Introduction
Male breast cancer (MBC) is a rare malignancy,
representing ~1% of all breast cancer cases worldwide and <1% of
all male cancer cases (1). Due to
the limited patient population, clinical randomized controlled
trials on MBC are scarce, with most studies being retrospective and
treatments for MBC are frequently based on protocols designed for
female MBC (2). Male breast cancer
differs significantly from female breast cancer (FBC) in several
aspects, primarily including differences in age at onset and
diagnostic stage, as well as differences in survival rate and
mortality rate (3). Males are
usually diagnosed with breast cancer at an advanced age, and the
disease is often at a later stage at the time of diagnosis. Their
5-year relative survival rate is 98.7% for localized disease, while
it is only 25.9% for distant metastatic disease. In contrast, FBC
has a higher rate of early diagnosis (4). Although there is no significant
difference in 10-year breast cancer-specific survival between males
and females, the overall survival of males is significantly lower
than that of females (68.0 vs. 79.0%) (3). Despite phenotypic similarities, there
are molecular-level differences between MBC and FBC (5). MBC is more frequently hormone receptor
(HR)-positive and it has a stronger association with genetic
susceptibility genes (e.g., BRCA2) (6). The current study presents a case of
advanced male invasive breast cancer and lung cancer of dual
primary origins, which was successfully treated using an integrated
approach combining traditional Chinese and Western medicine.
Case report
Presentation
A 59-year-old male patient with no family history of
cancer presented to Guang'anmen Hospital (Beijing, China) in June
2023 with a left breast mass. The mass had been present for 3 years
but had not received proper attention, and the patient had not
undergone any treatment for it prior to this presentation. Over the
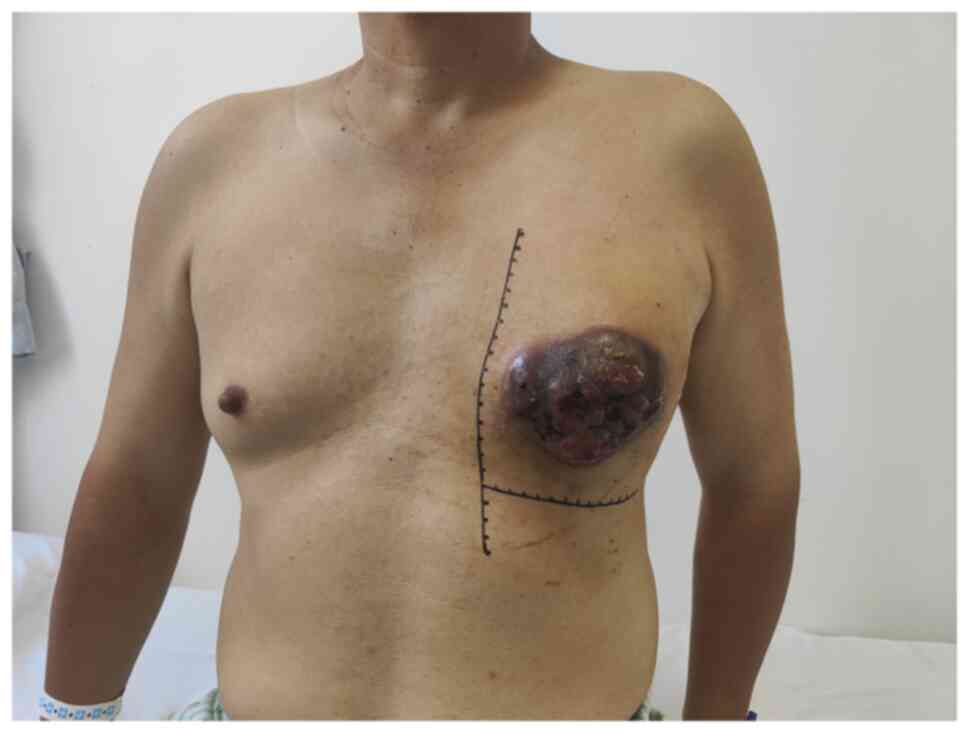
past 2 months, the mass had progressively enlarged and ruptured.
Initially, the 4-cm soft mass was mobile with indistinct borders,
causing no pain, itching or nipple retraction. Over time, the mass
hardened and eventually ulcerated, forming a cauliflower-like
lesion with yellowish-brown purulent discharge (Fig. 1).
Clinical examination
Asymmetry of the breasts was observed. The left
breast displayed a 12×10-cm ulcerated mass with a hard texture,
poor mobility and indistinct margins. No normal nipple or areolar
structure was visible, and enlarged lymph nodes were palpable in
the left axilla. The right breast showed no abnormalities.
