Introduction
Temporal bone malignant tumors are rare and
represent a small proportion (0.2%) of head and neck malignancies,
with an approximate annual incidence rate of 1–6 cases per million
individuals (1,2). Temporal bone squamous cell carcinoma
is the most common malignant tumor in this area, representing 80%
of all malignancies (2). The
remaining cases include adenoid cystic carcinoma and other rare
pathologies, such as sarcoma. The tumors can be primary (from the
external/middle ear) or secondary (from adjacent sites such as the
periauricular skin or parotid gland) and are chiefly diagnosed in
patients beyond the age of 60 years (3). These tumors often present with
non-specific symptoms such as otorrhea, otalgia, tinnitus, hearing
loss, and occasionally, facial paralysis, making early diagnosis
challenging. By the time a definitive diagnosis is made, the
disease tends to have expanded to a large extent, frequently
invading adjacent nerves, blood vessels and other vital organs
(1).
The persistent tissue injury stemming from ongoing
otitis media and external otitis is a predominant risk factor,
which serves as the primary mechanism driving the carcinogenesis of
temporal bone malignancies (4–6). Other
risk factors include exposure to ultraviolet radiation and prior
radiotherapy (RT), older age and immunodeficiency (7). Surgical resection is the mainstay of
curative treatment, typically in the form of temporal bone
resection with or without RT (8,9).
Despite multidisciplinary treatment, the prognosis
of temporal bone malignancies remains poor. The rarity of the
disease has impeded the collection of evidence from both clinical
and basic research, and there is currently no unified staging
system. The present retrospective analysis aimed to evaluate the
management strategies, survival outcomes and possible adverse
features of patients with temporal bone malignancies treated at
Beijing Tiantan Hospital (Beijing, China).
Materials and methods
Patient population
Patients with pathologically confirmed temporal bone
malignancies treated at Beijing Tiantan Hospital between March 2014
and March 2022 were retrospectively included in the current study.
The study was approved by the Ethics Committee of Beijing Tiantan
Hospital (approval no. 2022-255-01) and was carried out in
accordance with the principles of The Declaration of Helsinki.
Patients were excluded from the study if they had any of the
following: A prior carcinoma in another organ, metastatic disease,
a periauricular skin or parotid gland origin, or incomplete
treatment records. All patients routinely underwent parotid and
cervical lymph node ultrasound, and temporal bone computed
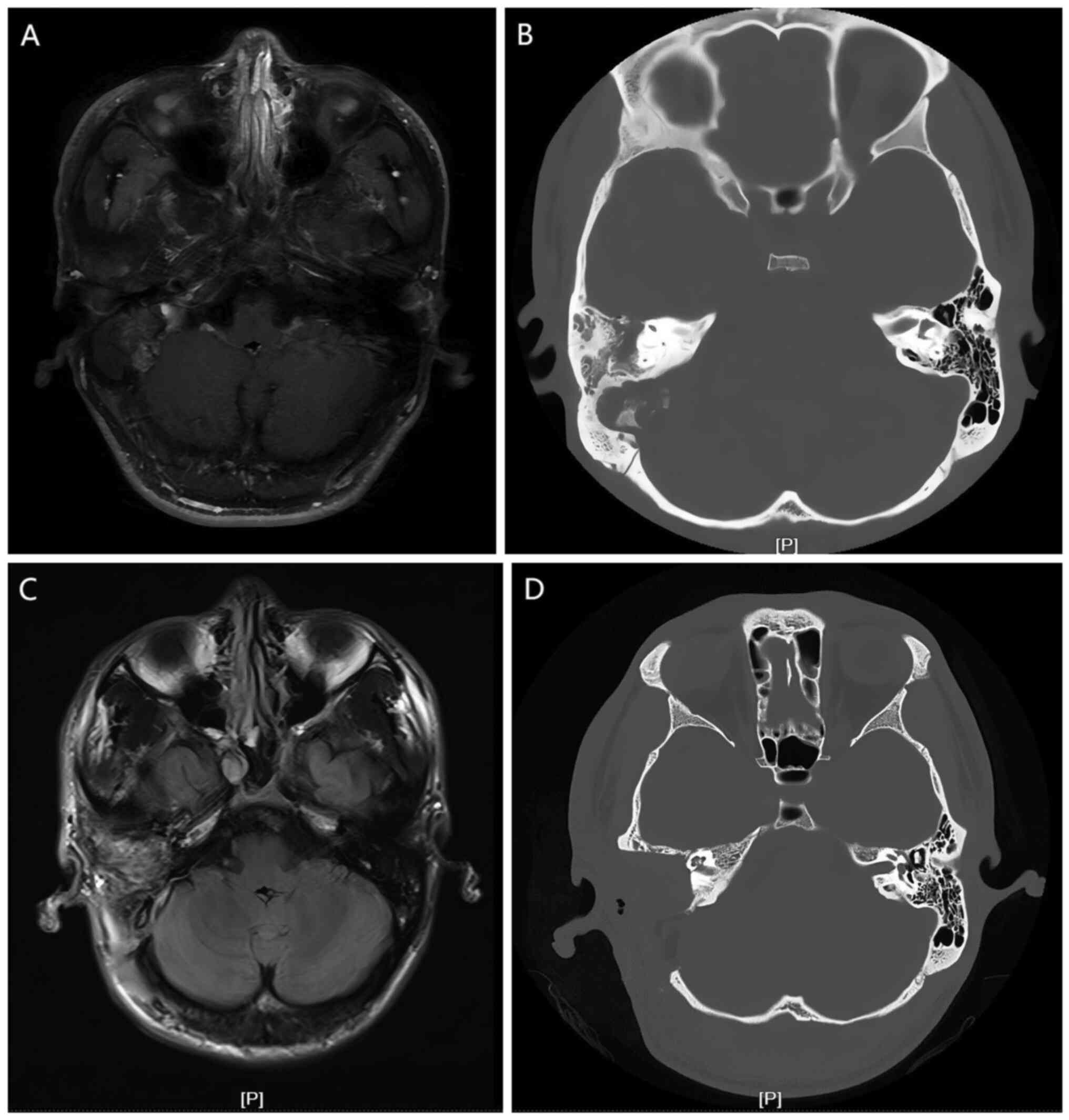
tomography (CT) and magnetic resonance imaging (MRI) (Fig. 1) examinations to determine the
extent of lesions and bone destruction, and the presence of
intracranial invasion before surgery. Positron emission tomography
was conducted in selected cases. Facial nerve function was
evaluated using the House-Brackmann (HB) grading system (10). Patients with squamous cell carcinoma
(SCC) and adenoid cystic carcinoma (ACC) were staged according to
the revised Pittsburgh classification system (11).
