Introduction
Adenoid cystic carcinoma (ACC) is a rare and
aggressive malignancy, comprising only ~1% of head and neck
malignancies (1) and ~10% of
salivary gland tumors (2). Most ACC
cases arise from the minor salivary glands, with the palate and
sinuses identified as the most common sites. A smaller proportion
of cases originate from the major salivary glands, primarily the
parotid gland (3–5). ACC is characterized by its indolent
growth, lymphovascular invasion, perineural invasion and a high
incidence of distant metastasis. The long-term prognosis for
patients with ACC is poor, with a 10-year overall survival (OS)
rate of ~50%. Age >50 years, T3-4, N+, M1, presence of
lymphovascular invasion, perineural/nerve invasion and
positive/close margin status are significant predictors for
decreased survival (6). Notably,
distant metastases occur in 40–50% of cases, and the median
survival time following metastasis is <3 years (7). Common metastatic sites include the
lungs, liver and bones, with lung metastases being the most
frequent, comprising 56–85.9% of metastases, whereas lymph node
metastases are very rare (6,8).
Initial treatment most commonly consists of surgery with adjuvant
radiation for the primary site (9),
but there is no consensus on the management of liver metastases.
The present report describes a rare case of submandibular gland ACC
with liver metastasis as the initial clinical manifestation, and
summarizes relevant literature to provide insights into the
diagnosis and management of rapidly advancing high-risk ACC.
Case report
A 69-year-old female patient presented at Beijing
Tongren Hospital (Beijing, China) in February 2022 with a complaint
of paroxysmal colicky pain in the mid-abdomen for a duration of 3
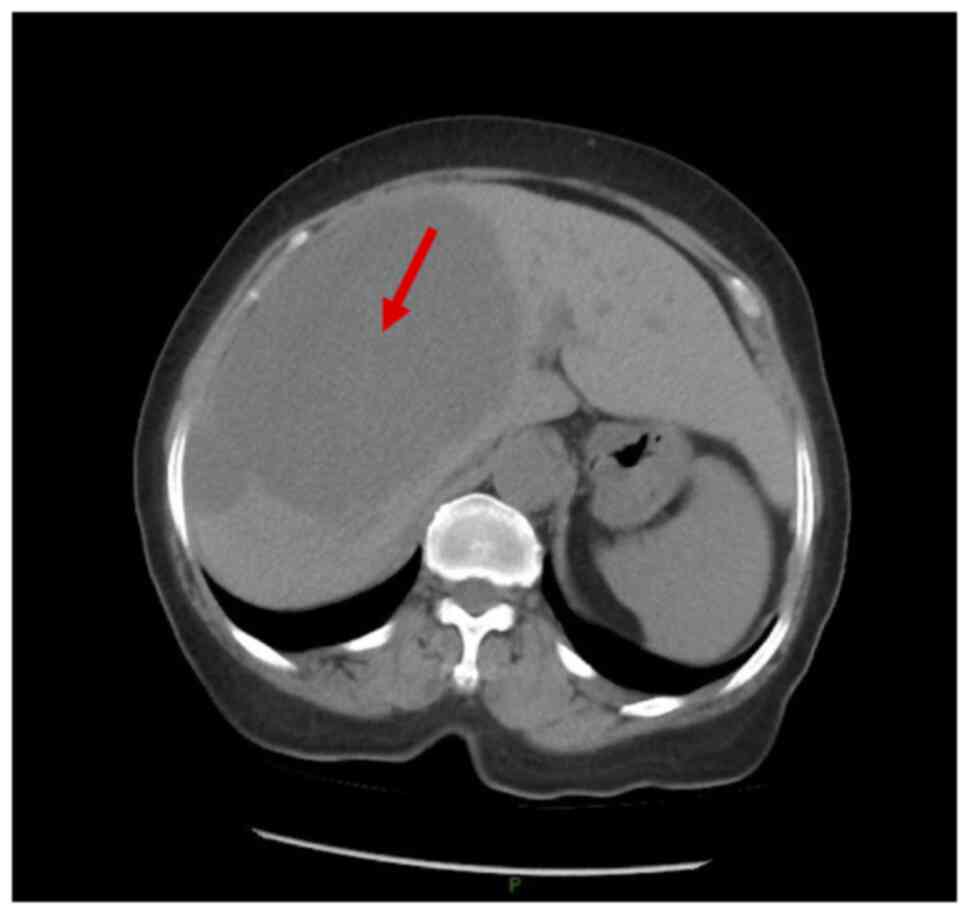
weeks. Abdominal CT revealed multiple irregularly shaped hepatic
masses, the largest measuring ~15.7×14.3 cm (Fig. 1). Cancer antigen 19-9 levels were
recorded as 41.8 U/ml (reference range: 0–37 U/ml), whereas
α-fetoprotein, carcinoembryonic antigen and other tumor biomarkers
were negative. PET/CT imaging was performed to assess both local
and systemic lesions, revealing a mildly hypermetabolic mass in the
left submandibular gland, hypermetabolic lymph nodes in the left
upper deep neck and multiple hepatic masses exhibiting unevenly
increased metabolism (Fig. S1);
these findings were highly suggestive of malignancy.
Following the exclusion of contraindications, a
right tri-lobe hepatectomy and regional lymph node dissection were
performed at Beijing Youan Hospital (Beijing, China) in April 2022.
Pathological analysis revealed multiple hepatic tumors, the largest
measuring ~19×14×10 cm, featuring a firm, gray-white cross-section
(data not shown). All specimens were fixed in 4% neutral buffered
formalin at room temperature for 24 h within 30 min of
devitalization, then grossed and subjected to routine dehydration,
clearing and paraffin embedding. Next, 4 µm sections were obtained
from each paraffin block using a microtome (MICROM HM 340E; Thermo
Fisher Scientific, Inc.) and stained with hematoxylin-eosin (HE).
Samples were de-waxed in two changes of xylene (5 min each),
rehydrated to water with graded alcohols and stained with
hematoxylin for 5 min. After washing the sections in water, the
samples were differentiated in 1% hydrochloric acid in 70% ethanol.
