Introduction
Prostate cancer remains a major public health issue
worldwide, being the second leading cause of death. In 2020, the
number of cancer patients exceeded 50 million. Moreover, it
accounts for >19 million new cases and nearly 10 million deaths
globally each (1). Approximately
10% of patients are diagnosed at the metastatic stage, according to
data from sources such as the French National Cancer Institute. The
therapeutic management of metastatic, hormone-sensitive prostate
cancer has undergone a drastic evolution in the past decade, with
intensification emerging as the standard therapeutic approach
(2–7).
Whilst the primary metastatic sites typically
involve the lymph nodes, bones, lungs and liver, reports of
metastatic involvement in the digestive system are scarce. The
prognosis and management of such cases are inadequately described
in the current literature (8). In
the present article, a rare case of prostate adenocarcinoma with
metastasis to the colon is presented, providing information on its
unique challenges and outlining the approach taken for its
management.
Case report
The patient, a 72-year-old man, was initially
asymptomatic but had a medical history of amebiasis and sigmoid
colectomy due to diverticulosis. The family history of the patient
included leukemia in a nephew and a brother, as well as
microsatellite stability colonic cancer in a daughter (diagnosed at
45 years old) and a brother (diagnosed at 50 years old). Due to
this familial predisposition, the patient underwent regular
colorectal cancer screenings, involving colonoscopies with
resection of low-grade dysplastic polyps.
In January 2020, during a routine screening at Bégin
Military Hospital (Saint-Mandé, France), three sub-centimeter
polyps were resected. A total of two were conventional colonic
adenomas with low-grade dysplasia, whilst the third consisted of
non-signet-ring isolated cells forming small clusters, occasionally
with small lumina, infiltrating the lamina propria between normal
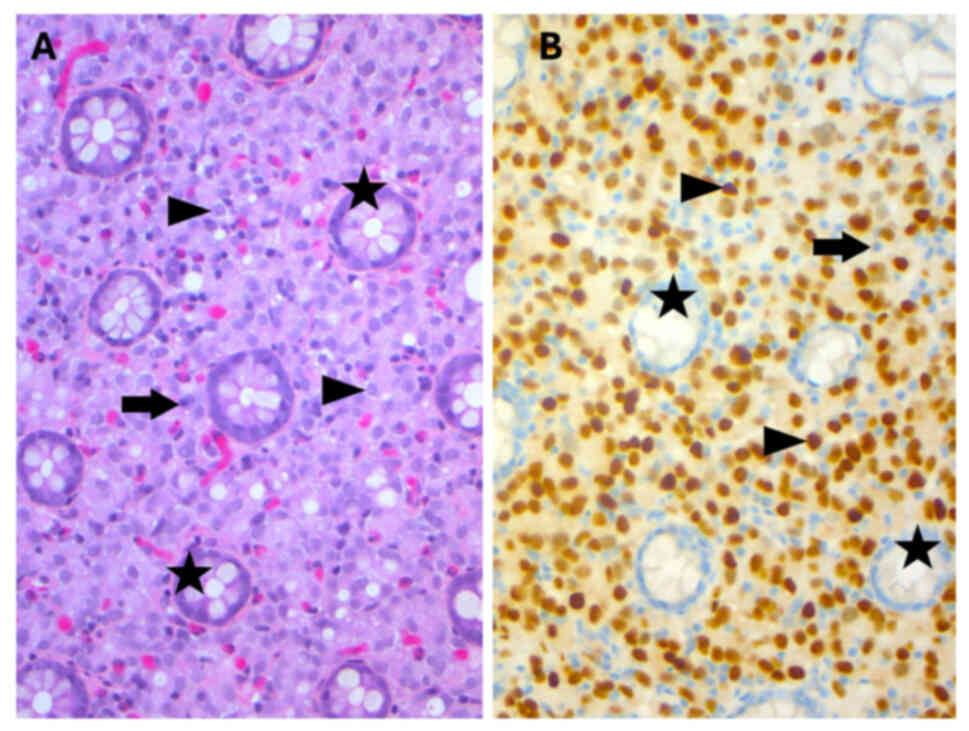
colonic glands (Fig. 1A).
Immunohistochemical staining demonstrated diffuse positivity for
NK3 homeobox 1 (NKX3.1; Fig. 1B),
cytokeratin AE1/AE3 and prosaposin (PSAP), and negativity for
caudal type homeobox 2, transcription termination factor 1, GATA
binding protein 3 and paired box 8 (Fig. 2). Due to the high specificity and
sensitivity of NKX3.1 for prostate adenocarcinoma, with negative
markers not suggesting intestinal, pulmonary, urothelial or renal
primary cancer, the presented staining profile required further
clinical investigation to identify if there was a prostatic primary
with metastasis to the colon (9).
