The incidence and mortality rates of pancreatic
cancer (PC) have been steadily increasing; there were 441,000 cases
of PC worldwide in 2017, compared with only 196,000 cases in 1990
(1). According to epidemiological
data, PC has the lowest 5-year relative survival rate among all
solid tumors, estimated at ~13% (2). Surgical resection combined with
adjuvant chemotherapy represents the current standard of care and
the most widely adopted clinical strategy for the treatment of PC
(3). However, due to the
asymptomatic nature of early-stage PC, the majority of patients are
diagnosed at an advanced stage. Consequently, only a small subset
of patients remains eligible for surgical intervention (4). Furthermore, the development of
chemotherapy resistance, often resulting from prolonged treatment,
notably impacts both the survival and quality of life of patients
with PC. The pathogenesis of PC remains incompletely elucidated;
however, in addition to modifiable risk factors such as smoking,
alcohol consumption, obesity and diabetes, genetic mutations,
familial inheritance and a complex tumor microenvironment (TME) are
also recognized as critical contributing factors (1,5,6). The
TME of PC is a complex ecosystem composed of cancer cells, stromal
cells, immune cells and various extracellular components. PC cells
actively drive genetic mutations; stromal cells produce abundant
collagen and extracellular matrix leading to tissue fibrosis and
highly expressed inflammatory factors create a pro-inflammatory
environment. This unique TME establishes a robust foundation for
the growth and metastasis of PC (7). Therefore, deepening the understanding
of the molecular mechanisms underlying PC, developing more
effective therapeutic strategies and overcoming chemotherapy
resistance represent critical research directions that require
further exploration.
Mesenchymal stem cells (MSCs) are multipotent stem
cells characterized by high differentiation potential and strong
self-renewal capacity, which originate from the mesoderm and
ectoderm during early development (8). Studies have indicated that MSCs
actively participate in the progression of PC, including tumor
growth and metastasis of cancer cells, as well as the modulation of
TME (9–11). Studies have reported that MSCs can
promote the progression of PC by secreting pro-tumorigenic factors
(12) and differentiating into
cancer-associated fibroblasts (CAFs) (13). However, other evidence suggests that
MSCs can also suppress the development of PC. For example, Mohr
et al (14) demonstrated
that systemic MSC-mediated delivery of soluble tumor necrosis
factor-related apoptosis inducing ligand, combined with X-linked
inhibitor of apoptosis protein inhibition, could inhibit PC growth
and metastasis. Additionally, IL-10-modified human MSCs were shown
to inhibit PC progression by suppressing the secretion of
pro-inflammatory cytokines IL-6 and TNF-α and inhibiting tumor
angiogenesis (15). The role of
MSCs in PC is closely associated with their derived EXOs (16).
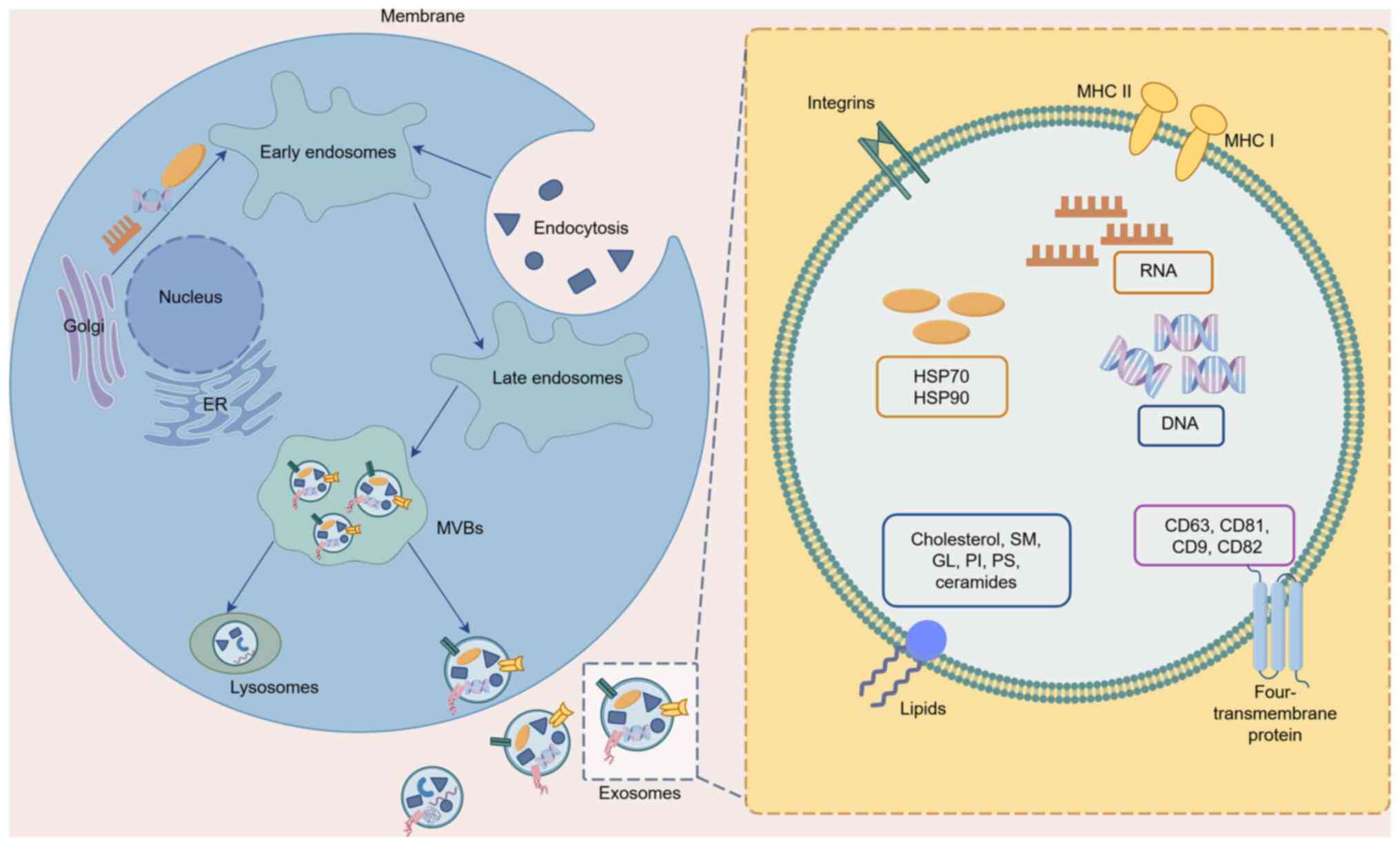
EXOs are extracellular vesicles (EVs) with a
diameter of 30–150 nm that are capable of transmitting complex
information between cells (17),
across distant tissues (18) and
between tumor and stromal compartments (19). They originate from a wide variety of
sources and are present in nearly all bodily fluids, and EXOs from
different origins exhibit distinct functions (20). MSC-derived EXOs (MSC-EXOs) share
numerous functional similarities with MSCs. However, compared with
MSCs, MSC-EXOs demonstrate enhanced safety, superior penetrability,
improved compatibility and higher stability when interacting with
tumor cells (21,22). In recent years, the role of MSC-EXOs
in the treatment of PC has been extensively investigated. For
instance, it has been reported that human umbilical cord MSC-EXOs
(hUC-MSC-EXOs) can promote the growth of pancreatic ductal
adenocarcinoma by transferring microRNA (miR/miRNA)-100-5p into the
PC tumor model (23). By contrast,
Xie et al (24) reported
opposing findings, demonstrating that hsa-miRNA-128-3p carried by
hUC-MSC-EXOs suppressed the proliferation, invasion and migration
of pancreatic ductal adenocarcinoma cells by targeting galectin-3.