Sections were stained with eosin for 3 min, re-immersed in alcohol
and xylene, dehydrated in ethanol, cleared in xylene and cover
slipped in a resinous mountant. Under an Olympus BX53 upright
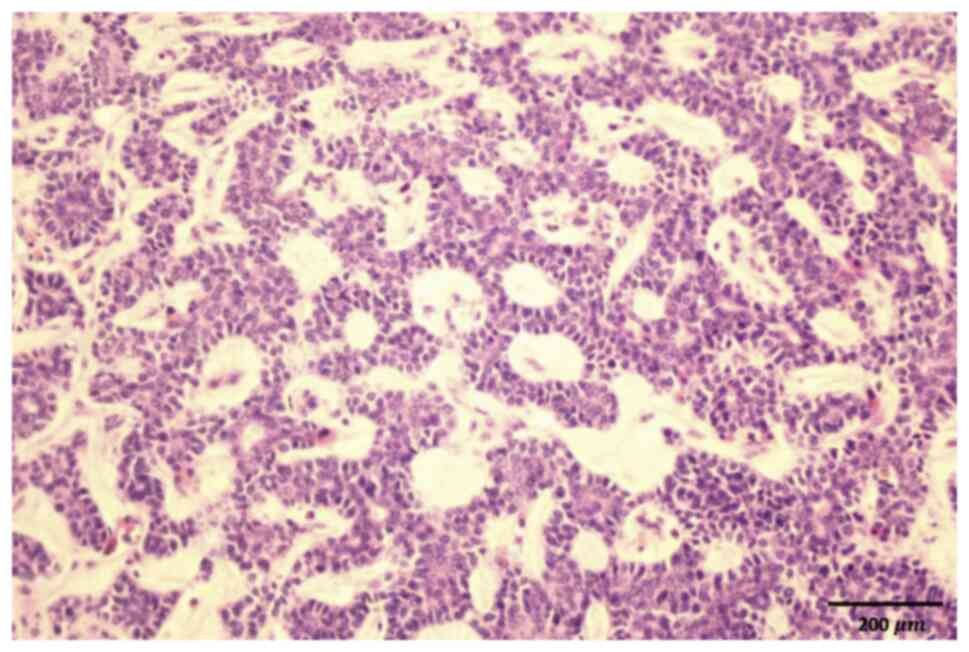
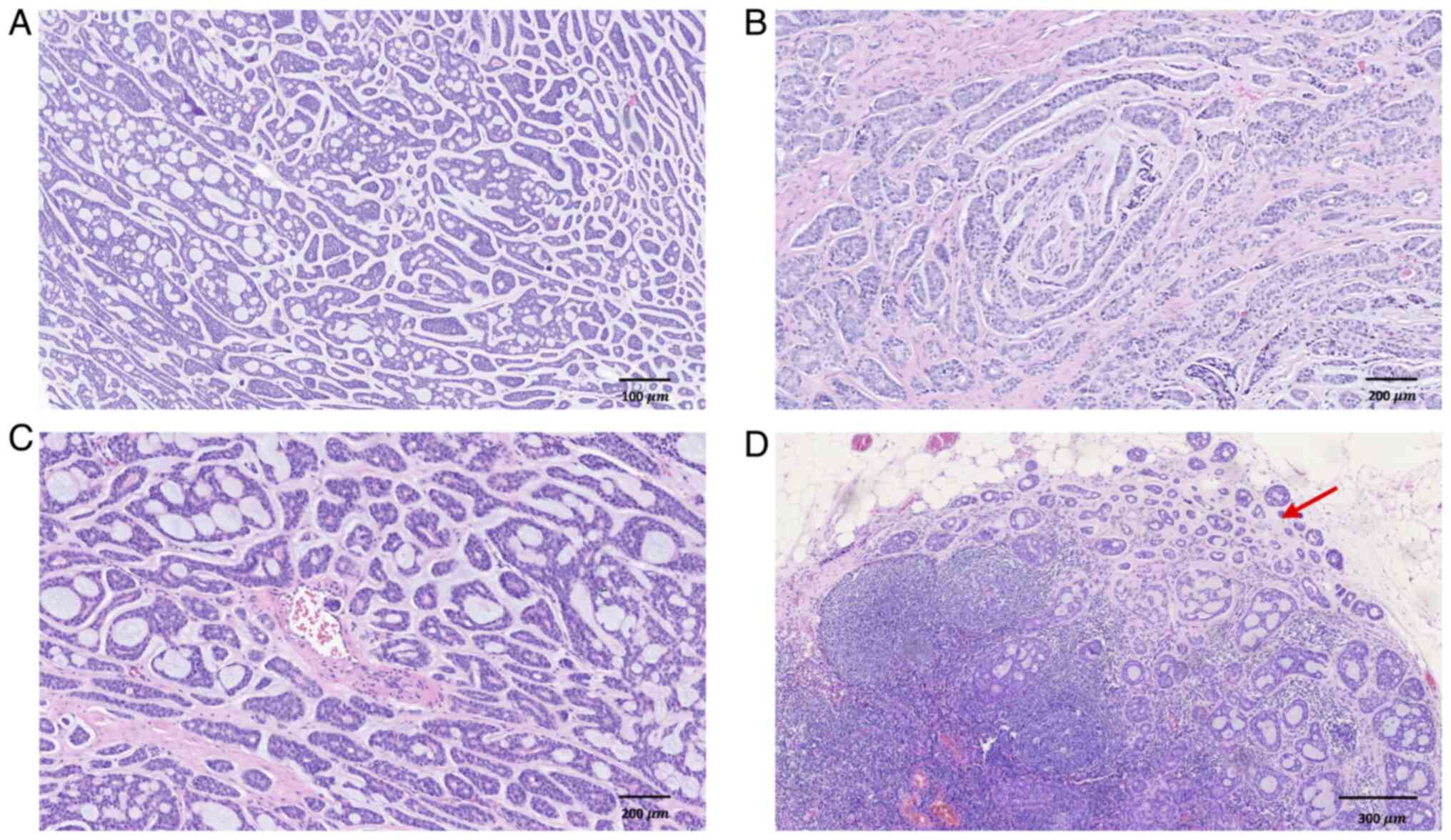
microscope, tumor cells were observed to be arranged in tubular,
cribriform and cord-like patterns, comprising epithelial and
myoepithelial cell components (Fig.
2). Based on the clinical history, the findings were indicative
of ACC with multiple liver metastases. Moreover, genomic profiling
was performed by Genetron Health (Beijing, China) using a
hybridization-capture-based targeted panel and Illumina
high-throughput sequencing. Genetic testing identified 15 gene
mutations, including ATP binding cassette subfamily C member 8,
A-kinase anchoring protein 10, α kinase 1, BCL6 corepressor,
carnosine synthase 1, cyclin dependent kinase inhibitor 1C, DEAF1
transcription factor, lysine demethylase 4B, keratin associated
protein 17-1, matrix metallopeptidase 24, phosphoinositide-3-kinase
regulatory subunit 1 (PIK3R1), solute carrier family 12 member 2,
SOX10, spermatogenesis and centriole associated 1 like and spectrin
α, erythrocytic 1 (Table I); three
gene copy number variations involving Achaete-Scute family BHLH
transcription factor 2, forkhead box L2 and NK2 homeobox 1; no gene
rearrangements; microsatellite stability; and a tumor mutational
burden of 0.49 mutations/mb.
 | Table I.Detected gene mutations and potential
clinical significance. |
Table I.
