Introduction
Angiosarcoma is a highly aggressive malignant tumor
of endothelial cells that arises from blood or lymphatic vessels
and accounts for ~2% of all soft tissue sarcomas (1). Primary pulmonary angiosarcoma is
uncommon and is usually identified only after it has spread to
other parts of the body (2). This
report describes a case of primary pulmonary angiosarcoma and
highlights the complexities of its diagnosis and treatment.
Primary pulmonary angiosarcoma is difficult to
diagnose because its clinical symptoms are nonspecific (3,4).
Patients with this condition frequently present with symptoms such
as hemoptysis, chest pain and shortness of breath (5,6).
However, these symptoms are often ascribed to other conditions,
resulting in delayed diagnosis. Furthermore, the radiological
features of pulmonary angiosarcoma are typically nonspecific and
may resemble those of other pulmonary disorders (7,8).
This case report presents an uncommon manifestation
of primary pulmonary angiosarcoma, delineates the typical
diagnostic challenges and investigates potential therapeutic
targets revealed through genetic analysis.
Materials and methods
Immunohistochemistry (IHC) and
hematoxylin-eosin (HE) staining
IHC analysis of programmed cell death ligand 1
(PD-L1), vimentin, CD31, CD34, ETS-related gene (ERG) and factor
VIII antibody (VIII-RA). Experiment was conducted using
formalin-fixed, paraffin-embedded tumor tissue sections. Staining
included routine dewaxing, antigen retrieval, inhibition of
endogenous peroxidase activity, incubation with primary and
secondary antibodies and counterstaining. The primary antibodies
included anti-vimentin antibody (cat. no. AB5733; 1:1,000
dilution), rabbit anti-CD31 antibody (cat. no. SAB5700639; 1:100
dilution), rabbit anti-CD34 antibody (cat. no. HPA036722, 1:200
dilution) and anti-CD274 (cat. no SAB4301882; 1:100 dilution; all
from Sigma-Aldrich; Merck KGaA), factor VIII-related antigen (cat.
no. 36B11; 1:50 dilution; Novocastra) and rabbit monoclonal ERG
antibody (cat. no. IPDX17045; 1:100 dilution; IPODIX). The
secondary antibody was goat anti-rabbit IgG H&L (cat. no.
ab150077; 1:2,000; Abcam). Subsequently, the samples were evaluated
using a microscope (DMi3000 B; Leica Microsystems) to calculate the
tumor proportion score and the comprehensive positive score.
HE staining was conducted to confirm the
histopathological diagnosis. The tissue sections were first
de-waxed using xylene to remove the paraffin. Subsequently, they
were made transparent through a series of alcohol treatments. Next,
hematoxylin (cat. no. H9627; Sigma-Aldrich; Merck KGaA) was used to
stain the nuclei of the sections. Subsequently, distilled water was
used to rinse off the excess hematoxylin. After that, eosin (cat.
no. E4009; Sigma-Aldrich; Merck KGaA) was used for counter-staining
to enhance cell differentiation. Finally, the sections were
dehydrated through a series of alcohol treatments and covered with
a coverslip for mounting. The sections were then observed using a
light microscope (DMi3000 B; Leica Microsystems) to evaluate the
tissue structure, cell morphology, and general pathological
features.
Genetic testing
Genomic DNA was isolated from the patient's blood
sample following established protocols (9), including proteinase K (cat. no.
P2308-5MG; Sigma-Aldrich; Merck KGaA) digestion and
phenol-chloroform extraction. Targeted next-generation sequencing
was then conducted using a custom-designed panel encompassing 1,238
clinically relevant genes, including exons 2–22 of PD-L1.
Next-generation sequencing library preparation involved genomic DNA
fragmentation, end repair, A-tailing, adapter ligation and size
selection (10). Sequencing was
performed on an Illumina platform, achieving a minimum depth of
30-fold coverage within the targeted regions. The library was
constructed using the Nextera XT kit (Illumina, Inc.), and
sequencing was performed on the NextSeq™ 500 (Illumina, Inc.)
platform using the 2×150 bp kit according to the manufacturer's
instructions (11). Sequence reads
were aligned using BWA-MEM (http://bio-bwa.sourceforge.net/bwa.shtml) and variant
calling was performed with the Genome Analysis Toolkit (GATK)
(https://gatk.broadinstitute.org/hc/en-us) (12). The identified mutations were
annotated and classified based on data from the Human Genome
Variation Database (ClinVar) (https://www.ncbi.nlm.nih.gov/clinvar/), Single
Nucleotide Polymorphism Database (dbSNP) (https://www.ncbi.nlm.nih.gov/snp) and Catalogue of
Somatic Mutations in Cancer (COSMIC) (https://cancer.sanger.ac.uk/cosmic/login). Filtering
steps were subsequently applied to remove low-quality reads and
false-positive variants. The final variant report provided
comprehensive clinical information, including the identification
and categorization of all clinically significant mutations, thereby
facilitating informed therapeutic decision-making. These data have
been uploaded to the SRA database, with the BioProject ID being
PRJNA1344604. They are available for download (https://www.ncbi.nlm.nih.gov/Traces/study/?acc=SRP636518&o=acc_s%3Aa).
Case report
A 71-year-old man presented with a history of
hypertension, cerebral infarction and coronary artery disease. The
patient had a smoking history of >50 years, with an average
daily consumption of 20 cigarettes. No significant family medical
history was noted. The patient was admitted to the Department of
Respiratory and Critical Care Medicine at the Banan Affiliated
Hospital of Chongqing Medical University (Chongqing, China) in
February 2023 after the onset of symptoms, including cough, sputum
production and unexplained dyspnea.
On admission, the patient was conscious and
cooperative. The patient exhibited a productive cough with
expectoration of phlegm containing dark red blood clots,
accompanied by fatigue and anorexia. The patient subsequently
reported pain localized to the right hemithorax, accompanied by
worsening cough. The pain radiated to the right shoulder and dorsal
region.
