Introduction
Natural killer/T-cell lymphoma (NKTCL) represents a
rare and highly aggressive subtype of non-Hodgkin lymphoma (NHL)
with Epstein-Barr virus (EBV) infection. The incidence of NKTCL
exhibits a predilection for certain geographical regions, such as
Asia and Latin America, with relatively fewer cases reported in
Europe and North America. Within the Chinese population, NKTCL
accounts for 12–17% of NHL cases and 55–61% of peripheral T-cell
lymphoma cases (1,2).
The therapeutic strategies for NKTCL vary based on
disease staging, and no standard regimen has been established.
Combined radiotherapy (RT) and chemotherapy are recommended for
patients with early-stage (Ann Arbor stage I/II) (3) disease, but systemic therapy is
recommended for patients with advanced-stage disease (4,5). The
success of early-stage NKTCL treatment hinges upon the irradiation
field and dosage, which are associated with local control rates and
prognosis (6). The optimal
chemotherapy regimens for NKTCL include those incorporating
pegaspargase, such as SMILE (dexamethasone, methotrexate,
ifosfamide, L-asparaginase and etoposide) (7), P-Gemox (gemcitabine, pegaspargase and
oxaliplatin) (8), P-GDP
(dexamethasone, cisplatin, gemcitabine and pegaspargase) (9) and AspMetDex (L-asparaginase,
methotrexate and dexamethasone) regimens (10). Largely variable effective results
have been reported for these regimens (11); however, a standard treatment for
NKTCL has not been established.
Several prognostic models have been developed for
patients with NKTCL. For example, the international prognostic
index (IPI) and Korean Prognostic Index are useful for patients
with anthracycline-containing response (12), and the prognostic index for NKTCL
(PINK) and the prognostic index for NKTCL with extended features
(PINK-E) were developed for patients treated with
non-anthracycline-containing regimens (13,14).
However, the most accurate model is yet to be determined.
The present study performed a retrospective analysis
of patients with NKTCL who received treatment at the Cancer Center,
Union Hospital, Huazhong University of Science and Technology
(Wuhan, China) between June 2013 and June 2022. The aim was to
assess the clinical features, treatment outcomes and prognostic
factors of NKTCL, thereby contributing to a deeper understanding of
this aggressive disease.
Materials and methods
Patients
Patients diagnosed with NKTCL between June 2013 and
June 2022 at the Cancer Center Union Hospital, Huazhong University
of Science and Technology, were identified, and those that met the
following criteria were included: i) Histologically confirmed NKTCL
according to the World Health Organization Classification of Tumors
of Hematopoietic and Lymphoid Tissues (15); ii) ≥1 type of antitumor therapy
administered; and iii) follow-up completed and clinical data
available. The sample size was determined by the total number of
eligible cases meeting the inclusion criteria during this period.
The clinical data were retrieved from electronic medical records,
including sex, age, Eastern Cooperative Oncology Group performance
status (ECOG PS) (16), disease
staging, involved anatomical sites, lactate dehydrogenase (LDH)
levels, prognostic indicators, EBV DNA levels, hematological
characteristics, presence of B symptoms and radiological
manifestations.
The present study was approved by the Ethics
Committee of Union Hospital, Tongji Medical College, Huazhong
University of Science and Technology (approval no. 20240986).
Written informed consent was waived, as there were no conflicts of
interest or potential harm to patients, and the confidentiality of
patient data was guaranteed in accordance with the requirements of
the Ethics Committee.
Treatments
Treatment strategies were implemented based on the
stage classification of each patient. For those presenting with
early-stage disease, a radiation regimen consisting of 50–54 Gy
delivered over 25–27 fractions was administered concurrently with
weekly intravenous cisplatin at a dose of 30 mg/m2.
Following completion of radiation therapy, patients underwent 3–4
cycles of pegaspargase-based chemotherapy (dosing schedule as
described for those with advanced-stage disease).
By contrast, patients diagnosed with advanced-stage
disease received chemotherapy as the primary treatment modality.
The selection of chemotherapy regimens was determined in alignment
with the patient's individual preferences. The combined
chemotherapy protocols employed included PIDE (2,500
IU/m2 pegaspargase with a maximum dose of 3,750 IU
administered intramuscularly on day 1; 1g/m2 ifosfamide
administered intravenously on days 2–4; 40 mg dexamethasone
administered orally on days 1–4; and 100 mg/m2 etoposide
administered intravenously on days 2–4), P-GDP (2,500
IU/m2 pegaspargase with a maximum dose of 3,750 IU
administered intramuscularly on day 1; 1,000 mg/m2
gemcitabine administered intravenously on days 1–8; 40 mg
dexamethasone administered orally on days 1–4; and 25
mg/m2 cisplatin administered intravenously on days 1–3),
P-Gemox (2,500 IU/m2 pegaspargase administered
intramuscularly on day 1; 1,000 mg/m2 gemcitabine
administered intravenously on days 1–8; and 100 mg/m2
oxaliplatin administered intravenously on days 1–8) and PPME (2,500
IU/m2 pegaspargase administered intramuscularly on day
1; 100 mg/m2 etoposide administered intravenously on
days 1–3; 3 g/m2 high-dose methotrexate administered
intravenously on day 4; and 200 mg camrelizumab administered
intravenously on day 7). The chemotherapy cycles were repeated
every 21 days. Regimens were designed by investigators based on
prior literature (6–9), with P-Gemox, PIDE and P-GDP adapted
from pegaspargase-based protocols and PPME incorporating immune
checkpoint blockade to reflect immunotherapy advances. Response
evaluation was performed following the completion of RT or after
every two cycles of chemotherapy.
Statistical analysis
The statistical analyses were performed using SPSS
26.0 software (IBM Corp.) and GraphPad Prism 9 software
(Dotmatics). Pearson's χ2 test was used for comparing
data from different groups. Kaplan-Meier curves and the log-rank
test were used to evaluate OS and PFS. To assess prognostic factors
associated with OS and PFS, Cox proportional hazards regression was
employed. Both univariate and multivariate Cox analyses were
performed to identify variables with significant prognostic values.
To account for multiple comparisons, the Benjamini-Hochberg false
discovery rate correction was applied separately to P-values from
multivariate Cox models for PFS and OS. Furthermore, an interaction
effect analysis was performed using R software (version 4.3.2;
Posit Software, PBC). Specifically, Cox proportional hazards
regression models (survival package) were fitted for both PFS and
OS, and multiplicative interaction terms (stage × age, stage × LDH,
stage × EBV DNA level and EBV DNA × age) were included in the
multivariate models. Wald tests were applied to evaluate the
statistical significance of the interaction terms, and hazard
ratios (HRs) with 95% confidence intervals (CIs) were calculated.
For the retrospective sample size, an events per covariate value of
5–10 was targeted to ensure reliable multivariate Cox model
estimates for PFS and OS, with sufficient power to detect a hazard
ratio of 2.0 (α=0.05). P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of patients
A retrospective analysis was performed on 200
individuals newly diagnosed with NKTCL who were admitted to the
Cancer Center at Union Hospital between June 2013 and June 2022.