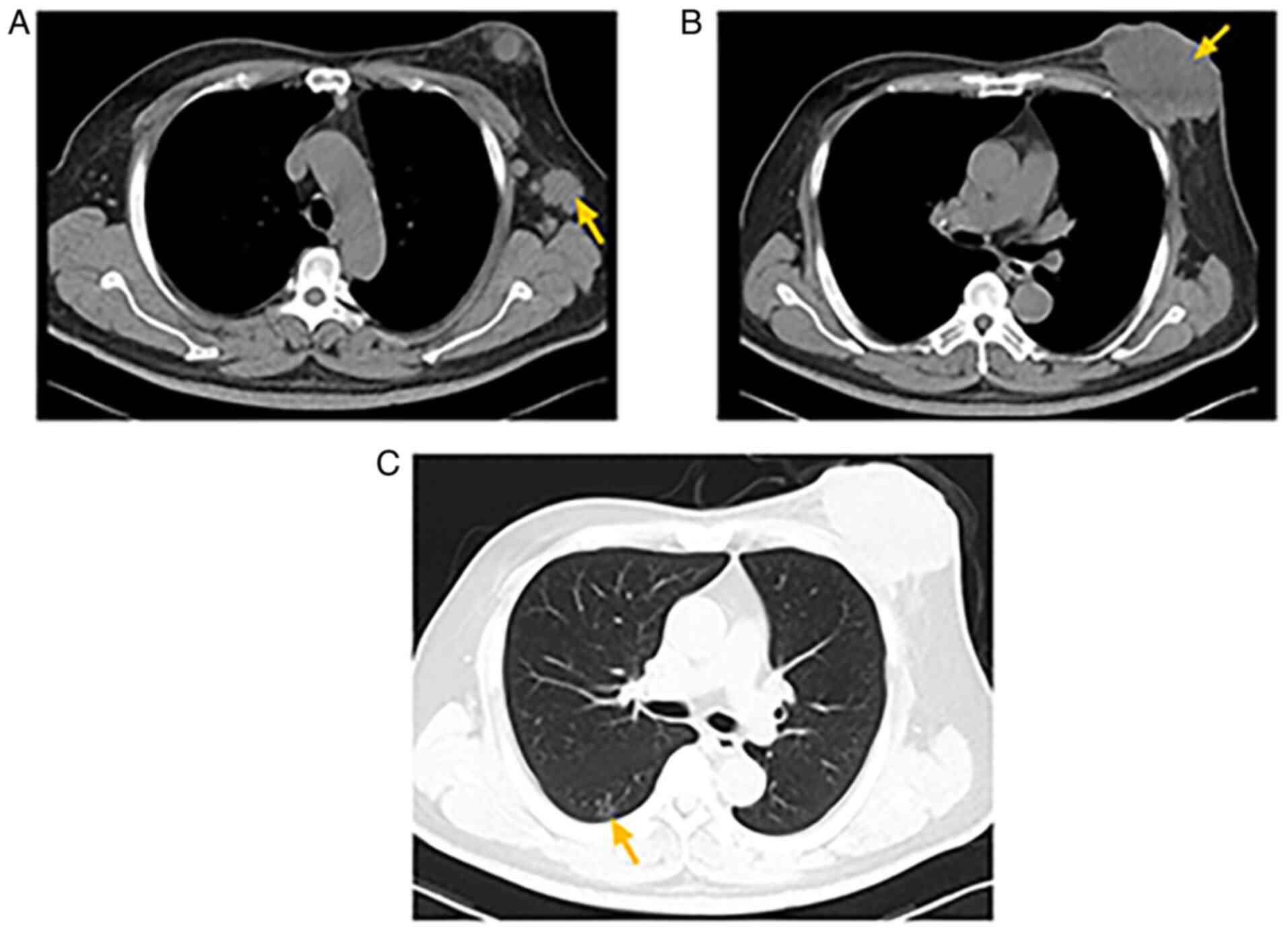
Auxiliary examinations included computed tomography (CT), which
showed a 62×93-mm soft-tissue mass invading the left chest wall,
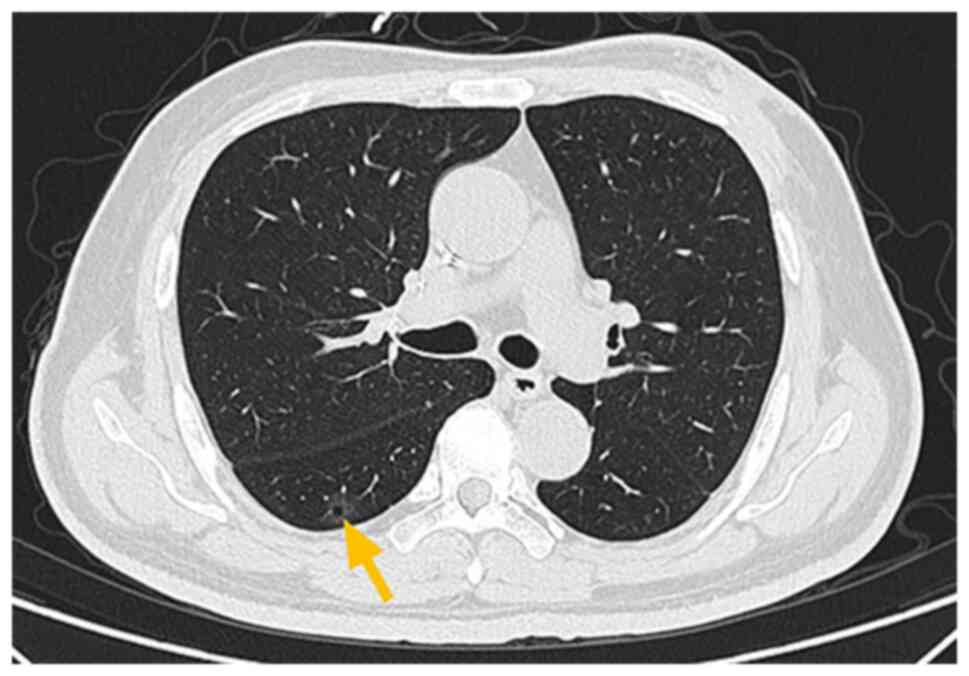
along with axillary lymphadenopathy (Fig. 2A and B). Ground-glass opacity and
calcified nodules were detected in the right lower lobe of the
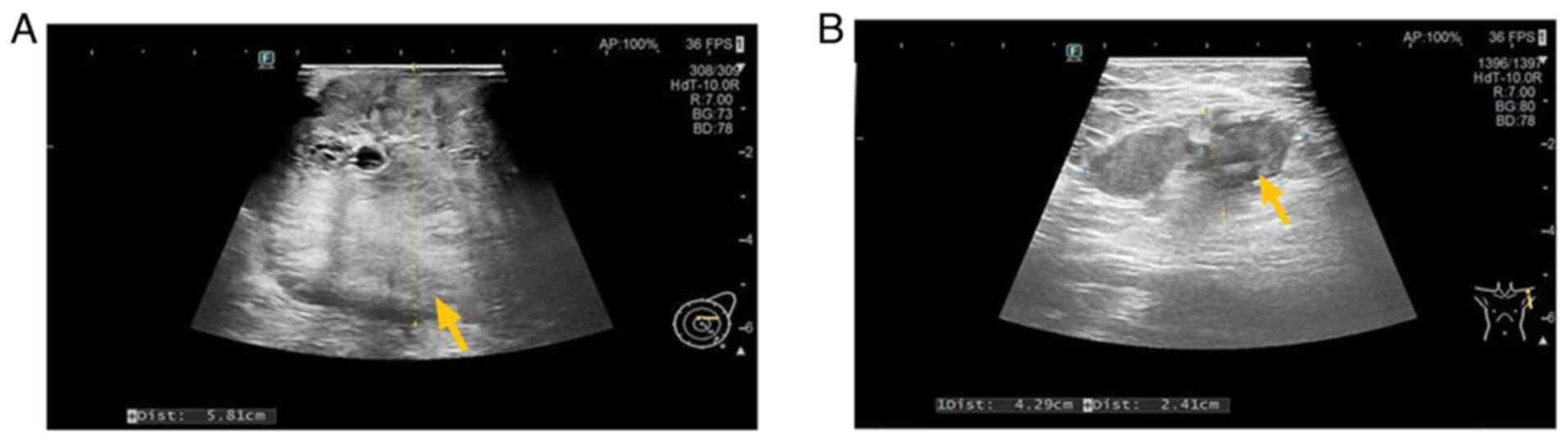
lung, the largest measuring 14×4 mm (Fig. 2C). Ultrasound revealed a
10.0×5.8×12.0-cm hypoechoic mass (Breast Imaging-Reporting and Data
System category 5) (7) in the left
breast and enlarged axillary lymph nodes (Fig. 3). Biopsy confirmed invasive ductal
carcinoma with Paget's-like spread of the left breast, grade III
(3+3+2=8). The scoring system used was the Nottingham histological
grading system (8).