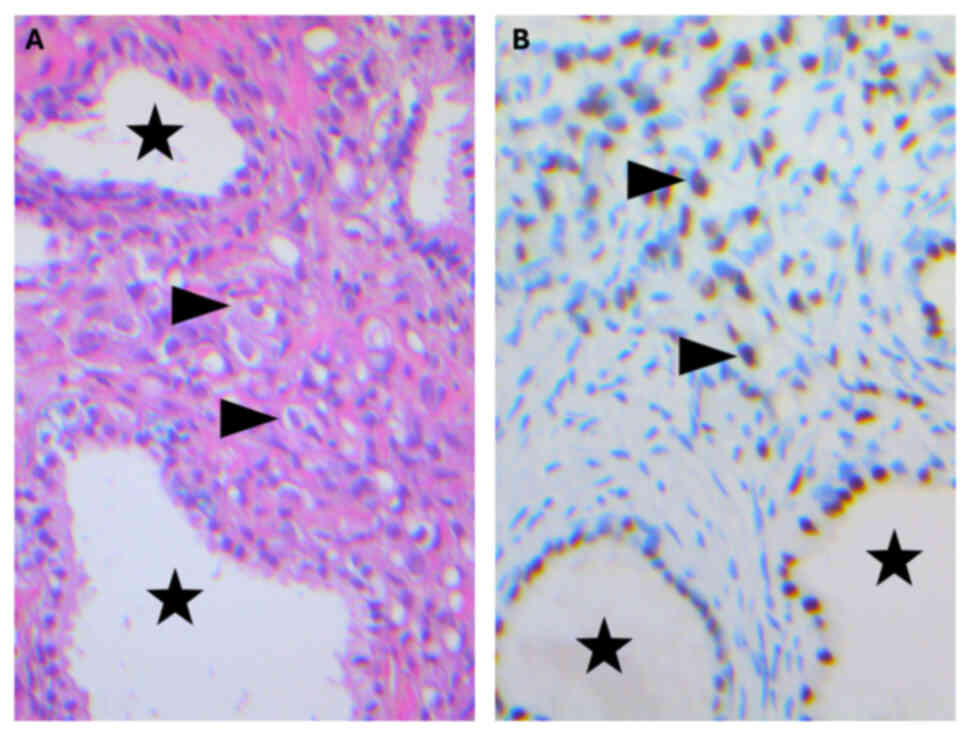
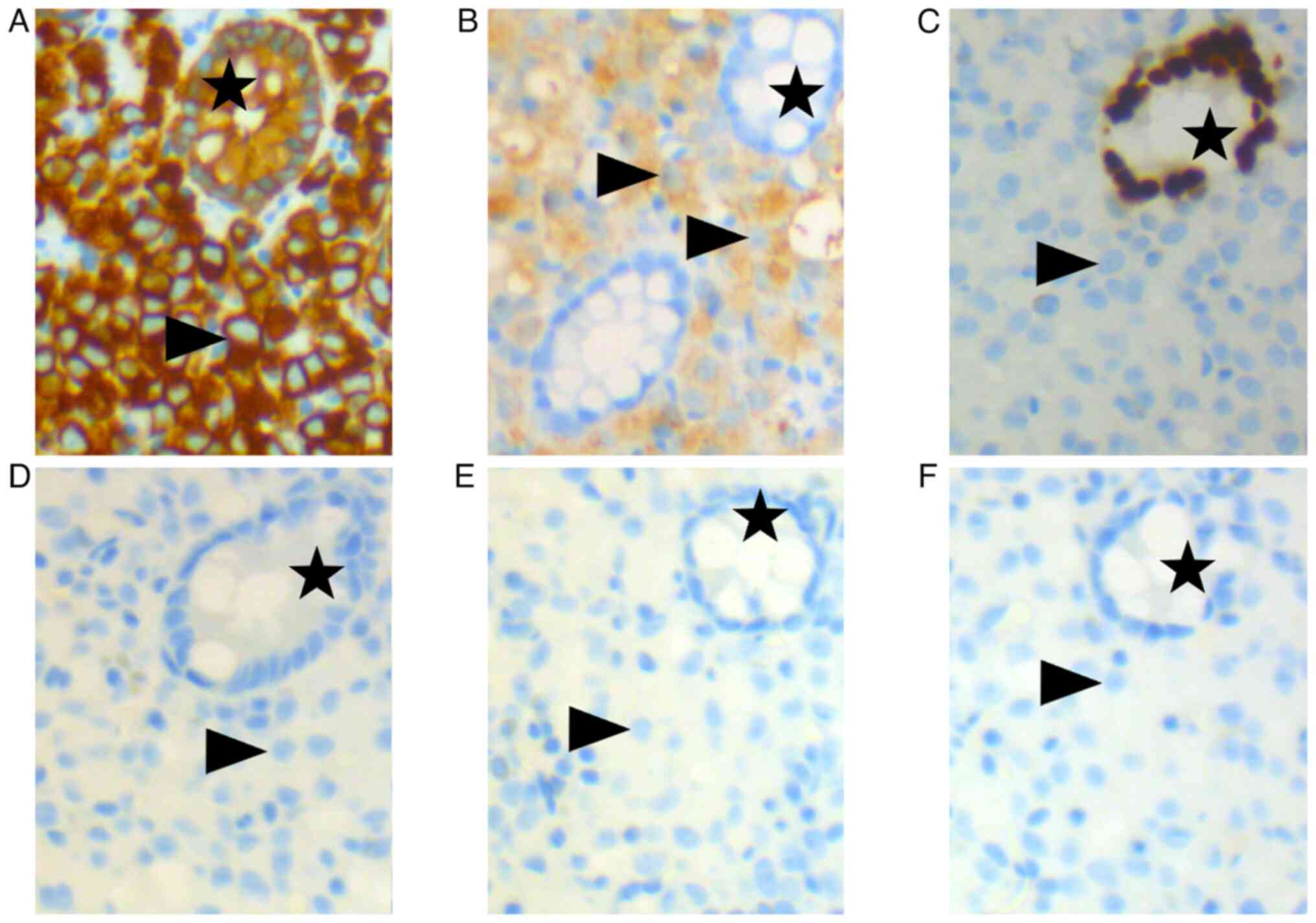 | Figure 2.Immunohistochemical staining performed
on a colonic polyp. (A) Positive, brown-colored cytoplasmic
staining of cytokeratin AE1/AE3 was observed in tumoral single
cells (arrowheads), indicating an epithelial nature, and in cells
of normal colonic crypts (stars), which serve as a positive
internal control. (B) Positive cytoplasmic staining of prosaposin
was observed in tumoral single cells (arrowheads), suggesting, in
addition to NK3 homeobox 1, a prostatic primary, whereas normal
blue-colored cells of normal colonic crypts (stars) were negative.
(C) No nuclear stain was observed in tumoral single cells
(arrowheads) stained with caudal type homeobox 2 antibodies,
suggesting this was not an intestinal primary, whereas cells of