Among the initial cohort, 29 patients were excluded from the study:
12 individuals did not undergo any form of treatment, whilst 17
received inadequate treatment. A total of 171 patients were
subsequently included in the final analysis. Within this cohort,
there were 114 male patients (66.7%) and 57 female patients
(33.3%), resulting in a male-to-female ratio of 2:1. The mean age
of the patients was 45 years, with an age range of 16–77 years. The
distribution of disease stages revealed that 117 patients presented
with early-stage disease (stage I/II; 68.4%), whilst 54 patients
were classified as having advanced-stage disease (stage III/IV;
31.6%). Among the patient population, 60.2% (103/171) exhibited B
symptoms, whilst elevated LDH levels were observed in 19.3% of
patients (33/171). ECOG PS scores of 0–1 were recorded for 93.6% of
individuals (160/171). Additionally, most patients were categorized
as having a low-to-moderate risk based on the IPI (86.0%; 147/171),
PINK (71.3%; 122/171) and PINK-E scores (65.5%; 112/171). Notably,
baseline assessments revealed the presence of EBV DNA in the
bloodstream of all patients except for 5 individuals (2.9%). A
detailed summary of the clinical characteristics of the patient
cohort is provided in Table I.
 | Table I.Clinical characteristics of patients
with natural killer/T-cell lymphoma (n=171). |
Table I.
Clinical characteristics of patients
with natural killer/T-cell lymphoma (n=171).
|
Characteristics | Patients, n
(%) |
|---|
| Sex |
|
|
Male | 114 (66.7) |
|
Female | 57 (33.3) |
| Age, years |
|
|
≥60 | 33 (19.3) |
|
<60 | 138 (80.7) |
| B symptoms |
|
|
Yes | 103 (60.2) |
| No | 68 (39.8) |
| Distant lymph node
involvement |
|
|
Yes | 49 (28.7) |
| No | 122 (71.3) |
| LDH |
|
|
Elevated | 33 (19.3) |
|
Normal | 138 (80.7) |
| Ann Arbor
Stage |
|
|
I/II | 117 (68.4) |
|
III/IV | 54 (31.6) |
| Bone marrow
involvement |
|
|
Yes | 18 (10.5) |
| No | 153 (89.5) |
| ECOG PS |
|
|
0-1 | 160 (93.6) |
| ≥2 | 11 (6.4) |
| IPI |
|
|
0-2 | 147 (86.0) |
|
3-5 | 24 (14.0) |
| PINK |
|
|
0-2 | 122 (71.3) |
|
3-5 | 49 (28.7) |
| PINK-E |
|
|
0-2 | 114 (66.7) |
|
3-5 | 57 (33.3) |
| Baseline serum EBV
DNA |
|
|
Positive | 106 (62.0) |
|
Negative | 65 (38.0) |
Treatment responses
Treatment response in patients with early-stage
NKTCL
A total of 117 patients diagnosed with early-stage
NKTCL were treated with combined RT and chemotherapy. The ORR
achieved through this combined treatment approach was 98.2%. In
terms of the chemotherapy regimens employed, the patient cohort was
stratified as follows: 66 individuals (56.4%) were subjected to the
PIDE protocol, 27 patients (23.1%) received P-GDP, 13 patients
(11.1%) were treated with the PPME and 11 patients (9.4%) underwent
P-Gemox treatment. Notably, patients administered PIDE and PPME
exhibited an ORR of 100%, whilst those receiving P-GDP and P-Gemox
demonstrated response rates of 96.4 and 90.9%, respectively.
Detailed findings pertaining to treatment responses are presented
in Table II.
 | Table II.Treatment response in patients with
early-stage natural killer/T-cell lymphoma. |
Table II.
Treatment response in patients with
early-stage natural killer/T-cell lymphoma.
| Treatment | CR, n (%) | PR, n (%) | SD, n (%) | PD, n (%) |
|---|
| PIDE (n=66) | 66 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| P-GDP (n=27) | 26 (96.3) | 0 (0.0) | 0 (0.0) | 1 (3.7) |
| PPME (n=13) | 13 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gemox (n=11) | 10 (90.9) | 0 (0.0) | 0 (0.0) | 1 (9.1) |
Treatment response in patients with
advanced-stage NKTCL
A total of 54 patients diagnosed with advanced-stage
NKTCL were subjected to chemotherapy, either as a standalone
treatment or in combination with RT. The choice of treatment
modality was made in consultation with each patient, considering
individual preferences. The ORR achieved through these
interventions was determined to be 92.5%. In terms of the specific
chemotherapy regimens employed, patient management encompassed the
following strategies: 17 individuals (31.5%) underwent treatment
with PPME, 13 patients (24.1%) were administered P-GDP, 12 patients
(22.2%) received PIDE, 8 individuals (14.8%) were treated with
P-Gemox, 3 patients (5.6%) received AspaMetDex and 1 patient (1.9%)
was enrolled in a clinical trial. A total of 18 patients underwent
RT after completing the chemotherapy regimen. Notably, patients
managed with PPME exhibited an ORR of 100%, with a complete
response (CR) rate of 94.1%. Those subjected to P-GDP demonstrated
an ORR of 92.3%, whilst individuals receiving PIDE achieved an ORR
of 100%. Patients treated with P-Gemox exhibited an ORR of 87.5%,
whilst those receiving AspaMetDex demonstrated an ORR of 66.7%. The
sole patient participating in the clinical trial experienced
progressive disease. Detailed findings regarding treatment
responses are presented in Table
III.
 | Table III.Treatment response in patients with
advanced-stage natural killer/T-cell lymphoma. |
Table III.
Treatment response in patients with
advanced-stage natural killer/T-cell lymphoma.
| Treatment | CR, n (%) | PR, n (%) | SD, n (%) | PD, n (%) |
|---|
| PPME (n=17) | 16 (94.1) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| P-GDP (n=13) | 12 (92.3) | 0 (0.0) | 1 (7.7) | 0 (0.0) |
| PIDE (n=12) | 12 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| P-Gemox (n=8) | 7 (87.5) | 0 (0.0) | 1 (12.5) | 0 (0.0) |
| AspaMetDex
(n=3) | 2 (66.7) | 0 (0.0) | 0 (0.0) | 1 (33.3) |
Survival outcomes
Over a median follow-up period of 47.5 months
(range, 2–122 months), the mortality rate among patients was 15.8%
(27/171). Of these cases, 26 deaths were attributed to disease
progression, whilst 1 patient death was associated with an
infection. Among the deceased, 11 patients were in the early-stage
category (11/117; 9.4%) and 16 were classified as advanced-stage
patients (16/54; 29.6%). A total of 39 patients experienced disease
progression, accounting for 22.8% of the total patient cohort.
Specifically, 20 cases of disease progression were observed within
the early-stage group (20/117; 17.1%) and 19 instances were
recorded among the advanced-stage patients (19/54; 35.2%). The
4-year PFS and OS rates for the entire patient cohort were 76.7%
(95% CI, 69.8–83.6%) and 83.3% (95% CI, 77.1–89.6%), respectively.