These findings indicate that MSC-EXOs serve a dual role in PC. The
present article systematically elaborates on the dual
tumor-promoting and tumor-suppressing mechanisms of MSC-EXOs in PC.
It also clarifies the potential factors influencing this duality
and provides direction for their application in targeted PC
therapy.
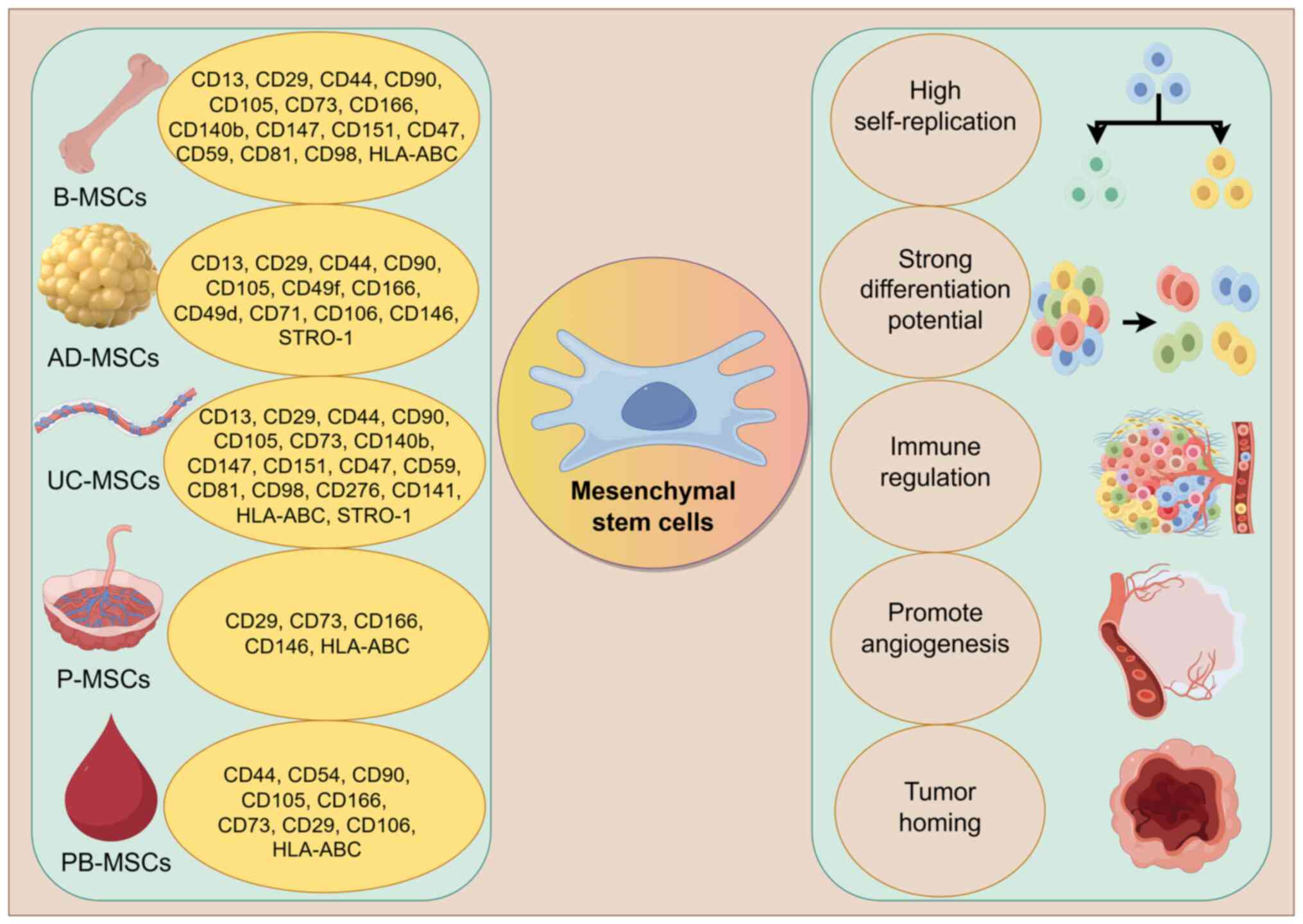
The International Society for Cell Therapy has
established the minimal defining criteria for MSCs: i) They must
exhibit plastic-adherence under standard culture conditions; ii)
they must express specific surface markers such as CD73, CD90 and
CD105; iii) they must possess the capacity to differentiate into
osteoblasts, chondrocytes and adipocytes in vitro; and iv)
they must lack expression of CD14, CD34, CD45, CD11b, CD79a, CD19
and human leukocyte antigen-DR (25,26).
MSCs can be isolated from a wide range of biological tissues,
including bone marrow, adipose tissue, umbilical cord, placenta and
peripheral blood (27–29). In previous years, MSCs have
demonstrated notable potential in the treatment of PC; however,
several limitations have also been identified. For example, studies
have indicated that MSCs may increase the risk of tumorigenicity
and cell death (30,31). To address these concerns,
researchers have proposed using MSC-EXOs as an alternative
therapeutic approach for PC. These EXOs exhibit a number of
functions similar to those of MSCs, offer improved safety and
stability profiles and can serve as excellent carriers for
delivering antitumor drugs (32).
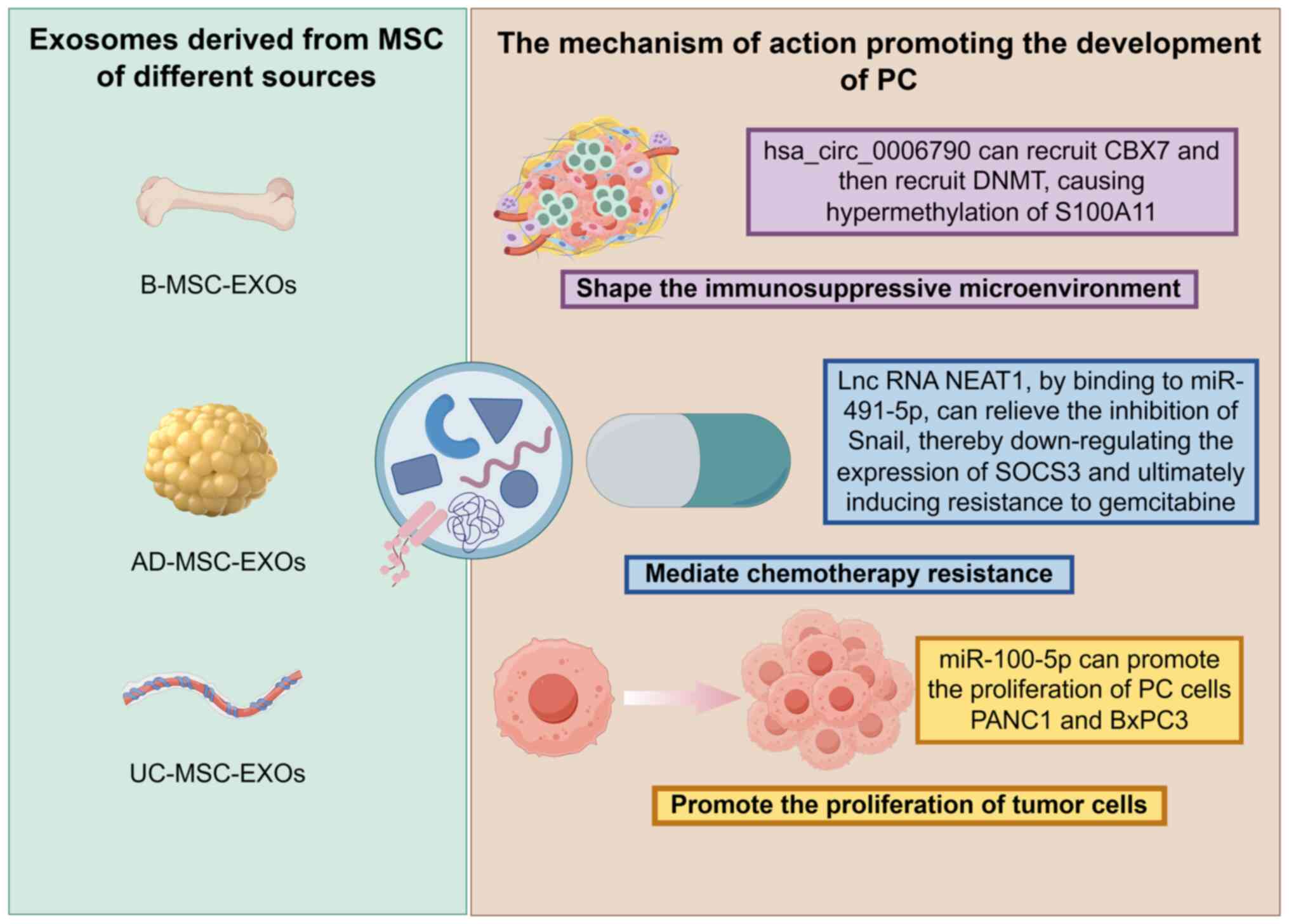
The tumor-promoting role of MSC-EXOs in PC has been
demonstrated in multiple studies (23,46,47).