Ultrasound examination of the thorax revealed
pleural effusion on the right side. Physical assessment
demonstrated diminished breath sounds in the right lung, but no
significant wet or dry adventitious sounds were detected in the
left lung. Thoracentesis yielded 350 ml of pleural fluid. Chest
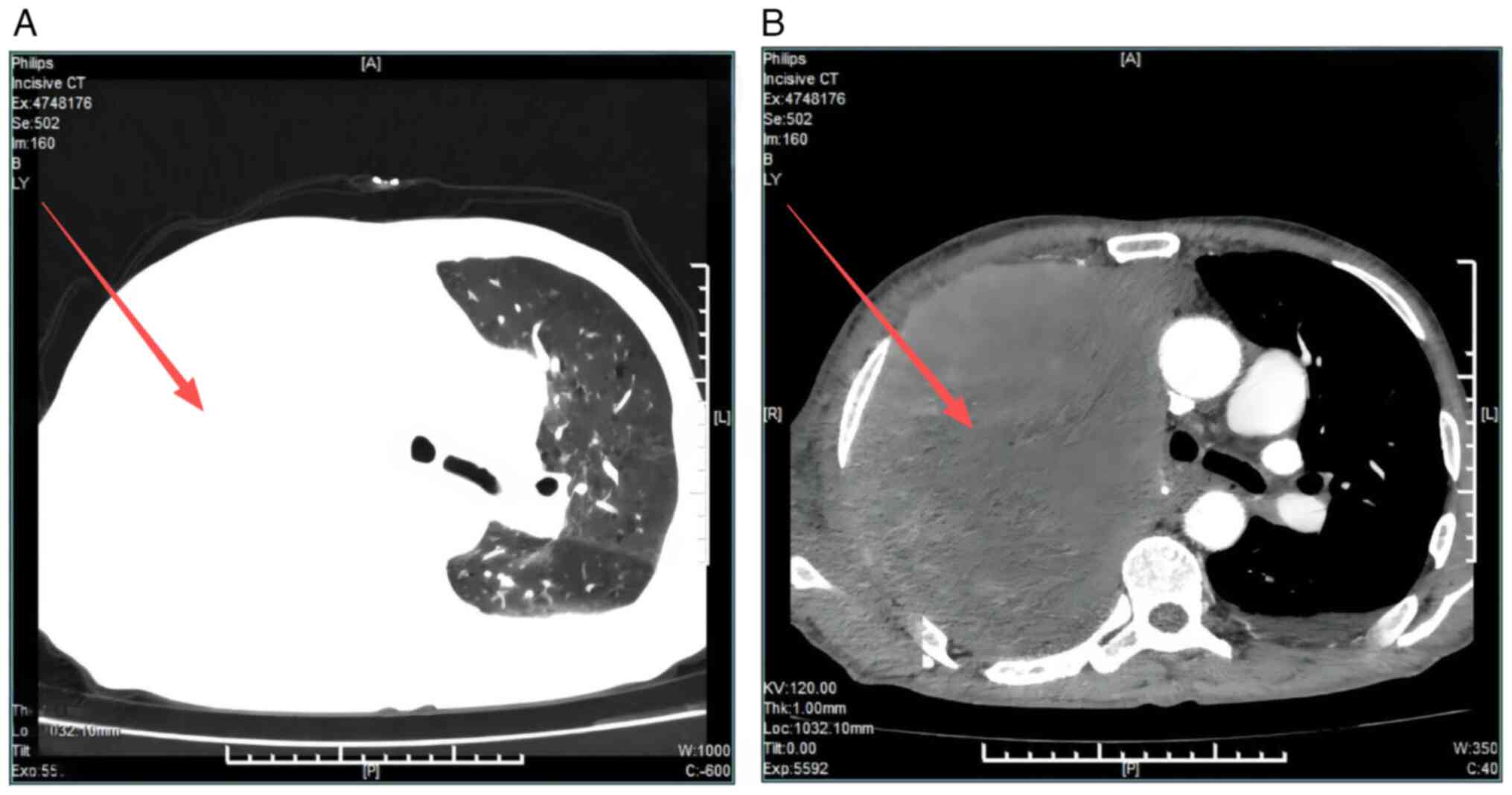
computed tomography (CT) imaging (Fig.
1, Videos S1 and S2) demonstrated the following findings:
i) A mass located in the right lung measuring ~147×113 mm, highly
suggestive of primary lung carcinoma with mediastinal invasion;
associated right pulmonary bronchial obstruction with resultant
atelectasis; involvement of the right pulmonary arterial trunk;
suspected mediastinal lymphadenopathy; evidence of right pleural
metastasis; and probable metastasis to the right ribs. ii) A small
volume of bilateral pleural effusions. iii) A nodule in the left
adrenal gland, with metastatic involvement excluded. iv) Mild
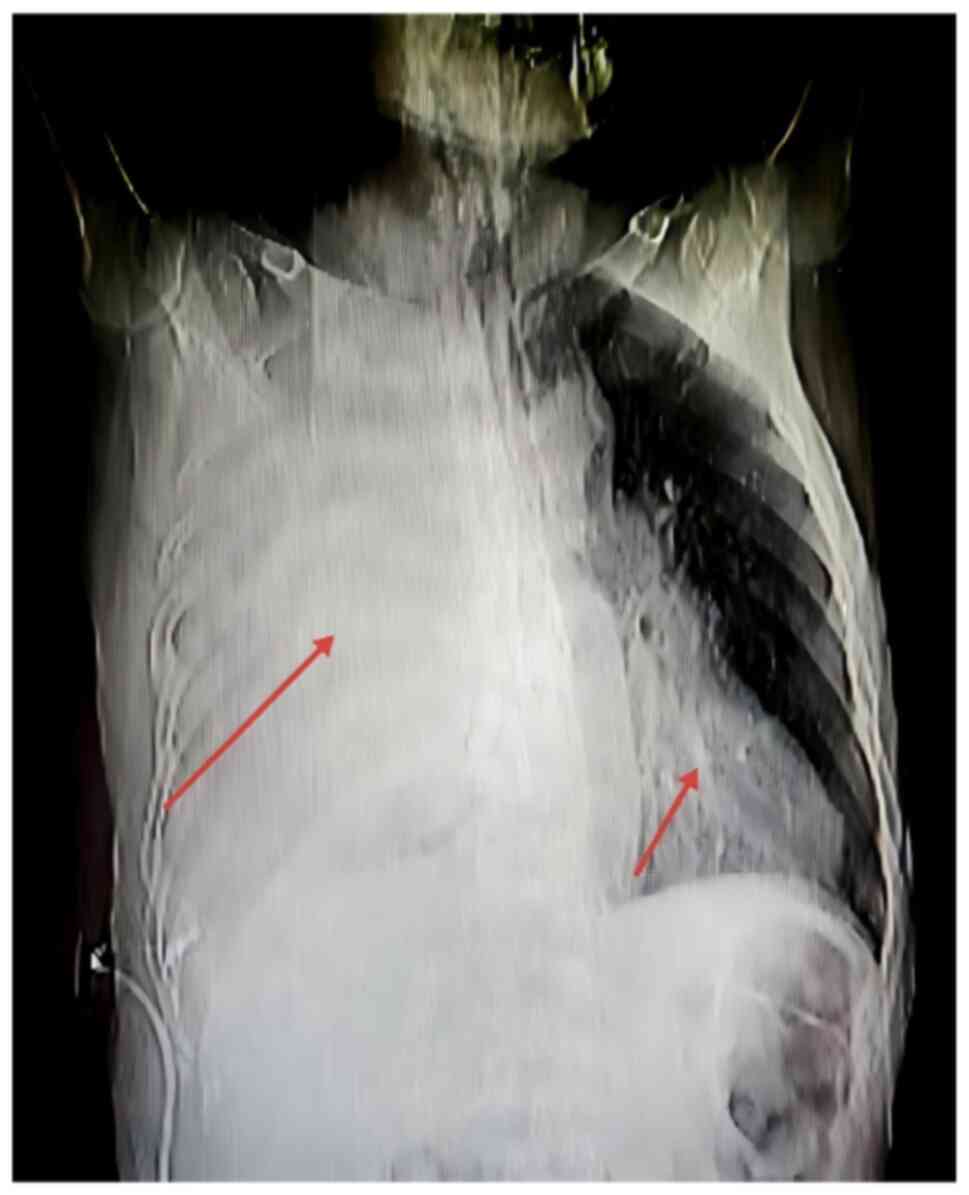
dilation of the common bile duct. Additionally, chest radiography
(Fig. 2) corroborated extensive
pathological involvement of the right lung with possible tumor
infiltration affecting the right lower lobe and extending into the
left lung, collectively manifesting as a generalized ‘white lung’
appearance.
