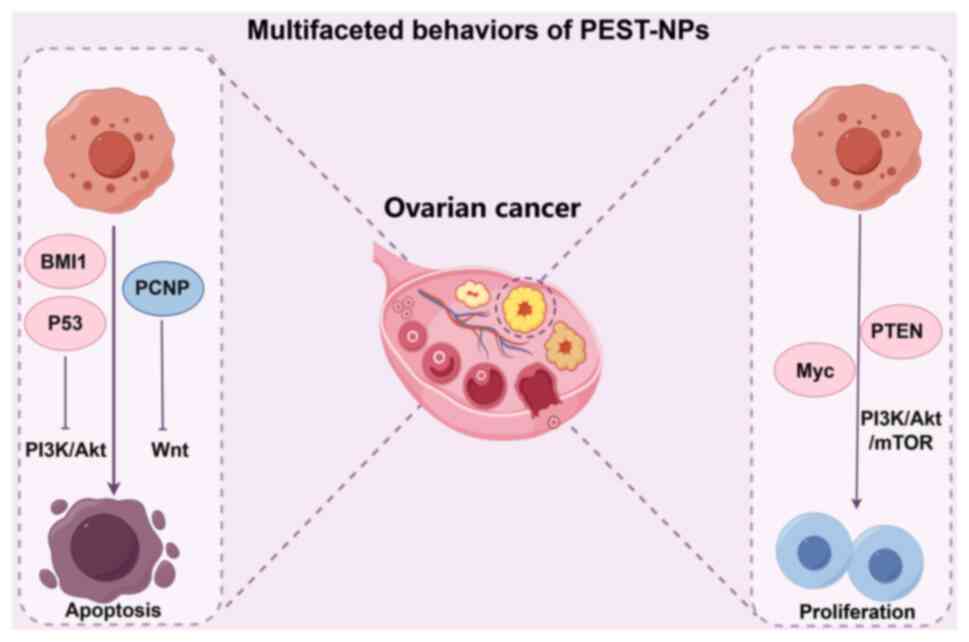
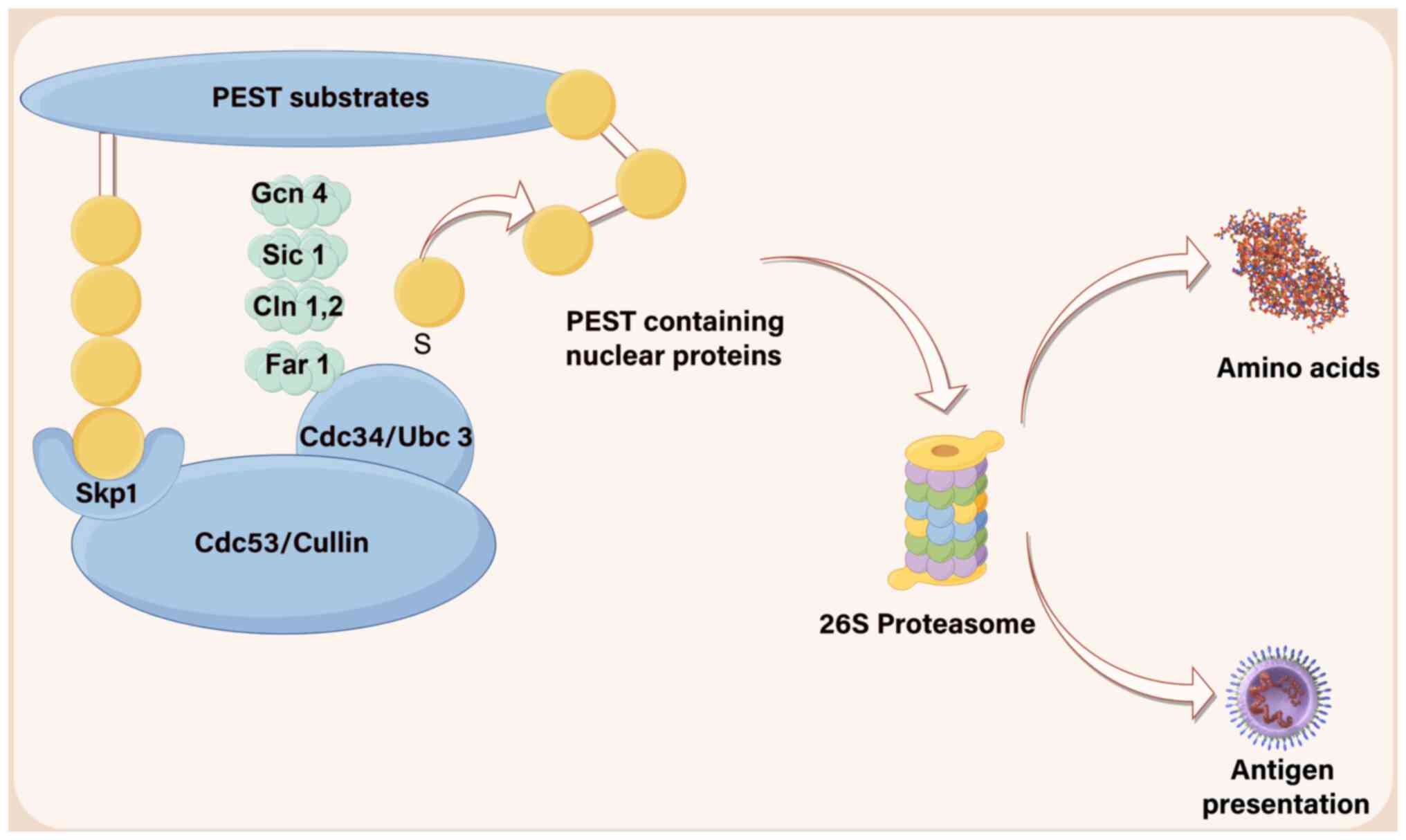
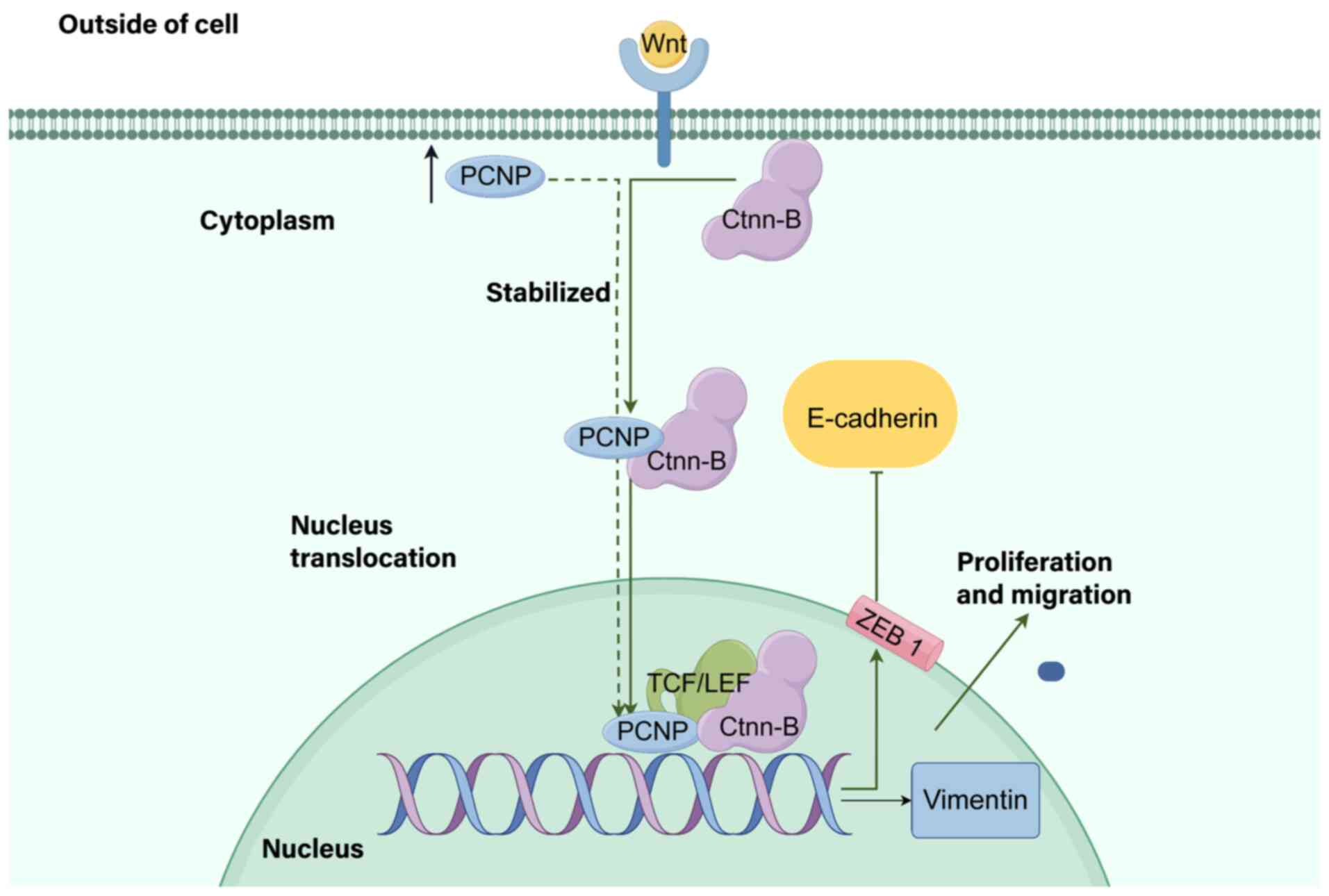
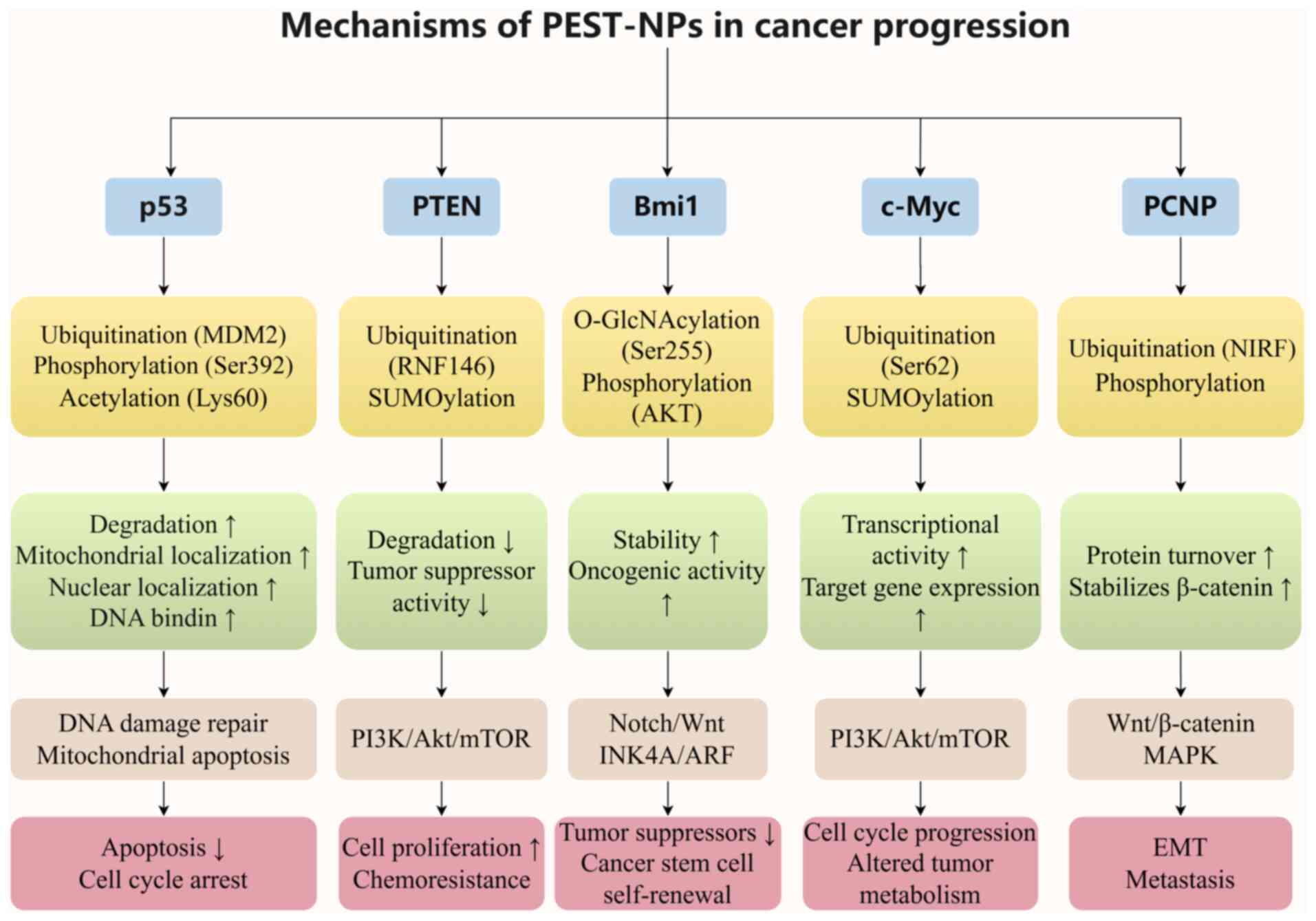
|
1
|
Menon U, Griffin M and Gentry-Maharaj A:
Ovarian cancer screening-current status, future directions. Gynecol
Oncol. 132:490–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta KK, Gupta VK and Naumann RW: Ovarian
cancer: Screening and future directions. Int J Gynecol Cancer.
29:195–200. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang MH, Zhang HH, Du XH, Gao J, Li C,
Shi HR and Li SZ: UCHL3 promotes ovarian cancer progression by
stabilizing TRAF2 to activate the NF-κB pathway. Oncogene.
