Lung cancer remains one of the most common and
lethal cancers worldwide, markedly contributing to cancer-related
illness and death (1,2). It is the leading cause of cancer
mortality, with >2 million new cases diagnosed each year and a
persistently low 5-year survival rate, especially in advanced
stages (3–5). The disease is mainly divided into two
subtypes based on histological and molecular features: Non-small
cell lung cancer (NSCLC), which accounts for ~85% of cases, and
small cell lung cancer (SCLC), representing the remaining ~15% and
known for its rapid growth and early spread (6). NSCLC includes adenocarcinoma, squamous
cell carcinoma and large cell carcinoma, each with unique molecular
profiles and clinical behaviors (7).
Lung cancer development and progression are driven
by complex signaling pathways that regulate fundamental cellular
processes such as proliferation, survival, metastasis and
resistance to therapy (8–10). Dysregulation of these pathways often
results from genetic mutations, epigenetic alterations or
environmental exposures, including tobacco smoke, air pollution and
carcinogens such as asbestos and radon (11–14).
Over the past decade, notable progress has been made in elucidating
the molecular mechanisms underlying lung cancer pathogenesis
(15,16). Advances in genomic sequencing have
enabled the identification of key oncogenic drivers, including
mutations in EGFR, rearrangements in anaplastic lymphoma kinase
(ALK), and mutations in KRAS (17–20).
These discoveries have led to the development of targeted therapies
that have fundamentally transformed the clinical management of lung
cancer (21). For example,
EGFR-tyrosine kinase inhibitors (TKIs), such as gefitinib,
erlotinib and osimertinib, have demonstrated robust efficacy in
patients with EGFR-mutant NSCLC, markedly improving both
progression-free survival (PFS) and overall survival (OS) compared
with conventional chemotherapy (22–25).
Similarly, ALK inhibitors, including crizotinib, alectinib and
lorlatinib, have markedly improved outcomes for patients with
ALK-rearranged NSCLC, offering durable responses and enhanced
quality of life (26–28). KRAS mutations are present in 25–30%
of lung adenocarcinomas and have historically been considered
untreatable with drugs due to the lack of suitable binding pockets
for therapeutic intervention (29,30).
However, recent breakthroughs, particularly the development of KRAS
G12C inhibitors such as sotorasib and adagrasib, have yielded
promising clinical results, opening new therapeutic avenues for
this patient population (30–32).
In addition to well-characterized signaling
pathways, the PI3K/AKT/mTOR axis serves a central role in tumor
growth and survival in lung cancer (33). Activation of this pathway is
frequently driven by mutations or amplifications in upstream
regulators such as EGFR, KRAS and PI3K itself (34). Dysregulation of the PI3K/AKT/mTOR
pathway contributes to resistance against both chemotherapy and
targeted therapies, thereby positioning it as a key focus of
ongoing research (35). Current
preclinical and clinical investigations are assessing inhibitors
targeting components of this pathway, including PI3K, AKT and mTOR,
both as monotherapies and in combination regimens, with the aim of
overcoming therapeutic resistance and improving patient outcomes
(36–38). The involvement of immune checkpoint
pathways, such as programmed cell death protein 1 (PD-1)/programmed
death receptor ligand-1 (PD-L1) and cytotoxic T
lymphocyte-associated antigen-4 (CTLA-4), in tumor immune evasion
has been established (39,40). These insights have facilitated the
development of immune checkpoint inhibitors (ICIs), which have
markedly reshaped treatment paradigms for both NSCLC and SCLC
(41). Therapeutic agents such as
pembrolizumab, nivolumab and atezolizumab have demonstrated
clinically meaningful improvements in survival, particularly among
patients with high PD-L1 expression or elevated tumor mutational
burden (42–44). Beyond enhancing clinical outcomes,
these agents have also redefined standard treatment practices, with
the combination of immunotherapy with chemotherapy now widely
adopted as a first-line therapy (45).
The present review provides an overview of key
signaling pathways involved in lung cancer pathogenesis, including
EGFR, ALK, KRAS, PI3K/AKT/mTOR and immune checkpoint pathways. It
also highlights recent advances in targeted therapies, such as
next-generation TKIs, monoclonal antibodies and combination
strategies, and their clinical implications.
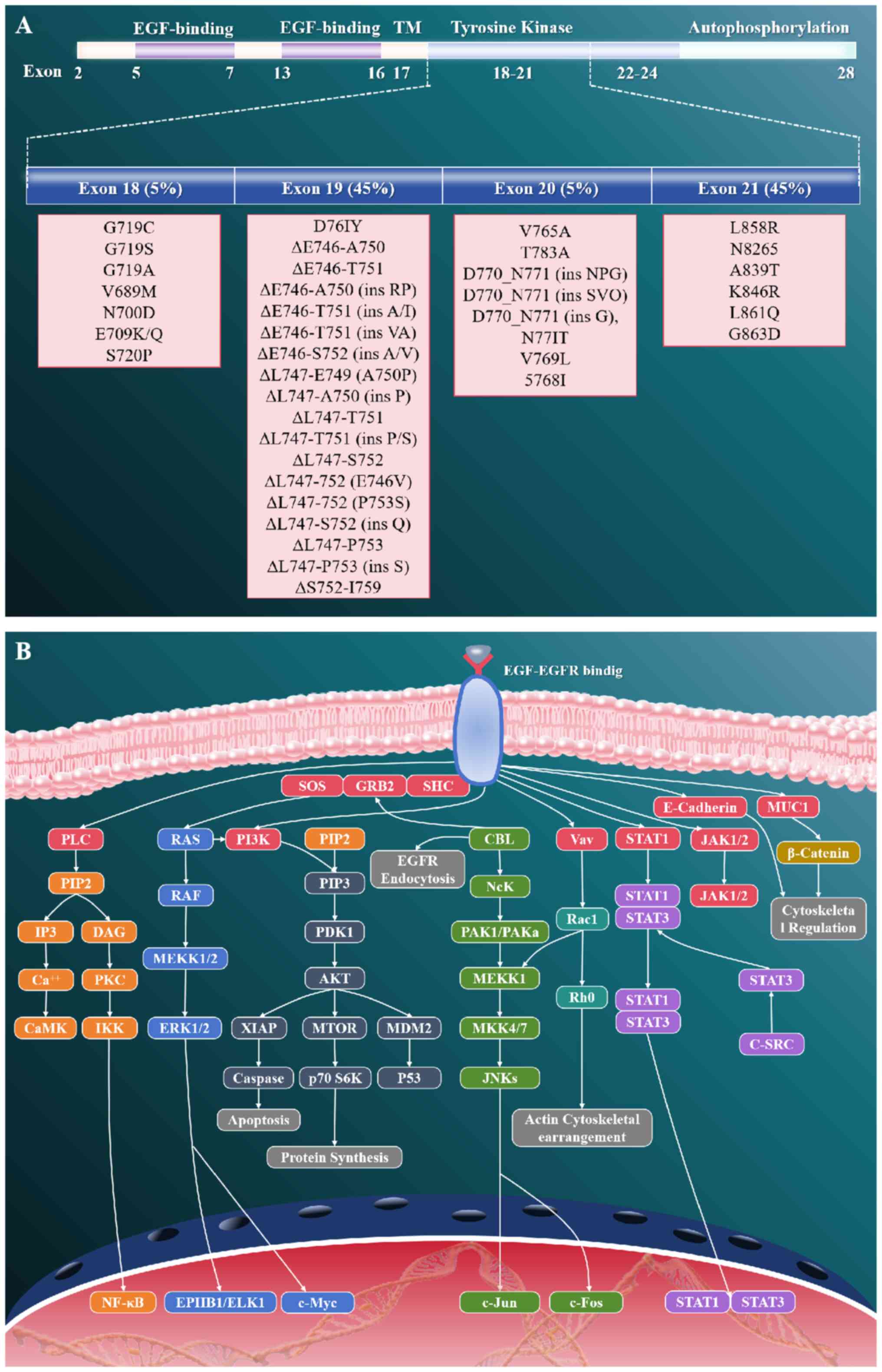
The EGFR pathway represents one of the most
extensively studied and clinically significant signaling cascades
in NSCLC (46). This pathway is
crucial for regulating essential cellular processes, including
proliferation, survival, differentiation and migration (47,48).
In NSCLC, specific genetic alterations in the EGFR gene, most
notably exon 19 deletions and the L858R point mutation in exon 21,
lead to constitutive activation of the receptor tyrosine kinase
(RTK) (49,50) (Fig.
1). Such activation results in ligand-independent dimerization
and subsequent autophosphorylation (48–50).
These oncogenic changes initiate downstream signal transduction
through key pathways including the PI3K/AKT/mTOR and
RAS/RAF/MEK/ERK cascades, ultimately driving uncontrolled cell
proliferation, suppression of apoptosis and enhanced tumor
viability (51,52). The recognition of EGFR mutations as
driver oncogenes in NSCLC has fundamentally reshaped the
therapeutic approach to lung cancer (49). First-generation EGFR-TKIs, including
gefitinib and erlotinib, have demonstrated notable efficacy in
patients harboring these mutations, leading to a substantial
improvement in PFS compared with conventional chemotherapy
(53). These small-molecule
inhibitors competitively bind to the ATP-binding site of the EGFR
tyrosine kinase domain (TKD), effectively obstructing downstream
signaling (54). The subsequent
development of second-generation TKIs (such as afatinib and
dacomitinib) alongside third-generation inhibitors (such as
osimertinib) has further enhanced clinical outcomes by addressing
resistance mechanisms whilst increasing target specificity
(55–57).
Despite these advancements, the development of
acquired resistance to EGFR-TKIs remains a major clinical
challenge. The most common mechanism of resistance is the T790M
gatekeeper mutation in exon 20, which increases the affinity of the
receptor for ATP and physically impedes TKI binding (58). Other mechanisms contributing to
resistance include MET amplification, human epidermal growth factor
receptor (HER)2 amplification, histologic transformation into SCLC,
and activation of alternative signaling pathways (59–64).
To overcome these challenges, ongoing research is focused on the
development of fourth-generation EGFR inhibitors, combination
therapies targeting parallel signaling pathways and novel
therapeutic approaches such as antibody-drug conjugates and
bispecific antibodies directed against EGFR (65,66).
The evolution of EGFR-targeted therapies in NSCLC
exemplifies a paradigm for precision medicine in oncology, wherein
tumor molecular profiling directly informs clinical
decision-making. Current clinical guidelines emphasize the
importance of comprehensive molecular testing at both initial
diagnosis and during disease progression to identify actionable
genetic alterations and mechanisms of therapeutic resistance
(66). Furthermore, the integration
of liquid biopsy methodologies for ctDNA analysis has facilitated
dynamic monitoring of EGFR mutation status and the detection of
emerging resistance mutations, thereby enabling more timely and
evidence-based treatment adjustments (67,68).
As the understanding of EGFR signaling biology continues to
advance, it is anticipated that novel therapeutic strategies will
further improve clinical outcomes for patients with EGFR-mutated
NSCLC.
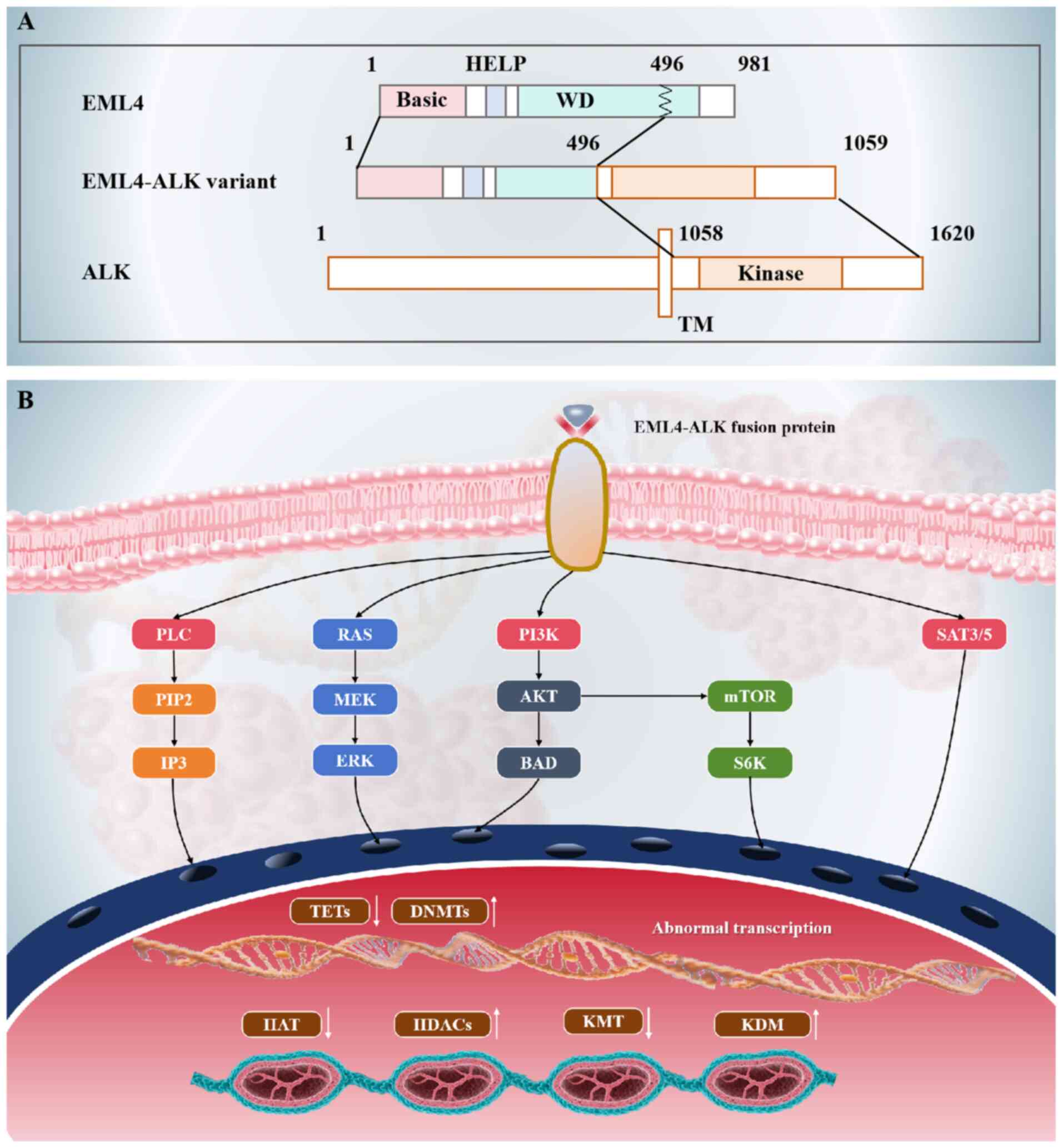
ALK rearrangements, particularly the echinoderm
microtubule-associated protein-like 4 (EML4)-ALK fusion, constitute
a clinically distinct molecular subgroup in NSCLC, occurring in
3–7% of cases (69,70). This genetic aberration originates
from a chromosomal inversion on chromosome 2p, leading to the
fusion of the EML4 gene with the ALK gene (71). The resulting EML4-ALK fusion protein
acts as a constitutively active tyrosine kinase that drives
oncogenic signaling primarily through the MAPK/ERK and PI3K/AKT
pathways, which are key regulators of cell proliferation, survival
and metastasis (72–74) (Fig.
2). The identification of ALK rearrangements has fundamentally
reshaped the therapeutic landscape for patients with ALK-positive
NSCLC (75). Targeted ALK
inhibitors, such as crizotinib, alectinib and lorlatinib, have
demonstrated substantial clinical benefits (76–78).
Crizotinib, as the first-generation ALK inhibitor, was the first to
show marked improvements in PFS and overall response rates compared
with conventional chemotherapy (76). However, despite its initial
efficacy, resistance to crizotinib commonly develops (typically
within 1 year of treatment initiation) due to secondary mutations
within the ALK kinase domain or activation of alternative signaling
pathways (79).
To overcome resistance mechanisms associated with
ALK inhibition, next-generation agents, such as alectinib and
lorlatinib, have been developed. Alectinib, classified as a
second-generation ALK inhibitor, has demonstrated superior clinical
efficacy in both treatment-naïve patients and those who have
developed resistance to crizotinib (77). Its enhanced central nervous system
(CNS) penetration markedly improves therapeutic effectiveness
against brain metastases (77).
Lorlatinib, a third-generation ALK inhibitor, is specifically
designed to target a broad spectrum of ALK resistance mutations and
has shown potent activity in patients who have progressed after
prior ALK inhibitor therapy (78).
Despite these advancements, resistance to ALK
inhibitors remains a notable clinical challenge. Resistance
mechanisms include on-target mutations within the ALK gene,
activation of alternative signaling pathways (referred to as
off-target resistance) and histological transformation (69,80,81).
Current research efforts are directed toward further characterizing
these resistance mechanisms and developing innovative therapeutic
strategies, including combination therapies and fourth-generation
ALK inhibitors-to enhance clinical outcomes for patients with
ALK-positive NSCLC (82–84). The ongoing advancement of
ALK-targeted therapies underscores the essential role of precision
medicine in the effective management of NSCLC.