Treatment strategy
The surgical methods mainly included lateral
temporal bone resection (LTBR) and subtotal temporal bone resection
(STBR). For patients with involvement of the lateral skull base,
the infratemporal fossa approach was used to remove the tumor. The
facial nerve was preserved as much as possible, unless it was
infiltrated by the tumor and could not be separated. Parotidectomy
and neck dissection were performed if clinically indicated, and the
temporomandibular joint was resected to achieve complete excision
of the tumor when necessary. Surgical margins were determined based
on postoperative pathological examination for patients with lateral
temporal bone resection applied. A clear margin was defined as a
distance of ≥5 mm from the invasive tumor front. However, since
early symptoms of malignant temporal bone tumors are often
non-specific, most patients presented with advanced disease at the
time of treatment. In such cases, subtotal temporal bone resection
rarely allows for en bloc excision. After tumor resection, an
otological drill is used to remove the diseased bone tissue, which
is not included in the histological examination. Therefore, margin
assessment requires a comprehensive evaluation that integrates
postoperative paraffin-section pathology with intraoperative
findings. Regular follow-up was routinely conducted every 3 months
for the first 3 years, every 6 months for years 4 and 5, and once a
year thereafter.
Statistical analysis
Data were analyzed using SPSS 26.0 software (IBM
Corp.). The survival rates were calculated using the Kaplan-Meier
method. A log-rank test was employed to compare the differences in
patient survival between groups. Survival analysis was confined to
univariate comparisons with the log-rank test, as the sample size
was too small for a reliable multivariate analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical data
In the group of 20 patients, there were 16 men and 4
women, aged between 9 and 90 years, with a mean age of 50.8 years.
The duration of illness varied from 1 month to 40 years. According
to the revised Pittsburgh classification system (11), 1 patient presented with a T1 tumor,
5 patients with T3 tumors and 8 patients with T4 tumors, among the
14 patients diagnosed with SCC and ACC. The follow-up period ranged
from 1 to 140 months, and 1 patient was lost to follow-up (5%). The
clinical symptoms mainly included hearing loss (n=15, 75%), otalgia
(n=13, 65%), chronic ear discharge (n=8, 40%), headaches (n=6,
30%), facial paralysis (n=5, 25%), dizziness (n=5, 25%) and
tinnitus (n=5, 25%). Regarding HB facial paralysis, 1 case
presented as grade II, 2 cases presented as grade V and 2 cases
presented as grade VI. A single case had previously undergone
surgery for chronic otitis media. Table
I summarizes the patient demographics, tumor characteristics
and survival outcomes.
 | Table I.Clinical characteristics of temporal
carcinoma (n=20). |
Table I.
Clinical characteristics of temporal
carcinoma (n=20).
