Introduction
Rectal cancer (RC) is among the most common
malignancies in humans, ranking third in global incidence. In
China, it is the second most prevalent cancer, with a standardized
incidence rate of 30.32 cases per 100,000 individuals in 2022
(1). Endometrial cancer (EC), a
leading malignancy of the female genital tract, ranks second among
cancers of the reproductive system, with an incidence rate of 11.25
cases per 100,000 individuals (1).
Multiple primary malignant tumors (MPMTs) are defined as the
occurrence of two or more independent malignancies in the same
individual; they are further categorized as synchronous cancers
(SCs) when diagnosed within 6 months of each other or metachronous
cancers (MCs) when diagnosed >6 months apart (2). While MPMTs frequently involve the
head, neck and digestive system (3), SCs affecting both the digestive and
genital systems are rare. The current report presents a case of
synchronous RC and EC, aiming to enhance therapeutic strategies for
similar presentations.
Case report
A 54-year-old postmenopausal woman was admitted to
the Department of Obstetrics and Gynaecology at Chongqing
University Central Hospital (Chonqing, China) on August 2024 with a
history of intermittent vaginal bleeding for 1 year, followed by
the development of tenesmus 6 months after bleeding onset. The
patient had entered menopause 2 years earlier and was not using
exogenous estrogen replacement therapy. A colposcopic biopsy
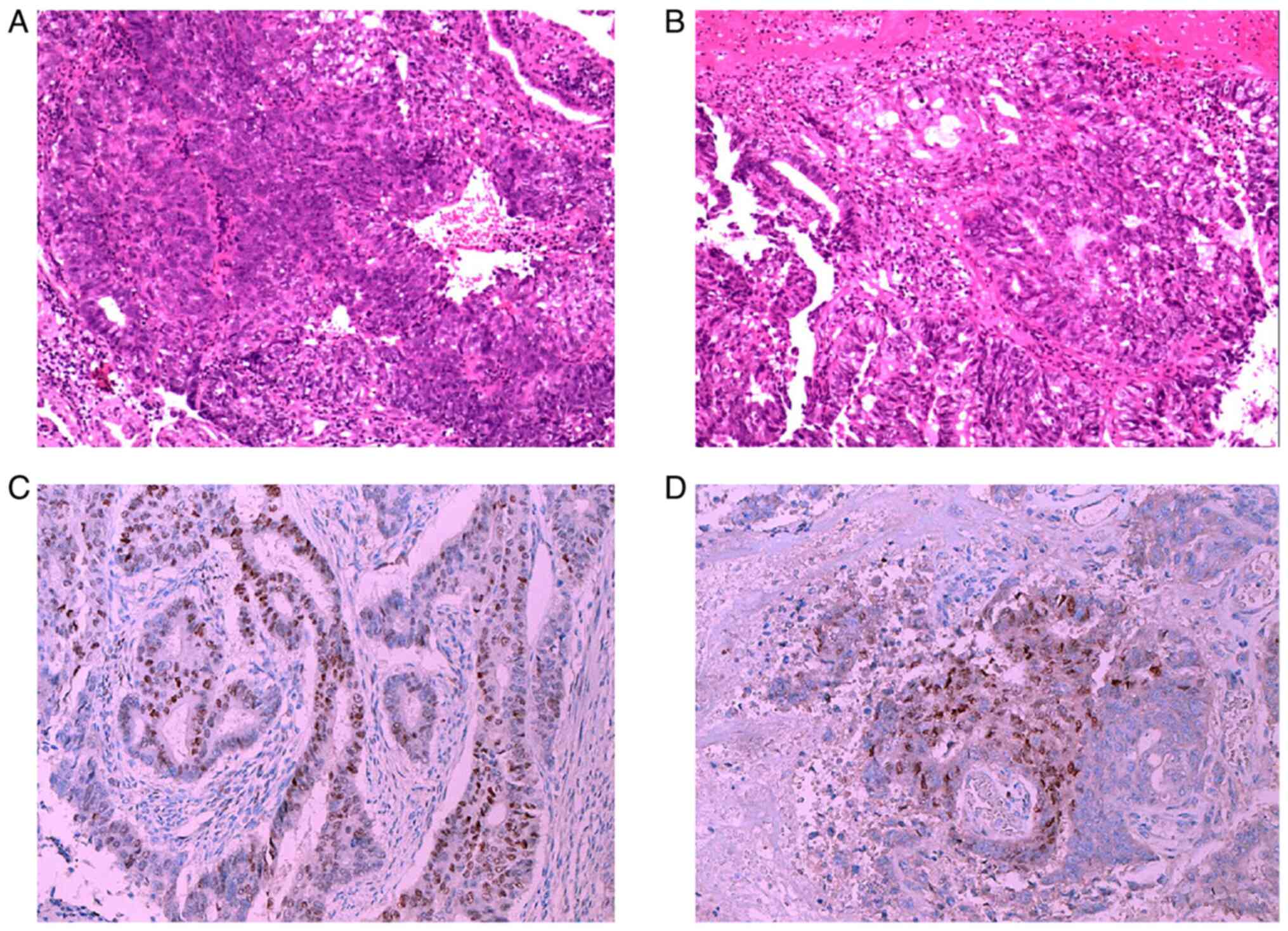
(Supplementary Methods) confirmed
a diagnosis of endometrioid carcinoma (Fig. 1), with immunohistochemistry (IHC)
analysis (Table SI) showing
positivity for cytokeratin (CK)7 (Fig.
2A), estrogen receptor (ER) (Fig.
1C) and progesterone receptor (PR) (Fig. 1D), as well as a Ki-67 proliferation
index >80% (Fig. 2B).
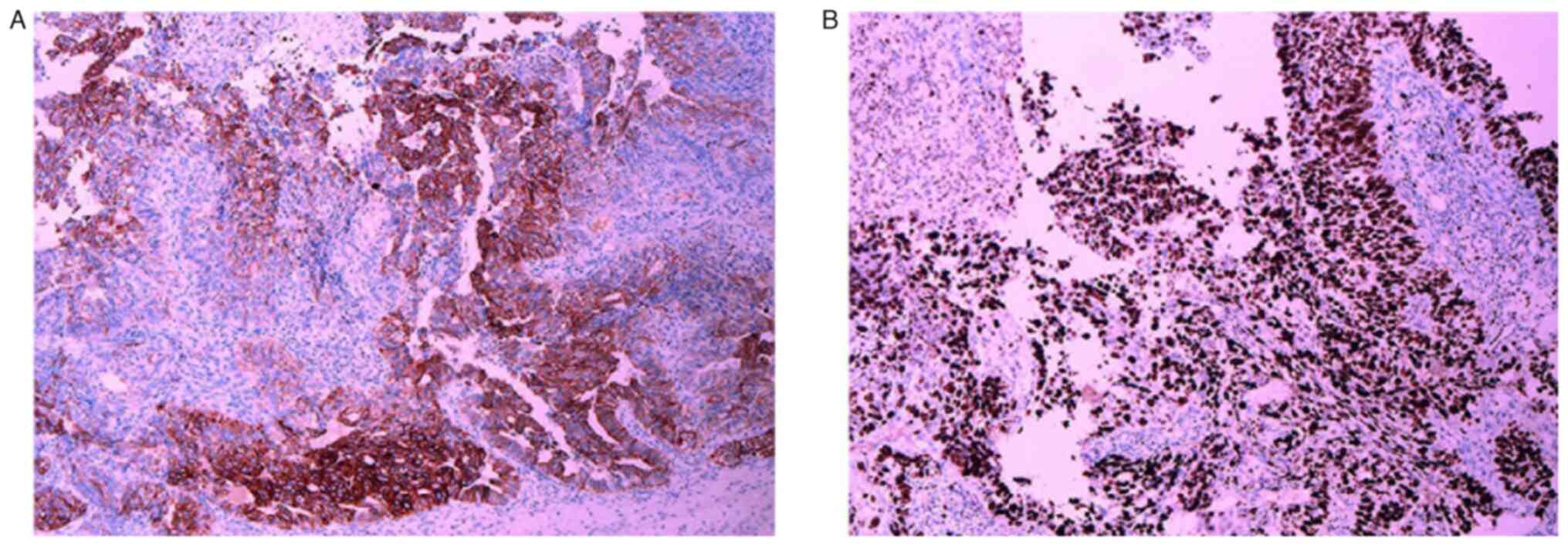
Concurrently, fibrocolonoscopic biopsy revealed rectal
adenocarcinoma (Fig. 3A), with IHC
analysis (Table SI) demonstrating
negativity for both ER (Fig. 3B)
and PR (Fig. 3C), and positivity
for CK20 (Fig. 4A) and
sequence-binding protein 2 (Fig.