normal colonic crypts (stars) were positive, serving as a positive
internal control. No nuclear stain was seen in tumoral single cells
(arrowheads) with (D) transcription termination factor 1, (E) GATA
binding protein 3 and (F) paired box 8 antibodies, nor in cells of
normal colonic crypts (stars); therefore not indicating a lung,
urothelial or kidney primary. Magnification, ×200. |
Following this unexpected potential diagnosis, a
prostate specific antigen (PSA) test revealed an elevated level of
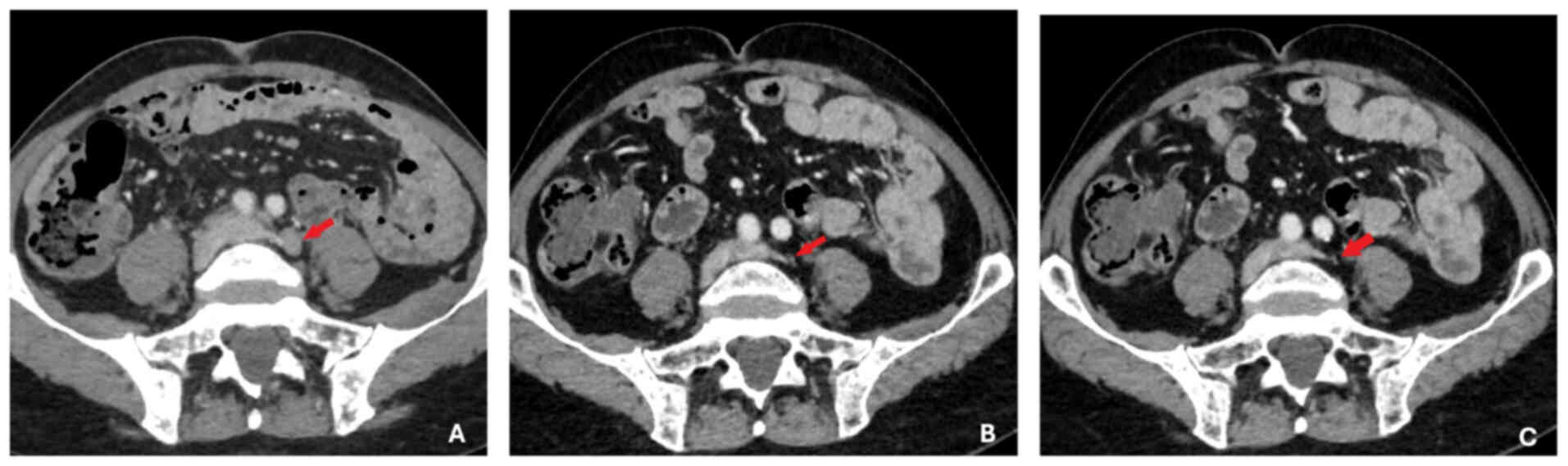
1,286 ng/ml (reference range, 0–4 ng/ml). Imaging studies,
including a thoraco-abdomino-pelvic CT scan, showed diffuse
secondary bone lesions, pelvic and retroperitoneal lymphadenopathy
and prostatic hypertrophy without identifiable suspicious lesions