| Patient no. | Pittsburgh stage | Age, years | Sex | Symptoms | Treatment | Pathology | Follow-up status
and survival time (months) |
|---|
| 1 | T3 | 56 | M | Hearing loss,
otorrhea, otalgia and headache | STBR | SCC | DOD (37) |
| 2 | T4 | 59 | M | Hearing loss,
otorrhea, ear bleeding, otalgia and facioplegia | STBR + CRT | SCC | DM (24); A (74) |
| 3 | T4 | 64 | M | Hearing loss,
otorrhea, otalgia, headache, dizziness and limited mouth
opening | STBR + RT | SCC | DOD (10) |
| 4 | T4 | 59 | M | Hearing loss,
otorrhea, otalgia, ear bleeding and tinnitus | STBR | SCC | DOD (8) |
| 5 | T1 | 56 | M | Headache | LTBR | ACC | A (54) |
| 6 | T3 | 56 | M | Otalgia | STBR + RT | ACC | A (54) |
| 7 | T4 | 76 | M | Hearing loss,
otorrhea, otalgia and tinnitus | STBR | ACC | A (73) |
| 8 | / | 53 | F | Hearing loss and
headache | LTBR (1st
treatment), STBR + END + RT (2nd treatment) | ACC | LR (64); A
(140) |
| 9 | T4 | 65 | M | Hearing loss,
otorrhea, otalgia and dizziness | ITF+RT | SCC | A (85) |
| 10 | T4 | 43 | M | Hearing loss and
limited mouth opening | STBR+RT | ACC | A (64) |
| 11 | / | 33 | F | Otalgia | LTBR (1st
treatment), STBR + CRT (2nd treatment) | ACC | LR (61); DOD
(70) |
| 12 | T3 | 33 | M | Hearing loss,
otorrhea, otalgia, facioplegia, tinnitus and dizziness | STBR + CRT | SCC | LFU |
| 13 | / | 54 | M | Vision loss,
headache, dizziness and hearing loss | STBR + RT | Fibrosarcoma | A (56) |
| 14 | / | 28 | M | Facioplegia and
mass | ITF | Chondrosarcoma | A (104) |
| 15 | / | 56 | M | Ear stuffy, hearing
loss, otorrhea, tinnitus and ear bleeding | STBR + CRT (1st
treatment), ITF (2nd treatment) | Endolymphatic
cystic papillary adenocarcinoma | LR (11); DWD (65) |
| 16 | / | 39 | M | Facioplegia,
otalgia, hearing loss and tinnitus | STBR (1st
treatment), ITF (2nd treatment) | Chondrosarcoma | LR (62); A
(140) |
| 17 | / | 9 | F | Headache, dizziness
and facioplegia | ITF |
Rhabdomyosarcoma | DOD (1) |
| 18 | / | 18 | M | Hearing loss and
otalgia | ITF + RT | LMPNST | LR (8); DOD (16) |
| 19 | T4 | 36 | M | Hearing loss and
otalgia | LTBR + RT (1st
treatment), STBR (2nd treatment) | ACC | LR (26); A (54) |
| 20 | T4 | 90 | M | Hearing loss and
otalgia | STBR | SCC | DWD (16) |
Pathological examination confirmed the diagnosis in
all 20 cases, with 7 patients diagnosed with SCC, 7 with ACC, 2
with chondrosarcoma, 1 with fibrosarcoma, 1 with endolymphatic
cystic papillary adenocarcinoma, 1 with rhabdomyosarcoma and 1 with
a low-grade malignant peripheral nerve sheath tumor.
Treatment
Among the 20 cases, as the first treatment, 10
underwent surgical excision only, 7 received surgery and RT, and 3
underwent surgery combined with chemoradiotherapy (CRT). RT with a
dose of 60–66 Gy in 30–33 fractions at 1.8–2 Gy per fraction was
administrated postoperatively for patients with an advanced tumor
or positive margin. Additional concurrent weekly cisplatin
chemotherapy (30–40 mg/m2) was administered in patients
with positive margins. For patients unsuitable to receive
cisplatin, either carboplatin or RT alone was used. A total of 4
cases underwent LTBR, 12 cases received STBR and 4 cases received
lateral skull base tumor resection via the infratemporal fossa
approach (Table I).
A parotidectomy was conducted in 8 patients, of
which 5 were pathologically confirmed with tumor invasion. A
partial temporomandibular joint cyst resection was performed in 8
patients, with tumor invasion in 4 patients. The facial nerve was
involved in 9 cases, among which the tumor was resected while
preserving the facial nerve in 3 patients. All 3 patients appeared
with varying degrees of facial nerve paralysis after surgery
(Table II).
 | Table II.Structures found to be invaded by the
tumor during surgery. |
Table II.
Structures found to be invaded by the
tumor during surgery.
|
|
Patient
no. |
|---|
|
|
|
|---|
| Operative
details | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
|---|
| SP/pathology
confirmed invasion | -/- | +/+ | +/- | +/+ | -/- | -/- | +/+ | -/- | +/- | +/+ | +/- | -/- | -/- | -/- | -/- | -/- | -/- | -/- | +/+ | -/- |
| TMJ
resection/pathology confirmed invasion | -/- | +/+ | +/+ | +/- | -/- | -/- | +/- | -/- | +/- | +/- | -/- | -/- | -/- | -/- | -/- | -/- | -/- | -/- | +/+ | +/+ |
| Posterior cranial
fossa meningeal involvement | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | + | + | - | - |
| Medial cranial
fossa meningeal involvement | + | - | - | + | - | - | - | - | + | + | - | - | + | + | - | - | + | + | - | - |
| Facial nerve
invasion/resection | -/- | +/+ | -/- | +/- | -/- | -/- | -/- | -/- | +/- | +/+ | -/- | -/- | -/- | +/+ | +/- | +/+ | +/+ | +/+ | -/- | -/- |
The tumor involved the cranial fossa meninges in 9
cases, including 5 cases of medial cranial fossa meninges
involvement, 1 case of post cranial fossa meninges involvement and
3 cases of both, among which tumors were resected while preserving
the integrity of the meninges without evident cerebrospinal fluid
leakage in 8 cases. In the remaining case, the tumor and affected
dura were excised, and repair was performed using the artificial
dura mater interposition method. The tumor invaded the cochlea and
penetrated deep into the skull in 1 case, and cerebrospinal fluid
leakage occurred after tumor resection. This was managed by
utilizing free fat grafting to fill the cavity, along with temporal
muscle flap reinforcement.
