Introduction
Bevacizumab is a recombinant humanized monoclonal
antibody targeting vascular endothelial growth factor (VEGF).
Numerous reports have shown that this drug exhibits significant
efficacy in the treatment of various cancer types, including
metastatic colorectal cancer, non-small cell lung cancer,
hepatocellular carcinoma, cervical cancer and ovarian cancer.
Bevacizumab can positively improve key indicators such as a
patient's overall survival (OS) time, progression-free survival
(PFS) time and objective response rate (ORR) (1–5). The
core mechanism of action lies in the binding of bevacizumab to VEGF
with high affinity, blocking the interaction between VEGF and
receptors on the surface of endothelial cells, thereby effectively
inhibiting tumor angiogenesis (6,7).
Although this drug has demonstrated notable efficacy in clinical
applications, its related adverse reactions cannot be ignored. A
phase III clinical trial evaluated the safety of continued
bevacizumab treatment in 245 breast cancer patients who experienced
disease progression after receiving bevacizumab combined with
chemotherapy. The most common grade 3 or higher adverse events were
hypertension (13%), neutropenia (12%) and hand-foot syndrome (11%)
(8). A phase II clinical trial
evaluated the efficacy of adding bevacizumab to the
cisplatin-paclitaxel treatment regimen in 150 patients with
advanced cervical cancer. Among these, the most common grade 3/4
adverse events were neutropenia (25%), anemia (19%), hypertension
(14%) and the occurrence of ≥1 perforation/fistula event (13%)
(4). However, a literature search
reveals that reports on esophageal perforation caused by
bevacizumab during the treatment of lung cancer are extremely rare
(9). The present report describes