Immunohistochemistry (IHC) was performed on paraffin-embedded
tissues. The tissue was fixed with 4% formaldehyde at 20°C for 12
h, embedded in paraffin and sectioned at a thickness of 4 µm. For
the staining procedure, hydrogen peroxide was used as the blocking
reagent and blocking was conducted at 37°C for 4 min. Primary and
secondary antibodies were used as working solutions (no dilution
required). All antibodies were supplied by Leica Biosystems
Newcastle Ltd. The catalogue numbers of the primary antibodies were
SN 136374, SN 129975 and SN 384122; the catalogue number of the
secondary antibody was DS9800. The results were as follows:
Estrogen receptor (ER) (80%, strong +), progesterone receptor (PR)
(5%, moderate +), human epidermal growth factor receptor-2 (HER-2)
(3+), Ki-67 (60%) and androgen receptor (AR) (80%, 2+) (Fig. 4A-E). Images were captured using a
Nikon ECLIPSE Ni-U light microscope at ×400 magnification. Genetic
testing indicated a PIK3CA gene mutation. Among them, Fig. 4A and B show hematoxylin-eosin
(H&E) staining. The relevant methodological details are as
follows: The tissue was fixed with 4% formaldehyde at 20°C for 12
h, processed through standard procedures including paraffinization
and deparaffinization, sectioned at a thickness of 4 µm and stained
with hematoxylin for 5 min and eosin for 1 min at room temperature.
Genetic testing, performed by an external institution (Beijing
GeneX Health Medical Laboratory Co., Ltd.) using targeted region
capture combined with next-generation sequencing technology,
indicated a PIK3CA gene mutation. The protocol followed targeted
capture of exonic regions and partial intronic regions of 794
genes, with sequencing conducted on an Illumina platform. The assay
was designed to detect single nucleotide variations, small
insertions/deletions (indels), copy number variations and partial
gene fusions. Quality control parameters included DNA extraction
yield (tissue ≥30 ng), average sequencing depth (tissue ≥1,000X),
sequence alignment rate (≥95%) and base quality Q30 ratio (≥80%),
as specified in the ‘Sample Quality Control’ section of the test
report. The reference genome used was GRCh37/hg19. This protocol
aligns with standard clinical practices for targeted panel
sequencing in oncology, as described in guidelines such as the
Chinese Expert Consensus on Tumor Mutation Burden Detection and
Clinical Application (2020 Edition) (9) and technical specifications for
clinical next-generation sequencing issued by regulatory
authorities.
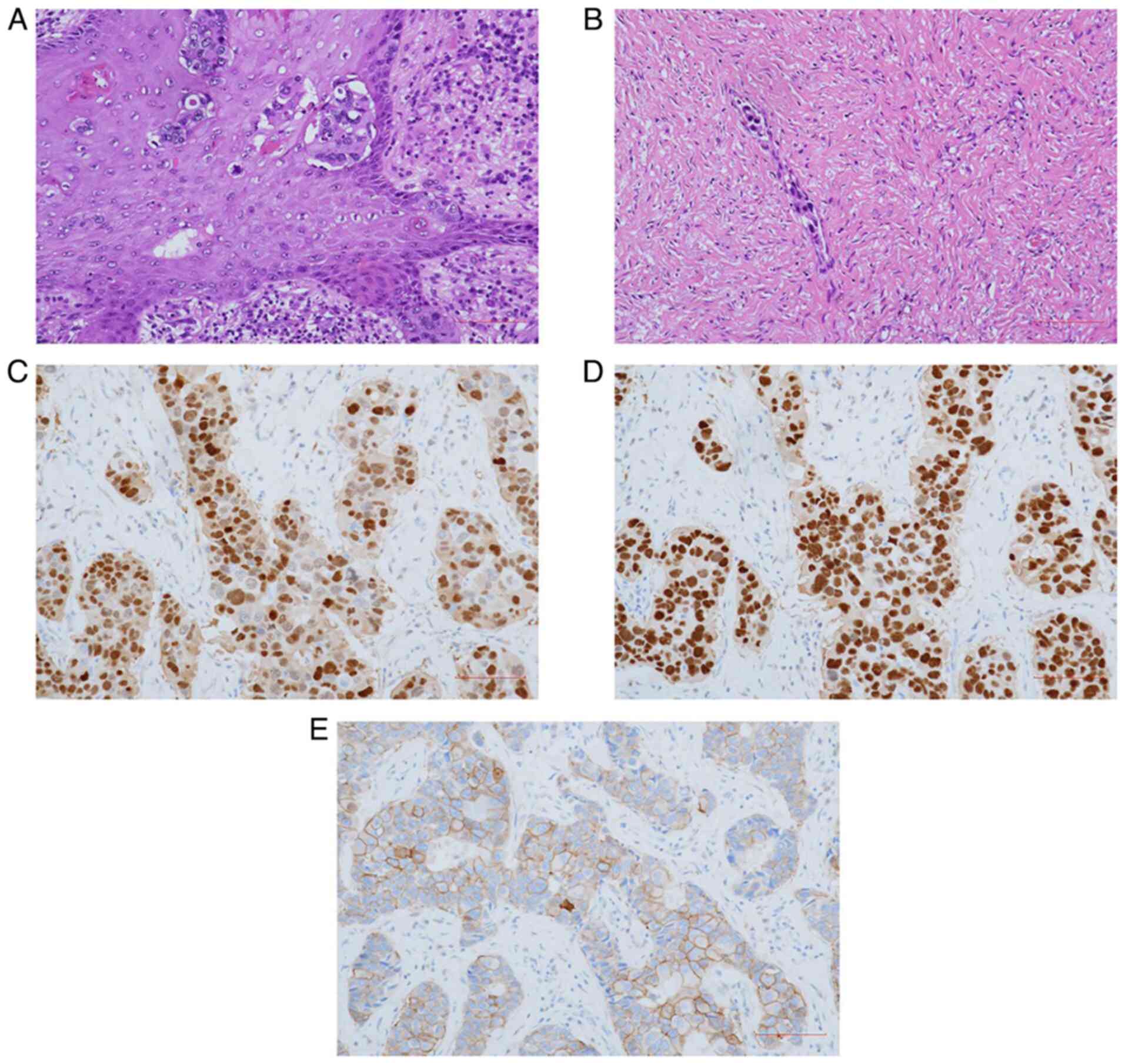 | Figure 4.Pathological sections diagnosing
invasive ductal carcinoma with labeled expression of specific
receptors. (A) Hematoxylin and eosin staining of the left breast
tissue showing invasive ductal carcinoma with Paget's-like spread
(magnification, ×400; scale bar, 100 µm). (B) Hematoxylin and eosin