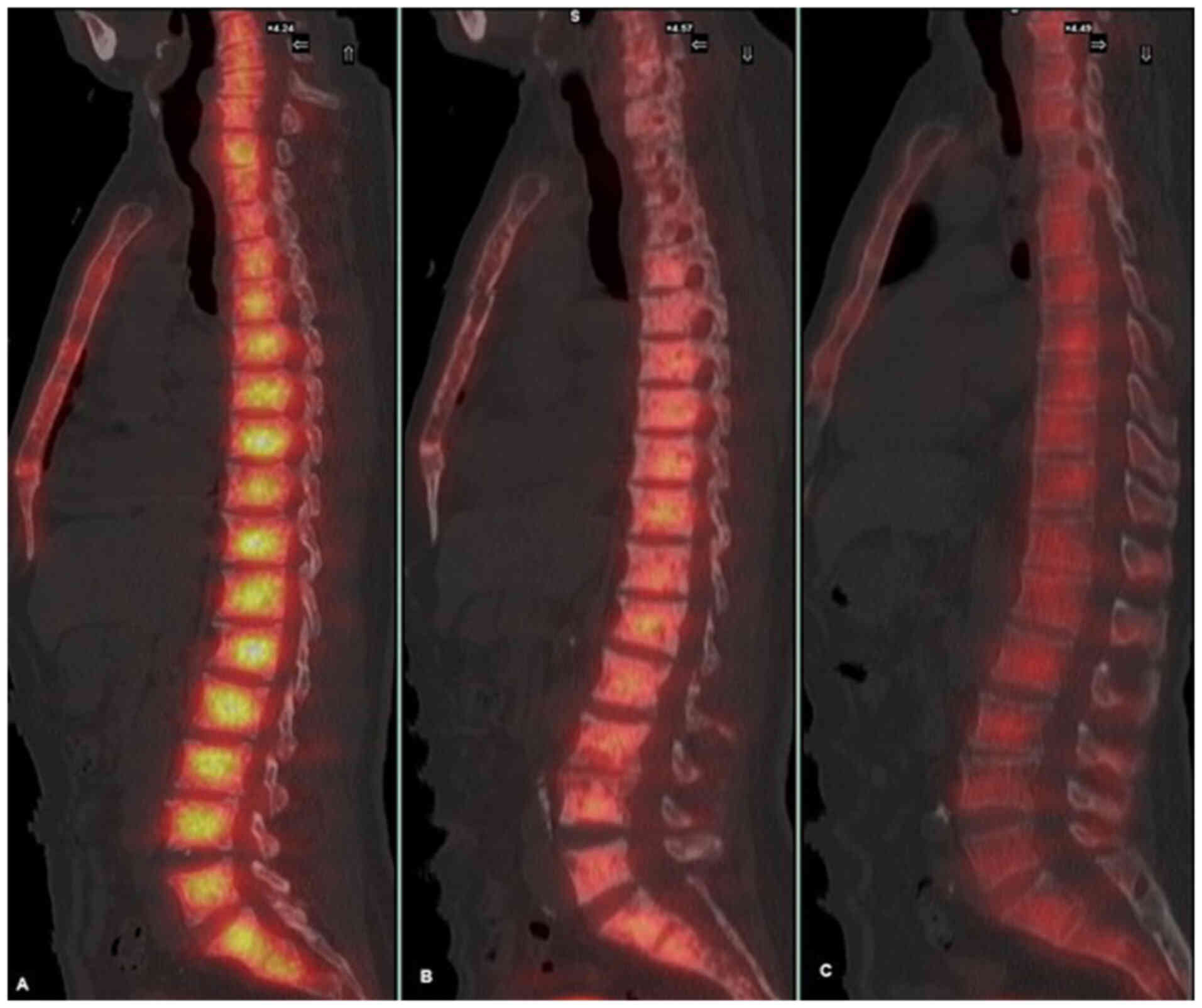
(Fig. 3). Bone scintigraphy
demonstrated a ‘super bone scan’, indicative of diffuse
osteomedullary involvement (Fig.
4).
Prostate needle biopsy (Fig. 5) showed a Gleason score of 4+5=9
adenocarcinoma [The International Society of Urological Pathology
(ISUP) grade group 5] (9). Gleason
pattern 5, consisting of single cells infiltrating prostatic
tissue, appeared morphologically similar to those seen in the
colonic lesion of the patient which stained positive for NKX3.1
(8). Finally, as subsequent
investigations into the initial histological observations revealed
an aggressive prostatic primary tumor by PSA test and prostate
needle biopsy, the clinical and histological data were consistent
with a metastatic prostatic adenocarcinoma with a rare colonic
metastasis.