Follow-up results
The 3- and 5-year disease-specific survival (DSS)
rates were 79 and 73%, and the 3- and 5-year overall survival (OS)
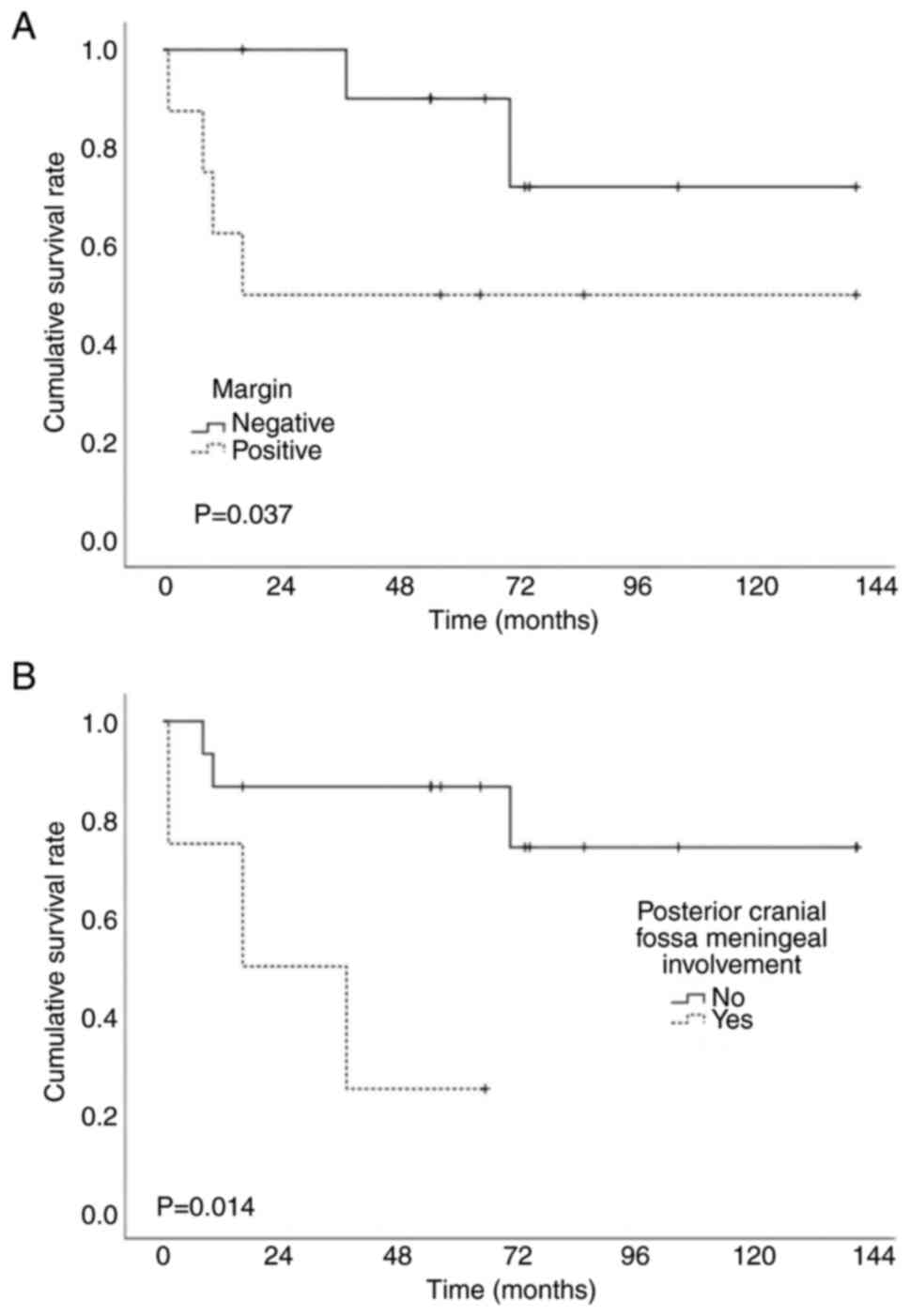
rates were 74 and 68%. Univariate analysis (Table III) results indicated that the
pathological type (both P<0.001) and posterior cranial fossa
meningeal involvement (P=0.005 and P=0.014, respectively) were
significantly associated with OS and DSS. Patients with a positive
surgical margin tended to have worse DSS (P=0.037) (Fig. 2). Due to the limited sample size,
further multivariate survival analysis was not conducted.
 | Table III.Variables associated with survival
(univariate analysis by log-rank test). |
Table III.