Detected gene mutations and potential
clinical significance.
| Gene | Protein function | Clinical
significance |
|---|
| ABCC8 | Modulator of
ATP-sensitive potassium channels and insulin release | Mutations have been
associated with non-insulin-dependent diabetes mellitus type II and
hyperinsulinemic hypoglycemia of infancy |
| AKAP10 | Differentially
targeted protein that binds to type I and II regulatory subunits of
protein kinase A and anchors them to the mitochondria or the plasma
membrane | Polymorphisms in this
gene may be associated with increased risk of arrhythmias and
sudden cardiac death |
| ALPK1 |
Serine/threonine-protein kinase that
detects bacterial PAMPs | Associated diseases
include retinal dystrophy, optic nerve edema, splenomegaly,
anhidrosis and migraine headache syndrome |
| BCOR | An interacting
corepressor of BCL6, a POZ/zinc finger transcription repressor may
influence apoptosis | BCOR is altered in
several solid and hematologic malignancies including acute myeloid
leukemia and kidney clear cell sarcoma |
| CARNS1 | Catalyzes the
formation of carnosine and homocarnosine | Associated diseases
include Persian Gulf syndrome |
| CDKN1C | A strong inhibitor of
several G1 cyclin/CDK complexes and a negative regulator of cell
proliferation | Mutations in this
gene are implicated in sporadic cancers and Beckwith-Wiedemann
syndrome |
| DEAF1 | A zinc finger
domain-containing protein that functions as a regulator of
transcription | Associated diseases
include neurodevelopmental disorder with hypotonia and impaired
expressive language and with or without seizures |
| KDM4B | Enables histone H3K36
demethylase activity and histone H3K9me2/H3K9me3 demethylase
activity | Biomarker of several
diseases, including alopecia areata, lung cancer, medulloblastoma,
prostate cancer and stomach cancer |
| KRTAP17-1 | KAP proteins form a
matrix of keratin intermediate filaments which contribute to the
structure of hair fibers | Associated diseases
include limited scleroderma |
| MMP24 | Breakdown of
extracellular matrix | Associated diseases
include cerebral arteriopathy, autosomal dominant, with subcortical
infarcts and leukoencephalopathy, type 1 and Waardenburg syndrome,
type 2E |
| PIK3R1 | This gene encodes the
regulatory subunit of PI3-kinase. PI3-kinase phosphorylates the
inositol ring of phosphatidylinositol at the 3′ position | Mutated in several
cancers, most frequently in glioma, endometrial and colorectal
cancers |
| SLC12A2 | The protein encoded
by this gene mediates sodium and chloride transport and
reabsorption | Associated diseases
include Delpire-Mcneill syndrome and Kilquist syndrome |
| SOX10 | A transcription
factor involved in embryonic development and cell fate | SOX10 amplification
has been identified in several cancers, including melanoma, bladder
cancer and nasopharyngeal carcinoma |
| SPATC1L | Predicted to be
involved in spermatogenesis | Associated diseases
include spermatogenic failure 16 and male infertility due to
acephalic spermatozoa |