The patient underwent molecular screening of the
prostate and colonic metastases tissues, as well as circulating
tumor DNA (ctDNA) and germinal screening, all of which were
negative (data not shown). Saliva and blood samples were analyzed
for germline alterations using the Invitae Multi-Cancer panel,
which includes the Fanconi anemia complementation group A gene.
Plasma derived from blood was also tested for cell-free DNA using
the Resolution ctDx HRD™ assay by Exact Sciences. Additionally,
tumor tissue was analyzed to detect tumor-derived alterations using
the FoundationOne® CDx test by Foundation Medicine,
Inc.
The treatment choice for the patient was based on
three key factors: i) The prognosis of the disease, which was poor
due to visceral involvement; ii) the patient and comorbidities,
particularly age; and iii) the maintenance of quality of life.
Based on these points, two options were possible: i)
Intensification with androgen deprivation therapy (ADT) + androgen
receptor pathway inhibitor (ARPI); or ii) a triplet with ADT +
abiraterone acetate (AA) + docetaxel or ADT + enzalutamide +
docetaxel. Data from PEACE 1 indicate that for patients aged >70
years old, there is no overall survival (OS) benefit from the
triplet, and it is associated with a marked increase in adverse
effects, such as cardiovascular issues and cytopenias (7). In the ENZAMET study, which evaluated
the combination of ADT + enzalutamide + docetaxel compared with the
standard arm of ADT +/- docetaxel, no benefit was demonstrated for
the enzalutamide + docetaxel combination (10). All trials evaluating intensification
with a doublet have demonstrated a clear OS benefit for this
population, along with a marked improvement in quality of life, as
measured by standardized scales (7,10).
Therefore, the doublet was chosen as the therapeutic option for the
patient in the present case.
The patient began anti-hormonal treatment with the
gonadotropin hormone-releasing hormone antagonist, degarelix, at an
initial dosage of 240 mg, administered only once, followed by an
80-mg injection 1 month later, which was then repeated monthly,
leading to a rapid decline in PSA levels to 687.5 ng/ml at 15 days.
After 1 month, an androgen synthesis inhibitor, abiraterone
acetate, was also administered at a dosage of 1,000 mg/day, orally.
The patient showed a rapid, clinical, biological (PSA <0.02
ng/ml at 6 months onwards) and radiological response over a 3-year
period, indicating successful disease management, 3 months of the
initiation of the association. The patient experienced no side
effects aside from grade 1 hot flashes, decreased libido and mild
grade 1 anxiety, according to the CTCAE version 5 (11).
Monitoring of efficacy and tolerance was conducted
through telemonitoring and an in-person medical visit every 3
months.
Histology
Specimens were fixed in 10% neutral buffered
formalin (4% formaldehyde solution) at room temperature (20–25°C)
for 24–48 h depending on the size and type of tissue. After
fixation, the tissue was processed using a series of alcohols for
dehydration (70, 80, 95 and 100%) and xylene for clearing, followed
by embedding in molten paraffin wax at 58–60°C. The paraffin block
was cut into 4- to 5-µm sections. The slides were then incubated in
xylene to remove paraffin wax, followed by rehydration through
graded alcohols (100, 95 and 70%) to water. The slides were
incubated in hematoxylin for 3–5 min at room temperature and then
rinsed under running tap water for 1–2 min. Differentiation was
performed in acid alcohol (optional) and bluing in alkaline water
or Scott's tap water substitute for 30 sec. Slides were immersed in
eosin for 1–2 min at room temperature. For dehydration, slides were
passed through graded alcohols (70, 95 and 100%) and cleared in
xylene. Finally, the slides were mounted with a coverslip using a
mounting medium. A brightfield microscope was used for
observation.
Immunohistochemical staining was performed using a
Ventana Benchmark Ultra stainer with UltraView Universal DAB
Detection Kit (Roche Diagnostics). Positive staining for NKX3.1
(EP356 clone; Bio SB, Inc) is highly indicative of a prostatic
primary tumor. Immunohistochemical staining was performed on a
colonic polyp. Positive, brown-colored cytoplasmic staining of
cytokeratin AE1/AE3 (PCK26 clone; Roche Diagnostics) was observed
in tumoral single cells, indicating an epithelial nature, and in
cells of normal colonic crypts (stars), which served as a positive
internal control (Fig. 2A).