staining of the intravascular tumor thrombus (magnification, ×400;
scale bar, 100 µm). (C) EnVision staining of progesterone receptor
(magnification, ×400; scale bar, 100 µm). (D) EnVision staining of
estrogen receptor (magnification, ×400; scale bar, 100 µm). (E)
EnVision staining of HER-2 (magnification, ×400; scale bar, 100
µm). |
Treatment plan
Due to the ulceration and significant impact on the
patient's quality of life, neoadjuvant chemotherapy for breast
cancer was initiated, consisting of four cycles of a paclitaxel,
carboplatin, trastuzumab and pertuzumab (TCbHP) regimen. The
specific dosage of each drug in the regimen is as follows:
Albumin-bound paclitaxel 0.5 g, carboplatin 70 ml, trastuzumab 440
mg and pertuzumab 420 mg. All drugs are administered via
intravenous infusion, with one chemotherapy cycle lasting 21 days.
After the first cycle, the left ventricular ejection fraction
dropped by over 30%, necessitating a switch to TCbH (no pertuzumab)
for the subsequent 4 cycles. During chemotherapy, the patient
received both oral and topical traditional Chinese medicine (TCM).
The oral formulation included Panax ginseng (15 g), Panax
notoginseng (6 g), Curcuma zedoaria (10 g), Pinellia
ternata (9 g), Rhodiola rosea (15 g) and honey-fried
Glycyrrhiza uralensis (10 g) ×21 doses (1 dose administered
twice daily). After the first cycle of chemotherapy, the patient's
WBC count was 3.21×109/l (reference range,
3.5–9.5×109/l). After 20 days of adjunctive herbal
treatment, prior to the second cycle, the WBC count increased to
4.62×109/l, indicating hematological improvement. In
terms of tumor markers, the α-fetoprotein level decreased from 11.6
to 2.99 IU/ml (normal range, 0–5.8 IU/ml), the carbohydrate antigen
(CA)125 level declined from 44.2 to 8.31 U/ml (normal range, 0–35
U/ml) and the CA724 level decreased from 68 to 13.9 U/ml (normal
range, 0–6.9 U/ml). According to the Response Evaluation Criteria
in Solid Tumors version 1.1 criteria (10), partial remission was achieved after
four cycles, with CT showing a ≥30% reduction in tumor size and
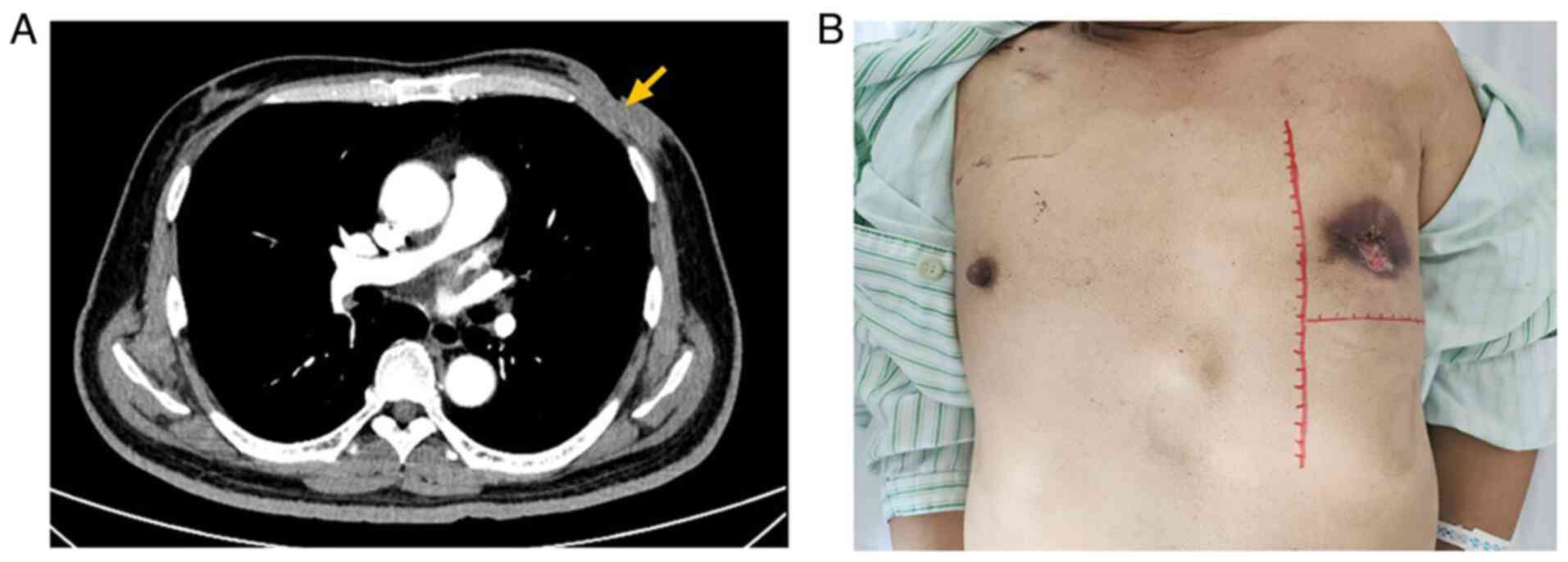
resolution of the axillary lymphadenopathy (Fig. 5A and B). After completion of 4
cycles of chemotherapy, the patient was admitted to the hospital,
and on the 2nd day post-admission in October 2023, the patient
underwent a modified radical mastectomy with latissimus dorsi flap
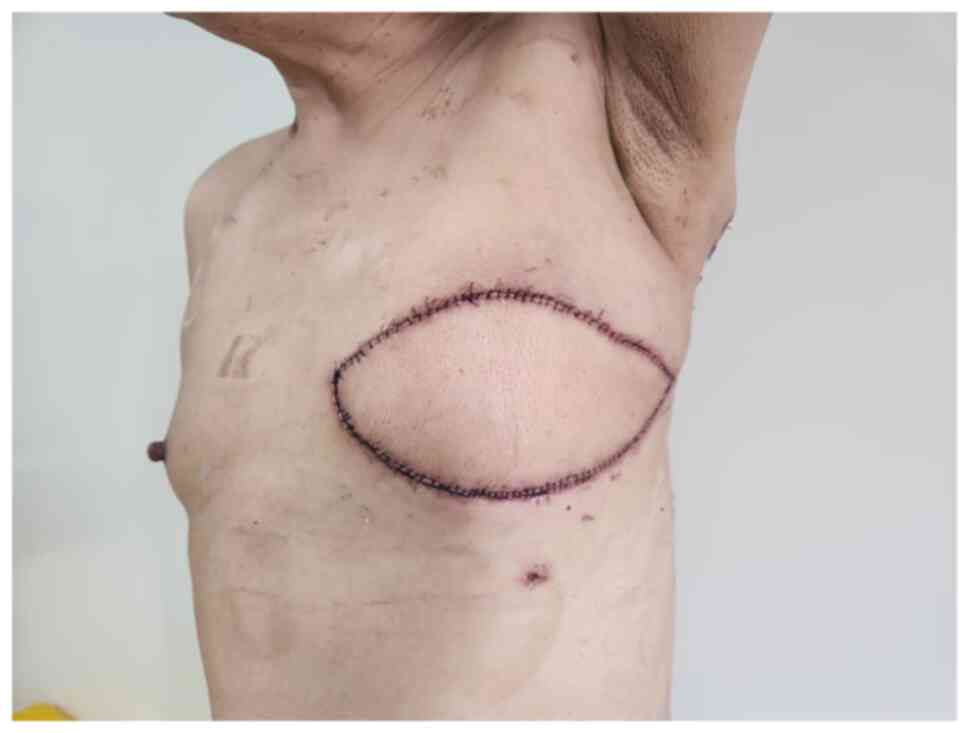
reconstruction, followed by postoperative recovery (Fig. 6). Two additional cycles of
chemotherapy were administered postoperatively, totaling six
cycles. The lung lesion remained unchanged throughout the
chemotherapy (Fig. 7). In December
2023, a thoracoscopic wedge resection of the lung was performed.
The diagnosis of minimally invasive adenocarcinoma with no vascular
or neural invasion was confirmed by postoperative paraffin
