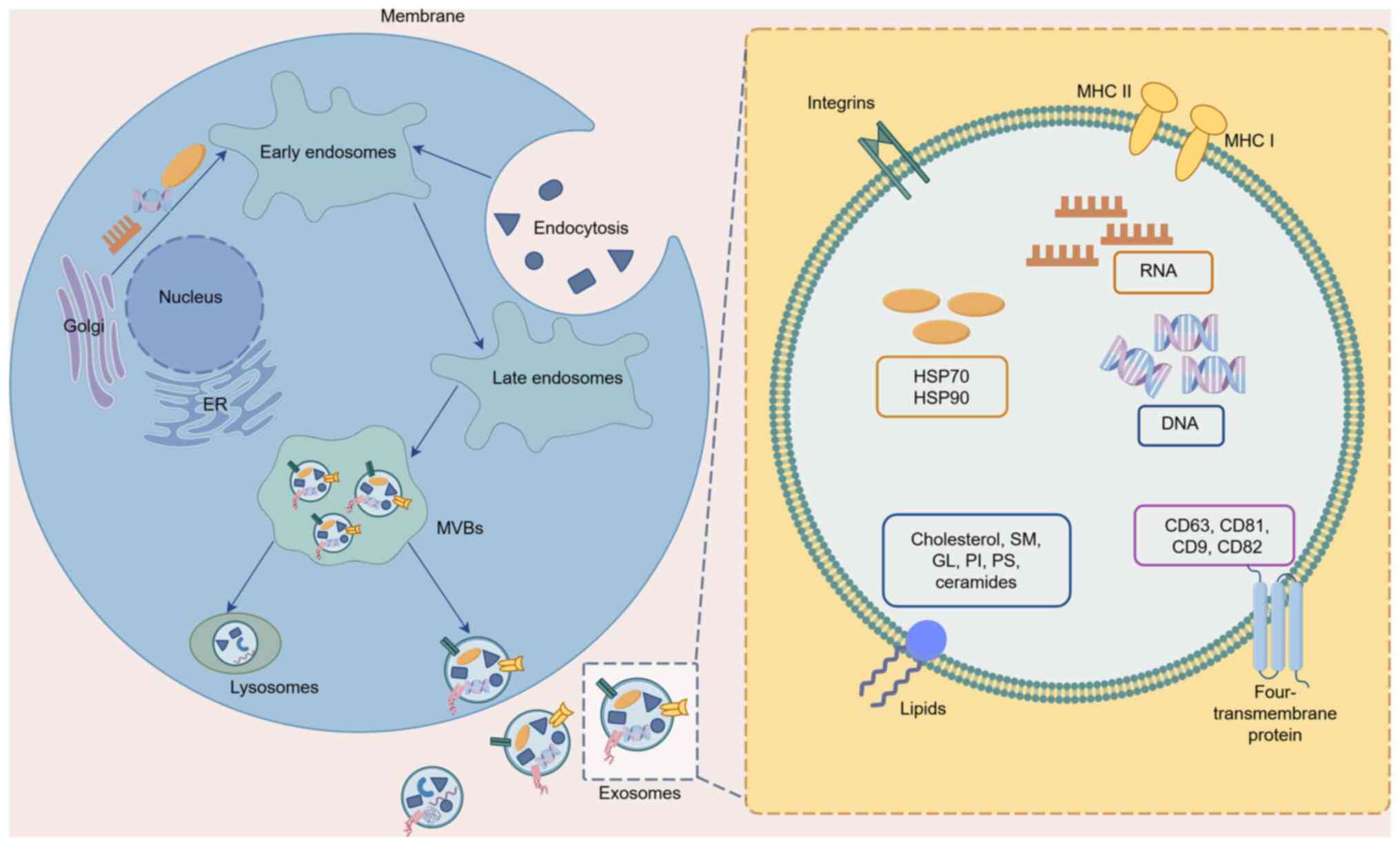
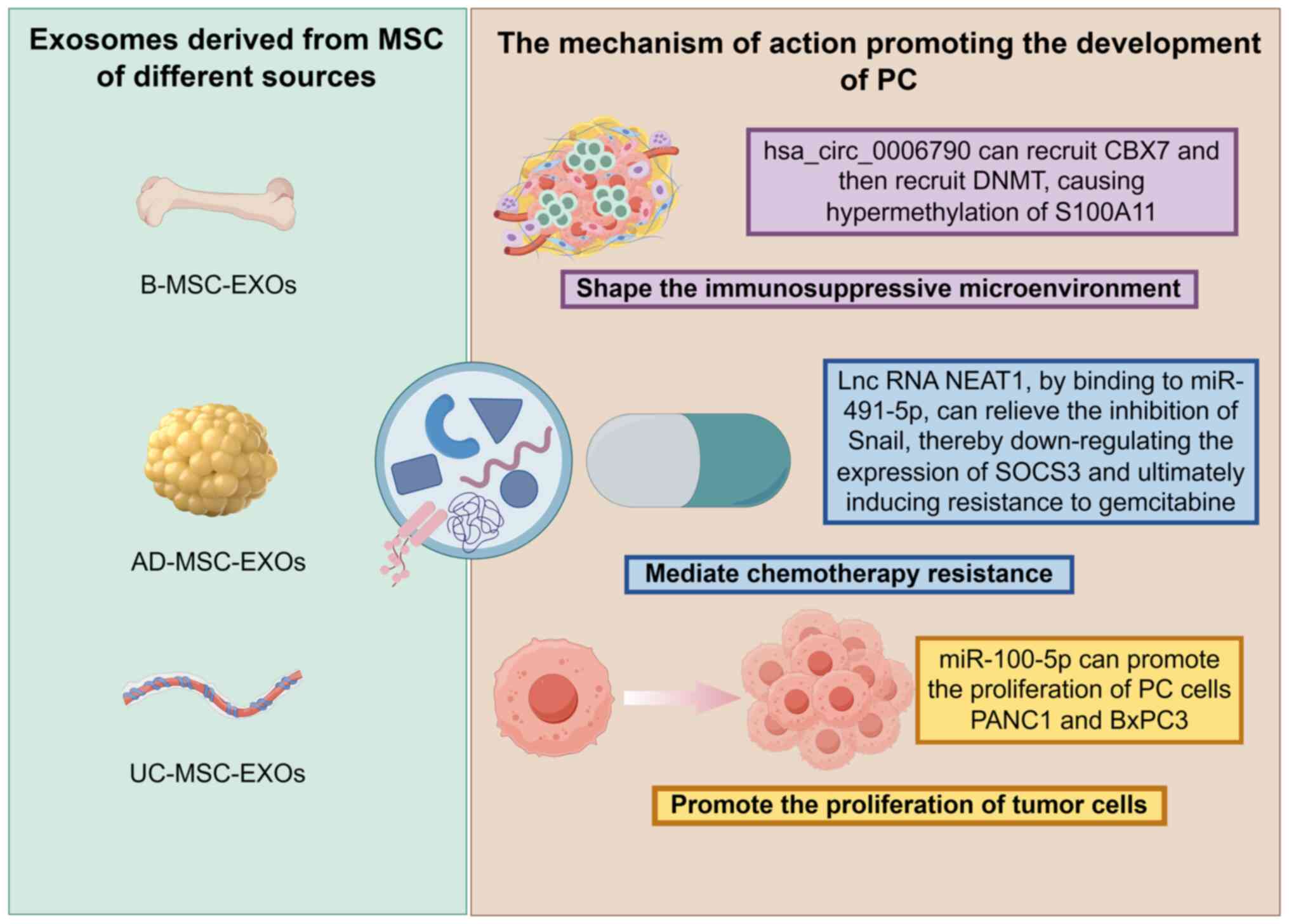
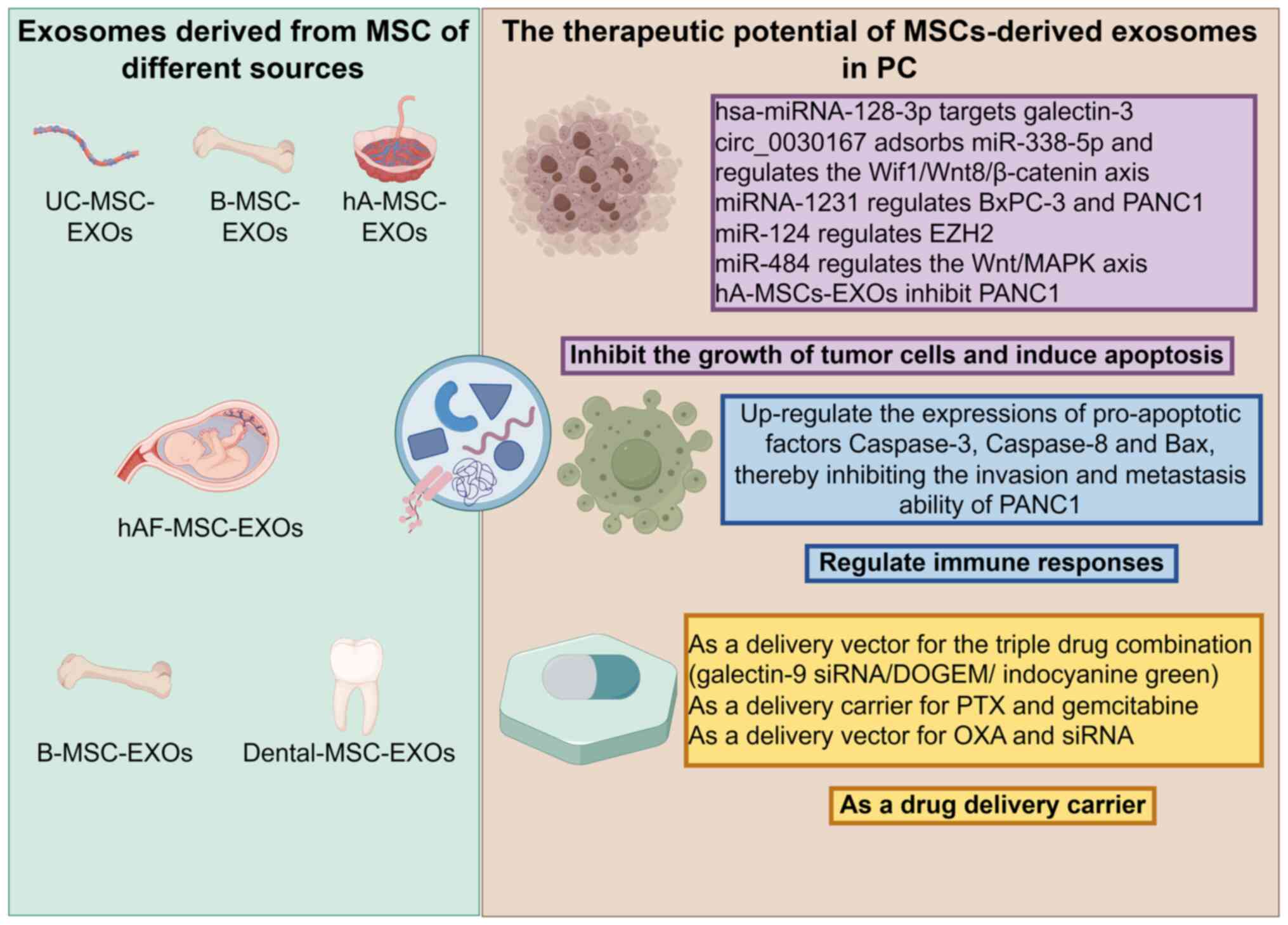
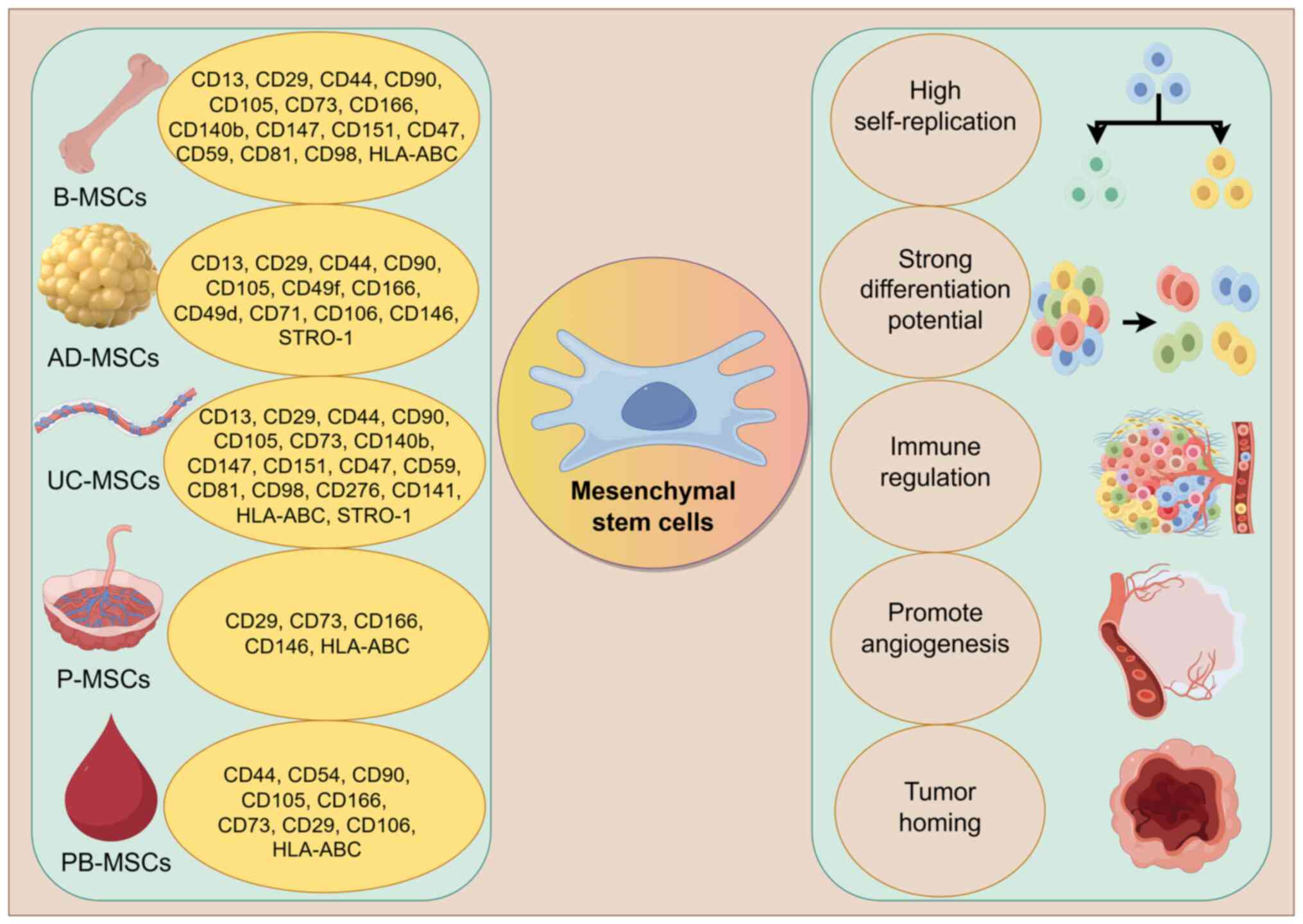
|
1
|
Klein AP: Pancreatic cancer epidemiology:
understanding the role of lifestyle and inherited risk factors. Nat
Rev Gastroenterol Hepatol. 18:493–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI
|
|
3
|
Park W, Chawla A and O'Reilly EM:
Pancreatic cancer: A review. JAMA. 326:851–862. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleeff J, Korc M, Apte M, La Vecchia C,
Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH
and Neoptolemos JP: Pancreatic cancer. Nat Rev Dis Primers.