in detail the clinical diagnosis and treatment process of a patient
with advanced lung cancer who developed esophageal perforation
while receiving bevacizumab treatment, and systematically analyzes
its occurrence mechanism and management strategies. The aim of the
study is to provide references for clinical diagnosis and
treatment, enhancing the awareness of and clinical management
ability for this rare adverse reaction of the drug, and optimizing
the risk early warning mechanism during the treatment process.
Case report
Disease diagnosis, treatment and
development
The patient in the present case was a 67-year-old
man, weighing 55 kg, with a history of chronic gastritis and a
long-term smoking history. In February 2023, the patient underwent
a wedge resection of the right lung tumor at Taizhou First People's
Hospital (Taizhou, China) due to a mass in the right lung.
Postoperative pathology indicated lung adenocarcinoma with a
staging of TxN3M1 [stage IV, with intrapulmonary metastasis;
American Joint Committee on Cancer (10)]. Genetic testing results were
negative, and no postoperative adjuvant antitumor treatment was
administered. In April 2024 (14 months after the surgery), due to
the delayed healing of the surgical incision, a chest CT
reexamination revealed multiple metastatic lesions in both lungs
and lymph node metastasis, and a 3-cm lesion in the upper lobe of
the left lung was found. Therefore, radiofrequency ablation of the
left lung lesion was performed under computed tomography (CT)
guidance. Starting in April 2024, the patient received a
chemotherapy regimen of 700 mg bevacizumab on day 1 + 0.8 g
pemetrexed intravenous infusion on day 1 + 0.4 g carboplatin
intravenous infusion on day 1, once every 3 weeks, for a total of 4