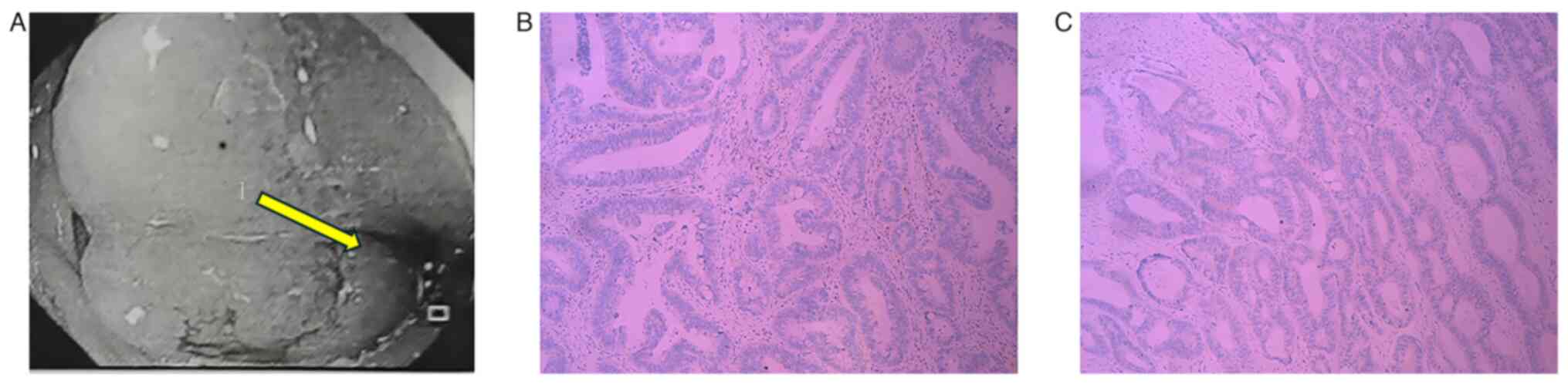
4B), alongside a Ki-67 proliferation index >70% (Fig. 4C). Enhanced magnetic resonance
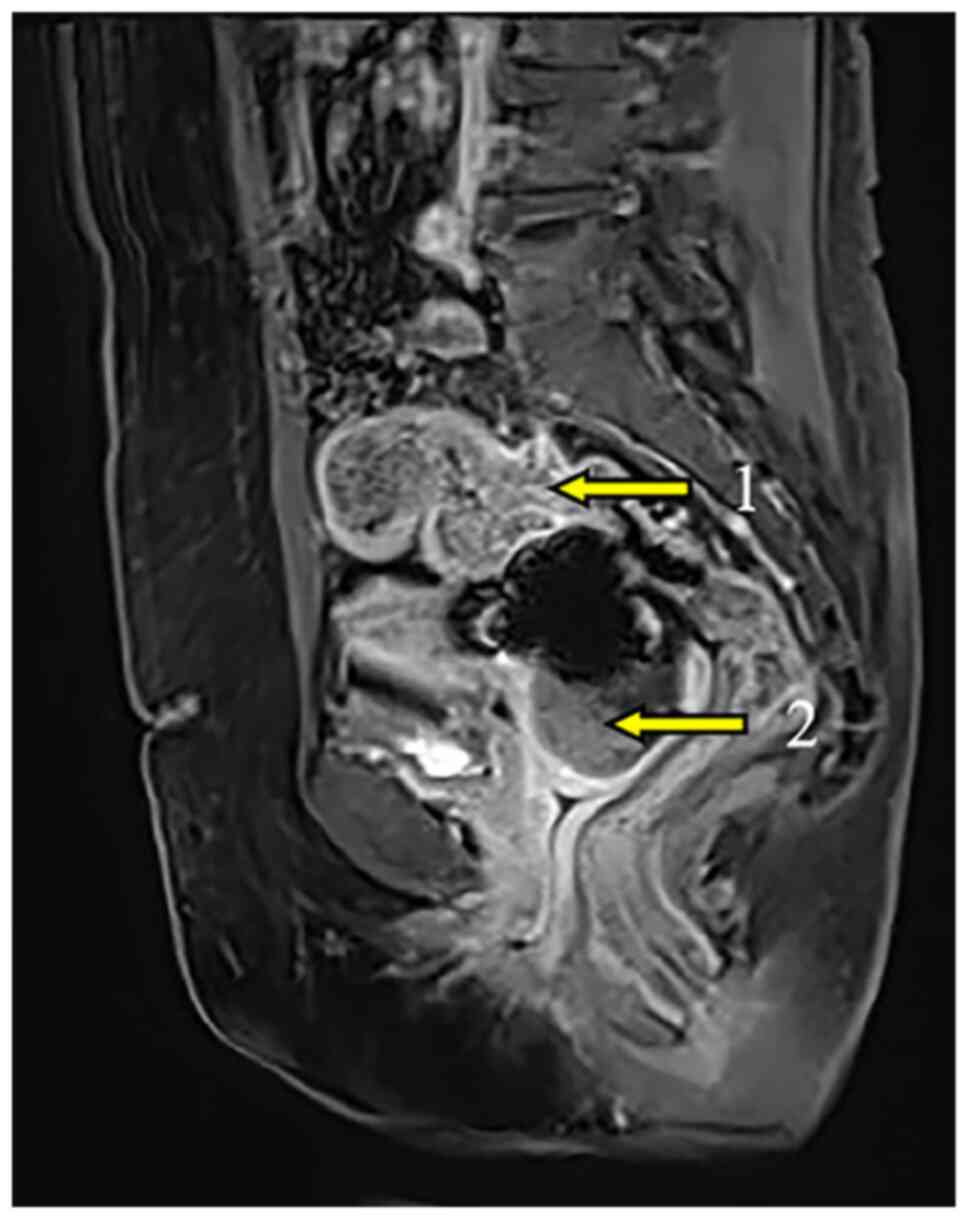
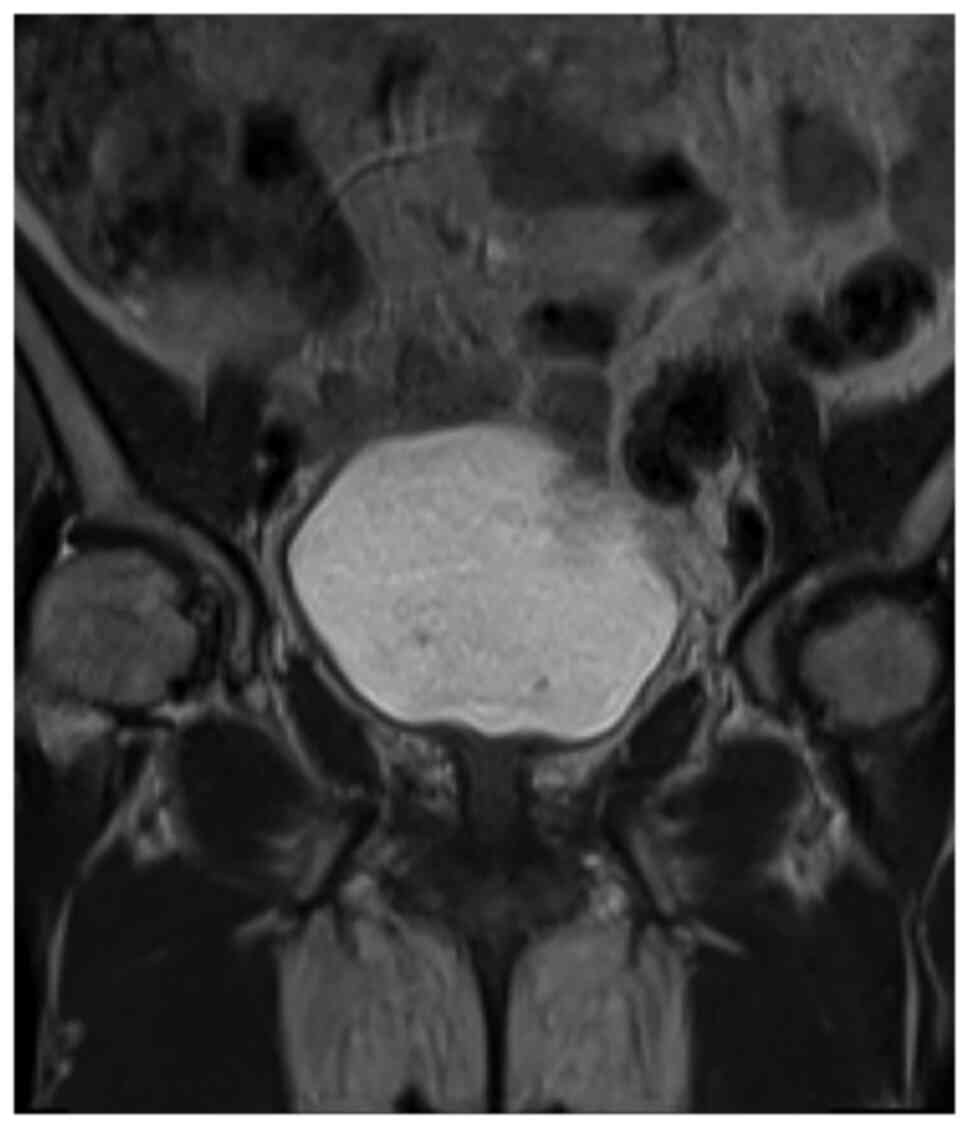
imaging (MRI) identified distinct lesions in the cervix and
rectosigmoid junction (Fig. 5),
confirming the presence of synchronous tumors.
Routine preoperative blood tests revealed severe
anemia (hemoglobin, 52 g/l; normal hemoglobin value without
pregnancy, ≥110 g/l) and hypoalbuminemia (33.1 g/l; normal albumin
range, 40–55 g/l), necessitating corrective interventions.
Following a MDT discussion, which included the Departments of
Pathology, Imaging, Oncology and Hematology, Obstetrics and
Gynecology, General Surgery, Urology, Blood Transfusion,
Anesthesiology and Nutrition, the diagnoses were established as
endometrial adenocarcinoma involving the cervix, staged as IB3
according to the International Federation of Gynecology and
Obstetrics (4), and rectal
adenocarcinoma, staged as T3N1M0 IIIb according to the TNM staging
criteria of the American Joint Committee on Cancer (5) and Union for International Cancer
Control (6). The MDT recommended
two potential courses of action: i) Initial pelvic external