Variables associated with survival
(univariate analysis by log-rank test).
| Variables | Ovall survival
P-value | Disease-specific
survival P-value |
|---|
| Sex
(male/female) | 0.301 | 0.442 |
| Age (≤55/>55
years) | 0.492 | 0.974 |
| Facial paralysis
(yes/no) | 0.421 | 0.703 |
| Medial cranial
fossa meningeal involvement (yes/no) | 0.369 | 0.121 |
| Posterior cranial
fossa meningeal involvement (yes/no) | 0.005 | 0.014 |
| Parotid gland/TMJ
involvement (yes/no) | 0.428 | 0.265 |
| T stage
(T4/T1-3) | 0.888 | 0.762 |
| Margin
(positive/negative) | 0.287 | 0.037a |
| Histology
(SCC/ACC/FSa/CS/ELST/RMS/LMPNST) | <0.001 | <0.001 |
| Treatment modality
(surgery/surgery + RT/CRT) | 0.630 | 0.513 |
Among the 14 patients diagnosed with ACC and SCC, 3
patients experienced local recurrence and underwent a second
surgery. A single patient with ACC died of disease at 70 months,
while another 2 patients with ACC were alive at 54 and 140 months
of follow-up. Only 1 out of the 14 patients had distant metastasis
to the lung at 24 months, and this patient received CRT and was
alive at 74 months. Another 3 patients died of the disease, at 8,
10 and 37 months postoperatively. A single patient died of
pulmonary infection at 16 months without evidence of disease.
Another patient diagnosed with a T3 tumor failed to be followed up
for unknown reason after receiving surgery and CRT. A further 5
patients survived without showing signs of disease at a mean of 66
months of follow-up (range, 54–85 months) (Table I).
Among the 6 patients with rare temporal bone
malignancies, 3 underwent lateral skull base tumor resection via
the infratemporal fossa approach and 2 experienced local relapse. A
single patient with a low-grade malignant peripheral nerve sheath
tumor died of disease at 16 months, while another patient with
endolymphatic cystic papillary adenocarcinoma died of glioma at 65
months. In addition, 1 patient with chondrosarcoma was alive at 140
months. Another 9-year-old child with rhabdomyosarcoma underwent
incomplete resection due to internal carotid artery invasion and
died of disease soon after the surgery. Furthermore, 2 patients
were alive at 56 and 104 months of follow-up.
Discussion
The present study conducted a thorough examination
of the survival rates and possible factors influencing the
prognosis for patients diagnosed with temporal bone malignancies at
Beijing Tiantan Hospital. The findings confirmed that the
pathological type and the presence of positive surgical margins may
be significant prognostic indicators of patients with temporal bone
malignancies.
At present, the International Union Against Cancer
and the American Joint Committee on Cancer have not proposed
staging criteria specifically for temporal bone malignancies. The
modified Pittsburgh staging criteria (11) is widely accepted for patients with
SCC of the temporal bone. However, Nabuurs et al (12) recruited 381 patients to assess the
prognostic predictive value of the modified Pittsburgh staging
system and demonstrated that T4 tumors with different directions of
invasion (anterior, posterior, superior, inferior, medial or
lateral) had different prognoses, but the staging system did not
distinguish between different directions of invasion of T4 tumors.
Zanoletti et al (13)
reported a hazard ratio of 1.13 (95% confidence interval,
0.98–1.32; P=0.089) for patients with tumors spreading in other
directions compared with patients with T4 external auditory canal
SCC tumors spreading anteriorly (preauricular region and parotid
space). Similarly, the results of the present study suggested that
patients with posterior cranial fossa meningeal involvement had a
worse prognosis. Patients without posterior cranial fossa disease
involvement had significantly superior 5-year OS and DSS rates of
80 and 87%, respectively, compared with those with involvement
(both 25%). Additionally, Zanoletti et al (13) built a new staging system based on
the modified Pittsburgh staging criteria combined with additional
T4 tumor prognostic factors, such as dural involvement, the
direction of invasion and histological grading. This new standard
was used to evaluate the prognosis of 44 cases of SCC of the
temporal bone, as well as other case series from different studies.
The results indicated that the new staging standard not only had
better prognostic predictive effects than the modified Pittsburgh
staging criteria, but also demonstrated good applicability
(13,14). Moreover, as demonstrated in the
present study, there are other types of temporal bone malignancies,
including ACC and other rare types, and more research is needed to
refine a staging system of temporal bone malignancies.
Depending on the area of tumor invasion, the
surgical approaches for temporal osteotomy include LTBR (resection
of the ear canal and tympanic membrane), STBR (resection of the
labyrinth/cochlea and internal auditory canal) and total temporal
bone resection (TTBR; resection of the petrous apex) (15). Typically, LTBR is recognized as a
preferred treatment for early-stage (T1-2) carcinoma, dispensing
with the need for postoperative RT, provided that the surgical
margins are clear of disease (15–19).
For advanced-stage tumors, treatment for the majority of patients
involves STBR with adjuvant RT, and occasionally, chemotherapy
(20). TTBR is now rarely used due
to associated surgical complications and lower survival rates
without an improvement in prognosis when compared with STBR plus
adjuvant therapy (16). In the
present study, 12 patients received STBR, 4 underwent LTBR, and 4
received lateral skull base tumor resection via the infratemporal
fossa approach.
The lymph node metastasis rate of temporal
malignancies is relatively low (21,22).