Positive cytoplasmic staining of prosaposin (PASE/4LJ clone; Cell
Marque™; Sigma-Aldrich; Merck KGaA) was observed in tumoral single
cells, suggesting, in addition to NK3 homeobox 1, a prostatic
primary, whereas normal blue-colored cells of normal colonic crypts
(stars) were negative (Fig. 2B). No
nuclear stain was observed in tumoral single stained with caudal
type homeobox 2 antibodies (PASE/4LJ clone; Cell Marque™;
Sigma-Aldrich; Merck KGaA), suggesting this was not an intestinal
primary, whereas cells of normal colonic crypts were positive,
serving as a positive internal control (Fig. 2C). No nuclear stain was seen in
tumoral single cells with transcription termination factor 1 (SP141
clone; Roche Diagnostics) (Fig.
2D), GATA binding protein 3 (L50-832 Clone; Zytomed Systems
GmbH) and paired box 8 (MRQ-50 clone; Cell Marque™; Sigma-Aldrich;
Merck KGaA) antibodies, nor in cells of normal colonic crypts;
therefore not indicating a lung, urothelial or kidney primary
(Fig. 2E). Magnification at ×200
was used.
Discussion
Globally, a total of ~10% of patients presenting
with prostate cancer are at a metastatic stage (12). A previous meta-analysis demonstrated
that the baseline presence of visceral disease is a negative
prognostic factor and the distribution of metastases markedly
influences OS (13). Most patients
with prostate cancer present with bone metastases, either with
(72.8%) or without lymph node involvement, followed by visceral
disease (20.8%) and lymph node-only disease (6.4%). Liver
metastases are associated with the shortest median OS (13.5
months), whilst lung metastases have shown an improved median OS
(19.4 months) compared with liver metastases, but worse than
non-visceral bone metastases (21.3 months). Lymph node-only disease
is associated with the longest median OS (31.6 months).
Furthermore, hazard ratios for mortality are markedly higher in men
with lung or liver metastases compared with those with bone
metastases (13). However, there is
limited data on other metastatic sites. Gandaglia et al
(8) reported that digestive
locations accounted for 2.7% of metastatic sites. Among patients
with exclusively digestive metastases, 52% had additional
concomitant metastatic sites; however, only 2.7% of patients with
digestive metastases presented a second metastatic site in bone
(8).
To the best of our knowledge, the present case
report is the 9th published case of a patient with prostate cancer
presenting with secondary digestive localization (13–21).
The majority of patients (n=7) presented with a metastatic
metachronous evolution with a long disease-free interval. Patients
presenting synchronous disease have histologically demonstrated a
more aggressive form (ISUP >4, Gleason score >7) (22). The circumstances of discovering
metastatic digestive disease of prostate cancer were often noisy
digestive symptoms, more closely resembling those of a primary
colorectal origin. Only a few patients reported urinary symptoms,
which, from an endoscopic perspective, could initially suggest a
primary digestive pathology. Nevertheless, immunohistochemical
analysis is essential, particularly with specific markers of
prostate cancer such as NKX3.1.
The present clinical case is one of the only cases
with molecular biology data at the tissue, germinal and ctDNA
levels. In the present case, no anomalies were identified. However,
Swami et al (23) presented
differences in the genomic, transcriptomic and immune landscape of
prostate cancer based on the site of metastasis at the American
Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium
(2024). Different molecular and immunological profiles between the
primary and metastatic sites were reported, explaining distinct
evolutions and providing avenues for therapeutic adaptation. For
these patients, a complete colonoscopy must be performed to assess
the volume of the disease, in addition to a bone and CT scan.
Moreover, molecular screening on the metastatic site should be
performed.
The management of patients with prostate cancer has
markedly evolved in previous years, with the addition of a
second-generation hormonal therapy +/- docetaxel to ADT. However,
to date, no study has included this digestive metastatic site, to
the best of our knowledge, and therapeutic management remains
challenging. Data from the literature suggests a notable role for
local treatment +/- ADT in patients with secondary digestive
localization (13–21).
Similar to the patient in the present case report,
patients with digestive localization, according to the CHAARTED
classification, are classified as high volume (visceral metastases
and/or 4 bone metastases, including at least one outside the pelvis
and spine) (2). Furthermore, if
there is the presence of >3 bone lesions and/or Gleason >7,
the patient will be classified as high risk, according to the
LATITUDE classification. The patient in the present case report was
thus classified as high volume and high risk, with >4 bone
locations, a Gleason score of 9 and the presence of visceral
localization (2,3).
The high-risk classification of the patient
according to the CHAARTED and LATITUDE criteria warranted a more
aggressive treatment approach (2,3). Based
on the data from STAMPEDE and PEACE 1, primary radiotherapy does
not provide a survival benefit to high risk patients (7,24). The
combination of ADT + ARPI has demonstrated a survival benefit for
high risk patients regardless of the molecule used (AA,
enzalutamide, apalutamide) (2–5). The
PEACE 1 and ARASENS studies (6,7)
reported a benefit from adding docetaxel to the ADT + ARPI doublet,
which could be an option for the present patient. For the patient
in the present study, a doublet was chosen to limit side effects.