Cytological examination revealed a small number of
atypical cells (data not shown) and the possibility of a malignant
tumor could not be ruled out. To further evaluate the patient's
condition, a thoracentesis was performed, which yielded 350 ml of
pleural effusion. Subsequently, a CT-guided percutaneous lung
biopsy was performed, resulting in the collection of three tissue
specimens. Laboratory assessments revealed elevated levels of tumor
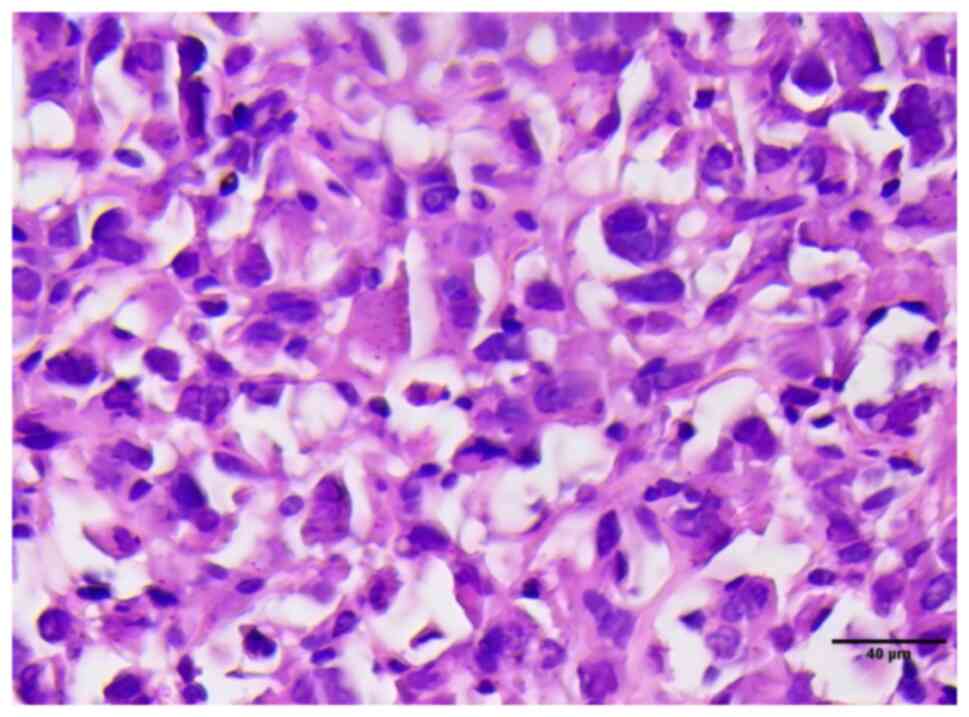
markers, notably Carcinoembryonic antigen (CEA). The HE staining
results showed that the cell nuclei were deeply stained, had
abnormal shapes, and there were numerous instances of cell division
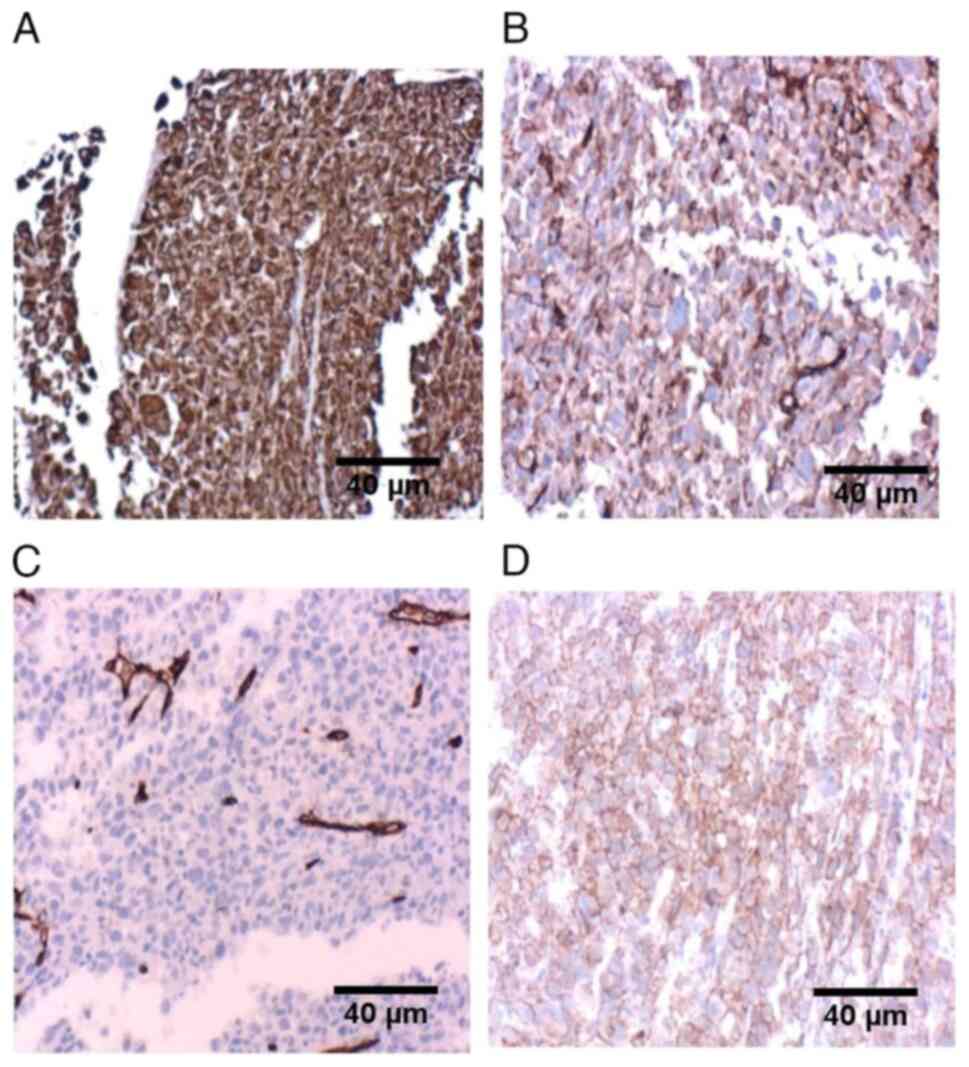
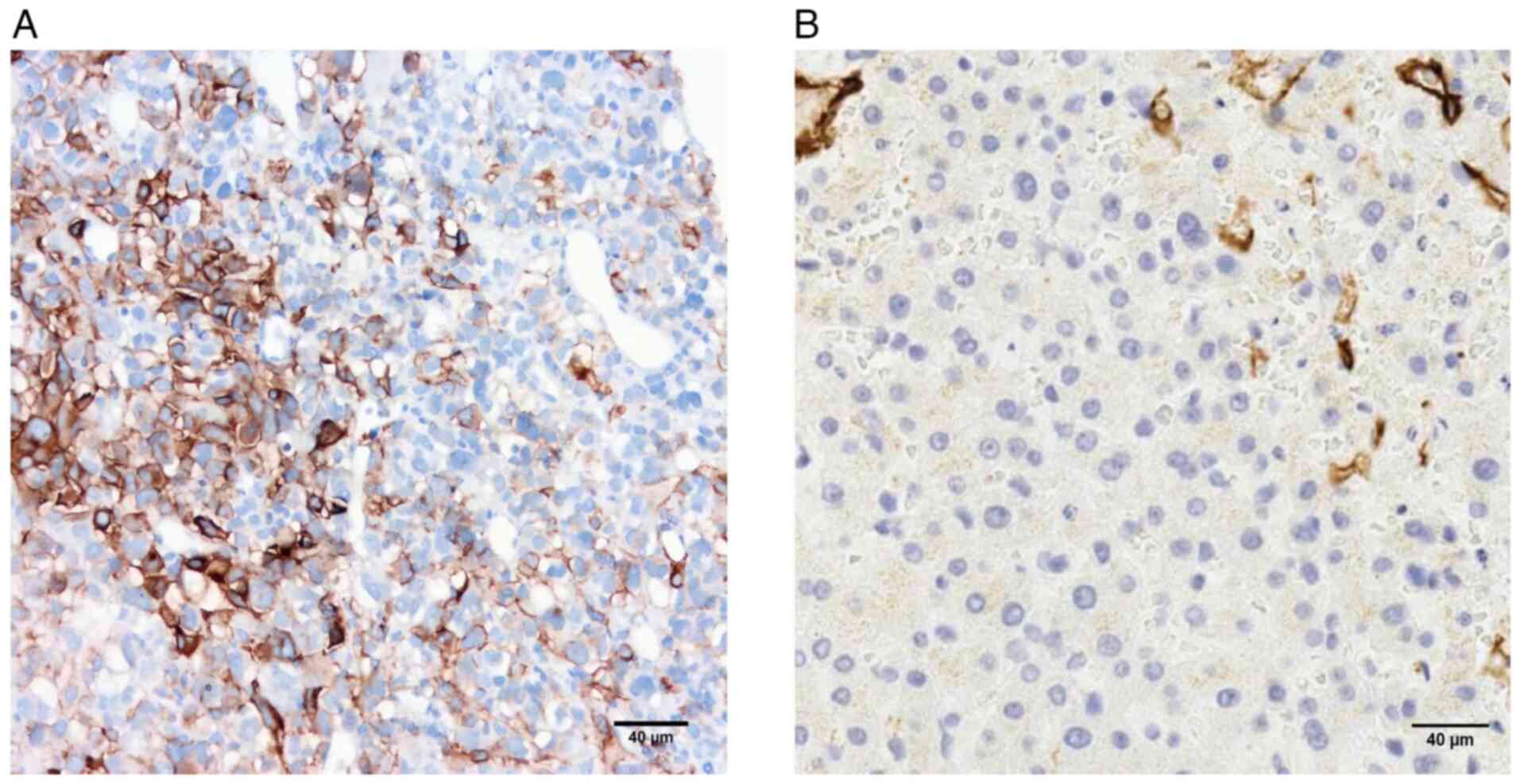
(Fig. 3). IHC examination indicated
positive expression of vimentin (Fig.
4A), CD31 (Fig. 4B), CD34
(Fig. 4C), and the ETS-related gene
(Fig. 4D), collectively suggesting
angiosarcoma. IHC analysis revealed positivity for both PD-L1 and
VIII-RA (Fig. 5). Based on the
ninth edition of the TNM classification system established by the
International Union Against Cancer (13), the patient was diagnosed with
pulmonary vascular sarcoma, staged as T2N1M1, corresponding to
overall clinical stage IV. This stage is characterized by a primary
tumor measuring between 5 and 10 cm in maximum diameter (T2), with
involvement of regional lymph nodes (N1) and the presence of
distant metastases (M1).
The patient's clinical condition progressively
deteriorated, ultimately precluding systemic chemotherapy.
Symptomatic treatment encompassed high-flow oxygen therapy,
hemostatic interventions and analgesic management. At the family's
request, genetic testing was performed to assess the feasibility of
targeted therapeutic options. Owing to the limited tissue obtained
from the initial lung puncture, a subsequent CT-guided percutaneous
lung biopsy was performed, yielding six specimens for comprehensive
multigene analysis pertinent to solid tumors. While awaiting
multigene test results, the patient demonstrated gradual
leukocytosis, suggestive of potential bone marrow involvement.
Concurrently, the patient's level of consciousness declined and
arterial blood gas analysis indicated hypercapnia. Nonsteroidal
anti-inflammatory drugs were administered for pain control and
local radiotherapy was provided. Regrettably, owing to the rapid
disease progression, the patient died before targeted therapy could
be initiated.