pathology. Further IHC staining and H&E staining were performed
for verification. The IHC staining procedure involved
antigen-antibody binding, followed by hematoxylin counterstaining
to visualize cell nuclei.
The patient is currently undergoing tamoxifen and
trastuzumab therapy. The dosing schedule is as follows: Tamoxifen
is administered at a dose of 10 mg per time, twice a day; the
trastuzumab-targeted therapy is maintained for 12 months, with an
administration frequency of once every 21 days. Routine follow-up
includes physical examinations and tumor marker testing every 3
months, as well as chest and abdominal CT scans every 6 months. As
of the last follow-up in May 2025, no signs of recurrence have been
observed.
Discussion
With continuous advances in tumor diagnosis and
treatment, the incidence of second primary cancers among patients
with breast cancer has steadily increased. Studies indicate that
lung cancer is one of the most common second primary malignancies
following breast cancer, with a median onset age of 50–59 years and
a median interval of 43.5–60.0 months. Lung cancer accounts for
0.8–1.4% of synchronous second primary cancers in patients with
breast cancer. Smoking, radiotherapy and chemotherapy are
established risk factors for dual primary breast and lung cancers
(11). MBC, accounting for <1%
of breast cancer cases, is exceedingly rare, and multiple primary
cancers in MBC are even more uncommon. Compared with women, men are
diagnosed with breast cancer at an older mean age of 67 years, with
a 25% higher mortality rate (12,13).
MBC is predominantly hormone-dependent. Research shows a strong
correlation between estrogen and lung cancer, with numerous lung
cancer cells overexpressing ER (14). While 90% of MBC cases are
ER-positive, only 8.7% are HER-2-positive. Most cases present with
painless sub-nipple nodules, with 40–50% involving the nipple, and
left-sided breast cancer is slightly more common (15,16).
Advanced cases may show skin changes, nipple retraction, ulcers or
masses fixed to underlying tissues, often with axillary
lymphadenopathy. Breast cancer susceptibility (BRCA) gene mutations
are the most recognized risk factors for MBC. Additional risk
factors include family history, chest radiation exposure, germline
mutations (e.g., BRCA2, BRCA1, checkpoint kinase 2, and partner and
localizer of BRCA2), and exogenous estrogen use. A large study of
2,175 cases of MBC revealed significantly higher AR/PR positivity
rates in MBC vs. female breast cancer, with HER-2 negativity being
more common (17). PIK3CA mutations
are prevalent in breast cancers and correlate with a poor prognosis
(18). The patient in the present
case exhibited advanced MBC with synchronous primary lung
adenocarcinoma, which is a rare combination. IHC demonstrated HR
and AR positivity, HER-2 positivity and PIK3CA mutations.