Moreover, there is no reported benefit of the triplet ADT + AA +
docetaxel in patients aged >70 years old. In addition,
considering the high metastatic volume and comorbidities of the
patient and potential side effects, the decision to avoid
chemotherapy and radiotherapy was taken to provide a tailored and
less burdensome therapeutic regimen. Despite the severe initial
prognosis of the patient, the sustained reduction of their PSA
levels markedly improved their prognosis. The use of PSA and its
reduction and duration are key tools in the management of the
patients.
At present, the triplet treatment with the
combination of ADT + darolutamide + ARPI could be considered, as it
has demonstrated a benefit in the high-volume hormone-sensitive
metastatic population, with this benefit maintained in patients
aged >70 years old (6). However,
this treatment option was not available at the time of the present
case.
Despite the valuable insights gained from the
present study, the small sample size, unique case presentation and
absence of randomized controlled trials limit the generalizability
of the findings, highlighting the need for larger studies and
further research to validate and expand upon these conclusions.
In conclusion, although digestive localizations are
infrequent, understanding their management is essential to maintain
the benefit in OS. Thus, a complete colonoscopy is an indispensable
complementary examination for diagnosis. Therapeutic options are
based on ADT + ARPI +/- docetaxel, considering the clinical status
and history of the patient. There is currently no role for local
treatment of prostate cancer with secondary digestive localization.
A molecular biology examination should also be performed to inform
the choice between doublet and triplet therapies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MAA, ATN, EP, JLL, DL, CH, MP, HP, ALR, AB, MD, HV
and AS made substantial contributions to the conception of the
work; the acquisition, analysis, interpretation of data; or the
creation of new software used in the work; drafted the work; and
approved the submitted version; and agreed to be personally
accountable for the their own contributions. MA and CH confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the local ethics
committee, Bégin Military Hospital and the national ethics
committee, Sud-Ouest et Outre-Mer II (approval no.
2020-A0003138).
Patient consent for publication
The patient, as a participant in a larger study,
provided written informed consent for the publication of their data
and gave additional verbal consent specifically for the publication
of the present case report, including the use of their data and
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sweeney CJ, Chen YH, Carducci M, Liu G,
Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et
al: Chemohormonal therapy in metastatic hormone-sensitive prostate
cancer. N Engl J Med. 373:737–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fizazi K, Tran N, Fein L, Matsubara N,
Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S,
Protheroe A, et al: Abiraterone acetate plus prednisone in patients
with newly diagnosed high-risk metastatic castration-sensitive
prostate cancer (LATITUDE): Final overall survival analysis of a
randomised, double-blind, phase 3 trial. Lancet Oncol. 20:686–700.
2019. View Article : Google Scholar
|
|
4
|
Armstrong AJ, Szmulewitz RZ, Petrylak DP,
Holzbeierlein J, Villers A, Azad A, Alcaraz A, Alekseev B, Iguchi
T, Shore ND, et al: ARCHES: A randomized, phase III study of
androgen deprivation therapy with enzalutamide or placebo in men
with metastatic hormone-sensitive prostate cancer. J Clin Oncol.
37:2974–2986. 2019. View Article : Google Scholar
|
|
5
|
Chi KN, Chowdhury S, Bjartell A, Chung BH,
Pereira de Santana Gomes AJ, Given R, Juárez A, Merseburger AS,
Özgüroğlu M, Uemura H, et al: Apalutamide in patients with
metastatic castration-sensitive prostate cancer: Final survival
analysis of the randomized, double-blind, phase III TITAN study. J
Clin Oncol. 39:2294–2303. 2021. View Article : Google Scholar
|
|
6
|
Smith MR, Hussain M, Saad F, Fizazi K,
Sternberg CN, Crawford ED, Kopyltsov E, Park CH, Alekseev B and
Montesa-Pino Á: Darolutamide and survival in metastatic,
hormone-sensitive prostate cancer. N Engl J Med. 386:1132–1142.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fizazi K, Foulon S, Carles J, Roubaud G,
McDermott R, Fléchon A, Tombal B, Supiot S, Berthold D, Ronchin P,
et al: Abiraterone plus prednisone added to androgen deprivation
therapy and docetaxel in de novo metastatic castration-sensitive
prostate cancer (PEACE-1): A multicentre, open-label, randomised,
phase 3 study with a 2 × 2 factorial design. Lancet. 399:1695–1707.