| SPTA1 | The encoded protein
is primarily composed of 22 spectrin repeats which are involved in
dimer formation. It forms a component of the erythrocyte plasma
membrane | Mutations in this
gene result in several hereditary red blood cell disorders |
After 3 months, the patient sought further diagnosis
and treatment for an anterior neck mass at Beijing Tongren
Hospital. This had been identified 6 years prior on the left
anterior neck and measured ~1 cm, without any apparent pain,
breathing difficulties, swallowing problems, choking or hoarseness.
Initially disregarded, the mass had recently exhibited gradual
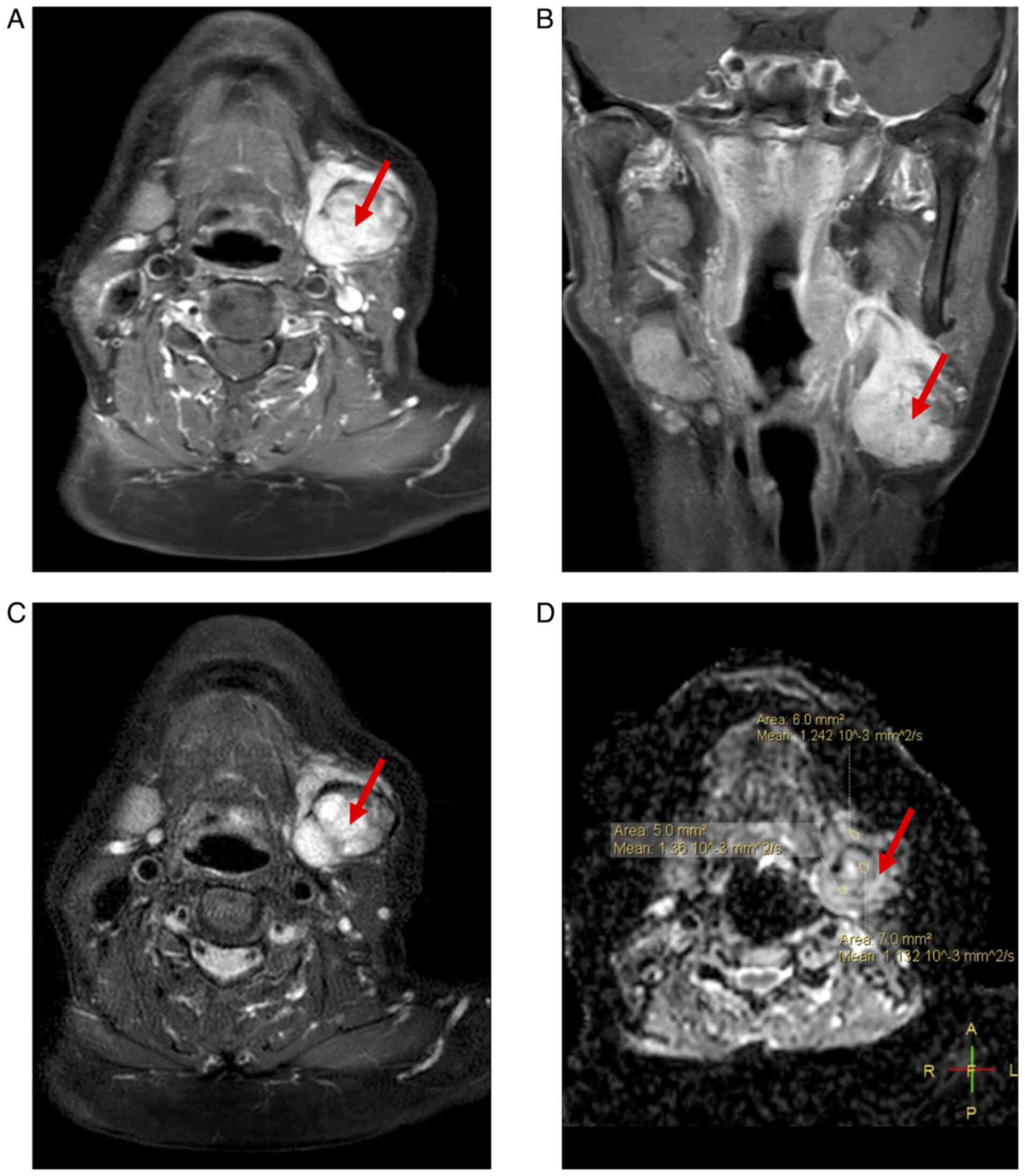
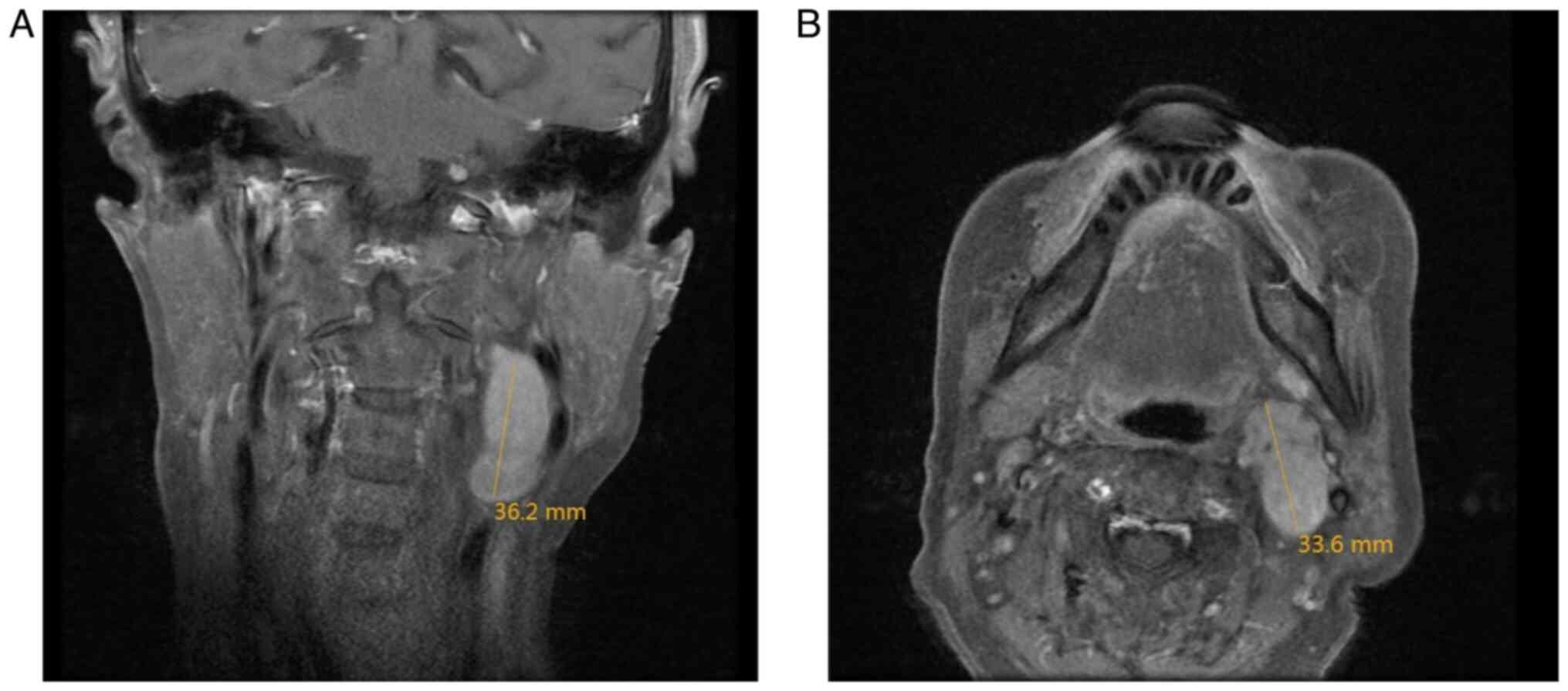
enlargement. MRI of the neck revealed a nodular lesion in the
posterior region of the left submandibular gland, displaying
slightly prolonged T1 and T2 signals with heterogeneous intensity,
a high signal on diffusion-weighted imaging and apparent diffusion
coefficient, and measuring ~3.3×2.3×3.4 cm. The lesion appeared
lobulated with indistinct boundaries and demonstrated notable
heterogeneous enhancement on contrast-enhanced scans (Fig. 3). Enlarged lymph nodes were observed
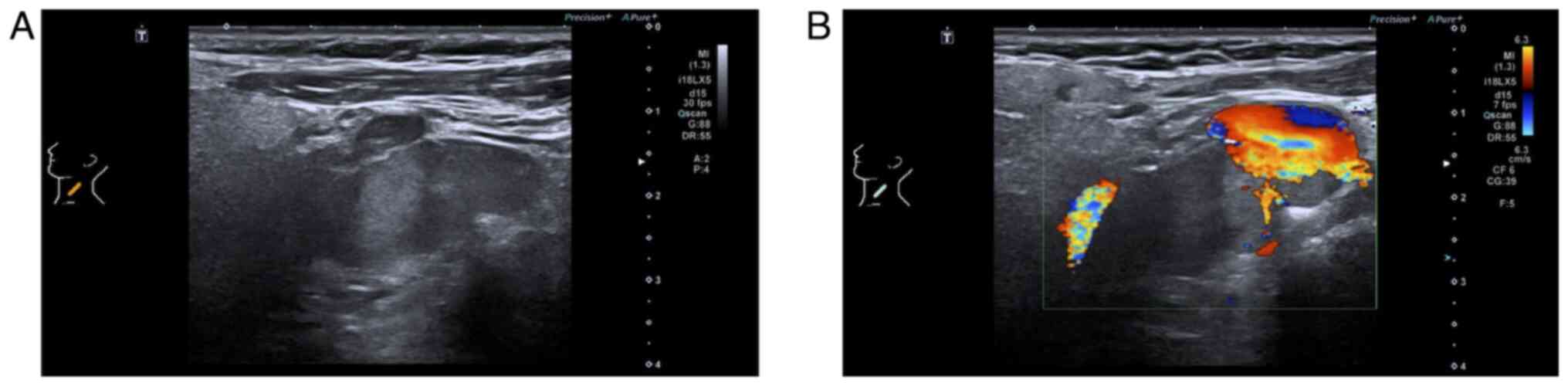
in the left cervical level II region. Ultrasonography revealed an
irregular hypoechoic lesion in the left submandibular area with
well-defined margins, protruding into the subcutaneous tissue,
exhibiting heterogeneous internal echoes, scattered calcifications
and minimal blood flow signals both around and within the lesion
(Fig. 4).
Following the exclusion of contraindications, the
patient underwent resection of the submandibular gland tumor and
cervical lymph node dissection at Beijing Tongren Hospital
(Beijing, China) in July 2022. Pathological examination following
surgery revealed a grayish-white, hard mass in the submandibular
gland, measuring ~4.5×2.5×3.2 cm (data not shown). HE staining was
performed as described above. The tumor was microscopically
diagnosed as ACC, exhibiting primarily cribriform structures (80%),
with tubular (15%) and solid (5%) patterns, demonstrating high
differentiation without high-grade transformation. Lymphovascular
invasion and perineural invasion were observed; one lymph node
exhibited metastasis, with the largest metastatic focus measuring
~2 mm and exhibiting extranodal extension (Fig. 5). Immunohistochemical staining was
performed with the envision two step method. All primary antibodies
were purchased from Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd. (OriGene Technologies, Inc.) in a ready-to-use format,
including Calponin (cat. no. ZA-0524; clone EP63), CD117 (cat. no.
ZA-0523; clone EP10), cytokeratin (CK; cat. no. ZM-0069; clone
AE1/AE3), CK7 (cat. no. ZM-0071; clone UMAB161), CK8/18 (cat. no.
ZM-0315; clone B22.1 and B23.1), p63 (cat. no. ZA-0553; clone B18),
S-100 (cat. no. ZM-0224; clone 15E2E2 and C4.9), SRY-box
transcription factor (SOX)10 (cat. no. ZA-0624; clone EP268),
Vimentin (cat. no. ZM-0260; clone UMAB159), β-catenin (cat. no.