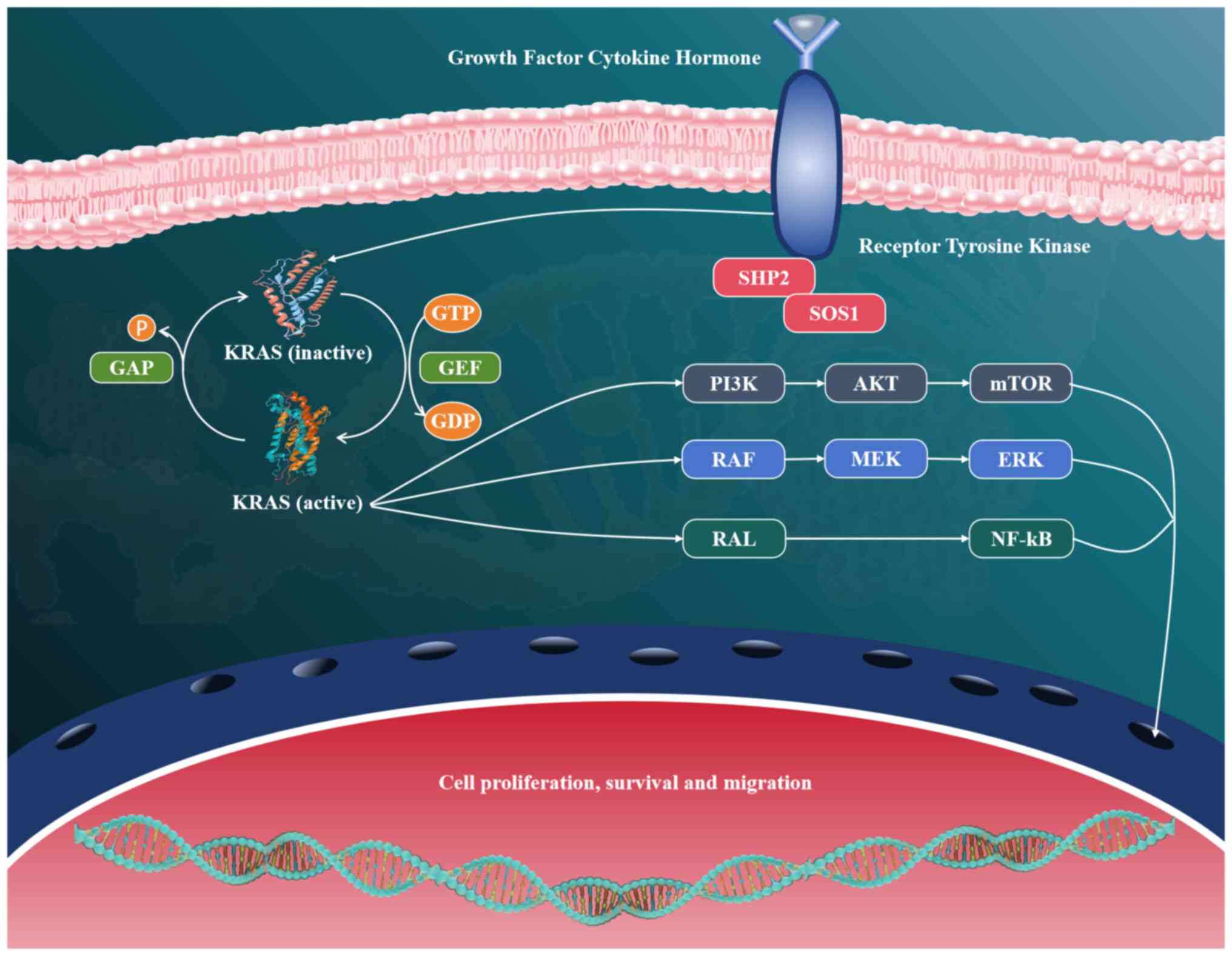
KRAS mutations represent one of the most prevalent
and clinically notable genetic alterations in NSCLC, occurring in
25–30% of cases, particularly among adenocarcinoma subtypes
(85). These mutations are
predominantly located at codon 12 (such as G12C, G12V and G12D),
with lower frequencies observed at codons 13 and 61 (86). They result in constitutive
activation of the KRAS protein, thereby promoting uncontrolled
cellular proliferation and survival (87). The oncogenic activity of mutant KRAS
is primarily mediated through sustained activation of downstream
signaling pathways, most notably the MAPK/ERK and PI3K/AKT cascades
(88). These pathways regulate
essential cellular functions such as cell cycle progression,
metabolic regulation and evasion of apoptosis, ultimately
contributing to tumor growth, metastasis and therapeutic resistance
(89,90) (Fig.
3).
For decades, KRAS was considered ‘undruggable’ due
to its lack of conventional binding pockets and its high affinity
for GTP/GDP, which posed substantial challenges in the development
of small-molecule inhibitors (91).
However, advances in structural biology and drug discovery have
fundamentally transformed the therapeutic landscape for KRAS-mutant
NSCLC (92). The identification of
a unique, druggable pocket adjacent to the G12C mutation site has
enabled the development of covalent inhibitors that selectively
target the mutant cysteine residue (93). Among these agents, sotorasib (AMG
510) and adagrasib (MRTX849) have emerged as promising therapeutic
options, demonstrating notable clinical efficacy in patients with
KRAS G12C-mutant NSCLC (85,94).
Sotorasib, recognized as the first US Food and Drug
Administration-approved KRAS G12C inhibitor, has demonstrated an
objective response rate of ~37% in pretreated patients (95). By contrast, adagrasib has shown both
systemic and intracranial antitumor activity, achieving a response
rate of ~43% in the KRYSTAL-1 trial (96).
These groundbreaking developments have not only
introduced novel therapeutic opportunities for patients with
KRAS-mutant NSCLC but have also spurred extensive research into
combination strategies and next-generation KRAS inhibitors. Current
clinical trials are actively evaluating the potential of combining
KRAS G12C inhibitors with ICIs, Src homology 2 domain-containing
protein tyrosine phosphatase 2 inhibitors or other targeted agents
to overcome resistance mechanisms and improve long-term clinical
outcomes (97–99). Additionally, ongoing efforts are
focused on developing inhibitors specifically targeting alternative
KRAS mutations (such as G12D and G12V), as well as pan-KRAS
inhibitors designed to benefit a broader patient population
(100). Despite these encouraging
advances, several challenges remain, most notably the emergence of
acquired resistance and the need for more effective therapeutic
approaches targeting non-G12C KRAS mutations, which highlights the
critical importance of continued research in this rapidly evolving
field (101,102).
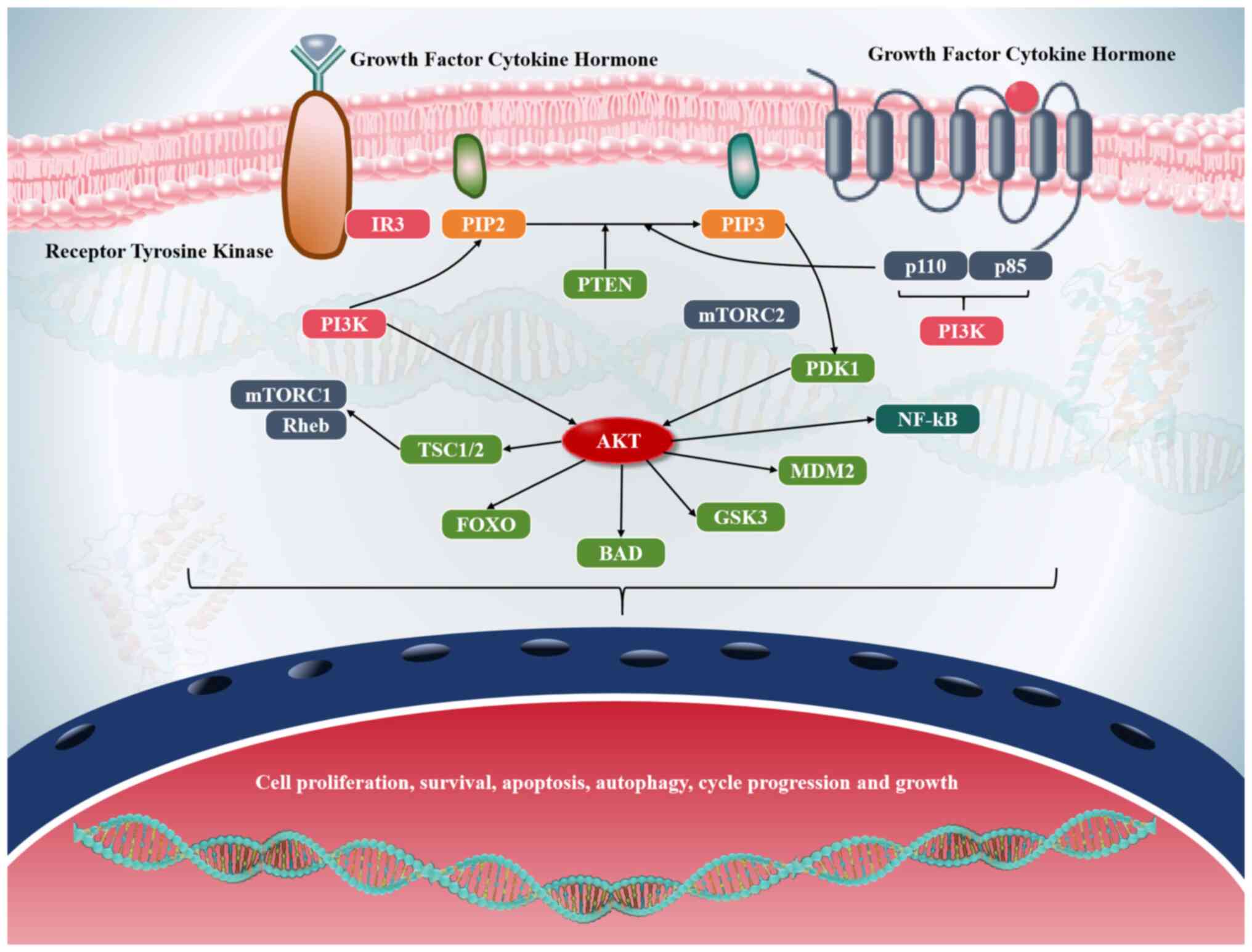
The PI3K/AKT/mTOR pathway is a highly conserved and
tightly regulated signaling cascade that governs essential cellular
functions such as growth, proliferation, metabolism and survival
(103–105). It integrates signals from diverse
extracellular stimuli, including growth factors, hormones and
nutrients, to coordinate critical cellular responses necessary for
maintaining homeostasis (106–108). Dysregulation of this pathway is
frequently observed in multiple malignancies, particularly lung
cancer, and is often driven by genetic alterations that result in
constitutive activation (109).
Among the most common are activating mutations in PI3K catalytic
subunit α, which encodes the catalytic subunit of PI3K and leads to
elevated PIP3 levels and enhanced signaling output (110). Concurrently, loss-of-function
mutations or deletions in the tumor suppressor gene PTEN, a
negative regulator of the pathway, further contribute to sustained
pathway activation (74).
Additionally, amplification or overexpression of AKT reinforces
oncogenic signaling, promoting aberrant cell survival and
proliferation (111). In lung
cancer, these molecular alterations are associated with aggressive
tumor phenotypes characterized by resistance to apoptosis,
increased angiogenesis and metabolic reprogramming-all of which
facilitate tumor progression and metastasis (112) (Fig.
4).
Despite compelling biological evidence supporting
the therapeutic targeting of the PI3K/AKT/mTOR pathway in lung
cancer, clinical development of inhibitors has encountered notable
obstacles. Although preclinical studies demonstrate that PI3K, AKT
and mTOR inhibitors can effectively suppress tumor growth and
induce apoptosis (73,113), their clinical utility has been
hampered by dose-limiting toxicities and the emergence of
resistance mechanisms (73,113). Common adverse effects, such as
hyperglycemia, rash, diarrhea and hepatotoxicity, frequently
necessitate dose reductions or treatment discontinuation, thereby
compromising therapeutic efficacy (51). Resistance typically develops through
compensatory activation of alternative pathways (such as MAPK/ERK),
feedback loops involving RTKs or mutations in downstream effectors
(114–116). These findings underscore the
complexity of pathway inhibition and highlight the urgent need for
innovative therapeutic strategies.
To overcome these limitations, combination therapies
targeting the PI3K/AKT/mTOR pathway are under intensive
investigation (117). One
promising strategy involves combining pathway inhibitors with other
targeted agents, such as EGFR or MEK inhibitors, to simultaneously
block multiple signaling nodes and prevent adaptive resistance
(118–120). For instance, dual inhibition of
EGFR and PI3K/AKT/mTOR has demonstrated robust synergistic effects
in preclinical models of lung cancer harboring both EGFR mutations
and pathway alterations (118).
Another emerging approach combines PI3K/AKT/mTOR inhibitors with
ICIs, such as anti-PD-1 or anti-PD-L1 antibodies, to enhance
antitumor immune responses (121,122). Preclinical evidence indicates that
pathway inhibition can modulate the tumor microenvironment by
reducing immunosuppressive signals and promoting T-cell
infiltration, thereby augmenting the efficacy of immunotherapy
(33,123). Ongoing clinical trials are
evaluating these combinatorial approaches with the goal of
improving clinical outcomes and overcoming resistance in patients
with lung cancer.
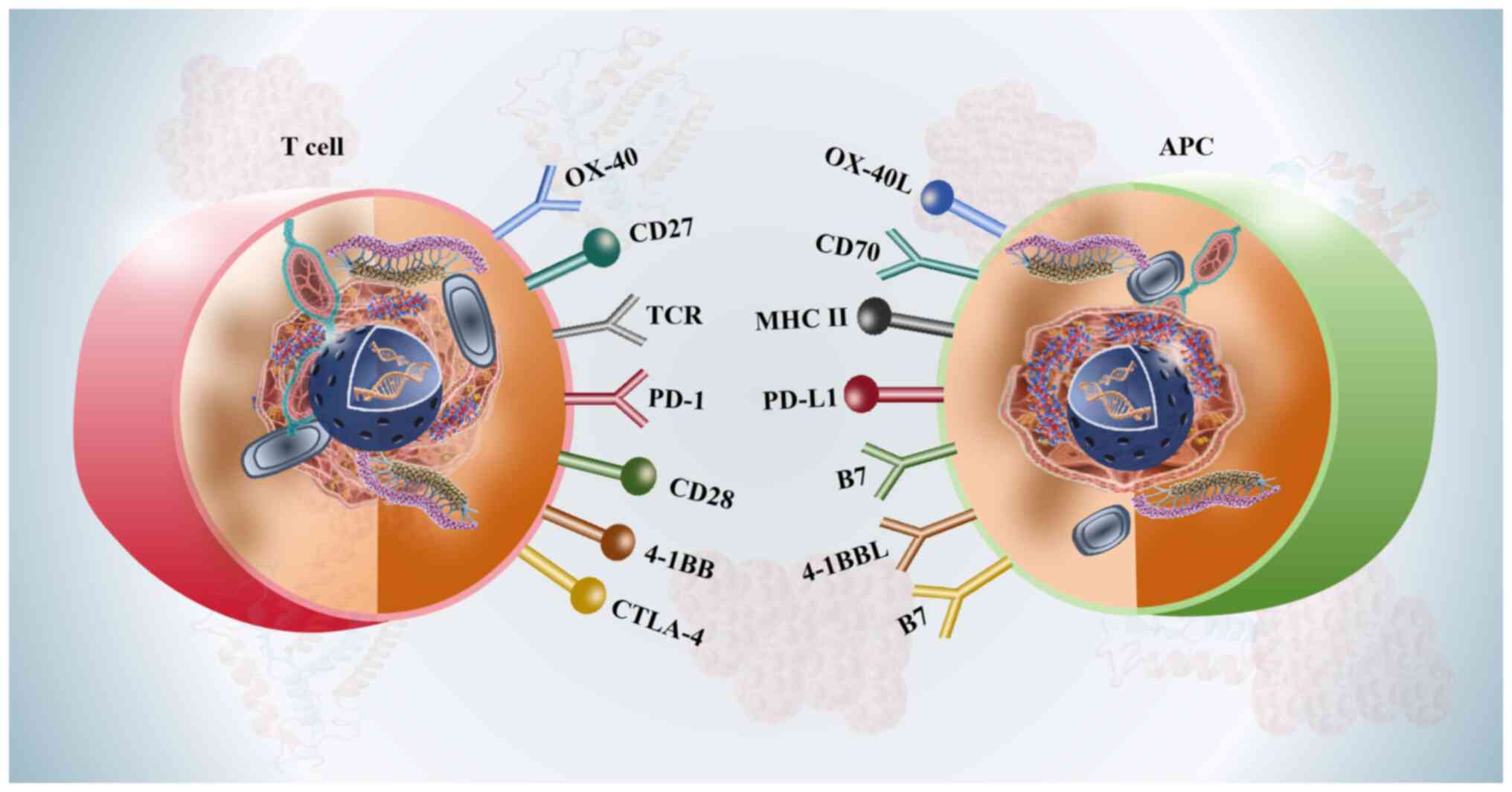
Immune checkpoint pathways, particularly the
PD-1/PD-L1 axis and CTLA-4, serve as critical mechanisms by which
tumors evade immune surveillance (124). Under normal physiological
conditions, these pathways regulate T-cell activation and maintain
immune self-tolerance (125).
However, cancer cells exploit these regulatory mechanisms to
suppress antitumor immune responses, thereby facilitating tumor
growth and metastasis (126). The
PD-1/PD-L1 interaction serves a central role in this immune evasion
process (127). PD-1 is expressed
on activated T cells and binds to PD-L1, which is frequently
overexpressed on tumor cells and within the tumor microenvironment
(124). This interaction inhibits
T-cell proliferation and effector functions, ultimately leading to
T-cell exhaustion and immune tolerance (128). Similarly, CTLA-4, expressed on
both regulatory T cells and activated effector T cells, competes
with CD28 for binding to B7 ligands on antigen-presenting cells,
thereby dampening early T-cell activation and promoting an
immunosuppressive environment (73,129)
(Fig. 5).
The advent of ICIs has markedly transformed the
treatment landscape for advanced NSCLC (130). Monoclonal antibodies targeting
PD-1 (such as pembrolizumab and nivolumab), PD-L1 (such as
atezolizumab) and CTLA-4 (such as ipilimumab) have demonstrated
robust clinical activity by reinvigorating antitumor immunity.
Pembrolizumab was the first ICI approved as a first-line therapy
for patients with NSCLC and high PD-L1 expression (≥50%), based on
the KEYNOTE-024 trial (131). It
notably improved PFS and OS compared with platinum-based
chemotherapy. Nivolumab and atezolizumab have also shown durable
clinical benefit, particularly in patients with high PD-L1
expression or elevated tumor mutational burden (TMB) (132,133). These therapies offer a favorable
toxicity profile relative to conventional chemotherapy,
contributing to improved patient quality of life (132).
Despite these therapeutic advances, both primary and
acquired resistance to ICIs remain notable clinical barriers
(134). Primary resistance refers
to the absence of an initial response, whereas acquired resistance
develops following an initial positive response (135). Resistance mechanisms can be
broadly categorized into tumor-intrinsic and tumor-extrinsic
factors. Tumor-intrinsic mechanisms include loss of antigen
presentation (such as mutations in human leukocyte antigen or
β2-microglobulin), activation of alternative immune checkpoints
(such as T-Cell immunoglobulin and mucin domain 3 or lymphocyte
activating 3) and upregulation of immunosuppressive signaling
pathways (such as TGF-β or indoleamine 2,3-dioxygenase) (136–138). Tumor-extrinsic mechanisms involve
an immunosuppressive tumor microenvironment enriched with
regulatory T cells, myeloid-derived suppressor cells and
M2-polarized macrophages (139).