cycles. Moreover, in April 2024, palliative radiotherapy with
intensity-modulated radiotherapy for the left lung mass was
sequentially performed (95% planning target volume, 40 Gy in 20
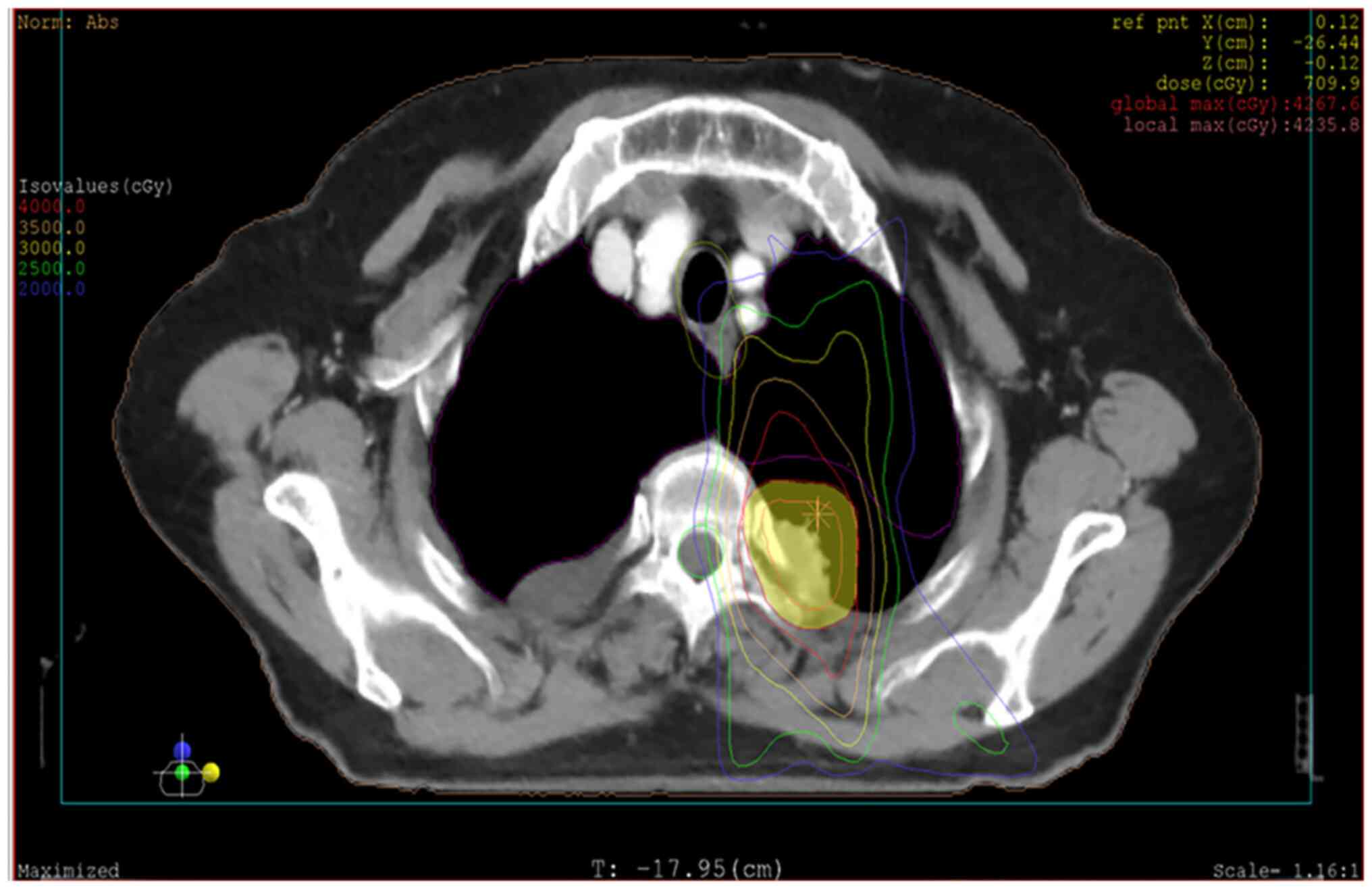
fractions). The radiation isodose curve is presented in Fig. 1 (XIO planning system; version 4.8;
Elekta AB). Grade I acute esophageal toxicity and grade I acute
skin toxicity [Common Terminology Criteria for Adverse Events
Version 5.0 (11)] were side
effects of the radiotherapy. In August 2024, the dose of
bevacizumab dose was adjusted to 800 mg, whilst the original
chemotherapy regimen was maintained (5th cycle). The last treatment
was in September 2024, and the efficacy evaluation revealed that
the disease was stable. In October 2024 (1 month after the last
treatment), the patient experienced a cough, with expectoration of
white sticky phlegm and shortness of breath without obvious
incentives. The patient experienced pain in the right chest and
hypochondrium when coughing, accompanied by dizziness, acid reflux,
heartburn and a poor appetite. The patient then visited the
Respiratory Department of Jiangyou Second People's Hospital
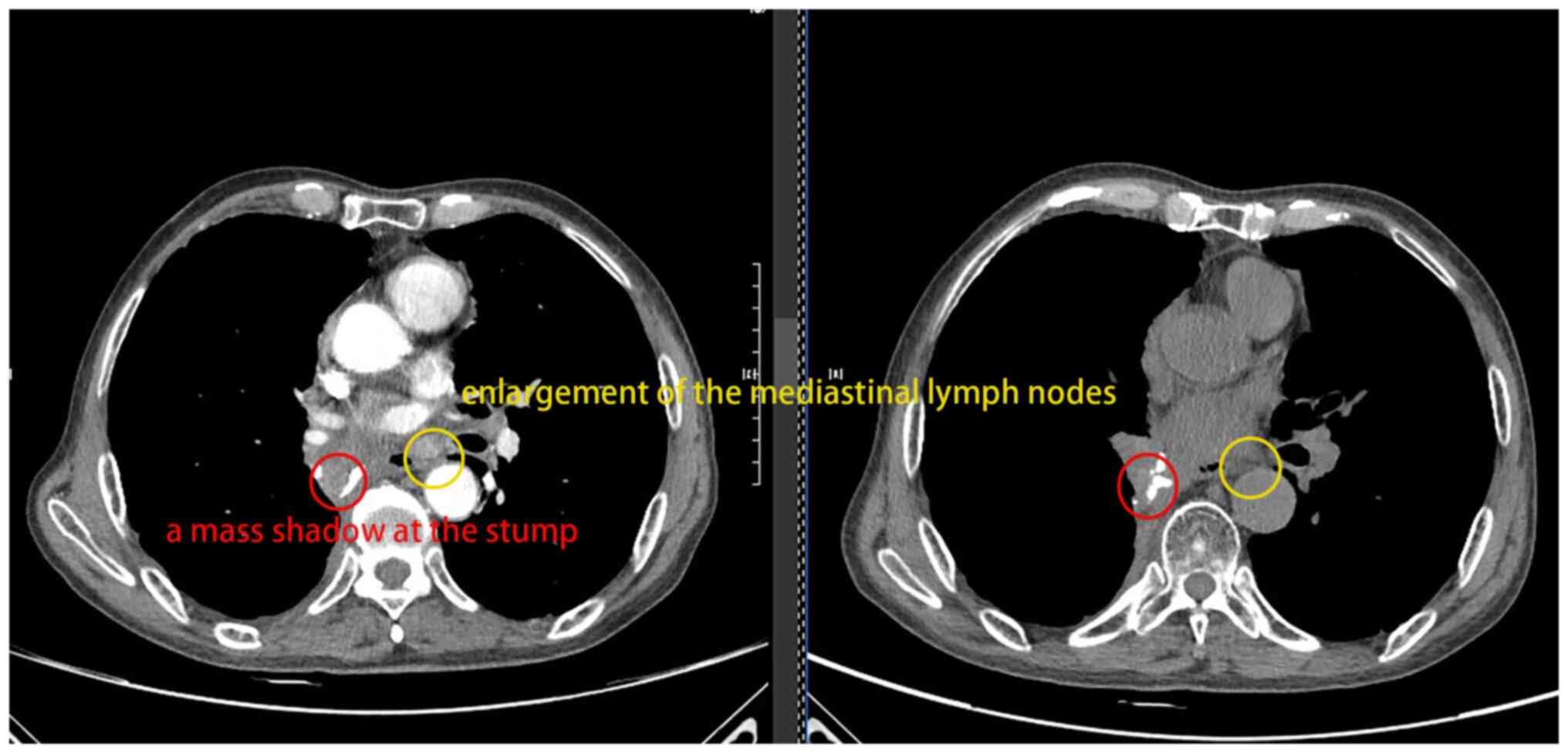
(Jiangyou, Mianyang, China). In November 2024 (Fig. 2), a chest CT (Ingenuity Core 128;
Philips Medical Systems, Inc.) revealed the following: i)
Postoperative changes in the right lung accompanied by a mass
shadow at the stump (no progression compared with the film from
October 2024); ii) multiple nodules in both lungs (stable compared
with October 2024); and iii) enlargement of the mediastinal lymph
nodes. After 2 weeks of anti-infection treatment (2 g cefoperazone
and sulbactam every 12 h + 0.4 g moxifloxacin every day;
