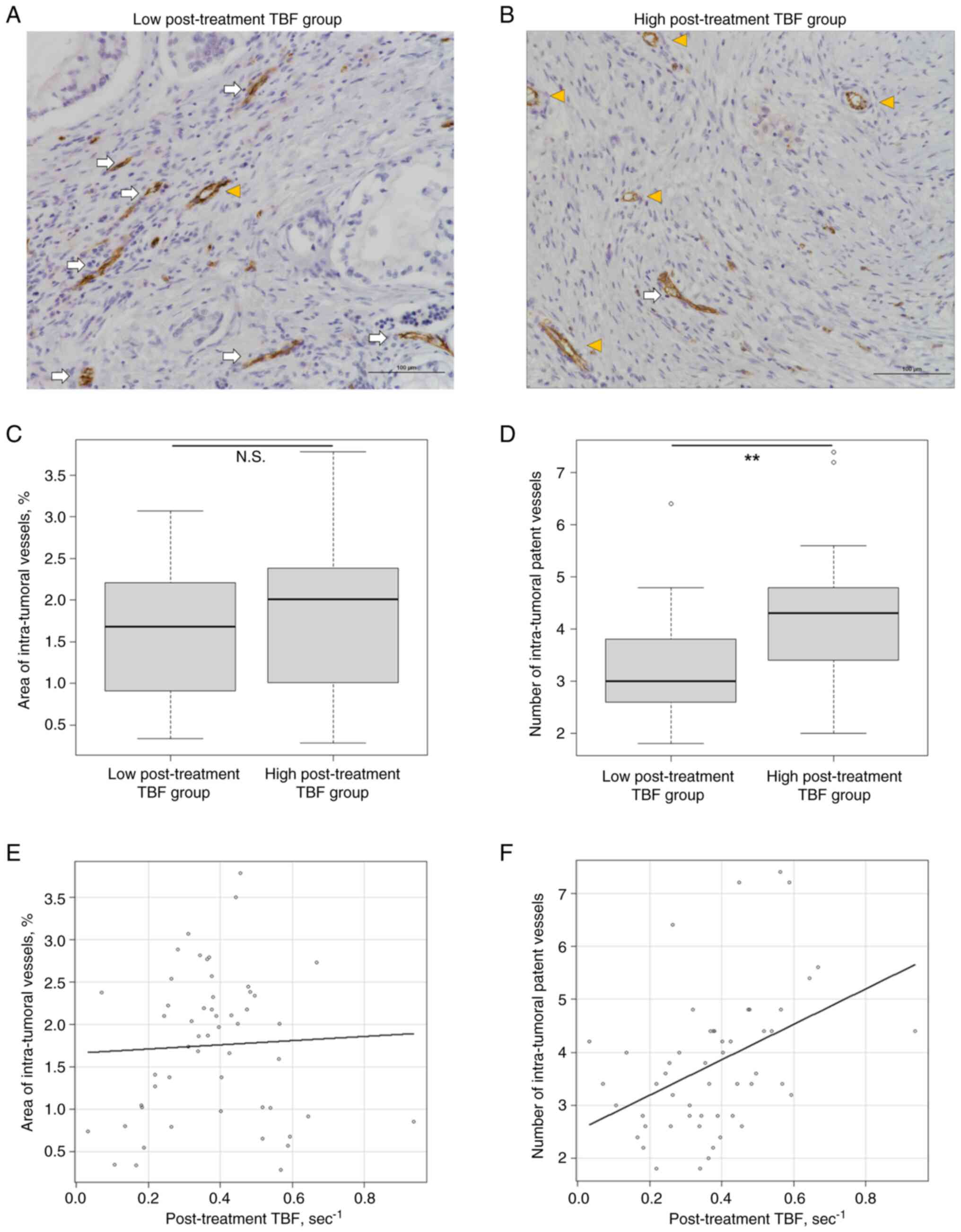
We further evaluated intra-tumoral vessels using
CD31 immunostaining (Fig. 4). The
number of intra-tumoral patent vessels was significantly higher in
the high post-treatment TBF group (P=0.006). However, the areas of
the intra-tumoral vessels were not significantly different between
the two groups (P=0.428). Regarding the correlation between
post-treatment TBF and intra-tumoral vessels, post-treatment TBF
did not correlate with the area of intra-tumoral vessels. However,
a higher post-treatment TBF was significantly associated with a
greater number of intra-tumoral patent vessels (Spearman rank
correlation coefficient, r=0.486; P<0.001).
