Introduction
Renal cell carcinoma (RCC) accounts for
approximately 2–3% of adult malignancies (1) and remains a notable cause of
cancer-related mortality worldwide. Clear-cell RCC (ccRCC) has been
identified as the predominant histological subtype, accounting for
approximately 75% of all RCC cases and the majority of RCC-specific
deaths (2,3).
Surgical resection remains the mainstay treatment
for localised RCC. Although patients with pT1 and pT2 tumours
typically exhibit favourable outcomes, a subset experiences ‘late
recurrence’ several years postoperatively, complicating follow-up
strategies (4). Several
inflammatory and nutritional blood biomarkers, including C-reactive
protein (CRP) and the neutrophil-to-lymphocyte ratio (NLR), have
been explored to improve risk stratification; however, none have
gained widespread clinical adoption (5,6). These
markers were selected based on their established relevance in
reflecting systemic inflammation (CRP and NLR) and nutritional
status (albumin and lymphocytes). As inflammation and immune
suppression are known contributors to the progression of RCC, their
integration into a composite index, such as the
CRP-albumin-lymphocyte (CALLY) index, offers a biologically
meaningful and clinically accessible prognostic tool.
The CALLY index has recently emerged as a novel
composite biomarker that reflects systemic inflammation,
nutritional status, and immune competence. Specifically, it has
been highlighted that these markers capture distinct but
interrelated aspects of cancer biology, inflammation (CRP, NLR),
nutritional status (albumin), and immune competence (lymphocyte
count), and that their composite use via the CALLY index may
provide a more comprehensive risk assessment in RCC (5–12). We
have previously demonstrated the prognostic value of the CALLY
index in patients with advanced RCC (pT3) (7). Nevertheless, whether the CALLY index
retains its clinical utility in lower-stage disease remains
unknown.
In the current study, we aimed to evaluate the
predictive relevance of the CALLY index for postoperative
recurrence and survival in patients with pT1/pT2 ccRCC, with an
emphasis on comparing preoperative and postoperative biomarker
values.
Materials and methods
Study population
This retrospective study included 253 patients with
pathologically confirmed ccRCC, staged as pT1 or pT2, who underwent
partial or radical nephrectomy at Yamaguchi University Hospital
between October 2005 and September 2023. Patient demographics,
clinical characteristics, and pathological features were extracted
from the institutional records. The median patient age was 67 years
(range, 28–92 years; 172 male, 81 female). The median follow-up
period was 37.9 months (range, 1–194 months). This study was
approved by the Institutional Review Board of the Graduate School
of Medicine, Yamaguchi University (IRB #2023-042), and all patients
provided written informed consent.
Data collection and biomarker
assessment
Data regarding clinical and laboratory parameters,
including age, sex, body mass index, tumour stage, Fuhrman grade,
and presence of sarcomatoid differentiation, were collected.
Laboratory values included serum albumin (g/dl), CRP (mg/dl),
absolute neutrophil count, and absolute lymphocyte count
(cells/µl). The CALLY index is calculated as follows: CALLY
index=(Albumin × Lymphocyte count)/CRP.
Peripheral blood samples were obtained at two time
points: Within two weeks prior to surgery (preoperative) and
approximately one month after surgery (postoperative). To minimise
the influence of perioperative physiological changes, only samples
collected at least one month postoperatively were used for
postoperative analysis.
Statistical analysis
Receiver operating characteristic (ROC) curve
analysis was performed to evaluate the predictive ability of CRP,
NLR, and CALLY index for progression-free survival (PFS) and
overall survival (OS). The optimal cut-off values were determined
using the Youden index. The sensitivity, specificity, positive
predictive value, and negative predictive value were also
calculated.
Kaplan-Meier survival analysis was used to estimate
PFS and OS, and survival distributions were compared using the
log-rank test. Univariate and multivariate Cox proportional hazards
regression analyses were performed to identify independent
predictors. Hazard ratios (HRs) with 95% confidence intervals (CIs)
are reported. P<0.05 was considered to indicate a statistically
significant difference. Regarding association with clinical
parameters, Wilcoxon rank-sum test was used. All statistical
analyses were performed using the JMP Pro software (version 16.0;
SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The patient characteristics are summarized in
Table I. The median age was 67
years, with 67.98% males and 32.02% females. Among the 253 patients
included in the study, 239 (94.5%) were classified as pT1, and 14
(5.5%) as pT2. Postoperative recurrence occurred in 21 (8.3%)
patients. At the time of the last follow-up, 236 (90.9%) patients
had survived (Table I).
 | Table I.Patient characteristics (T1/T2N0M0,
n=253). |
Table I.