radiotherapy, concurrent chemotherapy and vaginal brachytherapy,
with imaging evaluations every 2 to 3 months to monitor tumor
regression, followed by radical surgery; or ii) simultaneous
radical resection of both cancers. MRI showed that the uterine
lesion was mainly located in the cervix (Fig. 5), making cervical cancer a
possibility. According to previous studies and guidelines, the
progression-free survival (PFS) and overall survival rates
associated with minimally invasive radical hysterectomy for
cervical cancer are lower than those for open radical hysterectomy
(7,8). Meanwhile, the rectal tumor did not
affect the decision. Consequently, the patient opted for open
radical resection surgery and provided signed informed consent. The
operation was performed as scheduled and was smoothly completed in
260 min.
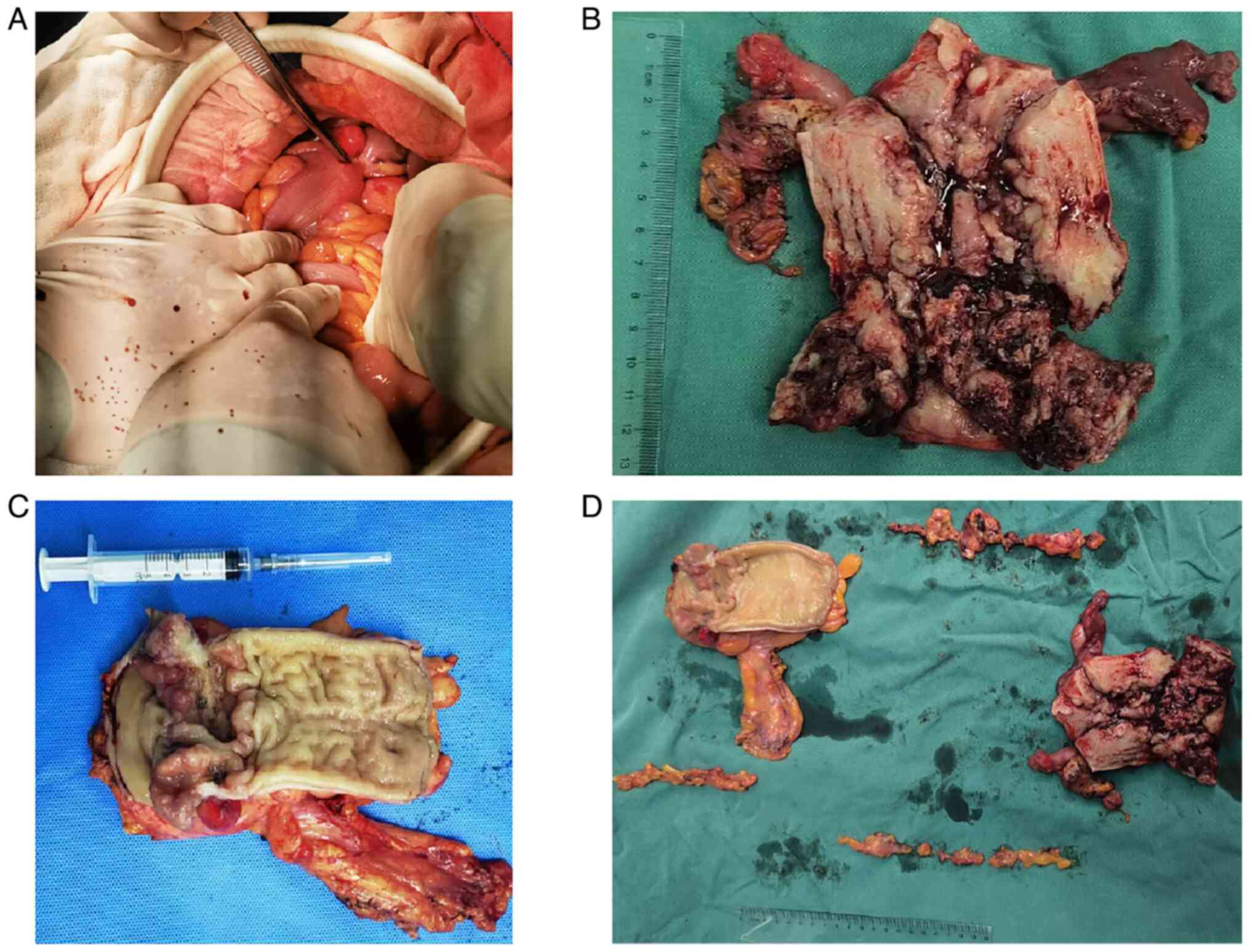
The postoperative gross specimen (Fig. 6) included a 12-cm segment of
resected rectum and sigmoid colon. An adenomatous tumor, ~2 cm in
diameter, encircled the rectal lumen. The uterine cavity was 10 cm
deep. A lesion was identified at the junction of the endometrium
and cervical mucosa, extending toward the cervix without
penetrating it. Portions of the endometrium were still smooth.