39:322–333. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hubackova M, Vaclavikova R, Ehrlichova M,
Mrhalova M, Kodet R, Kubackova K, Vrána D, Gut I and Soucek P:
Association of superoxide dismutases and NAD(P)H quinone
oxidoreductases with prognosis of patients with breast carcinomas.
Int J Cancer. 130:338–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ke Y, Chen X, Su Y, Chen C, Lei S, Xia L,
Wei D, Zhang H, Dong C, Liu X and Yin F: Low expression of SLC7A11
confers drug resistance and worse survival in ovarian cancer via
inhibition of cell autophagy as a competing endogenous RNA. Front
Oncol. 11:7449402021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li D, Hong X, Zhao F, Ci X and Zhang S:
Targeting Nrf2 may reverse the drug resistance in ovarian cancer.
Cancer Cell Int. 21:1162021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao Y, Liu X, Li T, Wei L, Yang A, Lu Y,
Zhang J, Li L, Wang S and Yin F: Cross-validation of genes
potentially associated with overall survival and drug resistance in
ovarian cancer. Oncol Rep. 37:3084–3092. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hydbring P, Castell A and Larsson LG: MYC
modulation around the CDK2/p27/SKP2 axis. Genes (Basel). 8:1742017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ammirante M, Kuraishy AI, Shalapour S,
Strasner A, Ramirez-Sanchez C, Zhang W, Shabaik A and Karin M: An
IKKα-E2F1-BMI1 cascade activated by infiltrating B cells controls
prostate regeneration and tumor recurrence. Genes Dev.
27:1435–1440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park JY, Wang PY, Matsumoto T, Sung HJ, Ma
W, Choi JW, Anderson SA, Leary SC, Balaban RS, Kang JG and Hwang
PM: p53 improves aerobic exercise capacity and augments skeletal
muscle mitochondrial DNA content. Circ Res. 105:705–712. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ho SR, Mahanic CS, Lee YJ and Lin WC:
RNF144A, an E3 ubiquitin ligase for DNA-PKcs, promotes apoptosis
during DNA damage. Proc Natl Acad Sci USA. 111:E2646–E2655. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bakhanashvili M, Grinberg S, Bonda E,
Simon AJ, Moshitch-Moshkovitz S and Rahav G: p53 in mitochondria
enhances the accuracy of DNA synthesis. Cell Death Differ.
15:1865–1874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nithipongvanitch R, Ittarat W, Velez JM,
Zhao R, St Clair DK and Oberley TD: Evidence for p53 as guardian of
the cardiomyocyte mitochondrial genome following acute adriamycin
treatment. J Histochem Cytochem. 55:629–639. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nie M, Moser BA, Nakamura TM and Boddy MN:
SUMO-targeted ubiquitin ligase activity can either suppress or
promote genome instability, depending on the nature of the DNA
lesion. PLoS Genet. 13:e10067762017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou ZD, Chan CH, Xiao ZC and Tan EK: Ring
finger protein 146/Iduna is a poly(ADP-ribose) polymer binding and
PARsylation dependent E3 ubiquitin ligase. Cell Adh Migr.
5:463–471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Freed-Pastor WA and Prives C: Mutant p53:
One name, many proteins. Genes Dev. 26:1268–2686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang HC, Lee YI, Shin JH, Andrabi SA, Chi
Z, Gagné JP, Lee Y, Ko HS, Lee BD, Poirier GG, et al: Iduna is a
poly(ADP-ribose) (PAR)-dependent E3 ubiquitin ligase that regulates
DNA damage. Proc Natl Acad Sci USA. 108:14103–14108. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu L, Zhang H, Bergholz J, Sun S and Xiao
ZX: MDM2/MDMX: Master negative regulators for p53 and RB. Mol Cell
Oncol. 3:e11066352016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tuna M, Ju Z, Yoshihara K, Amos CI, Tanyi
JL and Mills GB: Clinical relevance of TP53 hotspot mutations in
high-grade serous ovarian cancers. Br J Cancer. 122:405–412. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Afzal A, Sarfraz M, Li GL, Ji SP, Duan SF,
Khan NH, Wu DD and Ji XY: Taking a holistic view of PEST-containing
nuclear protein (PCNP) in cancer biology. Cancer Med. 8:6335–6343.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rogers S, Wells R and Rechsteiner M: Amino
acid sequences common to rapidly degraded proteins: The PEST
hypothesis. Science. 234:364–368. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roth AF, Sullivan DM and Davis NG: A large
PEST-like sequence directs the ubiquitination, endocytosis, and
vacuolar degradation of the yeast a-factor receptor. J Cell Biol.
142:949–961. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhuang X, Northup JK and Ray K: Large
putative PEST-like sequence motif at the carboxyl tail of human
calcium receptor directs lysosomal degradation and regulates cell
surface receptor level. J Biol Chem. 287:4165–4176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sekhar KR and Freeman ML: PEST sequences
in proteins involved in cyclic nucleotide signalling pathways. J
Recept Signal Transduct Res. 18:113–132. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bellini E, Pavesi G, Barbiero I, Bergo A,
Chandola C, Nawaz MS, Rusconi L, Stefanelli G, Strollo M, Valente
MM, et al: MeCP2 post-translational modifications: A mechanism to
control its involvement in synaptic plasticity and homeostasis?
Front Cell Neurosci. 8:2362014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bereshchenko OR, Gu W and Dalla-Favera R:
Acetylation inactivates the transcriptional repressor BCL6. Nat
Genet. 32:606–613. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao JF, Shyue SK and Lee TS: Excess
nitric oxide activates TRPV1-Ca(2+)-calpain signaling and promotes
PEST-dependent degradation of liver X receptor α. Int J Biol Sci.
12:18–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu
Y, Shi J, Zhao S, Liu W, Wang X, et al: Gut microbiome and serum
metabolome alterations in obesity and after weight-loss
intervention. Nat Med. 23:859–868. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pan S and Chen R: Pathological implication
of protein post-translational modifications in cancer. Mol Aspects
Med. 86:1010972022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Afzal A, Sarfraz M, Wu Z, Wang G and Sun
J: Integrated scientific data bases review on asulacrine and
associated toxicity. Crit Rev Oncol Hematol. 104:78–86. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lang V, Aillet F, Da Silva-Ferrada E,
Xolalpa W, Zabaleta L, Rivas C and Rodriguez MS: Analysis of PTEN
ubiquitylation and SUMOylation using molecular traps. Methods.
77–78. 112–118. 2015.
|
|
33
|
Li N, Zhang Y, Han X, Liang K, Wang J,
Feng L, Wang W, Songyang Z, Lin C, Yang L, et al: Poly-ADP
ribosylation of PTEN by tankyrases promotes PTEN degradation and
tumor growth. Genes Dev. 29:157–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shumway SD, Maki M and Miyamoto S: The
PEST domain of IkappaBalpha is necessary and sufficient for in
vitro degradation by mu-calpain. J Biol Chem. 274:30874–30881.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sarfraz M, Afzal A, Khattak S, Saddozai
UAK, Li HM, Zhang QQ, Madni A, Haleem KS, Duan SF, Wu DD, et al:
Multifaceted behavior of PEST sequence enriched nuclear proteins in
cancer biology and role in gene therapy. J Cell Physiol.