Patient characteristics (T1/T2N0M0,
n=253).
| Variable | Value |
|---|
| Sex, n (%) |
|
| Male | 172 (67.98) |
|
Female | 81 (32.02) |
| Age, years |
|
|
Median | 67 |
|
Range | 28-92 |
| BMI,
kg/m2 |
|
|
Median | 23.64 |
|
Range | 13.83–37.59 |
| T stage, n (%) |
|
| T1a | 191 (75.49) |
| T1b | 48 (18.97) |
| T2a | 11 (4.35) |
| T2b | 3 (1.19) |
| N stage, n (%) |
|
| N0 | 253 (100) |
| N1 | 0 (0) |
| M stage, n (%) |
|
| M0 | 253 (100) |
| M1 | 0 (0) |
| Fuhrman grade, n
(%) |
|
| G1 | 94 (37.15) |
| G2 | 140 (55.34) |
| G3 | 16 (6.32) |
| G4 | 3 (1.19) |
| Sarcomatoid feature,
n (%) |
|
|
No | 250 (98.8) |
|
Yes | 3 (1.2) |
| Outcome, n (%) |
|
|
Progression |
|
| No | 232 (91.70) |
| Yes | 21 (8.3) |
| Overall survival |
|
|
Survival | 236 (93.3) |
|
Death | 17 (6.7) |
ROC curve analysis
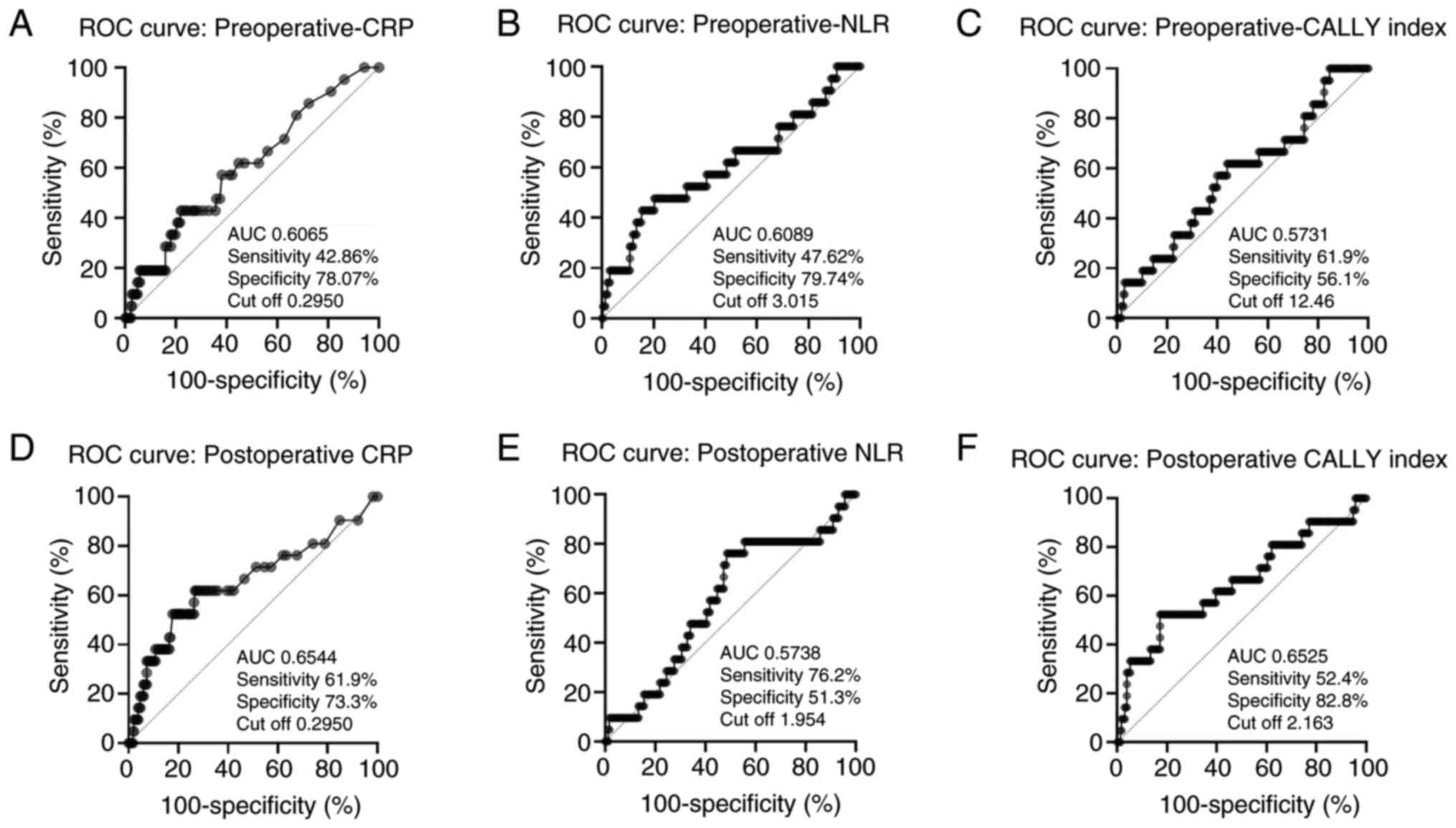
Figs. 1 and 2 showed that ROC analysis was used to
evaluate the predictive ability of CRP, NLR, and CALLY index for
PFS and OS. For PFS prediction using postoperative data, the CALLY
index showed the highest specificity (82.8%), with an area under
the curve (AUC) of 0.6525 and a cut-off value of 2.163. In
contrast, preoperative CRP (cut-off: 0.2950) and NLR (cut-off:
3.015) had lower discriminative power (AUCs=0.6965 and 0.6089,
respectively).