intravenous infusion, continuously for 7 days), preventive
antifungal treatment (0.2 g voriconazole every 12 h for 2 weeks),
bronchodilator treatment (0.2 g doxofylline every day for 2 weeks),
glucocorticoid treatment (40 mg methylprednisolone every day for 2
weeks) and nutritional support treatment, the symptoms were
relieved, and the patient was discharged from the hospital.
Esophageal perforation occurrence and
management
In November 2024, the patient was admitted to
Jiangyou Second People's Hospital again for a 6th cycle of
chemotherapy (800 mg bevacizumab + the original chemotherapy
regimen). After the treatment, the patient experienced a severe
cough accompanied by yellow sticky phlegm (which was difficult to
expectorate), decreased exercise tolerance and gastrointestinal
symptoms. In December 2024, Aspergillus flavus was detected
by targeted next-generation sequencing (tNGS) of bronchoalveolar
lavage fluid, and voriconazole (200 mg every 12 h for 40 days) was
administered as an antifungal treatment. During the treatment
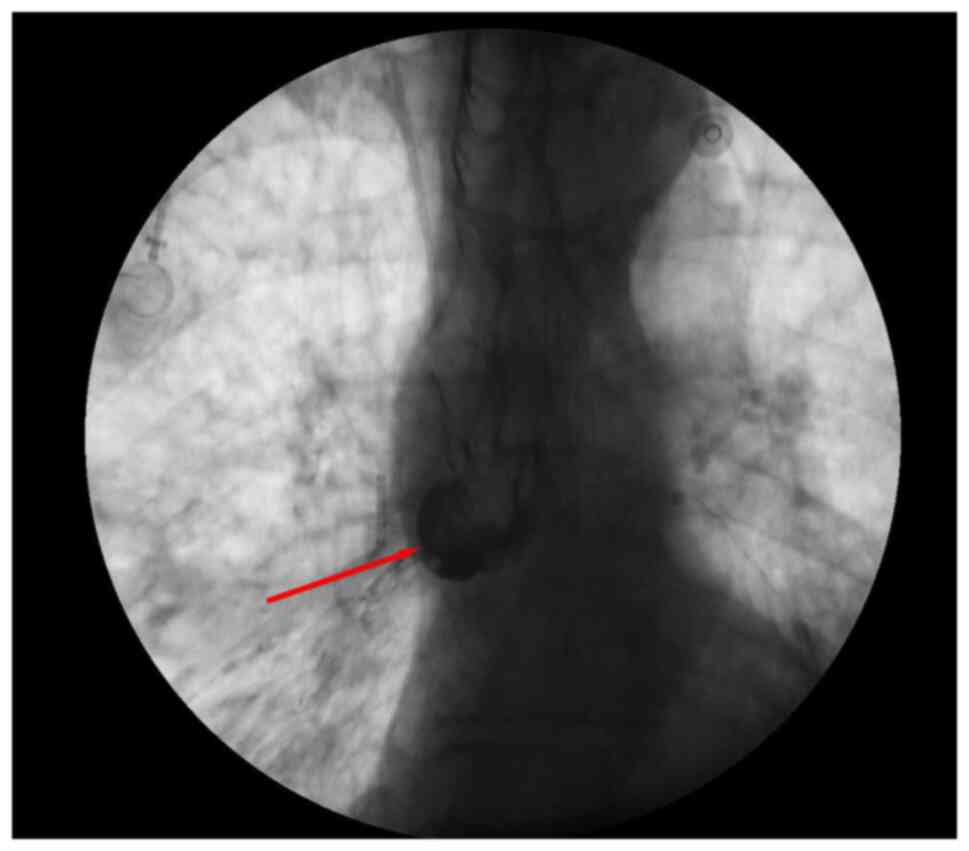
period, a new choking cough after eating occurred. A total of 4
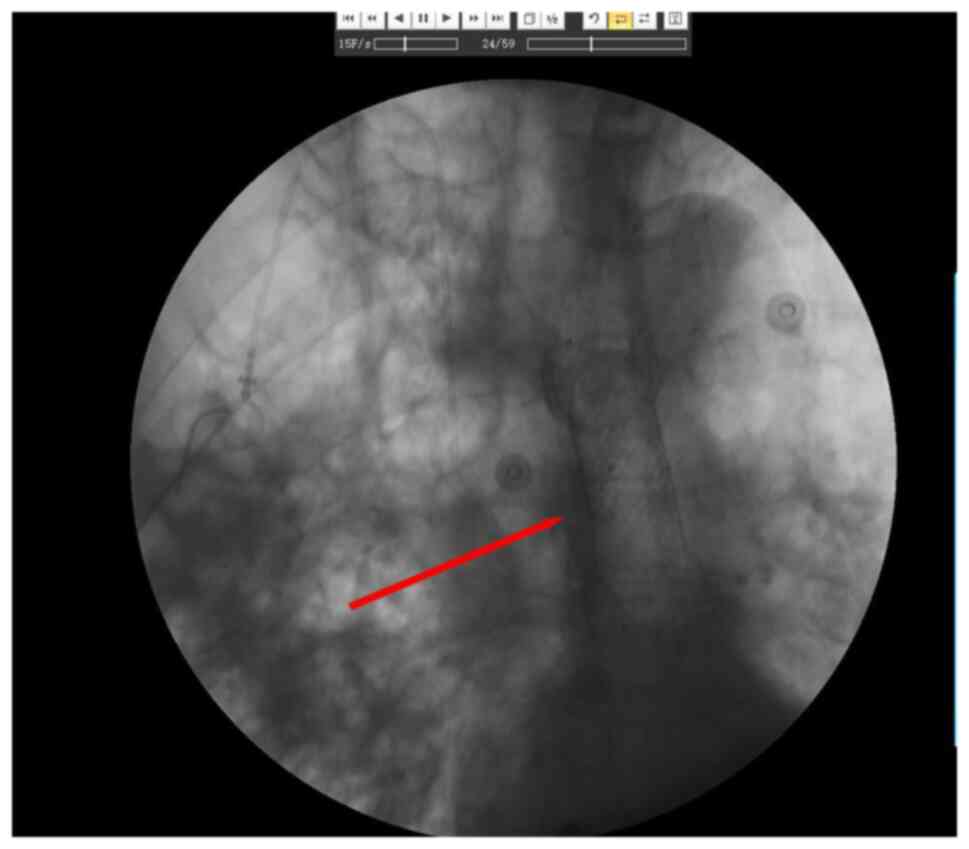
days later, both upper gastrointestinal contrast radiography
(Fig. 3) (X-ray fluoroscopic
diagnostic device; Ultimax-I; Canon Medical Systems Corporation)
and painless gastroscopy (Fig. 4)
(X-ray fluoroscopic diagnostic device; Ultimax-I; Canon Medical
Systems Corporation) confirmed a tracheoesophageal fistula in the
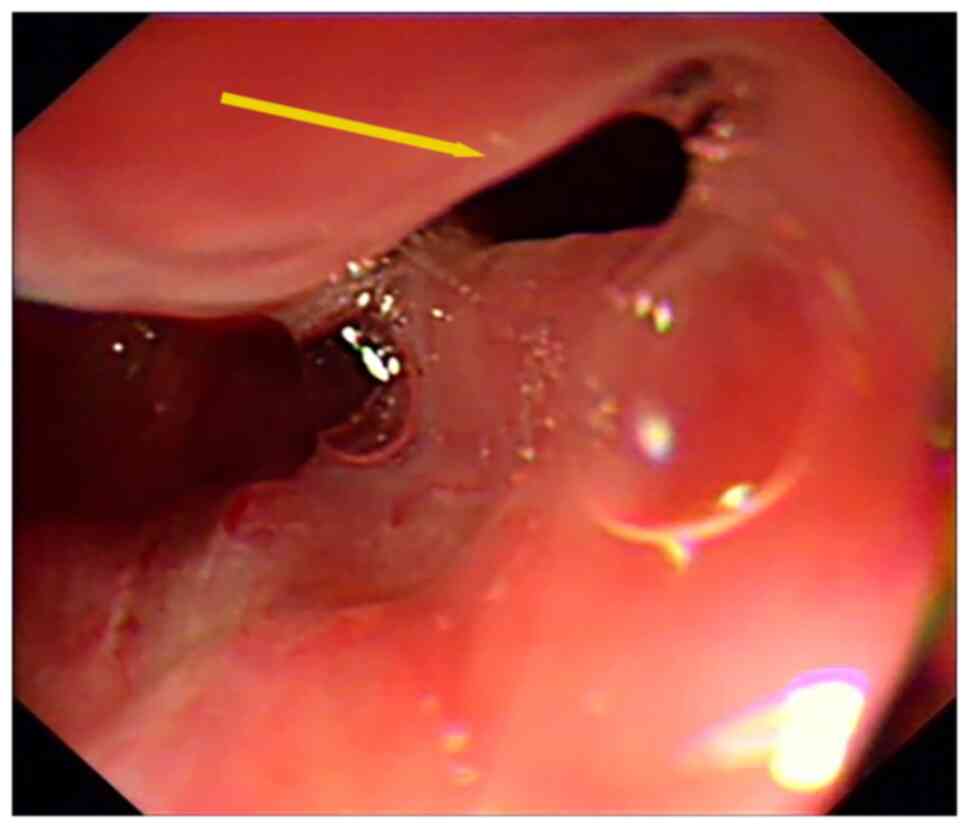
middle esophagus (35 cm from the incisors). Therefore, 2 days
later, esophageal covered stent implantation was performed under
endoscopy (Fig. 5) (X-ray
fluoroscopic diagnostic device; Ultimax-I; Canon Medical Systems