236:1658–1676. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chipuk JE, Bouchier-Hayes L, Kuwana T,
Newmeyer DD and Green DR: PUMA couples the nuclear and cytoplasmic
proapoptotic function of p53. Science. 309:1732–1735. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lisachev PD, Pustylnyak VO and Shtark MB:
Mdm2-dependent regulation of p53 expression during long-term
potentiation. Bull Exp Biol Med. 158:333–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Geyer RK, Yu ZK and Maki CG: The MDM2
RING-finger domain is required to promote p53 nuclear export. Nat
Cell Biol. 2:569–573. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu W, Pochampally R, Chen L, Traidej M,
Wang Y and Chen J: Nuclear exclusion of p53 in a subset of tumors
requires MDM2 function. Oncogene. 19:232–240. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang DY, Hong Y, Chen YG, Dong PZ, Liu SY,
Gao YR, Lu D, Li HM, Li T, Guo JC, et al: PEST-containing nuclear
protein regulates cell proliferation, migration, and invasion in
lung adenocarcinoma. Oncogenesis. 8:222019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ye J, Zhong L, Xiong L, Li J, Yu L, Dan W,
Yuan Z, Yao J, Zhong P, Liu J, et al: Nuclear import of NLS-RARα is
mediated by importin α/β. Cell Signal. 69:1095672020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Panagiotopoulos AA, Polioudaki C, Ntallis
SG, Dellis D, Notas G, Panagiotidis CA, Theodoropoulos PA, Castanas
E and Kampa M: The sequence [EKRKI(E/R)(K/L/R/S/T)] is a nuclear
localization signal for importin 7 binding (NLS7). Biochim Biophys
Acta Gen Subj. 1865:1298512021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng Y, Yu K, Lin JF, Liang Z, Zhang Q,
Li J, Wu QN, He CY, Lin M, Zhao Q, et al: Deep learning prioritizes
cancer mutations that alter protein nucleocytoplasmic shuttling to
drive tumorigenesis. Nat Commun. 16:25112025. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lang YD and Jou YS: PSPC1 is a new
contextual determinant of aberrant subcellular translocation of
oncogenes in tumor progression. J Biomed Sci. 28:572021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rubbi CP and Milner J: Disruption of the
nucleolus mediates stabilization of p53 in response to DNA damage
and other stresses. EMBO J. 22:6068–6077. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang X, Blaskovich MA, Forinash KD and
Sebti SM: Withacnistin inhibits recruitment of STAT3 and STAT5 to
growth factor and cytokine receptors and induces regression of
breast tumours. Br J Cancer. 111:894–902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Baugh EH, Ke H, Levine AJ, Bonneau RA and
Chan CS: Why are there hotspot mutations in the TP53 gene in human
cancers? Cell Death Differ. 25:154–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kastan MB and Berkovich E: p53: A
two-faced cancer gene. Nat Cell Biol. 9:489–491. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Levine AJ and Oren M: The first 30 years
of p53: Growing ever more complex. Nat Rev Cancer. 9:749–758. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wojnarowicz PM, Oros KK, Quinn MC, Arcand
SL, Gambaro K, Madore J, Birch AH, de Ladurantaye M, Rahimi K,
Provencher DM, et al: The genomic landscape of TP53 and p53
annotated high grade ovarian serous carcinomas from a defined
founder population associated with patient outcome. PLoS One.
7:e454842012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primers. 2:160612016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Joerger AC and Fersht AR: The tumor
suppressor p53: From structures to drug discovery. Cold Spring Harb
Perspect Biol. 2:a0009192010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhu G, Pan C, Bei JX, Li B, Liang C, Xu Y
and Fu X: Mutant p53 in cancer progression and targeted therapies.
Front Oncol. 10:5951872020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cole AJ, Dwight T, Gill AJ, Dickson KA,
Zhu Y, Clarkson A, Gard GB, Maidens J, Valmadre S, Clifton-Bligh R
and Marsh DJ: Assessing mutant p53 in primary high-grade serous
ovarian cancer using immunohistochemistry and massively parallel
sequencing. Sci Rep. 6:261912016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Flemming A: Cancer: Mutant p53 rescued by
aggregation inhibitor. Nat Rev Drug Discov. 15:852016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Duffy MJ, Synnott NC, O'Grady S and Crown
J: Targeting p53 for the treatment of cancer. Semin Cancer Biol.
79:58–67. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Okal A, Cornillie S, Matissek SJ, Matissek
KJ, Cheatham TE III and Lim CS: Re-engineered p53 chimera with
enhanced homo-oligomerization that maintains tumor suppressor
activity. Mol Pharm. 11:2442–2452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Okal A, Matissek KJ, Matissek SJ, Price R,
Salama ME, Janát-Amsbury MM and Lim CS: Re-engineered p53 activates
apoptosis in vivo and causes primary tumor regression in a dominant
negative breast cancer xenograft model. Gene Ther. 21:903–912.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Brady CA and Attardi LD: p53 at a glance.
J Cell Sci. 123:2527–2532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wei H, Qu L, Dai S, Li Y, Wang H, Feng Y,
Chen X, Jiang L, Guo M, Li J, et al: Structural insight into the
molecular mechanism of p53-mediated mitochondrial apoptosis. Nat
Commun. 12:22802021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Leu JI, Dumont P, Hafey M, Murphy ME and
George DL: Mitochondrial p53 activates Bak and causes disruption of
a Bak-Mcl1 complex. Nat Cell Biol. 6:443–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Heyne K, Schmitt K, Mueller D, Armbruester
V, Mestres P and Roemer K: Resistance of mitochondrial p53 to
dominant inhibition. Mol Cancer. 7:542008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen S, Cavazza E, Barlier C, Salleron J,
Filhine-Tresarrieu P, Gavoilles C, Merlin JL and Harlé A: Beside
P53 and PTEN: Identification of molecular alterations of the
RAS/MAPK and PI3K/AKT signaling pathways in high-grade serous
ovarian carcinomas to determine potential novel therapeutic
targets. Oncol Lett. 12:3264–3272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Toledo F and Wahl GM: MDM2 and MDM4: p53
regulators as targets in anticancer therapy. Int J Biochem Cell
Biol. 39:1476–1482. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Astanehe A, Arenillas D, Wasserman WW,
Leung PC, Dunn SE, Davies BR, Mills GB and Auersperg N: Mechanisms
underlying p53 regulation of PIK3CA transcription in ovarian
surface epithelium and in ovarian cancer. J Cell Sci. 121:664–674.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hu L, Zaloudek C, Mills GB, Gray J and
Jaffe RB: In vivo and in vitro ovarian carcinoma growth inhibition
by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin
Cancer Res. 6:880–886. 2000.PubMed/NCBI
|
|
68
|
Raab M, Kostova I, Peña-Llopis S, Fietz D,
Kressin M, Aberoumandi SM, Ullrich E, Becker S, Sanhaji M and
Strebhardt K: Rescue of p53 functions by in vitro-transcribed mRNA
impedes the growth of high-grade serous ovarian cancer. Cancer
Commun (Lond). 44:101–126. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Faramarzi L, Dadashpour M, Sadeghzadeh H,
Mahdavi M and Zarghami N: Enhanced anti-proliferative and
pro-apoptotic effects of metformin encapsulated PLGA-PEG
nanoparticles on SKOV3 human ovarian carcinoma cells. Artif Cells
Nanomed Biotechnol. 47:737–746. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Guo X, Fang Z, Zhang M, Yang D, Wang S and
Liu K: A Co-delivery system of curcumin and p53 for enhancing the
sensitivity of drug-resistant ovarian cancer cells to cisplatin.