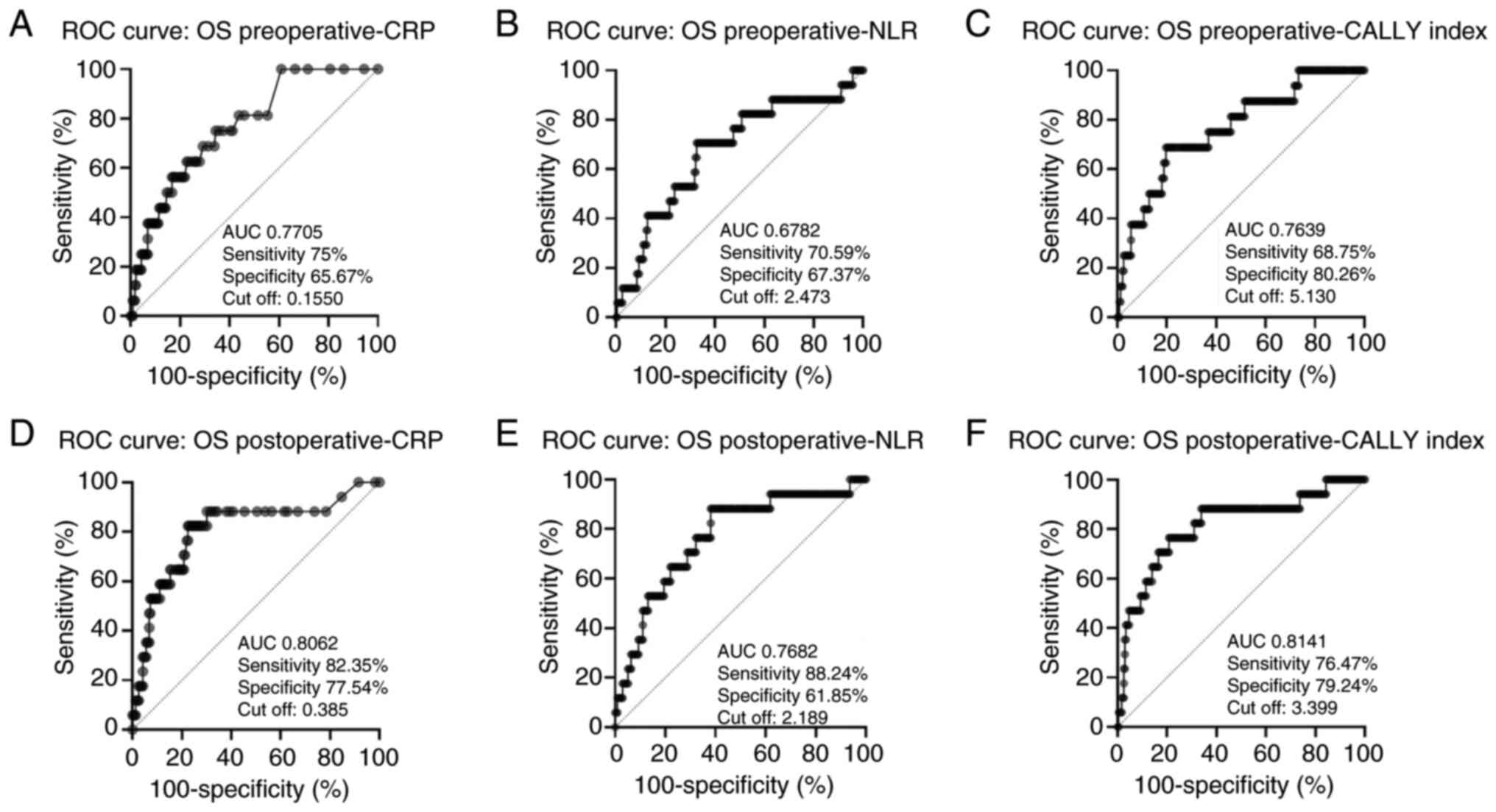 | Figure 2.ROC curve analysis for OS using pre-
and postoperative biomarkers: (A) Preoperative CRP, (B)
preoperative NLR, (C) preoperative CALLY index, (D) postoperative
CRP, (E) postoperative NLR, (F) postoperative CALLY index. ROC,
receiver operating characteristic; CRP, C-reactive protein; NLR,
neutrophil-to-lymphocyte ratio; CALLY, CRP-albumin-lymphocyte; OS,
overall survival. |
Likewise, the postoperative CALLY index exhibited
the highest performance for OS prediction, with an AUC of 0.8141,
sensitivity of 76.5%, and specificity of 79.2% (cut-off: 3.399).
Postoperative CRP level and NLR also exhibited notable
performances, with AUCs of 0.8062 and 0.7682, respectively.
Kaplan-Meier survival analysis
Kaplan-Meier survival curves revealed that patients
with a postoperative CALLY index ≤2.163 had significantly shorter
PFS and OS than those with higher values (log-rank P=0.0026 and
P=0.002, respectively) (Figs. 3 and
4). Similarly, high preoperative
NLR (≥3.015) and high postoperative CRP (≥0.3850) were associated
with significantly shorter PFS and OS.