2:160222016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Halbrook CJ, Lyssiotis CA, Pasca di
Magliano M and Maitra A: Pancreatic cancer: Advances and
challenges. Cell. 186:1729–1754. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klatte DCF, Wallace MB, Löhr M, Bruno MJ
and van Leerdam ME: Hereditary pancreatic cancer. Best Pract Res
Clin Gastroenterol. 58–59. 1017832022.PubMed/NCBI
|
|
7
|
Wood LD, Canto MI, Jaffee EM and Simeone
DM: Pancreatic cancer: Pathogenesis, screening, diagnosis, and
treatment. Gastroenterology. 163:386–402.e1. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai CC, Su PF, Huang YF, Yew TL and Hung
SC: Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal
and undifferentiated state in mesenchymal stem cells. Mol Cell.
47:169–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YC, Lan YW, Huang SM, Yen CC, Chen W,
Wu WJ, Staniczek T, Chong KY and Chen CM: Human amniotic fluid
mesenchymal stem cells attenuate pancreatic cancer cell
proliferation and tumor growth in an orthotopic xenograft mouse
model. Stem Cell Res Ther. 13:2352022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kabashima-Niibe A, Higuchi H, Takaishi H,
Masugi Y, Matsuzaki Y, Mabuchi Y, Funakoshi S, Adachi M, Hamamoto
Y, Kawachi S, et al: Mesenchymal stem cells regulate
epithelial-mesenchymal transition and tumor progression of
pancreatic cancer cells. Cancer Sci. 104:157–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beckermann BM, Kallifatidis G, Groth A,
Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner
W, Diehlmann A, et al: VEGF expression by mesenchymal stem cells
contributes to angiogenesis in pancreatic carcinoma. Br J Cancer.
99:622–631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito K, Sakaguchi M, Maruyama S, Iioka H,
Putranto EW, Sumardika IW, Tomonobu N, Kawasaki T, Homma K and
Kondo E: Stromal mesenchymal stem cells facilitate pancreatic
cancer progression by regulating specific secretory molecules
through mutual cellular interaction. J Cancer. 9:2916–2929. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyazaki Y, Oda T, Inagaki Y, Kushige H,
Saito Y, Mori N, Takayama Y, Kumagai Y, Mitsuyama T and Kida YS:
Adipose-derived mesenchymal stem cells differentiate into
heterogeneous cancer-associated fibroblasts in a stroma-rich
xenograft model. Sci Rep. 11:46902021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohr A, Albarenque SM, Deedigan L, Yu R,
Reidy M, Fulda S and Zwacka RM: Targeting of XIAP combined with
systemic mesenchymal stem cell-mediated delivery of sTRAIL ligand
inhibits metastatic growth of pancreatic carcinoma cells. Stem
Cells. 28:2109–2120. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao C, Pu Y, Zhang H, Hu X, Zhang R, He
S, Zhao Q and Mu B: IL10-modified human mesenchymal stem cells
inhibit pancreatic cancer growth through angiogenesis inhibition. J
Cancer. 11:5345–5352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keshtkar S, Azarpira N and Ghahremani MH:
Mesenchymal stem cell-derived extracellular vesicles: Novel
frontiers in regenerative medicine. Stem Cell Res Ther. 9:632018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou J, Tan X, Tan Y, Li Q, Ma J and Wang
G: Mesenchymal stem cell derived exosomes in cancer progression,
metastasis and drug delivery: A comprehensive review. J Cancer.