Discussion
In this study, we identified three clinically
significant findings. First, higher TBF before treatment was
associated with better therapeutic efficacy of mFOLFIRINOX, whereas
pre-treatment TBF did not influence outcomes in patients receiving
GnP or GS. Second, in cases with low pre-treatment TBF, TBF
improved after treatment with GnP, yet remained unchanged in
patients treated with mFOLFIRINOX or GS. Finally, the TBF was
positively correlated with the number of intra-tumoral patent
vessels. Recently, neoadjuvant chemotherapy has become the standard
therapeutic option for PDAC in recent years (3–7).
However, the optimal pre-operative therapeutic regimen remains
unclear. Considering the heterogeneous nature of the pancreatic
tumor microenvironment (2),
personalizing chemotherapy based on individual tumor
characteristics may improve clinical outcomes. Our findings suggest
that TBF may serve as a useful indicator for selecting an
appropriate chemotherapy regimen for PDAC.
First, we found that patients with higher
pretreatment TBF showed better pathological responses to
mFOLFIRINOX. Previous study has reported that therapeutic
responders showed significantly higher TBF when assessed using
perfusion computed tomography, which allows accurate evaluation of
TBF (14). The findings of our
study are consistent with these results, supporting the notion that
higher TBF is associated with a better response to therapy in PDAC.
Although our study evaluated TBF using conventional
contrast-enhanced CT, which is considered less accurate than
perfusion CT for assessing TBF, we obtained similar findings.
Importantly, conventional contrast-enhanced CT is more widely
available and routinely performed in clinical practice compared
with perfusion CT, which highlights the potential clinical
applicability of our results. Furthermore, prior study did not
perform analyses according to treatment regimen, and their cohorts
included patients treated with both gemcitabine- and 5-fluorouracil
(5-Fu)-based therapies. In contrast, our study demonstrated the
relationship between TBF and preoperative therapy according to each
regimen, which may provide new information for guiding the
selection of personalized optimal therapy for patients with PDAC.
Nevertheless, both previous report and our study are limited by
relatively small sample sizes, and further large-scale
investigations are needed to validate these findings and to clarify
the role of TBF in treatment stratification. One of the hallmark
pathological features of PDAC is the dense desmoplastic stroma
surrounding tumor cells (2,11), which increases intra-tumoral
pressure, compresses blood vessels, and reduces perfusion. This, in
turn, impairs drug delivery to tumor (12). Previous studies have also reported a
relationship between stromal density and TBF (18), supporting the notion that tumors
with high TBF levels may permit more effective drug delivery,
leading to improved therapeutic outcomes.
Conversely, no correlation was observed between
pre-treatment TBF and therapeutic response in patients receiving
GnP or GS. This led us to hypothesize that changes in TBF during
treatment may contribute to therapeutic response. Indeed, when we
compared TBF before and after treatment, we observed that TBF
improved in GnP-treated patients with an initially low TBF, but
remained unchanged in those treated with mFOLFIRINOX or GS. These
results suggest that GnP may improve tumor perfusion during
treatment, potentially enhancing drug delivery and efficacy, even
in poorly perfused tumors. The previous study showed GnP reduce
fibrillar collagen matrix (19).
Thus, GnP may reduce intra-tumoral pressure and compresses blood
vessels, subsequently improve tumor perfusion. On the other hand,
in the nude mice gastric cancer model, conventional dose of 5-Fu
and prodrug of 5-Fu did not reduce cancer-associated fibroblast
(20). Thus, mFOLFIRINOX and GS may
not improve tumor perfusion. Therefore, mFOLFIRINOX may be more
suitable for tumors with a high pre-treatment TBF, whereas GnP may
be preferable for tumors with a low pre-treatment TBF. As for GS,
there were differences in clinical background compared to
mFOLFIRINOX or GnP group, such as shorter treatment duration and a
greater number of patients with low pre-treatment CA19-9 levels.
However, it was difficult to describe the reason behind the lack of
correlation between TBF and treatment efficacy in GS group only
from these factors. In the previous report, molecular and cellular
alterations in PDAC were shown to occur during treatment,
suggesting that a single time-point analysis of tumor
characteristics may be insufficient (13). To achieve personalized treatment, it
may be important to repeatedly assess molecular and cellular
information and intervene promptly in accordance with tumor
evolution. Therefore, future investigations are warranted.