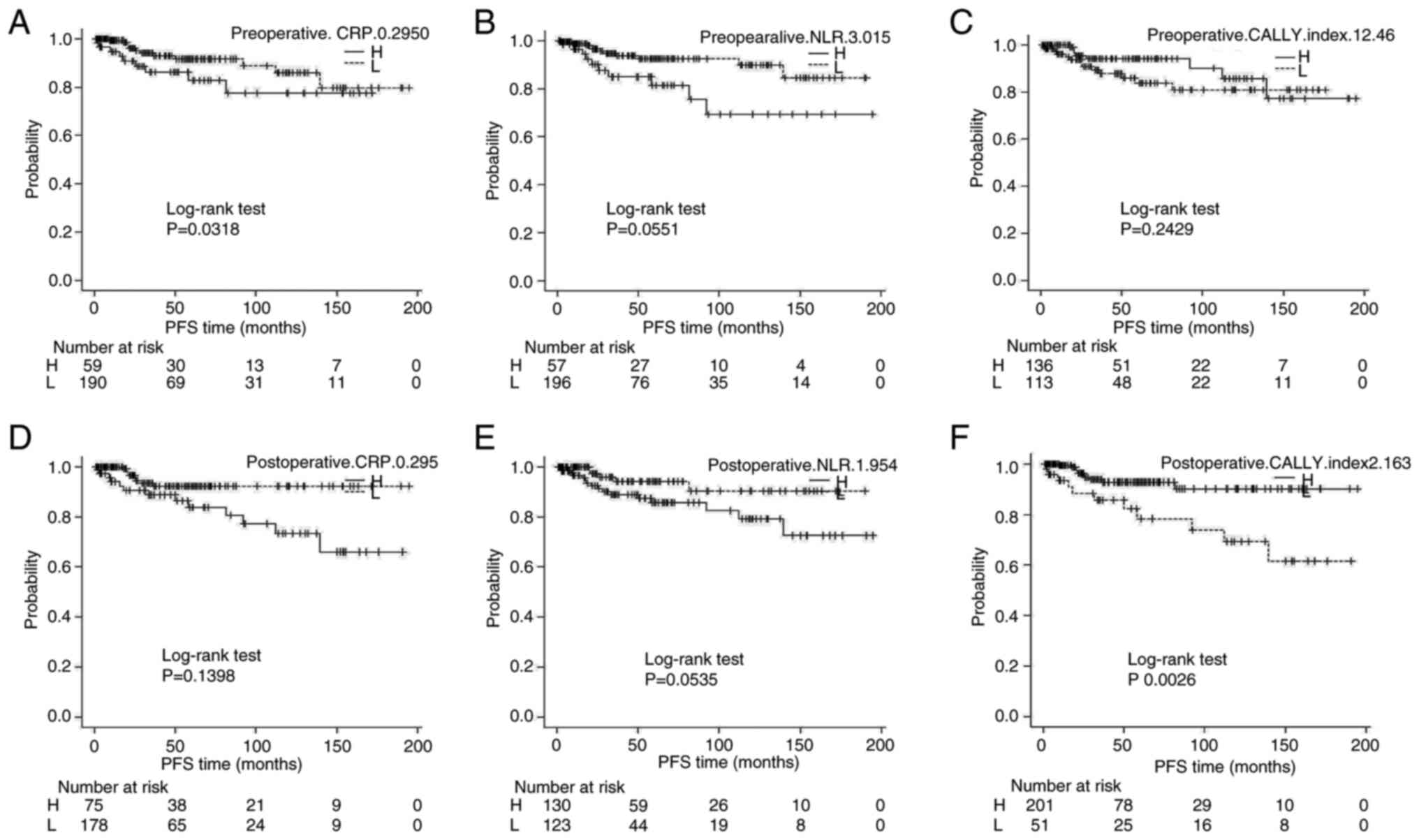 | Figure 3.Kaplan-Meier survival analysis for PFS
stratified by biomarker cut-offs: (A) Preoperative CRP, (B)
preoperative NLR, (C) preoperative CALLY index, (D) postoperative
CRP, (E) postoperative NLR, (F) postoperative CALLY index. Patients
were divided into H and L groups according to the cut-off value
determined by receiver operating characteristic curve analysis.
CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio;
CALLY, CRP-albumin-lymphocyte; PFS, progression-free survival; H,
high; L, low. |
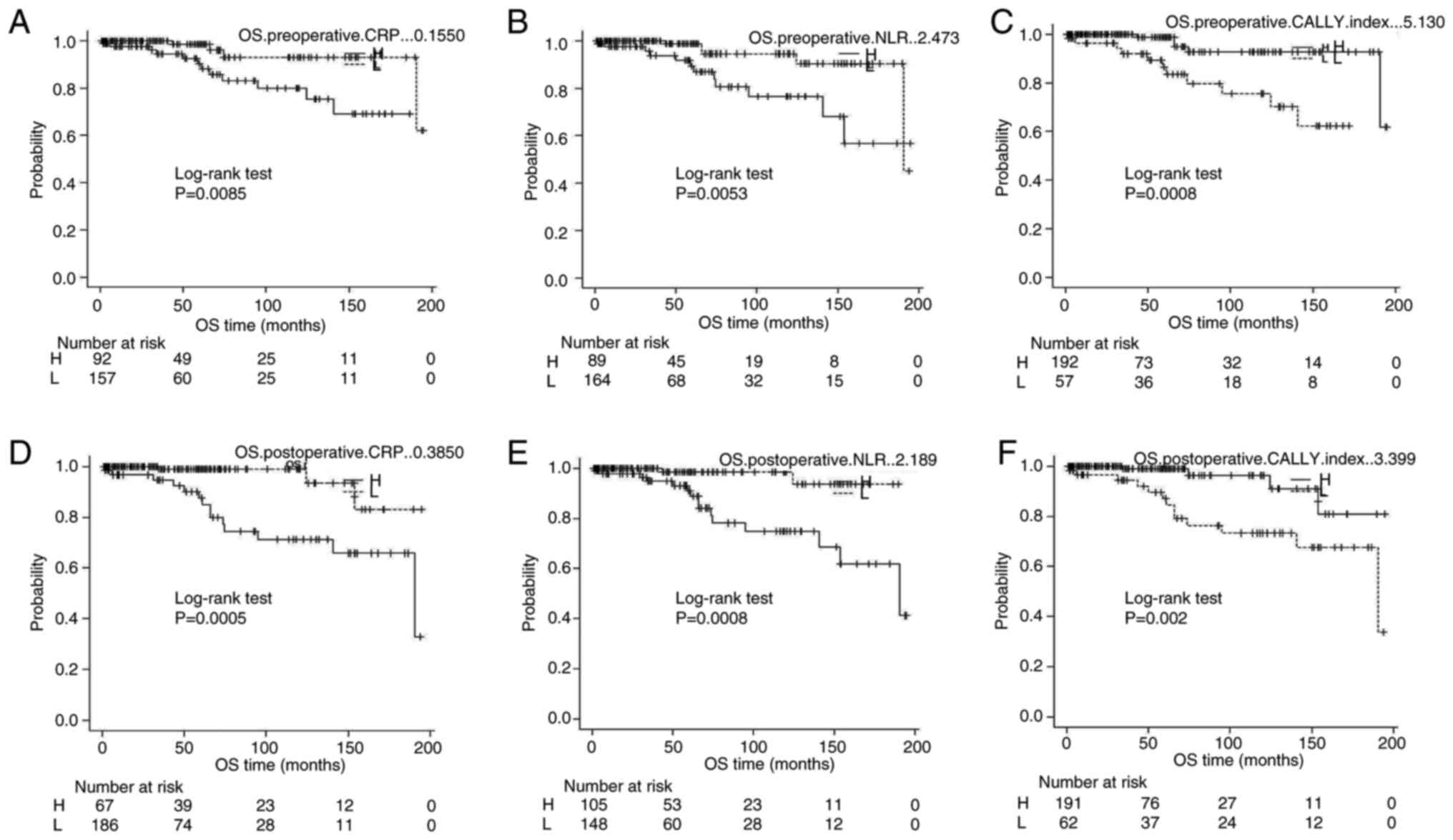 | Figure 4.Kaplan-Meier survival analysis for OS
stratified by biomarker cut-offs: (A) Preoperative CRP, (B)
preoperative NLR, (C) preoperative CALLY index, (D) postoperative
CRP, (E) postoperative NLR, (F) postoperative CALLY index. Patients
were divided into H and L groups according to the cut-off value
determined by receiver operating characteristic curve analysis.
CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio;
CALLY, CRP-albumin-lymphocyte; OS, overall survival; H, high; L,
low. |
Cox proportional hazards
regression
Univariate analysis identified preoperative NLR
≥3.015 (HR=2.74; 95% CI: 1.16–6.47; P=0.0208) and postoperative
CALLY index ≤2.163 (HR=3.64; 95% CI: 1.45–8.25; P=0.005) as
significant predictors of recurrence (Table II). For OS, postoperative Fuhrman
grade ≥3 (HR=6.07; 95% CI: 1.28–28.79; P=0.023) and postoperative
CALLY index ≤2.163 (HR=4.98; 95% CI: 1.59–15.56; P=0.0057) were
identified as significant predictors associated with recurrence
(Table IIIA).
 | Table II.Univariate analysis to predict
progression-free survival in pT1/pT2 patients. |
Table II.
Univariate analysis to predict
progression-free survival in pT1/pT2 patients.
| Variable | Cut-off | Comparison | HR (95% CI) | P-value |
|---|
| Sex | - | Male vs. Female | 1.292572
(0.973913–1.715494) | 0.0756 |
| Age, years | 67a | Older vs.
Younger | 1.321941
(0.544308–3.21055) | 0.5376 |
| BMI,
kg/m2 | 23.64a | High vs. Low | 1.064536
(0.822657–1.378381) | 0.6312 |
| Stage | - | T2 vs. T1 | 3.00129
(0.881974–10.21316) | 0.0786 |
| Fuhrman grade | - | 34 vs. 1 + 2 | 1.473568
(0.194606–11.15796) | 0.7074 |
| Preoperative CRP | 0.295 | High vs. Low | 1.919245
(0.807844–4.559671) | 0.1398 |
| Preoperative NLR | 3.015 | High vs. Low | 2.748875
(1.166315–6.478798) | 0.0208 |
| Preoperative CALLY
index | 12.46 | Low vs. High | 1.79135
(0.741854–4.325562) | 0.1949 |
| Postoperative
CRP | 0.2950 | High vs. Low | 1.919245
(0.807844–4.559671) | 0.1398 |
| Postoperative
NLR | 1.954 | High vs. Low | 2.690201
(0.985032–7.347159) | 0.0535 |
| Postoperative CALLY
index | 2.163 | Low vs. High | 3.461179
(1.454691–8.235263) | 0.005 |
 | Table III.Univariate and multivariate analyses
to predict overall survival in pT1/pT2 patients. |
Table III.