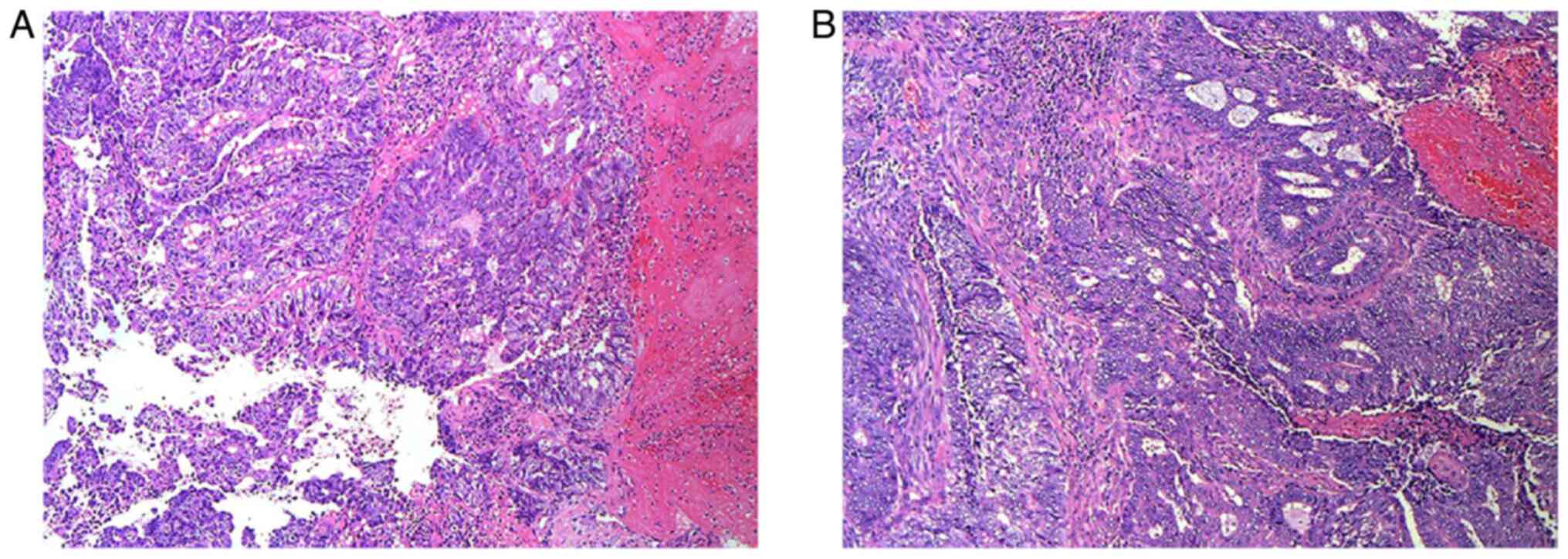
Postoperative pathological examination (Supplementary Methods) revealed
endometrial adenocarcinoma involving the cervix (Fig. 7B). The preoperative (Fig. 7A) and postoperative pathological
results (Fig. 7B) were the same.
The cancerous tissue had invaded the entire uterine cavity and
extended downward to the cervical wall. Evidence of cancer thrombus
formation was visible within blood vessels and lymphatic ducts;
however, no invasion of nerve tissue was detected. Metastasis was
found in the left and right pelvic lymph nodes (5/14 and 2/13,
respectively). The rectum contained a moderately differentiated
adenocarcinoma that had infiltrated the entire thickness of the
rectal wall and perienteric adipose tissue, accompanied by lymph
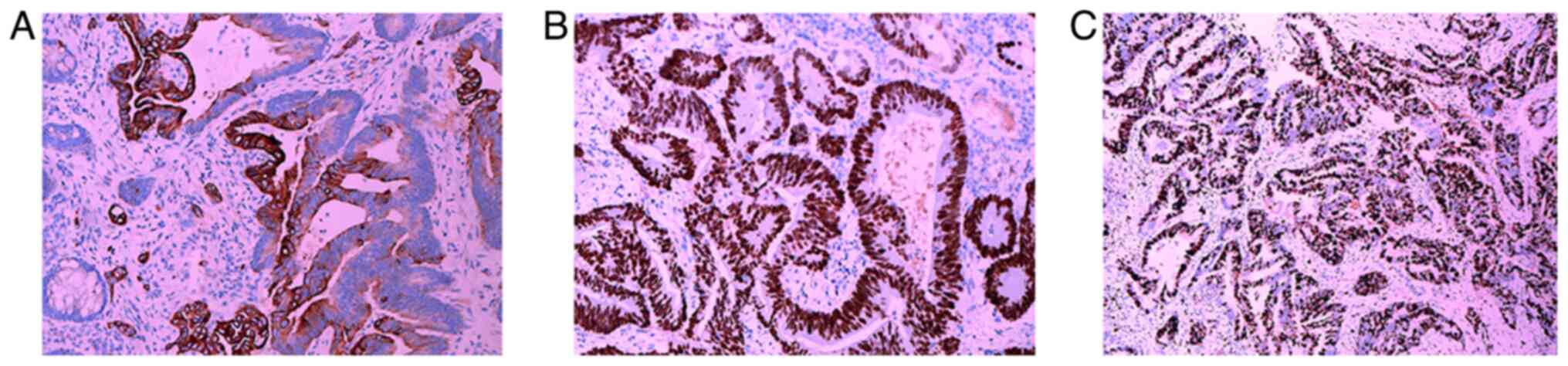
node metastasis (3/12), and was staged as T4aN1M0. The IHC results
(Table SI) for the endometrioid
adenocarcinoma were consistent with the preoperative biopsy. IHC
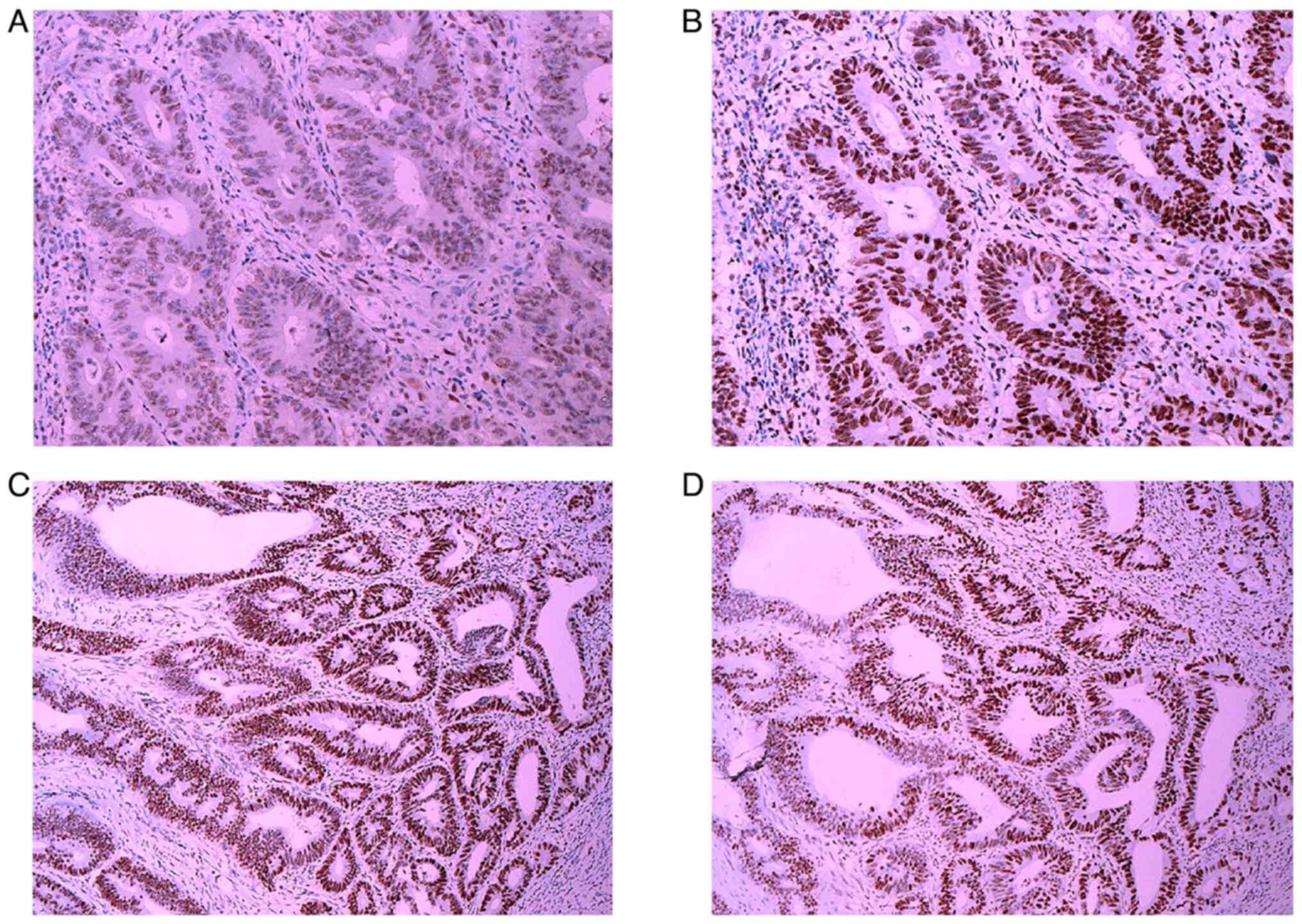
analysis of the RC showed elevated expression of Postmeiotic
Segregation Increased 2 (PMS2), MutL Homolog 1 (MLH1), MutS Homolog
(MSH)2 and MSH6 (Fig. 8). Gene
expression analysis (Table SII)
using quantitative (q)PCR combined with amplification refractory
mutation system (ARMS) showed that programmed death-ligand 1
(PD-L1) was expressed in <1% of tumor cells (tumor proportion
score <1) and <1% of immune cells (immune cell score <1).
Additionally, joint detection of KRAS NRAS, BRAF and
PIK3CA (KNBP) genes in the RC sample identified a G12V
mutation in codon 12 of exon 2 of the KRAS gene. No
mutations were detected in the NRAS, BRAF or PIK3CA
genes.
After an MDT discussion, the patient was scheduled
to receive paclitaxel (175 mg/m2) + carboplatin (AUC=5)
+ capecitabine (1,250 mg/m2) chemotherapy and pelvic
external beam radiotherapy lasting for 3 weeks. Presently, the
patient has completed five cycles of chemotherapy, each lasting 3
weeks. Paclitaxel caused hair loss but no cardiotoxicity.
Peripheral neuritis, nausea and mild diarrhea also occurred. A
subcutaneous injection of 150 mg of granulocyte colony-stimulating
factor was administered after the white blood cell count dropped to
2.1×109/l (normal white blood cell count,