9:3129–3137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han S, Gonzalo DH, Feely M, Rinaldi C,
Belsare S, Zhai H, Kalra K, Gerber MH, Forsmark CE and Hughes SJ:
Stroma-derived extracellular vesicles deliver tumor-suppressive
miRNAs to pancreatic cancer cells. Oncotarget. 9:5764–5777. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Freitas D, Kim HS, Fabijanic K,
Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, et al:
Identification of distinct nanoparticles and subsets of
extracellular vesicles by asymmetric flow field-flow fractionation.
Nat Cell Biol. 20:332–343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zagrean AM, Hermann DM, Opris I, Zagrean L
and Popa-Wagner A: Multicellular crosstalk between exosomes and the
neurovascular unit after cerebral ischemia. therapeutic
implications. Front Neurosci. 12:8112018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JR, Park BW, Kim J, Choo YW, Kim HY,
Yoon JK, Kim H, Hwang JW, Kang M, Kwon SP, et al: Nanovesicles
derived from iron oxide nanoparticles-incorporated mesenchymal stem
cells for cardiac repair. Sci Adv. 6:eaaz09522020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding Y, Mei W, Zheng Z, Cao F, Liang K,
Jia Y, Wang Y, Liu D, Li J and Li F: Exosomes secreted from human
umbilical cord mesenchymal stem cells promote pancreatic ductal
adenocarcinoma growth by transferring miR-100-5p. Tissue Cell.
73:1016232021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie X, Ji J, Chen X, Xu W, Chen H, Zhu S,
Wu J, Wu Y, Sun Y, Sai W, et al: Human umbilical cord mesenchymal
stem cell-derived exosomes carrying hsa-miRNA-128-3p suppress
pancreatic ductal cell carcinoma by inhibiting Galectin-3. Clin
Transl Oncol. 24:517–531. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv FJ, Tuan RS, Cheung KM and Leung VY:
Concise review: The surface markers and identity of human
mesenchymal stem cells. Stem Cells. 32:1408–1419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teixeira FG, Panchalingam KM, Anjo SI,
Manadas B, Pereira R, Sousa N, Salgado AJ and Behie LA: Do
hypoxia/normoxia culturing conditions change the neuroregulatory
profile of Wharton Jelly mesenchymal stem cell secretome? Stem Cell
Res Ther. 6:1332015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kisiel AH, McDuffee LA, Masaoud E, Bailey
TR, Esparza Gonzalez BP and Nino-Fong R: Isolation,
characterization, and in vitro proliferation of canine mesenchymal
stem cells derived from bone marrow, adipose tissue, muscle, and
periosteum. Am J Vet Res. 73:1305–1317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murphy MB, Moncivais K and Caplan AI:
Mesenchymal stem cells: Environmentally responsive therapeutics for
regenerative medicine. Exp Mol Med. 45:e542013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trounson A and McDonald C: Stem cell
therapies in clinical trials: Progress and challenges. Cell Stem
Cell. 17:11–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He Z, Wang J, Zhu C, Xu J, Chen P, Jiang
X, Chen Y, Jiang J and Sun C: Exosome-derived FGD5-AS1 promotes
tumor-associated macrophage M2 polarization-mediated pancreatic
cancer cell proliferation and metastasis. Cancer Lett.
548:2157512022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai J, Wu J, Wang J, Li Y, Hu X, Luo S and
Xiang D: Extracellular vesicles derived from different sources of
mesenchymal stem cells: Therapeutic effects and translational
potential. Cell Biosci. 10:692020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh S, Dansby C, Agarwal D, Bhat PD,
Dubey PK and Krishnamurthy P: Exosomes: Methods for isolation and
characterization in biological samples. Methods Mol Biol.
2835:181–213. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Henne WM, Buchkovich NJ and Emr SD: The
ESCRT pathway. Dev Cell. 21:77–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tschuschke M, Kocherova I, Bryja A,
Mozdziak P, Angelova Volponi A, Janowicz K, Sibiak R,
Piotrowska-Kempisty H, Iżycki D, Bukowska D, et al: Inclusion
biogenesis, methods of isolation and clinical application of human
cellular exosomes. J Clin Med. 9:4362020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gurung S, Perocheau D, Touramanidou L and
Baruteau J: The exosome journey: From biogenesis to uptake and
intracellular signalling. Cell Commun Signal. 19:472021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Joo HS, Suh JH, Lee HJ, Bang ES and Lee
JM: Current knowledge and future perspectives on mesenchymal stem
cell-derived exosomes as a new therapeutic agent. Int J Mol Sci.
21:7272020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kowal J, Tkach M and Théry C: Biogenesis
and secretion of exosomes. Curr Opin Cell Biol. 29:116–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wortzel I, Dror S, Kenific CM and Lyden D:
Exosome-mediated metastasis: Communication from a distance. Dev
Cell. 49:347–360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Doyle LM and Wang MZ: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells. 8:7272019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Y, Hong Y, Cho E, Kim GB and Kim IS:
Extracellular vesicles as a platform for membrane-associated
therapeutic protein delivery. J Extracell Vesicles. 7:14401312018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kugeratski FG, Hodge K, Lilla S, McAndrews
KM, Zhou X, Hwang RF, Zanivan S and Kalluri R: Quantitative
proteomics identifies the core proteome of exosomes with syntenin-1
as the highest abundant protein and a putative universal biomarker.