Additionally, we investigated the association
between TBF and intra-tumoral vessels using CD31 immunostaining.
Although TBF did not correlate with the area of the intra-tumoral
vessels, a higher TBF was associated with a greater number of
intra-tumoral patent vessels. Generally, PDAC are characterized by
dense desmoplastic stroma (2,11).
Therefore, both patent and occlusive vessels were observed in these
tumors. However, a previous report evaluated only the area of
intra-tumoral vessels (18). Our
study was the first, to the best of our knowledge, to evaluate
patent intra-tumoral vessels. In GnP-treated patients, TBF improved
post-treatment, suggesting dynamic changes in intra-tumoral
vascular patency. The predictive value of TBF for chemotherapeutic
response is likely due to its effect on the efficiency of
intra-tumoral drug delivery.
This study had several limitations. First, this was
a retrospective analysis with a limited small sample size. Second,
variations in neoadjuvant treatment duration among patients could
not be controlled. Third, owing to the lack of pre-treatment
specimens, we were unable to directly evaluate the correlation
between pre-treatment TBF and pathological features. Because all
tissue analyses were performed on post-treatment specimens,
treatment-induced changes could not be ruled out. Nonetheless, we
believe that the correlations observed between post-treatment TBF
and pathological features are biologically plausible. Future in
vivo studies are warranted to investigate these associations
under controlled conditions.
In conclusion, we demonstrated an association
between TBF and the therapeutic response to each chemotherapy
regimen for PDAC. Furthermore, we observed that the changes in TBF
during treatment varied depending on the chemotherapy regimen.
These findings suggest that TBF may serve as a useful biomarker for
guiding the selection of neoadjuvant chemotherapy for PDAC.
However, larger prospective studies are needed to validate these
results.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HMa, TMa, YT, HMo, NN, TS, RO, ST, KT, MK, SK, TMi
and MT contributed to the study conception and design. HMa
performed data analysis and interpretation. HMa and TMa assessed
the immunostaining. HMa and YT evaluated tumor blood flow using
computed tomography. HMo, NN, TMa and TS sectioned the pathological
tissue blocks, prepared the slides and performed the
immunostaining. HMo, NN, TMa, TS, RO, ST, KT, MK, SK, TMi and MT
performed surgery and postoperative management. The first draft of
the manuscript was written by HMa, and TMa, YT, HMo, NN, TMa, TS,
RO, ST, KT, MK, SK, TMi and MT commented on previous versions of
the manuscript. HMa, TMa and MT confirmed the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shiga University of Medical Science (approval no.
R2017-171; Otsu, Japan) and was conducted in accordance with the
principles outlined in The Declaration of Helsinki. Informed
consent was obtained from all the patients or their legal
representatives on an opt-out basis.
Patient consent for publication
Consent for publication of the study was obtained
via the opt-out approach.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Kratzer TB, Giaquinto AN, Sung
H and Jemal A: Cancer statistics, 2025. CA Cancer J Clin. 75:10–45.
2025.PubMed/NCBI
|
|
2
|
Maehira H, Miyake T, Iida H, Tokuda A,
Mori H, Yasukawa D, Mukaisho KI, Shimizu T and Tani M: Vimentin
expression in tumor microenvironment predicts survival in
pancreatic ductal adenocarcinoma: heterogeneity in fibroblast
population. Ann Surg Oncol. 26:4791–4804. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andriulli A, Festa V, Botteri E, Valvano
MR, Koch M, Bassi C, Maisonneuve P and Sebastiano PD:
Neoadjuvant/preoperative gemcitabine for patients with localized
pancreatic cancer: A meta-analysis of prospective studies. Ann Surg
Oncol. 19:1644–1662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kato H, Usui M, Isaji S, Nagakawa T, Wada
K, Unno M, Nakao A, Miyakawa S and Ohta T: Clinical features and
treatment outcome of borderline resectable pancreatic head/body
cancer: A multi-institutional survey by the Japanese society of
pancreatic surgery. J Hepatobiliary Pancreat Sci. 20:601–610. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Angelo F, Antolino L, Farcomeni A,
Sirimarco D, Kazemi Nava A, De Siena M, Petrucciani N, Nigri G,
Valabrega S, Aurello P and Ramacciato G: Neoadjuvant treatment in
pancreatic cancer: Evidence-based medicine? A systematic review and
meta-analysis. Med Oncol. 34:852017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Satoi S, Yamaue H, Kato K, Takahashi S,
Hirono S, Takeda S, Eguchi H, Sho M, Wada K, Shinchi H, et al: Role
of adjuvant surgery for patients with initially unresectable
pancreatic cancer with a long-term favorable response to
non-surgical anti-cancer treatments: Results of a project study for
pancreatic surgery by the Japanese society of
hepato-biliary-pancreatic surgery. J Hepatobiliary Pancreat Sci.