Furthermore, the 4-year PFS rate was 82.5% (95% CI, 75.1–89.9%) for
patients in the early-stage group and 62.3% (95% CI, 47.4–77.2%)
for those in the advanced-stage group. The 4-year OS rate was
recorded as 92.2% (95% CI, 86.9–97.5%) for the early-stage patients
and 63.5% (95% CI, 48.2–78.8%) for the advanced-stage cohort.
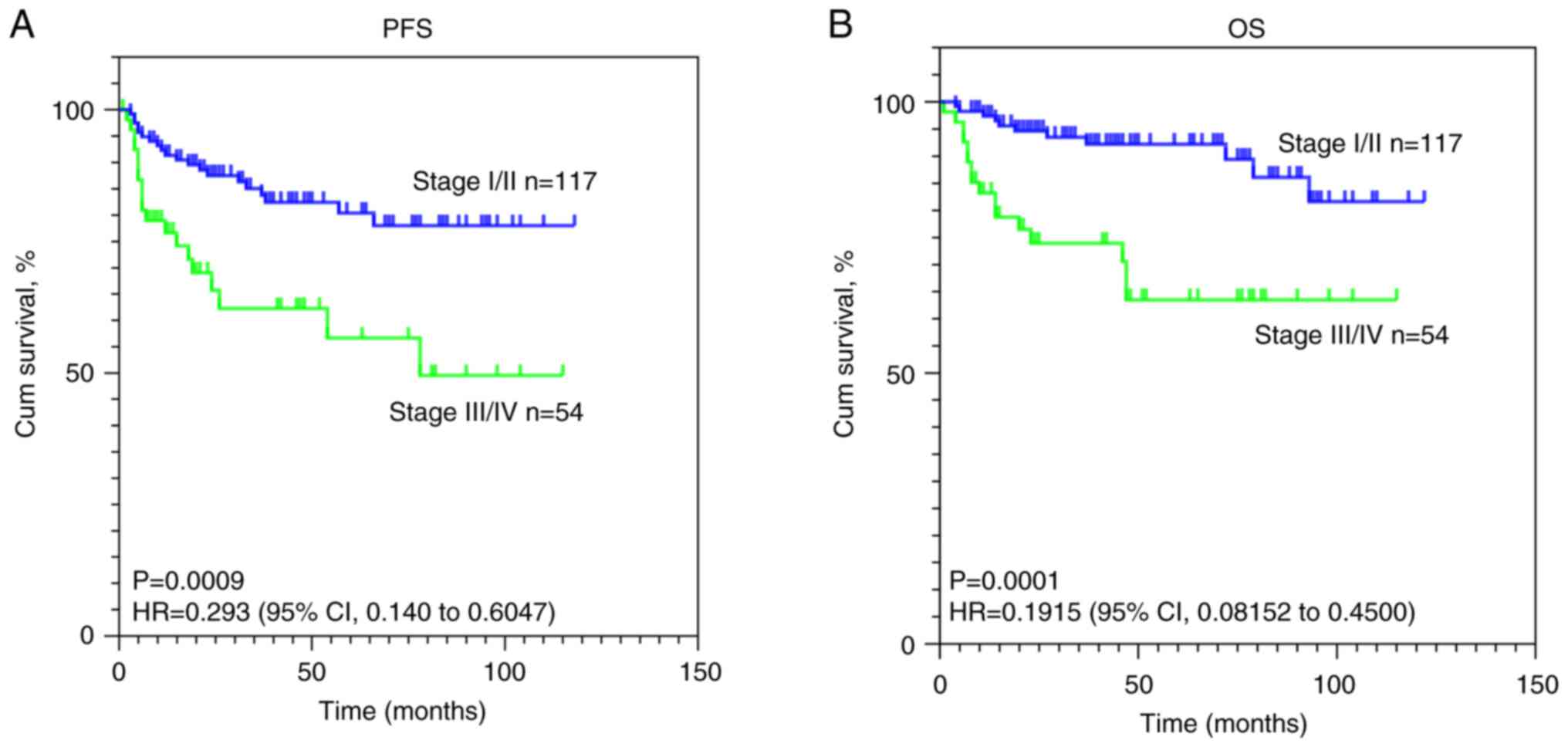
Kaplan-Meier estimates of PFS and OS are visually depicted in
Fig. 1. The statistical analysis
using the log-rank test indicated significant differences in
survival curves (both P<0.001) for both OS and PFS based on
stage.
To determine if treatment regimen choice influenced
survival outcomes in NKTCL, Kaplan-Meier survival curves were
generated for four chemotherapy regimens (P-Gemox, PIDE, P-GDP and
PPME) and compared using log-rank tests. The analysis revealed no
significant differences in PFS (P>0.05) or OS (P>0.05) across
the regimens (Fig. 2), indicating
