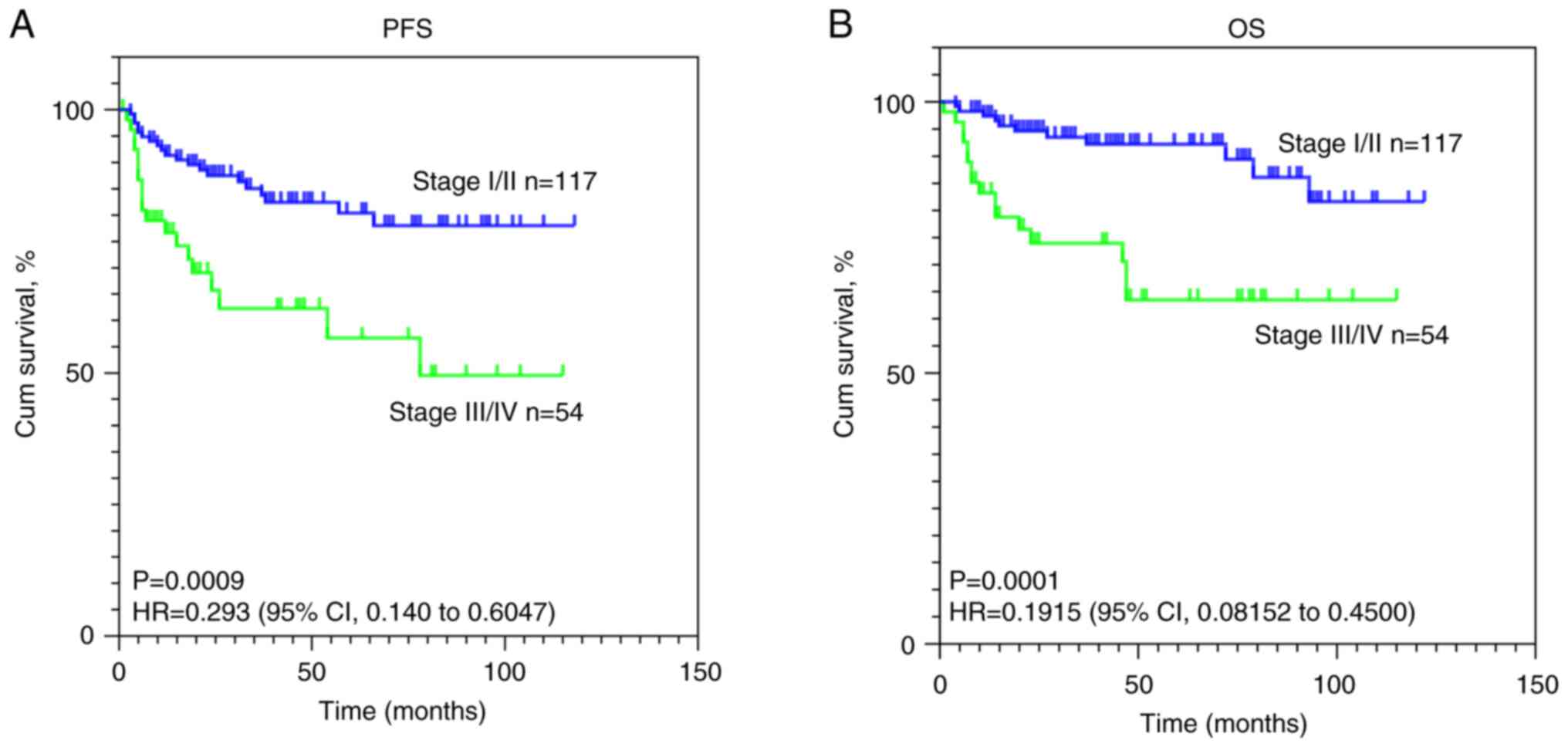
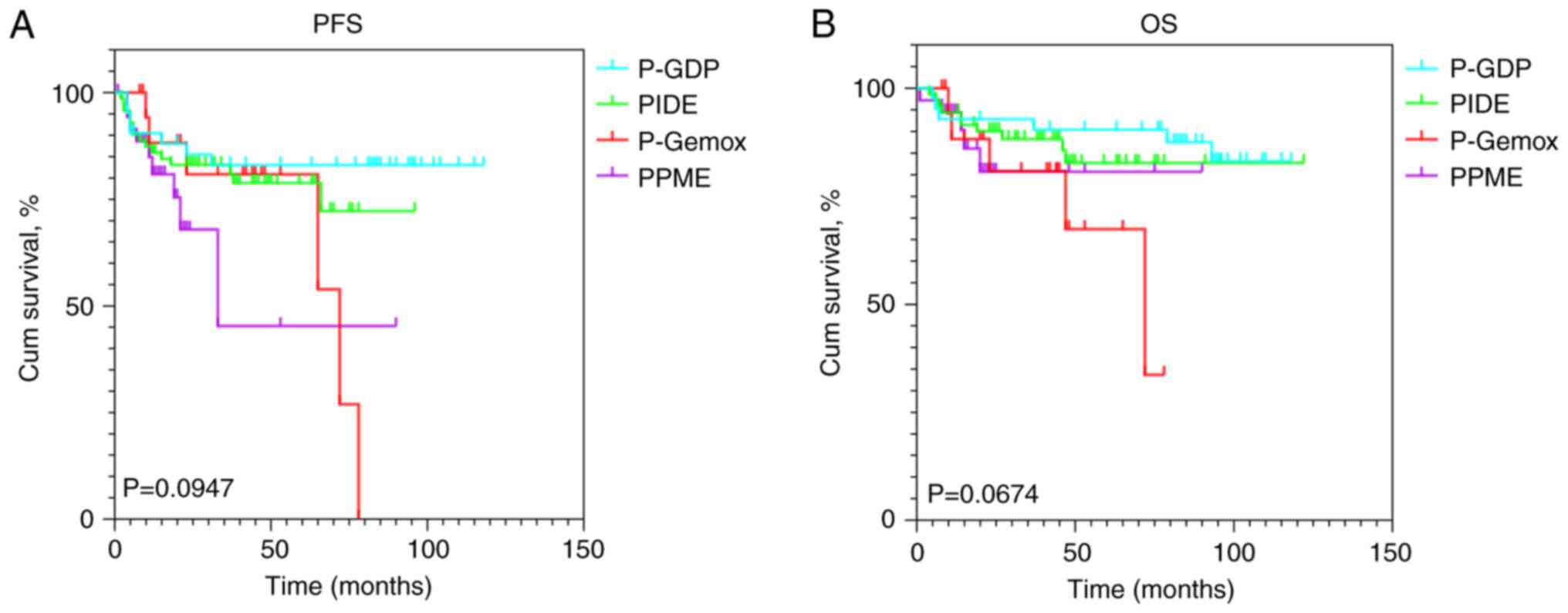
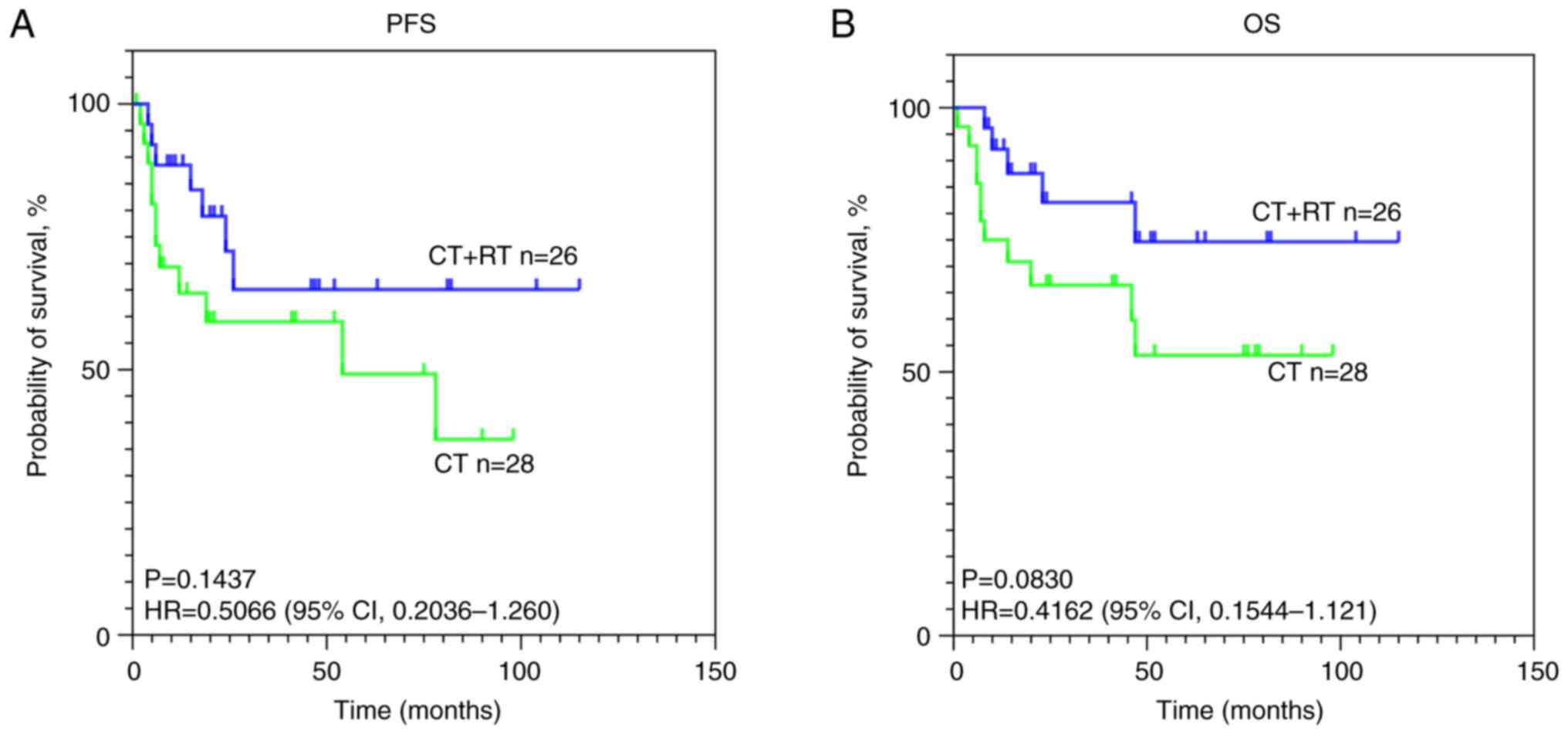
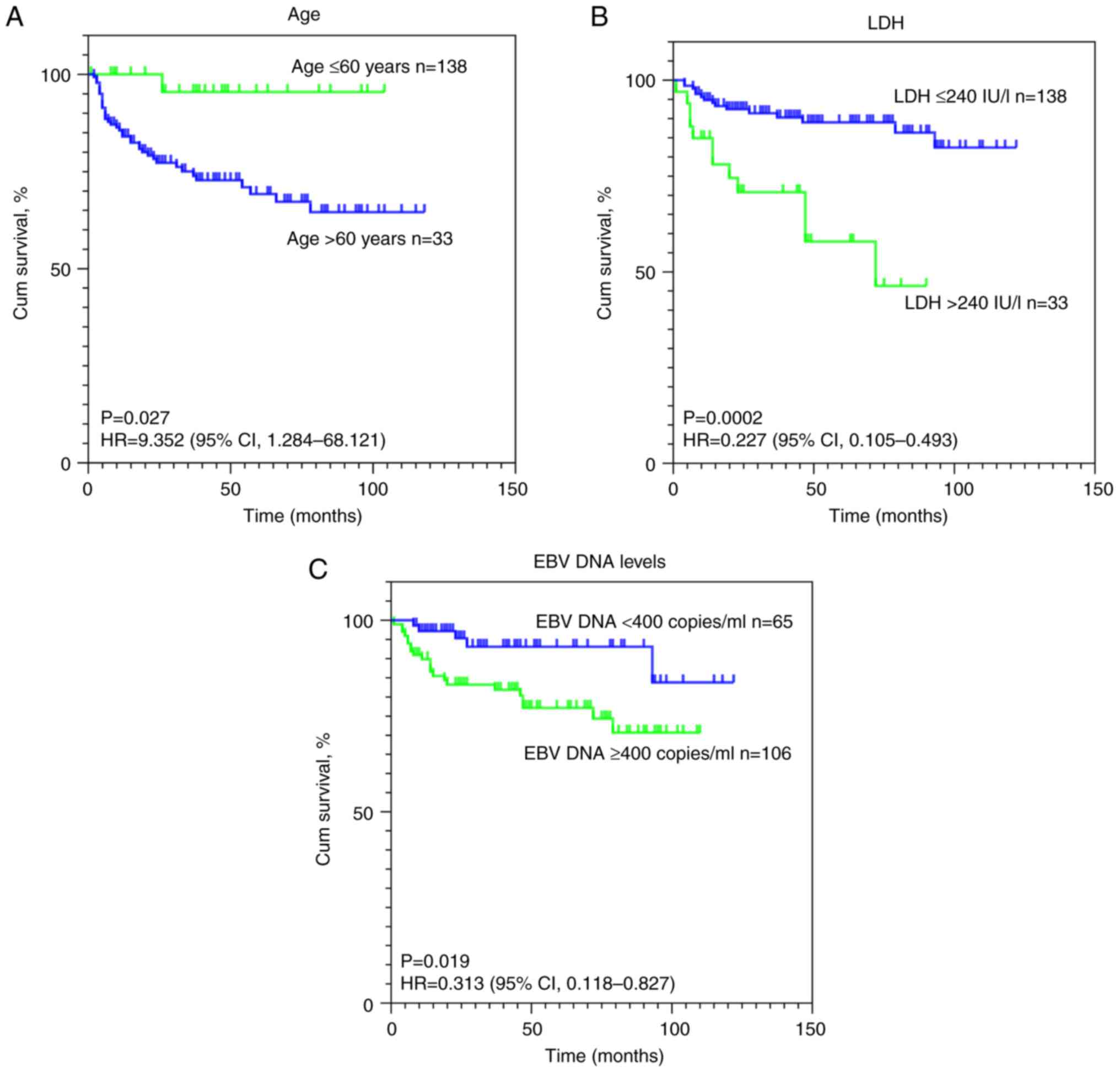
|
1
|
Wei YC, Qi F, Zheng BM, Zhang CG, Xie Y,
Chen B, Liu WX, Liu WP, Fang H, Qi SN, et al: Intensive therapy can
improve long-term survival in newly diagnosed, advanced-stage
extranodal NK/T-cell lymphoma: A multi-institutional, real-world
study. Int J Cancer. 153:1643–1657. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen JJ, Tokumori FC, Del Guzzo C, Kim J
and Ruan J: Update on T-cell lymphoma epidemiology. Curr Hematol
Malig Rep. 19:93–103. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian
Leukaemia; Lymphoma Group and Eastern Cooperative Oncology Group, ;
et al: Recommendations for initial evaluation, staging, and