Corporation). After the operation, parenteral nutrition and
anti-infection management were administered (1 g meropenem every 8
h for 17 days). A total of 19 days after stent implantation, a
reexamination revealed that the position of the stent was good, and
the patient was discharged from the hospital after their condition
gradually improved. Due to advanced malignant tumors complicated by
cardiopulmonary and respiratory circulatory system failure, the
patient was followed up until January 2025 when they passed away
due to advanced lung cancer.
Discussion
The present report describes the case of a patient
with advanced lung cancer who had a history of chronic gastritis.
In the early stage, the patient received 4 cycles of chemotherapy
with 700 mg bevacizumab combined with pemetrexed and carboplatin,
as well as intensity-modulated radiotherapy (total dose of 40 Gy/20
fractions). Later, the dose of bevacizumab was adjusted to 800 mg +
the original chemotherapy regimen. Esophageal perforation occurred
during the 6th cycle of treatment. After comprehensive treatments,
such as esophageal covered stent implantation under endoscopy, the
condition of the patient improved in the short term.
From the results of the present report, combined
with a literature analysis, we hypothesize that the pathogenesis is
associated with the synergistic effect of multiple risk factors.
The anti-angiogenic effect of bevacizumab as a key therapeutic
drug, the duality of the vascular normalization effect and the
toxic effect of bevacizumab are worthy of in-depth discussion. This
drug blocks the phosphorylation of VEGFR-2 by binding to VEGF-A
with high affinity, which inhibits tumor angiogenesis whilst also
impairing the physiological vascular repair mechanism (12,13).