ZM-0442; clone UMAB15), C-Myc (cat. no. ZA-0555; clone EP121), EGFR
(cat. no. ZM-0093; clone UMAB95), VEGF (cat. no. ZA-0287), Ki67
(cat. no. ZM-0166; clone UMAB107), Her-2 (cat. no. ZM-0065; clone
UMAB36), GFAP (cat. no. ZM-0118; clone UMAB129), SOX-2 (cat. no.
ZA-0571; clone EP103), p53 (cat. no. ZM-0408; clone DO-7) and PD-L1
(cat. no. ZA-0629; clone SP142). Tissue sections were
deparaffinized and rehydrated. Antigen retrieval was achieved by
pressure cooking in 0.1 M citrate buffer pH 6 for 10 min followed
by cooling at room temperature. The endogenous peroxidase was
inactivated with 3% hydrogen peroxide for 10 min. Sections were
pre-incubated with 10% goat serum (cat. no. E07-100, OriGene
Technologies, Inc.) at room temperature for 30 min to block
non-specific antibody binding. Subsequently, the sections were
incubated overnight at 4°C with specific primary antibodies
followed by horseradish peroxidase-linked goat anti-mouse/rabbit
secondary antibody (cat. No. TA373082/TA373083; 1:500; OriGene
Technologies, Inc.) at room temperature for 60 min. Controls were
carried out by omitting the primary antibody. The reactions were
visualized by 3,3-Diaminobenzidine. The slides were then
counterstained with hematoxylin as aforementioned and mounted using
a synthetic resin. Immunohistochemical analysis under an Olympus
BX53 upright microscope revealed positive expressions of Calponin,
CD117, creatine kinase, CK7, CK8/18, P63, S-100, SOX10, Vimentin,
β-catenin, C-Myc, EGFR, VEGF, Ki67 index in hot-spot (10–15%),
alongside negative expression of Her-2, glial fibrillary acidic
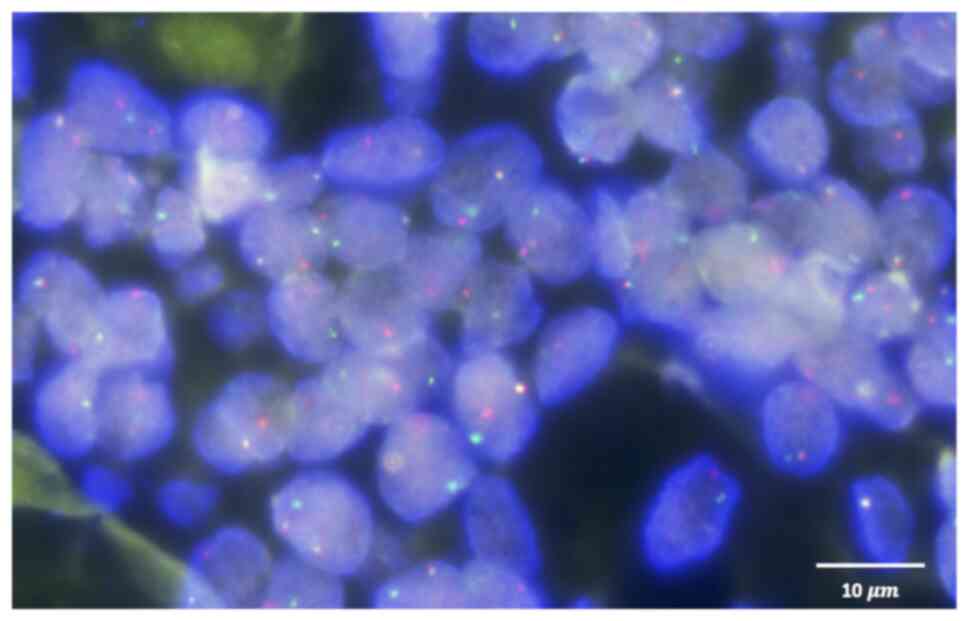
protein, SOX-2, p53 and programmed death-ligand 1 (PD-L1) (Fig. 6). MYB fluorescence in situ
hybridization (FISH) was carried out on 4-µm formalin-fixed
paraffin-embedded sections using an Anbiping MYB break apart probe
(cat. no. F.01247–01; Guangzhou Anbiping Pharmaceutical Technology
Co., Ltd.). After deparaffinization, rehydration and heat-mediated
antigen retrieval (100°C, 25 min), tissue was digested with
proteinase K (37°C, 15 min) to enhance probe penetration,
dehydrated and air-dried. Slides were co-denatured with the MYB
break-apart probe (red 455 kb 5′/green 577 kb 3′) at 85°C for 5
min, hybridized overnight at 37°C, stringency-washed with saline
sodium citrate buffer and then counterstained with DAPI at room
temperature for 10 min. Signals were scored under a fluorescence
microscope with appropriate single-band filters. FISH analysis of
the primary ACC tumor specimen revealed no MYB gene rearrangement
(Fig. 7).
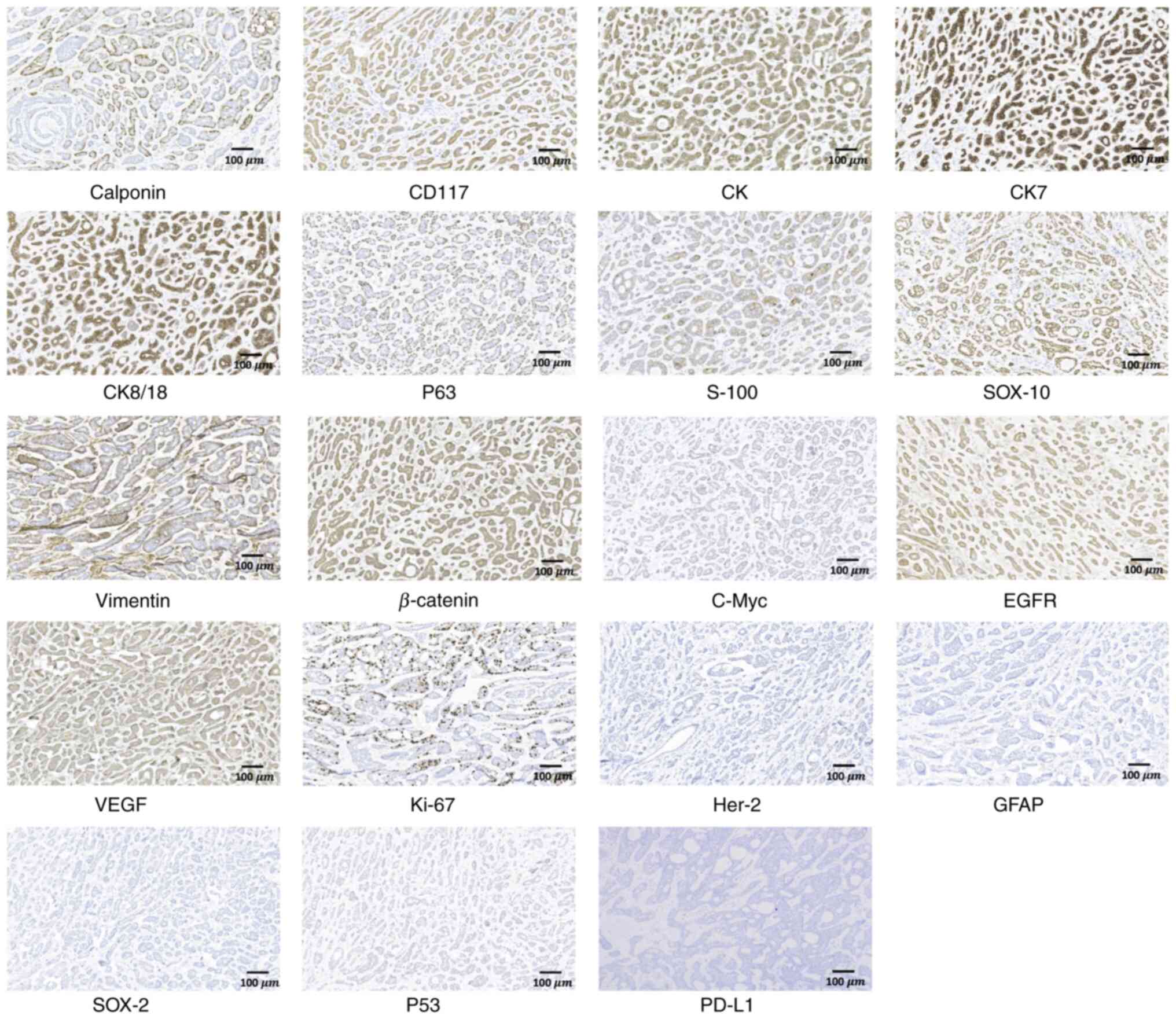 | Figure 6.Immunohistochemical images of
submandibular gland adenoid cystic carcinoma, revealing positive
expression of Calponin, CD117, CK, CK7, CK8/18, P63, S-100, SOX-10,
Vimentin, β-catenin, C-Myc, EGFR, VEGF, and Ki-67 index in hot-spot
(10–15%), alongside negative expression of Her-2, GFAP, SOX-2, P53
and PD-L1 (magnification, ×100). CK, creatine kinase; CK7,
cytokeratin 7; CK8/18, cytokeratin 8/18; SOX, SRY-box transcription
factor; GFAP, glial fibrillary acidic protein; PD-L1, programmed
death-ligand 1. |
A total of 4 months following liver metastasis
surgery, a new lesion in the left hepatic lobe was identified (data
not shown) and treated with ultrasound-guided percutaneous
microwave ablation, resulting in a successful surgical outcome and
recovery. Follow-up concluded at the end of May 2024, by which time
cervical lymph node metastasis in the left level Ib and II regions
(Fig. 8) and multiple hepatic
masses were observed. The patient declined any additional
interventions due to concerns of pain and treatment burden.
Subsequent follow-ups were unsuccessful despite repeated contact
efforts.
Discussion
ACC is a rare malignant tumor that represents ~1% of
all head and neck cancer cases and ~10% of salivary gland
malignancies. ACC characteristically grows slowly, with a 5-year OS
rate of 68–90%. Long-term outcomes indicate that the 10- and
15-year OS rates decrease to 52 and 28%, respectively, with neural
invasion, failure of local control and distant metastasis
identified as the primary causes (4). Distant metastasis occurs in 16.1–72.7%
of cases, typically within ~8 years post-treatment but earlier in
salivary gland ACC (10,11). The most common metastatic sites are
the lungs, followed by bones, the liver and the brain (12).