For instance, B-MSC-EXOs, AD-MSC-EXOs and UC-MSC-EXOs can jointly
exert cancer-promoting effects through different mechanisms of
action (Fig. 2). The current
section will focus on elucidating the specific molecular mechanisms
and signaling pathways underlying their pro-tumorigenic effects,
including promoting tumor cell proliferation, shaping an
immunosuppressive microenvironment and mediating chemotherapy
resistance (Table I). By
systematically elaborating these mechanisms, the present study
aimed to provide researchers with a clear understanding of the
tumor-promoting functions of MSC-EXOs in PC and establish a
theoretical foundation for developing future exosome-targeted
therapeutic strategies.
MSC-EXOs can directly promote the proliferation of
PC cells, thereby exerting tumor-promoting effects. In the study by
Ding et al (23),
hUC-MSC-EXOs carrying miR-100-5p were demonstrated to promote the
proliferation of PC cells PANC1 and BxPC3 in both in vivo
and in vitro studies, consequently accelerating the growth
of pancreatic ductal adenocarcinoma. This finding not only reveals
the key mechanism by which hUC-MSC-EXOs contribute to the
development and progression of pancreatic ductal adenocarcinoma
through the transfer of miR-100-5p, but also provides new insights
for targeted intervention. Inhibiting miR-100-5p may represent a
potential therapeutic strategy to block EXO-mediated
pro-tumorigenic effects, thereby opening new avenues for precision
therapy in pancreatic ductal adenocarcinoma.
MSC-EXOs can participate in shaping the
immunosuppressive microenvironment of PC through complex
mechanisms, thereby providing favorable conditions for tumor
initiation and progression. For example, Gao et al (46) found that bone marrow MSC-EXOs
(B-MSC-EXOs) carrying hsa_circ_0006790 can recruit chromobox
protein homolog 7, which subsequently recruits DNA
methyltransferases, leading to hypermethylation of the promoter
region of the downstream target gene S100A11 and resulting in its
downregulation. As a key molecule involved in immunoregulation, the
decreased expression of S100A11 ultimately induces immune escape in
PC cells. This study by Gao et al (46) not only reveals the specific
mechanism by which B-MSC-EXOs contribute to the formation of the
immunosuppressive microenvironment in PC but also provides a
potential target for developing novel immunotherapy strategies
targeting the exosomal signaling axis.
MSC-EXOs can participate in mediating chemotherapy
resistance in PC. For example, a study showed that human adipose
MSC-EXOs (hAD-MSC-EXOs) are enriched with the lncRNA NEAT1, which
can competitively bind to miR-491-5p, thereby relieving its
inhibition of the transcription factor Snail. This subsequently
leads to downregulation of suppressor of cytokine signaling 3
(SOCS3) expression, ultimately inducing resistance to gemcitabine
in PC cells (47). This mechanism
highlights the critical role of exosome-carried lncRNA in mediating
chemotherapy resistance, not only providing a new molecular
perspective for understanding drug resistance in PC but also
offering potential therapeutic strategies for reversing resistance,
such as targeting the NEAT1/miR-491-5p/Snail/SOCS3 signaling axis
to reduce chemoresistance.
In conclusion, although MSC-EXOs derived from
different tissues such as umbilical cord, bone marrow and adipose
can promote the progression of PC through multiple mechanisms,
related research is still relatively limited, and more experimental
evidence is needed to verify the specific cancer-promoting
mechanisms. In addition, apart from gemcitabine, there are
currently numerous drugs used to inhibit the development of PC.
Therefore, whether hAD-MSC-EXOs still exhibit cancer-promoting
effects when used in conjuction with other anticancer drugs remains
to be elucidated. In addition, whether MSC-EXOs derived from the
same tissue only serve a promoting role in PC also remains to be
elucidated. Through in-depth sorting and analysis, a more complex
fact has been demonstrated: Even MSC-EXOs derived from the same
tissue exhibit a dual role in PC and may either promote tumor
development or inhibit its progression.