that the treatment regimen did not significantly impact survival
outcomes in this cohort.
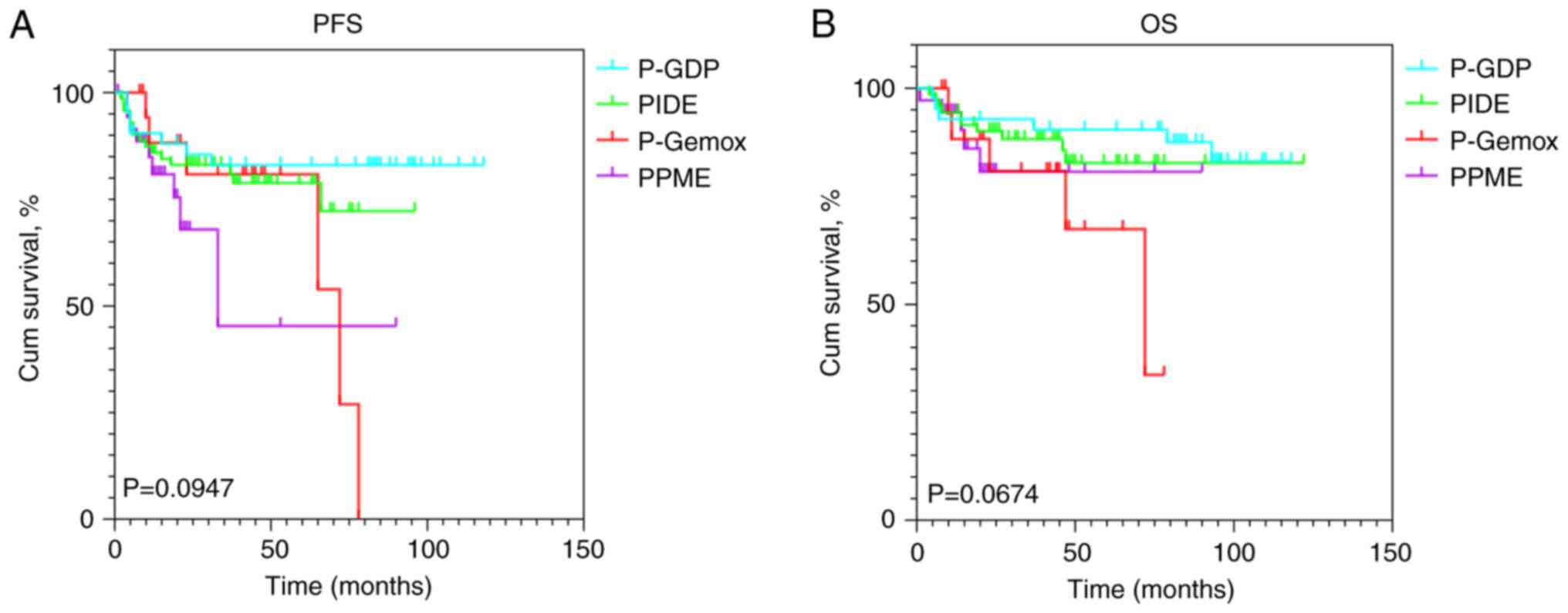 | Figure 2.Kaplan-Meier survival curves of (A)
PFS and (B) OS stratified by chemotherapy regimens, including PIDE,
P-GDP, P-Gemox and PPME. P-GDP, pegaspargase, gemcitabine,
dexamethasone, and cisplatin; PIDE, pegaspargase, ifosfamide,
dexamethasone, and etoposide; P-Gemox, pegaspargase, gemcitabine,
and oxaliplatin; PPME: pegaspargase, etoposide, high-dose
methotrexate, and camrelizumab; PFS, progression-free survival; OS,
overall survival; cum, cumulative. |
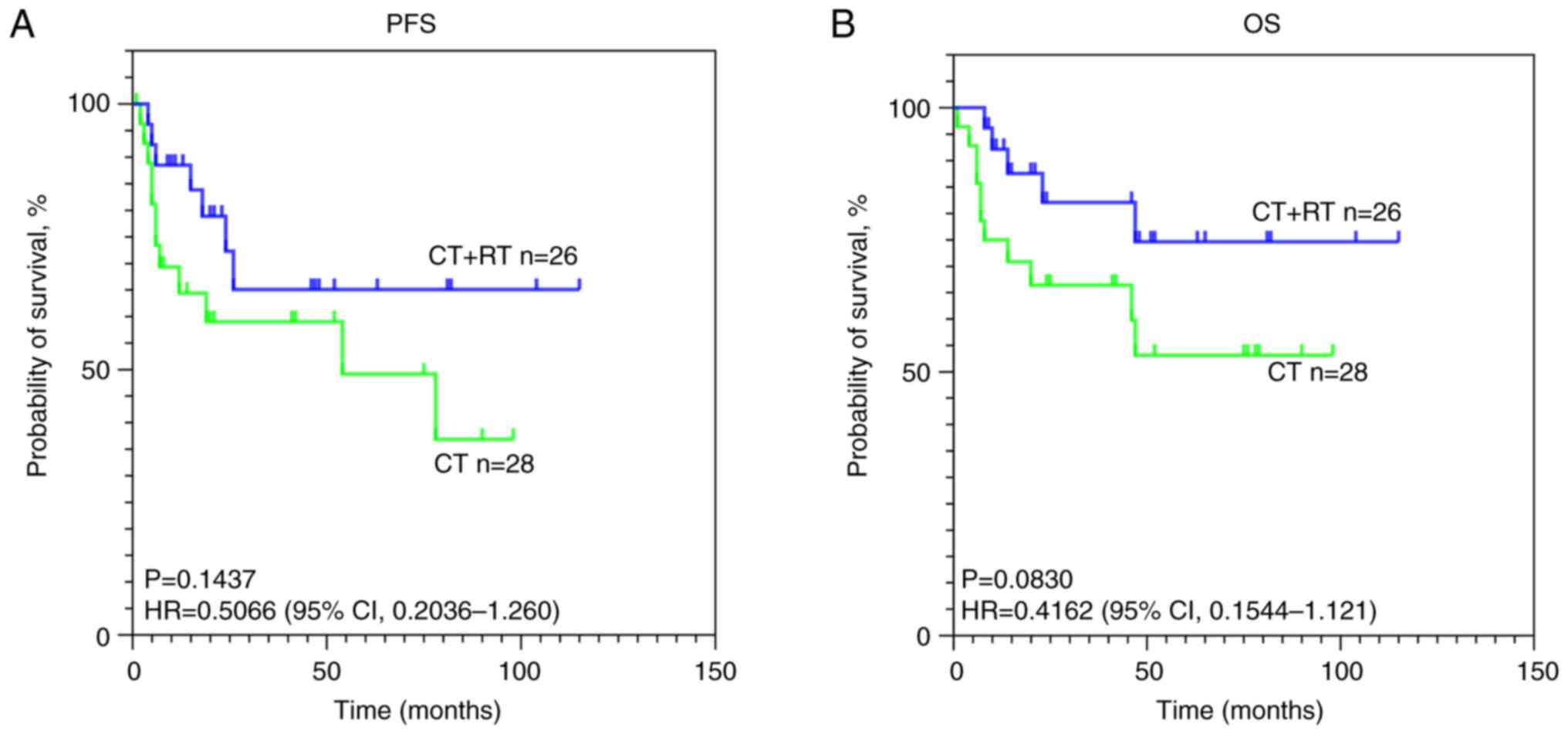
In patients with advanced-stage disease, whether
chemotherapy combined with RT could improve PFS and OS was
analyzed. Although the differences did not reach statistical
significance, a clear trend toward improved prognosis was observed
in patients receiving chemoradiotherapy. Kaplan-Meier estimates of
PFS and OS are presented in Fig.
3.