ACC of the head and neck with liver metastasis is
infrequently documented and lacks a standardized treatment
consensus. To date, only seven cases of ACC with liver metastasis
as the initial clinical manifestation have been documented, to the
best of our knowledge (13–19) (Table
II). Most of these cases identified the submandibular gland as
the primary site, with liver metastasis potentially occurring in
both early- and late-stage patients based on tumor (T) staging.
Only Spolverato et al (16)
and the patient described in the present report exhibited
simultaneous lymph node metastasis, to the best of our
knowledge.
 | Table II.Literature review of adenoid cystic
carcinoma presenting with liver metastasis as the initial
symptom. |
Table II.
Literature review of adenoid cystic
carcinoma presenting with liver metastasis as the initial
symptom.
| First author/s,
year | Age, years | Sex | Primary site | Initial
presentation | Stage | Histopathological
characteristics | Imaging features
(liver metastasis) | Management | Follow-up | (Refs.) |
|---|
| Walker et
al, 2025 | 29 | Male | Parotid gland | Right upper
quadrant abdominal pain and early satiety | T4N0M1 | Tubiform and
cribriform | A bulky mass (left
lobe, 14 cm) | TACE, chemotherapy
and radiotherapy | Alive with disease
after 5 months | (13) |
| Brincat and Cassar,
2024 | 50 | Female | Submandibular
gland | Asymptomatic | pT1N0 | Tubiform and
cribriform | Solitary (left
lobe) | Primary excision +
SND (IA and IB) + locoregional radiotherapy; and left lateral liver
sectionectomy (liver segments II/III) | No recurrence after
4.5 years | (14) |
| Li et al,
2021 | 51 | Female | Sublingual
gland | Asymptomatic | NA | Tubiform and
cribriform; MYB-NFIB fusion positive | Solitary (right
lobe) | Partial right
hepatic lobectomy and primary excision | Recurrence
after | (15) |
| Spolverato et
al, 2014 | 59 | Female | Submandibular
gland | Mild, vague
abdominal pain | T3N2bM1 | Solid architecture
with certain areas characterized by a cribriform pattern;
t(6;9)(q22-23; p23-24) | Solitary | Extended left
hepatectomy with concomitant lymphadenectomy; and primary excision
+ ND + adjuvant systemic therapy | No recurrence after
5 months | (16) |
| Garg et al,
2014 | 75 | Male | Submandibular
gland | Pain and a lump in
the right hypochondrium associated with loss of appetite and
weakness | NA | NA | Multiple (both
lobes) | Treatment
declined | NA | (17) |
| Karatzas et
al, 2011 | 51 | Female | Submandibular
gland | Asymptomatic | NA | NA | Multiple (both
lobes) | TACE + atypical
extended right hepatectomy with right liver lobe and segment IVA
liver resection; intraoperative RFA (segment II); and percutaneous
RFA (segment III) | No recurrence after
1 year | (18) |
| Deshpande and
Kelkar, 2009 | 68 | Male | Submandibular
gland | Weight loss, an
upper abdominal lump, and itching all over the body | NA | NA | Multiple (both
lobes) | Chemotherapy
declined | NA | (19) |
| Present study | 69 | Female | Submandibular
gland | Paroxysmal colicky
pain in the mid-abdomen | T3N2bM1 | Cribriform
structures (80%) with tubular (15%) and solid (5%) patterns | Multiple (right
lobe) | Right hepatectomy;
RFA; and primary excision + SND | Alive with disease
after 28 months | - |
Beyond the clinical rarity, the propensity for
submandibular ACC liver metastases may be related to anatomical
location, lymph node metastases and molecular alterations. Patients
with submandibular gland ACC exhibit a notably higher rate of neck
lymph node metastasis and distant metastasis within the first year
of diagnosis, as well as more rapid disease progression, compared
with those with parotid gland ACC (20). Furthermore, lymph node involvement
may increase the risk of distant metastasis. Lymph node metastasis
is infrequent in ACC, with a rate of ~17% in the salivary gland
(21). The patient in the current
report presented with positive lymph node metastasis and was
classified as being at a locally advanced stage; therefore,
selective neck lymph node dissection was performed. Patients with
advanced-stage submandibular gland ACC have a markedly lower
survival rate in comparison with those with ACC located in other
regions (22). Critically,
molecular alterations may also drive metastasis. The patient had
EGFR and VEGF upregulation and a PIK3R1 mutation. PI3K
signaling serves an important role in cancer cell survival,
angiogenesis and metastasis (23).