Nat Cell Biol. 23:631–641. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hánělová K, Raudenská M, Masařík M and
Balvan J: Protein cargo in extracellular vesicles as the key
mediator in the progression of cancer. Cell Commun Signal.
22:252024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Choi DS, Kim DK, Kim YK and Gho YS:
Proteomics, transcriptomics and lipidomics of exosomes and
ectosomes. Proteomics. 13:1554–1571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao G, Wang L and Li C: Circ_0006790
carried by bone marrow mesenchymal stem cell-derived exosomes
regulates S100A11 DNA methylation through binding to CBX7 in
pancreatic ductal adenocarcinoma. Am J Cancer Res. 12:1934–1959.
2022.PubMed/NCBI
|
|
47
|
Wu R, Su Z, Zhao L, Pei R, Ding Y, Li D,
Zhu S, Xu L, Zhao W and Zhou W: Extracellular vesicle-loaded
oncogenic lncRNA NEAT1 from adipose-derived mesenchymal stem cells
confers gemcitabine resistance in pancreatic cancer via
miR-491-5p/Snail/SOCS3 axis. Stem Cells Int. 2023:65105712023.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang R, Zhang Y, Hao F, Su Z, Duan X and
Song X: Exosome-mediated triple drug delivery enhances apoptosis in
pancreatic cancer cells. Apoptosis. 30:1893–1911. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen
Q, Zhang Y, Lu Y, Ding X and Jiang C: Bone marrow mesenchymal stem
cells-derived exosomes for penetrating and targeted chemotherapy of
pancreatic cancer. Acta Pharm Sin B. 10:1563–1575. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yao X, Mao Y, Wu D, Zhu Y, Lu J, Huang Y,
Guo Y, Wang Z, Zhu S, Li X and Lu Y: Exosomal circ_0030167 derived
from BM-MSCs inhibits the invasion, migration, proliferation and
stemness of pancreatic cancer cells by sponging miR-338-5p and
targeting the Wif1/Wnt8/β-catenin axis. Cancer Lett. 512:38–50.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shang S, Wang J, Chen S, Tian R, Zeng H,
Wang L, Xia M, Zhu H and Zuo C: Exosomal miRNA-1231 derived from
bone marrow mesenchymal stem cells inhibits the activity of
pancreatic cancer. Cancer Med. 8:7728–7740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu Y, Liu N, Wei Y, Zhou D, Lin R, Wang X
and Shi B: Anticancer effects of miR-124 delivered by BM-MSC
derived exosomes on cell proliferation, epithelial mesenchymal
transition, and chemotherapy sensitivity of pancreatic cancer
cells. Aging (Albany NY). 12:19660–19676. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin T, Pu X, Zhou S, Huang Z, Chen Q,
Zhang Y, Mao Q, Liang Y and Ding G: Identification of exosomal
miR-484 role in reprogramming mitochondrial metabolism in
pancreatic cancer through Wnt/MAPK axis control. Pharmacol Res.
197:1069802023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Alidoust Saharkhiz Lahiji M and Safari F:
Potential therapeutic effects of hAMSCs secretome on Panc1
pancreatic cancer cells through downregulation of SgK269,
E-cadherin, vimentin, and snail expression. Biologicals. 76:24–30.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Safari F and Dadvar F: In vitro evaluation
of autophagy and cell death induction in Panc1 pancreatic cancer by
secretome of hAMSCs through downregulation of p-AKT/p-mTOR and
upregulation of p-AMPK/ULK1 signal transduction pathways. Tissue
Cell. 84:1021602023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Safari F, Shafiee Nejad N and Aghaei Nejad
A: The inhibition of Panc1 cancer cells invasion by hAMSCs
secretome through suppression of tyrosine phosphorylation of SGK223
(at Y411 site), c-Src (at Y416, Y530 sites), AKT activity, and
JAK1/Stat3 signaling. Med Oncol. 39:282022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou W, Zhou Y, Chen X, Ning T, Chen H,
Guo Q, Zhang Y, Liu P, Zhang Y, Li C, et al: Pancreatic
cancer-targeting exosomes for enhancing immunotherapy and
reprogramming tumor microenvironment. Biomaterials. 268:1205462021.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Klimova D, Jakubechova J, Altanerova U,
Nicodemou A, Styk J, Szemes T, Repiska V and Altaner C:
Extracellular vesicles derived from dental mesenchymal stem/stromal
cells with gemcitabine as a cargo have an inhibitory effect on the
growth of pancreatic carcinoma cell lines in vitro. Mol Cell
Probes. 67:1018942023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hu X, Zhou W, Pi R, Zhao X and Wang W:
Genetically modified cancer vaccines: Current status and future
prospects. Med Res Rev. 42:1492–1517. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dunbar CE, High KA, Joung JK, Kohn DB,
Ozawa K and Sadelain M: Gene therapy comes of age. Science.