Prognosis
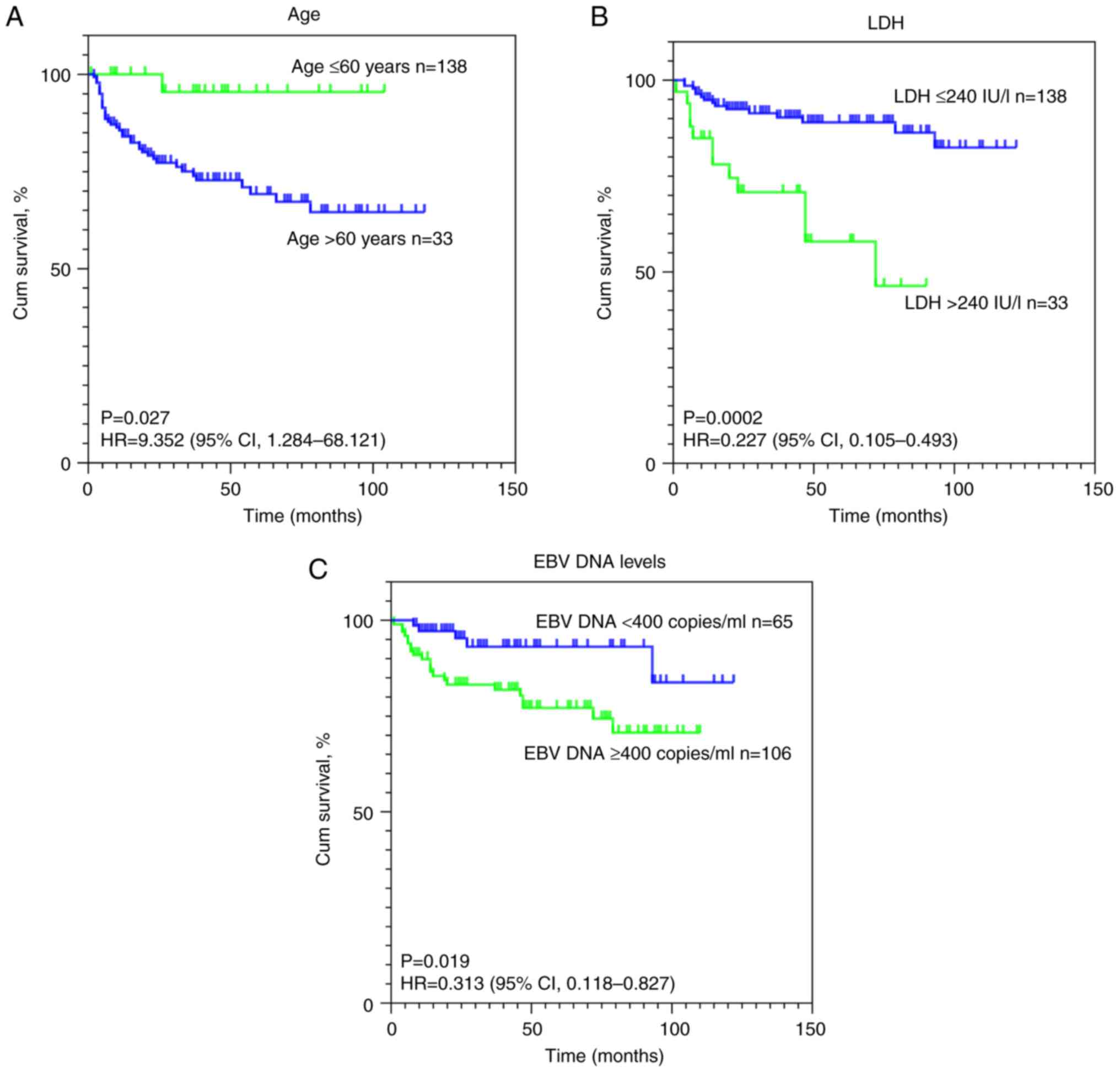
Univariate and multivariate analyses were performed
to identify the prognostic factors influencing OS and PFS in
patients with NKTCL. The univariate analysis revealed that an age
of >60 years and stage III/IV disease were adverse prognostic
indicators for PFS in patients with NKTCL, whilst elevated LDH
levels, stage III/IV disease and high EBV DNA levels were
identified as adverse prognostic factors for OS (Table IV). Subsequent multivariate
analysis demonstrated that an age of >60 years and stage III/IV
disease were independent unfavorable prognostic factors for PFS,
whilst elevated LDH levels and stage III/IV disease were
established as independent adverse prognostic factors for OS
(Table V). Given that treatment
modality had been previously demonstrated to exert no significant
impact on patient prognosis in both the univariate analyses and
Kaplan-Meier survival assessments, analysis of chemotherapy
regimens as a prognostic factor was excluded from the subsequent
multivariate analysis. The survival curves delineating the impact
of these prognostic factors are visually presented in Fig. 4. Interaction effects among clinical
variables (stage × age, stage × LDH, stage × EBV DNA and EBV DNA ×
age) were evaluated in multivariate Cox models. No significant
interactions were observed, as shown in Fig. S1 and Table SI. The multivariate Cox models,
adjusting for five covariates, yielded 7.8 (PFS) and 5.4 (OS)
events per covariate, supporting reliable estimates based on the
current sample size.
 | Table IV.Univariate analysis of prognosis
factors associated with survival. |
Table IV.
Univariate analysis of prognosis
factors associated with survival.
|
| PFS | OS |
|---|
|
|
|
|
|---|
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (male vs.
female) | 1.249
(0.655–2.383) | 0.499 | 1.193
(0.546–2.607) | 0.658 |
| Age (>60 vs. ≤60
years) | 9.352
(1.284–68.121) | 0.027a | 1.293
(0.447–3.740) | 0.636 |
| Stage (I/II vs.
III/IV) | 0.362
(0.192–0.677) | 0.002a | 0.251
(0.116–0.544) |
<0.001a |
| ECOG PS (0/1 vs.
≥2) | 0.910
(0.279–2.965) | 0.876 | 0.597
(0.179–1.989) | 0.401 |
| B symptoms (no vs.
yes) | 0.729
(0.374–1.420) | 0.353 | 0.623
(0.273–1.424) | 0.262 |
| LDH (≤240 vs.
>240 IU/l) | 0.662
(0.314–1.395) | 0.278 | 0.227
(0.105–0.493) |
<0.001a |
| EBV DNA levels
(<400 vs. ≥400 copies/ml) | 0.669
(0.344–1.304) | 0.802 | 0.313
(0.118–0.827) | 0.019a |
| Chemotherapy
regimen | - | 0.053 | - | 0.297 |
| PGDP
vs. PIDE | 1.771
(0.697–4.504) | 0.230 | 1.697
(0.565–5.098) | 0.346 |
| PGDP
vs. P-Gemox | 2.914
(0.944–8.997) | 0.063 | 3.611
(1.005–12.97) | 0.051 |
| PGDP
vs. PPME | 3.431
(1.196–9.841) | 0.052 | 2.500
(0.685–9.118) | 0.165 |
 | Table V.Multivariate analysis of prognosis
factors associated with survival. |
Table V.
Multivariate analysis of prognosis
factors associated with survival.
|
| PFS | OS |
|---|
|
|
|
|
|---|
| Factors | HR (95% CI) | P-value | Bonferroni-Hochberg
adjusted P-value | HR (95% CI) | P-value | Bonferroni-Hochberg
adjusted P-value |
|---|
| Sex (female vs.
male) | 1.399
(0.692–2.828) | 0.350 | - | 1.194
(0.525–2.712) | 0.672 | - |
| Age (>60 vs. ≤60
years) | 9.877
(1.305–74.732) | 0.027a | 0.036a | 1.113
(0.364–3.405) | 0.851 | - |
| Stage (I/II vs.
III/IV) | 0.311
(0.143–0.676) | 0.003a | 0.012a | 0.292
(0.112–0.761) | 0.012a | 0.024a |
| ECOG PS (0/1 vs.
2) | 1.070
(0.266–4.312) | 0.924 | - | 1.106
(0.284–4.315) | 0.884 | - |
| B symptoms (no vs.
yes) | 0.656
(0.333–1.292) | 0.223 | - | 0.570
(0.247–1.315) | 0.188 | - |
| LDH (≤240 vs.