A previous study reported that inhibitors of EGFR and PI3K/AKT
could suppress the proliferation, migration and neural invasion of
SACC-83 cells (24). Collectively,
the anatomical predisposition, nodal metastasis and activation of
pro-metastatic pathways may have converged to drive the occult
distant metastasis and rapid progression observed in the present
submandibular ACC case.
Upon initial examination, an ultrasound revealed
multiple liver lesions in the patient in the present report. To
further evaluate the local and systemic condition, PET/CT was
performed, revealing a metabolically increased mass in the left
submandibular gland. The diagnosis of multiple liver metastases
originating from left submandibular gland ACC was confirmed through
a combination of clinical examination, imaging studies, biopsy and
postoperative histopathological analysis. The patient tested
negative for MYB gene breakage and rearrangement, and the
absence of the ACC-specific molecular marker,
MYB-NFIB, removed a key molecular diagnostic
clue.
The patient was diagnosed with both submandibular
gland ACC and liver metastases, necessitating distinct therapeutic
approaches for the primary site and the liver metastases. Whilst no
specific treatment consensus exists for head and neck ACC with
multiple liver metastases, hepatectomy for liver metastases
originating from other malignancies has been recognized as an
effective treatment modality (25).
Given the substantial size of the liver tumor, which had already
invaded the right lobe, continued tumor growth could have resulted
in liver failure. The patient had a favorable evaluation of liver
reserve, with a remnant liver volume to standard liver volume ratio
of ~57%, indicating potential surgical tolerance. Consequently, a
right hepatectomy was performed to address the liver metastasis
prior to excising the primary tumor. The primary tumor was
subsequently treated in accordance with American Society of
Clinical Oncology guidelines, which recommend surgery combined with
postoperative radiotherapy as the optimal strategy for localized
ACC (26). Research suggests that
patients with advanced submandibular gland ACC derive the most
benefit from adjuvant radiotherapy (22). The surgical and postoperative
radiotherapy regimen was adopted; however, the patient discontinued
standard postoperative radiotherapy due to perceived pain during
treatment, which may have contributed to local recurrence 21 months
post-surgery.
Particle radiotherapy, chemotherapy, targeted
therapy and immunotherapy may offer survival benefits and enhance
the quality of life for patients with ACC experiencing local
recurrence and refractory distant metastasis (7). The PD-L1 immunohistochemical result of
the patient in the present case was negative, indicating that the
patient was not eligible for treatment with immune checkpoint
inhibitors as part of a novel immunotherapy approach. However, the
patient tested positive for EGFR and VEGF, permitting consideration
of targeted therapies such as cetuximab, lapatinib and pazopanib.
An earlier study involving patients with ACC indicated that
cetuximab treatment in a cohort of 23 individuals did not yield a
meaningful response; however, 12 patients experienced disease
stabilization for >6 months (27). Another study involving 19 patients
with advanced ACC reported no response to lapatinib; however, 15
patients experienced disease stabilization for ≥6 months (28). Furthermore, in a clinical trial
involving 46 patients treated with pazopanib, only one case
demonstrated a partial response, with a median progression-free
survival of 5.9 months and OS of 16.6 months (29). Genetic testing of the patient in the
present case indicated that the mutations clustered in genes
associated with cell growth and developmental disorders (such as
CDKN1C, BCOR, PIK3R1 and SOX10). Among the mutated
genes, PIK3R1 was the only gene associated with potential
clinical targeted therapies (30),
although its clinical efficacy in patients with ACC remains
unclear.
In conclusion, to the best of our knowledge, the
present case represents the sixth report in the literature in which
submandibular gland ACC initially manifests with atypical liver
metastasis as the first symptom, without involvement of other
common metastatic sites. The present case demonstrates a rare,
high-risk variant of rapidly progressing ACC, with liver metastasis
serving as the initial presenting symptom. Immunohistochemistry and
genetic testing may facilitate the identification of novel
therapeutic strategies, including immunotherapy and targeted
therapy, beyond traditional chemotherapy and radiotherapy.
Moreover, a multidisciplinary approach is essential for the
effective management of complex metastatic ACC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82173312 and 82372967) and
Capital's Funds for Health Improvement and Research (grant no.
2022-2-2057).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TL was a major contributor to the conception of the
study, as well as to the literature search for related studies. TM
and XLW were involved in the literature review, study design and
obtaining medical images. YZ, MW, XZ and XDW were involved in the
processing of the figures and analyzing patient data. SZ, GY, HL
and JW participated in the clinical discussion and patient
management. XLZ and XC contributed to the acquisition and
interpretation of data. XLZ and XC confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki, and approved by the Ethics Committee
of Beijing Tongren Hospital, Capital Medical University (approval
no. TREC2022-KY023).
Patient consent for publication
Written informed consent was obtained from the
participant for the publication of the details of the medical case
and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spiro RH, Huvos AG and Strong EW: Adenoid
cystic carcinoma of salivary origin. A clinicopathologic study of
242 cases. Am J Surg. 128:512–520. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ciccolallo L, Licitra L, Cantú G and Gatta
G; EUROCARE Working Group, : Survival from salivary glands adenoid
cystic carcinoma in European populations. Oral Oncol. 45:669–674.
2009. View Article : Google Scholar
|
|
3
|
Bjørndal K, Krogdahl A, Therkildsen MH,
Overgaard J, Johansen J, Kristensen CA, Homøe P, Sørensen CH,
Andersen E, Bundgaard T, et al: Salivary gland carcinoma in Denmark
1990–2005: A national study of incidence, site and histology.
Results of the Danish head and neck cancer group (DAHANCA). Oral
Oncol. 47:677–682. 2011. View Article : Google Scholar
|
|
4
|
Atallah S, Casiraghi O, Fakhry N, Wassef
M, Uro-Coste E, Espitalier F, Sudaka A, Kaminsky MC, Dakpe S, Digue
L, et al: A prospective multicentre REFCOR study of 470 cases of
head and neck adenoid cystic carcinoma: Epidemiology and prognostic
factors. Eur J Cancer. 130:241–249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coca-Pelaz A, Rodrigo JP, Bradley PJ,
Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A,
Haigentz M Jr, Takes RP, et al: Adenoid cystic carcinoma of the
head and neck-An update. Oral Oncol. 51:652–661. 2015. View Article : Google Scholar
|
|
6
|
Ouyang DQ, Liang LZ, Zheng GS, Ke ZF, Weng
DS, Yang WF, Su YX and Liao GQ: Risk factors and prognosis for
salivary gland adenoid cystic carcinoma in southern China: A
25-year retrospective study. Medicine (Baltimore). 96:e59642017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nightingale J, Lum B, Ladwa R, Simpson F
and Panizza B: Adenoid cystic carcinoma: A review of clinical
features, treatment targets and advances in improving the immune
response to monoclonal antibody therapy. Biochim Biophys Acta Rev
Cancer. 1875:1885232021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Weert S, Bloemena E, van der Waal I,
de Bree R, Rietveld DH, Kuik JD and Leemans CR: Adenoid cystic
carcinoma of the head and neck: A single-center analysis of 105
consecutive cases over a 30-year period. Oral Oncol. 49:824–829.