3.5-9.5×109/l). With the aforementioned symptomatic
treatment, the symptoms were relieved, and the patient was able to
continue chemotherapy. At 3-months post-surgery, imaging results
indicated a favorable response to treatment (Fig. 9) and this patient received an
imaging examination every 3 months for follow-up. The patient has a
favorable prognosis.
Discussion
The present report describes the case of a
postmenopausal woman diagnosed with synchronous EC
(ER+/PR+) and RC
(ER−/PR−), meeting the Warren and Gates
criteria for MPMTs (2). First,
distinct IHC profiles (ER/PR discordance) and the presence of
separate lesions confirmed by imaging supported the diagnosis of
two separate tumors rather than metastatic disease. Moreover, both
malignancies were identified within a 6-month interval, aligning
with the SC classification. The patient had no known risk factors
such as smoking, alcohol use, chemical or radiation exposure
(9,10), circadian rhythm disruption (11), or specific factors such as
infection, endocrine factors (12–14) or
Lynch syndrome (LS). LS is caused by germline mutations in mismatch
repair (MMR) genes, including MLH1, MSH2, MSH6 and
PMS2, which manifest as a loss of protein expression on IHC
analysis. Obesity may be a risk factor for both EC and RC (2). The present patient was overweight,
with a body mass index of 27.5 kg/m2 and an abdominal
wall fat thickness of 8 cm, which may have contributed to
carcinogenesis, as reported in previous studies linking obesity to
elevated EC and RC risks (2,15).
Notably, while intrauterine devices (IUDs) are associated with a
reduced EC risk (16), this
patient's longstanding IUD use did not prevent the malignancy,
suggesting that the etiologies leading to EC and the simultaneous
occurrence of EC and RC may be multifactorial.
While most MPMT reports describe dual primaries,
cases involving three or more malignancies are exceedingly rare
(17). The present report adds to
the limited body of literature on synchronous EC and RC,
particularly in patients who do not have LS or a history of
environmental carcinogen exposure. Studies (18,19)
have emphasized the role of genetic syndromes in the synchronous
development of EC and RC; for instance, the EC risk in women with
LS is as high as 40 to 60% (18).