At three days after the patient's death, four
potential therapeutic target genes were identified within the
pan-cancer 1,238-gene panel, each of which exhibited an
amplification mutation. Specifically, CD274 demonstrated a
mutation abundance of 6.3-fold and atezolizumab was identified as a
potentially effective therapeutic agent. Kinase insert domain
receptor (KDR) showed a mutation abundance of 2.2-fold, with
regorafenib, sunitinib and sorafenib being potentially beneficial
drugs. Similarly, KIT exhibited a 2.2-fold mutation
abundance, with pazopanib as a potential treatment option. Lastly,
platelet-derived growth factor receptor α (PDGFRA) exhibited
a 2.2-fold mutation abundance, with pazopanib and sunitinib
considered potential therapeutic agents.
Discussion
The mechanisms underlying angiosarcoma have remained
to be fully elucidated. Patients who have undergone breast cancer
surgery and radiotherapy are prone to developing angiosarcoma of
the skin and chest (14). Pulmonary
angiosarcomas are even rarer, with most reports being case reports.
In the present case, the patient had been smoking for a long time,
similar to a previously reported case (15). However, the patient also had a
history of hypertension, cerebral infarction and coronary artery
disease. Currently, there is no direct evidence that pulmonary
angiosarcoma is associated with these factors, to the best of our
knowledge.
The clinical manifestations of pulmonary
angiosarcomas are often nonspecific, making them difficult to
detect. The most common clinical manifestation is hemoptysis, along
with symptoms such as dyspnea, chest pain and cough (5,16).
Patients often present with systemic manifestations such as weight
loss, fatigue and fever. In the present case, the patient presented
with a cough and hemoptysis. After examination, a mass was found in
the right lung and the pathological diagnosis was pulmonary
angiosarcoma. It can be seen that in clinical practice, common
respiratory symptoms, such as hemoptysis, chest pain and shortness
of breath, should be taken seriously and should not be readily
dismissed, so as not to overlook the diagnosis of pulmonary
angiosarcoma.
Currently, imaging examinations for pulmonary
angiosarcoma are mainly based on chest radiography and CT. In the
case reported by Yu et al (17), the main imaging manifestations were
multiple nodules, ground-glass patches and diffuse infiltration.
Imaging examination of this patient suggested a mass in the right
lung, which is consistent with previous reports. However, the
imaging features of pulmonary angiosarcoma lack specificity and are
often similar to those of other lesions, such as metastatic tumors
(including pulmonary tuberculosis), making differential diagnosis
difficult. Therefore, imaging data may provide imited reference
value for the diagnosis of this disease (5,17).
In the current clinical diagnosis of pulmonary
angiosarcoma, immunomarkers, such as CD34, CD31, VIII-RA, Fli-1
proto-oncogene and vimentin, are commonly used (3–6,17), all
of which are specific markers for endothelial cells of vascular
origin. Certain studies have reported that VIII-RA and CD31 are the
most specific marker antibodies for angiosarcoma, particularly in
poorly differentiated tumors (18–23).
In the present case, the IHC markers CD34, CD31 and vimentin were
all positive, which may have helped confirm the diagnosis.
The Italian Sarcoma Organization and Japanese
Sarcoma Association published consensus documents and guidelines
for angiosarcoma treatment (24,25).
The treatment plans typically include surgery, radiotherapy and
chemotherapy. For localized angiosarcomas, extensive resection is
the standard treatment method and a clean surgical margin is one of
the factors affecting prognosis (26). Surgery is the primary treatment for
localized disease; however, the postoperative recurrence rate is
high. Bartakke and Saez de Ibarra Sanchez (27) reported a case of pulmonary
angiosarcoma that was successfully treated surgically. Adjuvant
radiotherapy after radical surgery has been proven to be an
effective combination treatment for angiosarcoma; however, no
formal observational trial has demonstrated that radiotherapy alone
is effective. Chemotherapy is the primary treatment for patients
who have lost the opportunity to undergo surgery. The main
chemotherapeutic drugs include taxanes, doxorubicin, liposomal
doxorubicin and ifosfamide. Among these, taxanes are considered
effective agents for the clinical treatment of angiosarcoma and are
often used as first- or second-line treatments for metastatic
disease (25,28,29).
In addition, recombinant IL-2 may have a positive effect on
angiosarcoma treatment (30). In
recent years, new approaches have provided novel strategies for the
treatment of angiosarcoma. Angiosarcoma cells with high VEGFR-2
expression are sensitive to anti-VEGFR antibody drugs, such as
sorafenib and sunitinib (31). A
similar drug, pazopanib, has entered clinical trials for cutaneous
angiosarcoma as a second-line standard therapy, but its overall
efficacy has not met the primary endpoint (32). Further trials are required before
these drugs can be adopted clinically. As shown in Table I, there are various current
treatment methods; however, most patients present with multiple
metastases at diagnosis, resulting in a poor prognosis. In the
present case, after diagnosis, the patient had multiple metastases
to the mediastinum, right pulmonary artery trunk, mediastinal lymph
nodes, right pleura and right third rib and eventually died. The
patient therefore missed the opportunity to receive targeted
therapy.
 | Table I.Summary of reported cases of
pulmonary angiosarcoma. |
Table I.
Summary of reported cases of
pulmonary angiosarcoma.
| Patient
details | Clinical
presentation | Diagnostic
basis | Treatment | Outcome | (Refs.) |
|---|
| 71-year-old
male | Recurrent cough,
expectoration, hemoptysis, chest pain, dyspnea | Large right lung
mass, pleural effusion, IHC (CD34+, CD31+,
vimentin+, Ki67 60%) | Symptomatic
support, genetic testing identified actionable mutations (CD274,
KDR, KIT, PDGF) | Died prior to
targeted therapy initiation | Present case
report |
| 70-year-old
male | Hemoptysis, weight
loss, general weakness and mild recurrent epistaxis | Pulmonary nodules,
hemorrhagic pleural effusion, pleural cavity masses, IHC
(CD31+, vimentin+) | Discharged from the
hospital and referred to the tumor center to consider any further
treatment options | Died five days
after discharge | (3) |
| 11 cases | Chest pain, cough,
breathing difficulties, hemoptysis | All patients were
eventually diagnosed by pathology, and the positive rates of CD31
and CD34 were as high as 60% | Three cases
received surgical treatment and the remaining eight patients
received chemotherapy or immunotherapy | Surgical treatment
OS: 23 months; Non-surgical treatment OS: 9.7 months | (4) |
| 34-year-old
woman | Cough, hemoptysis
and breathing difficulties | Multiple small
nodules and ground-glass patches in both lungs; Pleural bloody
effusion; IHC (CD34+, CD31+, Ki67 70%,
ERG+, FLI-1+) | Hypovolemic shock
occurred after thoracoscopic lung biopsy and was transferred to the
ICU for resuscitation | Died of respiratory
failure 19 days later | (6) |
| 72-year-old
male | Shortness of breath
worsens | Impaired right
ventricular function, pulmonary artery mass | Endarterectomy for
pulmonary thrombosis, paclitaxel chemotherapy | Died of acute
pulmonary thrombosis after 4 months | (16) |
Most literature reports cases of pulmonary vascular
sarcoma (4,5,33), as
well as related studies (34,35).