Additionally, genomic alterations, such as mutations in
serine/threonine kinase 11 or kelch-like ECH associated protein 1,
have been associated with reduced responsiveness to ICIs in NSCLC
(140).
To enhance patient selection and optimize treatment
outcomes, there is a need for reliable predictive biomarkers.
Currently, PD-L1 expression assessed using immunohistochemistry is
the most widely used biomarker; however, its predictive value is
limited. Certain patients with low PD-L1 expression demonstrate
durable responses, whilst others with high expression fail to
respond. TMB, which reflects the total number of somatic mutations
per megabase of genome sequenced, has emerged as a complementary
biomarker (141). Higher TMB
levels are associated with increased neoantigen production and
enhanced immune recognition. Nevertheless, standardization of TMB
assessment remains a challenge. Other emerging biomarkers under
investigation include gene expression signatures, spatial patterns
of immune infiltration and ctDNA analysis (142).
To overcome resistance and improve ICI efficacy,
several combination strategies are being evaluated in clinical
trials. Dual checkpoint inhibition, such as combined PD-1 and
CTLA-4 blockade, has demonstrated synergistic effects in NSCLC
(143). Combinations of ICIs with
targeted therapies (such as EGFR or ALK inhibitors) are also being
explored in molecularly-defined NSCLC subpopulations (144). Another promising approach involves
combining ICIs with agents that modulate the tumor
microenvironment, including angiogenesis inhibitors (such as
bevacizumab) and drugs targeting immunosuppressive myeloid cells
[such as colony-stimulating factor 1 receptor inhibitors (CSF1R)]
(145). Furthermore, integrating
ICIs with conventional modalities such as chemotherapy or
radiotherapy has shown potential to enhance antitumor immunity
through mechanisms such as immunogenic cell death and increased
antigen release (146–148).
Lung cancer is a heterogeneous disease that arises
from complex interactions among multiple signaling pathways.
Although targeted therapies have markedly transformed the treatment
landscape for certain patient populations, the development of
resistance mechanisms and pathway redundancies continue to pose
substantial challenges. In addition to the well-established
oncogenic drivers such as EGFR, ALK and KRAS, a multitude of other
signaling pathways contribute to lung cancer pathogenesis, disease
progression and therapeutic resistance. These pathways frequently
interact with known oncogenic drivers, thereby modulating tumor
proliferation, survival, metastasis and immune evasion. The
following section provides an overview of several key signaling
pathways implicated in lung cancer (Table I).
HER2, also known as ErbB2, is a transmembrane
glycoprotein with intrinsic tyrosine kinase activity (149). It is encoded by the HER2/neu
proto-oncogene located on chromosome 17q21 (149). As a core member of the EGFR/ErbB
family, HER2 serves a pivotal role in regulating fundamental
cellular processes such as proliferation, differentiation and
survival (150). These functions
underscore its significance in normal embryonic development as well
as in the pathogenesis of several malignancies, particularly NSCLC
(149,150). In lung tumorigenesis, HER2
alterations, including point mutations, gene amplifications and
protein overexpression, drive uncontrolled tumor cell
proliferation, enhance metastatic potential and contribute to
resistance to conventional therapeutic strategies (151). Consequently, HER2 has emerged as a
key therapeutic target in oncology, leading to the development of
targeted agents such as monoclonal antibodies (such as trastuzumab)
and TKIs (such as lapatinib), which are specifically designed to
inhibit its oncogenic signaling (152).
FLT3, also known as CD135 or Flk-2, is a member of
the RTK III family, which includes KIT proto-oncogene, receptor
tyrosine kinase (KIT), platelet-derived growth factor receptor
(PDGFR) and CSF1R (153). The
receptor comprises an extracellular ligand-binding domain, a
transmembrane region and an intracellular TKD (153). FLT3 is predominantly expressed in
hematopoietic stem and progenitor cells within the bone marrow,
thymus and lymph nodes, where it regulates myeloid and lymphoid
cell proliferation, survival and differentiation (152,153). Although primarily associated with
hematologic malignancies such as acute myeloid leukemia (AML), FLT3
dysregulation has also been implicated in solid tumors, including
NSCLC (154,155). FLT3 aberrations in lung cancer
occur through three major mechanisms: i) Overexpression associated
with aggressive disease and poor clinical prognosis (155); ii) rare internal tandem
duplication and TKD mutations that may drive tumor progression
(155); and iii) crosstalk with
EGFR, MET or ALK signaling pathways, contributing to drug
resistance in lung adenocarcinoma (155). Given its oncogenic potential, FLT3
is currently under investigation as a therapeutic target in lung
cancer. Small-molecule inhibitors such as midostaurin and
quizartinib, originally developed for AML, are now being evaluated
in clinical trials involving FLT3-altered NSCLC (156). To overcome resistance, combination
therapies incorporating MEK or PI3K/AKT inhibitors are actively
being explored (156).
The PDGFR is a single-chain transmembrane
glycoprotein belonging to the Type III RTK family (157). It is widely expressed in several
cell types, including smooth muscle cells, fibroblasts, endothelial
cells, glial cells and chondrocytes (158). PDGFRα, one of its major isoforms,
promotes tumor cell proliferation, invasion and neovascularization
(159). Activation of the PDGFR
signaling cascade engages key downstream effectors such as the
RAS-MAPK and PI3K pathways (160).
PDGFRα contributes to lung cancer progression through four
well-defined mechanisms (159): i)
Enhancing cyclin D1 expression and accelerating the G1/S phase
transition; ii) increasing MMP-2 and MMP-9 levels to promote tumor
invasion; iii) inducing VEGF secretion and recruiting
tumor-associated fibroblasts to support angiogenesis; and iv)
activating cancer-associated fibroblasts that release
pro-tumorigenic cytokines. Dysregulation of PDGFR in lung cancer
arises from multiple molecular alterations, including gene
amplification, activating mutations, chromosomal rearrangements,
and autocrine or paracrine activation loops (157–159). Therapeutic strategies targeting
PDGFR include multitargeted TKIs (such as imatinib, sunitinib and
sorafenib), selective inhibitors (such as crenolanib and
olaratumab) and combination regimens with chemotherapy,
anti-angiogenic agents or immunotherapy (159,160). Despite promising preclinical and
clinical evidence, the development of drug resistance and
challenges in patient stratification remain notable barriers
(160). Further understanding of
PDGFR biology may enable the design of more effective therapeutic
strategies for PDGFR-driven lung cancers.
The KIT receptor (CD117) serves a notable role in
lung cancer, particularly in neuroendocrine subtypes such as SCLC
and large cell neuroendocrine carcinoma (161). As a type III RTK, KIT has a unique
structural organization: Its extracellular domain binds stem cell
factor (SCF) through five immunoglobulin-like loops, whilst the
intracellular region contains a split TKD responsible for
downstream signal transduction (161). This architecture enables KIT to
regulate essential cellular functions, including proliferation,
survival, differentiation and migration (162). A total of 15–20% of SCLC cases
exhibit KIT overexpression coupled with autocrine SCF production,
establishing a self-sustaining growth loop (162). Although TKIs, such as imatinib,
demonstrate robust efficacy in other KIT-driven malignancies, most
notably gastrointestinal stromal tumors, their clinical benefit in
lung cancer is restricted to patients harboring specific activating
mutations or gene amplifications (163). Therefore, comprehensive molecular
profiling is crucial for identifying patients who may derive
therapeutic benefit. Research efforts have been directed toward
developing next-generation KIT inhibitors with enhanced CNS
penetration, such as avapritinib, as well as exploring combination
strategies involving ICIs or epigenetic modulators (162,163).
FGFR, a member of the RTK family, comprises four
principal isoforms: FGFR1, FGFR2, FGFR3 and FGFR4 (164). Upon binding to its ligand,
fibroblast growth factor, FGFR undergoes dimerization and
autophosphorylation, leading to the activation of multiple
downstream signaling pathways, including JAK/STAT, phospholipase
Cγ, PI3K and MAPK (165). These
signaling cascades regulate fundamental cellular processes such as
chemotaxis, differentiation and proliferation, and also serve roles
in the development of nerve cells and fibroblasts (166). Notably, the FGFR pathway functions
as a key oncogenic driver in a subset of lung cancers, particularly
squamous cell carcinomas (167).
Although FGFR-targeted therapies show preclinical efficacy, their
clinical translation requires more precise patient stratification
and strategies to overcome resistance mechanisms (168). Further investigation into FGFR
biology may facilitate the development of novel, molecularly
tailored therapeutic approaches for distinct subtypes of lung
cancer.
The p53 signaling pathway interacts with multiple
intracellular networks and is central to maintaining cellular
homeostasis and normal physiological functions (173). It activates in response to DNA
damage or abnormal proliferation, causing cell cycle arrest and DNA
repair (174). If damage cannot be
repaired, p53 induces apoptosis by activating pro-apoptotic genes
(173). Normally, p53 mRNA levels
are high, but protein levels stay low due to rapid mouse double
minute 2 (MDM2)-mediated ubiquitination and degradation (173–175). MDM2 binds to p53 and promotes its
breakdown, forming a key negative feedback loop (176). Due to its tumor-suppressive
function, p53 pathway dysregulation serves a major role in lung
cancer development (177).
Targeting this pathway may offer potential for personalized
treatment, though challenges remain due to mutations and complex
regulation (178). Advances in
targeted therapy have created new opportunities for leveraging p53
in precision oncology (179).
Integrating comprehensive molecular profiling with the development
of novel agents and rational combination strategies will be
critical to unlocking the therapeutic potential of p53.
Abnormal activation of the Wnt signaling pathway is
strongly associated with lung cancer development (180). Upon binding of Wnt ligands to the
Frizzled-LDL receptor related protein receptor complex on the cell
membrane, Axin translocation is initiated, allowing β-catenin to
accumulate in the nucleus (181).
There, β-catenin interacts with Tcf/Lef transcription factors and
recruits co-activators to drive the expression of Wnt target genes
(182). In the absence of Wnt
signals, β-catenin is phosphorylated and subsequently degraded by a
destruction complex composed of adenomatous polyposis coli protein,
GSK3β and casein kinase 1 α1, thereby maintaining low intracellular
levels of β-catenin (183–185). Under these conditions, Tcf/Lef
functions as a transcriptional repressor, inhibiting gene
expression (186). Mutations in
β-catenin that disrupt the regulation of Wnt signaling have been
identified in several malignancies, including lung, liver, ovarian,
skin, prostate cancer and melanoma (187–191). The Wnt/β-catenin pathway serves a
pivotal role in lung cancer pathogenesis, particularly in tumor
initiation and the maintenance of cancer stem cell properties
(184). Although therapeutic
targeting of this pathway remains challenging due to its
pleiotropic functions and intricate regulatory mechanisms, advances
in molecular profiling and targeted therapies offer promising
avenues for intervention (186,187). Future progress will depend on
integrating molecular stratification with novel agents and rational
combination strategies to fully exploit the therapeutic potential
of the Wnt/β-catenin pathway in lung cancer treatment (187–191).
The TGFβ family includes cytokines that inhibit the
growth of normal epithelial cells (199). It consists of TGFβ isoforms,
Activins, Inhibins, Nodal, bone morphogenetic proteins,
anti-Müllerian hormone, and growth and differentiation factors
(200–202). This pathway regulates key cellular
processes such as proliferation, apoptosis, invasion, metastasis,
extracellular matrix remodeling, differentiation and immune
responses (203). Dysregulated
TGFβ signaling disrupts cellular homeostasis and is associated with
tumor initiation, progression and immune evasion (203). TGFβ initiates signaling by binding
to two transmembrane receptors, TβRI and TβRII, that activate a
downstream signaling cascade (199,203). Upon binding to TβRII, TGFβ
recruits and phosphorylates TβRI (203). Activated TβRI subsequently
phosphorylates SMAD2 and SMAD3, which associate with SMAD4 to form
a transcriptional complex that translocates into the nucleus to
regulate gene expression (204).
As a central regulator of lung cancer progression, aberrant TGFβ
signaling serves a critical role in promoting metastasis and
suppressing antitumor immunity (205). Although targeting this pathway
holds therapeutic promise, further research is needed to clarify
its context-dependent functions and optimize combination therapies
(206). A deeper understanding of
how TGFβ interacts with other oncogenic pathways-such as EGFR and
KRAS-is essential for developing effective precision treatments in
lung cancer (207).
The NF-κB family consists of transcription factors
that regulate essential biological processes such as immunity,
inflammation, cell survival, growth and differentiation (208–210). The mammalian NF-κB family consists
of five members: NF-κB1 (p50), NF-κB2 (p65), RelA (p65), RelB and
c-Rel (211). These proteins form
homodimers or heterodimers, translocate into the nucleus, bind to
κB sites in target genes and control their expression (212). Under normal conditions, NF-κB is
sequestered in the cytoplasm by the inhibitory protein IκB,
remaining in an inactive state (213). Aberrant NF-κB activation, driven
by overexpression or mutations in pathway components, is strongly
associated with lung cancer initiation and progression (214,215). As a central regulator of
inflammation and oncogenesis, NF-κB promotes tumor survival, immune
evasion and metastasis (216).
Although therapeutic targeting is complicated by its essential
physiological roles, precision strategies, such as selective
inhibition and combination therapies, offer potential for improving
lung cancer treatment (217).
Further research on biomarker-guided modulation of NF-κB signaling
is crucial for advancing clinical applications (218).
Insulin and IGF signaling pathways are central
regulators of metabolic processes (219). Previous research demonstrated that
these pathways contribute to tumor initiation and progression,
positioning them as key therapeutic targets (220). The human insulin receptor and IGF1
receptor both have tetrameric structures with intrinsic tyrosine
kinase activity (221). Upon
ligand binding, they phosphorylate insulin receptor substrate
proteins, which activate downstream pathways such as PI3K/AKT and
MAPK, thereby promoting oncogenic processes (220,221). Studies have reported that insulin
and IGF1 stimulate tumor cell proliferation, whereas inhibition of
either pathway suppresses tumor growth (222). The insulin/IGF axis serves as a
critical driver of lung cancer metabolism and progression,
integrating metabolic dysregulation with oncogenic signaling
(223,224). Although the clinical application
of IGF-targeted therapies has encountered challenges, combination
approaches and precision metabolic interventions offer promising
avenues (225). Future research
should focus on overcoming resistance mechanisms and targeting
metabolic vulnerabilities in lung cancer (226).
The development of next-generation TKIs marks a
notable advancement in the treatment of oncogene-driven NSCLC,
particularly for patients exhibiting resistance to
earlier-generation inhibitors (227). These next-generation TKIs,
including osimertinib for EGFR-mutant NSCLC and lorlatinib for
ALK-positive NSCLC, have been specifically designed to overcome the
limitations associated with their predecessors (18,228).
They offer enhanced efficacy, broader activity against resistance
mutations and improved penetration into the CNS (229,230). Such advancements have transformed
the therapeutic landscape, providing renewed hope for patients who
develop resistance to initial therapies.
Osimertinib, a third-generation EGFR-TKI, has
emerged as a cornerstone in the treatment of NSCLC with EGFR
mutations (228). First- and
second-generation EGFR TKIs, including erlotinib, gefitinib and
afatinib, have demonstrated marked clinical benefits for patients
harboring activating EGFR mutations such as exon 19 deletions and
L858R mutations (53–57). However, resistance to these agents
inevitably develops, often due to the acquisition of the EGFR T790M
mutation, which diminishes the binding affinity of
earlier-generation TKIs (228).
Osimertinib was specifically designed to target both activating
EGFR mutations and the T790M resistance mutation, rendering it an
effective option for overcoming this resistance (231). Clinical trials, including the AURA
series and the FLAURA study, have reported that osimertinib offers
superior PFS and OS compared with earlier-generation TKIs, even in
first-line settings (232–234). Furthermore, osimertinib
demonstrates enhanced CNS penetration, making it particularly
effective against brain metastases, a common and challenging
complication associated with EGFR-mutant NSCLC (229). This CNS activity is especially
critical given that 25–40% of patients with EGFR-mutant NSCLC
develop brain metastases during their disease course (235).
Similarly, lorlatinib, a third-generation ALK-TKI,
has emerged as a groundbreaking therapy for ALK-positive NSCLC
(18). Earlier-generation ALK
inhibitors, including crizotinib, ceritinib and alectinib, have
demonstrated notable efficacy in treating ALK-rearranged NSCLC
(78). However, resistance
frequently develops due to secondary mutations in the ALK gene or
activation of bypass signaling pathways (69). Lorlatinib was specifically designed
to address these resistance mechanisms and exhibits potent activity
against a broad spectrum of ALK resistance mutations, notably the
highly resistant G1202R mutation (236). The CROWN trial provided compelling
evidence of the superior efficacy of lorlatinib compared with
crizotinib in the first-line treatment setting, showing notably
prolonged PFS and higher intracranial response rates (237). Similar to osimertinib, lorlatinib
also demonstrates marked CNS penetration, rendering it particularly
effective in managing brain metastases, a complication that affects
≤60% of patients with ALK-positive NSCLC throughout their disease
trajectory (230,238).
The enhanced CNS activity of next-generation TKIs
represents a critical feature, particularly given that the brain is
a common site for metastasis in oncogene-driven NSCLC (239). Traditional chemotherapy and
earlier-generation TKIs frequently fail to achieve sufficient drug
concentrations within the CNS due to the presence of the
blood-brain barrier, resulting in inadequate control over brain
metastases (240). By contrast,
next-generation TKIs such as osimertinib and lorlatinib are
specifically designed to penetrate the blood-brain barrier more
effectively, thereby facilitating robust intracranial responses and
delaying the progression of CNS disease (229,230). This capability not only enhances
survival rates but also preserves neurological function and
improves quality of life for patients.
Despite these advancements, resistance to
next-generation TKIs continues to pose a significant challenge. In
the case of osimertinib, resistance mechanisms include the
emergence of EGFR C797S mutations, MET amplification and
histological transformation to SCLC (57). For lorlatinib, resistance may arise
through compound ALK mutations or activation of alternative
signaling pathways (241). To
address these challenges, ongoing research is focused on developing
fourth-generation TKIs, exploring combination therapies, and
investigating novel strategies, such as antibody-drug conjugates
and immunotherapy in oncogene-driven NSCLC (242–244).