Thawani et al (14) reported
that even if patients did not receive local treatments
(radiotherapy or surgery) and only used bevacizumab, it could also
cause a tracheoesophageal fistula. When perforation occurred in the
patients, the median dose of bevacizumab used was 733 mg, which was
close to the median dose of 800 mg for the risk threshold of
gastrointestinal perforation reported in the literature (15). The odds ratio was 2.8 (P=0.03),
suggesting that high-dose medication may be an important
predisposing factor. ii) The synergistic damage effect of combined
radiotherapy and chemotherapy is another risk factor. During the
radiotherapy process for lung cancer, there is an association
between the length of time after the completion of radiotherapy and
the occurrence of esophageal fistula. According to previous
studies, the time span from the end of radiotherapy to the
appearance of esophageal fistula varies greatly. The condition may
occur as soon as ~4 months after the end of radiotherapy (16) or as late as 21 months (17). Previous case reports by Nishie et
al (18) and Wang et al
(19) have indicated that during
the treatment of lung cancer, esophageal perforation may be induced
in patients who did not receive radiotherapy when treated with
bevacizumab combined with chemotherapy. However, in the present
case, the situation was more complex, as the patient not only
received bevacizumab combined with chemotherapy but also
sequentially underwent radiotherapy. Specifically, the mean
esophageal dose of the patient was 18 Gy, and the maximum
esophageal dose was 36 Gy. The mean dose at the location of the
later esophageal perforation was 21 Gy, and the maximum esophageal
dose was 33 Gy. Radiotherapy is likely to cause chronic
inflammation and fibrosis of the esophageal mucosa (20). When the adverse effects caused by
radiotherapy are superimposed on the anti-angiogenic effect of
bevacizumab, the tolerance of tissues may be further weakened
(21). Furthermore, the regimen of
pemetrexed combined with carboplatin used in the patient in the
present case may have exacerbated the damage to the esophageal
mucosa (22). The Aspergillus
flavus infection (confirmed by tNGS) that occurred in the
patient later could have caused pulmonary inflammation and the
severe cough (23,24). This may have increased the pressure
on the esophageal wall through mechanical stress and become a
direct predisposing factor for perforation (25). Spigel et al (26) performed two independent phase II
clinical trials on 34 patients with small cell lung cancer (29
patients) and non-small cell lung cancer (5 patients). The research
revealed that the combination of bevacizumab with radiotherapy and
chemotherapy markedly increased the risk of a tracheoesophageal
fistula in patients with lung cancer (incidence rate, 2.3 vs. 0.0%,
respectively). The treatment regimen of the patient in the present
case included the combination of bevacizumab with radiotherapy and
chemotherapy, which may have increased the risk of
tracheoesophageal fistula. Another risk factor associated with the
pathogenesis of perforation is the superimposed impact of
underlying diseases and predisposing factors. The previous chronic
gastritis of the patient in the present case may have led to acid
reflux, which could have chronically stimulated the esophageal
mucosa to form chronic inflammation and reduce the resistance of
local tissues (27). Zhou et
al (15) performed a
retrospective analysis of 8 cases of bevacizumab-related
gastrointestinal perforation, reporting that 62.5% of the patients
had underlying digestive tract diseases, which was consistent with
the situation in the present case. Moreover, when the patient in
the present case coughed, the intrathoracic pressure suddenly
increased (up to 50–100 mmHg) (28,29),
which may have caused damage to the weak areas of the esophageal
wall (25). Especially when the
mucosa has already been damaged by radiotherapy or drugs, it is
easy for a full-thickness rupture to form and create a fistula.
In view of the serious consequences of
tracheoesophageal fistula (the patient in the present case
eventually died due to advanced cancer), the following management
strategies should be considered when bevacizumab is used
clinically: i) Screening of high-risk factors: Patient medical
history should be collected in detail, with a focus on basic
conditions such as the history of digestive tract diseases (such as
chronic gastritis and ulcers), radiotherapy history and chronic
cough. For patients who are due to receive bevacizumab combined
with radiotherapy, the radiation dose received by the esophagus
should be strictly evaluated. Furthermore, the superimposed use of
high-dose drugs (such as ≥800 mg per time) plus radiotherapy and
chemotherapy should be avoided if possible. ii) Baseline assessment
and monitoring: For patients at moderate-to-high risk of esophageal
perforation (such as those with combined radiotherapy, chemotherapy
and bevacizumab, or those with a history of digestive tract
diseases), gastroscopy is recommended before treatment (30) to exclude esophageal mucosal damage.
During the treatment, swallowing functions (such as swallowing
pain, a foreign body sensation and a choking cough) and respiratory
symptoms (an aggravated cough and shortness of breath) should be
closely monitored. When abnormalities occur, an esophageal fistula
should be immediately screened for (upper gastrointestinal contrast
radiography or endoscopy is preferred). iii) Principles of
emergency treatment: Once a tracheoesophageal fistula is diagnosed,
bevacizumab should be immediately stopped, fasting started, and
anti-infection and nutritional support treatments should be
administered. Minimally invasive methods such as endoscopic stent
implantation should be prioritized to close the fistula and improve
the quality of life of the patient.