Similarly, an International MBC Program analysis of
1,483 MBC cases showed 99% ER-positivity and only 9%
HER2-positivity. However, the present case exhibited
HER2-positivity (3+), resembling the molecular profile more common
in female breast cancer. This observation aligns with a 2023 New
England Journal of Medicine review stating ‘the HER2-positivity
rate in men is comparable to that in older postmenopausal women’
(15). The high ER and AR
expression in the present study and its potential link to lung
adenocarcinoma also support Fentiman's ‘estrogen cross-talk’ theory
(19). Treatment-wise, a real-world
study from China retrospectively collected data from patients with
MBC across 36 centers in the country. The study suggested that an
anthracycline combined with taxane regimen is a protective factor
for disease-free survival in these patients (20). The present case adopted a
neoadjuvant TCbHP regimen, using taxane-based drugs. The stable
pulmonary lesion in the present study also aligns with the study by
Peng et al (21), which
reported a 74.2% 5-year overall survival rate after surgical
resection of synchronous multiple primary lung adenocarcinomas.
Research suggests 22.9–70.0% of patients with breast
cancer and lung nodules have primary lung cancer. The epidermal
growth factor receptor (EGFR) signaling pathway is vital in
tumorigenesis, progression and metastasis. EGFR mutations are the
most common driver mutations in lung cancer and are associated with
apoptosis inhibition, angiogenesis and tumor vasculature formation
(22,23). Evidence shows cross-signaling
between ER/PR and EGFR pathways in patients with breast cancer and
second primary lung cancer, suggesting shared biological mechanisms
and overlapping risk factors such as elevated hormone levels.
Clinical data indicate that EGFR mutation rates in patients with
dual primary breast and lung cancers are twice those in patients
with non-small cell lung cancer (NSCLC). EGFR signaling may thus be
critical in lung cancer development as a second primary malignancy
in patients with breast cancer (24).
Additionally, epithelial cell adhesion molecule
(EpCAM), a single-pass transmembrane glycoprotein, is upregulated
in epithelial-derived malignancies such as breast and lung cancers.
EpCAM interferes with key tumorigenic signaling pathways,
contributing to progression. A study has demonstrated its
association with metastasis, drug resistance and prognosis in
breast cancer, and its correlation with Tumor-Node-Metastasis
staging (25) in squamous cell lung
carcinoma (26). Weak EpCAM
expression in normal epithelial cells vs. strong expression in
cancer tissues highlights its carcinogenic role.
Immunohistochemical analysis of EpCAM expression and exploration of
anti-EpCAM immunotherapy may provide new treatment directions
(27).
The rhodopsin family is closely associated with
tumor biology, including cellular growth, differentiation and
migration; its role in the initiation, progression, metastasis and
drug resistance of breast and lung cancers is well-studied. Ras
homolog family member A (RhoA) and RhoC regulate cytoskeletal
reorganization, contributing to tumor cell invasion and metastasis.
High RhoC expression promotes breast cancer cell invasion and
metastasis by enhancing actin remodeling and extracellular matrix
degradation through increased matrix metalloproteinase secretion.
Similarly, RhoC is significantly upregulated in NSCLC, facilitating
distant metastasis (28). Rac
family small GTPase 1 (Rac1) modulates the tumor microenvironment,
promoting invasive migration of breast cancer cells. Rac1 also
activates the shared phosphatidylinositol 3-kinase (PI3K)/Akt
pathway in breast and lung cancers, which is implicated in
chemotherapy resistance in breast cancer and targeted therapy
resistance in lung cancer (29).
Both RhoA and Rac1 activate the mitogen-activated protein kinase
(MAPK) signaling pathway in these cancer types, driving cell
proliferation and anti-apoptotic processes (30). Additionally, RhoA and RhoC
collaboratively reshape the tumor microenvironment, bolstering
tumor cell immune evasion (31,32).
Targeted inhibition of oncogenes such as Rac1, RhoA and RhoC
presents a promising strategy to reduce the invasive potential of
breast and lung cancer cells. This approach underscores the
therapeutic potential of targeting shared molecular mechanisms to
manage dual malignancies effectively.
Furthermore, in the present report of synchronous
primary MBC and lung adenocarcinoma, potential biological
associations are explored. Previous studies suggest that these
cancers may share common risk factors and oncogenic pathways, such
as chronic smoking, radiation exposure and elevated hormone levels
(33,34). Based on large-scale genome-wide
association study data, researchers have analyzed shared pathogenic
mechanisms between breast and lung cancers genome-wide. Findings
indicate that both malignancies converge on the erb-b2 receptor
tyrosine kinase 2 signaling pathway, toll-like receptor 2 signaling
cascade, and nuclear factor-κB and MAPK pathways, suggesting
possible common genetic origins (35). As breast cancer is an
estrogen-dependent tumor, its development and progression are
closely linked to ER status. In normal breast tissue, ER mediates
downstream cascades, including Ras/Raf/MAPK and PI3K/AKT/mTOR
pathways, regulating proliferation, metabolism, survival and
apoptosis. Dysregulated estrogen metabolism may impair ER function
and contribute to tumorigenesis (36,37).
Emerging evidence also indicates that ER status may influence lung
cancer pathogenesis. In a study of 110 female patients with both
cancer types, 80% of breast tumors were ER-negative, suggesting a
potential association (38). This
supports a significant association between ER-negative breast
cancer and primary lung adenocarcinoma occurrence. In the present
study, the patient exhibited HER2 positivity, hormone receptor
positivity and a PIK3CA mutation, indicating possible molecular
involvement of multiple co-activated oncogenic pathways. Based on
this profile, we hypothesize the synchronous tumors may be
biologically linked, although larger studies are needed for
validation.
Standard MBC treatment follows female breast cancer
protocols, including mastectomy and endocrine therapy for hormone
receptor-positive cases (39).
Currently, in clinical practice, the application of traditional
Chinese medicine as an adjunct to radiotherapy and chemotherapy in
the treatment of breast cancer is widespread. This addition can
enhance therapeutic efficacy, reduce side effects, promote the
recovery of physical function after cancer treatment, decrease
recurrence and metastasis, and improve quality of life (40). Research has found that Huangqi Sijun
Decoction can alleviate fatigue in patients with breast cancer
after chemotherapy (41), while
Wenshen Zhuanggu Formula can reduce leukopenia, nausea, vomiting,
gastrointestinal reactions, hair loss and bone marrow suppression
(42). Adverse events were
evaluated according to the Common Terminology Criteria for Adverse
Events version 5.0 (43).