An ADC represents a sophisticated and innovative
class of therapeutic agents that integrates the precision of
monoclonal antibodies with the potent cytotoxic effects
characteristic of chemotherapy (245). Structurally, an ADC consists of
three core components: i) A monoclonal antibody engineered to
selectively recognize and bind to a specific antigen expressed on
the surface of tumor cells; ii) a cytotoxic agent, commonly
referred to as the payload, which induces cell death in targeted
cancer cells; and iii) a chemical linker that stably connects the
antibody to the payload (245).
The monoclonal antibody functions as a ‘guided missile’,
selectively delivering the cytotoxic payload directly to tumor
cells whilst minimizing damage to healthy tissues, thereby reducing
off-target toxicity (246). This
targeted delivery mechanism represents one of the key advantages of
ADCs, enabling the use of highly potent cytotoxic agents that would
be too toxic for systemic administration (246).
ADCs are increasingly being adopted in oncology,
particularly in the treatment of lung cancer, where early clinical
trials have shown encouraging results (247–249). In both NSCLC and SCLC, ADCs have
demonstrated notable response rates and improved survival outcomes,
especially among patients who have limited remaining treatment
options (250,251). For example, ADCs targeting
proteins such as HER2, HER3, trophoblast cell-surface antigen 2
(TROP2), carcinoembryonic antigen-related cell adhesion molecule 5
(CEACAM5) and MET in NSCLC, and delta-like ligand 3 (DLL3) in SCLC,
have attracted considerable attention due to their ability to
produce durable responses in heavily pretreated populations
(251–254). These findings highlight the
potential of ADCs to address notable unmet needs in lung cancer
therapy, particularly for patients with limited therapeutic
alternatives.
In NSCLC, several ADCs are currently under
investigation, each targeting distinct tumor-associated antigens.
HER2-targeted ADCs, such as trastuzumab deruxtecan, have
demonstrated notable efficacy in NSCLC characterized by HER2
mutations or overexpression (255). Although HER2 mutations are rare in
NSCLC, they are associated with aggressive disease and poor
prognosis; therefore, HER2-targeted ADCs represent a promising
therapeutic strategy for this specific patient population (256). Similarly, HER3-targeted ADCs,
including patritumab deruxtecan, are being evaluated in EGFR-mutant
NSCLC, particularly in patients who have developed resistance to
EGFR-TKIs (257). HER3 is
frequently overexpressed in EGFR-mutant NSCLC and serves a key role
in mediating resistance to targeted therapies, making it an
attractive target for ADC development (258).
TROP2 is another promising target in NSCLC, as it
is overexpressed in a substantial proportion of lung
adenocarcinomas and is associated with poor prognosis (259). Sacituzumab govitecan, a
TROP2-targeted ADC, has demonstrated encouraging activity in NSCLC,
with durable responses observed in patients with advanced disease
(260). In addition, CEACAM5 and
MET are actively being investigated as ADC targets in NSCLC
(246). CEACAM5 is overexpressed
in a subset of lung adenocarcinomas, whilst MET amplification or
overexpression is a well-established driver of tumor growth and
therapeutic resistance in NSCLC (246,261). ADCs directed against these
proteins aim to deliver potent cytotoxic payloads specifically to
tumor cells, thereby overcoming resistance mechanisms and improving
clinical outcomes (262). In SCLC,
DLL3 has emerged as a compelling target for ADC development
(263). DLL3 is highly expressed
on the surface of SCLC cells but is scarcely present in normal
tissues, making it an ideal candidate for targeted therapy
(263). Rovalpituzumab tesirine, a
DLL3-targeted ADC, has shown promising activity in SCLC,
particularly among patients with high DLL3 expression (264). Although early clinical trials
encountered certain challenges, ongoing research is focused on
optimizing the use of DLL3-targeted ADCs and identifying biomarkers
that can predict treatment response (265–267).
However, the development of ADCs for lung cancer
presents multiple challenges. Key considerations include the
optimization of target selection, improvement of linker stability
to prevent premature release of the cytotoxic payload, and
effective management of toxicities such as interstitial lung
disease, which has been reported with certain ADCs (268–270). Moreover, the identification of
predictive biomarkers is crucial for selecting patients most likely
to benefit from ADC therapy, thereby enhancing therapeutic efficacy
(253).
Combination strategies, such as dual inhibition of
EGFR and MET or concurrent targeting of the PI3K/AKT/mTOR pathway
along with immune checkpoints, are currently under active
investigation to overcome resistance and improve therapeutic
outcomes (147,271,272). These approaches aim to address the
complex and heterogeneous nature of cancer biology, where
single-agent therapies often fail due to the development of
resistance mechanisms and tumor heterogeneity (273).
For example, dual inhibition of EGFR and MET
represents a promising therapeutic approach in cancers
characterized by co-activation of these pathways or in cases where
resistance to EGFR inhibitors arises from MET amplification or
signaling activation (274). EGFR
is a well-characterized oncogenic driver involved in tumor
proliferation across multiple malignancies, including NSCLC
(46). However, resistance to
EGFR-TKIs frequently emerges through mechanisms such as activation
of the MET pathway (275). By
simultaneously targeting both EGFR and MET, researchers seek to
block compensatory signaling pathways that tumors utilize to evade
therapeutic pressure, thereby prolonging the duration of treatment
response (276).
Similarly, the co-targeting of the PI3K/AKT/mTOR
pathway and immune checkpoints represents a synergistic strategy in
cancer therapy (277). The
PI3K/AKT/mTOR pathway functions as a central regulator of cell
growth, survival and metabolic processes, with its dysregulation
commonly observed across multiple tumor types (120). However, inhibitors targeting this
pathway frequently trigger feedback activation of compensatory
signaling mechanisms, which can compromise their therapeutic
effectiveness (122). Combining
PI3K/AKT/mTOR inhibitors with ICIs, such as anti-PD-1 or
anti-CTLA-4 antibodies, aims not only to suppress tumor
proliferation directly but also to enhance the ability of the
immune system to recognize and eradicate malignant cells (278). This combinatorial strategy
leverages the potential of immunotherapy to counteract the
immunosuppressive tumor microenvironment often reinforced by
aberrant PI3K/AKT/mTOR signaling (279).
These combination strategies are supported by
preclinical evidence and early-phase clinical trials, which have
shown encouraging results in terms of tumor regression and
prolonged PFS (280–282). Nonetheless, several challenges
remain, including the optimization of dosing regimens, mitigation
of overlapping toxicities and the identification of predictive
biomarkers to guide patient selection for those most likely to
derive clinical benefit (283). As
research progresses, these combination therapies hold promise to
transform the treatment paradigm for cancers that currently present
major therapeutic challenges when managed with monotherapies.
Liquid biopsies, which analyze ctDNA, have emerged
as a transformative, non-invasive tool for detecting targetable
mutations and monitoring treatment responses in patients with
cancer (284). By contrast to
traditional tissue biopsies, often requiring invasive procedures
and potentially failing to capture the full genetic heterogeneity
of tumors, liquid biopsies offer a minimally invasive alternative
that can be serially performed over time (285). This approach involves isolating
ctDNA, which consists of DNA fragments shed by tumor cells into the
bloodstream, and analyzing them for clinically relevant genetic
alterations such as mutations, amplifications or gene fusions that
drive tumorigenesis (286). By
providing a real-time molecular profile of the tumor, liquid
biopsies enable clinicians to identify actionable mutations,
including EGFR, ALK or BRAF variants, and guide personalized
therapeutic strategies accordingly (287).
One of the most notable advantages of liquid
biopsies is their ability to monitor treatment response and detect
emerging resistance mechanisms (288). For example, in patients receiving
targeted therapies such as EGFR inhibitors, resistance mutations,
such as T790M in NSCLC, can be detected early through ctDNA
analysis (289). Such early
identification allows for timely adjustments to treatment regimens.
Moreover, liquid biopsies can assess tumor burden and minimal
residual disease following surgical resection or systemic therapy,
thereby offering valuable prognostic insights that support clinical
decision-making regarding adjuvant or maintenance treatments
(290).
Biomarker-driven approaches, which rely on the
identification of specific molecular alterations, serve a
fundamental role in personalizing cancer therapy and improving
patient outcomes (291). By
integrating liquid biopsy data with clinical and pathological
information, clinicians can stratify patients into distinct
molecular subgroups and select therapies most likely to achieve
favorable outcomes (292,293). For instance, patients with
HER2-positive breast cancer or KRAS-mutated colorectal cancer can
be matched to targeted therapies or clinical trials evaluating
novel agents specifically designed to target their unique genetic
profiles (294,295). This precision medicine strategy
not only enhances therapeutic efficacy but also minimizes
unnecessary exposure to ineffective treatments and their associated
adverse effects (296).
Furthermore, liquid biopsies serve an increasingly
central role in clinical trials by facilitating patient selection,
monitoring pharmacodynamic responses and evaluating the impact of
investigational therapies on tumor genetics (297). As technological advancements,
marked by improvements in sensitivity, specificity and the ability
to detect low-frequency mutations, continue to evolve, liquid
biopsies are poised to become a cornerstone of modern oncology care
(298). These innovations hold the
potential to transform how cancer is diagnosed, treated and
monitored, ultimately advancing the development of more
personalized, effective and patient-centered therapeutic strategies
(298,299).
Despite marked advancements in targeted therapy for
lung cancer, several critical challenges persist that require
focused attention and innovative strategies. One of the most
pressing concerns is the inevitable emergence of resistance to
targeted therapies, which can occur through both on-target and
off-target mechanisms (300).
Primary resistance affects 20–30% of patients, whilst acquired
resistance typically develops within 9–14 months after treatment
initiation, substantially limiting the duration of clinical benefit
(301). The underlying mechanisms
of resistance are complex and heterogeneous, encompassing secondary
mutations in the target gene (such as EGFR T790M and C797S),
activation of bypass signaling pathways (such as MET amplification
and HER2 activation) and histological transformations (such as
conversion to SCLC) (302–304).
The identification and validation of predictive
biomarkers remain a major challenge in optimizing targeted
therapeutic strategies (305).
Although considerable progress has been achieved in detecting
common driver mutations (such as EGFR, ALK and ROS1), there is an
urgent need for more sensitive and comprehensive biomarker
platforms capable of detecting rare mutations, gene fusions and
intricate molecular profiles (306–308). Liquid biopsy technologies,
particularly ctDNA analysis, have shown promise in this regard;
however, their application remains limited by issues of sensitivity
and specificity, particularly in the context of early-stage disease
and minimal residual disease monitoring (309).
Moreover, the development of effective therapies
for rare molecular subtypes, collectively representing 10–15% of
NSCLC cases, remains a notable unmet clinical need (227). These subtypes encompass rare
genetic alterations such as EGFR exon 20 insertions, HER2
mutations, RET fusions, NTRK fusions and MET exon 14 skipping
mutations (64–67). Although targeted therapies have been
developed for several alterations, certain patients still lack
effective treatment options (71).
Furthermore, the limited size of patient populations associated
with these rare subtypes poses substantial challenges for clinical
trial enrollment and pharmaceutical development.
Future research should focus on several key areas
to effectively address the aforementioned challenges: i)
Elucidating the molecular mechanisms of resistance through
comprehensive genomic profiling and functional investigations; ii)
developing novel therapeutic agents, including fourth-generation
EGFR inhibitors, covalent KRAS inhibitors and selective RET
inhibitors; iii) optimizing combination strategies that target
multiple pathways concurrently whilst maintaining acceptable
toxicity profiles; iv) advancing biomarker discovery through
multi-omics approaches and artificial intelligence-assisted data
analysis; and v) innovating clinical trial designs, such as basket
trials and platform trials, to accelerate drug development for rare
molecular subtypes.
Additionally, there is an increasing necessity to
integrate targeted therapies with other treatment modalities, such
as immunotherapy and radiotherapy, to develop more comprehensive
treatment strategies. The investigation of novel drug delivery
systems, including antibody-drug conjugates and nanoparticle-based
therapies, may also present new opportunities to enhance
therapeutic efficacy whilst minimizing systemic toxicity.
Signaling pathways serve a crucial role in the
pathogenesis of lung cancer, driving essential processes such as
cell proliferation, survival, invasion and metastasis. The
dysregulation of these pathways, often resulting from genetic
mutations or amplifications, serves as a hallmark of lung cancer
and contributes to its aggressive behavior and resistance to
conventional therapies. Targeted therapies that specifically
inhibit these aberrant signaling pathways have transformed the
treatment landscape for lung cancer, particularly in NSCLC, which
constitutes the majority of cases. Advancements in understanding of
these signaling pathways have also facilitated the development of
innovative therapeutic strategies, including combination therapies
and next-generation inhibitors aimed at overcoming resistance
mechanisms. Ongoing research and clinical trials are imperative to
address existing challenges and further propel advancements in the
field of precision oncology.
Not applicable.
Funding: No funding was received.
Not applicable.
ZT, WS and HaZ were responsible for conception and
design, acquisition of data, and analysis and interpretation of
data. SX, JZ and JR revised the article. XW and HuZ completed the
final version of the manuscript. All authors read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Li Y, Wu X, Yang P, Jiang G and Luo Y:
Machine learning for lung cancer diagnosis, treatment, and
prognosis. Genomics Proteomics Bioinformatics. 20:850–866. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bade BC and Dela Cruz CS: Lung cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harðardottir H, Jonsson S, Gunnarsson O,
Hilmarsdottir B, Asmundsson J, Gudmundsdottir I, Saevarsdottir VY,
Hansdottir S, Hannesson P and Gudbjartsson T: Advances in lung
cancer diagnosis and treatment-a review. Laeknabladid. 108:17–29.
2020.(In Icelandic). View Article : Google Scholar
|
|
4
|
Abu Rous F, Singhi EK, Sridhar A, Faisal
MS and Desai A: Lung cancer treatment advances in 2022. Cancer
Invest. 41:12–24. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu F, Wang L and Zhou C: Lung cancer in
China: Current and prospect. Curr Opin Oncol. 33:40–46. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodriguez-Canales J, Parra-Cuentas E and
Wistuba II: Diagnosis and molecular classification of lung cancer.
Cancer Treat Res. 170:25–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Sousa VML and Carvalho L: Heterogeneity
in lung cancer. Pathobiology. 85:96–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abolfathi H, Arabi M and Sheikhpour M: A
literature review of microRNA and gene signaling pathways involved
in the apoptosis pathway of lung cancer. Respir Res. 24:552023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niu Z, Jin R, Zhang Y and Li H: Signaling
pathways and targeted therapies in lung squamous cell carcinoma:
Mechanisms and clinical trials. Signal Transduct Target Ther.
7:3532022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan M, Zhao Y, Arkenau HT, Lao T, Chu L
and Xu Q: Signal pathways and precision therapy of small-cell lung
cancer. Signal Transduct Target Ther. 7:1872022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masciale V, Banchelli F, Grisendi G,
Samarelli AV, Raineri G, Rossi T, Zanoni M, Cortesi M, Bandini S,
Ulivi P, et al: The molecular features of lung cancer stem cells in
dedifferentiation process-driven epigenetic alterations. J Biol
Chem. 300:1079942024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoque MO, Brait M, Rosenbaum E, Poeta ML,
Pal P, Begum S, Dasgupta S, Carvalho AL, Ahrendt SA, Westra WH and
Sidransky D: Genetic and epigenetic analysis of erbB signaling
pathway genes in lung cancer. J Thorac Oncol. 5:1887–1893. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He H, He MM, Wang H, Qiu W, Liu L, Long L,
Shen Q, Zhang S, Qin S, Lu Z, et al: In utero and
childhood/adolescence exposure to tobacco smoke, genetic risk, and
lung cancer incidence and mortality in adulthood. Am J Respir Crit
Care Med. 207:173–182. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CY, Huang KY, Chen CC, Chang YH, Li
HJ, Wang TH and Yang PC: The role of PM2.5 exposure in lung cancer:
Mechanisms, genetic factors, and clinical implications. EMBO Mol
Med. 17:31–40. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagano T, Tachihara M and Nishimura Y:
Molecular mechanisms and targeted therapies including immunotherapy
for non-small cell lung cancer. Curr Cancer Drug Targets.
19:595–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samarelli AV, Masciale V, Aramini B, Coló
GP, Tonelli R, Marchioni A, Bruzzi G, Gozzi F, Andrisani D,
Castaniere I, et al: Molecular mechanisms and cellular contribution
from lung fibrosis to lung cancer development. Int J Mol Sci.
22:121792021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohmori T, Yamaoka T, Ando K, Kusumoto S,
Kishino Y, Manabe R and Sagara H: Molecular and clinical features
of EGFR-TKI-associated lung injury. Int J Mol Sci. 22:7922021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schneider JL, Lin JJ and Shaw AT:
ALK-positive lung cancer: A moving target. Nat Cancer. 4:330–343.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reck M, Carbone DP, Garassino M and
Barlesi F: Targeting KRAS in non-small-cell lung cancer: Recent
progress and new approaches. Ann Oncol. 32:1101–1110. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoda S, Dagogo-Jack I and Hata AN:
Targeting oncogenic drivers in lung cancer: Recent progress,
current challenges and future opportunities. Pharmacol Ther.
193:20–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herrera-Juárez M, Serrano-Gómez C,
Bote-de-Cabo H and Paz-Ares L: Targeted therapy for lung cancer:
Beyond EGFR and ALK. Cancer. 129:1803–1820. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoneda K, Imanishi N, Ichiki Y and Tanaka
F: Treatment of non-small cell lung cancer with EGFR-mutations. J
UOEH. 41:153–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hosomi Y, Morita S, Sugawara S, Kato T,
Fukuhara T, Gemma A, Takahashi K, Fujita Y, Harada T, Minato K, et
al: Gefitinib alone versus gefitinib plus chemotherapy for
non-small-cell lung cancer with mutated epidermal growth factor
receptor: NEJ009 study. J Clin Oncol. 38:115–123. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Greenhalgh J, Bagust A, Boland A, Dwan K,
Beale S, Hockenhull J, Proudlove C, Dundar Y, Richardson M, Dickson
R, et al: Erlotinib and gefitinib for treating non-small cell lung
cancer that has progressed following prior chemotherapy (review of
NICE technology appraisals 162 and 175): A systematic review and
economic evaluation. Health Technol Assess. 19:1–134. 2015.