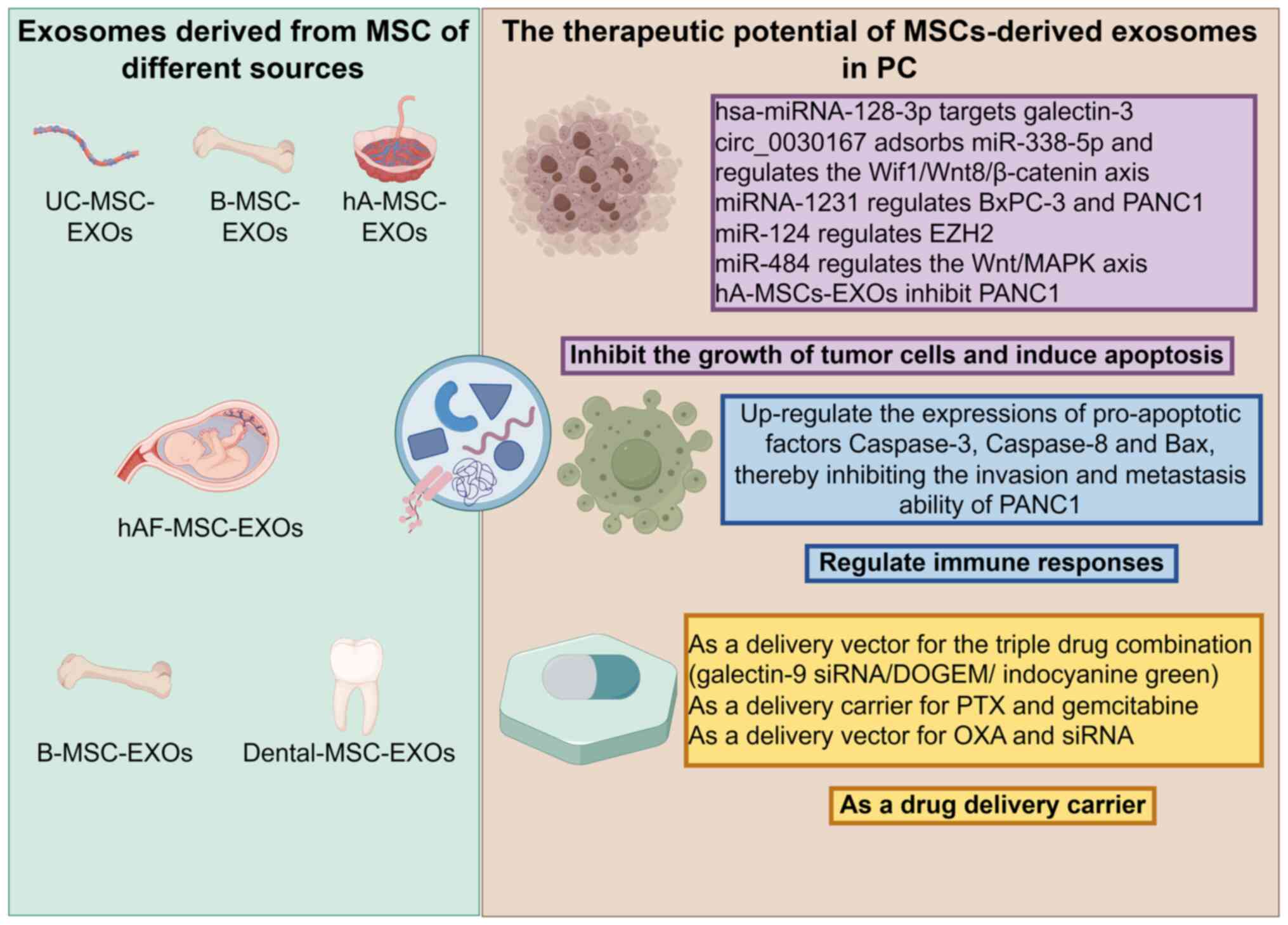
Although MSC-EXOs can promote the initiation and
progression of PC through various mechanisms, current research
focuses more on their tumor-suppressive roles. For instance,
UC-MSC-EXOs, B-MSC-EXOs, hA-MSC-EXOs, hAF-MSC-EXOs and
Dental-MSC-EXOs can jointly exert anti-cancer effects through
different mechanisms of action (Fig.
3). In fact, owing to their notable immunomodulatory
capabilities and tumor-homing properties, MSC-EXOs exhibit
multifaceted functions in tumor suppression: On the one hand, they
can directly inhibit the proliferation of PC cells and induce
apoptosis; on the other hand, they can indirectly exert antitumor
effects by modulating immune responses. Furthermore, due to their
high biocompatibility, low immunogenicity and efficient targeted
delivery capacity, MSC-EXOs serve as a highly promising drug
delivery system and are widely used for loading antitumor agents,
thereby further enhancing their value in suppressing PC (Table II) (48,49).
Therefore, despite reports indicating that MSC-EXOs may promote the
growth and development of PC, their functions in tumor suppression
remain more comprehensive and hold greater translational
potential.
MSC-EXOs exert notable tumor-suppressive effects by
inhibiting tumor cell growth and inducing apoptosis. Multiple
studies support this conclusion through various mechanisms. For
instance, hsa-miRNA-128-3p in hUC-MSC-EXOs can effectively inhibit
the proliferation, invasion and migration of PC PANC1 cells by
targeting galectin-3 (24).
Meanwhile, circ_0030167 from human (h)B-MSC-EXOs suppresses the
proliferation, invasion and metastasis of PC cells by sponging
miR-338-5p and regulating the Wnt inhibitory factor
1/Wnt8/β-catenin axis (50).
Additionally, miRNA-1231 from B-MSC-EXOs negatively regulates the
proliferation, migration, invasion and matrix adhesion of both
BxPC-3 and PANC1 cells (51). Xu
et al (52) demonstrated
that B-MSC-EXOs carrying miR-124 inhibit proliferation, invasion
and metastasis, while promoting apoptosis in AsPC1 and PANC1 cells
by modulating enhancer of zeste homolog 2 expression. Another study
indicated that miR-484, carried by hB-MSC-EXOs, suppresses
proliferation and migration and induces apoptosis in PC cells via
regulation of the Wnt/MAPK axis (53). Furthermore, human amniotic MSC-EXOs
inhibit the proliferation of PANC1 cells by downregulating the
expression of Sugen kinase 269, E-cadherin, vimentin and the Snail
transcription factor (54), a
finding further supported by studies from Safari and Dadvar
(55) and Safari et al
(56). Collectively, these findings
demonstrate that MSC-EXOs inhibit PC cell proliferation and promote
apoptosis through multiple molecular mechanisms, serving a crucial
role in suppressing PC progression.
MSC-EXOs can exert antitumor effects by modulating
immune responses. For example, a study by Chen et al
(9) demonstrated that human
amniotic fluid MSC-EXOs (hAF-MSC-EXOs) markedly upregulate the
expression of pro-apoptotic factors caspase-3, caspase-8 and Bax,
thereby inhibiting the invasion and metastatic capabilities of
PANC1 cells. This finding suggests that hAF-MSC-EXOs may enhance
immune effector mechanisms by upregulating pro-apoptotic factors,
consequently suppressing the progression of PC.
MSC-EXOs are regarded as a highly promising drug
delivery vehicle for anti-PC therapy due to their excellent
biocompatibility, low immunogenicity and inherent targeting
capability. A study has shown that B-MSC-EXOs can serve as carriers
for a triple-drug combination [galectin-9 small interfering
(si)RNA/dodecyloxybenzyl gemcitabine/indocyanine green],
demonstrating favorable tumor-targeting ability and pH-responsive
release characteristics both in vivo and in vitro,
notably enhancing the anti-PC efficacy (48). Furthermore, B-MSC-EXOs, as delivery
vehicles for paclitaxel and gemcitabine, effectively overcome
chemotherapy resistance in PC owing to their superior homing and
penetration capabilities during delivery (49). Zhou et al (57) developed a dual-delivery biosystem
using B-MSC-EXOs capable of co-loading oxaliplatin and siRNA. This
system not only elicits an antitumor immune response by inhibiting
tumor-associated macrophage polarization, promoting T lymphocyte
recruitment and downregulating regulatory T cell activity, but also
exhibits higher stability and lower side effects compared with
conventional synthetic carriers. An in vitro study by
Klimova et al (58) found
that EXOs derived from human dental pulp MSCs can act as carriers
for gemcitabine, significantly inhibiting the growth of PC cells.
In summary, the multiple advantages demonstrated by MSC-EXOs in
drug delivery indicate their potential as an efficient and safe
nanoscale platform for the treatment of PC.
Over time, genetic engineering has evolved into a
pivotal approach for treating various diseases, including
hematological disorders, genetic conditions, and cancers (59–61).
Particularly in the field of oncology, continuous technological
advancements and expanding applications have notably enhanced the
efficacy and safety of genetic engineering-based therapies,
demonstrating broad prospects for clinical application. MSC-EXOs
have garnered considerable attention in the treatment of PC due to
their unique biocompatibility, low immunogenicity and targeted
delivery capabilities. However, natural EXOs face limitations such
as insufficient targeting specificity, limited efficacy of their
cargo and rapid clearance, which substantially restrict their
clinical translation and therapeutic effectiveness. Consequently,
combining them with genetic engineering strategies can markedly
enhance their tumor-targeting ability, treatment specificity and
immunomodulatory functions in PC.