Neck dissection is undertaken mainly for patients exhibiting
radiographic or clinical signs of cervical lymph node involvement.
In the present group of patients, with the exception of 2 patients
who underwent parotid and postauricular lymph node excision with
negative pathology, no patient underwent an elective regional neck
dissection. Moreover, no local lymph node recurrence was observed
in any patient during the follow-up period. There is still
considerable debate over whether patients with malignant tumors of
the temporal bone should routinely undergo parotidectomy (23,24).
Currently, a parotidectomy is performed in cases with notable
involvement of the parotid gland or suspicion of facial nerve
invasion, and it is also utilized in specific cases to achieve
negative margins. In the present study, a parotidectomy was
conducted in 8 patients, and in 5 of these cases, tumor invasion
was confirmed pathologically.
In terms of functionality, the goal when treating
temporal bone malignancies is to completely remove the tumor with
negative margin while preserving the integrity of the facial nerve
as much as possible. However, temporal bone malignancies are often
covert, and by the time they are diagnosed, the extent of the
disease can be quite high. Compared with LTBR, STBR often requires
consideration as to whether to preserve or sacrifice the facial
nerve, and it may be necessary to sacrifice it. Since malignant
tumors of the external auditory canal and middle ear have a high
degree of malignancy and pose a threat to life, the focus is on
completely removing the lesion and extending survival, rather than
emphasizing the preservation of hearing. In the present group of
patients, the facial nerve was resected in 6 cases, and the tumor
was peeled off the surface of the facial nerve in 3 cases. All
patients had varying degrees of hearing loss after surgery.
Surgery combined with postoperative supplementary RT
is an effective intervention for controlling temporal bone tumors,
even though ACC and tumors of mesenchymal origin are not
particularly sensitive to RT (25–28).
Generally, RT is applied postoperatively for advanced/recurrent
disease, as well as for individuals exhibiting positive surgical
margins, lymph node metastasis, nerve invasion and extracapsular
spread. However, definitive CRT is emerging as a primary treatment
for advanced or unresectable disease. Morita et al (19) observed comparable 5-year OS rates
among patients with T3-4 tumors who underwent definitive CRT
(52.1%) in contrast to patients who received surgery followed by
postoperative RT, with or without chemotherapy (55.6%).
Limited research shows that molecular-targeting
agents, such as cetuximab and isocitrate dehydrogenase 1
inhibitors, exhibit promising antitumor activity in patients with
advanced temporal bone malignancies (29,30).
Additionally, Yan and Sui (31)
reported the long-term survival of a 47-year-old man with
uncontrolled locally advanced temporal bone SCC after immunotherapy
followed by CRT. Addressing these challenges will lead to improved
prognosis for patients with unresectable, residual, or recurrent
temporal bone malignancies.
The 5-year OS rate has been reported to vary in
different studies, ranging from 51.7 to 66.8% (6,32–34),
while the DSS rate for patients with T1 and T2 temporal bone tumors
has been shown to range from 92 to 100%, compared with 48 to 65%
for those with T3 and T4 tumors (21). The majority of patients included in
the present study presented as advanced and recurrent cases, and
the 5-year DSS and OS rates were 73 and 68%, respectively.
A positive margin is often considered a poor
prognostic factor (20,35), as reported in the present study. The
5-year DSS rates of patients with negative and positive margins
were 89 and 50%, respectively. However, what should be noted is
that the correct judgment of tumor margins must take into account
both histological examinations and surgical records, distinguishing
between ‘false-positives’ and ‘true-positives’ due to piecemeal
resection. Other reported factors associated with a poor prognosis
include clinical facial nerve palsy at presentation and advanced
stage disease (11,36–39).
However, a recent multivariate analysis revealed the sole poor
prognostic indicator with statistical significance was surgery on
patients with recurrent disease (2). Dean et al (40) also found no difference between local
control rates for cases that required facial nerve resection
(67.6%) compared with those that did not (76.9%) (P=0.13), a
finding that aligns with the results of the current study.
Manolidis et al (41) noted
that tumors of mesenchymal origin were generally less invasive and
tended to exhibit better local control rates in comparison to other
types of malignancies. Similar results were observed in the present
study, since pathological type statistically affected prognosis
(P<0.001). Notably, the SCC pathological type was associated
with worse OS and DSS rates compared with the ACC (P=0.028
and P=0.073, respectively). However, given the small number
of patients with other pathological types, interpretation of the
prognostic data requires caution.
The present study has some limitations. Owing to the
rarity of temporal bone malignancies, conducting large-scale
prospective studies within a single institution has been
challenging. Thus, the present study is a single-centre
retrospective case series, which may have potential selection bias.
The small sample size precluded statistical analysis of several
adverse features and further multivariable survival analysis to
rule out the influence of confounding factors. Multicenter
collaboration is warranted to validate these findings in a larger,
more diverse cohort. In addition, the retrospective study design
relied on the accuracy and completeness of medical records, which
may result in potential information bias. Moreover, with the
progression of technology, surgical techniques and adjuvant
treatment strategies, it may be challenging to compare treatment
results with those from other studies and to determine the optimal
therapeutic approach.