Univariate and multivariate analyses
to predict overall survival in pT1/pT2 patients.
| A, Analyses based on
postoperative data |
|---|
|
|---|
| Variable | Cut-off | Comparison | Univariate HR (95%
CI) | Univariate
P-value | Multivariate HR (95%
CI) | Multivariate
P-value |
|---|
| Sex | - | Male vs.
Female | 1.863947 | 0.2832 | - | - |
|
|
|
|
(0.597746–5.812336) |
|
|
|
| Age, years | 67a | Older vs.
Younger | 1.725438 | 0.2819 | - | - |
|
|
|
|
(0.638896–4.65981) |
|
|
|
| BMI,
kg/m2 | 23.64a | High vs. Low | 2.207195 | 0.138 | - | - |
|
|
|
|
(0.775425–6.282635) |
|
|
|
| Stage | - | T2 vs. T1 | 5.324408 | 0.002 | 3.225972 | 0.0463 |
|
|
|
|
(1.84181–15.3921) |
|
(1.019327–10.20957) |
|
| Fuhrman grade | - | 34 vs. 1+2 | 6.075185 | 0.023 | 3.657307 | 0.1216 |
|
|
|
|
(1.281854–28.79258) |
|
(0.708305–18.88436) |
|
| CRP | 0.385 | High vs. Low | 6.889604 | 0.0027 | 5.828419 | 0.1371 |
|
|
|
|
(1.951844–24.31887) |
|
(0.570453–59.54996) |
|
| NLR | 2.189 | High vs. Low | 8.108854 | 0.0057 | 4.462875 | 0.0652 |
|
|
|
|
(1.840612–35.72372) |
|
(0.910259–21.88086) |
|
| CALLY index | 3.399 | Low vs. High | 4.981984 | 0.0057 | 1.771683 | 0.5931 |
|
|
|
|
(1.594653–15.56462) |
|
(0.217442–14.43542) |
|
|
| B, Analyses
based on preoperative data |
|
|
Variable | Cut-off |
Comparison | Univariate HR
(95% CI) | Univariate
P-value | Multivariate HR
(95% CI) | Multivariate
P-value |
|
| Sex | - | Male vs.
Female | 1.863947 | 0.2832 | - | - |
|
|
|
|
(0.597746–5.812336) |
|
|
|
| Age, years | 67a | Older vs.
Younger | 1.725438 | 0.2819 | - | - |
|
|
|
|
(0.638896–4.65981) |
|
|
|
| BMI,
kg/m2 | 23.64a | High vs. Low | 2.207195 | 0.138 | - | - |
|
|
|
|
(0.775425–6.282635) |
|
|
|
| Stage | - | T2 vs. T1 | 5.324408 | 0.002 | 2.46747 | 0.1357 |
|
|
|
|
(1.84181–15.3921) |
|
(0.753225–8.083124) |
|
| Fuhrman grade | - | 34 vs. 1+2 | 6.075185 | 0.023 | 3.878816 | 0.1004 |
|
|
|
|
(1.281854–28.79258) |
|
(0.769975–19.53987) |
|
| CRP | 0.155 | High vs. Low | 4.698866 | 0.0168 | 1.033698 | 0.9773 |
|
|
|
|
(1.321837–16.70353) |
|
(0.105675–10.11152) |
|
| NLR | 2.473 | High vs. Low | 3.957946 | 0.0099 | 2.330853 | 0.1416 |
|
|
|
|
(1.392138–11.25272) |
|
(0.754118–7.204278) |
|
| CALLY index | 5.130 | Low vs. High | 5.675808 | 0.0031 | 3.058945 | 0.3013 |
|
|
|
|
(1.799224–17.90483) |
|
(0.367179–25.48385) |
|
In multivariate analysis, a low postoperative CALLY
index (≤2.163) and high Fuhrman grade remained independent
predictors of poor OS. In contrast, the CALLY index showed the
strongest association with both recurrence and survival
outcomes.
Association with clinical
parameters
Subgroup analysis revealed that the CALLY index was
significantly lower in patients with pT2 tumours and higher Fuhrman
grades (P<0.05) (Table IVA).
Although similar trends were observed postoperatively, they did not
reach statistical significance (Table
IVB).
 | Table IV.Association of CALLY index with
several clinical factors. |
Table IV.
