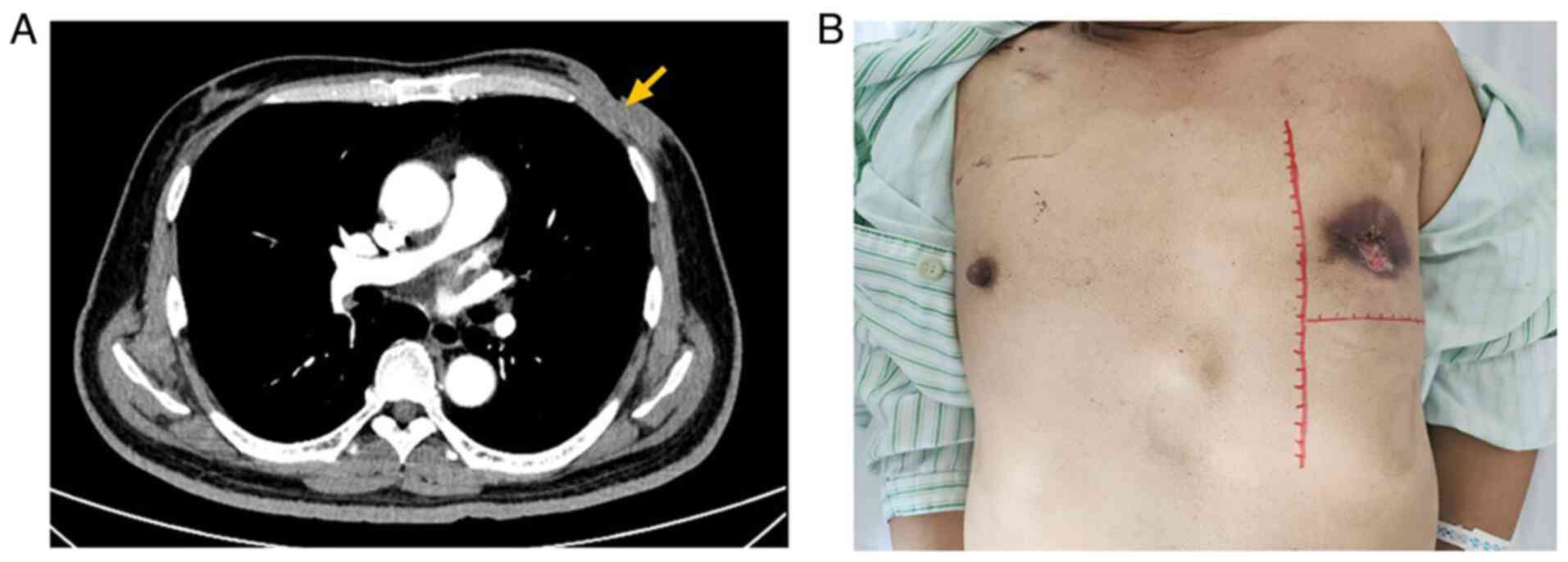
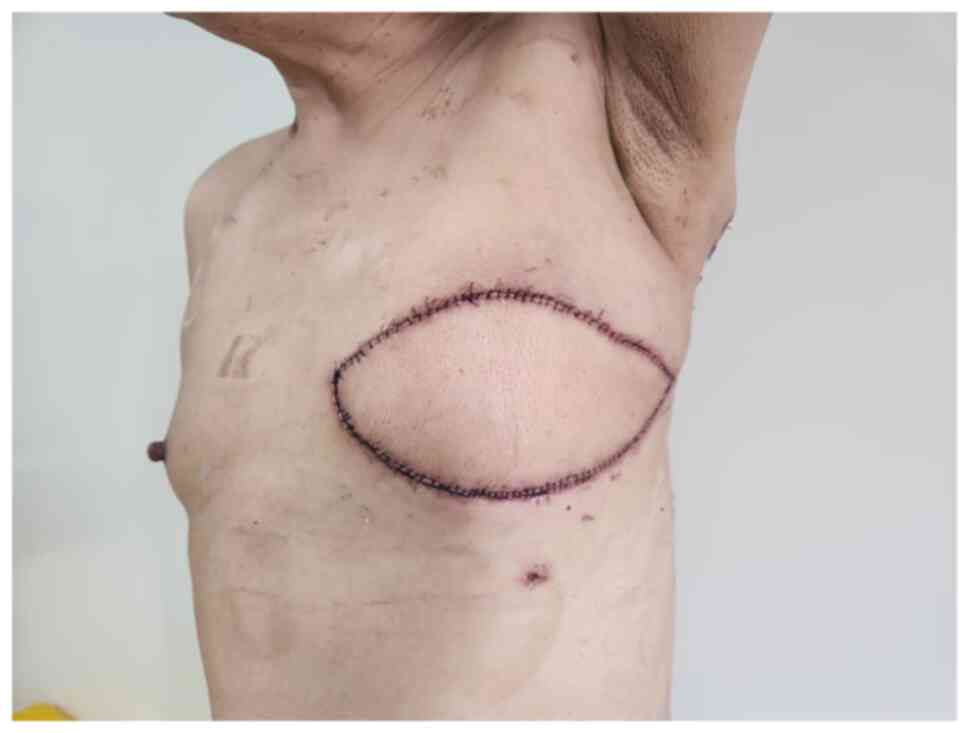
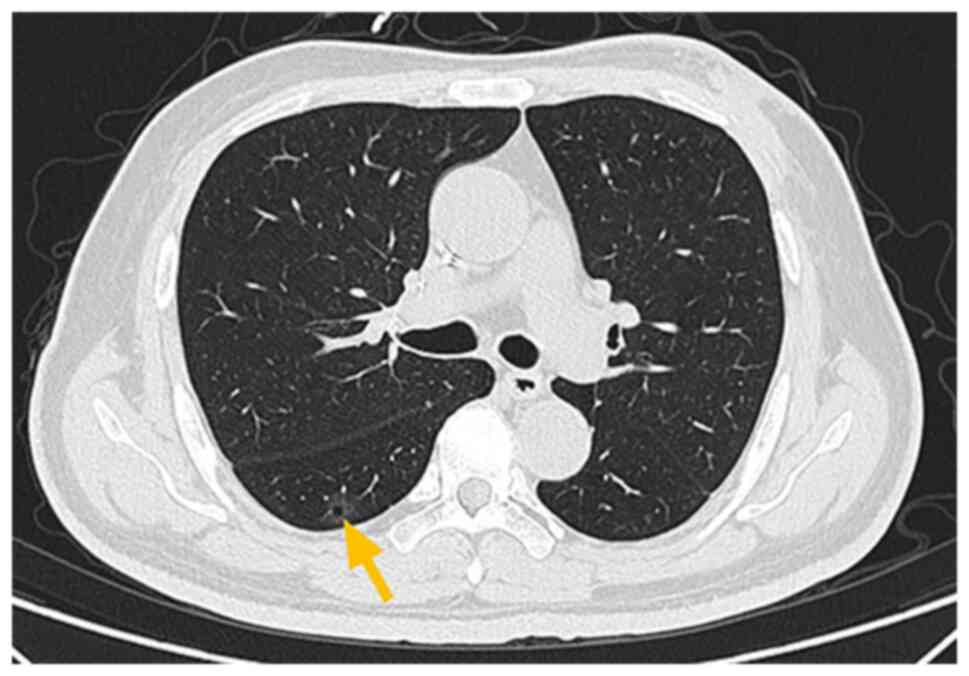
|
1
|
Gómez-Raposo C, Zambrana Tévar F, Sereno
Moyano M, López Gómez M and Casado A: Male breast cancer. Cancer
Treat Rev. 36:451–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li SY and Liu XF: A case report of Luminal
B type male breast cancer and literature review. Chin J Curr Adv
Gen Surg. 27:325–329. 2024.(In Chinese).
|
|
3
|
Choi N, Moon S, Kim JS, Kim AY, Ahn JH,
Han Y, Woo J, Kim H, Chung MS and Cha CD: Long-term survival
outcomes of male breast cancer: The propensity score matching
analysis of nationwide registry database. Breast. 83:1045562025.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ellington TD, Henley SJ, Wilson RJ and
Miller JW: Breast cancer survival among males by race, ethnicity,
age, geographic region, and Stage-United States, 2007–2016. MMWR
Morb Mortal Wkly Rep. 69:1481–1484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chatterji S, Krzoska E, Thoroughgood CW,
Saganty J, Liu P, Elsberger B, Abu-Eid R and Speirs V: Defining
genomic, transcriptomic, proteomic, epigenetic, and phenotypic
biomarkers with prognostic capability in male breast cancer: A
systematic review. Lancet Oncol. 24:e74–e85. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maguire S, Perraki E, Tomczyk K, Jones ME,
Fletcher O, Pugh M, Winter T, Thompson K, Cooke R; kConFab
Consortium, ; et al: common susceptibility loci for male breast
cancer. J Natl Cancer Inst. 113:453–461. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao MM, Joe BN, Sickles EA and Lee CS:
BI-RADS category 5 assessments at diagnostic breast
imaging:outcomes analysis based on lesion descriptors. Acad Radiol.