However, most of these articles focused on the clinical symptoms,
diagnostic markers and conventional treatments of pulmonary
vascular sarcoma, while in the present study, gene sequencing was
conducted, thus paying more attention to gene mutations and related
targeted therapies.
Regarding gene-targeted therapy, an angiosarcoma
research project conducted by Painter et al (36) indicated that anti-PD-1 therapy may
be an effective approach for head and neck, face and scalp
angiosarcoma. Another study has shown that PD-L1 is positively
expressed in 29% of angiosarcomas (37). PD-L1 is highly expressed in
angiosarcoma (38–41). Research conducted by Kösemehmetoğlu
et al (41) indicated that a
higher tumor grade and a worse patient prognosis are associated
with a higher expression of PD-L1 in soft tissue sarcomas.
Atezolizumab can be used as an antibody targeting CD274 for cancer
treatment (42). However, the
therapeutic effects of anti-PD-1 drugs in primary pulmonary
vascular sarcomas have not yet been confirmed. KDR mutations
are present in pancreatic, cardiac, breast and renal angiosarcomas
(43–46). In angiosarcoma, the incidence of KDR
mutations is ~7–10% (47).
Amplification of KDR is associated with activation of mTOR
and p38, as well as infiltration of tumor cells caused by VEGF
(48). The clinical trials
NCT02693535, NCT03297606 and NCT02029001 have reported that
regorafenib, sunitinib and sorafenib, respectively, can target and
treat tumors caused by KDR amplification. Relatively few
studies have reported significant mutation characteristics of
KIT and PDGFRA in angiosarcoma (49–54).
Research conducted by Xu et al (55) reported that KIT mutations are
widespread in angiosarcoma, occurring in ~25% of cases. Because
PDGFRA mutations are relatively rare in angiosarcoma, there
are no published studies specifically reporting the incidence of
these mutations, to the best of our knowledge. However, studies on
the chromosome 4q11-q13.1 region (containing KIT, PDGFRA and
VEGFR2) and the 4q12 amp region (containing KIT,
PDGFRA and KDR) have shown that PDGFRA mutations
are present in angiosarcomas (54,56),
with 4q12 amp being detected in 4.8% of angiosarcomas (56). Since primary pulmonary angiosarcoma
is relatively rare, KIT and PDGFRA mutations are more
often found in other angiosarcomas, whereas pulmonary angiosarcoma
cases are rarely reported. The clinical trial NCT02029001
demonstrated that pazopanib may be effective in treating advanced
malignant solid tumors caused by KIT amplification. In
addition, apatinib can be used to treat angiosarcomas caused by
KIT and KDR gene amplification (50). Pazopanib may be applicable to
gastrointestinal stromal tumors (57) and the US Food and Drug
Administration has approved its use for soft tissue sarcomas.
Sunitinib is used for the treatment of gastrointestinal stromal
tumors and renal cell carcinomas (58,59).
It may also inhibit the activity of various receptor tyrosine
kinases, including PDGFR (38). The
clinical trial NCT03297606 indicated that sunitinib may be
applicable to various tumors caused by PDGFRA amplification.
Because of the rarity and nonspecific clinical
symptoms of primary pulmonary angiosarcoma, early identification
and treatment remain important challenges. This case highlights the
diagnostic and therapeutic difficulties associated with primary
pulmonary angiosarcoma and underscores the potential role of
genomic findings in guiding future treatment strategies.
In conclusion, this study reports the case of
primary pulmonary vascular sarcoma in a 71-year-old man with
hemoptysis, chest pain and shortness of breath. Immunological
marker testing confirmed the diagnosis of primary pulmonary
vascular sarcoma. Genetic testing revealed mutations that may be
targeted for therapy. However, the rapid progression of the tumor
hindered treatment implementation, highlighting the need for early
diagnosis and personalized treatment.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The raw sequence reads were uploaded to the SRA
database, with the BioProject ID PRJNA1344604. They are available
for download from the following URL: https://www.ncbi.nlm.nih.gov/Traces/study/?acc=SRP636518&o=acc_s%3Aa.
Authors' contributions
YZ conceived and designed the study, performed the
experiments, analyzed the data and wrote the manuscript. CG
collected clinical data, performed pathological analyses and
contributed to manuscript preparation. YZ and CG confirm the
authenticity of all the raw data. Both authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present research followed the Declaration of
Helsinki and was approved by the Banan Hospitals Ethics Committee
(Chongqing, China; approval no. BNLLKY2025074). As the patient's
condition deteriorated and he lost consciousness, written informed
consent was obtained from the patient's daughter for the patient to
undergo imaging examinations, pathological examinations and genetic
testing.
Patient consent for publication
Written informed consent was obtained from the
patient's daughter for the publication of this manuscript and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Young RJ, Brown NJ, Reed MW, Hughes D and
Woll PJ: Angiosarcoma. Lancet Oncol. 11:983–991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khalid K, Khan A, Lomiguen CM and Chin J:
Clinical detection of primary pulmonary angiosarcoma. Cureus.
13:e170592021.PubMed/NCBI
|
|
3
|
Rana MK, Rahman O and O'Brien A: Primary
pulmonary angiosarcoma. BMJ Case Rep. 14:e2445782021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ren Y, Zhu M, Liu Y, Diao X and Zhang Y:
Primary pulmonary angiosarcoma: Three case reports and literature
review. Thorac Cancer. 7:607–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luan T, Hao J, Gu Y, He P, Li Y, Wang L,
Deng H, Guan W, Lin X, Xie X, et al: A clinical analysis and
literature review of eleven cases with primary pulmonary
angiosarcoma. BMC Cancer. 24:15972024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Huang X, Peng C, Wang Y, Wu Q, Wu
Z, Shao H and Wang W: Primary pulmonary epithelioid angiosarcoma: A
case report and literature review. J Cancer Res Ther. 14
(Suppl):S533–S535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang X, Zhu J, Zhu F, Tu H, Deng A, Lu J,
Yang M, Dai L, Huang K and Zhang L: Case report: Primary pulmonary
angiosarcoma with brain metastasis. Front Bioeng Biotechnol.
9:8038682021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Basiri R, Ziaei Moghaddam A, Rikhtegar A
and Jafarian AH: Primary pulmonary angiosarcoma found incidentally
in a complicated patient: A rare case report. Clin Respir J.