View Article : Google Scholar
|
|
25
|
Remon J, Besse B, Aix SP, Callejo A,
Al-Rabi K, Bernabe R, Greillier L, Majem M, Reguart N, Monnet I, et
al: Osimertinib treatment based on plasma T790M monitoring in
patients with EGFR-mutant non-small-cell lung cancer (NSCLC): EORTC
lung cancer group 1613 APPLE phase II randomized clinical trial.
Ann Oncol. 34:468–476. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abdelgalil AA and Alkahtani HM:
Crizotinib: A comprehensive profile. Profiles Drug Subst Excip
Relat Methodol. 48:39–69. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peters S, Camidge DR, Shaw AT, Gadgeel S,
Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, et al:
Alectinib versus crizotinib in untreated ALK-positive
non-small-cell lung cancer. N Engl J Med. 377:829–838. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Solomon BJ, Liu G, Felip E, Mok TSK, Soo
RA, Mazieres J, Shaw AT, de Marinis F, Goto Y, Wu YL, et al:
Lorlatinib versus crizotinib in patients with advanced ALK-positive
non-small cell lung cancer: 5-Year outcomes from the phase III
CROWN study. J Clin Oncol. 42:3400–3409. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo J, Ostrem J, Pellini B, Imbody D,
Stern Y, Solanki HS, Haura EB and Villaruz LC: Overcoming
KRAS-mutant lung cancer. Am Soc Clin Oncol Educ Book. 42:1–11.
2022.PubMed/NCBI
|
|
30
|
Jänne PA, Riely GJ, Gadgeel SM, Heist RS,
Ou SI, Pacheco JM, Johnson ML, Sabari JK, Leventakos K, Yau E, et
al: Adagrasib in non-small-cell lung cancer harboring a
KRASG12C mutation. N Engl J Med. 387:120–131. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Skoulidis F, Li BT, Dy GK, Price TJ,
Falchook GS, Wolf J, Italiano A, Schuler M, Borghaei H, Barlesi F,
et al: Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl
J Med. 384:2371–2381. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Olivier T and Prasad V: Sotorasib in
KRAS(G12C) mutated lung cancer. Lancet. 403:1452024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sanaei MJ, Razi S, Pourbagheri-Sigaroodi A
and Bashash D: The PI3K/Akt/mTOR pathway in lung cancer; oncogenic
alterations, therapeutic opportunities, challenges, and a glance at
the application of nanoparticles. Transl Oncol. 18:1013642022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iksen, Pothongsrisit S and Pongrakhananon
V: Targeting the PI3K/AKT/mTOR signaling pathway in lung cancer: An
update regarding potential drugs and natural products. Molecules.
26:41002021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghareghomi S, Atabaki V, Abdollahzadeh N,
Ahmadian S and Hafez Ghoran S: Bioactive PI3-kinase/Akt/mTOR
inhibitors in targeted lung cancer therapy. Adv Pharm Bull.
13:24–35. 2023.PubMed/NCBI
|
|
36
|
Tan AC: Targeting the PI3K/Akt/mTOR
pathway in non-small cell lung cancer (NSCLC). Thorac Cancer.
11:511–518. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen B, Song Y, Zhan Y, Zhou S, Ke J, Ao
W, Zhang Y, Liang Q, He M, Li S, et al: Fangchinoline inhibits
non-small cell lung cancer metastasis by reversing
epithelial-mesenchymal transition and suppressing the cytosolic
ROS-related Akt-mTOR signaling pathway. Cancer Lett.
543:2157832022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, Zhang D, Wang S, Yu P, Sun J, Zhang
Y, Meng X, Li J and Xiang L: Baicalein induces apoptosis by
inhibiting the glutamine-mTOR metabolic pathway in lung cancer. J
Adv Res. 68:341–357. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng W, Kang K, Zhao A and Wu Y: Dual
blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung
cancer. J Hematol Oncol. 17:542024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen X, Huang S, Xiao H, Zeng S, Liu J,
Ran Z and Xiong B: Efficacy and safety of PD-1/PD-L1 plus CTLA-4
antibodies ± other therapies in lung cancer: A systematic review
and meta-analysis. Eur J Hosp Pharm. 30:3–8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vergnenegre A and Chouaid C: Economic
analyses of immune-checkpoint inhibitors to treat lung cancer.
Expert Rev Pharmacoecon Outcomes Res. 21:365–371. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cascone T, Awad MM, Spicer JD, He J, Lu S,
Sepesi B, Tanaka F, Taube JM, Cornelissen R, Havel L, et al:
Perioperative nivolumab in resectable lung cancer. N Engl J Med.
390:1756–1769. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Felip E, Altorki N, Zhou C, Vallières E,
Martínez-Martí A, Rittmeyer A, Chella A, Reck M, Goloborodko O,
Huang M, et al: Overall survival with adjuvant atezolizumab after
chemotherapy in resected stage II–IIIA non-small-cell lung cancer
(IMpower010): A randomised, multicentre, open-label, phase III
trial. Ann Oncol. 34:907–919. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang T, Li W, Diwu D, Chen L, Chen X and
Wang H: Efficacy and safety of first-line immunotherapy plus
chemotherapy in treating patients with extensive-stage small cell
lung cancer: A Bayesian network meta-analysis. Front Immunol.
14:11970442023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
da Cunha Santos G, Shepherd FA and Tsao
MS: EGFR mutations and lung cancer. Annu Rev Pathol. 6:49–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu X, Wang P, Zhang C and Ma Z: Epidermal
growth factor receptor (EGFR): A rising star in the era of
precision medicine of lung cancer. Oncotarget. 8:50209–50220. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Agraso S, Lázaro M, Firvida XL, Santomé L,
Fernández N, Azpitarte C, Leon L, Garcia C, Hudobro G, Areses MC,
et al: Real-world data with afatinib in Spanish patients with
treatment-naïve non-small-cell lung cancer harboring exon 19
deletions in epidermal growth factor receptor (Del19 EGFR):
Clinical experience of the Galician lung cancer group. Cancer Treat
Res Commun. 33:1006462022.PubMed/NCBI
|
|
50
|
Matsui T, Tanizawa Y and Enatsu S: Exon 19
deletion and exon 21 L858R point mutation in EGFR Mutation-positive
non-small cell lung cancer. Gan To Kagaku Ryoho. 48:673–676.
2021.(In Japanese). PubMed/NCBI
|
|
51
|
Yu J, Zhang L, Peng J, Ward R, Hao P, Wang
J, Zhang N, Yang Y, Guo X, Xiang C, et al: Dictamnine, a novel
c-Met inhibitor, suppresses the proliferation of lung cancer cells
by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways.
Biochem Pharmacol. 195:1148642022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wen Z, Jiang R, Huang Y, Wen Z, Rui D,
Liao X and Ling Z: Inhibition of lung cancer cells and
Ras/Raf/MEK/ERK signal transduction by ectonucleoside triphosphate
phosphohydrolase-7 (ENTPD7). Respir Res. 20:1942019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qin BM, Chen X, Zhu JD and Pei DQ:
Identification of EGFR kinase domain mutations among lung cancer
patients in China: Implication for targeted cancer therapy. Cell
Res. 15:212–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang Q, Dai HH, Dong HY, Sun CT, Yang Z
and Han JQ: EGFR mutations and clinical outcomes of chemotherapy
for advanced non-small cell lung cancer: A meta-analysis. Lung
Cancer. 85:339–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang C, Zhao K, Hu S, Dong W, Gong Y and
Xie C: Clinical outcomes of afatinib versus osimertinib in patients
with non-small cell lung cancer with uncommon EGFR mutations: A
pooled analysis. Oncologist. 28:e397–e405. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sun H and Wu YL: Dacomitinib in
non-small-cell lung cancer: A comprehensive review for clinical
application. Future Oncol. 15:2769–2777. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Leonetti A, Sharma S, Minari R, Perego P,
Giovannetti E and Tiseo M: Resistance mechanisms to osimertinib in
EGFR-mutated non-small cell lung cancer. Br J Cancer. 121:725–737.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Skoulidis F and Papadimitrakopoulou VA:
Targeting the gatekeeper: Osimertinib in EGFR T790M
mutation-positive non-small cell lung cancer. Clin Cancer Res.
23:618–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Spagnolo CC, Ciappina G, Giovannetti E,
Squeri A, Granata B, Lazzari C, Pretelli G, Pasello G and Santarpia
M: Targeting MET in non-small cell lung cancer (NSCLC): A new old
story? Int J Mol Sci. 24:101192023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Oh DY and Bang YJ: HER2-targeted
therapies-a role beyond breast cancer. Nat Rev Clin Oncol.
17:33–48. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yin X, Li Y, Wang H, Jia T, Wang E, Luo Y,
Wei Y, Qin Z and Ma X: Small cell lung cancer transformation: From
pathogenesis to treatment. Semin Cancer Biol. 86:595–606. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cheng WL, Feng PH, Lee KY, Chen KY, Sun
WL, Van Hiep N, Luo CS and Wu SM: The role of EREG/EGFR pathway in
tumor progression. Int J Mol Sci. 22:128282021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Iqbal MA, Arora S, Prakasam G, Calin GA
and Syed MA: MicroRNA in lung cancer: Role, mechanisms, pathways
and therapeutic relevance. Mol Aspects Med. 70:3–20. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lin L, Lu Q, Cao R, Ou Q, Ma Y, Bao H, Wu
X, Shao Y, Wang Z and Shen B: Acquired rare recurrent EGFR
mutations as mechanisms of resistance to osimertinib in lung cancer
and in silico structural modelling. Am J Cancer Res. 10:4005–4015.
2020.PubMed/NCBI
|
|
65
|
Mansour MA, AboulMagd AM, Abbas SH,
Abdel-Rahman HM and Abdel-Aziz M: Insights into fourth generation
selective inhibitors of (C797S) EGFR mutation combating non-small
cell lung cancer resistance: A critical review. RSC Adv.
13:18825–18853. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Singh D: Revolutionizing lung cancer
treatment: Innovative CRISPR-Cas9 delivery strategies. AAPS
PharmSciTech. 25:1292024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li L, Jiang H, Zeng B, Wang X, Bao Y, Chen
C, Ma L and Yuan J: Liquid biopsy in lung cancer. Clin Chim Acta.
554:1177572024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hsu PC, Jablons DM, Yang CT and You L:
Epidermal growth factor receptor (EGFR) pathway, yes-associated
protein (YAP) and the regulation of programmed death-ligand 1
(PD-L1) in non-small cell lung cancer (NSCLC). Int J Mol Sci.
20:38212019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Elshatlawy M, Sampson J, Clarke K and
Bayliss R: EML4-ALK biology and drug resistance in non-small cell
lung cancer: A new phase of discoveries. Mol Oncol. 17:950–963.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Camidge DR, Dziadziuszko R, Peters S, Mok
T, Noe J, Nowicka M, Gadgeel SM, Cheema P, Pavlakis N, de Marinis
F, et al: Updated efficacy and safety data and impact of the
EML4-ALK fusion variant on the efficacy of alectinib in untreated
ALK-positive advanced non-small cell lung cancer in the global
phase III ALEX study. J Thorac Oncol. 14:1233–1243. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Paliouras AR, Buzzetti M, Shi L, Donaldson
IJ, Magee P, Sahoo S, Leong HS, Fassan M, Carter M, Di Leva G, et
al: Vulnerability of drug-resistant EML4-ALK rearranged lung cancer
to transcriptional inhibition. EMBO Mol Med. 12:e110992020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li K, Liu Y, Ding Y, Zhang Z, Feng J, Hu
J, Chen J, Lian Z, Chen Y, Hu K, et al: BCL6 is regulated by the
MAPK/ELK1 axis and promotes KRAS-driven lung cancer. J Clin Invest.
132:e1613082022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gadgeel SM and Wozniak A: Preclinical
rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for
epidermal growth factor receptor inhibitor-resistant non-small-cell
lung cancer. Clin Lung Cancer. 14:322–332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shi L, Zhu W, Huang Y, Zhuo L, Wang S,
Chen S, Zhang B and Ke B: Cancer-associated fibroblast-derived
exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to
promote the progression and chemoresistance of non-small cell lung
cancer. Clin Transl Med. 12:e9892022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tan AC and Tan DSW: Targeted therapies for
lung cancer patients with oncogenic driver molecular alterations. J
Clin Oncol. 40:611–625. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ahn MJ, Kim HR, Yang JCH, Han JY, Li JYC,
Hochmair MJ, Chang GC, Delmonte A, Lee KH, Campelo RG, et al:
Efficacy and safety of brigatinib compared with crizotinib in asian
vs non-asian patients with locally advanced or metastatic
ALK-inhibitor-naive ALK+ non-small cell lung cancer: Final results
from the phase III ALTA-1L study. Clin Lung Cancer. 23:720–730.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mok T, Camidge DR, Gadgeel SM, Rosell R,
Dziadziuszko R, Kim DW, Pérol M, Ou SHI, Ahn JS, Shaw AT, et al:
Updated overall survival and final progression-free survival data
for patients with treatment-naive advanced ALK-positive
non-small-cell lung cancer in the ALEX study. Ann Oncol.
31:1056–1064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Baba K and Goto Y: Lorlatinib as a
treatment for ALK-positive lung cancer. Future Oncol. 18:2745–2766.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lin JJ, Zhu VW, Yoda S, Yeap BY, Schrock
AB, Dagogo-Jack I, Jessop NA, Jiang GY, Le LP, Gowen K, et al:
Impact of EML4-ALK variant on resistance mechanisms and clinical
outcomes in ALK-positive lung cancer. J Clin Oncol. 36:1199–1206.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pinto JA, Raez LE and Domingo G: Clinical
consequences of resistance to ALK inhibitors in non-small cell lung
cancer. Expert Rev Respir Med. 14:385–390. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shaw AT, Solomon BJ, Besse B, Bauer TM,
Lin CC, Soo RA, Riely GJ, Ou SHI, Clancy JS, Li S, et al: ALK
resistance mutations and efficacy of lorlatinib in advanced
anaplastic lymphoma kinase-positive non-small-cell lung cancer. J
Clin Oncol. 37:1370–1379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Desai A and Lovly CM: Strategies to
overcome resistance to ALK inhibitors in non-small cell lung
cancer: A narrative review. Transl Lung Cancer Res. 12:615–628.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Balasundaram A and Doss GPC: A
computational examination of the therapeutic advantages of
fourth-generation ALK inhibitors TPX-0131 and repotrectinib over
third-generation lorlatinib for NSCLC with ALK F1174C/L/V
mutations. Front Mol Biosci. 10:13060462024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Golding B, Luu A, Jones R and
Viloria-Petit AM: The function and therapeutic targeting of
anaplastic lymphoma kinase (ALK) in non-small cell lung cancer
(NSCLC). Mol Cancer. 17:522018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Torres-Jiménez J, Espinar JB, de Cabo HB,
Berjaga MZ, Esteban-Villarrubia J, Fraile JZ and Paz-Ares L:
Targeting KRAS(G12C) in non-small-cell lung cancer: Current
standards and developments. Drugs. 84:527–548. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chang YS, Tu SJ, Chen YC, Liu TY, Lee YT,
Yen JC, Fang HY and Chang JG: Mutation profile of non-small cell
lung cancer revealed by next generation sequencing. Respir Res.
22:32021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Mugarza E, van Maldegem F, Boumelha J,
Moore C, Rana S, Llorian Sopena M, East P, Ambler R, Anastasiou P,
Romero-Clavijo P, et al: Therapeutic KRASG12C inhibition
drives effective interferon-mediated antitumor immunity in
immunogenic lung cancers. Sci Adv. 8:eabm87802022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ceddia S, Landi L and Cappuzzo F:
KRAS-mutant non-small-cell lung cancer: From past efforts to future
challenges. Int J Mol Sci. 23:93912022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Bironzo P, Cani M, Jacobs F, Napoli VM,
Listì A, Passiglia F, Righi L, Di Maio M, Novello S and Scagliotti
GV: Real-world retrospective study of KRAS mutations in advanced
non-small cell lung cancer in the era of immunotherapy. Cancer.
129:1662–1671. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ferrer I, Zugazagoitia J, Herbertz S, John
W, Paz-Ares L and Schmid-Bindert G: KRAS-mutant non-small cell lung
cancer: From biology to therapy. Lung Cancer. 124:53–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xu K, Park D, Magis AT, Zhang J, Zhou W,
Sica GL, Ramalingam SS, Curran WJ and Deng X: Small molecule KRAS
agonist for mutant KRAS cancer therapy. Mol Cancer. 18:852019.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Brazel D, Arter Z and Nagasaka M: A long
overdue targeted treatment for KRAS mutations in NSCLC: Spotlight
on adagrasib. Lung Cancer (Auckl). 13:75–80. 2022.PubMed/NCBI
|
|
93
|
Di Federico A, Ricciotti I, Favorito V,
Michelina SV, Scaparone P, Metro G, De Giglio A, Pecci F, Lamberti
G, Ambrogio C and Ricciuti B: Resistance to KRAS G12C inhibition in
non-small cell lung cancer. Curr Oncol Rep. 25:1017–1029. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Canon J, Rex K, Saiki AY, Mohr C, Cooke K,
Bagal D, Gaida K, Holt T, Knutson CG, Koppada N, et al: The
clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity.
Nature. 575:217–223. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lee A: Sotorasib: A review in KRAS G12C
mutation-positive non-small cell lung cancer. Target Oncol.
17:727–733. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Mausey N and Halford Z: Targeted therapies
for previously ‘undruggable’ KRAS-mutated non-small cell lung
cancer: A review of sotorasib and adagrasib. Ann Pharmacother.