The present case demonstrates that mutations in the KRAS
gene can be detected through combined KNBP gene testing. The
present case also suggests that obesity may be a potential driver
for MPMTs, offering a distinct perspective on their
pathogenesis.
Unopposed estrogen exposure due to progesterone
deficiency is a well-established risk factor for EC in
postmenopausal women (20).
However, emerging evidence suggests that estrogen may also
influence RC progression. Mouse models have demonstrated that
estrogen deprivation accelerates RC growth by altering the tumor
immune microenvironment (21).
Although ERβ is expressed in rectal tissues and may exert a
protective effect, its expression levels are low (22). However, hormone replacement therapy
is not recommended for the sole purpose of preventing RC, as the
literature demonstrated that there were no statistically
significant associations between hormone replacement therapy use
and overall RC risk (23).
Furthermore, some RC cells synthesize estradiol via the G
protein-coupled estrogen receptor (GPER) pathway, which may
potentially induce endometrial hyperplasia (24). In the present patient, the EC was
ER+, while the RC was ER−, suggesting that
estrogen signaling is tissue-specific. Although EC proliferation
was likely estrogen-driven, RC may have been influenced by
paracrine or systemic estrogen derived from adipose tissue, given
the patient's obese status. In other estrogen-independent pathways,
human epidermal growth factor receptor 2 (HER2) status shows no
significant association with the clinicopathological features of RC
(25). However, the activation of
HER2 signaling can lead to resistance to anti-epidermal growth
factor receptor therapy in a subset of patients with RC and RAS
wild-type tumors (25,26).
Adipose tissue serves as an extragonadal estrogen
source through aromatase activity. Obesity increases EC risk
[relative risk (RR), 7.1] and RC risk (RR, 1.5-1.8) (15), likely via chronic inflammation,
insulin resistance and adipokine dysregulation. In the present
study, the patient's abdominal adiposity (8 cm fat thickness) may
have created a pro-tumorigenic milieu that synergized with hormonal
pathways, thereby promoting the development of synchronous
malignancies.
Gene mutations can lead to the occurrence of MPMTs
(27). Studies (28–30)
have identified several genetic and epigenetic changes that affect
synchronous RC and EC. For instance, elevated expression of the
gene encoding tetratricopeptide repeat protein 9A in RC correlates
with a poor prognosis, whereas its hypermethylation in EC predicts
worse outcomes (28). Additionally,
the presence of elevated Tac2-N transcript levels in RC and
promoter hypermethylation in both RC and EC point to epigenetic
dysregulation as an underlying cause of synchronous RC and EC;
however, the prognostic relevance of these alterations remains
unclear (29). Meanwhile, rare
germline variations in the dicer 1 ribonuclease III-encoding gene
may be linked to both EC and RC (30). More cases are needed to support the
aforementioned genetic and epigenetic findings, and to increase the
understanding of the mechanisms involved in synchronous RC and EC
occurrence. In this context, expanded genetic testing, including
the use of next-generation sequencing (NGS), qPCR, microsatellite
instability detection, circulating tumor cell (CTC) and circulating
tumor DNA (ctDNA) detection, and microarray comparative genomic
hybridization, could uncover novel variants, thereby providing more
accurate diagnostic tools and more precise, individualized
treatment schemes for SCs.
The incidence of MPMTs ranges from 2.4 to 8.0%
globally, with dual primaries predominating (17). In China, the incidence of MPMTs was
reported to range from 0.52 to 3.66% in 2017 (31). Sex-specific patterns are notable,
with prostate, colorectal and bladder cancer predominating in
males, and breast cancer, colorectal cancer and EC being most
common in females (32). The
present case aligns with the female-predominant co-occurrence of
EC/RC but is atypical given the absence of breast cancer, a
frequent component of female MPMTs. This observation highlights the
anatomical and hormonal heterogeneity underlying such
occurrences.
SCs are under-reported relative to MCs (33), partly due to overlapping symptoms
such as vaginal bleeding and tenesmus. The present patient's
delayed tenesmus diagnosis stresses the need for holistic symptom
assessment. Although positron emission tomography-computed
tomography (PET-CT) is not routinely performed for RC, it has
previously proven valuable in distinguishing synchronous lesions
(34), a finding consistent with
recent literature advocating the use of advanced imaging for the
diagnosis of MPMTs (35). The
present patient had no clinical manifestations of distant
metastasis, and no distant metastasis was found on CT or MRI;
therefore, the added diagnostic value of PET-CT was considered low.
Furthermore, PET-CT was not used in this case due to the higher
costs compared with other imaging modalities.
Although the therapeutic options for MPMTs are
usually limited, radical resection remains the cornerstone of SC
management (3,36). Laparoscopy combined with resection
has been used for synchronous EC and rectal adenocarcinoma in women
with obesity (37). Patients
recover better after minimally invasive laparoscopic surgery, which
is associated with less trauma. The enhanced recovery after surgery
(ERAS) program can be implemented to shorten hospital stay and
costs, reduce postoperative complications and improve
health-related quality of life (38,39).
However, the technical requirements for laparoscopy are typically
high. If MPMTs cannot be radically cured by surgery alone,
neoadjuvant (40) and conversion
therapies can be used to reduce the tumor stage, thus providing an
opportunity for radical surgery. According to European Society for
Medical Oncology guidelines for RC and EC, adjuvant chemotherapy is
required after RC surgery (41),
whereas concurrent chemotherapy is required after EC surgery
(42).
Patients with SCs generally have a worse prognosis
compared with patients with MCs. The median PFS time for patients
with synchronous RC and EC without defective MMR expression is 27
months, while the median PFS time of SCs linked with LS is 30
months. Thus, functional MMR proteins may confer a survival
advantage. However, the prognosis of LS-associated SCs does not
significantly differ from that of sporadic cases (43).
The early detection and diagnosis of MPMTs in
clinical practice are of great importance for guiding treatment and
predicting the prognosis of patients. The present case reinforces
the necessity of thorough symptom evaluation. Secondary symptoms,
such as tenesmus, must not be overlooked, particularly in high-risk
groups such as postmenopausal women with obesity, as in the present
case. Treatment planning was optimized through MDT discussions,
emphasizing the need for institutional MDT protocols in MPMT
management. Precision medicine and genetic testing may improve
diagnosis and help monitor treatment responses and recurrence.
While routine MMR testing excludes LS, broader panels, such as NGS
and ctDNA detection, can identify targetable mutations such as
those in PI3K and KRAS (44). Liquid biopsies (CTC/ctDNA) can also
be used to monitor treatment responses and recurrence, although
their utility in MPMTs requires validation (45). While open surgery ensured a complete
resection in this case, minimally invasive surgery with ERAS should
be considered in future cases to reduce morbidity (38). Although the patient's adjuvant
regimen (paclitaxel/carboplatin/capecitabine + radiotherapy)
adhered to guidelines, there is an evident need for studies
comparing its efficacy in SCs vs. single malignancies.
Following a series of diagnostic procedures, MDT
discussions and treatments, the patient in the present study
experienced a smooth recovery. The patient was discharged after
surgery and received regular follow-up examinations to monitor SC
recurrence. Despite this positive outcome, this report has some
limitations. As a single-case report, it inherently lacks
generalizability due to a lack of statistical power. The absence of
genetic/epigenetic profiling also limited mechanistic insights.