2022. View Article : Google Scholar
|
|
8
|
Gandaglia G, Abdollah F, Schiffmann J,
Trudeau V, Shariat SF, Kim SP, Perrotte P, Montorsi F, Briganti A,
Trinh QD, et al: Distribution of metastatic sites in patients with
prostate cancer: A population-based analysis. Prostate. 74:210–216.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gurel B, Ali TZ, Montgomery EA, Begum S,
Hicks J, Goggins M, Eberhart CG, Clark DP, Bieberich CJ, Epstein JI
and De Marzo AM: NKX3.1 as a marker of prostatic origin in
metastatic tumors. Am J Surg Pathol. 34:1097–1105. 2010. View Article : Google Scholar
|
|
10
|
Sweeney CJ, Martin AJ, Stockler MR, Begbie
S, Cheung L, Chi KN, Chowdhury S, Frydenberg M, Horvath LG and
Joshua AM: Testosterone suppression plus enzalutamide versus
testosterone suppression plus standard antiandrogen therapy for
metastatic hormone-sensitive prostate cancer (ENZAMET): An
international, open-label, randomised, phase 3 trial. Lancet Oncol.
24:323–334. 2023. View Article : Google Scholar
|
|
11
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events (CTCAE). 2017.
|
|
12
|
Jegu J, Tretarre B, Velten M, Guizard AV,
Danzon A, Buemi A, Colonna M, Kadi-Hanifi AM, Ganry O, Molinie F,
et al: Prostate cancer management and factors associated with
radical prostatectomy in France in 2001. Prog Urol. 20:56–64.
2010.(In French). View Article : Google Scholar
|
|
13
|
Halabi S, Kelly WK, Ma H, Zhou H, Solomon
NC, Fizazi K, Tangen CM, Rosenthal M, Petrylak DP, Hussain M, et
al: Meta-analysis evaluating the impact of site of metastasis on
overall survival in men with castration-resistant prostate cancer.
J Clin Oncol. 34:1652–1659. 2016. View Article : Google Scholar
|
|
14
|
Vaghefi H, Magi-Galluzzi C and Klein EA:
Local recurrence of prostate cancer in rectal submucosa after
transrectal needle biopsy and radical prostatectomy. Urology.
66:8812005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Venara A, Thibaudeau E, Lebdai S, Mucci S,
Ridereau-Zins C, Azzouzi R and Hamy A: Rectal metastasis of
prostate cancer: About a case. J Clin Med Res. 2:137–139.
2010.PubMed/NCBI
|
|
16
|
Morita T, Meguro N, Tomooka Y, Maeda O,
Saiki S, Kuroda M, Miki T, Usami M and Kotake T: Rectal metastasis
of prostatic cancer causing annular stricture: A case report.
Hinyokika Kiyo. 37:295–298. 1991.(In Japanese). PubMed/NCBI
|
|
17
|
Tunio MA, Agamy AM, Fenn N, Hanratty D and
Williams NW: An unusual delayed rectal metastasis from prostate
cancer masquerading as primary rectal cancer. Int J Surg Case Rep.
100:1077322022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nwankwo N, Mirrakhimov AE, Zdunek T and
Bucher N: Prostate adenocarcinoma with a rectal metastasis. BMJ
Case Rep. 2013:bcr20130095032013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abbas TO, Al-Naimi AR, Yakoob RA, Al-Bozom
IA and Alobaidly AM: Prostate cancer metastases to the rectum: A
case report. World J Surg Oncol. 9:562011. View Article : Google Scholar
|
|
20
|
Liu ZH, Li C, Kang L, Zhou ZY, Situ S and
Wang JP: Prostate cancer incorrectly diagnosed as a rectal tumor: A
case report. Oncol Lett. 9:2647–2650. 2015. View Article : Google Scholar
|
|
21
|
Dulskas A, Cereska V, Zurauskas E,
Stratilatovas E and Jankevicius F: Prostate cancer solitary
metastasis to anal canal: Case report and review of literature. BMC
Cancer. 19:3742019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Epstein JI, Egevad L, Amin MB, Delahunt B,
Srigley JR and Humphrey PA; Grading Committee, : The 2014
International Society of Urological Pathology (ISUP) consensus
conference on gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a new grading system. Am J Surg
Pathol. 40:244–252. 2016. View Article : Google Scholar
|
|
23
|
Swami U, Nazari S, Gebrael G, Elliott A,
Bagrodia A, Puri D, Nabhan C, McKay RR, Antonarakis ES and Agarwal
N: Differences in genomic, transcriptomic, and immune landscape of
prostate cancer (PCa) based on site of metastasis (mets). J Clin
Oncol. 42:212024. View Article : Google Scholar
|
|
24
|
Attard G, Murphy L, Clarke NW, Cross W,
Jones RJ, Parker CC, Gillessen S, Cook A, Brawley C, Amos CL, et
al: Abiraterone acetate and prednisolone with or without
enzalutamide for high-risk non-metastatic prostate cancer: A
meta-analysis of primary results from two randomised controlled
phase 3 trials of the STAMPEDE platform protocol. Lancet.
399:447–460. 2022. View Article : Google Scholar
|