PC CAFs serve a critical role in the initiation and
progression of PC. Targeted reprogramming of CAFs may represent a
promising therapeutic strategy for PC. Zhou et al (62) employed a genetic engineering
approach to endogenously modify B-MSC-EXOs, enabling surface
display of integrin α5 targeting peptides and concomitant
overexpression of miR-148a-3p, thereby achieving precise
reprogramming of CAFs and providing new insights for clinical
translation of this strategy. On the other hand, Buocikova et
al (63) genetically engineered
EXOs from placental MSCs to express the yeast cytosine
deaminase::uracil phosphoribosyltransferase fusion enzyme. In a
model of pancreatic ductal adenocarcinoma, this approach
demonstrated potent cytotoxicity-reducing effects. Therefore, it
offers a promising cell-free therapeutic strategy for PC. Although
genetically engineered MSC-EXOs are considered a promising strategy
for cancer treatment, their current application in PC remains in
its early stages and requires further in-depth research to advance
their development. The development of more efficient and safer
engineered exosome-based therapeutic strategies remains a notable
challenge in the current treatment of PC.
MSC-EXOs exhibit a dual role in PC, a phenomenon
that warrants in-depth consideration. The underlying mechanisms are
likely closely associated with the following factors. Firstly, the
functional properties of EXOs are notably influenced by their
cellular origin. Although EXOs derived from different sources of
MSCs share common characteristics, such as high self-renewal
capacity, multipotent differentiation potential, immunomodulatory
activity, pro-angiogenic effects and tumor-homing ability (64–74)
(Fig. 4), the specific molecules
present on their surface and their cargo compositions may vary
considerably, potentially leading to distinct functional
orientations (75–79) (Table
III). MSC-EXOs from different tissue sources have demonstrated
a dual role in PC, which has been discussed in the present review.
On the other hand, the stage of PC progression may also affect the
functional outcomes of EXOs. For instance, early-stage PC may
respond more favorably to therapy with MSC-EXOs compared with
advanced-stage disease; however, this hypothesis still lacks robust
experimental evidence and thus represents a critical question that
urgently requires validation in future research. In addition, the
differences in experimental design within this research field are
another reason for the contradictions among different research
results. Specifically, the differences in the extraction and
identification methods of EXOs, cell co-culture systems and animal
models (such as mouse strains, quantities and tumor-bearing sites)
may all introduce notable heterogeneity, thereby affecting the
comparability and repeatability of research conclusions.
In summary, MSC-EXOs exhibit a notable dual role in
PC. The present review has systematically discussed their specific
molecular mechanisms and functional characteristics in PC, and
summarized the latest research advances in engineered modification
of MSC-EXOs. In-depth elucidation of the key molecules, proteins
and signaling pathways involved will provide new research
directions and intervention strategies for the treatment of PC.
Despite the considerable tumor-suppressive potential
of MSC-EXOs in PC, several unresolved issues and challenges remain.
First, the techniques for isolating and extracting MSC-EXOs are
still inadequate. Although multiple methods have been developed for
exosome isolation and extraction, such as ultracentrifugation
(80,81), ultrafiltration (82–84),
flushing separation (85),
precipitation (86–88), immunoaffinity capture (89–91),
microfluidics (92–95) and mass spectrometry (96), each of these approaches has its own
limitations. There is currently a lack of a simple, efficient,
cost-effective and standardized extraction protocol suitable for
clinical application. Second, research on and preparation of
MSC-EXOs lack unified standards. No authoritative institutions have
yet established clear guidelines for their isolation, preparation,
characterization and functional evaluation. Third, large-scale
production, storage and transportation systems remain
underdeveloped. Systematic research and solutions are still needed
regarding large-scale preparation, long-term stable storage methods
and potential loss of activity and integrity during transportation.
Fourth, evidence for clinical translation is insufficient. Most
current studies are limited to animal experiments; future research
should focus on clinical trials to validate the safety and efficacy
of these EXOs in human applications. Fifth, the immunogenicity
risks brought about by the engineering modification of MSC-EXOs
need to be systematically evaluated. For example, whether the
natural low immunogenicity advantage of MSC-EXOs will be altered
after engineering. Or, whether it may trigger an immune response in
the body, especially in the context of repeated administration for
PC, which would be a core issue regarding treatment safety. Sixth,
the dense fibrotic matrix of PC may severely impede the penetration
ability of EXOs, and their pharmacokinetic performance in this
environment requires further research and confirmation. Finally,
there is a lack of horizontal efficacy comparisons among various
treatment methods for PC. For instance, compared with traditional
nanoparticles (such as liposomes) that have entered clinical
application, MSC-EXOs may have inherent advantages in active
targeting and biocompatibility, but they are slightly inferior in
drug loading capacity and the maturity of production processes
(97). However, future research
should prospectively explore strategies for combining the two to
achieve synergistic therapeutic effects.
Although the present article systematically expounds
the research progress of MSC-EXOs in PC, there are still certain
limitations. Firstly, due to the rapid development of research in
this field, this article may not be able to cover all the latest
released research achievements. Secondly, the discussion on the
mechanism of MSC-EXOs in promoting cancer still needs to be further
clarified. In addition, the analysis of the potential and
challenges of EXOs derived from engineered MSCs in this field needs
to be strengthened. Looking to the future, first of all,
researchers should focus on developing a simple, efficient and
low-cost standardized extraction scheme for the separation and
extraction of EXOs. Secondly, more in-depth exploration and
research should be performed on the role of MSC-EXOs in PC to
address the potential loss issues that may arise during their
large-scale production, storage and transportation. In addition, a
systematic safety assessment of the immunogenicity risk and in
vivo pharmacokinetic behavior of engineered modified EXOs is
necessary, which is a prerequisite for their clinical
transformation. Another important task lies in performing a
horizontal efficacy comparison between exosome therapy and other
treatment methods, with the aim of providing the optimal treatment
strategy for patients with PC. Finally, accelerating the
transformation of MSC-EXOs from basic research to clinical practice
makes it possible for them to become an important component of the
comprehensive treatment system for PC.
MSC-EXOs serve as highly plastic messengers in PC,
where their role in promoting or suppressing tumor progression is
not fixed but rather determined by their specific surface molecules
and cargo contents, which are closely associated with the diverse
origins of MSCs. Future research should focus on transforming
MSC-EXOs from a ‘double-edged sword’ into a precise weapon against
PC. The primary task is to deeply explore its specific active
ingredients and their precise regulatory mechanisms in PC and, on
this basis, strategies for engineering and modifying MSC-EXOs
should be established. At the same time, establishing standardized,
repeatable, low-cost yet high-purity separation and purification
technologies is the core step for it to move towards clinical
application. In addition, it is important to actively explore the
combined application of EXOs therapy and existing treatment
methods. Only through in-depth exploration at multiple levels can
this promising treatment method be advanced from the laboratory to
clinical practice, bringing new treatment options for patients with
PC.
Not applicable.
Funding: No funding was received.
Not applicable.
HZ contributed to the conception and overall design
of the study. HZ drafted the manuscript and prepared the figures
and tables. XS reviewed and revised the manuscript. All authors
read and approved the final version of the manuscript. Data
authentication was not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Klein AP: Pancreatic cancer epidemiology:
understanding the role of lifestyle and inherited risk factors. Nat
Rev Gastroenterol Hepatol. 18:493–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI
|
|
3
|
Park W, Chawla A and O'Reilly EM:
Pancreatic cancer: A review. JAMA. 326:851–862. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleeff J, Korc M, Apte M, La Vecchia C,
Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH
and Neoptolemos JP: Pancreatic cancer. Nat Rev Dis Primers.