In conclusion, temporal bone malignancies are rare
and are associated with a poor prognosis. Factors predictive of
poor survival outcomes include SCC pathology, a positive surgical
margin and posterior cranial fossa meningeal involvement. Treatment
is complex, and surgery combined with RT/CRT is currently the main
effective treatment plan. Nevertheless, further multi-institutional
prospective investigations are required to specify the biological
behavior of temporal malignancies, and to identify unified staging
and optimal treatment strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
All authors had full access to the data in the study
and take responsibility for the integrity of the data and the
accuracy of the data analysis. LG and YX were responsible for
conceptualization. LG and RG were responsible for the methodology.
Study investigation was performed by LG and TY. Formal data
analysis and interpretation was performed by LG, TY, RG and WZ. The
original draft was written by LG. YX and RG reviewed and revised
the manuscript. RG supervised the study. Data curation was the
responsibility of LG and TY. Project administration was performed
by RG, and validation was performed by YX and RG. LG and YX confirm
the authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Beijing Tiantan Hospital (Beijing, China; approval no.
2022-255-01). Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lechner M, Sutton L, Murkin C, Masterson
L, O'Flynn P, Wareing MJ, Tatla T and Saeed S: Squamous cell cancer
of the temporal bone: A review of the literature. Eur Arch
Otorhinolaryngol. 278:2225–2228. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCracken M, Pai K, Cabrera CI, Johnson
BR, Tamaki A, Gidley PW and Manzoor NF: Temporal bone resection for
squamous cell carcinoma of the lateral skull base: Systematic
review and meta-analysis. Otolaryngol Head Neck Surg. 168:154–164.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gidley PW, Thompson CR, Roberts DB,
DeMonte F and Hanna EY: The oncology of otology. Laryngoscope.
122:393–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allanson BM, Low TH, Clark JR and Gupta R:
Squamous cell carcinoma of the external auditory canal and temporal
bone: An update. Head Neck Pathol. 12:407–418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato K, Komune N, Hongo T, Koike K, Niida
A, Uchi R, Noda T, Kogo R, Matsumoto N, Yamamoto H, et al: Genetic
landscape of external auditory canal squamous cell carcinoma.
Cancer Sci. 111:3010–3019. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin M, Ishikawa K, Honda K, Arakawa T,
Harabuchi Y, Nagabashi T, Fukuda S, Taira A, Himi T, Nakamura N, et
al: Analysis of 95 cases of squamous cell carcinoma of the external
and middle ear. Auris Nasus Larynx. 33:251–257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Acharya PP, Sarma D and McKinnon B: Trends
of temporal bone cancer: SEER database. Am J Otolaryngol.
41:1022972020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hongo T, Kuga R, Miyazaki M, Komune N,
Nakano T, Yamamoto H, Koike K, Sato K, Kogo R, Nabeshima K, et al:
Programmed death-ligand 1 expression and tumor-infiltrating
lymphocytes in temporal bone squamous cell carcinoma. Laryngoscope.
131:2674–2683. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Komune N, Noda T, Kogo R, Miyazaki M,
Tsuchihashi NA, Hongo T, Koike K, Sato K, Uchi R, Wakasaki T, et
al: Primary advanced squamous cell carcinoma of the temporal bone:
A single-center clinical study. Laryngoscope. 131:E583–E589. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
House JW: Facial nerve grading systems.
Laryngoscope. 93:1056–1069. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moody SA, Hirsch BE and Myers EN: Squamous
cell carcinoma of the external auditory canal: An evaluation of a
staging system. Am J Otol. 21:582–588. 2000.PubMed/NCBI
|
|
12
|
Nabuurs CH, Kievit W, Labbe N, Leemans CR,
Smit CFGM, van den Brekel MWM, Pauw RJ, van der Laan BFAM, Jansen
JC, Lacko M, et al: Evaluation of the modified Pittsburgh
classification for predicting the disease-free survival outcome of
squamous cell carcinoma of the external auditory canal. Head Neck.
42:3609–3622. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zanoletti E, Franz L, Cazzador D,
Franchella S, Calvanese L, Nicolai P, Mazzoni A and Marioni G:
Temporal bone carcinoma: Novel prognostic score based on clinical
and histological features. Head Neck. 42:3693–3701. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Franz L, Zanoletti E, Franchella S,
Cazzador D, Favaretto N, Calvanese L, Mazzoni A, Nicolai P and
Marioni G: Temporal bone carcinoma: Testing the prognostic value of
a novel clinical and histological scoring system. Eur Arch
Otorhinolaryngol. 278:4179–4186. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shinomiya H, Uehara N, Teshima M, Kakigi
A, Otsuki N and Nibu KI: Clinical management for T1 and T2 external
auditory canal cancer. Auris Nasus Larynx. 46:785–789. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piras G, Grinblat G, Albertini R,
Sykopetrites V, Zhong SX, Lauda L and Sanna M: Management of
squamous cell carcinoma of the temporal bone: Long-term results and
factors influencing outcomes. Eur Arch Otorhinolaryngol.