Conclusions regarding long-term survival or recurrence are
precluded by the short-term nature of the recovery data.
Furthermore, the patient's incomplete genetic workup limited the
understanding of the underlying mechanisms. For instance, while
programmed cell death protein 1/PD-L1 negativity excludes the
patient as a candidate for immunotherapy, it does not explain how
the tumor evades the immune system.
Future research should prioritize four
interconnected domains to advance SC management. First, large-scale
genomic initiatives, building on The Cancer Genome Atlas, should
employ multi-omics approaches to identify conserved mutations and
carcinogenic pathways underlying synchronous tumorigenesis. Second,
the clinical validation of integrated PET-CT and liquid biopsy
protocols requires the standardization of biomarker thresholds for
the screening of multiple primary tumors. Third, for therapeutic
optimization, comparative studies are needed to evaluate surgical
outcomes (open vs. minimally invasive), along with targeted
immunotherapy trials focusing on tumor microenvironment
heterogeneity in PD-L1-positive subgroups. Finally, preventive
strategies require mechanistic studies on adipokine-driven
carcinogenesis, coupled with trials evaluating structured
weight-loss interventions and GPER antagonists in genetically
predisposed obese populations. This integrated approach, employing
molecular profiling, diagnostic refinement, therapeutic
personalization and precision prevention, could lead to the
establishment of a translational framework that addresses the
complexity of SCs through combined genomic, clinical and metabolic
stratification, ultimately enabling tailored management for
high-risk cohorts.
In conclusion, the present case of synchronous EC
and RC illustrates the complex interplay among hormonal, metabolic
and potential genetic factors in MPMT pathogenesis. The report also
underscores the necessity for comprehensive diagnostic approaches,
MDT collaboration and patient-tailored therapies. Despite existing
limitations, the present findings contribute to the evolving field
of precision oncology, prompting further research into SC-specific
mechanisms and management frameworks for SCs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding was received from the Chongqing Key Laboratory of
Emergency Medicine Open Project (grant no. 2022KFKT09).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
ZL and BZ made substantial contributions to
conception and design. LP and QS acquired the data and performed
its analysis and interpretation. ZL and BZ were involved in
drafting the manuscript and revising it critically for important
intellectual content. BZ gave final approval of the version to be
published and agreed to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of
any part of the work are appropriately investigated and resolved.
All authors have read and approved the final manuscript. ZL, LP, QS
and BZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The patient provided written informed consent to
participate. The requirement for ethical approval for this case
report was exempted by Chongqing University Central Hospital
(Chonqing, China).
Patient consent for publication
Written informed consent was provided by the patient
for publication of the present study and the accompanying
images.
Competing interests
The authors declare that they do not have any
competing interests.
References
|
1
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Cent. 4:47–53. 2024.PubMed/NCBI
|
|
2
|
Yang XB, Zhang LH, Xue JN, Wang YC, Yang
X, Zhang N, Liu D, Wang YY, Xun ZY, Li YR, et al: High incidence
combination of multiple primary malignant tumors of the digestive
system. World J Gastroenterol. 28:5982–5992. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wan Y, Wang Z, Yang N and Liu F: Treatment
of multiple primary malignancies with PD-1 inhibitor camrelizumab:
A case report and brief literature review. Front Oncol.
12:9119612022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berek JS, Matias-Guiu X, Creutzberg C,
Fotopoulou C, Gaffney D, Kehoe S, Lindemann K, Mutch D and Concin
N; Endometrial Cancer Staging Subcommittee and FIGO Women's Cancer
Committee, : FIGO staging of endometrial cancer: 2023. Int J
Gynaecol Obstet. 162:383–394. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nicholls RJ, Zinicola R and Haboubi N:
Extramural spread of rectal cancer and the AJCC Cancer Staging
Manual 8th edition, 2017. Ann Oncol. 30:1394–1395. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Waldmann A, Borchers P and Katalinic A:
Temporal trends in age- and stage-specific incidence of colorectal
adenocarcinomas in Germany. BMC Cancer. 23:11802023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong BH, Song K and Yin AJ: Prevention and
treatment of endometrial cancer. Zhonghua Yi Xue Za Zhi.
104:715–720. 2024.(In Chinese). PubMed/NCBI
|
|
8
|
Ramirez PT, Frumovitz M, Pareja R, Lopez
A, Vieira M, Ribeiro R, Buda A, Yan X, Shuzhong Y, Chetty N, et al:
Minimally invasive versus abdominal radical hysterectomy for
cervical cancer. N Engl J Med. 379:1895–1904. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi HJ and Lee JH: Multiple human
papilloma virus 16 infection presenting as various skin lesions. J
Craniofac Surg. 27:e379–e381. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ribeiro MF, Peretz Soroka H, Bhura Z,
Hirsch I, Wunder J, Ferguson P, Tsoi K, Brar S, Gladdy R, Swallow
C, et al: Clinico-demographic characteristics and outcomes of
radiation-induced sarcomas (RIS): A CanSaRCC study. Ther Adv Med
Oncol. 15:175883592311989432023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roberts NT, MacDonald CR, Mohammadpour H,
Antoch MP and Repasky EA: Circadian rhythm disruption increases
tumor growth rate and accumulation of Myeloid-derived suppressor
cells. Adv Biol (Weinh). 6:e22000312022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim TH, Kim JH, Kang CH, Keam B and Kim
HJ: Treatment of Fanconi anemia patient with synchronous esophageal
and tongue cancer in COVID-19 era: A case report. Radiat Oncol J.
42:83–87. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yatsuoka T, Fukumitsu H, Kita T, Kitaoka
M, Otsuki T and Suzuki S: A case of synchronous papillary thyroid
cancer and breast ductal cancer. Gan To Kagaku Ryoho. 51:220–222.
2024.(In Japanese). PubMed/NCBI
|
|
14
|
Damaskos C, Dimitroulis D, Garmpi A,
Antoniou EA, Kouraklis G, Psilopatis I, Mavri M, Diamantis E,
Marinos G and Kyriakos G: Synchronous insulinoma and glucagonoma: A
Review of the literature. In Vivo. 37:2402–2408. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang F, Xu P, Yue Z, Song Y, Hu K, Zhao
X, Gao M and Chong Z: Body weight correlates with molecular
variances in patients with cancer. Cancer Res. 84:757–770. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Minalt N, Caldwell A, Yedlicka GM, Joseph
S, Robertson SE, Landrum LM and Peipert JF: Association between
intrauterine device use and endometrial, cervical, and ovarian
cancer: An expert review. Am J Obstet Gynecol. 229:93–100. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Jin C, Zhang Y, Wang J and Zheng L:
Identification of BRAF, CCND1, and MYC mutations in a patient with
multiple primary malignant tumors: A case report and review of the
literature. World J Surg Oncol. 21:1582023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bogani G, Tibiletti MG, Ricci MT,
Carnevali I, Liberale V, Paolini B, Milione M, Vitellaro M, Murgia
F and Chiappa V: Lynch syndrome-related non-endometrioid
endometrial cancer: Analysis of outcomes. Int J Gynecol Cancer.