response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano
classification. J Clin Oncol. 32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan Z, Yao S, Wang Z, Zhou W, Yao Z and
Liu Y: Treatment of extranodal NK/T-cell lymphoma: From past to
future. Front Immunol. 14:10886852023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang C and Wang L: Resistance mechanisms
and potential therapeutic strategies in relapsed or refractory
natural killer/T cell lymphoma. Chin Med J (Engl). 137:2308–2324.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng XW, Wu JX, Wu T, Zhu SY, Shi M, Su H,
Wang Y, He X, Xu LM, Yuan ZY, et al: Radiotherapy is essential
after complete response to asparaginase-containing chemotherapy in
early-stage extranodal nasal-type NK/T-cell lymphoma: A multicenter
study from the China Lymphoma Collaborative Group (CLCG). Radiother
Oncol. 129:3–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaguchi M, Suzuki R, Miyazaki K, Amaki
J, Takizawa J, Sekiguchi N, Kinoshita S, Tomita N, Wada H,
Kobayashi Y, et al: Improved prognosis of extranodal NK/T cell
lymphoma, nasal type of nasal origin but not extranasal origin. Ann
Hematol. 98:1647–1655. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng D, Bai S, Chen G, Fu B, Song C, Tang
H, Wang L and Wang H: Comparison of pegaspargase with concurrent
radiation vs. P-GEMOX with sequential radiation in early-stage
NK/T-cell lymphoma. Oncol Res. 33:965–974. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu F, Liu T, Pan H, Xiao Y, Li Q, Liu X,
Chen W, Wu G and Zhang L: Long-term outcomes of upfront concurrent
chemoradiotherapy followed by P-GDP regimen in newly diagnosed
early stage extranodal nasal-type NK/T cell lymphoma: A prospective
single-center phase II study. Medicine (Baltimore). 99:e217052020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horwitz SM, Ansell S, Ai WZ, Barnes J,
Barta SK, Clemens MW, Dogan A, Goodman AM, Goyal G, Guitart J, et
al: NCCN guidelines insights: T-cell lymphomas, version 1.2021. J
Natl Compr Canc Netw. 18:1460–1467. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang H, Xun Y, Ke C, Tateishi K and You H:
Extranodal lymphoma: Pathogenesis, diagnosis and treatment. Mol
Biomed. 4:292023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Zhang YJ, Zhu Y, Cao JZ, Yuan ZY,
Xu LM, Wu JX, Wang W, Wu T, Lu B, et al: Prognostic nomogram for
overall survival in previously untreated patients with extranodal
NK/T-cell lymphoma, nasal-type: A multicenter study. Leukemia.