Association of CALLY index with
several clinical factors.
| A, Preoperative
(n=249) |
|---|
|
|---|
| Parameter | Group | n | CALLY index
(mean) |
P-valuea |
|---|
| Sex | Male | 169 | 120.698 | 0.1707 |
|
| Female | 80 | 134.088 | Reference |
| Age (median, 67
years) | Low | 125 | 121.344 | Reference |
|
| High | 124 | 128.685 | 0.4213 |
| BMI (median, 23.64
kg/m2) | Low | 125 | 128.688 | Reference |
|
| High | 124 | 121.282 | 0.4172 |
| TNM | T1 | 235 | 129.017 | Reference |
|
| T2 | 14 | 57.571 | 0.0003 |
| Fuhrman grade | G1 + G2 | 232 | 127.563 | Reference |
|
| G3 + G4 | z17 | 90.029 | 0.0381 |
|
| B, Postoperative
(n=253) |
|
|
Parameter | Group | n | CALLY index
(mean) |
P-valuea |
|
| Sex | Male | 172 | 123.267 | 0.2311 |
|
| Female | 81 | 134.926 | Reference |
| Age (median, 67
years) | Low | 127 | 118.386 | 0.0601 |
|
| High | 126 | 135.683 | Reference |
| BMI (median, 23.64
kg/m2) | Low | 126 | 134.992 | Reference |
|
| High | 127 | 119.071 | 0.0836 |
| TNM | T1 | 239 | 128.264 | Reference |
|
| T2 | 14 | 105.429 | 0.2565 |
| Fuhrman grade | G1 + G2 | 235 | 128.815 | Reference |
|
| G3 + G4 | 18 | 103.306 | 0.1541 |
Discussion
In this study, we evaluated the prognostic relevance
of the CALLY index in patients with pathological T1/T2 ccRCC,
focusing on its ability to predict postoperative recurrence and
OS.
Our findings demonstrate that the postoperative
CALLY index is a superior prognostic marker compared with
individual haematologic biomarkers, such as CRP and NLR. Notably, a
low postoperative CALLY index (≤2.163) was independently associated
with disease recurrence and poor OS, underscoring its clinical
relevance in postoperative risk stratification.
Previous studies primarily examined the prognostic
value of the CALLY index in advanced RCC (7) and other malignancies (8–10). Our
previous study highlighted the significance of this index in
patients with pT3 RCC (7). By
extending this study to a lower-risk cohort, we demonstrated that
the CALLY index remained a robust indicator of prognosis,
suggesting broader applicability across disease stages.
Importantly, the enhanced prognostic accuracy of postoperative
values, compared with preoperative measures, indicates that the
resolution of perioperative inflammation may be necessary to reveal
the true baseline immune and nutritional status.
The components of the CALLY index, serum albumin
level, CRP level, and lymphocyte count have been individually
recognised for their prognostic relevance. Hypoalbuminaemia
reflects poor nutritional status and systemic inflammation
(11,12), CRP is a marker of the acute-phase
response and tumour-related inflammation, and lymphopenia indicates
impaired immune surveillance (13–15).
The integration of these variables into a composite index allows
for a more holistic assessment of host-tumour interactions. Our
data reinforce the utility of the CALLY index as a composite
measure, particularly when assessed in a stable postoperative
setting.
The postoperative CALLY index demonstrated stronger
predictive power for OS (AUC=0.8141) than either CRP or NLR alone.
This supports previous findings observed in patients with
gastrointestinal and hepatocellular cancers, in which postoperative
inflammatory indices provided improved prognostic resolution
(8–10). In RCC, systemic inflammation is
associated with disease progression and resistance to therapies
such as immune checkpoint inhibitors (ICIs) (16–18). A
persistently low postoperative CALLY index may signal sustained
inflammation or immune dysfunction, making patients less likely to
benefit from ICI monotherapy (19).
Our findings have several important clinical
implications. First, the postoperative CALLY index may serve as a
cost-effective and non-invasive tool to guide surveillance
intensity. Second, patients with low postoperative CALLY index
values may benefit from closer follow-up or earlier introduction of
adjuvant therapy. Third, this index may aid in the selection of
patients for combined ICI and tyrosine kinase inhibitor regimens in
future prospective trials.
Nevertheless, the limitations of this study need to
be addressed. The retrospective design and single-institution
cohort may have introduced selection bias. Additionally, the
limited number of recurrence events restricts the statistical
power, particularly in multivariate models. Although the Fuhrman
grade and tumour stage were included in the analysis, other
molecular or genomic features were not assessed. Future studies
should incorporate molecular profiling to enhance prognostic
modelling and validate our findings in independent cohorts.
Furthermore, the retrospective design, single-centre setting, and
lack of external validation may limit the generalisability of our
findings. In addition, the absence of dynamic postoperative
biomarker trends or radiological confirmation of the timing of
recurrence warrants cautious interpretation. Future prospective
studies incorporating multicentre data and longer follow-up periods
are necessary.
To further validate the prognostic role of the CALLY
index, future multicentre prospective studies incorporating diverse
populations and standardised postoperative sampling protocols are
warranted.
Overall, the postoperative CALLY index is a valuable
biomarker for identifying patients with T1/T2 ccRCC who have an
increased risk of recurrence and mortality. Its incorporation into
clinical decision-making may improve individualised follow-up
strategies and support the selection of appropriate therapeutic
interventions.