>240 IU/l) | 1.150
(0.416–3.182) | 0.788 | - | 0.361
(0.136–0.956) | 0.040a | 0.040a |
| EBV DNA levels
(<400 vs. ≥400 copies/ml) | 0.654
(0.324–1.321) | 0.236 | - | 0.420
(0.152–1.164) | 0.095 | - |
Discussion
NKTCL is a rare and aggressive subtype of NHL,
typically characterized by a poor prognosis. The present study
performed a retrospective review of a cohort comprising 171
patients with NKTCL, aiming to analyze their clinical
characteristics, evaluate the efficacy of asparaginase-based
treatment regimens in improving survival rates, and investigate
determinants influencing the survival. The findings demonstrated
that the median age of onset for NKTCL was 45 years, with a
predominance of men with the disease. The etiology of NKTCL was
significantly associated with EBV infection. Moreover, a
preliminary assessment revealed that, except for a minority of 5
patients, all patients exhibited positive serum EBV DNA levels.
Notably, the number of cases classified as early-stage exceeded
those classified as late-stage.
Retrospective comparative studies have reported that
asparaginase-based or pegaspargase-based regimens are associated
with superior efficacy compared with conventional
anthracycline-based regimens. In a retrospective study by Wang
et al (17), the efficacy of
chemotherapy regimen GELOX (gemcitabine, l-asparaginase and
oxaliplatin) was compared with those of EPOCH (etoposide,
vincristine, doxorubicin, cyclophosphamide and prednisone) and CHOP
(cyclophosphamide, doxorubicin, vincristine and prednisone) in the
treatment of NKTCL. The results demonstrated that the 3-year OS and
PFS rates in the GELOX group were significantly superior to those
in the EPOCH or CHOP groups (OS, 87.0 vs. 54.0 vs. 54.0%,
respectively; P<0.05; PFS, 72.0 vs. 50.0 vs. 43.0%,
respectively; P<0.05). However, no significant differences in
PFS (P=0.421) and OS (P=0.765) were observed between the EPOCH and
CHOP groups, indicating the effectiveness of asparaginase-based
chemotherapy regimens. The present study also demonstrated that
asparaginase-based chemotherapy exhibited promising efficacy.
Several studies have demonstrated the value of
combined RT and chemotherapy rather than chemotherapy alone in
early-stage patients. The study by Cao et al (18) reported that sequential
RT-chemotherapy combination therapy or concomitant chemo-RT (CCRT)
produced a greater response than CHOP chemotherapy alone, with a CR
of ~70% (RT-chemotherapy) and 77% (CCRT) compared with 60% (CHOP),
and a 5-year OS rate of 42% (RT-chemotherapy) and 71% (CCRT)
compared with 35% (CHOP), respectively. Huang et al
(19) reported that in 44 patients
treated with gemcitabine, cisplatin and dexamethasone, the ORR was
~95% after intensity-adjusted RT (50 Gy for primary tumor and
additional 3–6 Gy for residual tumor area), with ~89% of patients
achieving a CR, and the 3-year OS and PFS rates were 85 and 77%,
respectively. Zhu et al (9)
performed a study involving 30 patients who underwent concurrent
chemo-RT (RT at 56 Gy/27F and chemotherapy using 25
mg/m2 cisplatin), followed by 3 cycles of DDGP
chemotherapy (dexamethasone, cisplatin, gemcitabine and
pegaspargase). The ORR was 93.3% (28/30), with all 28 patients
achieving a CR at the end of treatment. The findings of the present
study for early-stage patients are consistent with the results
reported in the aforementioned study.
Numerous studies have also indicated that
chemotherapy containing pegaspargase is superior to CHOP
chemotherapy in terms of efficacy and survival rates. In a study by
Bi et al (20), compared
with the regimen without pegaspargase, the P-Gemox/GELOX regimen
was associated with a significantly higher ORR in early-stage
patients (92.9 vs. 51.6%; P=0.009) and demonstrated significantly
improved 5-year OS rates (78.6 vs. 23.9%; P=0.010). Dong et
al (21) reported that,
compared with RT alone, the combination of L-asparaginase,
cisplatin, etoposide, cyclophosphamide and dexamethasone with RT
showed no significant difference in CR rates (90.9 vs. 77.8%;
P=0.124). Nevertheless, the group receiving chemotherapy combined
with RT exhibited higher 5-year PFS and OS rates compared with the
RT-alone group (89.2 vs. 49.6%, P=0.024; and 82.9% vs. 48%,
P=0.039; respectively). Huang et al (19) reported that the ORRs for the
combination of L-asparaginase, vincristine and dexamethasone (LOP)
or CHOP with RT were 89.6 and 65.6%, respectively (P=0.009), with
CR rates of 68.8 vs. 50%, respectively. Furthermore, the 3-year OS
and PFS rates significantly increased with LOP treatment (87.5 vs.
62.5%, P=0.006; and 79.2 vs. 50%, P=0.007; respectively),
indicating the superiority of asparaginase-based chemotherapy.
The dosage of RT for NKTCL is controversial, as
increasing the radiation dose may enhance efficacy, but it also
increases the risk of radiation-related complications. A literature
review revealed variability across different clinical centers, with
doses ranging from 40–65 Gy (22,23).
In the study by Wang et al (24), a planned prescription dose of 50 Gy
was administered, with an additional 5–10 Gy of radiation dose for
residual primary disease, resulting in actual doses ranging from
49.6–64 Gy, with a mean dose of 55.5 Gy. Jiang et al
(25) utilized a dose of 56 Gy,
achieving a reasonable ORR (88.5%) whilst maintaining tolerable
radiation toxicity. In the study by Oh et al (26), which involved 62 patients diagnosed
with stage I/II NKTCL, a median dose of 40 Gy of radiation therapy
was administered, resulting in a total response rate of 97% and a
CR rate of 90%. Among this cohort, 58 patients continued with
consolidation chemotherapy, achieving 3-year OS and PFS rates of
83.1 and 77.1%, respectively. The study by Niu et al
(27) involved 60 patients with
stage I/II nasal-type extranodal NKTCL. After induction
chemotherapy with pegaspargase, the patients received radiation
therapy with a dose of 54.6 Gy to the tumor and positive lymph
nodes, 50.7 Gy to the high-risk clinical target volume (CTV) and
45.5 Gy to the low-risk CTV, delivered in 26 fractions. The median
follow-up time was 95.8 months, and the 5-year locoregional
recurrence-free survival, PFS and OS rates were 83.3, 81.7 and
88.3%, respectively. In the present study, combined
chemoradiotherapy with a dose of 54 Gy demonstrated favorable
outcomes, with an ORR of 98.2%, a 4-year PFS rate of 82.5% (95% CI,
75.1–89.9%) and an OS rate of 92.2% (95% CI, 86.9–97.5%) for
early-stage patients. For advanced-stage patients treated with
pegaspargase-based chemotherapy, the 4-year PFS rate was 62.3% (95%
CI, 47.4–77.2%) and the OS rate was 63.5% (95% CI, 48.2–78.8%).
Both early and advanced-stage patients treated with
pegaspargase-based chemotherapy demonstrated high response rates
and long-term survival, consistent with the present study
findings.
The EBV load is an important consideration in NKTCL.