Molecules. 25:26212020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang X, Cao Z, Qian J, Ding T, Wu Y,
Zhang H, Zhong S, Wang X, Ren X, Zhang W, et al: Nanoreceptors
promote mutant p53 protein degradation by mimicking selective
autophagy receptors. Nat Nanotechnol. 19:545–553. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang XF and Gurunathan S: Combination of
salinomycin and silver nanoparticles enhances apoptosis and
autophagy in human ovarian cancer cells: An effective anticancer
therapy. Int J Nanomedicine. 11:3655–3675. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang J, Qu C, Shao X, Song G, Sun J, Shi
D, Jia R, An H and Wang H: Carrier-free nanoprodrug for p53-mutated
tumor therapy via concurrent delivery of zinc-manganese dual ions
and ROS. Bioact Mater. 20:404–417. 2022.PubMed/NCBI
|
|
74
|
Lee JM and Johnson JA: An important role
of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem
Mol Biol. 37:139–143. 2004.PubMed/NCBI
|
|
75
|
Castrogiovanni C, Waterschoot B, De Backer
O and Dumont P: Serine 392 phosphorylation modulates p53
mitochondrial translocation and transcription-independent
apoptosis. Cell Death Differ. 25:190–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ai G, Dachineni R, Kumar DR, Marimuthu S,
Alfonso LF and Bhat GJ: Aspirin acetylates wild type and mutant p53
in colon cancer cells: Identification of aspirin acetylated sites
on recombinant p53. Tumour Biol. 37:6007–6016. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Oreskes N: Beyond the ivory tower. The
scientific consensus on climate change. Science. 306:16862004.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Haddadi N, Lin Y, Travis G, Simpson AM,
Nassif NT and McGowan EM: PTEN/PTENP1: ‘Regulating the regulator of
RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy.
Mol Cancer. 17:372018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jiang C, Song Y, Rorive S, Allard J, Tika
E, Zahedi Z, Dubois C, Salmon I, Sifrim A and Blanpain C: Innate
immunity and the NF-κB pathway control prostate stem cell
plasticity, reprogramming and tumor initiation. Nat Cancer.
6:1537–1558. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ren G, Chen J, Pu Y, Yang EJ, Tao S, Mou
PK, Chen LJ, Zhu W, Chan KL, Luo G, et al: BET inhibition induces
synthetic lethality in PTEN deficient colorectal cancers via dual
action on p21CIP1/WAF1. Int J Biol Sci. 20:1978–1991.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Georgescu MM, Kirsch KH, Akagi T, Shishido
T and Hanafusa H: The tumor-suppressor activity of PTEN is
regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA.
96:10182–10187. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Meyer RD, Srinivasan S, Singh AJ, Mahoney
JE, Gharahassanlou KR and Rahimi N: PEST motif serine and tyrosine
phosphorylation controls vascular endothelial growth factor
receptor 2 stability and downregulation. Mol Cell Biol.
31:2010–2025. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Trotman LC, Wang X, Alimonti A, Chen Z,
Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo
C, Erdjument-Bromage H, et al: Ubiquitination regulates PTEN
nuclear import and tumor suppression. Cell. 128:141–156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hu X, Xu X, Zeng X, Jin R, Wang S, Jiang
H, Tang Y, Chen G, Wei J, Chen T and Chen Q: Gut microbiota
dysbiosis promotes the development of epithelial ovarian cancer via
regulating Hedgehog signaling pathway. Gut Microbes.
15:22210932023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Willner J, Wurz K, Allison KH, Galic V,
Garcia RL, Goff BA and Swisher EM: Alternate molecular genetic
pathways in ovarian carcinomas of common histological types. Hum
Pathol. 38:607–613. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Karlsson T, Krakstad C, Tangen IL, Hoivik
EA, Pollock PM, Salvesen HB and Lewis AE: Endometrial cancer cells
exhibit high expression of p110β and its selective inhibition
induces variable responses on PI3K signaling, cell survival and
proliferation. Oncotarget. 8:3881–3894. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Singer G, Oldt R III, Cohen Y, Wang BG,
Sidransky D, Kurman RJ and Shih IeM: Mutations in BRAF and KRAS
characterize the development of low-grade ovarian serous carcinoma.
J Natl Cancer Inst. 95:484–486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dinulescu DM, Ince TA, Quade BJ, Shafer
SA, Crowley D and Jacks T: Role of K-ras and Pten in the
development of mouse models of endometriosis and endometrioid
ovarian cancer. Nat Med. 11:63–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhao X, Lai H, Li G, Qin Y, Chen R, Labrie
M, Stommel JM, Mills GB, Ma D, Gao Q and Fang Y: Rictor
orchestrates β-catenin/FOXO balance by maintaining redox
homeostasis during development of ovarian cancer. Oncogene.
44:1820–1832. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tamura T, Nagai S, Masuda K, Imaeda K,
Sugihara E, Yamasaki J, Kawaida M, Otsuki Y, Suina K, Nobusue H, et
al: mTOR-mediated p62/SQSTM1 stabilization confers a robust
survival mechanism for ovarian cancer. Cancer Lett. 616:2175652025.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Trillsch F, Czogalla B, Mahner S, Loidl V,
Reuss A, du Bois A, Sehouli J, Raspagliesi F, Meier W, Cibula D, et
al: Risk factors for anastomotic leakage and its impact on survival
outcomes in radical multivisceral surgery for advanced ovarian
cancer: An AGO-OVAR.OP3/LION exploratory analysis. Int J Surg.
111:2914–2922. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Russo A, Czarnecki AA, Dean M, Modi DA,
Lantvit DD, Hardy L, Baligod S, Davis DA, Wei JJ and Burdette JE:
PTEN loss in the fallopian tube induces hyperplasia and ovarian
tumor formation. Oncogene. 37:1976–1990. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Coscia F, Watters KM, Curtis M, Eckert MA,
Chiang CY, Tyanova S, Montag A, Lastra RR, Lengyel E and Mann M:
Integrative proteomic profiling of ovarian cancer cell lines
reveals precursor cell associated proteins and functional status.