58:622–635. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Briere DM, Li S, Calinisan A, Sudhakar N,
Aranda R, Hargis L, Peng DH, Deng J, Engstrom LD, Hallin J, et al:
The KRASG12C inhibitor MRTX849 reconditions the tumor
immune microenvironment and sensitizes tumors to checkpoint
inhibitor therapy. Mol Cancer Ther. 20:975–985. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Shi Z, Weng J, Niu H, Yang H, Liu R, Weng
Y, Zhu Q, Zhang Y, Tao L, Wang Z, et al: D-1553: A novel
KRASG12C inhibitor with potent and selective cellular
and in vivo antitumor activity. Cancer Sci. 114:2951–2960. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Santarpia M, Ciappina G, Spagnolo CC,
Squeri A, Passalacqua MI, Aguilar A, Gonzalez-Cao M, Giovannetti E,
Silvestris N and Rosell R: Targeted therapies for KRAS-mutant
non-small cell lung cancer: From preclinical studies to clinical
development-a narrative review. Transl Lung Cancer Res. 12:346–368.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yun J, Nakagawa R and Tham K:
KRAS-targeted therapy in the treatment of non-small cell lung
cancer. J Oncol Pharm Pract. 29:422–430. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Corral de la Fuente E, Olmedo Garcia ME,
Gomez Rueda A, Lage Y and Garrido P: Targeting KRAS in non-small
cell lung cancer. Front Oncol. 11:7926352022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Tomasini P, Walia P, Labbe C, Jao K and
Leighl NB: Targeting the KRAS pathway in non-small cell lung
cancer. Oncologist. 21:1450–1460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Li XQ, Cheng XJ, Wu J, Wu KF and Liu T:
Targeted inhibition of the PI3K/AKT/mTOR pathway by
(+)-anthrabenzoxocinone induces cell cycle arrest, apoptosis, and
autophagy in non-small cell lung cancer. Cell Mol Biol Lett.
29:582024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Gong G, Ganesan K, Xiong Q and Zheng Y:
Antitumor effects of ononin by modulation of apoptosis in
non-small-cell lung cancer through inhibiting PI3K/Akt/mTOR
pathway. Oxid Med Cell Longev. 2022:51224482022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chandarlapaty S, Sawai A, Scaltriti M,
Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK,
Baselga J and Rosen N: AKT inhibition relieves feedback suppression
of receptor tyrosine kinase expression and activity. Cancer Cell.
19:58–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bellmunt J, Maroto P, Bonfill T, Vazquez
F, Perez-Gracia JL, Juanpere N, Hernandez-Prat A, Hernandez-Llodra
S, Rovira A, Juan O and Rodriguez-Vida A: Dual mTOR1/2 inhibitor
sapanisertib (FTH-003/TAK-228) in combination with weekly
paclitaxel in patients with previously treated metastatic
urothelial carcinoma: A phase II open-label study: A phase II
open-label study. Clin Genitourin Cancer. 22:1021232024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Al-Bustany HA, Muhammad HA, Chawsheen MA
and Dash PR: Fenretinide induces apoptosis and synergises the
apoptosis inducing effect of gemcitabine through inhibition of key
signalling molecules involved in A549 cell survival in in silico
and in vitro analyses. Cell Signal. 111:1108852023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Curless BP, Uko NE and Matesic DF:
Modulator of the PI3K/Akt oncogenic pathway affects mTOR complex 2
in human adenocarcinoma cells. Invest New Drugs. 37:902–911. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Fang W, Huang Y, Gu W, Gan J, Wang W,
Zhang S, Wang K, Zhan J, Yang Y, Huang Y, et al: PI3K-AKT-mTOR
pathway alterations in advanced NSCLC patients after progression on
EGFR-TKI and clinical response to EGFR-TKI plus everolimus
combination therapy. Transl Lung Cancer Res. 9:1258–1267. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Pal I and Mandal M: PI3K and Akt as
molecular targets for cancer therapy: Current clinical outcomes.
Acta Pharmacol Sin. 33:1441–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xu Y, Hu Y, Xu T, Yan K, Zhang T, Li Q,
Chang F, Guo X, Peng J, Li M, et al: RNF8-mediated regulation of
Akt promotes lung cancer cell survival and resistance to DNA
damage. Cell Rep. 37:1098542021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
He YM, Zhou XM, Jiang SY, Zhang ZB, Cao
BY, Liu JB, Zeng YY, Zhao J and Mao XL: TRIM25 activates AKT/mTOR
by inhibiting PTEN via K63-linked polyubiquitination in non-small
cell lung cancer. Acta Pharmacol Sin. 43:681–691. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Alharbi KS, Shaikh MAJ, Almalki WH, Kazmi
I, Al-Abbasi FA, Alzarea SI, Imam SS, Alshehri S, Ghoneim MM, Singh
SK, et al: PI3K/Akt/mTOR pathways inhibitors with potential
prospects in non-small-cell lung cancer. J Environ Pathol Toxicol
Oncol. 41:85–102. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yan D, Huelse JM, Kireev D, Tan Z, Chen L,
Goyal S, Wang X, Frye SV, Behera M, Schneider F, et al: MERTK
activation drives osimertinib resistance in EGFR-mutant non-small
cell lung cancer. J Clin Invest. 132:e1505172022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Coco S, Truini A, Alama A, Dal Bello MG,
Venè R, Garuti A, Carminati E, Rijavec E, Genova C, Barletta G, et
al: Afatinib resistance in non-small cell lung cancer involves the
PI3K/AKT and MAPK/ERK signalling pathways and
epithelial-to-mesenchymal transition. Target Oncol. 10:393–404.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Adachi Y, Watanabe K, Kita K, Kitai H,
Kotani H, Sato Y, Inase N, Yano S and Ebi H: Resistance mediated by
alternative receptor tyrosine kinases in FGFR1-amplified lung
cancer. Carcinogenesis. 38:1063–1072. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yu Y, Shang Y, Shi S, He Y, Shi W, Wang M,
Wang Q, Xu D, Shi C and Chen H: Combination of arsenic trioxide and
apatinib synergistically inhibits small cell lung cancer by
down-regulating VEGFR2/mTOR and Akt/c-Myc signaling pathway via
GRB10. Hereditas. 161:292024. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wu YY, Wu HC, Wu JE, Huang KY, Yang SC,
Chen SX, Tsao CJ, Hsu KF, Chen YL and Hong TM: The dual PI3K/mTOR
inhibitor BEZ235 restricts the growth of lung cancer tumors
regardless of EGFR status, as a potent accompanist in combined
therapeutic regimens. J Exp Clin Cancer Res. 38:2822019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang J, Hong Y and Shen J: Combination
treatment with perifosine and MEK-162 demonstrates synergism
against lung cancer cells in vitro and in vivo. Tumour Biol.
36:5699–5706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Quan Z, Yang Y, Zheng H, Zhan Y, Luo J,
Ning Y and Fan S: Clinical implications of the interaction between
PD-1/PD-L1 and PI3K/AKT/mTOR pathway in progression and treatment
of non-small cell lung cancer. J Cancer. 13:3434–3443. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wu J, Zhao X, Sun Q, Jiang Y, Zhang W, Luo
J and Li Y: Synergic effect of PD-1 blockade and endostar on the
PI3K/AKT/mTOR-mediated autophagy and angiogenesis in Lewis lung
carcinoma mouse model. Biomed Pharmacother. 125:1097462020.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Liu Q, Chen X, Qi M, Li Y, Chen W and
Zhang C, Wang J, Han Z and Zhang C: Combined cryoablation and PD-1
inhibitor synergistically enhance antitumor immune responses in
Lewis lung adenocarcinoma mice via the PI3K/AKT/mTOR pathway.
Biochim Biophys Acta Mol Basis Dis. 1870:1672622024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Shi Z, Shen Y, Liu X and Zhang S:
Sinensetin inhibits the movement ability and tumor immune
microenvironment of non-small cell lung cancer through the
inactivation of AKT/β-catenin axis. J Biochem Mol Toxicol.
38:e700242024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Santini FC and Hellmann MD: PD-1/PD-L1
axis in lung cancer. Cancer J. 24:15–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kumar R, Collins D, Dolly S, McDonald F,
O'Brien MER and Yap TA: Targeting the PD-1/PD-L1 axis in non-small
cell lung cancer. Curr Probl Cancer. 41:111–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yadav R, Khatkar R, Yap KCH, Kang CYH, Lyu
J, Singh RK, Mandal S, Mohanta A, Lam HY, Okina E, et al: The miRNA
and PD-1/PD-L1 signaling axis: An arsenal of immunotherapeutic
targets against lung cancer. Cell Death Discov. 10:4142024.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lu M, Wang K, Ji W, Yu Y, Li Z, Xia W and
Lu S: FGFR1 promotes tumor immune evasion via YAP-mediated PD-L1
expression upregulation in lung squamous cell carcinoma. Cell
Immunol. 379:1045772022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Qiao M, Jiang T, Liu X, Mao S, Zhou F, Li
X, Zhao C, Chen X, Su C, Ren S and Zhou C: Immune checkpoint
inhibitors in EGFR-mutated NSCLC: Dusk or dawn? J Thorac Oncol.
16:1267–1288. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Genova C, Dellepiane C, Carrega P,
Sommariva S, Ferlazzo G, Pronzato P, Gangemi R, Filaci G, Coco S
and Croce M: Therapeutic implications of tumor microenvironment in
lung cancer: Focus on immune checkpoint blockade. Front Immunol.
12:7994552022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Sholl LM: Biomarkers of response to
checkpoint inhibitors beyond PD-L1 in lung cancer. Mod Pathol. 35
(Suppl 1):S66–S74. 2022. View Article : Google Scholar
|
|
131
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Updated analysis of KEYNOTE-024: Pembrolizumab versus
platinum-based chemotherapy for advanced non-small-cell lung cancer
with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol.
37:537–546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ready NE, Ott PA, Hellmann MD,
Zugazagoitia J, Hann CL, de Braud F, Antonia SJ, Ascierto PA,
Moreno V, Atmaca A, et al: Nivolumab monotherapy and nivolumab plus
ipilimumab in recurrent small cell lung cancer: Results from the
checkmate 032 randomized cohort. J Thorac Oncol. 15:426–435. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liu SV, Reck M, Mansfield AS, Mok T,
Scherpereel A, Reinmuth N, Garassino MC, De Castro Carpeno J,
Califano R, Nishio M, et al: Updated overall survival and PD-L1
subgroup analysis of patients with extensive-stage small-cell lung
cancer treated with atezolizumab, carboplatin, and etoposide
(IMpower133). J Clin Oncol. 39:619–630. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Tang S, Qin C, Hu H, Liu T, He Y, Guo H,
Yan H, Zhang J, Tang S and Zhou H: Immune checkpoint inhibitors in
non-small cell lung cancer: Progress, challenges, and prospects.
Cells. 11:3202022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Passaro A, Brahmer J, Antonia S, Mok T and
Peters S: Managing resistance to immune checkpoint inhibitors in
lung cancer: Treatment and novel strategies. J Clin Oncol.
40:598–610. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kejamurthy P and Devi KTR: Immune
checkpoint inhibitors and cancer immunotherapy by aptamers: An
overview. Med Oncol. 41:402023. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Li Y, Jiang M, Aye L, Luo L, Zhang Y, Xu
F, Wei Y, Peng D, He X, Gu J, et al: UPP1 promotes lung
adenocarcinoma progression through the induction of an
immunosuppressive microenvironment. Nat Commun. 15:12002024.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ghorani E, Swanton C and Quezada SA:
Cancer cell-intrinsic mechanisms driving acquired immune tolerance.
Immunity. 56:2270–2295. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Giatromanolaki A, Kouroupi M, Pouliliou S,
Mitrakas A, Hasan F, Pappa A and Koukourakis MI: Ectonucleotidase
CD73 and CD39 expression in non-small cell lung cancer relates to
hypoxia and immunosuppressive pathways. Life Sci. 259:1183892020.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Best SA, Gubser PM, Sethumadhavan S,
Kersbergen A, Negrón Abril YL, Goldford J, Sellers K, Abeysekera W,
Garnham AL, McDonald JA, et al: Glutaminase inhibition impairs CD8
T cell activation in STK11-/Lkb1-deficient lung cancer. Cell Metab.
34:874–887.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhu M, Kim J, Deng Q, Ricciuti B, Alessi
JV, Eglenen-Polat B, Bender ME, Huang HC, Kowash RR, Cuevas I, et
al: Loss of p53 and mutational heterogeneity drives immune
resistance in an autochthonous mouse lung cancer model with high
tumor mutational burden. Cancer Cell. 41:1731–1748.e8. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zhu C, Zhuang W, Chen L, Yang W and Ou WB:
Frontiers of ctDNA, targeted therapies, and immunotherapy in
non-small-cell lung cancer. Transl Lung Cancer Res. 9:111–138.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Chu X, Tian W, Wang Z, Zhang J and Zhou R:
Co-inhibition of TIGIT and PD-1/PD-L1 in cancer immunotherapy:
Mechanisms and clinical trials. Mol Cancer. 22:932023. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chen Y, Chen Z, Chen R, Fang C, Zhang C,
Ji M and Yang X: Immunotherapy-based combination strategies for
treatment of EGFR-TKI-resistant non-small-cell lung cancer. Future
Oncol. 18:1757–1775. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
West HJ, McCleland M, Cappuzzo F, Reck M,
Mok TS, Jotte RM, Nishio M, Kim E, Morris S, Zou W, et al: Clinical
efficacy of atezolizumab plus bevacizumab and chemotherapy in
KRAS-mutated non-small cell lung cancer with STK11, KEAP1, or TP53
comutations: Subgroup results from the phase III IMpower150 trial.
J Immunother Cancer. 10:e0030272022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Judd J and Borghaei H: Combining
immunotherapy and chemotherapy for non-small cell lung cancer.
Thorac Surg Clin. 30:199–206. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Shang S, Liu J, Verma V, Wu M, Welsh J, Yu
J and Chen D: Combined treatment of non-small cell lung cancer
using radiotherapy and immunotherapy: Challenges and updates.
Cancer Commun (Lond). 41:1086–1099. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Chae YK, Arya A, Iams W, Cruz M, Mohindra
N, Villaflor V and Giles FJ: Immune checkpoint pathways in
non-small cell lung cancer. Ann Transl Med. 6:882018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Raghav KPS and Moasser MM: Molecular
pathways and mechanisms of HER2 in cancer therapy. Clin Cancer Res.
29:2351–2361. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Nützinger J, Bum Lee J, Li Low J, Ling
Chia P, Talisa Wijaya S, Chul Cho B, Min Lim S and Soo RA:
Management of HER2 alterations in non-small cell lung cancer-the
past, present, and future. Lung Cancer. 186:1073852023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Riudavets M, Sullivan I, Abdayem P and
Planchard D: Targeting HER2 in non-small-cell lung cancer (NSCLC):
A glimpse of hope? An updated review on therapeutic strategies in
NSCLC harbouring HER2 alterations. ESMO Open. 6:1002602021.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ghezzi C, Chen BY, Damoiseaux R and Clark
PM: Pacritinib inhibits glucose consumption in squamous cell lung
cancer cells by targeting. FLT3.Sci Rep. 13:14422023. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Kuncman Ł, Orzechowska M, Milecki T,
Kucharz J and Fijuth J: High FLT3 expression increases immune-cell
infiltration in the tumor microenvironment and correlates with
prolonged disease-free survival in patients with non-small cell
lung cancer. Mol Oncol. 18:1316–1326. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Dhillon S: Gilteritinib: First global
approval. Drugs. 79:331–339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Hu C, Zhang Y, Yang J, Xu Y, Deng T, Li Y,
Xu S, Wang S and Wang P: Ningetinib, a novel FLT3 inhibitor,
overcomes secondary drug resistance in acute myeloid leukemia. Cell
Commun Signal. 22:3552024. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Bruner JK, Ma HS, Li L, Qin ACR, Rudek MA,
Jones RJ, Levis MJ, Pratz KW, Pratilas CA and Small D: Adaptation
to TKI treatment reactivates ERK signaling in tyrosine
kinase-driven leukemias and other malignancies. Cancer Res.
77:5554–5563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
He L, Wang X, Liu K, Wu X, Yang X, Song G,
Zhang B and Zhong L: Integrative PDGF/PDGFR and focal adhesion
pathways are downregulated in ERCC1-defective non-small cell lung
cancer undergoing sodium glycididazole-sensitized cisplatin
treatment. Gene. 691:70–76. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Catena R, Luis-Ravelo D, Antón I, Zandueta
C, Salazar-Colocho P, Larzábal L, Calvo A and Lecanda F: PDGFR
signaling blockade in marrow stroma impairs lung cancer bone
metastasis. Cancer Res. 71:164–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Riess JW and Neal JW: Targeting FGFR,
ephrins, Mer, MET, and PDGFR-α in non-small cell lung cancer. J
Thorac Oncol. 6 (11 Suppl 4):S1797–S1798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Kranthi Reddy S, Reddy SVG and Hussain
Basha S: Discovery of novel PDGFR inhibitors targeting non-small
cell lung cancer using a multistep machine learning assisted hybrid
virtual screening approach. RSC Adv. 15:851–869. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Xuan H, Jingshu G, Fang Y, Na L, Xiaolin
S, Zhaoyang Y, Meng W and Gongyan C: Somatic mutation of KIT is
rare in small cell lung cancer patients from Northeast China.
Histol Histopathol. 29:273–278. 2014.PubMed/NCBI
|
|
162
|
Miettinen M and Lasota J: KIT (CD117): A
review on expression in normal and neoplastic tissues, and
mutations and their clinicopathologic correlation. Appl
Immunohistochem Mol Morphol. 13:205–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Funkhouser AT, Strigenz AM, Blair BB,
Miller AP, Shealy JC, Ewing JA, Martin JC, Funk CR, Edenfield WJ
and Blenda AV: KIT mutations correlate with higher galectin levels
and brain metastasis in breast and non-small cell lung cancer.
Cancers (Basel). 14:27812022. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Yang L, Zhou F, Zheng D, Wang D, Li X,
Zhao C and Huang X: FGF/FGFR signaling: From lung development to
respiratory diseases. Cytokine Growth Factor Rev. 62:94–104. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Pacini L, Jenks AD, Lima NC and Huang PH:
Targeting the fibroblast growth factor receptor (FGFR) family in
lung cancer. Cells. 10:11542021. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Desai A and Adjei AA: FGFR signaling as a
target for lung cancer therapy. J Thorac Oncol. 11:9–20. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Peng M, Deng J and Li X: Clinical advances
and challenges in targeting FGF/FGFR signaling in lung cancer. Mol
Cancer. 23:2562024. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Biello F, Burrafato G, Rijavec E, Genova
C, Barletta G, Truini A, Coco S, Bello MG, Alama A, Boccardo F and
Grossi F: Fibroblast growth factor receptor (FGFR): A new target
for non-small cell lung cancer therapy. Anticancer Agents Med Chem.