2:160222016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Halbrook CJ, Lyssiotis CA, Pasca di
Magliano M and Maitra A: Pancreatic cancer: Advances and
challenges. Cell. 186:1729–1754. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klatte DCF, Wallace MB, Löhr M, Bruno MJ
and van Leerdam ME: Hereditary pancreatic cancer. Best Pract Res
Clin Gastroenterol. 58–59. 1017832022.PubMed/NCBI
|
|
7
|
Wood LD, Canto MI, Jaffee EM and Simeone
DM: Pancreatic cancer: Pathogenesis, screening, diagnosis, and
treatment. Gastroenterology. 163:386–402.e1. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai CC, Su PF, Huang YF, Yew TL and Hung
SC: Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal
and undifferentiated state in mesenchymal stem cells. Mol Cell.
47:169–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YC, Lan YW, Huang SM, Yen CC, Chen W,
Wu WJ, Staniczek T, Chong KY and Chen CM: Human amniotic fluid
mesenchymal stem cells attenuate pancreatic cancer cell
proliferation and tumor growth in an orthotopic xenograft mouse
model. Stem Cell Res Ther. 13:2352022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kabashima-Niibe A, Higuchi H, Takaishi H,
Masugi Y, Matsuzaki Y, Mabuchi Y, Funakoshi S, Adachi M, Hamamoto
Y, Kawachi S, et al: Mesenchymal stem cells regulate
epithelial-mesenchymal transition and tumor progression of
pancreatic cancer cells. Cancer Sci. 104:157–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beckermann BM, Kallifatidis G, Groth A,
Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner
W, Diehlmann A, et al: VEGF expression by mesenchymal stem cells
contributes to angiogenesis in pancreatic carcinoma. Br J Cancer.
99:622–631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito K, Sakaguchi M, Maruyama S, Iioka H,
Putranto EW, Sumardika IW, Tomonobu N, Kawasaki T, Homma K and
Kondo E: Stromal mesenchymal stem cells facilitate pancreatic
cancer progression by regulating specific secretory molecules
through mutual cellular interaction. J Cancer. 9:2916–2929. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyazaki Y, Oda T, Inagaki Y, Kushige H,
Saito Y, Mori N, Takayama Y, Kumagai Y, Mitsuyama T and Kida YS:
Adipose-derived mesenchymal stem cells differentiate into
heterogeneous cancer-associated fibroblasts in a stroma-rich
xenograft model. Sci Rep. 11:46902021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohr A, Albarenque SM, Deedigan L, Yu R,
Reidy M, Fulda S and Zwacka RM: Targeting of XIAP combined with
systemic mesenchymal stem cell-mediated delivery of sTRAIL ligand
inhibits metastatic growth of pancreatic carcinoma cells. Stem
Cells. 28:2109–2120. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao C, Pu Y, Zhang H, Hu X, Zhang R, He
S, Zhao Q and Mu B: IL10-modified human mesenchymal stem cells
inhibit pancreatic cancer growth through angiogenesis inhibition. J
Cancer. 11:5345–5352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keshtkar S, Azarpira N and Ghahremani MH:
Mesenchymal stem cell-derived extracellular vesicles: Novel
frontiers in regenerative medicine. Stem Cell Res Ther. 9:632018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou J, Tan X, Tan Y, Li Q, Ma J and Wang
G: Mesenchymal stem cell derived exosomes in cancer progression,
metastasis and drug delivery: A comprehensive review. J Cancer.
9:3129–3137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han S, Gonzalo DH, Feely M, Rinaldi C,
Belsare S, Zhai H, Kalra K, Gerber MH, Forsmark CE and Hughes SJ:
Stroma-derived extracellular vesicles deliver tumor-suppressive
miRNAs to pancreatic cancer cells. Oncotarget. 9:5764–5777. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Freitas D, Kim HS, Fabijanic K,
Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, et al:
Identification of distinct nanoparticles and subsets of
extracellular vesicles by asymmetric flow field-flow fractionation.
Nat Cell Biol. 20:332–343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zagrean AM, Hermann DM, Opris I, Zagrean L
and Popa-Wagner A: Multicellular crosstalk between exosomes and the
neurovascular unit after cerebral ischemia. therapeutic
implications. Front Neurosci. 12:8112018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JR, Park BW, Kim J, Choo YW, Kim HY,
Yoon JK, Kim H, Hwang JW, Kang M, Kwon SP, et al: Nanovesicles
derived from iron oxide nanoparticles-incorporated mesenchymal stem
cells for cardiac repair. Sci Adv. 6:eaaz09522020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding Y, Mei W, Zheng Z, Cao F, Liang K,
Jia Y, Wang Y, Liu D, Li J and Li F: Exosomes secreted from human
umbilical cord mesenchymal stem cells promote pancreatic ductal
adenocarcinoma growth by transferring miR-100-5p. Tissue Cell.
73:1016232021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie X, Ji J, Chen X, Xu W, Chen H, Zhu S,
Wu J, Wu Y, Sun Y, Sai W, et al: Human umbilical cord mesenchymal
stem cell-derived exosomes carrying hsa-miRNA-128-3p suppress
pancreatic ductal cell carcinoma by inhibiting Galectin-3. Clin
Transl Oncol. 24:517–531. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv FJ, Tuan RS, Cheung KM and Leung VY:
Concise review: The surface markers and identity of human
mesenchymal stem cells. Stem Cells. 32:1408–1419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teixeira FG, Panchalingam KM, Anjo SI,
Manadas B, Pereira R, Sousa N, Salgado AJ and Behie LA: Do
hypoxia/normoxia culturing conditions change the neuroregulatory
profile of Wharton Jelly mesenchymal stem cell secretome? Stem Cell
Res Ther. 6:1332015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kisiel AH, McDuffee LA, Masaoud E, Bailey
TR, Esparza Gonzalez BP and Nino-Fong R: Isolation,
characterization, and in vitro proliferation of canine mesenchymal
stem cells derived from bone marrow, adipose tissue, muscle, and
periosteum. Am J Vet Res. 73:1305–1317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murphy MB, Moncivais K and Caplan AI:
Mesenchymal stem cells: Environmentally responsive therapeutics for
regenerative medicine. Exp Mol Med. 45:e542013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trounson A and McDonald C: Stem cell
therapies in clinical trials: Progress and challenges. Cell Stem
Cell. 17:11–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He Z, Wang J, Zhu C, Xu J, Chen P, Jiang
X, Chen Y, Jiang J and Sun C: Exosome-derived FGD5-AS1 promotes
tumor-associated macrophage M2 polarization-mediated pancreatic
cancer cell proliferation and metastasis. Cancer Lett.
548:2157512022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai J, Wu J, Wang J, Li Y, Hu X, Luo S and
Xiang D: Extracellular vesicles derived from different sources of
mesenchymal stem cells: Therapeutic effects and translational
potential. Cell Biosci. 10:692020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh S, Dansby C, Agarwal D, Bhat PD,
Dubey PK and Krishnamurthy P: Exosomes: Methods for isolation and
characterization in biological samples. Methods Mol Biol.
2835:181–213. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Henne WM, Buchkovich NJ and Emr SD: The
ESCRT pathway. Dev Cell. 21:77–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tschuschke M, Kocherova I, Bryja A,
Mozdziak P, Angelova Volponi A, Janowicz K, Sibiak R,
Piotrowska-Kempisty H, Iżycki D, Bukowska D, et al: Inclusion
biogenesis, methods of isolation and clinical application of human
cellular exosomes. J Clin Med. 9:4362020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gurung S, Perocheau D, Touramanidou L and
Baruteau J: The exosome journey: From biogenesis to uptake and
intracellular signalling. Cell Commun Signal. 19:472021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Joo HS, Suh JH, Lee HJ, Bang ES and Lee
JM: Current knowledge and future perspectives on mesenchymal stem
cell-derived exosomes as a new therapeutic agent. Int J Mol Sci.