26:1048–1052. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mei F, Liu JY and Xue WC: Histological
grading of invasive breast carcinoma: Nottingham histological
grading system. Zhonghua Bing Li Xue Za Zhi. 48:659–664. 2019.(In
Chinese). PubMed/NCBI
|
|
9
|
Genetic Tumor Markers Collaboration Group,
Tumor Biomarker Committee, China Anti-cancer Association, Molecular
Pathology Collaboration Group, Tumor Pathology Committee, China
Anti-Cancer Association, . Chinese expert consensus on tumor
mutational burden testing and clinical application (2020 edition).
Chin J Oncol Prev Treat. 12:485–493. 2020.(In Chinese).
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen D, Xiao Y and Zhong K: Risk factors
and pathogenic mechanism for secondary primary lung cancer in
breast cancer patients: A review. Zhongguo Fei Ai Za Zhi.
25:750–755. 2022.(In Chinese). PubMed/NCBI
|
|
12
|
Hassett MJ, Somerfield MR, Baker ER,
Cardoso F, Kansal KJ, Kwait DC, Plichta JK, Ricker C, Roshal A,
Ruddy KJ, et al: Management of male breast cancer: ASCO guideline.
J Clin Oncol. 38:1849–1863. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qavi Q, Alkistawi F, Kumar S, Ahmed R and
Saad Abdalla Al-Zawi A: Male triple-negative breast cancer. Cureus.
13:e145422021.PubMed/NCBI
|
|
14
|
Pang L, Cao C, Hua Y, Zeng T, Yang F, Sun
C, Huang X and Li W: A case report of breast and lung dual primary
cancers. J Nanjing Med Univ (Nat Sci). 39:1696–1698. 2019.(In
Chinese).
|
|
15
|
Giordano SH, Buzdar AU and Hortobagyi GN:
Breast cancer in men. Ann Intern Med. 137:678–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goss PE, Reid C, Pintilie M, Lim R and
Miller N: Male breast carcinoma: A review of 229 patients who
presented to the Princess Margaret Hospital during 40 years:
1955–1996. Cancer. 85:629–636. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mangone L, Ferrari F, Mancuso P, Carrozzi
G, Michiara M, Falcini F, Piffer S, Filiberti RA, Caldarella A,
Vitale F, et al: Epidemiology and biological characteristics of
male breast cancer in Italy. Breast Cancer. 27:724–731. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi Q, Xuhong J, Tian H, Qu M, Zhang Y,
Jiang J and Qi X: Predictive and prognostic value of PIK3CA
mutations in HER2-positive breast cancer treated with tyrosine
kinase inhibitors: A systematic review and meta-analysis. Biochim
Biophys Acta Rev Cancer. 1878:1888472023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye Z and Liang M: The dilemma of breast
cancer in males and research progress. Chin Gen Pract.
22:3260–3264. 2019.(In Chinese).
|
|
20
|
Gao Y, Zhang M, Sun G, Ma L, Nie J, Yuan
Z, Liu Z, Cao Y, Li J, Liu Q, et al: The features of male breast
cancer in China: A real-world study. Breast. 76:1037622024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng Y, Wang H, Xie H, Ren W, Feng Z, Li M
and Peng Z: Surgical treatment and prognosis for patients with
synchronous multiple primary lung adenocarcinomas. Zhongguo Fei Ai
Za Zhi. 20:107–113. 2017.(In Chinese). PubMed/NCBI
|
|
22
|
Hu Z, Zou X, Qin S, Li Y, Wang H, Yu H,
Sun S, Wu X, Wang J and Chang J: Hormone receptor expression
correlates with EGFR gene mutation in lung cancer in patients with
simultaneous primary breast cancer. Transl Lung Cancer Res.
9:325–336. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Zeng Z, Zou Y, Pan X and Ouyang C:
A case report of dual primary cancers: Bilateral breast and lung
cancer. J Mod Med Health. 38:3954–3958. 2022.(In Chinese).
|
|
24
|
Hao Y, Zhang X, Cui G, Qi X, Jiang Z and
Yu L: Clinicopathological features, prognostic factor analysis, and
survival nomogram of patients with double primary cancers involving
lung cancer. Cancer Med. 13:e72962024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye B and Zhao H: Revision of the TNM stage
grouping in the forthcoming eighth edition of the TNM
classification for lung cancer. Zhongguo Fei Ai Za Zhi. 19:337–342.