18:e138182024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Ma X, Chen J, Wang H and Yu Z:
Nontuberculous mycobacteria by metagenomic next-generation
sequencing: Three cases reports and literature review. Front Public
Health. 10:9722802022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu W, Miller S and Chiu CY: Clinical
metagenomic next-generation sequencing for pathogen detection. Annu
Rev Pathol. 14:319–338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bejaoui S, Nielsen SH, Rasmussen A, Coia
JE, Andersen DT, Pedersen TB, Møller MV, Kusk Nielsen MT, Frees D
and Persson S: Comparison of Illumina and Oxford Nanopore
sequencing data quality for Clostridioides difficile genome
analysis and their application for epidemiological surveillance.
BMC Genomics. 26:922025. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McKenna A, Hanna M, Banks E, Sivachenko A,
Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly
M and DePristo MA: The genome analysis toolkit: A MapReduce
framework for analyzing next-generation DNA sequencing data. Genome
Res. 20:1297–1303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang B, Fang WT and Zhong H: Introduction
to the 9th edition of TNM classification for lung
cancer. Zhonghua Zhong Liu Za Zhi. 46:206–210. 2024.(In Chinese).
PubMed/NCBI
|
|
14
|
Bonito FJP, de Almeida Cerejeira D,
Dahlstedt-Ferreira C, Oliveira Coelho H and Rosas R:
Radiation-induced angiosarcoma of the breast: A review. Breast J.
26:458–463. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piechuta A, Przybyłowski T, Szołkowska M
and Krenke R: Hemoptysis in a patient with multifocal primary
pulmonary angiosarcoma. Pneumonol Alergol Pol. 84:283–289.
2016.PubMed/NCBI
|
|
16
|
Lim R, Harper L and Swiston J: Clinical
manifestations and diagnostic methods in pulmonary angiosarcoma:
Protocol for a scoping review. Syst Rev. 6:1362017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu M, Huang W, Wang Y, Wang G, Wang L, Tao
W, Faiz SA, Ng FH and Li H: Pulmonary angiosarcoma presenting with
diffuse alveolar hemorrhage: A case report. Ann Transl Med.
9:742021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Studer LL and Selby DM: Hepatic
Epithelioid Hemangioendothelioma. Arch Pathol Lab Med. 142:263–267.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giuffrida MA, Bacon NJ and Kamstock DA:
Use of routine histopathology and factor VIII-related antigen/von
Willebrand factor immunohistochemistry to differentiate primary
hemangiosarcoma of bone from telangiectatic osteosarcoma in 54
dogs. Vet Comp Oncol. 15:1232–1239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Silwal A, B R S, Rehman S, Daing A,
Gopagoni R, Alyas Akram M, Saeed A, Sadiq KO, Farrukh AM and
Harikrishna A: Bladder angiosarcoma: A systematic literature review
and survival analysis. Proc (Bayl Univ Med Cent). 38:305–312.
2025.PubMed/NCBI
|
|
21
|
Kapagan T, Bulut N, Erdem GU, Yıldırım S,
Erdem ZB and Sahin H: Synchronous double Primary Angiosarcoma
originating from the stomach and rectum: A case report and a
literature review. Arch Iran Med. 27:168–173. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salehi M, Rehman S, Davis S and Jafari HR:
Angiosarcoma of gallbladder, a literature review. J Med Case Rep.
18:622024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma XM, Yang BS, Yang Y, Wu GZ, Li YW, Yu
X, Ma XL, Wang YP, Hou XD and Guo QH: Small intestinal angiosarcoma
on clinical presentation, diagnosis, management and prognosis: A
case report and review of the literature. World J Gastroenterol.
29:561–578. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palassini E, Baldi GG, Sulfaro S,
Barisella M, Bianchi G, Campanacci D, Fiore M, Gambarotti M,
Gennaro M, Morosi C, et al: Clinical recommendations for treatment
of localized angiosarcoma: A consensus paper by the Italian Sarcoma
Group. Cancer Treat Rev. 126:1027222024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujimura T, Maekawa T, Kato H, Ito T,
Matsushita S, Yoshino K, Fujisawa Y, Ishizuki S, Segawa K, Yamamoto
J, et al: Treatment for taxane-resistant cutaneous angiosarcoma: A
multicenter study of 50 Japanese cases. J Dermatol. 50:912–916.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou
C, Smith KD, Phung TL, Pollock RE, Benjamin R, Hunt KK, et al:
Angiosarcoma: Clinical and molecular insights. Ann Surg.
251:1098–1106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bartakke AA and Saez de Ibarra Sanchez JI:
Successful perioperative management of a primary pulmonary arterial
angiosarcoma: Case report. Braz J Anesthesiol.
74:7441862024.PubMed/NCBI
|
|
28
|
Cao J, Wang J, He C and Fang M:
Angiosarcoma: A review of diagnosis and current treatment. Am J
Cancer Res. 9:2303–2313. 2019.PubMed/NCBI
|
|
29
|
Ito T, Uchi H, Nakahara T, Tsuji G, Oda Y,
Hagihara A and Furue M: Cutaneous angiosarcoma of the head and
face: A single-center analysis of treatment outcomes in 43 patients
in Japan. J Cancer Res Clin Oncol. 142:1387–1394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Noguchi G, Ota J, Ishigaki H, Onuki T,
Kato Y and Moriyama M: A case of retroperitoneal angiosarcoma
effectively treated with recombinant interleukin-2. Nihon Hinyokika
Gakkai Zasshi. 103:697–703. 2012.(In Japanese). PubMed/NCBI
|
|
31
|
Park MS, Ravi V and Araujo DM: Inhibiting
the VEGF-VEGFR pathway in angiosarcoma, epithelioid
hemangioendothelioma, and hemangiopericytoma/solitary fibrous
tumor. Curr Opin Oncol. 22:351–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jones RL, Ravi V, Brohl AS, Chawla S,
Ganjoo KN, Italiano A, Attia S, Burgess MA, Thornton K, Cranmer LD,
et al: Efficacy and safety of TRC105 Plus Pazopanib vs Pazopanib
alone for treatment of patients with advanced angiosarcoma: A
randomized clinical trial. JAMA Oncol. 8:740–747. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edemskiy A, Vasiltseva O, Kliver E,
Novikova N, Sirota D and Chernyavskiy A: Surgical treatment of
pulmonary artery Angiosarcoma-a ten-year experience. Braz J
Cardiovasc Surg. 40:e202304412025. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin Y, Yu H, Wang C and Zhang D: Primary
pulmonary angiosarcoma mimicking diffuse pulmonary hemorrhage: A
case report. Oncol Lett. 25:2112023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu L, Sun Y, Wang M, Yuan L, Wang Q and
Qian X: Primary pulmonary epithelioid angiosarcoma with thyroid
tumor history: A case report and literature review. Exp Ther Med.