16:1142–1154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Cervantes-Villagrana RD, Mendoza V, Hinck
CS, de la Fuente-León RL, Hinck AP, Reyes-Cruz G, Vázquez-Prado J
and López-Casillas F: Betaglycan sustains HGF/Met signaling in lung
cancer and endothelial cells promoting cell migration and tumor
growth. Heliyon. 10:e305202024. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Huang G, Liu X, Jiang T, Cao Y, Sang M,
Song X, Zhou B, Qu H, Cai H, Xing D, et al: Luteolin overcomes
acquired resistance to osimertinib in non-small cell lung cancer
cells by targeting the HGF-MET-Akt pathway. Am J Cancer Res.
13:4145–4162. 2023.PubMed/NCBI
|
|
171
|
Moosavi F, Giovannetti E, Peters GJ and
Firuzi O: Combination of HGF/MET-targeting agents and other
therapeutic strategies in cancer. Crit Rev Oncol Hematol.
160:1032342021. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Yin J, Hu W, Fu W, Dai L, Jiang Z, Zhong
S, Deng B and Zhao J: HGF/MET regulated epithelial-mesenchymal
transitions and metastasis by FOSL2 in non-small cell lung cancer.
Onco Targets Ther. 12:9227–9237. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Fregni M, Ciribilli Y and Zawacka-Pankau
JE: The therapeutic potential of the restoration of the p53 protein
family members in the EGFR-mutated lung cancer. Int J Mol Sci.
23:72132022. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Lin CC, Liao WT, Yang TY, Lu HJ, Hsu SL
and Wu CC: MicroRNA-10b modulates cisplatin tolerance by targeting
p53 directly in lung cancer cells. Oncol Rep. 46:1672021.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zhang H, Zhang G, Xiao M, Cui S, Jin C,
Yang J, Wu S and Lu X: Two-polarized roles of transcription factor
FOSB in lung cancer progression and prognosis: Dependent on p53
status. J Exp Clin Cancer Res. 43:2372024. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Chantarawong W, Kuncharoen N, Tanasupawat
S and Chanvorachote P: Lumichrome inhibits human lung cancer cell
growth and induces apoptosis via a p53-dependent mechanism. Nutr
Cancer. 71:1390–1402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Li L, Li P, Ma X, Zeng S, Peng Y and Zhang
G: Therapeutic restoring p53 function with small molecule for
oncogene-driven non-small cell lung cancer by targeting serine 392
phosphorylation. Biochem Pharmacol. 203:1151882022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Wang J, Liu D, Sun Z, Ye T, Li J, Zeng B,
Zhao Q and Rosie Xing H: Autophagy augments the self-renewal of
lung cancer stem cells by the degradation of ubiquitinated p53.
Cell Death Dis. 12:982021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Tang X, Li Y, Liu L, Guo R, Zhang P, Zhang
Y, Zhang Y, Zhao J, Su J, Sun L and Liu Y: Sirtuin 3 induces
apoptosis and necroptosis by regulating mutant p53 expression in
small-cell lung cancer. Oncol Rep. 43:591–600. 2020.PubMed/NCBI
|
|
180
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
He Y, Jiang X, Duan L, Xiong Q, Yuan Y,
Liu P, Jiang L, Shen Q, Zhao S, Yang C and Chen Y: LncRNA PKMYT1AR
promotes cancer stem cell maintenance in non-small cell lung cancer
via activating Wnt signaling pathway. Mol Cancer. 20:1562021.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Li HJ, Ke FY, Lin CC, Lu MY, Kuo YH, Wang
YP, Liang KH, Lin SC, Chang YH, Chen HY, et al: ENO1 promotes lung
cancer metastasis via HGFR and WNT signaling-driven
epithelial-to-mesenchymal transition. Cancer Res. 81:4094–4109.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Li Z, Wu S and Liu W: Advances of
Wnt/β-catenin signaling pathway in lung cancer: A review. Altern
Ther Health Med. 30:238–247. 2024.
|
|
184
|
Chen J, Wang D, Chen H, Gu J, Jiang X, Han
F, Cao J, Liu W and Liu J: TMEM196 inhibits lung cancer metastasis
by regulating the Wnt/β-catenin signaling pathway. J Cancer Res
Clin Oncol. 149:653–667. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Shen Y, Yang Y, Zhao Y, Nuerlan S, Zhan Y
and Liu C: YY1/circCTNNB1/miR-186-5p/YY1 positive loop aggravates
lung cancer progression through the Wnt pathway. Epigenetics.
19:23690062024. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Malyla V, Paudel KR, De Rubis G, Hansbro
NG, Hansbro PM and Dua K: Cigarette smoking induces lung cancer
tumorigenesis via upregulation of the WNT/β-catenin signaling
pathway. Life Sci. 326:1217872023. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Yang J, Chen J, He J, Li J, Shi J, Cho WC
and Liu X: Wnt signaling as potential therapeutic target in lung
cancer. Expert Opin Ther Targets. 20:999–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Zhang Y and Wang X: Targeting the
Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol.
13:1652020. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Bugter JM, Fenderico N and Maurice MM:
Mutations and mechanisms of WNT pathway tumour suppressors in
cancer. Nat Rev Cancer. 21:5–21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Neiheisel A, Kaur M, Ma N, Havard P and
Shenoy AK: Wnt pathway modulators in cancer therapeutics: An update
on completed and ongoing clinical trials. Int J Cancer.
150:727–740. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Song P, Gao Z, Bao Y, Chen L, Huang Y, Liu
Y, Dong Q and Wei X: Wnt/β-catenin signaling pathway in
carcinogenesis and cancer therapy. J Hematol Oncol. 17:462024.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Xue C, Yao Q, Gu X, Shi Q, Yuan X, Chu Q,
Bao Z, Lu J and Li L: Evolving cognition of the JAK-STAT signaling
pathway: Autoimmune disorders and cancer. Signal Transduct Target
Ther. 8:2042023. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Hu X, Li J, Fu M, Zhao X and Wang W: The
JAK/STAT signaling pathway: From bench to clinic. Signal Transduct
Target Ther. 6:4022021. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Erdogan F, Radu TB, Orlova A, Qadree AK,
de Araujo ED, Israelian J, Valent P, Mustjoki SM, Herling M,
Moriggl R and Gunning PT: JAK-STAT core cancer pathway: An
integrative cancer interactome analysis. J Cell Mol Med.
26:2049–2062. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Shen M, Xu Z, Xu W, Jiang K, Zhang F, Ding
Q, Xu Z and Chen Y: Inhibition of ATM reverses EMT and decreases
metastatic potential of cisplatin-resistant lung cancer cells
through JAK/STAT3/PD-L1 pathway. J Exp Clin Cancer Res. 38:1492019.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Wu Q, Wu W, Fu B, Shi L, Wang X and Kuca
K: JNK signaling in cancer cell survival. Med Res Rev.
39:2082–2104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Patel MR, Dash A, Jacobson BA, Ji Y,
Baumann D, Ismail K and Kratzke RA: JAK/STAT inhibition with
ruxolitinib enhances oncolytic virotherapy in non-small cell lung
cancer models. Cancer Gene Ther. 26:411–418. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Ding Y, Yuan X, Wang Y and Yan J: CASQ2
alleviates lung cancer by inhibiting M2 tumor-associated macrophage
polarization and JAK/STAT pathway. J Biochem Mol Toxicol.
38:e238012024. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Saito A, Horie M and Nagase T: TGF-β
signaling in lung health and disease. Int J Mol Sci. 19:24602018.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Kim BN, Ahn DH, Kang N, Yeo CD, Kim YK,
Lee KY, Kim TJ, Lee SH, Park MS, Yim HW, et al: TGF-β induced EMT
and stemness characteristics are associated with epigenetic
regulation in lung cancer. Sci Rep. 10:105972020. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Huang Y, Chen Z, Lu T, Bi G, Li M, Liang
J, Hu Z, Zheng Y, Yin J, Xi J, et al: HIF-1α switches the
functionality of TGF-β signaling via changing the partners of smads
to drive glucose metabolic reprogramming in non-small cell lung
cancer. J Exp Clin Cancer Res. 40:3982021. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Deng X, Ma N, He J, Xu F and Zou G: The
role of TGFBR3 in the development of lung cancer. Protein Pept
Lett. 31:491–503. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Li J, Shen C, Wang X, Lai Y, Zhou K, Li P,
Liu L and Che G: Prognostic value of TGF-beta in lung cancer:
Systematic review and meta-analysis. BMC Cancer. 19:6912019.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Lai XN, Li J, Tang LB, Chen WT, Zhang L
and Xiong LX: MiRNAs and LncRNAs: Dual roles in TGF-β
signaling-regulated metastasis in lung cancer. Int J Mol Sci.
21:11932020. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Cui Z, Ruan Z, Li M, Ren R, Ma Y, Zeng J,
Sun J, Ye W, Xu W, Guo X, et al: Obstructive sleep apnea promotes
the progression of lung cancer by modulating cancer cell invasion
and cancer-associated fibroblast activation via TGFβ signaling.
Redox Rep. 28:22798132023. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Hedrick E, Mohankumar K and Safe S:
TGFβ-induced lung cancer cell migration is NR4A1-dependent. Mol
Cancer Res. 16:1991–2002. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Saito A, Horie M, Micke P and Nagase T:
The role of TGF-β signaling in lung cancer associated with
idiopathic pulmonary fibrosis. Int J Mol Sci. 19:36112018.
View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Yin Y, Dai H, Sun X, Xi Z, Zhang J, Pan Y,
Huang Y, Ma X, Xia Q and He K: HRG inhibits liver cancer lung
metastasis by suppressing neutrophil extracellular trap formation.
Clin Transl Med. 13:e12832023. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Liu W, Wang H, Bai F, Ding L, Huang Y, Lu
C, Chen S, Li C, Yue X, Liang X, et al: IL-6 promotes metastasis of
non-small-cell lung cancer by up-regulating TIM-4 via NF-κB. Cell
Prolif. 53:e127762020. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Wang Y, Liu F, Chen L, Fang C, Li S, Yuan
S, Qian X, Yin Y, Yu B, Fu B, et al: Neutrophil extracellular traps
(NETs) promote non-small cell lung cancer metastasis by suppressing
lncRNA MIR503HG to activate the NF-κB/NLRP3 inflammasome pathway.
Front Immunol. 13:8675162022. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Zhang J, Zhao K, Zhou W, Kang R, Wei S,
Shu Y, Yu C, Ku Y, Mao Y, Luo H, et al: Tet methylcytosine
dioxygenase 2 (TET2) deficiency elicits EGFR-TKI (tyrosine kinase
inhibitors) resistance in non-small cell lung cancer. Signal
Transduct Target Ther. 9:652024. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Rasmi RR, Sakthivel KM and Guruvayoorappan
C: NF-κB inhibitors in treatment and prevention of lung cancer.
Biomed Pharmacother. 130:1105692020. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Liu X, Liu X, Zhuo C, Shen J, Lu K, Sha M,
Ye J, Huang J, Han H and Yu H: NAT10 promotes malignant progression
of lung cancer via the NF-κB signaling pathway. Discov Med.
35:936–945. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Wei X, Liu Z, Shen Y, Dong H, Chen K, Shi
X, Chen Y, Wang B and Dong S: Semaphorin4A promotes lung cancer by
activation of NF-κB pathway mediated by PlexinB1. PeerJ.
11:e162922023. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Dimitrakopoulos FD, Kottorou AE, Kalofonou
M and Kalofonos HP: The fire within: NF-κB involvement in non-small
cell lung cancer. Cancer Res. 80:4025–4036. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Li Y, Zu L, Wu H, Zhang F, Fan Y, Pan H,
Du X, Guo F and Zhou Q: MiR-192/NKRF axis confers lung cancer cell
chemoresistance to cisplatin via the NF-κB pathway. Thorac Cancer.
13:430–441. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Jin Y, Zhang Y, Huang A, Chen Y, Wang J,
Liu N, Wang X, Gong Y, Wang W and Pan J: Overexpression of SERPINA3
suppresses tumor progression by modulating SPOP/NF-κB in lung
cancer. Int J Oncol. 63:962023. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Wang Y, Zhang J, Li YJ, Yu NN, Liu WT,
Liang JZ, Xu WW, Sun ZH, Li B and He QY: MEST promotes lung cancer
invasion and metastasis by interacting with VCP to activate NF-κB
signaling. J Exp Clin Cancer Res. 40:3012021. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Xu X, Qiu Y, Chen S, Wang S, Yang R, Liu
B, Li Y, Deng J, Su Y, Lin Z, et al: Different roles of the
insulin-like growth factor (IGF) axis in non-small cell lung
cancer. Curr Pharm Des. 28:2052–2064. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Remsing Rix LL, Sumi NJ, Hu Q, Desai B,
Bryant AT, Li X, Welsh EA, Fang B, Kinose F, Kuenzi BM, et al:
IGF-binding proteins secreted by cancer-associated fibroblasts
induce context-dependent drug sensitization of lung cancer cells.
Sci Signal. 15:eabj58792022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Jiang S, Xu Z, Shi Y, Liang S, Jiang X,
Xiao M, Wang K and Ding L: Circulating insulin-like growth factor-1
and risk of lung diseases: A Mendelian randomization analysis.
Front Endocrinol (Lausanne). 14:11263972023. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Xu J, Bie F, Wang Y, Chen X, Yan T and Du
J: Prognostic value of IGF-1R in lung cancer: A PRISMA-compliant
meta-analysis. Medicine (Baltimore). 98:e154672019. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Wang Z, Li W, Guo Q, Wang Y, Ma L and
Zhang X: Insulin-like growth factor-1 signaling in lung development
and inflammatory lung diseases. Biomed Res Int.
2018:60575892018.PubMed/NCBI
|
|
224
|
Peng Y and Tan J: The relationship between
IGF pathway and acquired resistance to tyrosine kinase inhibitors
in cancer therapy. Front Biosci (Landmark Ed). 28:1632023.
View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Pal S, Yadav P, Sainis KB and Shankar BS:
TNF-α and IGF-1 differentially modulate ionizing radiation
responses of lung cancer cell lines. Cytokine. 101:89–98. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Sun L, Yuan W, Wen G, Yu B, Xu F, Gan X,
Tang J, Zeng Q, Zhu L, Chen C and Zhang W: Parthenolide inhibits
human lung cancer cell growth by modulating the IGF-1R/PI3K/Akt
signaling pathway. Oncol Rep. 44:1184–1193. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Das D, Xie L and Hong J: Next-generation
EGFR tyrosine kinase inhibitors to overcome C797S mutation in
non-small cell lung cancer (2019–2024). RSC Med Chem. 15:3371–3394.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Das D, Wang J and Hong J: Next-generation
kinase inhibitors targeting specific biomarkers in non-small cell
lung cancer (NSCLC): A recent overview. ChemMedChem. 16:2459–2479.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Wang N, Zhang Y, Mi Y, Deng H, Chen G,
Tang Z, Mao J, Cui S, Zhang Y and Wang L: Osimertinib for
EGFR-mutant lung cancer with central nervous system metastases: A
meta-analysis and systematic review. Ann Palliat Med. 9:3038–3047.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Solomon BJ, Besse B, Bauer TM, Felip E,
Soo RA, Camidge DR, Chiari R, Bearz A, Lin CC, Gadgeel SM, et al:
Lorlatinib in patients with ALK-positive non-small-cell lung
cancer: Results from a global phase 2 study. Lancet Oncol.
19:1654–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Soejima K, Yasuda H and Hirano T:
Osimertinib for EGFR T790M mutation-positive non-small cell lung
cancer. Expert Rev Clin Pharmacol. 10:31–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Yang JCH, Ahn MJ, Kim DW, Ramalingam SS,
Sequist LV, Su WC, Kim SW, Kim JH, Planchard D, Felip E, et al:
Osimertinib in pretreated T790M-positive advanced non-small-cell
lung cancer: AURA study phase II extension component. J Clin Oncol.
35:1288–1296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Ramalingam SS, Yang JCH, Lee CK, Kurata T,
Kim DW, John T, Nogami N, Ohe Y, Mann H, Rukazenkov Y, et al:
Osimertinib as first-line treatment of EGFR mutation-positive
advanced non-small-cell lung cancer. J Clin Oncol. 36:841–849.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Araki T, Kanda S, Horinouchi H and Ohe Y:
Current treatment strategies for EGFR-mutated non-small cell lung
cancer: From first line to beyond osimertinib resistance. Jpn J
Clin Oncol. 53:547–561. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Jänne PA, Planchard D, Kobayashi K, Cheng
Y, Lee CK, Valdiviezo N, Laktionov K, Yang TY, Yu Y, Kato T, et al:
CNS efficacy of osimertinib with or without chemotherapy in
epidermal growth factor receptor-mutated advanced non-small-cell
lung cancer. J Clin Oncol. 42:808–820. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Ye Z and Guo J: Acquired ALK G1202R-, ALK
I1171N-, or EML4-ALK-mediated resistance to ensartinib in lung
adenocarcinoma but responded to lorlatinib: A case report. Front
Oncol. 13:10821152023. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Solomon BJ, Bauer TM, Mok TSK, Liu G,
Mazieres J, de Marinis F, Goto Y, Kim DW, Wu YL, Jassem J, et al:
Efficacy and safety of first-line lorlatinib versus crizotinib in
patients with advanced, ALK-positive non-small-cell lung cancer:
Updated analysis of data from the phase 3, randomised, open-label
CROWN study. Lancet Respir Med. 11:354–366. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Bauer TM, Shaw AT, Johnson ML, Navarro A,
Gainor JF, Thurm H, Pithavala YK, Abbattista A, Peltz G and Felip
E: Brain penetration of lorlatinib: cumulative incidences of CNS
and non-CNS progression with lorlatinib in patients with previously
treated ALK-positive non-small-cell lung cancer. Target Oncol.
15:55–65. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Rybarczyk-Kasiuchnicz A, Ramlau R and
Stencel K: Treatment of brain metastases of non-small cell lung
carcinoma. Int J Mol Sci. 22:5932021. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Ernani V and Stinchcombe TE: Management of
brain metastases in non-small-cell lung cancer. J Oncol Pract.