21:7272020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kowal J, Tkach M and Théry C: Biogenesis
and secretion of exosomes. Curr Opin Cell Biol. 29:116–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wortzel I, Dror S, Kenific CM and Lyden D:
Exosome-mediated metastasis: Communication from a distance. Dev
Cell. 49:347–360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Doyle LM and Wang MZ: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells. 8:7272019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Y, Hong Y, Cho E, Kim GB and Kim IS:
Extracellular vesicles as a platform for membrane-associated
therapeutic protein delivery. J Extracell Vesicles. 7:14401312018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kugeratski FG, Hodge K, Lilla S, McAndrews
KM, Zhou X, Hwang RF, Zanivan S and Kalluri R: Quantitative
proteomics identifies the core proteome of exosomes with syntenin-1
as the highest abundant protein and a putative universal biomarker.
Nat Cell Biol. 23:631–641. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hánělová K, Raudenská M, Masařík M and
Balvan J: Protein cargo in extracellular vesicles as the key
mediator in the progression of cancer. Cell Commun Signal.
22:252024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Choi DS, Kim DK, Kim YK and Gho YS:
Proteomics, transcriptomics and lipidomics of exosomes and
ectosomes. Proteomics. 13:1554–1571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao G, Wang L and Li C: Circ_0006790
carried by bone marrow mesenchymal stem cell-derived exosomes
regulates S100A11 DNA methylation through binding to CBX7 in
pancreatic ductal adenocarcinoma. Am J Cancer Res. 12:1934–1959.
2022.PubMed/NCBI
|
|
47
|
Wu R, Su Z, Zhao L, Pei R, Ding Y, Li D,
Zhu S, Xu L, Zhao W and Zhou W: Extracellular vesicle-loaded
oncogenic lncRNA NEAT1 from adipose-derived mesenchymal stem cells
confers gemcitabine resistance in pancreatic cancer via
miR-491-5p/Snail/SOCS3 axis. Stem Cells Int. 2023:65105712023.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang R, Zhang Y, Hao F, Su Z, Duan X and
Song X: Exosome-mediated triple drug delivery enhances apoptosis in
pancreatic cancer cells. Apoptosis. 30:1893–1911. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen
Q, Zhang Y, Lu Y, Ding X and Jiang C: Bone marrow mesenchymal stem
cells-derived exosomes for penetrating and targeted chemotherapy of
pancreatic cancer. Acta Pharm Sin B. 10:1563–1575. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yao X, Mao Y, Wu D, Zhu Y, Lu J, Huang Y,
Guo Y, Wang Z, Zhu S, Li X and Lu Y: Exosomal circ_0030167 derived
from BM-MSCs inhibits the invasion, migration, proliferation and
stemness of pancreatic cancer cells by sponging miR-338-5p and
targeting the Wif1/Wnt8/β-catenin axis. Cancer Lett. 512:38–50.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shang S, Wang J, Chen S, Tian R, Zeng H,
Wang L, Xia M, Zhu H and Zuo C: Exosomal miRNA-1231 derived from
bone marrow mesenchymal stem cells inhibits the activity of
pancreatic cancer. Cancer Med. 8:7728–7740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu Y, Liu N, Wei Y, Zhou D, Lin R, Wang X
and Shi B: Anticancer effects of miR-124 delivered by BM-MSC
derived exosomes on cell proliferation, epithelial mesenchymal
transition, and chemotherapy sensitivity of pancreatic cancer
cells. Aging (Albany NY). 12:19660–19676. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin T, Pu X, Zhou S, Huang Z, Chen Q,
Zhang Y, Mao Q, Liang Y and Ding G: Identification of exosomal
miR-484 role in reprogramming mitochondrial metabolism in
pancreatic cancer through Wnt/MAPK axis control. Pharmacol Res.
197:1069802023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Alidoust Saharkhiz Lahiji M and Safari F:
Potential therapeutic effects of hAMSCs secretome on Panc1
pancreatic cancer cells through downregulation of SgK269,
E-cadherin, vimentin, and snail expression. Biologicals. 76:24–30.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Safari F and Dadvar F: In vitro evaluation
of autophagy and cell death induction in Panc1 pancreatic cancer by
secretome of hAMSCs through downregulation of p-AKT/p-mTOR and
upregulation of p-AMPK/ULK1 signal transduction pathways. Tissue
Cell. 84:1021602023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Safari F, Shafiee Nejad N and Aghaei Nejad
A: The inhibition of Panc1 cancer cells invasion by hAMSCs
secretome through suppression of tyrosine phosphorylation of SGK223
(at Y411 site), c-Src (at Y416, Y530 sites), AKT activity, and
JAK1/Stat3 signaling. Med Oncol. 39:282022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou W, Zhou Y, Chen X, Ning T, Chen H,
Guo Q, Zhang Y, Liu P, Zhang Y, Li C, et al: Pancreatic
cancer-targeting exosomes for enhancing immunotherapy and
reprogramming tumor microenvironment. Biomaterials. 268:1205462021.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Klimova D, Jakubechova J, Altanerova U,
Nicodemou A, Styk J, Szemes T, Repiska V and Altaner C:
Extracellular vesicles derived from dental mesenchymal stem/stromal
cells with gemcitabine as a cargo have an inhibitory effect on the
growth of pancreatic carcinoma cell lines in vitro. Mol Cell
Probes. 67:1018942023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hu X, Zhou W, Pi R, Zhao X and Wang W:
Genetically modified cancer vaccines: Current status and future
prospects. Med Res Rev. 42:1492–1517. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dunbar CE, High KA, Joung JK, Kohn DB,
Ozawa K and Sadelain M: Gene therapy comes of age. Science.
359:eaan46722018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Doering CB, Archer D and Spencer HT:
Delivery of nucleic acid therapeutics by genetically engineered
hematopoietic stem cells. Adv Drug Deliv Rev. 62:1204–1212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhou P, Ding X, Du X, Wang L and Zhang Y:
Targeting reprogrammed cancer-associated fibroblasts with
engineered mesenchymal stem cell extracellular vesicles for
pancreatic cancer treatment. Biomater Res. 28:00502024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Buocikova V, Altanerova U, Soltysova A,
Andrezal M, Vanova D, Jakubechova J, Cihova M, Burikova M, Urbanova
M, Juhasikova L, et al: Placental mesenchymal stem cells: A
promising platform for advancing gene therapy in pancreatic ductal
adenocarcinoma. Biomed Pharmacother. 190:1184282025. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Amati E, Perbellini O, Rotta G, Bernardi
M, Chieregato K, Sella S, Rodeghiero F, Ruggeri M and Astori G:
High-throughput immunophenotypic characterization of bone marrow-
and cord blood-derived mesenchymal stromal cells reveals common and
differentially expressed markers: Identification of
angiotensin-converting enzyme (CD143) as a marker differentially
expressed between adult and perinatal tissue sources. Stem Cell Res
Ther. 9:102018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang J, Liu Y, Yin W and Hu X:
Adipose-derived stromal cells in regulation of hematopoiesis. Cell
Mol Biol Lett. 25:162020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xu T, Yu X, Yang Q, Liu X, Fang J and Dai
X: Autologous micro-fragmented adipose tissue as stem cell-based
natural scaffold for cartilage defect repair. Cell Transplant.