In conclusion, we hypothesize that the esophageal
perforation in the present case was the result of the synergistic
effect of multiple factors, including drug dosage, radiation dose
to the esophagus, esophageal damage caused by chemotherapy,
underlying digestive tract diseases and mechanical stress damage.
Clinically, individualized risk assessments for high-risk groups
are needed, and treatment indications and dosages should be
strictly controlled and combined with close symptom monitoring and
imaging evaluations, in the hope of the early identification and
management of this rare but potentially fatal complication.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QJ, YLZ, and GWH were responsible for the conception
and design of this study. QJ undertook the analysis and summary of
patient clinical data and drafted the initial manuscript. YLZ and
GWH conducted rigorous revisions on important intellectual content
of the manuscript (such as the relevance between the discussion
section and literature) and provided critical comments. HCL and LYD
were responsible for the acquisition of medical imaging data and
collection of patient treatment records, participated in the
preliminary analysis and interpretation of data, and also took part
in manuscript review. QJ, YLZ, LYD, HCL and GWH confirm the
authenticity of all the raw data and take responsibility for all
aspects of the work, ensuring that any questions regarding the
accuracy or integrity of any part of the work were appropriately
investigated and resolved. All authors read and approved the final
version of the manuscript and unanimously agreed that the
manuscript could be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Informed consent for the publication of the
manuscript was obtained from the patient's wife.
Competing interests
The authors declares that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Grothey A, Hedrick EE, Mass RD, Sarkar S,
Suzuki S, Ramanathan RK, Hurwitz HI, Goldberg RM and Sargent DJ:
Response-independent survival benefit in metastatic colorectal
cancer: A comparative analysis of N9741 and AVF2107. J Clin Oncol.
26:183–189. 2008. View Article : Google Scholar
|
|
2
|
Langer CJ, Socinski MA, Patel JD, Sandler
AB, Schiller JH, Leon L, Hazard SJ and Ramalingam SS: Isolating the
role of bevacizumab in elderly patients with previously untreated
nonsquamous non-small cell lung cancer: Secondary analyses of the
ECOG 4599 and pointbreak trials. Am J Clin Oncol. 39:441–447. 2016.
View Article : Google Scholar
|
|
3
|
Cheng AL, Qin S, Ikeda M, Galle PR,
Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, et al:
Updated efficacy and safety data from IMbrave150: Atezolizumab plus
bevacizumab vs. sorafenib for unresectable hepatocellular
carcinoma. J Hepatol. 76:862–873. 2022. View Article : Google Scholar
|
|
4
|
Redondo A, Colombo N, McCormack M, Dreosti
L, Nogueira-Rodrigues A, Scambia G, Lorusso D, Joly F, Schenker M,
Ruff P, et al: Primary results from CECILIA, a global single-arm
phase II study evaluating bevacizumab, carboplatin and paclitaxel
for advanced cervical cancer. Gynecol Oncol. 159:142–149. 2020.
View Article : Google Scholar
|
|
5
|
You B, Purdy C, Copeland LJ, Swisher EM,
Bookman MA, Fleming G, Coleman R, Randall LM, Tewari KS, Monk BJ,
et al: Identification of patients with ovarian cancer experiencing
the highest benefit from bevacizumab in the first-line setting on
the basis of their tumor-intrinsic chemosensitivity (KELIM): The
GOG-0218 validation study. J Clin Oncol. 40:3965–3974. 2022.
View Article : Google Scholar
|
|
6
|
Holash J, Davis S, Papadopoulos N, Croll
SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, et
al: VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc
Natl Acad Sci USA. 99:11393–11398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar
|
|
8
|
Von Minckwitz G, Puglisi F, Cortes J,
Vrdoljak E, Marschner N, Zielinski C, Villanueva C, Romieu G, Lang
I, Ciruelos E, et al: Bevacizumab plus chemotherapy versus
chemotherapy alone as second-line treatment for patients with
HER2-negative locally recurrent or metastatic breast cancer after
first-line treatment with bevacizumab plus chemotherapy (TANIA): An
open-label, randomised phase 3 trial. Lancet Oncol. 15:1269–1278.
2014. View Article : Google Scholar
|
|
9
|
Zhang T, Yang Y, Cheng G, Chen P and Bi N:
Tracheoesophageal fistula associated with bevacizumab after
thoracic radiotherapy in non-small cell lung cancer: A case report.
Medicine (Baltimore). 99:e198782020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olawaiye AB, Baker TP, Washington MK and
Mutch DG: The new (version 9) American joint committee on cancer
tumor, node, metastasis staging for cervical cancer. CA Cancer J
Clin. 71:287–298. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niu G and Chen X: Vascular endothelial
growth factor as an anti-angiogenic target for cancer therapy. Curr
Drug Targets. 11:1000–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sullivan LA and Brekken RA: The VEGF
family in cancer and antibody-based strategies for their
inhibition. MAbs. 2:165–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thawani R, Thomas A and Thakur K:
Tracheomediastinal fistula: Rare complication of treatment with
bevacizumab. Cureus. 10:e24192018.PubMed/NCBI
|
|
15
|
Zhou Y, Rui Y, Xu P, Yang Q, Luo X, Yi B
and Zheng Y: Clinical analysis of 8 cases of gastrointestinal
perforation caused by bevacizumab. Chin J Bases Clin Gen Surg.