278:3193–3202. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang T, Li W, Dai C, Chi F, Wang S and
Wang Z: Evidence-based surgical management of T1 or T2 temporal
bone malignancies. Laryngoscope. 123:244–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Homer JJ, Lesser T, Moffat D, Slevin N,
Price R and Blackburn T: Management of lateral skull base cancer:
United Kingdom national multidisciplinary guidelines. J Laryngol
Otol. 130:S119–S124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morita S, Homma A, Nakamaru Y, Sakashita
T, Hatakeyama H, Kano S, Fukuda A and Fukuda S: The outcomes of
surgery and chemoradiotherapy for temporal bone cancer. Otol
Neurotol. 37:1174–1182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seligman KL, Sun DQ, Eyck PP, Schularick
NM and Hansen MR: Temporal bone carcinoma: Treatment patterns and
survival. Laryngoscope. 130:E11–E20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lovin BD and Gidley PW: Squamous cell
carcinoma of the temporal bone: A current review. Laryngoscope
Investig Otolaryngol. 4:684–692. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borsetto D, Vijendren A, Franchin G,
Donnelly N, Axon P, Smith M, Masterson L, Bance M, Saratziotis A,
Polesel J, et al: Prevalence of occult nodal metastases in squamous
cell carcinoma of the temporal bone: A systematic review and
meta-analysis. Eur Arch Otorhinolaryngol. 279:5573–5581. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JM, Joo JW, Kim SH, Choi JY and Moon
IS: Evidence based tailored parotidectomy in treating external
auditory canal carcinoma. Sci Rep. 8:121122018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie B, Wang M, Zhang S and Liu Y:
Parotidectomy in the management of squamous cell carcinoma of the
external auditory canal. Eur Arch Otorhinolaryngol. 278:1355–1364.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhen S, Fu T, Qi J and Wen J: Adenoid
cystic carcinoma of external auditory canal: 8 cases report. Lin
Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 29:343–345. 2015.(In
Chinese). PubMed/NCBI
|
|
26
|
Wang M, Song Y, Liu S and Sun W: Effect of
surgery and radiotherapy on overall survival in patients with
chondrosarcoma: A SEER-based study. J Orthop Surg (Hong Kong).
30:102255362210863192022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu VM, Wang S, Daniels DJ, Spinner RJ,
Levi AD and Niazi TN: The clinical course and role of surgery in
pediatric malignant peripheral nerve sheath tumors: A database
study. J Neurosurg Pediatr. 29:92–99. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frankart AJ, Breneman JC and Pater LE:
Radiation therapy in the treatment of head and neck
rhabdomyosarcoma. Cancers (Basel). 13:35672021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agosti E, Zeppieri M, Antonietti S, Ius T,
Fontanella MM and Panciani PP: Advancing the management of skull
base chondrosarcomas: A systematic review of targeted therapies. J
Pers Med. 14:2612024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ebisumoto K, Okami K, Hamada M, Maki D,
Sakai A, Saito K, Shimizu F, Kaneda S and Iida M: Cetuximab with
radiotherapy as an alternative treatment for advanced squamous cell
carcinoma of the temporal bone. Auris Nasus Larynx. 45:637–639.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan J and Sui JD: Long-term survival after
immunotherapy for uncontrolled locally advanced temporal bone
squamous cell carcinoma followed by chemoradiotherapy: A case
report. Precis Radiat Oncol. 28:99–105. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Testa JR, Fukuda Y and Kowalski LP:
Prognostic factors in carcinoma of the external auditory canal.
Arch Otolaryngol Head Neck Surg. 123:720–724. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang B, Tu G, Xu G, Tang P and Hu Y:
Squamous cell carcinoma of temporal bone: reported on 33 patients.
Head Neck. 21:461–466. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nyrop M and Grontved A: Cancer of the
external auditory canal. Arch Otolaryngol Head Neck Surg.
128:834–837. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Komune N, Kuga R, Hongo T, Kuga D, Sato K
and Nakagawa T: Impact of positive-margin resection of external
auditory canal squamous cell carcinoma. Cancers (Basel).
15:42892023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gidley PW and DeMonte F: Temporal bone
malignancies. Neurosurg Clin N Am. 24:97–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bacciu A, Clemente IA, Piccirillo E,
Ferrari S and Sanna M: Guidelines for treating temporal bone
carcinoma based on long-term outcomes. Otol Neurotol. 34:898–907.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morris LG, Mehra S, Shah JP, Bilsky MH,
Selesnick SH and Kraus DH: Predictors of survival and recurrence
after temporal bone resection for cancer. Head Neck. 34:1231–1239.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Omura G, Ando M, Saito Y, Fukuoka O,
Akashi K, Yoshida M, Kakigi A, Asakage T and Yamasoba T: Survival
impact of local extension sites in surgically treated patients with
temporal bone squamous cell carcinoma. Int J Clin Oncol.
22:431–437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dean NR, White HN, Carter DS, Desmond RA,
Carroll WR, McGrew BM and Rosenthal EL: Outcomes following temporal
bone resection. Laryngoscope. 120:1516–1522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Manolidis S, Pappas D Jr, Von Doersten P,
Jackson CG and Glasscock ME III: Temporal bone and lateral skull
base malignancy: Experience and results with 81 patients. Am J
Otol. 19:S1–S15. 1998.PubMed/NCBI
|