Numerous studies have reported an association between EBV viral
load and prognosis. A meta-analysis performed by Liu et al
(28) indicated that high EBV viral
load before and after treatment is associated with decreased
survival rates. High levels of baseline EBV DNA were significantly
associated with a poor OS, with an HR of 3.45 (95% CI, 1.63–7.31;
P=0.001), and a poor PFS, with an HR of 2.29 (95% CI, 1.50–3.51;
P=0.0001). Similarly, patients with high EBV DNA levels
post-treatment had poor PFS, with an HR of 2.36 (95% CI, 1.40–3.98;
P=0.001). These findings have been corroborated by subsequent
studies (29,30). Wang et al (31) also demonstrated that pre- and
post-treatment EBV DNA positivity were associated with worse OS and
PFS. Moreover, Suzuki et al (32) stratified patients into three
prognostic groups based on post-treatment plasma EBV viral load,
namely, negative, <100 copies/µg and >100 copies/µg, with
significantly decreased survival rates observed in the latter two
groups (P=0.001). The study by Zhong et al (33) also reported that the presence of EBV
DNA positivity during treatment is an independent predictor of
worse PFS and OS. EBV DNA positivity is associated with
upregulation of chromatin remodeling changes, immune
evasion-related genes and a reduction in infiltrating monocytes/M1
macrophages (34). The present
study also demonstrated that EBV load is an essential factor
influencing prognosis. Currently, due to the high sensitivity of
quantitative-PCR and its routine applicability in several medical
facilities, assessing EBV viral load in plasma samples using
quantitative-PCR is crucial throughout the diagnostic, treatment
and long-term follow-up phases (35). An increase in viral load levels may
indicate a recurrence of lymphoma, underscoring the importance of
evaluating EBV during follow-up (36).
The present study has several limitations related to
its retrospective, single-center design. Reliance on incomplete or
inconsistent medical records may introduce selection and
information bias, potentially obscuring true treatment effects and
compromising outcome assessment. Patient preference in choosing
chemotherapy regimens was not controlled, which may confound
survival outcomes. The single-institution setting may reflect local
practices, limiting generalizability. Additionally, variable
follow-up durations may affect prognostic analyses, as shorter
follow-up increases censoring, while longer follow-up may
overrepresent favorable outcomes. These limitations highlight the
need for future prospective, multi-center studies with standardized
protocols and larger, more diverse populations to validate and
strengthen the findings.
In summary, NKTCL predominantly affects young men
and is often associated with B symptoms. Staging is a crucial
prognostic factor for both PFS and OS. Frontline therapy with
pegaspargase-based chemotherapy, either alone or in combination
with concurrent RT, has demonstrated promising efficacy. Future
efforts should focus on optimizing treatment strategies.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LiZ conceived the study. Data was collected,
organized, validated, and managed to ensure data quality and
consistency for statistical analysis by QL, XL, FZ and TL. Data
collection was performed by PL, GA, LuZ, QL and XL. Data analysis
was performed by PL, GA, LuZ, FZ, TL and LiZ. QL and XL were also
involved in the statistical analysis. PL and GA wrote the original
draft. LiZ reviewed and edited the manuscript. TL and LiZ confirm
the authenticity of all the raw data. All authors contributed to
the article and approved the submitted version.
Ethics approval and consent to
participate
This study was conducted in accordance with the
principles of the Declaration of Helsinki. The study was approved
by the Ethical Review Committee of the Union Hospital, Tongji
Medical College, Huazhong University of Science and Technology
(Wuhan, China; approval no. 20240986). The requirement for patient
approval or written informed consent to participate was waived due
to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei YC, Qi F, Zheng BM, Zhang CG, Xie Y,
Chen B, Liu WX, Liu WP, Fang H, Qi SN, et al: Intensive therapy can
improve long-term survival in newly diagnosed, advanced-stage
extranodal NK/T-cell lymphoma: A multi-institutional, real-world
study. Int J Cancer. 153:1643–1657. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen JJ, Tokumori FC, Del Guzzo C, Kim J
and Ruan J: Update on T-cell lymphoma epidemiology. Curr Hematol
Malig Rep. 19:93–103. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian
Leukaemia; Lymphoma Group and Eastern Cooperative Oncology Group, ;
et al: Recommendations for initial evaluation, staging, and
response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano
classification. J Clin Oncol. 32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan Z, Yao S, Wang Z, Zhou W, Yao Z and
Liu Y: Treatment of extranodal NK/T-cell lymphoma: From past to
future. Front Immunol. 14:10886852023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang C and Wang L: Resistance mechanisms
and potential therapeutic strategies in relapsed or refractory
natural killer/T cell lymphoma. Chin Med J (Engl). 137:2308–2324.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng XW, Wu JX, Wu T, Zhu SY, Shi M, Su H,
Wang Y, He X, Xu LM, Yuan ZY, et al: Radiotherapy is essential
after complete response to asparaginase-containing chemotherapy in
early-stage extranodal nasal-type NK/T-cell lymphoma: A multicenter
study from the China Lymphoma Collaborative Group (CLCG). Radiother
Oncol. 129:3–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaguchi M, Suzuki R, Miyazaki K, Amaki
J, Takizawa J, Sekiguchi N, Kinoshita S, Tomita N, Wada H,
Kobayashi Y, et al: Improved prognosis of extranodal NK/T cell
lymphoma, nasal type of nasal origin but not extranasal origin. Ann
Hematol. 98:1647–1655. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng D, Bai S, Chen G, Fu B, Song C, Tang
H, Wang L and Wang H: Comparison of pegaspargase with concurrent
radiation vs. P-GEMOX with sequential radiation in early-stage
NK/T-cell lymphoma. Oncol Res. 33:965–974. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu F, Liu T, Pan H, Xiao Y, Li Q, Liu X,
Chen W, Wu G and Zhang L: Long-term outcomes of upfront concurrent
chemoradiotherapy followed by P-GDP regimen in newly diagnosed
early stage extranodal nasal-type NK/T cell lymphoma: A prospective
single-center phase II study. Medicine (Baltimore). 99:e217052020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horwitz SM, Ansell S, Ai WZ, Barnes J,
Barta SK, Clemens MW, Dogan A, Goodman AM, Goyal G, Guitart J, et
al: NCCN guidelines insights: T-cell lymphomas, version 1.2021. J
Natl Compr Canc Netw. 18:1460–1467. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang H, Xun Y, Ke C, Tateishi K and You H:
Extranodal lymphoma: Pathogenesis, diagnosis and treatment. Mol
Biomed. 4:292023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Zhang YJ, Zhu Y, Cao JZ, Yuan ZY,
Xu LM, Wu JX, Wang W, Wu T, Lu B, et al: Prognostic nomogram for
overall survival in previously untreated patients with extranodal
NK/T-cell lymphoma, nasal-type: A multicenter study. Leukemia.