Nat Commun. 7:126452016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Labidi-Galy SI, Papp E, Hallberg D,
Niknafs N, Adleff V, Noe M, Bhattacharya R, Novak M, Jones S,
Phallen J, et al: High grade serous ovarian carcinomas originate in
the fallopian tube. Nat Commun. 8:10932017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Roh MH, Yassin Y, Miron A, Mehra KK,
Mehrad M, Monte NM, Mutter GL, Nucci MR, Ning G, Mckeon FD, et al:
High-grade fimbrial-ovarian carcinomas are unified by altered p53,
PTEN and PAX2 expression. Mod Pathol. 23:1316–1324. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Dean M, Jin V, Bergsten TM, Austin JR,
Lantvit DD, Russo A and Burdette JE: Loss of PTEN in fallopian tube
epithelium results in multicellular tumor spheroid formation and
metastasis to the ovary. Cancers (Basel). 11:8842019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang L, Ma T, Brozick J, Babalola K,
Budiu R, Tseng G and Vlad AM: Effects of Kras activation and Pten
deletion alone or in combination on MUC1 biology and
epithelial-to-mesenchymal transition in ovarian cancer. Oncogene.
35:5010–5020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Alkema MJ, Wiegant J, Raap AK, Berns A and
van Lohuizen M: Characterization and chromosomal localization of
the human proto-oncogene BMI-1. Hum Mol Genet. 2:1597–1603. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Calao M, Sekyere EO, Cui HJ, Cheung BB,
Thomas WD, Keating J, Chen JB, Raif A, Jankowski K, Davies NP, et
al: Direct effects of Bmi1 on p53 protein stability inactivates
oncoprotein stress responses in embryonal cancer precursor cells at
tumor initiation. Oncogene. 32:3616–3626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Su WJ, Fang JS, Cheng F, Liu C, Zhou F and
Zhang J: RNF2/Ring1b negatively regulates p53 expression in
selective cancer cell types to promote tumor development. Proc Natl
Acad Sci USA. 110:1720–1725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ginjala V, Nacerddine K, Kulkarni A, Oza
J, Hill SJ, Yao M, Citterio E, van Lohuizen M and Ganesan S: BMI1
is recruited to DNA breaks and contributes to DNA damage-induced
H2A ubiquitination and repair. Mol Cell Biol. 31:1972–1982. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Itahana K, Zou Y, Itahana Y, Martinez JL,
Beausejour C, Jacobs JJ, Van Lohuizen M, Band V, Campisi J and
Dimri GP: Control of the replicative life span of human fibroblasts
by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 23:389–401.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yadav AK, Sahasrabuddhe AA, Dimri M, Bommi
PV, Sainger R and Dimri GP: Deletion analysis of BMI1 oncoprotein
identifies its negative regulatory domain. Mol Cancer. 9:1582010.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Jacobs JJ, Scheijen B, Voncken JW, Kieboom
K, Berns A and van Lohuizen M: Bmi-1 collaborates with c-Myc in
tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF.
Genes Dev. 13:2678–2690. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu
LJ, Kong QL, Xu LH, Zhang X, Liu WL, et al: The polycomb group
protein Bmi-1 represses the tumor suppressor PTEN and induces
epithelial-mesenchymal transition in human nasopharyngeal
epithelial cells. J Clin Invest. 119:3626–3636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bhattacharyya J, Mihara K, Ohtsubo M,
Yasunaga S, Takei Y, Yanagihara K, Sakai A, Hoshi M, Takihara Y and
Kimura A: Overexpression of BMI-1 correlates with drug resistance
in B-cell lymphoma cells through the stabilization of survivin
expression. Cancer Sci. 103:34–41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fan C, He L, Kapoor A, Gillis A, Rybak AP,
Cutz JC and Tang D: Bmi1 promotes prostate tumorigenesis via
inhibiting p16(INK4A) and p14(ARF) expression. Biochim Biophys
Acta. 1782:642–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Vonlanthen S, Heighway J, Altermatt HJ,
Gugger M, Kappeler A, Borner MM, van Lohuizen M and Betticher DC:
The bmi-1 oncoprotein is differentially expressed in non-small cell
lung cancer and correlates with INK4A-ARF locus expression. Br J
Cancer. 84:1372–1376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Song LB, Zeng MS, Liao WT, Zhang L, Mo HY,
Liu WL, Shao JY, Wu QL, Li MZ, Xia YF, et al: Bmi-1 is a novel
molecular marker of nasopharyngeal carcinoma progression and
immortalizes primary human nasopharyngeal epithelial cells. Cancer
Res. 66:6225–6232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Fasano CA, Dimos JT, Ivanova NB, Lowry N,
Lemischka IR and Temple S: shRNA knockdown of Bmi-1 reveals a
critical role for p21-Rb pathway in NSC self-renewal during
development. Cell Stem Cell. 1:87–99. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mao L, Ding J, Perdue A, Yang L, Zha Y,
Ren M, Huang S, Cui H and Ding HF: Cyclin E1 is a common target of
BMI1 and MYCN and a prognostic marker for neuroblastoma
progression. Oncogene. 31:3785–3795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Dimri GP, Martinez JL, Jacobs JJ, Keblusek
P, Itahana K, Van Lohuizen M, Campisi J, Wazer DE and Band V: The
Bmi-1 oncogene induces telomerase activity and immortalizes human
mammary epithelial cells. Cancer Res. 62:4736–4745. 2002.PubMed/NCBI
|
|
113
|
Ismail IH, Gagné JP, Caron MC, McDonald D,
Xu Z, Masson JY, Poirier GG and Hendzel MJ: CBX4-mediated SUMO
modification regulates BMI1 recruitment at sites of DNA damage.
Nucleic Acids Res. 40:5497–5510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Voncken JW, Niessen H, Neufeld B,
Rennefahrt U, Dahlmans V, Kubben N, Holzer B, Ludwig S and Rapp UR:
MAPKAP kinase 3pK phosphorylates and regulates chromatin
association of the polycomb group protein Bmi1. J Biol Chem.
280:5178–5187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bruggeman SW, Hulsman D, Tanger E, Buckle
T, Blom M, Zevenhoven J, van Tellingen O and van Lohuizen M: Bmi1
controls tumor development in an Ink4a/Arf-independent manner in a
mouse model for glioma. Cancer Cell. 12:328–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Xu CR, Lee S, Ho C, Bommi P, Huang SA,
Cheung ST, Dimri GP and Chen X: Bmi1 functions as an oncogene
independent of Ink4A/Arf repression in hepatic carcinogenesis. Mol
Cancer Res. 7:1937–1945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Liu Y, Liu F, Yu H, Zhao X, Sashida G,
Deblasio A, Harr M, She QB, Chen Z, Lin HK, et al: Akt
phosphorylates the transcriptional repressor bmi1 to block its
effects on the tumor-suppressing ink4a-arf locus. Sci Signal.