20:590–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Motoi F, Satoi S, Honda G, Wada K, Shinchi
H, Matsumoto I, Sho M, Tsuchida A and Unno M; Study Group of
Preoperative therapy for Pancreatic cancer (PREP), : A single-arm,
phase II trial of neoadjuvant gemcitabine and S1 in patients with
resectable and borderline resectable pancreatic adenocarcinoma:
PREP-01 study. J Gastroenterol. 54:194–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Unno M, Motoi F, Matsuyama Y, Satoi S,
Toyama H, Matsumoto I, Aosasa S, Shirakawa H, Wada K, Fujii T, et
al: Neoadjuvant chemotherapy with gemcitabine and S-1 versus
upfront surgery for resectable pancreatic cancer: Results of the
randomized phase II/III Prep-02/JSAP05 trial. Ann Surg. April
16–2025.(Epub ahead of print). View Article : Google Scholar
|
|
9
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maehira H, Tsuji Y, Iida H, Mori H, Nitta
N, Maekawa T, Kaida S, Miyake T and Tani M: Estimated tumor blood
flow as a predictive imaging indicator of therapeutic response in
pancreatic ductal adenocarcinoma: Use of three-phase
contrast-enhanced computed tomography. Int J Clin Oncol.
27:373–382. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neesse A, Michl P, Frese KK, Feig C, Cook
N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM and
Tuveson DA: Stromal biology and therapy in pancreatic cancer. Gut.
60:861–868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neesse A, Bauer CA, Öhlund D, Lauth M,
Buchholz M, Michl P, Tuveson DA and Gress TM: Stromal biology and
therapy in pancreatic cancer: Ready for clinical translation? Gut.
68:159–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wolff RA, Wang-Gillam A, Alvarez H, Tiriac
H, Engle D, Hou S, Groff AF, San Lucas A, Bernard V, Allenson K, et
al: Dynamic changes during the treatment of pancreatic cancer.
Oncotarget. 9:14764–14790. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamdy A, Ichikawa Y, Toyomasu Y, Nagata M,
Nagasawa N, Nomoto Y, Sami H and Sakuma H: Perfusion CT to assess
response to neoadjuvant chemotherapy and radiation therapy in
pancreatic ductal adenocarcinoma: Initial experience. Radiology.
292:628–635. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miles KA: Measurement of tissue perfusion
by dynamic computed tomography. Br J Radiol. 64:409–412. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishida M, Fujii T, Kishiwada M, Shibuya K,
Satoi S, Ueno M, Nakata K, Takano S, Uchida K, Ohike N, et al:
Japanese classification of pancreatic carcinoma by the Japan
pancreas society: Eight edition. J Hepatobiliary Pancreat Sci.
31:755–768. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koyasu S, Tsuji Y, Harada H, Nakamoto Y,
Nobashi T, Kimura H, Sano K, Koizumi K, Hamaji M and Togashi K:
Evaluation of tumor associated stroma and its relationship with
tumor hypoxia using dynamic contrast-enhanced CT and (18)F
misonidazole PET in murine tumor models. Radiology. 278:734–741.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alvarez R, Musteanu M, Garcia-Garcia E,
Lopez-Casas PP, Megias D, Guerra C, Muñoz M, Quijano Y, Cubillo A,
Rodriguez-Pascual J, et al: Stromal disrupting effects of
nab-paclitaxel in pancreatic cancer. Br J Cancer. 109:926–933.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Xi W, Jiang J, Ji J, Yu Y, Zhu Z
and Zhang J: Metronomic chemotherapy remodel cancer-associated
fibroblasts to decrease chemoresistance of gastric cancer in nude
mice. Oncol Lett. 14:7903–7909. 2017.PubMed/NCBI
|