15:563–570. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Lin JJ, Choudhury NJ, Yoda S, Zhu VW,
Johnson TW, Sakhtemani R, Dagogo-Jack I, Digumarthy SR, Lee C, Do
A, et al: Spectrum of mechanisms of resistance to crizotinib and
lorlatinib in ROS1 fusion-positive lung cancer. Clin Cancer Res.
27:2899–2909. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Corvaja C, Passaro A, Attili I, Aliaga PT,
Spitaleri G, Signore ED and de Marinis F: Advancements in
fourth-generation EGFR TKIs in EGFR-mutant NSCLC: Bridging
biological insights and therapeutic development. Cancer Treat Rev.
130:1028242024. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Desai A and Peters S: Immunotherapy-based
combinations in metastatic NSCLC. Cancer Treat Rev. 116:1025452023.
View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Passaro A, Jänne PA and Peters S:
Antibody-drug conjugates in lung cancer: Recent advances and
implementing strategies. J Clin Oncol. 41:3747–3761. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Desai A, Abdayem P, Adjei AA and Planchard
D: Antibody-drug conjugates: A promising novel therapeutic approach
in lung cancer. Lung Cancer. 163:96–106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Merle G, Friedlaender A, Desai A and Addeo
A: Antibody drug conjugates in lung cancer. Cancer J. 28:429–435.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Rosner S, Valdivia A, Hoe HJ, Murray JC,
Levy B, Felip E and Solomon BJ: Antibody-drug conjugates for lung
cancer: Payloads and progress. Am Soc Clin Oncol Educ Book.
43:e3899682023. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Marks S and Naidoo J: Antibody drug
conjugates in non-small cell lung cancer: An emerging therapeutic
approach. Lung Cancer. 163:59–68. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Tarantino P, Carmagnani Pestana R, Corti
C, Modi S, Bardia A, Tolaney SM, Cortes J, Soria JC and Curigliano
G: Antibody-drug conjugates: Smart chemotherapy delivery across
tumor histologies. CA Cancer J Clin. 72:165–182. 2022.PubMed/NCBI
|
|
250
|
Weng W, Meng T, Zhao Q, Shen Y, Fu G, Shi
J, Zhang Y, Wang Z, Wang M, Pan R, et al: Antibody-exatecan
conjugates with a novel self-immolative moiety overcome resistance
in colon and lung cancer. Cancer Discov. 13:950–973. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Verma S, Breadner D and Raphael J:
‘Targeting’ improved outcomes with antibody-drug conjugates in
non-small cell lung cancer-an updated review. Curr Oncol.
30:4329–4350. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Parisi C, Mahjoubi L, Gazzah A and Barlesi
F: TROP-2 directed antibody-drug conjugates (ADCs): The revolution
of smart drug delivery in advanced non-small cell lung cancer
(NSCLC). Cancer Treat Rev. 118:1025722023. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Peters S, Loi S, André F, Chandarlapaty S,
Felip E, Finn SP, Jänne PA, Kerr KM, Munzone E, Passaro A, et al:
Antibody-drug conjugates in lung and breast cancer: Current
evidence and future directions-a position statement from the ETOP
IBCSG partners foundation. Ann Oncol. 35:607–629. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Ricciuti B, Lamberti G, Andrini E, Genova
C, De Giglio A, Bianconi V, Sahebkar A, Chiari R and Pirro M:
Antibody-drug conjugates for lung cancer in the era of personalized
oncology. Semin Cancer Biol. 69:268–278. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Pourjamal N, Yazdi N, Halme A, Joncour VL,
Laakkonen P, Saharinen P, Joensuu H and Barok M: Comparison of
trastuzumab emtansine, trastuzumab deruxtecan, and disitamab
vedotin in a multiresistant HER2-positive breast cancer lung
metastasis model. Clin Exp Metastasis. 41:91–102. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Fu Z, Gao C, Xie J, Zhang C, Li S, Gu M
and Shi C: Incidence and risk of fatal adverse events in cancer
patients treated with HER2-targeted antibody-drug conjugates: A
systematic review and meta-analysis of randomized controlled
trials. BMC Cancer. 23:9602023. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Larsen ME, Lyu H and Liu B: HER3-targeted
therapeutic antibodies and antibody-drug conjugates in non-small
cell lung cancer refractory to EGFR-tyrosine kinase inhibitors.
Chin Med J Pulm Crit Care Med. 1:11–17. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Chen Q, Jia G, Zhang X and Ma W: Targeting
HER3 to overcome EGFR TKI resistance in NSCLC. Front Immunol.
14:13320572024. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Belluomini L, Avancini A, Sposito M,
Milella M, Rossi A and Pilotto S: Antibody-drug conjugates (ADCs)
targeting TROP-2 in lung cancer. Expert Opin Biol Ther.
23:1077–1087. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Paz-Ares LG, Juan-Vidal O, Mountzios GS,
Felip E, Reinmuth N, de Marinis F, Girard N, Patel VM, Takahama T,
Owen SP, et al: Sacituzumab govitecan versus docetaxel for
previously treated advanced or metastatic non-small cell lung
cancer: The randomized, open-label phase III EVOKE-01 study. J Clin
Oncol. 42:2860–2872. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Kim YJ, Li W, Zhelev DV, Mellors JW,
Dimitrov DS and Baek DS: Chimeric antigen receptor-T cells are
effective against CEACAM5 expressing non-small cell lung cancer
cells resistant to antibody-drug conjugates. Front Oncol.
13:11240392023. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Zanchetta C, De Marchi L, Macerelli M,
Pelizzari G, Costa J, Aprile G and Cortiula F: Antibody-drug
conjugates in non-small cell lung cancer: state of the art and
future perspectives. Int J Mol Sci. 26:2212024. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Belluomini L, Sposito M, Avancini A,
Insolda J, Milella M, Rossi A and Pilotto S: Unlocking new horizons
in small-cell lung cancer treatment: The onset of antibody-drug
conjugates. Cancers (Basel). 15:53682023. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Blackhall F, Jao K, Greillier L, Cho BC,
Penkov K, Reguart N, Majem M, Nackaerts K, Syrigos K, Hansen K, et
al: Efficacy and safety of rovalpituzumab tesirine compared with
topotecan as second-line therapy in DLL3-high SCLC: Results from
the phase 3 TAHOE study. J Thorac Oncol. 16:1547–1558. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Owen DH, Giffin MJ, Bailis JM, Smit MAD,
Carbone DP and He K: DLL3: An emerging target in small cell lung
cancer. J Hematol Oncol. 12:612019. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Morgensztern D, Besse B, Greillier L,
Santana-Davila R, Ready N, Hann CL, Glisson BS, Farago AF, Dowlati
A, Rudin CM, et al: Efficacy and safety of rovalpituzumab tesirine
in third-line and beyond patients with DLL3-expressing,
relapsed/refractory small-cell lung cancer: Results from the phase
II TRINITY study. Clin Cancer Res. 25:6958–6966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Lashari BH, Vallatharasu Y, Kolandra L,
Hamid M and Uprety D: Rovalpituzumab tesirine: A novel
DLL3-targeting antibody-drug conjugate. Drugs R D. 18:255–258.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Zhao C, Zhang R, Yang H, Gao Y, Zou Y and
Zhang X: Antibody-drug conjugates for non-small cell lung cancer:
Advantages and challenges in clinical translation. Biochem
Pharmacol. 226:1163782024. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Neupane N, Thapa S, Bhattarai A, Ahuja K,
Schlam I, Mittal A, Tolaney SM and Tarantino P: Opportunities and
challenges for a histology-agnostic utilization of trastuzumab
deruxtecan. Curr Oncol Rep. 25:1467–1482. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Desai A, Subbiah V, Roy-Chowdhuri S,
Sheshadri A, Deshmukh S and Peters S: Association of antibody-drug
conjugate (ADC) target expression and interstitial lung disease
(ILD) in non-small-cell lung cancer (NSCLC): Association or
causation or neither? Cancers (Basel). 16:37532024. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Attili I, Passaro A, Pavan A, Conte P, De
Marinis F and Bonanno L: Combination immunotherapy strategies in
advanced non-small cell lung cancer (NSCLC): Does biological
rationale meet clinical needs? Crit Rev Oncol Hematol. 119:30–39.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Tagliamento M, Genova C, Rossi G, Coco S,
Rijavec E, Dal Bello MG, Boccardo S, Grossi F and Alama A:
Microtubule-targeting agents in the treatment of non-small cell
lung cancer: Insights on new combination strategies and
investigational compounds. Expert Opin Investig Drugs. 28:513–523.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Girard N: New strategies and novel
combinations in EGFR TKI-resistant non-small cell lung cancer. Curr
Treat Options Oncol. 23:1626–1644. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Wu YL, Guarneri V, Voon PJ, Lim BK, Yang
JJ, Wislez M, Huang C, Liam CK, Mazieres J, Tho LM, et al:
Tepotinib plus osimertinib in patients with EGFR-mutated
non-small-cell lung cancer with MET amplification following
progression on first-line osimertinib (INSIGHT 2): A multicentre,
open-label, phase 2 trial. Lancet Oncol. 25:989–1002. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Yoshida R, Saigi M, Tani T, Springer BF,
Shibata H, Kitajima S, Mahadevan NR, Campisi M, Kim W, Kobayashi Y,
et al: MET-induced CD73 restrains STING-mediated immunogenicity of
EGFR-mutant lung cancer. Cancer Res. 82:4079–4092. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Zhao S, Ma Y, Liu L, Fang J, Ma H, Feng G,
Xie B, Zeng S, Chang J, Ren J, et al: Ningetinib plus gefitinib in
EGFR-mutant non-small-cell lung cancer with MET and AXL
dysregulations: A phase 1b clinical trial and biomarker analysis.
Lung Cancer. 188:1074682024. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Wang S, Liu C, Yang C, Jin Y, Cui Q, Wang
D, Ge T, He G, Li W, Zhang G, et al: PI3K/AKT/mTOR and
PD-1/CTLA-4/CD28 pathways as key targets of cancer immunotherapy
(review). Oncol Lett. 28:5672024. View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Fu ZY, Huang Y, Lian LS, Huang HT, Zhan
SF, Cai Y, Li JX and Liu XH: Potential of semen coicis in enhancing
the anti-tumor effects of PD-1 inhibitor on A549 cell lines by
blocking the PI3K-AKT-mTOR pathway. Clin Transl Oncol.
26:2250–2261. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Li C, Tian C, Liu Y, Liang J, Zeng Y, Yang
Q, Liu Y, Wu D, Wu J, Wang J, et al: Comprehensive profiling
reveals distinct microenvironment and metabolism characterization
of lung adenocarcinoma. Front Genet. 12:6198212021. View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Li Y, Tuerxun H and Zhao Y, Liu X, Li X,
Wen S and Zhao Y: The new era of lung cancer therapy: Combining
immunotherapy with ferroptosis. Crit Rev Oncol Hematol.
198:1043592024. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Nair NU, Greninger P, Zhang X, Friedman
AA, Amzallag A, Cortez E, Sahu AD, Lee JS, Dastur A, Egan RK, et
al: A landscape of response to drug combinations in non-small cell
lung cancer. Nat Commun. 14:38302023. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Zheng H, Zeltsman M, Zauderer MG, Eguchi
T, Vaghjiani RG and Adusumilli PS: Chemotherapy-induced
immunomodulation in non-small-cell lung cancer: A rationale for
combination chemoimmunotherapy. Immunotherapy. 9:913–927. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Corke L and Sacher A: New strategies and
combinations to improve outcomes in immunotherapy in metastatic
non-small-cell lung cancer. Curr Oncol. 29:38–55. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Casagrande GMS, Silva MO, Reis RM and Leal
LF: Liquid biopsy for lung cancer: Up-to-date and perspectives for
screening programs. Int J Mol Sci. 24:25052023. View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Pellini B and Chaudhuri AA: ctDNA
monitoring for small cell lung cancer: Ready for prime time? Clin
Cancer Res. 29:2176–2178. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Pan Y, Zhang JT, Gao X, Chen ZY, Yan B,
Tan PX, Yang XR, Gao W, Gong Y, Tian Z, et al: Dynamic circulating
tumor DNA during chemoradiotherapy predicts clinical outcomes for
locally advanced non-small cell lung cancer patients. Cancer Cell.
41:1763–1773 .e4. 2023.
|
|
287
|
Sun X, Abrahamson P, Ballew N, Kalilani L,
Phiri K, Bell KF, Slowley A, Zajac M, Hofstatter E, Stojadinovic A,
et al: The utility of ctDNA in lung cancer clinical research and
practice: A systematic review and meta-analysis of clinical
studies. Cancer Invest. 41:571–592. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Ren F, Fei Q, Qiu K, Zhang Y, Zhang H and
Sun L: Liquid biopsy techniques and lung cancer: Diagnosis,
monitoring and evaluation. J Exp Clin Cancer Res. 43:962024.
View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Sands J, Li Q and Hornberger J: Urine
circulating-tumor DNA (ctDNA) detection of acquired EGFR T790M
mutation in non-small-cell lung cancer: An outcomes and total
cost-of-care analysis. Lung Cancer. 110:19–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
290
|
Chen K, Yang F, Shen H, Wang C, Li X,
Chervova O, Wu S, Qiu F, Peng D, Zhu X, et al: Individualized
tumor-informed circulating tumor DNA analysis for postoperative
monitoring of non-small cell lung cancer. Cancer Cell.
41:1749–1762.e6. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
291
|
Yan X and Liu C: Clinical application and
prospect of MRD evaluation in lung cancer based on ctDNA level: A
review. Tumori. 109:356–362. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
292
|
Chae YK and Oh MS: Detection of minimal
residual disease using ctDNA in lung cancer: Current evidence and
future directions. J Thorac Oncol. 14:16–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
293
|
Reina C, Šabanović B, Lazzari C, Gregorc V
and Heeschen C: Unlocking the future of cancer diagnosis-promises
and challenges of ctDNA-based liquid biopsies in non-small cell
lung cancer. Transl Res. 272:41–53. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
294
|
Medford AJ, Moy B, Spring LM, Hurvitz SA,
Turner NC and Bardia A: Molecular residual disease in breast
cancer: Detection and therapeutic interception. Clin Cancer Res.
29:4540–4548. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
295
|
Lavacchi D, Gelmini S, Calabri A, Rossi G,
Simi L, Caliman E, Mancini I, Salvianti F, Petroni G, Guidolin A,
et al: Early changes in circulating tumor DNA (ctDNA) predict
treatment response in metastatic KRAS-mutated colorectal cancer
(mCRC) patients. Heliyon. 9:e218532023. View Article : Google Scholar : PubMed/NCBI
|
|
296
|
Pellini B and Chaudhuri AA: Circulating
tumor DNA minimal residual disease detection of non-small-cell lung
cancer treated with curative intent. J Clin Oncol. 40:567–575.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
297
|
Dong S, Wang Z, Zhang JT, Yan B, Zhang C,
Gao X, Sun H, Li YS, Yan HH, Tu HY, et al: Circulating tumor
DNA-guided de-escalation targeted therapy for advanced non-small
cell lung cancer: A nonrandomized controlled trial. JAMA Oncol.
10:932–940. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
298
|
Yao H, Wen L, Li Z and Xia C: Analysis of
diagnostic value of CTC and CTDNA in early lung cancer. Cell Mol
Biol (Noisy-le-grand). 69:57–62. 2023. View Article : Google Scholar
|
|
299
|
Xie J, Hu B, Gong Y, He S, Lin J, Huang Q
and Cheng J: A comparative study on ctDNA and tumor DNA mutations
in lung cancer and benign cases with a high number of CTCs and
CTECs. J Transl Med. 21:8732023. View Article : Google Scholar : PubMed/NCBI
|
|
300
|
Xiang Y, Liu X, Wang Y, Zheng D, Meng Q,
Jiang L, Yang S, Zhang S, Zhang X, Liu Y and Wang B: Mechanisms of
resistance to targeted therapy and immunotherapy in non-small cell
lung cancer: Promising strategies to overcoming challenges. Front
Immunol. 15:13662602024. View Article : Google Scholar : PubMed/NCBI
|
|
301
|
Zhang KR, Zhang YF, Lei HM, Tang YB, Ma
CS, Lv QM, Wang SY, Lu LM, Shen Y, Chen HZ and Zhu L: Targeting
AKR1B1 inhibits glutathione de novo synthesis to overcome acquired
resistance to EGFR-targeted therapy in lung cancer. Sci Transl Med.
13:eabg64282021. View Article : Google Scholar : PubMed/NCBI
|
|
302
|
Roys A, Chang X, Liu Y, Xu X, Wu Y and Zuo
D: Resistance mechanisms and potent-targeted therapies of
ROS1-positive lung cancer. Cancer Chemother Pharmacol. 84:679–688.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
303
|
Liu WJ, Du Y, Wen R, Yang M and Xu J: Drug
resistance to targeted therapeutic strategies in non-small cell
lung cancer. Pharmacol Ther. 206:1074382020. View Article : Google Scholar : PubMed/NCBI
|
|
304
|
Lim ZF and Ma PC: Emerging insights of
tumor heterogeneity and drug resistance mechanisms in lung cancer
targeted therapy. J Hematol Oncol. 12:1342019. View Article : Google Scholar : PubMed/NCBI
|
|
305
|
Meyer ML, Fitzgerald BG, Paz-Ares L,
Cappuzzo F, Jänne PA, Peters S and Hirsch FR: New promises and
challenges in the treatment of advanced non-small-cell lung cancer.
Lancet. 404:803–822. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
306
|
Koga T, Suda K and Mitsudomi T: Utility of
the Ba/F3 cell system for exploring on-target mechanisms of
resistance to targeted therapies for lung cancer. Cancer Sci.
113:815–827. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
307
|
Makarem M and Jänne PA: Top advances of
the year: Targeted therapy for lung cancer. Cancer. 130:3239–3250.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
308
|
Li S, Wang A, Wu Y, He S, Shuai W, Zhao M,
Zhu Y, Hu X, Luo Y and Wang G: Targeted therapy for non-small-cell
lung cancer: New insights into regulated cell death combined with
immunotherapy. Immunol Rev. 321:300–334. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
309
|
Zullo L, Dall'Olio FG, Rossi G, Dellepiane
C, Barletta G, Bennicelli E, Ingaliso M, Tagliamento M and Genova
C: Molecular and genetic advances in small cell lung cancer
landscape: From homogeneity to diversity. Int J Mol Sci.
25:2242023. View Article : Google Scholar : PubMed/NCBI
|