30:56–61. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valencia Cardona AF, Cruz Barbosa JS and
Cortés Buelvas A: Synchronous adenocarcinoma of the endometrium and
colon in a woman with Lynch syndrome associated with a mutation of
the MSH6 gene. Rev Esp Patol. 58:1008262025.PubMed/NCBI
|
|
20
|
Wang L, Wei W and Cai M: A Review of the
risk factors associated with endometrial hyperplasia during
perimenopause. Int J Womens Health. 16:1475–1482. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang L, Fei H, Yang A, Zhu J, Sun J, Liu
X, Xu W, Yang J and Zhang S: Estrogen inhibits the growth of colon
cancer in mice through reversing extracellular Vesicle-mediated
immunosuppressive tumor microenvironment. Cancer Lett. 520:332–343.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Williams C, DiLeo A, Niv Y and Gustafsson
JÅ: Estrogen receptor beta as target for colorectal cancer
prevention. Cancer Lett. 372:48–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brändstedt J, Wangefjord S, Nodin B,
Eberhard J, Jirström K and Manjer J: Associations of hormone
replacement therapy and oral contraceptives with risk of colorectal
cancer defined by clinicopathological factors, beta-catenin
alterations, expression of cyclin D1, p53, and
microsatellite-instability. BMC Cancer. 14:3712014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gilligan LC, Rahman HP, Hewitt AM, Sitch
AJ, Gondal A, Arvaniti A, Taylor AE, Read ML, Morton DG and Foster
PA: Estrogen activation by steroid sulfatase increases colorectal
cancer proliferation via GPER. J Clin Endocrinol Metab.
102:4435–4447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ni S, Wang X, Chang J, Sun H, Weng W, Wang
X, Tan C, Zhang M, Wang L, Huang Z, et al: Human epidermal growth
factor receptor 2 overexpression and amplification in patients with
colorectal cancer: A Large-scale retrospective study in Chinese
population. Front Oncol. 12:8427872022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Underwood PW, Ruff SM and Pawlik TM:
Update on targeted therapy and immunotherapy for metastatic
colorectal cancer. Cells. 13:2452024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akizawa Y, Kanno T, Horibe Y, Shimizu Y,
Noguchi E, Yamamoto T, Okamoto T, Nagashima Y and Tabata T: Ovarian
metastasis from breast cancer mimicking a primary ovarian neoplasm:
A case report. Mol Clin Oncol. 15:1352021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao Y, Liu J and Zhou X, Liu Z, Qiu S, He
Y and Zhou X: A pan-cancer analysis of TTC9A expression level and
its correlation with prognosis and immune microenvironment. Nan
Fang Yi Ke Da Xue Xue Bao. 44:70–82. 2024.(In Chinese). PubMed/NCBI
|
|
29
|
Qureshi MA, Khan S, Tauheed MS, Syed SA,
Ujjan ID, Lail A and Sharafat S: Pan-cancer multiomics analysis of
TC2N gene suggests its important role(s) in tumourigenesis of many
cancers. Asian Pac J Cancer Prev. 21:3199–3209. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Munro D, Ghersi D and Singh M: Two
critical positions in zinc finger domains are heavily mutated in
three human cancer types. PLoS Comput Biol. 14:e10062902018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lv M, Zhang X, Shen Y, Wang F and Yang J,
Wang B, Chen Z, Li P, Zhang X, Li S and Yang J: Clinical analysis
and prognosis of synchronous and metachronous multiple primary
malignant tumors. Medicine (Baltimore). 96:e67992017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Jiao F, Yao J, Zhou X, Zhang X and
Wang L: Clinical features of multiple primary malignant tumors: A
retrospective clinical analysis of 213 Chinese patients at two
centers. Discov Med. 32:65–78. 2021.PubMed/NCBI
|
|
33
|
Mir AW, Parveen S, Ahmad I, Naveed S, Syed
NA, Mohmad HM and Dar N: Multiple primary malignancies: A
clinicopathological profile of patients at a tertiary center of
North India-A retrospective Hospital-Based observational study.
Indian J Med Paediatr Oncol. 45:052–060. 2023.
|
|
34
|
Edamadaka Y, Parghane RV and Basu S:
Complimentary Role of [18F]FDG and [18F]NaF-PET/CT in evaluating
synchronous thyroid carcinoma and parathyroid adenoma with brown
tumors. World J Nucl Med. 23:220–224. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu N, Gao Y, Pan Y, Song L, Yang Y, Yin
Y, Wang Y, Zhang L, Wu S and Yu G: Clinical analysis of lymphoma
with malignant solid tumor simultaneously: A retrospective case
series. Diagn Pathol. 20:542025. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kremzer T, Pete I, Ruttner P, Csucska M
and Lóderer Z: Synchronous tumor treatment in the presence of
gynecologycal cancer in three patients. Orv Hetil. 164:70–75. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Macciò A, Lavra F, Chiappe G, Kotsonis P,
Sollai G, Zamboni F and Madeddu C: Combined laparoscopic excisional
surgery for synchronous endometrial and rectal adenocarcinoma in an
obese woman. J Obstet Gynaecol. 36:1012–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ferrari F, Soleymani Majd H, Giannini A,
Favilli A, Laganà AS, Gozzini E and Odicino F: Health-related
quality of life after hysterectomy for endometrial cancer: The
impact of enhanced recovery after surgery shifting paradigm.
Gynecol Obstet Invest. 89:304–310. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mihăilescu AA, Onisâi M, Alexandru A,
Teodorescu M, Aliuș C, Blendea CD, Neagu ȘI, Șerban D and Grădinaru
S: A comparative analysis between enhanced recovery after surgery
and traditional care in the management of obstructive colorectal
cancer. Medicina (Kaunas). 60:13192024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakanishi K, Goto W, Ishihara A, Tauchi J,
Kashiwagi S, Amano R, Kubo S and Ohira M: A case of synchronous
double cancer including borderline resectable pancreatic body
cancer and breast carcinoma with Osseous/cartilaginous
differentiation treated with neoadjuvant chemotherapy and radical
resection. Gan To Kagaku Ryoho. 48:2005–2007. 2021.(In Japanese).
PubMed/NCBI
|
|
41
|
Di Franco DS, Chiloiro G, Savino M,
Costamagna I, Romano A, Meldolesi E, Damiani A and Valentini V:
3057: Clinical impact of ESMO guidelines adherence in rectal
cancer: A process mining of real world. Radiotherapy Oncol. 194
(Suppl):S1566–S1568. 2024. View Article : Google Scholar
|
|
42
|
Oaknin A, Bosse TJ, Creutzberg CL,
Giornelli G, Harter P, Joly F, Lorusso D, Marth C, Makker V, Mirza
MR, et al: Endometrial cancer: ESMO clinical practice guideline for
diagnosis, treatment and follow-up. Ann Oncol. 33:860–877. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ye S, Zhou S, Zhong S, Shan B, Jiang W,
Yang W, Cai X and Yang H: The frequency and clinical implication of
mismatch repair protein deficiency in Chinese patients with ovarian
clear cell carcinoma. BMC Cancer. 22:4492022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Virga A, Gianni C, Palleschi M, Angeli D,
Merloni F, Maltoni R, Ulivi P, Martinelli G, De Giorgi U and
Bravaccini S: A Novel AKT1, ERBB2, ESR1, KRAS, PIK3CA, and TP53 NGS
Assay: A Non-invasive tool to monitor resistance mechanisms to
hormonal therapy and CDK4/6 inhibitors. Biomedicines. 12:21832024.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yin H, Zhang M, Zhang Y, Zhang X, Zhang X
and Zhang B: Liquid biopsies in cancer. Mol Biomed. 6:182025.
View Article : Google Scholar : PubMed/NCBI
|