28:1709–1720. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Schäffler A and Büchler C: Concise review:
Adipose tissue-derived stromal cells-basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kozlowska U, Krawczenko A, Futoma K, Jurek
T, Rorat M, Patrzalek D and Klimczak A: Similarities and
differences between mesenchymal stem/progenitor cells derived from
various human tissues. World J Stem Cells. 11:347–374. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dubey NK, Mishra VK, Dubey R, Deng YH,
Tsai FC and Deng WP: Revisiting the advances in isolation,
characterization and secretome of adipose-derived stromal/stem
cells. Int J Mol Sci. 19:22002018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fukuchi Y, Nakajima H, Sugiyama D, Hirose
I, Kitamura T and Tsuji K: Human placenta-derived cells have
mesenchymal stem/progenitor cell potential. Stem Cells. 22:649–658.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Oliveira MS and Barreto-Filho JB:
Placental-derived stem cells: Culture, differentiation and
challenges. World J Stem Cells. 7:769–775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ulrich C, Abruzzese T, Maerz JK, Ruh M,
Amend B, Benz K, Rolauffs B, Abele H, Hart ML and Aicher WK: Human
placenta-derived CD146-positive mesenchymal stromal cells display a
distinct osteogenic differentiation potential. Stem Cells Dev.
24:1558–1569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li S, Huang KJ, Wu JC, Hu MS, Sanyal M, Hu
M, Longaker MT and Lorenz HP: Peripheral blood-derived mesenchymal
stem cells: Candidate cells responsible for healing critical-sized
calvarial bone defects. Stem Cells Transl Med. 4:359–368. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cao C and Dong Y and Dong Y: Study on
culture and in vitro osteogenesis of blood-derived human
mesenchymal stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
19:642–647. 2005.(In Chinese). PubMed/NCBI
|
|
75
|
Tracy SA, Ahmed A, Tigges JC, Ericsson M,
Pal AK, Zurakowski D and Fauza DO: A comparison of clinically
relevant sources of mesenchymal stem cell-derived exosomes: Bone
marrow and amniotic fluid. J Pediatr Surg. 54:86–90. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang Z, He Z, Liang S, Yang Q, Cheng P and
Chen A: Comprehensive proteomic analysis of exosomes derived from
human bone marrow, adipose tissue, and umbilical cord mesenchymal
stem cells. Stem Cell Res Ther. 11:5112020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ji L, Bao L, Gu Z, Zhou Q, Liang Y, Zheng
Y, Xu Y, Zhang X and Feng X: Comparison of immunomodulatory
properties of exosomes derived from bone marrow mesenchymal stem
cells and dental pulp stem cells. Immunol Res. 67:432–442. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Katsuda T, Tsuchiya R, Kosaka N, Yoshioka
Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M and Ochiya T:
Human adipose tissue-derived mesenchymal stem cells secrete
functional neprilysin-bound exosomes. Sci Rep. 3:11972013.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pomatto M, Gai C, Negro F, Cedrino M,
Grange C, Ceccotti E, Togliatto G, Collino F, Tapparo M, Figliolini
F, et al: Differential therapeutic effect of extracellular vesicles
derived by bone marrow and adipose mesenchymal stem cells on wound
healing of diabetic ulcers and correlation to their cargoes. Int J
Mol Sci. 22:38512021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Langevin SM, Kuhnell D, Orr-Asman MA,
Biesiada J, Zhang X, Medvedovic M and Thomas HE: Balancing yield,
purity and practicality: A modified differential
ultracentrifugation protocol for efficient isolation of small
extracellular vesicles from human serum. RNA Biol. 16:5–12. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gardiner C, Di Vizio D, Sahoo S, Théry C,
Witwer KW, Wauben M and Hill AF: Techniques used for the isolation
and characterization of extracellular vesicles: Results of a
worldwide survey. J Extracell Vesicles. 5:329452016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Van Deun J, Mestdagh P, Sormunen R,
Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O and
Hendrix A: The impact of disparate isolation methods for
extracellular vesicles on downstream RNA profiling. J Extracell
Vesicles. 3:2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lobb RJ, Becker M, Wen SW, Wong CS,
Wiegmans AP, Leimgruber A and Möller A: Optimized exosome isolation
protocol for cell culture supernatant and human plasma. J Extracell
Vesicles. 4:270312015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bhattacharjee C and Singh M: Studies on
the applicability of artificial neural network (ANN) in continuous
stirred ultrafiltration. Chem Eng Technol. 25:1187–1192. 2002.
View Article : Google Scholar
|
|
85
|
Cheng H, Fang H, Xu RD, Fu MQ, Chen L,
Song XY, Qian JY, Zou YZ, Ma JY and Ge JB: Development of a rinsing
separation method for exosome isolation and comparison to
conventional methods. Eur Rev Med Pharmacol Sci. 23:5074–5083.
2019.PubMed/NCBI
|
|
86
|
Soares Martins T, Catita J, Martins Rosa
I, AB da Cruz E, Silva O and Henriques AG: Exosome isolation from
distinct biofluids using precipitation and column-based approaches.
PLoS One. 13:e01988202018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Buschmann D, Kirchner B, Hermann S, Märte
M, Wurmser C, Brandes F, Kotschote S, Bonin M, Steinlein OK, Pfaffl
MW, et al: Evaluation of serum extracellular vesicle isolation
methods for profiling miRNAs by next-generation sequencing. J
Extracell Vesicles. 7:14813212018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhao L, Yu J, Wang J, Li H, Che J and Cao
B: Isolation and identification of miRNAs in exosomes derived from
serum of colon cancer patients. J Cancer. 8:1145–1152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Popovic M, Mazzega E, Toffoletto B and De
Marco A: Isolation of anti-extra-cellular vesicle single-domain
antibodies by direct panning on vesicle-enriched fractions. Microb
Cell Fact. 17:62018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhu L, Sun HT, Wang S, Huang SL, Zheng Y,
Wang CQ, Hu BY, Qin W, Zou TT, Fu Y, et al: Isolation and
characterization of exosomes for cancer research. J Hematol Oncol.
13:1522020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pei S, Sun W, Han Q, Wang H and Liang Q:
Bifunctional immunoaffinity magnetic nanoparticles for
high-efficiency separation of exosomes based on host-guest
interaction. Talanta. 272:1257902024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Iliescu FS, Vrtačnik D, Neuzil P and
Iliescu C: Microfluidic technology for clinical applications of
exosomes. Micromachines (Basel). 10:3922019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang P, Zhou X, He M, Shang Y, Tetlow AL,
Godwin AK and Zeng Y: Ultrasensitive detection of circulating
exosomes with a 3D-nanopatterned microfluidic chip. Nat Biomed Eng.
3:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hua X, Zhu Q, Liu Y, Zhou S, Huang P, Li Q
and Liu S: A double tangential flow filtration-based microfluidic
device for highly efficient separation and enrichment of exosomes.
Anal Chim Acta. 1258:3411602023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ding L, Liu X, Zhang Z, Liu LE, He S, Wu
Y, Effah CY, Yang R, Zhang A, Chen W, et al:
Magnetic-nanowaxberry-based microfluidic ExoSIC for affinity and
continuous separation of circulating exosomes towards cancer
diagnosis. Lab Chip. 23:1694–1702. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Abramowicz A, Marczak L, Wojakowska A,
Zapotoczny S, Whiteside TL, Widlak P and Pietrowska M:
Harmonization of exosome isolation from culture supernatants for
optimized proteomics analysis. PLoS One. 13:e02054962018.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Iqbal Z, Rehman K, Mahmood A, Shabbir M,
Liang Y, Duan L and Zeng H: Exosome for mRNA delivery: Strategies
and therapeutic applications. J Nanobiotechnology. 22:3952024.
View Article : Google Scholar : PubMed/NCBI
|