In the present study, integrating traditional
Chinese medicine with Western cancer treatment alleviated the
patient's discomfort and chemotherapy-induced bone marrow
suppression. Both traditional decoctions and Chinese herbal
medicine serve as important approaches in the treatment of breast
cancer, and hold important implications for breast cancer therapy.
During chemotherapy, no grade 3 or greater nausea/vomiting occurred
(43). Multidisciplinary management
effectively controlled both malignancies, with the patient
remaining recurrence-free for >1 year post-treatment. This
underscores the importance of comprehensive, integrative strategies
and long-term follow-up for managing rare, complex cancer
cases.
Acknowledgements
Not applicable.
Funding
This research was funded by the Major Innovation Project of
Science and Technology of the China Academy of Chinese Medical
Sciences (grant no. 2024-KY-082).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
XX was responsible for writing the original draft,
provided advice on patient treatment and analyzed patient data. SZ
was responsible for conception. JYL, JL and DZ confirm the
authenticity of all the raw data, and made contributions to the
conception and design of the study. YueW designed the study. YunW
obtained the patient's medical pathological smears. DL made
substantial contributions to conception and design, acquisition of
data, and analysis and interpretation of data. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
Guang'anmen Hospital, China Academy of Chinese Medical Sciences
(Beijing, China). Written informed consent was obtained from the
patient for participation in this study.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the present study, including medical
case information and images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MBC
|
male breast cancer
|
|
CT
|
computed tomography
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER-2
|
human epidermal growth factor
receptor-2
|
|
AR
|
androgen receptor
|
|
PIK3CA
|
phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α
|
|
EGFR
|
epidermal growth factor receptor
|
|
NSCLC
|
non-small cell lung cancer
|
|
EpCAM
|
epithelial cell adhesion molecule
|
|
RhoA
|
ras homolog family member A
|
|
RhoC
|
ras homolog family member C
|
|
Rac1
|
rac family small GTPase 1
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
TCM
|
traditional Chinese medicine
|
|
WBC
|
white blood cell
|
References
|
1
|
Gómez-Raposo C, Zambrana Tévar F, Sereno
Moyano M, López Gómez M and Casado A: Male breast cancer. Cancer
Treat Rev. 36:451–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li SY and Liu XF: A case report of Luminal
B type male breast cancer and literature review. Chin J Curr Adv
Gen Surg. 27:325–329. 2024.(In Chinese).
|
|
3
|
Choi N, Moon S, Kim JS, Kim AY, Ahn JH,
Han Y, Woo J, Kim H, Chung MS and Cha CD: Long-term survival
outcomes of male breast cancer: The propensity score matching
analysis of nationwide registry database. Breast. 83:1045562025.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ellington TD, Henley SJ, Wilson RJ and
Miller JW: Breast cancer survival among males by race, ethnicity,
age, geographic region, and Stage-United States, 2007–2016. MMWR
Morb Mortal Wkly Rep. 69:1481–1484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chatterji S, Krzoska E, Thoroughgood CW,
Saganty J, Liu P, Elsberger B, Abu-Eid R and Speirs V: Defining
genomic, transcriptomic, proteomic, epigenetic, and phenotypic
biomarkers with prognostic capability in male breast cancer: A
systematic review. Lancet Oncol. 24:e74–e85. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maguire S, Perraki E, Tomczyk K, Jones ME,
Fletcher O, Pugh M, Winter T, Thompson K, Cooke R; kConFab
Consortium, ; et al: common susceptibility loci for male breast
cancer. J Natl Cancer Inst. 113:453–461. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao MM, Joe BN, Sickles EA and Lee CS:
BI-RADS category 5 assessments at diagnostic breast
imaging:outcomes analysis based on lesion descriptors. Acad Radiol.
26:1048–1052. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mei F, Liu JY and Xue WC: Histological
grading of invasive breast carcinoma: Nottingham histological
grading system. Zhonghua Bing Li Xue Za Zhi. 48:659–664. 2019.(In
Chinese). PubMed/NCBI
|
|
9
|
Genetic Tumor Markers Collaboration Group,
Tumor Biomarker Committee, China Anti-cancer Association, Molecular
Pathology Collaboration Group, Tumor Pathology Committee, China
Anti-Cancer Association, . Chinese expert consensus on tumor
mutational burden testing and clinical application (2020 edition).
Chin J Oncol Prev Treat. 12:485–493. 2020.(In Chinese).
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen D, Xiao Y and Zhong K: Risk factors
and pathogenic mechanism for secondary primary lung cancer in
breast cancer patients: A review. Zhongguo Fei Ai Za Zhi.
25:750–755. 2022.(In Chinese). PubMed/NCBI
|
|
12
|
Hassett MJ, Somerfield MR, Baker ER,
Cardoso F, Kansal KJ, Kwait DC, Plichta JK, Ricker C, Roshal A,
Ruddy KJ, et al: Management of male breast cancer: ASCO guideline.
J Clin Oncol. 38:1849–1863. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qavi Q, Alkistawi F, Kumar S, Ahmed R and
Saad Abdalla Al-Zawi A: Male triple-negative breast cancer. Cureus.
13:e145422021.PubMed/NCBI
|
|
14
|
Pang L, Cao C, Hua Y, Zeng T, Yang F, Sun
C, Huang X and Li W: A case report of breast and lung dual primary
cancers. J Nanjing Med Univ (Nat Sci). 39:1696–1698. 2019.(In
Chinese).
|
|
15
|
Giordano SH, Buzdar AU and Hortobagyi GN:
Breast cancer in men. Ann Intern Med. 137:678–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goss PE, Reid C, Pintilie M, Lim R and
Miller N: Male breast carcinoma: A review of 229 patients who
presented to the Princess Margaret Hospital during 40 years:
1955–1996. Cancer. 85:629–636. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mangone L, Ferrari F, Mancuso P, Carrozzi
G, Michiara M, Falcini F, Piffer S, Filiberti RA, Caldarella A,
Vitale F, et al: Epidemiology and biological characteristics of
male breast cancer in Italy. Breast Cancer. 27:724–731. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi Q, Xuhong J, Tian H, Qu M, Zhang Y,
Jiang J and Qi X: Predictive and prognostic value of PIK3CA
mutations in HER2-positive breast cancer treated with tyrosine
kinase inhibitors: A systematic review and meta-analysis. Biochim
Biophys Acta Rev Cancer. 1878:1888472023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye Z and Liang M: The dilemma of breast
cancer in males and research progress. Chin Gen Pract.