359:eaan46722018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Doering CB, Archer D and Spencer HT:
Delivery of nucleic acid therapeutics by genetically engineered
hematopoietic stem cells. Adv Drug Deliv Rev. 62:1204–1212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhou P, Ding X, Du X, Wang L and Zhang Y:
Targeting reprogrammed cancer-associated fibroblasts with
engineered mesenchymal stem cell extracellular vesicles for
pancreatic cancer treatment. Biomater Res. 28:00502024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Buocikova V, Altanerova U, Soltysova A,
Andrezal M, Vanova D, Jakubechova J, Cihova M, Burikova M, Urbanova
M, Juhasikova L, et al: Placental mesenchymal stem cells: A
promising platform for advancing gene therapy in pancreatic ductal
adenocarcinoma. Biomed Pharmacother. 190:1184282025. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Amati E, Perbellini O, Rotta G, Bernardi
M, Chieregato K, Sella S, Rodeghiero F, Ruggeri M and Astori G:
High-throughput immunophenotypic characterization of bone marrow-
and cord blood-derived mesenchymal stromal cells reveals common and
differentially expressed markers: Identification of
angiotensin-converting enzyme (CD143) as a marker differentially
expressed between adult and perinatal tissue sources. Stem Cell Res
Ther. 9:102018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang J, Liu Y, Yin W and Hu X:
Adipose-derived stromal cells in regulation of hematopoiesis. Cell
Mol Biol Lett. 25:162020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xu T, Yu X, Yang Q, Liu X, Fang J and Dai
X: Autologous micro-fragmented adipose tissue as stem cell-based
natural scaffold for cartilage defect repair. Cell Transplant.
28:1709–1720. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Schäffler A and Büchler C: Concise review:
Adipose tissue-derived stromal cells-basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kozlowska U, Krawczenko A, Futoma K, Jurek
T, Rorat M, Patrzalek D and Klimczak A: Similarities and
differences between mesenchymal stem/progenitor cells derived from
various human tissues. World J Stem Cells. 11:347–374. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dubey NK, Mishra VK, Dubey R, Deng YH,
Tsai FC and Deng WP: Revisiting the advances in isolation,
characterization and secretome of adipose-derived stromal/stem
cells. Int J Mol Sci. 19:22002018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fukuchi Y, Nakajima H, Sugiyama D, Hirose
I, Kitamura T and Tsuji K: Human placenta-derived cells have
mesenchymal stem/progenitor cell potential. Stem Cells. 22:649–658.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Oliveira MS and Barreto-Filho JB:
Placental-derived stem cells: Culture, differentiation and
challenges. World J Stem Cells. 7:769–775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ulrich C, Abruzzese T, Maerz JK, Ruh M,
Amend B, Benz K, Rolauffs B, Abele H, Hart ML and Aicher WK: Human
placenta-derived CD146-positive mesenchymal stromal cells display a
distinct osteogenic differentiation potential. Stem Cells Dev.
24:1558–1569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li S, Huang KJ, Wu JC, Hu MS, Sanyal M, Hu
M, Longaker MT and Lorenz HP: Peripheral blood-derived mesenchymal
stem cells: Candidate cells responsible for healing critical-sized
calvarial bone defects. Stem Cells Transl Med. 4:359–368. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cao C and Dong Y and Dong Y: Study on
culture and in vitro osteogenesis of blood-derived human
mesenchymal stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
19:642–647. 2005.(In Chinese). PubMed/NCBI
|
|
75
|
Tracy SA, Ahmed A, Tigges JC, Ericsson M,
Pal AK, Zurakowski D and Fauza DO: A comparison of clinically
relevant sources of mesenchymal stem cell-derived exosomes: Bone
marrow and amniotic fluid. J Pediatr Surg. 54:86–90. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang Z, He Z, Liang S, Yang Q, Cheng P and
Chen A: Comprehensive proteomic analysis of exosomes derived from
human bone marrow, adipose tissue, and umbilical cord mesenchymal
stem cells. Stem Cell Res Ther. 11:5112020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ji L, Bao L, Gu Z, Zhou Q, Liang Y, Zheng
Y, Xu Y, Zhang X and Feng X: Comparison of immunomodulatory
properties of exosomes derived from bone marrow mesenchymal stem
cells and dental pulp stem cells. Immunol Res. 67:432–442. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Katsuda T, Tsuchiya R, Kosaka N, Yoshioka
Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M and Ochiya T:
Human adipose tissue-derived mesenchymal stem cells secrete
functional neprilysin-bound exosomes. Sci Rep. 3:11972013.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pomatto M, Gai C, Negro F, Cedrino M,
Grange C, Ceccotti E, Togliatto G, Collino F, Tapparo M, Figliolini