29:1571–1577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin HN, Liu CY, Pai JT, Chang FP, Yang CF,
Yu YB, Hsiao LT, Chiou TJ, Liu JH, Gau JP, et al: How to predict
the outcome in mature T and NK cell lymphoma by currently used
prognostic models? Blood Cancer J. 2:e932012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim JQ, Huang D, Chan JY, Laurensia Y,
Wong EKY, Cheah DMZ, Chia BKH, Chuang WY, Kuo MC, Su YJ, et al: A
genomic-augmented multivariate prognostic model for the survival of
natural-killer/T-cell lymphoma patients from an international
cohort. Am J Hematol. 97:1159–1169. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alaggio R, Amador C, Anagnostopoulos I,
Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D,
Calaminici M, et al: The 5th edition of the World Health
Organization classification of haematolymphoid tumours: Lymphoid
neoplasms. Leukemia. 36:1720–1748. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang F, Markovic SN, Molina JR,
Halfdanarson TR, Pagliaro LC, Chintakuntlawar AV, Li R, Wei J, Wang
L, Liu B, et al: Association of sex, age, and eastern cooperative
oncology group performance status with survival benefit of cancer
immunotherapy in randomized clinical trials: A systematic review
and meta-analysis. JAMA Netw Open. 3:e20125342020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Wang WD, Xia ZJ, Zhang YJ, Xiang J
and Lu Y: Combination of gemcitabine, L-asparaginase, and
oxaliplatin (GELOX) is superior to EPOCH or CHOP in the treatment
of patients with stage IE/IIE extranodal natural killer/T cell
lymphoma: A retrospective study in a cohort of 227 patients with
long-term follow-up. Med Oncol. 31:8602014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao J, Lan S, Shen L, Si H, Zhang N, Li H
and Guo R: A comparison of treatment modalities for nasal
extranodal natural killer/T-cell lymphoma in early stages: The
efficacy of CHOP regimen based concurrent chemoradiotherapy.
Oncotarget. 8:20362–20370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Yang J, Liu P, Zhou S, Gui L, He
X, Qin Y, Zhang C, Yang S, Xing P, et al: Intensity-modulated
radiation therapy followed by GDP chemotherapy for newly diagnosed
stage I/II extranodal natural killer/T cell lymphoma, nasal type.
Ann Hematol. 96:1477–1483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bi XW, Xia Y, Zhang WW, Sun P, Liu PP,
Wang Y, Huang JJ, Jiang WQ and Li ZM: Radiotherapy and PGEMOX/GELOX
regimen improved prognosis in elderly patients with early-stage
extranodal NK/T-cell lymphoma. Ann Hematol. 94:1525–1533. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong LH, Zhang LJ, Wang WJ, Lei W, Sun X,
Du JW, Gao X, Li GP and Li YF: Sequential DICE combined with
l-asparaginase chemotherapy followed by involved field radiation in
newly diagnosed, stage IE to IIE, nasal and extranodal NK/T-cell
lymphoma. Leuk Lymphoma. 57:1600–1606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh
J, Lee SW, Kim JS, Cho J, Lee GW, et al: Phase II trial of
concurrent radiation and weekly cisplatin followed by VIPD
chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal
NK/T-Cell Lymphoma: Consortium for improving survival of lymphoma
study. J Clin Oncol. 27:6027–6032. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li YX, Yao B, Jin J, Wang WH, Liu YP, Song
YW, Wang SL, Liu XF, Zhou LQ, He XH, et al: Radiotherapy as primary
treatment for stage IE and IIE nasal natural killer/T-cell
lymphoma. J Clin Oncol. 24:181–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Li YX, Wang WH, Jin J, Dai JR,
Wang SL, Liu YP, Song YW, Wang ZY, Liu QF, et al: Mild toxicity and
favorable prognosis of high-dose and extended involved-field
intensity-modulated radiotherapy for patients with early-stage
nasal NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys.
82:1115–1121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang M, Zhang L, Xie L, Zhang H, Jiang Y,
Liu WP, Zhang WY, Tian R, Deng YT, Zhao S and Zou LQ: A phase II
prospective study of the ‘Sandwich’ protocol, L-asparaginase,
cisplatin, dexamethasone and etoposide chemotherapy combined with
concurrent radiation and cisplatin, in newly diagnosed, I/II stage,
nasal type, extranodal natural killer/T-cell lymphoma. Oncotarget.
8:50155–50163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oh D, Ahn YC, Kim SJ, Kim WS and Ko YH:
Concurrent chemoradiation therapy followed by consolidation
chemotherapy for localized extranodal natural killer/t-cell
lymphoma, nasal type. Int J Radiat Oncol Biol Phys. 93:677–683.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niu S, Li Y, Shao H, Hu J, Wang J, Wang H
and Zhang Y: Phase 2 clinical trial of simultaneous boost intensity
modulated radiation therapy with 3 dose gradients in patients with
stage I–II nasal type natural killer/T-cell lymphoma: Long-term
outcomes of survival and quality of life. Int J Radiat Oncol Biol
Phys. 118:770–780. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu ZL, Bi XW, Liu PP, Lei DX, Jiang WQ
and Xia Y: The clinical utility of circulating epstein-barr virus
DNA concentrations in NK/T-cell lymphoma: A meta-analysis. Dis
Markers. 2018:19610582018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimura H and Kwong YL: EBV viral loads in
diagnosis, monitoring, and response assessment. Front Oncol.
9:622019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Porte J, Hennequin C, Krizch D, Vercellino
L, Guillerm S, Thieblemont C and Quéro L: Extranodal nasal-type
NK/T lymphoma treated with chemotherapy and radiotherapy: Case
series from a European tertiary referral center and review of the
literature. Strahlenther Onkol. 200:434–443. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Hu J, Dong M, Ding M, Zhu L, Wu J,
Sun Z, Li X, Zhang L, Li L, et al: DDGP vs. SMILE in
relapsed/refractory extranodal natural killer/T-cell lymphoma,
nasal type: A retrospective study of 54 patients. Clin Transl Sci.
14:405–411. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suzuki R, Yamaguchi M, Izutsu K, Yamamoto
G, Takada K, Harabuchi Y, Isobe Y, Gomyo H, Koike T, Okamoto M, et
al: Prospective measurement of Epstein-Barr virus-DNA in plasma and
peripheral blood mononuclear cells of extranodal NK/T-cell
lymphoma, nasal type. Blood. 118:6018–6022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhong H, Cheng S, Xiong J, Chen J, Mu R,
Yang H, Yi H, Song Q, Zhang H, Hu Y, et al: Dynamic change in
Epstein-Barr virus DNA predicts prognosis in early stage natural
killer/T-cell lymphoma with pegaspargase-based treatment: long-term
follow-up and biomarker analysis from the NHL-004 multicenter
randomized study. Haematologica. 2025.(Epub ahead of print).
View Article : Google Scholar
|
|
34
|
Damania B, Kenney SC and Raab-Traub N:
Epstein-Barr virus: Biology and clinical disease. Cell.
185:3652–3670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stelzl E, Kessler HH, Parulekar AD, Bier
C, Nörz D, Schneider T, Kumar S, Simon CO and Lütgehetmann M:
Comparison of four commercial EBV DNA quantitative tests to a new
test at an early stage of development. J Clin Virol.
161:1054002023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shannon-Lowe C, Rickinson AB and Bell AI:
Epstein-Barr virus-associated lymphomas. Philos Trans R Soc Lond B
Biol Sci. 372:201602712017. View Article : Google Scholar : PubMed/NCBI
|