2013. View Article : Google Scholar
|
|
9
|
Dalal P, Dermody S, Brummel C, Brenner C,
Casper K, Chinn SB, Malloy KM, Mierzwa ML, Neal MH, Prince MEP, et
al: Clinical outcomes in salivary adenoid cystic carcinoma. J Clin
Oncol. 42 (Suppl 16):S6102. 2024. View Article : Google Scholar
|
|
10
|
Gao M, Hao Y, Huang MX, Ma DQ, Luo HY, Gao
Y, Peng X and Yu GY: Clinicopathological study of distant
metastases of salivary adenoid cystic carcinoma. Int J Oral
Maxillofac Surg. 42:923–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang S, Patel PN, Kimple RJ and McCulloch
TM: Clinical outcomes and prognostic factors of adenoid cystic
carcinoma of the head and neck. Anticancer Res. 37:3045–3052.
2017.PubMed/NCBI
|
|
12
|
Fang Y, Peng Z, Wang Y, Gao K, Liu Y, Fan
R, Zhang H, Xie Z and Jiang W: Current opinions on diagnosis and
treatment of adenoid cystic carcinoma. Oral Oncol. 130:1059452022.
View Article : Google Scholar
|
|
13
|
Walker M, Dewald A, Refaey A, Berezowski
I, Newman J, Younes M and Gray S: Salivary gland adenoid cystic
carcinoma presenting as a large metastatic hepatic mass: A case
report. J Med Case Rep. 19:1262025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brincat SD and Cassar N: Solitary liver
metastasis from adenoid cystic carcinoma of the submandibular
gland. BMJ Case Rep. 17:e2589232024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li XH, Zhang YT and Feng H: Liver
metastasis as the initial clinical manifestation of sublingual
gland adenoid cystic carcinoma: A case report. World J Clin Cases.
9:5238–5244. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spolverato G, Fite J, Bishop J, Argani P
and Pawlik TM: Liver metastasis as the initial presentation of
adenoid cystic carcinoma. Dig Dis Sci. 59:2004–2006. 2014.
View Article : Google Scholar
|
|
17
|
Garg N, Tomar R, Goyal S and Singh UR:
Isolated liver metastases in an adenoid cystic carcinoma of the
submandibular gland on fine needle aspiration cytology: An unusual
presentation. Cytopathology. 25:137–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karatzas A, Katsanos K, Maroulis I,
Kalogeropoulou C, Tzorakoleftherakis E and Karnabatidis D:
Multi-modality curative treatment of salivary gland cancer liver
metastases with drug-eluting bead chemoembolization, radiofrequency
ablation, and surgical resection: A case report. J Med Case Rep.
5:4162011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deshpande AH and Kelkar AA: Hepatic
metastasis as an initial manifestation of salivary adenoid cystic
carcinoma: Cytologic diagnosis. Diagn Cytopathol. 37:45–47. 2009.
View Article : Google Scholar
|
|
20
|
Zhou M, Ma T, Wang X, Zhang S, Yang G,
Song R and Chen X: High-risk subtype: Clinical manifestations and
molecular characteristics of submandibular gland adenoid cystic
carcinoma. Front Oncol. 12:10211692022. View Article : Google Scholar
|
|
21
|
Megwalu UC and Sirjani D: Risk of nodal
metastasis in major salivary gland adenoid cystic carcinoma.
Otolaryngol Head Neck Surg. 156:660–664. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tasoulas J, Divaris K, Theocharis S,
Farquhar D, Shen C, Hackman T and Amelio AL: Impact of tumor site
and adjuvant radiotherapy on survival of patients with adenoid
cystic carcinoma: A SEER database analysis. Cancers (Basel).
13:5892021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mishra R, Patel H, Alanazi S, Kilroy MK
and Garrett JT: PI3K inhibitors in cancer: Clinical implications
and adverse effects. Int J Mol Sci. 22:34642021. View Article : Google Scholar
|
|
24
|
Ren Y, Hong Y, He W, Liu Y, Chen W, Wen S
and Sun M: EGF/EGFR promotes salivary adenoid cystic carcinoma cell
malignant neural invasion via activation of PI3K/AKT and MEK/ERK
signaling. Curr Cancer Drug Targets. 22:603–616. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Zhang Y, Zhang Y and Li B: A
bibliometric analysis of gastric cancer liver metastases: Advances
in mechanisms of occurrence and treatment options. Int J Surg.
110:2288–2299. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geiger JL, Ismaila N, Beadle B, Caudell
JJ, Chau N, Deschler D, Glastonbury C, Kaufman M, Lamarre E, Lau
HY, et al: Management of salivary gland malignancy: ASCO guideline.
J Clin Oncol. 39:1909–1941. 2021. View Article : Google Scholar
|
|
27
|
Locati LD, Bossi P, Perrone F, Potepan P,
Crippa F, Mariani L, Casieri P, Orsenigo M, Losa M, Bergamini C, et
al: Cetuximab in recurrent and/or metastatic salivary gland
carcinomas: A phase II study. Oral Oncol. 45:574–578. 2009.
View Article : Google Scholar
|
|
28
|
Agulnik M, Cohen EWE, Cohen RB, Chen EX,
Vokes EE, Hotte SJ, Winquist E, Laurie S, Hayes DN, Dancey JE, et
al: Phase II study of lapatinib in recurrent or metastatic
epidermal growth factor receptor and/or erbB2 expressing adenoid
cystic carcinoma and non-adenoid cystic carcinoma malignant tumors
of the salivary glands. J Clin Oncol. 25:3978–3984. 2007.
View Article : Google Scholar
|
|
29
|
Guigay J, Fayette J, Even C, Cupissol D,
Rolland F, Peyrade F, Laguerre B, Le Tourneau C, Zanetta S, Le Moal
LB, et al: PACSA: Phase II study of pazopanib in patients with
progressive recurrent or metastatic (R/M) salivary gland carcinoma
(SGC). J Clin Oncol. 34 (Suppl 15):S60862016. View Article : Google Scholar
|
|
30
|
Cheung LWT, Yu S, Zhang D, Li J, Ng PK,
Panupinthu N, Mitra S, Ju Z, Yu Q, Liang H, et al: Naturally
occurring neomorphic PIK3R1 mutations activate the MAPK pathway,
dictating therapeutic response to MAPK pathway inhibitors. Cancer
Cell. 26:479–494. 2014. View Article : Google Scholar
|