24:4712022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Painter CA, Jain E, Tomson BN, Dunphy M,
Stoddard RE, Thomas BS, Damon AL, Shah S, Kim D, Gómez Tejeda
Zañudo J, et al: The angiosarcoma project: Enabling genomic and
clinical discoveries in a rare cancer through patient-partnered
research. Nat Med. 26:181–187. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vargas AC, Maclean FM, Sioson L, Tran D,
Bonar F, Mahar A, Cheah AL, Russell P, Grimison P, Richardson L and
Gill AJ: Prevalence of PD-L1 expression in matched recurrent and/or
metastatic sarcoma samples and in a range of selected sarcomas
subtypes. PLoS One. 15:e02225512020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin J, Xie Y, Zhang JS, Wang JQ, Dai SJ,
He WF, Li SY, Ashby CR Jr, Chen ZS and He Q: Sunitinib resistance
in renal cell carcinoma: From molecular mechanisms to predictive
biomarkers. Drug Resist Updat. 67:1009292023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee AQ, Hao C, Pan M, Ganjoo KN and Bui
NQ: Histologic and immunologic factors associated with response to
immune checkpoint inhibitors in advanced sarcoma. Clin Cancer Res.
31:678–684. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Googe PB, Flores K, Jenkins F, Merritt B,
Moschos SJ and Grilley-Olson JE: Immune Checkpoint markers in
superficial angiosarcomas: PD-L1, PD-1, CD8, LAG-3, and
tumor-infiltrating lymphocytes. Am J Dermatopathol. 43:556–559.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kösemehmetoğlu K, Özoğul E, Babaoğlu B,
Tezel GG and Gedikoğlu G: Programmed death Ligand 1 (PD-L1)
expression in malignant mesenchymal tumors. Turk Patoloji Derg.
1:192–197. 2017.PubMed/NCBI
|
|
42
|
Yi M, Zheng X, Niu M, Zhu S, Ge H and Wu
K: Combination strategies with PD-1/PD-L1 blockade: Current
advances and future directions. Mol Cancer. 21:282022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Whitlock RS, Ebare K, Cheng LS, Fishman
DS, Mills JL, Nguyen HN, Nuchtern JG, Ruan W, Smith VE, Patel KA,
et al: Angiosarcoma of the pancreas in a pediatric patient with an
activating KDR-internal tandem duplication: A case report and
review of the literature. J Pediatr Hematol Oncol. 44:e751–e755.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Odintsov I, Papke DJ, George S, Padera RF,
Hornick JL and Siegmund SE: Genomic profiling of cardiac
angiosarcoma reveals novel targetable KDR variants, recurrent MED12
mutations, and a high burden of Germline POT1 alterations. Clin
Cancer Res. 31:1091–1102. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Omiyale AO: Primary vascular tumours of
the kidney. World J Clin Oncol. 12:1157–1168. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Beca F, Krings G, Chen YY, Hosfield EM,
Vohra P, Sibley RK, Troxell ML, West RB, Allison KH and Bean GR:
Primary mammary angiosarcomas harbor frequent mutations in KDR and
PIK3CA and show evidence of distinct pathogenesis. Mod Pathol.
33:1518–1526. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Torrence D and Antonescu CR: The genetics
of vascular tumours: An update. Histopathology. 80:19–32. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nilsson MB, Giri U, Gudikote J, Tang X, Lu
W, Tran H, Fan Y, Koo A, Diao L, Tong P, et al: KDR amplification
is associated with VEGF-induced activation of the mTOR and invasion
pathways but does not predict clinical benefit to the VEGFR TKI
Vandetanib. Clin Cancer Res. 22:1940–1950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Styring E, Seinen J, Dominguez-Valentin M,
Domanski HA, Jönsson M, von Steyern FV, Hoekstra HJ, Suurmeijer AJ
and Nilbert M: Key roles for MYC, KIT and RET signaling in
secondary angiosarcomas. Br J Cancer. 111:407–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang L, Liu L, Han B, Han W and Zhao M:
Apatinib treatment for KIT- and KDR-amplified angiosarcoma: A case
report. BMC Cancer. 18:6182018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dickerson EB, Marley K, Edris W, Tyner JW,
Schalk V, Macdonald V, Loriaux M, Druker BJ and Helfand SC:
Imatinib and dasatinib inhibit hemangiosarcoma and implicate
PDGFR-β and Src in tumor growth. Transl Oncol. 6:158–168. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Homsi J and Daud AI: Spectrum of activity
and mechanism of action of VEGF/PDGF inhibitors. Cancer Control.
14:285–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Terada T: Angiosarcoma of the oral cavity.
Head Neck Pathol. 5:67–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Prenen H, Smeets D, Mazzone M, Lambrechts
D, Sagaert X, Sciot R and Debiec-Rychter M: Phospholipase C gamma 1
(PLCG1) R707Q mutation is counterselected under targeted therapy in
a patient with hepatic angiosarcoma. Oncotarget. 6:36418–34425.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xu L, Xie X, Shi X, Zhang P, Liu A, Wang J
and Zhang B: Potential application of genomic profiling for the
diagnosis and treatment of patients with sarcoma. Oncol Lett.
21:3532021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Disel U, Madison R, Abhishek K, Chung JH,
Trabucco SE, Matos AO, Frampton GM, Albacker LA, Reddy V,
Karadurmus N, et al: The Pan-cancer landscape of coamplification of
the tyrosine kinases KIT, KDR, and PDGFRA. Oncologist. 25:e39–e47.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mir O, Cropet C, Toulmonde M, Cesne AL,
Molimard M, Bompas E, Cassier P, Ray-Coquard I, Rios M, Adenis A,
et al: Pazopanib plus best supportive care versus best supportive
care alone in advanced gastrointestinal stromal tumours resistant
to imatinib and sunitinib (PAZOGIST): A randomised, multicentre,
open-label phase 2 trial. Lancet Oncol. 17:632–641. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Demlová R, Turjap M, Peš O, Kostolanská K
and Juřica J: Therapeutic drug monitoring of sunitinib in
gastrointestinal stromal tumors and metastatic renal cell carcinoma
in adults-a review. Ther Drug Monit. 42:20–32. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dufies M, Giuliano S, Viotti J,
Borchiellini D, Cooley LS, Ambrosetti D, Guyot M, Ndiaye PD, Parola
J, Claren A, et al: CXCL7 is a predictive marker of sunitinib
efficacy in clear cell renal cell carcinomas. Br J Cancer.
117:947–953. 2017. View Article : Google Scholar : PubMed/NCBI
|