22:3260–3264. 2019.(In Chinese).
|
|
20
|
Gao Y, Zhang M, Sun G, Ma L, Nie J, Yuan
Z, Liu Z, Cao Y, Li J, Liu Q, et al: The features of male breast
cancer in China: A real-world study. Breast. 76:1037622024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng Y, Wang H, Xie H, Ren W, Feng Z, Li M
and Peng Z: Surgical treatment and prognosis for patients with
synchronous multiple primary lung adenocarcinomas. Zhongguo Fei Ai
Za Zhi. 20:107–113. 2017.(In Chinese). PubMed/NCBI
|
|
22
|
Hu Z, Zou X, Qin S, Li Y, Wang H, Yu H,
Sun S, Wu X, Wang J and Chang J: Hormone receptor expression
correlates with EGFR gene mutation in lung cancer in patients with
simultaneous primary breast cancer. Transl Lung Cancer Res.
9:325–336. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Zeng Z, Zou Y, Pan X and Ouyang C:
A case report of dual primary cancers: Bilateral breast and lung
cancer. J Mod Med Health. 38:3954–3958. 2022.(In Chinese).
|
|
24
|
Hao Y, Zhang X, Cui G, Qi X, Jiang Z and
Yu L: Clinicopathological features, prognostic factor analysis, and
survival nomogram of patients with double primary cancers involving
lung cancer. Cancer Med. 13:e72962024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye B and Zhao H: Revision of the TNM stage
grouping in the forthcoming eighth edition of the TNM
classification for lung cancer. Zhongguo Fei Ai Za Zhi. 19:337–342.
2016.(In Chinese). PubMed/NCBI
|
|
26
|
Cui JL and Wang Q: Research progress on
EpCAM in lung cancer, breast cancer, and digestive system tumors. J
Precis Med. 39:367–370. 2024.(In Chinese).
|
|
27
|
Pak MG, Shin DH, Lee CH and Lee MK:
Significance of EpCAM and TROP2 expression in non-small cell lung
cancer. World J Surg Oncol. 10:532012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lou Y, Jiang Y, Liang Z, Liu B, Li T and
Zhang D: Role of RhoC in cancer cell migration. Cancer Cell Int.
21:5272021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Castellano E, Molina-Arcas M, Krygowska
AA, East P, Warne P, Nicol A and Downward J: RAS signalling through
PI3-Kinase controls cell migration via modulation of Reelin
expression. Nat Commun. 7:112452016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borgoño CA, Fracchioli S, Yousef GM,
Rigault de la Longrais IA, Luo LY, Soosaipillai A, Puopolo M, Grass
L, Scorilas A, Diamandis EP and Katsaros D: Favorable prognostic
value of tissue human kallikrein 11 (hK11) in patients with ovarian
carcinoma. Int J Cancer. 106:605–610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jaffe AB and Hall A: Rho GTPases:
Biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rathinam R, Berrier A and Alahari SK: Role
of Rho GTPases and their regulators in cancer progression. Front
Biosci (Landmark Ed). 16:2561–2571. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hao Y, Jiang H, Thapa P, Ding N,
Alshahrani A, Fujii J, Toledano MB and Wei Q: Critical role of the
sulfiredoxin-peroxiredoxin IV axis in urethane-induced non-small
cell lung cancer. Antioxidants (Basel). 12:3672023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Song W, Wang H, Zhu G, Li Y, Wang
Z, Li W and Che G: Increased risk of subsequent primary lung cancer
among female hormone-related cancer patients: A meta-analysis based
on over four million cases. Chin Med J (Engl). 137:1790–1801. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qian DC, Byun J, Han Y, Greene CS, Field
JK, Hung RJ, Brhane Y, Mclaughlin JR, Fehringer G, Landi MT, et al:
Identification of shared and unique susceptibility pathways among
cancers of the lung, breast, and prostate from genome-wide
association studies and tissue-specific protein interactions. Hum
Mol Genet. 24:7406–7420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Loboda A, Nebozhyn M, Klinghoffer R,
Frazier J, Chastain M, Arthur W, Roberts B, Zhang T, Chenard M,
Hager J, et al: A gene expression signature of RAS pathway
dependence predicts response to PI3K and RAS pathway inhibitors and
expands the population of RAS pathway activated tumors. BMC Med
Genomics. 3:262010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dey N, De P and Leyland-Jones B:
PI3K-AKT-mTOR inhibitors in breast cancers: From tumor cell
signaling to clinical trials. Pharmacol Ther. 175:91–106. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tennis M, Singh B, Hjerpe A, Prochazka M,
Czene K, Hall P and Shields PG: Pathological confirmation of
primary lung cancer following breast cancer. Lung Cancer. 69:40–45.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giunta G, Rossi M, Toia F, Rinaldi G and
Cordova A: Male breast cancer: Modified radical mastectomy or
breast conservation surgery? A case report and review of the
literature. Int J Surg Case Rep. 30:89–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng RQ, Li DH, Liu XK, Zhao XH, Wen QE
and Yang Y: Traditional Chinese medicine for breast cancer: A
review. Breast Cancer (Dove Med Press). 15:747–759. 2023.PubMed/NCBI
|
|
41
|
Cui Y, Mi J, Feng Y, Li L, Wang Y, Hu J
and Wang H: Huangqi Sijunzi decoction for treating cancer-related
fatigue in breast cancer patients: a randomized trial and network
pharmacology study. Nan Fang Yi Ke Da Xue Xue Bao. 42:649–657.
2022.(In Chinese). PubMed/NCBI
|
|
42
|
Jiang H, Li M, Du K, Ma C, Cheng Y, Wang
S, Nie X, Fu C and He Y: Traditional Chinese medicine for adjuvant
treatment of breast cancer: Taohong Siwu decoction. Chin Med.
16:1292021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|