In conclusion, the postoperative CALLY index is a
reliable and accessible biomarker for predicting recurrence and
survival in patients with pathological T1/T2 ccRCC. This index
provides superior prognostic accuracy compared with individual
haematologic markers and may enhance current risk stratification
protocols. Incorporating the CALLY index into postoperative
monitoring could improve individualised patient management and
inform clinical decision-making regarding adjuvant therapy. The
postoperative CALLY index may assist in identifying patients who
require close surveillance or early systemic therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HH conceived the study and wrote the manuscript.
KosS and TT contributed to the data acquisition. NF and KK
conducted the statistical analyses. KojS contributed to the
analysis of data. HH and TT confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of the Graduate School of Medicine, Yamaguchi University (IRB
#2023-042), and written informed consent was obtained from all
participants.
Patient consent for publication
Written informed consent for publication was
obtained from all patients prior to inclusion in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI
|
|
2
|
Haake SM and Rathmell WK: Renal cancer
subtypes: Should we be lumping or splitting for therapeutic
decision making? Cancer. 123:200–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheaib JG, Patel HD, Johnson MH, Gorin MA,
Haut ER, Canner JK, Allaf ME and Pierorazio PM: Stage-specific
conditional survival in renal cell carcinoma after nephrectomy.
Urol Oncol. 38:6.e1–6.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pichler M, Hutterer GC, Stoeckigt C,
Chromecki TF, Stojakovic T, Golbeck S, Eberhard K, Gerger A,
Mannweiler S, Pummer K, et al: Validation of the pre-treatment
neutrophil-lymphocyte ratio as a prognostic factor in RCC. Br J
Cancer. 108:901–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jagdev SP, Gregory W, Vasudev NS, Harnden
P, Sim S, Thompson D, Cartledge J, Selby PJ and Banks RE: Improving
the accuracy of pre-operative survival prediction in RCC with CRP.
Br J Cancer. 103:1649–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirata H, Fujii N, Oka S, Nakamura K,
Shimizu K, Kobayashi K, Hiroyoshi T, Isoyama N and Shiraishi K:
CALLY index as a novel biomarker for progression in RCC. Cancer
Diagn Progn. 4:748–753. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iida H, Tani M, Komeda K, Nomi T,
Matsushima H, Tanaka S, Ueno M, Nakai T, Maehira H, Mori H, et al:
Superiority of CRP-albumin-lymphocyte index (CALLY index) as a
non-invasive prognostic biomarker after hepatectomy for
hepatocellular carcinoma. HPB (Oxford). 24:101–115. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng J, Wang L, Yang X and Chen Q:
Clinical significance of preoperative CALLY index in esophageal
SCC. Sci Rep. 14:7132024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakurai K, Kubo N, Hasegawa T, Nishimura
J, Iseki Y, Nishii T, Inoue T, Yashiro M, Nishiguchi Y and Maeda K:
CALLY index in gastric cancer prognosis. World J Surg.
48:2749–2759. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu MT, He SY, Chen SL, Li LF, He ZQ, Zhu
YY, He X and Chen H: Clinical and prognostic implications of
pretreatment albumin to C-reactive protein ratio in patients with
hepatocellular carcinoma. BMC Cancer. 19:5382019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishizuka M, Ishizuka M, Nagata H, Takagi
K, Horie T and Kubota K: Inflammation-based prognostic score is a
novel predictor of postoperative outcome in patients with
colorectal cancer. Ann Surg. 246:1047–1051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou L, Cai X, Liu Q, Jian ZY, Li H and
Wang KJ: Prognostic role of C-reactive protein in urological
cancers: A meta-analysis. Sci Rep. 5:127332015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel SH, Derweesh IH, Saito K, Patil D,
Meagher MF, Bindayi A, Eldefrawy A, Patel DN, Nasseri R, Yasuda Y,
et al: Preoperative elevation of C-reactive protein is a predictor
for adverse oncologic survival outcomes for renal cell carcinoma:
Analysis from the International Marker Consortium Renal Cancer
(INMARC). Clin Genitourin Cancer. 19:e206–e215. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McMillan DC: The systemic
inflammation-based Glasgow Prognostic Score: A decade of experience
in patients with cancer. Cancer Treat Rev. 39:534–540. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W and Wang J: The current state of
inflammation-related research in prostate cancer: a bibliometric
analysis and systematic review. Front Oncol. 14:14328572024.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iivanainen S, Ahvonen J, Knuuttila A,
Tiainen S and Koivunen JP: Elevated CRP levels indicate poor
progression-free and overall survival on cancer patients treated
with PD-1 inhibitors. ESMO Open. 4:e0005312019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson TV, Abbasi A, Owen-Smith A, Young
AN, Kucuk O, Harris WB, Osunkoya AO, Ogan K, Pattaras J, et al:
Postoperative better than preoperative C-reactive protein at
predicting outcome after potentially curative nephrectomy for renal
cell carcinoma. Urology. 76:766–e1. 2010. View Article : Google Scholar
|
|
19
|
Onodera R, Chiba S, Nihei S, Fujimura I,
Akiyama M, Utsumi Y, Nagashima H, Kudo K and Maemondo M: High level
of C-reactive protein as a predictive factor for immune-related
adverse events of immune checkpoint inhibitors in non-small cell
lung cancer: A retrospective study. J Thorac Dis. 15:4237–4247.
2023. View Article : Google Scholar : PubMed/NCBI
|