5:ra772012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cui H, Hu B, Li T, Ma J, Alam G, Gunning
WT and Ding HF: Bmi-1 is essential for the tumorigenicity of
neuroblastoma cells. Am J Pathol. 170:1370–1378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sahasrabuddhe AA, Dimri M, Bommi PV and
Dimri GP: βTrCP regulates BMI1 protein turnover via ubiquitination
and degradation. Cell Cycle. 10:1322–1330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
López-Arribillaga E, Rodilla V,
Pellegrinet L, Guiu J, Iglesias M, Roman AC, Gutarra S, González S,
Muñoz-Cánoves P, Fernández-Salguero P, et al: Bmi1 regulates murine
intestinal stem cell proliferation and self-renewal downstream of
Notch. Development. 142:41–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Goel HL, Chang C, Pursell B, Leav I, Lyle
S, Xi HS, Hsieh CC, Adisetiyo H, Roy-Burman P, Coleman IM, et al:
VEGF/neuropilin-2 regulation of Bmi-1 and consequent repression of
IGF-IR define a novel mechanism of aggressive prostate cancer.
Cancer Discov. 2:906–921. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang J and Sarge KD: Identification of a
polymorphism in the RING finger of human Bmi-1 that causes its
degradation by the ubiquitin-proteasome system. FEBS Lett.
583:960–964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhao Q, Qian Q, Cao D, Yang J, Gui T and
Shen K: Role of BMI1 in epithelial ovarian cancer: Investigated via
the CRISPR/Cas9 system and RNA sequencing. J Ovarian Res.
11:312018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Gui T, Bai H, Zeng J, Zhong Z, Cao D, Cui
Q, Chen J, Yang J and Shen K: Tumor heterogeneity in the recurrence
of epithelial ovarian cancer demonstrated by polycomb group
proteins. Onco Targets Ther. 7:1705–1716. 2014.PubMed/NCBI
|
|
125
|
Bhattacharya R, Nicoloso M, Arvizo R, Wang
E, Cortez A, Rossi S, Calin GA and Mukherjee P: MiR-15a and MiR-16
control Bmi-1 expression in ovarian cancer. Cancer Res.
69:9090–9095. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Koren A, Rijavec M, Kern I, Sodja E,
Korosec P and Cufer T: BMI1, ALDH1A1, and CD133 transcripts connect
epithelial-mesenchymal transition to cancer stem cells in lung
carcinoma. Stem Cells Int. 2016:97143152016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wang H, Liu H, Li X, Zhao J, Zhang H, Mao
J, Zou Y, Zhang H, Zhang S, Hou W, et al: Estrogen receptor
α-coupled Bmi1 regulation pathway in breast cancer and its clinical
implications. BMC Cancer. 14:1222014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Qiao B, Chen Z, Hu F, Tao Q and Lam AK:
BMI-1 activation is crucial in hTERT-induced epithelial-mesenchymal
transition of oral epithelial cells. Exp Mol Pathol. 95:57–61.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kim BR, Kwon Y and Rho SB: BMI-1 interacts
with sMEK1 and inactivates sMEK1-induced apoptotic cell death.
Oncol Rep. 37:579–586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang E, Bhattacharyya S, Szabolcs A,
Rodriguez-Aguayo C, Jennings NB, Lopez-Berestein G, Mukherjee P,
Sood AK and Bhattacharya R: Enhancing chemotherapy response with
Bmi-1 silencing in ovarian cancer. PLoS One. 6:e179182011.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Chen H, Liu H and Qing G: Targeting
oncogenic Myc as a strategy for cancer treatment. Signal Transduct
Target Ther. 3:52018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Dang CV, O'Donnell KA, Zeller KI, Nguyen
T, Osthus RC and Li F: The c-Myc target gene network. Semin Cancer
Biol. 16:253–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Adhikary S and Eilers M: Transcriptional
regulation and transformation by Myc proteins. Nat Rev Mol Cell
Biol. 6:635–645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Alexander WS, Adams JM and Cory S:
Oncogene cooperation in lymphocyte transformation: Malignant
conversion of E mu-myc transgenic pre-B cells in vitro is enhanced
by v-H-ras or v-raf but not v-abl. Mol Cell Biol. 9:67–73. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Kessler JD, Kahle KT, Sun T, Meerbrey KL,
Schlabach MR, Schmitt EM, Skinner SO, Xu Q, Li MZ, Hartman ZC, et
al: A SUMOylation-dependent transcriptional subprogram is required
for Myc-driven tumorigenesis. Science. 335:348–353. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Conacci-Sorrell M, McFerrin L and Eisenman
RN: An overview of MYC and its interactome. Cold Spring Harb
Perspect Med. 4:a0143572014. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ward PS and Thompson CB: Signaling in
control of cell growth and metabolism. Cold Spring Harb Perspect
Biol. 4:a0067832012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Souza JL, Martins-Cardoso K, Guimarães IS,
de Melo AC, Lopes AH, Monteiro RQ and Almeida VH: Interplay between
EGFR and the platelet-activating factor/PAF receptor signaling axis
mediates aggressive behavior of cervical cancer. Front Oncol.
10:5572802020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Skírnisdóttir IA, Sorbe B, Lindborg K and
Seidal T: Prognostic impact of p53, p27, and C-MYC on
clinicopathological features and outcome in early-stage (FIGO I–II)
epithelial ovarian cancer. Int J Gynecol Cancer. 21:236–244. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Chen CH, Shen J, Lee WJ and Chow SN:
Overexpression of cyclin D1 and c-Myc gene products in human
primary epithelial ovarian cancer. Int J Gynecol Cancer.
15:878–883. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Plisiecka-Hałasa J, Karpińska G, Szymańska
T, Ziółkowska I, Madry R, Timorek A, Debniak J, Ułańska M, Jedryka
M, Chudecka-Głaz A, et al: P21WAF1, P27KIP1, TP53 and C-MYC
analysis in 204 ovarian carcinomas treated with platinum-based
regimens. Ann Oncol. 14:1078–1085. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
van Dam PA, Vergote IB, Lowe DG, Watson
JV, van Damme P, van der Auwera JC and Shepherd JH: Expression of
c-erbB-2, c-myc, and c-ras oncoproteins, insulin-like growth factor
receptor I, and epidermal growth factor receptor in ovarian
carcinoma. J Clin Pathol. 47:914–919. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Watson JV, Curling OM, Munn CF and Hudson
CN: Oncogene expression in ovarian cancer: A pilot study of c-myc
oncoprotein in serous papillary ovarian cancer. Gynecol Oncol.