2016.(In Chinese). PubMed/NCBI
|
|
26
|
Cui JL and Wang Q: Research progress on
EpCAM in lung cancer, breast cancer, and digestive system tumors. J
Precis Med. 39:367–370. 2024.(In Chinese).
|
|
27
|
Pak MG, Shin DH, Lee CH and Lee MK:
Significance of EpCAM and TROP2 expression in non-small cell lung
cancer. World J Surg Oncol. 10:532012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lou Y, Jiang Y, Liang Z, Liu B, Li T and
Zhang D: Role of RhoC in cancer cell migration. Cancer Cell Int.
21:5272021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Castellano E, Molina-Arcas M, Krygowska
AA, East P, Warne P, Nicol A and Downward J: RAS signalling through
PI3-Kinase controls cell migration via modulation of Reelin
expression. Nat Commun. 7:112452016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borgoño CA, Fracchioli S, Yousef GM,
Rigault de la Longrais IA, Luo LY, Soosaipillai A, Puopolo M, Grass
L, Scorilas A, Diamandis EP and Katsaros D: Favorable prognostic
value of tissue human kallikrein 11 (hK11) in patients with ovarian
carcinoma. Int J Cancer. 106:605–610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jaffe AB and Hall A: Rho GTPases:
Biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rathinam R, Berrier A and Alahari SK: Role
of Rho GTPases and their regulators in cancer progression. Front
Biosci (Landmark Ed). 16:2561–2571. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hao Y, Jiang H, Thapa P, Ding N,
Alshahrani A, Fujii J, Toledano MB and Wei Q: Critical role of the
sulfiredoxin-peroxiredoxin IV axis in urethane-induced non-small
cell lung cancer. Antioxidants (Basel). 12:3672023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Song W, Wang H, Zhu G, Li Y, Wang
Z, Li W and Che G: Increased risk of subsequent primary lung cancer
among female hormone-related cancer patients: A meta-analysis based
on over four million cases. Chin Med J (Engl). 137:1790–1801. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qian DC, Byun J, Han Y, Greene CS, Field
JK, Hung RJ, Brhane Y, Mclaughlin JR, Fehringer G, Landi MT, et al:
Identification of shared and unique susceptibility pathways among
cancers of the lung, breast, and prostate from genome-wide
association studies and tissue-specific protein interactions. Hum
Mol Genet. 24:7406–7420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Loboda A, Nebozhyn M, Klinghoffer R,
Frazier J, Chastain M, Arthur W, Roberts B, Zhang T, Chenard M,
Hager J, et al: A gene expression signature of RAS pathway
dependence predicts response to PI3K and RAS pathway inhibitors and
expands the population of RAS pathway activated tumors. BMC Med
Genomics. 3:262010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dey N, De P and Leyland-Jones B:
PI3K-AKT-mTOR inhibitors in breast cancers: From tumor cell
signaling to clinical trials. Pharmacol Ther. 175:91–106. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tennis M, Singh B, Hjerpe A, Prochazka M,
Czene K, Hall P and Shields PG: Pathological confirmation of
primary lung cancer following breast cancer. Lung Cancer. 69:40–45.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giunta G, Rossi M, Toia F, Rinaldi G and
Cordova A: Male breast cancer: Modified radical mastectomy or
breast conservation surgery? A case report and review of the
literature. Int J Surg Case Rep. 30:89–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng RQ, Li DH, Liu XK, Zhao XH, Wen QE
and Yang Y: Traditional Chinese medicine for breast cancer: A
review. Breast Cancer (Dove Med Press). 15:747–759. 2023.PubMed/NCBI
|
|
41
|
Cui Y, Mi J, Feng Y, Li L, Wang Y, Hu J
and Wang H: Huangqi Sijunzi decoction for treating cancer-related
fatigue in breast cancer patients: a randomized trial and network
pharmacology study. Nan Fang Yi Ke Da Xue Xue Bao. 42:649–657.
2022.(In Chinese). PubMed/NCBI
|
|
42
|
Jiang H, Li M, Du K, Ma C, Cheng Y, Wang
S, Nie X, Fu C and He Y: Traditional Chinese medicine for adjuvant
treatment of breast cancer: Taohong Siwu decoction. Chin Med.
16:1292021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|