F, et al: Differential therapeutic effect of extracellular vesicles
derived by bone marrow and adipose mesenchymal stem cells on wound
healing of diabetic ulcers and correlation to their cargoes. Int J
Mol Sci. 22:38512021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Langevin SM, Kuhnell D, Orr-Asman MA,
Biesiada J, Zhang X, Medvedovic M and Thomas HE: Balancing yield,
purity and practicality: A modified differential
ultracentrifugation protocol for efficient isolation of small
extracellular vesicles from human serum. RNA Biol. 16:5–12. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gardiner C, Di Vizio D, Sahoo S, Théry C,
Witwer KW, Wauben M and Hill AF: Techniques used for the isolation
and characterization of extracellular vesicles: Results of a
worldwide survey. J Extracell Vesicles. 5:329452016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Van Deun J, Mestdagh P, Sormunen R,
Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O and
Hendrix A: The impact of disparate isolation methods for
extracellular vesicles on downstream RNA profiling. J Extracell
Vesicles. 3:2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lobb RJ, Becker M, Wen SW, Wong CS,
Wiegmans AP, Leimgruber A and Möller A: Optimized exosome isolation
protocol for cell culture supernatant and human plasma. J Extracell
Vesicles. 4:270312015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bhattacharjee C and Singh M: Studies on
the applicability of artificial neural network (ANN) in continuous
stirred ultrafiltration. Chem Eng Technol. 25:1187–1192. 2002.
View Article : Google Scholar
|
|
85
|
Cheng H, Fang H, Xu RD, Fu MQ, Chen L,
Song XY, Qian JY, Zou YZ, Ma JY and Ge JB: Development of a rinsing
separation method for exosome isolation and comparison to
conventional methods. Eur Rev Med Pharmacol Sci. 23:5074–5083.
2019.PubMed/NCBI
|
|
86
|
Soares Martins T, Catita J, Martins Rosa
I, AB da Cruz E, Silva O and Henriques AG: Exosome isolation from
distinct biofluids using precipitation and column-based approaches.
PLoS One. 13:e01988202018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Buschmann D, Kirchner B, Hermann S, Märte
M, Wurmser C, Brandes F, Kotschote S, Bonin M, Steinlein OK, Pfaffl
MW, et al: Evaluation of serum extracellular vesicle isolation
methods for profiling miRNAs by next-generation sequencing. J
Extracell Vesicles. 7:14813212018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhao L, Yu J, Wang J, Li H, Che J and Cao
B: Isolation and identification of miRNAs in exosomes derived from
serum of colon cancer patients. J Cancer. 8:1145–1152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Popovic M, Mazzega E, Toffoletto B and De
Marco A: Isolation of anti-extra-cellular vesicle single-domain
antibodies by direct panning on vesicle-enriched fractions. Microb
Cell Fact. 17:62018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhu L, Sun HT, Wang S, Huang SL, Zheng Y,
Wang CQ, Hu BY, Qin W, Zou TT, Fu Y, et al: Isolation and
characterization of exosomes for cancer research. J Hematol Oncol.
13:1522020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pei S, Sun W, Han Q, Wang H and Liang Q:
Bifunctional immunoaffinity magnetic nanoparticles for
high-efficiency separation of exosomes based on host-guest
interaction. Talanta. 272:1257902024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Iliescu FS, Vrtačnik D, Neuzil P and
Iliescu C: Microfluidic technology for clinical applications of
exosomes. Micromachines (Basel). 10:3922019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang P, Zhou X, He M, Shang Y, Tetlow AL,
Godwin AK and Zeng Y: Ultrasensitive detection of circulating
exosomes with a 3D-nanopatterned microfluidic chip. Nat Biomed Eng.
3:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hua X, Zhu Q, Liu Y, Zhou S, Huang P, Li Q
and Liu S: A double tangential flow filtration-based microfluidic
device for highly efficient separation and enrichment of exosomes.
Anal Chim Acta. 1258:3411602023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ding L, Liu X, Zhang Z, Liu LE, He S, Wu
Y, Effah CY, Yang R, Zhang A, Chen W, et al:
Magnetic-nanowaxberry-based microfluidic ExoSIC for affinity and
continuous separation of circulating exosomes towards cancer
diagnosis. Lab Chip. 23:1694–1702. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Abramowicz A, Marczak L, Wojakowska A,
Zapotoczny S, Whiteside TL, Widlak P and Pietrowska M:
Harmonization of exosome isolation from culture supernatants for
optimized proteomics analysis. PLoS One. 13:e02054962018.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Iqbal Z, Rehman K, Mahmood A, Shabbir M,
Liang Y, Duan L and Zeng H: Exosome for mRNA delivery: Strategies
and therapeutic applications. J Nanobiotechnology. 22:3952024.
View Article : Google Scholar : PubMed/NCBI
|