28:516–519. 2021.(In Chinese).
|
|
16
|
Goodgame B, Veeramachaneni N, Patterson A
and Govindan R: Tracheo-esophageal fistula with bevacizumab after
mediastinal radiation. J Thorac Oncol. 3:1080–1081. 2008.
View Article : Google Scholar
|
|
17
|
Gore E, Currey A and Choong N:
Tracheoesophageal fistula associated with bevacizumab 21 months
after completion of radiation therapy. J Thorac Oncol. 4:1590–1591.
2009. View Article : Google Scholar
|
|
18
|
Nishie K, Yasuo M, Kitaguchi Y, Kobayashi
N, Tateishi K, Ushiki A, Urushihata K, Yamamoto H, Ideura G and
Hanaoka M: Bevacizumab-induced tracheoesophageal fistula in a
patient suffering from lung cancer with bulky subcarinal lymph
node: A case report. Nagoya J Med Sci. 80:129–134. 2018.
|
|
19
|
Wang T, Thakur A and Chen B:
Bevacizumab-induced esophageal pleural fistula during maintenance
therapy without radiation in lung cancer. BMC Pulm Med. 21:3842021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Feng W, Gao LT, Cai XW, Liu Q,
Zhu ZF, Fu XL and Yu W: Long-term follow-up of a phase I/II trial
of radiation dose escalation by simultaneous integrated boost for
locally advanced esophageal squamous cell carcinoma. Radiother
Oncol. 159:190–196. 2021. View Article : Google Scholar
|
|
21
|
Mangoni M, Vozenin MC, Biti G and Deutsch
E: Normal tissues toxicities triggered by combined anti-angiogenic
and radiation therapies: Hurdles might be ahead. Br J Cancer.
107:308–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seiwert TY, Connell PP, Mauer AM, Hoffman
PC, George CM, Szeto L, Salgia R, Posther KE, Nguyen B, Haraf DJ
and Vokes EE: A phase I study of pemetrexed, carboplatin, and
concurrent radiotherapy in patients with locally advanced or
metastatic non-small cell lung or esophageal cancer. Clin Cancer
Res. 13:515–522. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee RJ, Workman AD, Carey RM, Chen B,
Rosen PL, Doghramji L, Adappa ND, Palmer JN, Kennedy DW and Cohen
NA: Fungal aflatoxins reduce respiratory mucosal ciliary function.
Sci Rep. 6:332212016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Banz M, Stallmach A, Gaßler N, Schulze PC,
Fritzenwanger M, Cornely O, Kurzai O and Pletz MW: Fatal pulmonary
hemorrhage, pneumothorax and skin necrosis caused by IRIS to an
Aspergillus flavus infection in a young patient with
metamizole associated agranulocytosis. Infection. 52:685–690. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ladna M and George J: Large esophageal
intramural hematoma after solid food ingestion in a patient without
identifiable inherited or acquired coagulopathy. ACG Case Rep J.
10:e010672023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spigel DR, Hainsworth JD, Yardley DA,
Raefsky E, Patton J, Peacock N, Farley C, Burris HA III and Greco
FA: Tracheoesophageal fistula formation in patients with lung
cancer treated with chemoradiation and bevacizumab. J Clin Oncol.
28:43–48. 2010. View Article : Google Scholar
|
|
27
|
McColl KEL: What is causing the rising
incidence of esophageal adenocarcinoma in the West and will it also
happen in the East? J Gastroenterol. 54:669–673. 2019. View Article : Google Scholar
|
|
28
|
Hines MT, Crisman MV, Kohn CW, Hansen B,
Hinchcliff KW, Foreman JH, Beard LA and Rush BR: Clinical Approach
to Commonly Encountered Problems. Equine Internal Medicine. 2nd
edition. Elsevier Inc; pp. 111–168. 2004, View Article : Google Scholar
|
|
29
|
Dwivedi S, Schrickel EB, Siddiqui F,
O'Brien J and Kruer J: Esophageal microperforation due to calcified
mediastinal lymph node leading to tracheoesophageal fistula. Case
Rep Gastrointest Med. 2016:97471932016.PubMed/NCBI
|
|
30
|
Haanen J, Obeid M, Spain L, Carbonnel F,
Wang Y, Robert C, Lyon AR, Wick W, Kostine M, Peters S, et al:
Management of toxicities from immunotherapy: ESMO clinical practice
guideline for diagnosis, treatment and follow-up. Ann Oncol.
33:1217–1238. 2022. View Article : Google Scholar
|