28:137–150. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Sasano H, Nagura H and Silverberg SG:
Immunolocalization of c-myc oncoprotein in mucinous and serous
adenocarcinomas of the ovary. Hum Pathol. 23:491–495. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Li XS, Sun J and He XL: Expression of
c-myc and mutation of the KRAS gene in patients with ovarian
mucinous tumors. Genet Mol Res. 14:10752–10759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Polacarz SV, Hey NA, Stephenson TJ and
Hill AS: C-myc oncogene product P62c-myc in ovarian mucinous
neoplasms: Immunohistochemical study correlated with malignancy. J
Clin Pathol. 42:148–152. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Ning YX, Luo X, Xu M, Feng X and Wang J:
Let-7d increases ovarian cancer cell sensitivity to a genistein
analog by targeting c-Myc. Oncotarget. 8:74836–74845. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Curling M, Stenning S, Hudson CN and
Watson JV: Multivariate analyses of DNA index, p62c-myc, and
clinicopathological status of patients with ovarian cancer. J Clin
Pathol. 51:455–461. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Jung M, Russell AJ, Kennedy C, Gifford AJ;
Australian Ovarian Cancer Study Group, ; Mallitt KA, Sivarajasingam
S, Bowtell DD, DeFazio A, Haber M, et al: Clinical importance of
Myc family oncogene aberrations in epithelial ovarian cancer. JNCI
Cancer Spectr. 2:pky0472018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Yamamoto A, Kurata M, Yamamoto K, Nogawa
D, Inoue M, Ishibashi S, Ikeda M, Miyasaka N and Kitagawa M: High
amplification of PVT1 and MYC predict favorable prognosis in early
ovarian carcinoma. Pathol Res Pract. 216:1531752020. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Mori T, Li Y, Hata H, Ono K and Kochi H:
NIRF, a novel RING finger protein, is involved in cell-cycle
regulation. Biochem Biophys Res Commun. 296:530–536. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Khan NH, Chen HJ, Fan Y, Surfaraz M,
Ahammad MF, Qin YZ, Shahid M, Virk R, Jiang E, Wu DD and Ji XY:
Biology of PEST-containing nuclear protein: A potential molecular
target for cancer research. Front Oncol. 12:7845972022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Xu T, Wu K, Shi J, Ji L, Song X, Tao G,
Zheng S, Zhang L and Jiang B: LINC00858 promotes colon cancer
progression through activation of STAT3/5 signaling by recruiting
transcription factor RAD21 to upregulate PCNP. Cell Death Discov.
8:2282022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Dong P, Fu H, Chen L, Zhang S, Zhang X, Li
H, Wu D and Ji X: PCNP promotes ovarian cancer progression by
accelerating β-catenin nuclear accumulation and triggering EMT
transition. J Cell Mol Med. 24:8221–8235. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Alhosin M, Sharif T, Mousli M,
Etienne-Selloum N, Fuhrmann G, Schini-Kerth VB and Bronner C:
Down-regulation of UHRF1, associated with re-expression of tumor
suppressor genes, is a common feature of natural compounds
exhibiting anti-cancer properties. J Exp Clin Cancer Res.
30:412011. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Wu DD, Gao YR, Li T, Wang DY, Lu D, Liu
SY, Hong Y, Ning HB, Liu JP, Shang J, et al: PEST-containing
nuclear protein mediates the proliferation, migration, and invasion
of human neuroblastoma cells through MAPK and PI3K/AKT/mTOR
signaling pathways. BMC Cancer. 18:4992018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Ghahhari NM and Babashah S: Interplay
between microRNAs and WNT/β-catenin signalling pathway regulates
epithelial-mesenchymal transition in cancer. Eur J Cancer.
51:1638–1649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Shi-Bai Z, Rui-Min L, Ying-Chuan S, Jie Z,
Chao J, Can-Hua Y, Xi C and Wen-Wei Q: TIPE2 expression is
increased in peripheral blood mononuclear cells from patients with
rheumatoid arthritis. Oncotarget. 8:87472–87479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Rivlin N, Brosh R, Oren M and Rotter V:
Mutations in the p53 tumor suppressor gene: important milestones at
the various steps of tumorigenesis. Genes Cancer. 2:466–474. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Momand J, Zambetti GP, Olson DC, George D
and Levine AJ: The mdm-2 oncogene product forms a complex with the
p53 protein and inhibits p53-mediated transactivation. Cell.
69:1237–1245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Lin J, Chen J, Elenbaas B and Levine AJ:
Several hydrophobic amino acids in the p53 amino-terminal domain
are required for transcriptional activation, binding to mdm-2 and
the adenovirus 5 E1B 55-kD protein. Genes Dev. 8:1235–1346. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Stramucci L, Pranteda A and Bossi G:
Insights of crosstalk between p53 protein and the MKK3/MKK6/p38
MAPK signaling pathway in cancer. Cancers (Basel). 10:1312018.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357:eaan25072017. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Thul PJ, Åkesson L, Wiking M, Mahdessian
D, Geladaki A, Ait Blal H, Alm T, Asplund A, Björk L, Breckels LM,
et al: A subcellular map of the human proteome. Science.
356:eaal33212017. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Cheaib B, Auguste A and Leary A: The
PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities
and challenges. Chin J Cancer. 34:4–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Hopkins BD, Hodakoski C, Barrows D, Mense
SM and Parsons RE: PTEN function: The long and the short of it.
Trends Biochem Sci. 39:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Mabuchi S, Kuroda H, Takahashi R and
Sasano T: The PI3K/AKT/mTOR pathway as a therapeutic target in
ovarian cancer. Gynecol Oncol. 137:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Siddique HR, Parray A, Tarapore RS, Wang
L, Mukhtar H, Karnes RJ, Deng Y, Konety BR and Saleem M: BMI1
polycomb group protein acts as a master switch for growth and death
of tumor cells: Regulates TCF4-transcriptional factor-induced BCL2
signaling. PLoS One. 8:e606642013. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Wang J, Guo Y, Chu H, Guan Y, Bi J and
Wang B: Multiple functions of the RNA-binding protein HuR in cancer
progression, treatment responses and prognosis. Int J Mol Sci.
14:10015–10041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Cao L, Bombard J, Cintron K, Sheedy J,
Weetall ML and Davis TW: BMI1 as a novel target for drug discovery
in cancer. J Cell Biochem. 112:2729–2741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Glinsky GV, Berezovska O and Glinskii AB:
Microarray analysis identifies a death-from-cancer signature
predicting therapy failure in patients with multiple types of
cancer. J Clin Invest. 115:1503–1521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Gray F, Cho HJ, Shukla S, He S, Harris A,
Boytsov B, Jaremko Ł, Jaremko M, Demeler B, Lawlor ER, et al: BMI1
regulates PRC1 architecture and activity through homo- and
hetero-oligomerization. Nat Commun. 7:133432016. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Luo W, Chen J, Li L, Ren X, Cheng T, Lu S,
Lawal RA, Nie Q, Zhang X and Hanotte O: c-Myc inhibits myoblast
differentiation and promotes myoblast proliferation and muscle
fibre hypertrophy by regulating the expression of its target genes,
miRNAs and lincRNAs. Cell Death Differ. 26:426–442. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Rechsteiner M and Rogers SW: PEST
sequences and regulation by proteolysis. Trends Biochem Sci.
21:267–271. 1996. View Article : Google Scholar : PubMed/NCBI
|