29:1571–1577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin HN, Liu CY, Pai JT, Chang FP, Yang CF,
Yu YB, Hsiao LT, Chiou TJ, Liu JH, Gau JP, et al: How to predict
the outcome in mature T and NK cell lymphoma by currently used
prognostic models? Blood Cancer J. 2:e932012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim JQ, Huang D, Chan JY, Laurensia Y,
Wong EKY, Cheah DMZ, Chia BKH, Chuang WY, Kuo MC, Su YJ, et al: A
genomic-augmented multivariate prognostic model for the survival of
natural-killer/T-cell lymphoma patients from an international
cohort. Am J Hematol. 97:1159–1169. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alaggio R, Amador C, Anagnostopoulos I,
Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D,
Calaminici M, et al: The 5th edition of the World Health
Organization classification of haematolymphoid tumours: Lymphoid
neoplasms. Leukemia. 36:1720–1748. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang F, Markovic SN, Molina JR,
Halfdanarson TR, Pagliaro LC, Chintakuntlawar AV, Li R, Wei J, Wang
L, Liu B, et al: Association of sex, age, and eastern cooperative
oncology group performance status with survival benefit of cancer
immunotherapy in randomized clinical trials: A systematic review
and meta-analysis. JAMA Netw Open. 3:e20125342020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Wang WD, Xia ZJ, Zhang YJ, Xiang J
and Lu Y: Combination of gemcitabine, L-asparaginase, and
oxaliplatin (GELOX) is superior to EPOCH or CHOP in the treatment
of patients with stage IE/IIE extranodal natural killer/T cell
lymphoma: A retrospective study in a cohort of 227 patients with
long-term follow-up. Med Oncol. 31:8602014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao J, Lan S, Shen L, Si H, Zhang N, Li H
and Guo R: A comparison of treatment modalities for nasal
extranodal natural killer/T-cell lymphoma in early stages: The
efficacy of CHOP regimen based concurrent chemoradiotherapy.
Oncotarget. 8:20362–20370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Yang J, Liu P, Zhou S, Gui L, He
X, Qin Y, Zhang C, Yang S, Xing P, et al: Intensity-modulated
radiation therapy followed by GDP chemotherapy for newly diagnosed
stage I/II extranodal natural killer/T cell lymphoma, nasal type.
Ann Hematol. 96:1477–1483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bi XW, Xia Y, Zhang WW, Sun P, Liu PP,
Wang Y, Huang JJ, Jiang WQ and Li ZM: Radiotherapy and PGEMOX/GELOX
regimen improved prognosis in elderly patients with early-stage
extranodal NK/T-cell lymphoma. Ann Hematol. 94:1525–1533. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong LH, Zhang LJ, Wang WJ, Lei W, Sun X,
Du JW, Gao X, Li GP and Li YF: Sequential DICE combined with
l-asparaginase chemotherapy followed by involved field radiation in
newly diagnosed, stage IE to IIE, nasal and extranodal NK/T-cell
lymphoma. Leuk Lymphoma. 57:1600–1606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh
J, Lee SW, Kim JS, Cho J, Lee GW, et al: Phase II trial of
concurrent radiation and weekly cisplatin followed by VIPD
chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal
NK/T-Cell Lymphoma: Consortium for improving survival of lymphoma
study. J Clin Oncol. 27:6027–6032. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li YX, Yao B, Jin J, Wang WH, Liu YP, Song
YW, Wang SL, Liu XF, Zhou LQ, He XH, et al: Radiotherapy as primary
treatment for stage IE and IIE nasal natural killer/T-cell
lymphoma. J Clin Oncol. 24:181–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Li YX, Wang WH, Jin J, Dai JR,
Wang SL, Liu YP, Song YW, Wang ZY, Liu QF, et al: Mild toxicity and
favorable prognosis of high-dose and extended involved-field
intensity-modulated radiotherapy for patients with early-stage
nasal NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys.
82:1115–1121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang M, Zhang L, Xie L, Zhang H, Jiang Y,
Liu WP, Zhang WY, Tian R, Deng YT, Zhao S and Zou LQ: A phase II
prospective study of the ‘Sandwich’ protocol, L-asparaginase,
cisplatin, dexamethasone and etoposide chemotherapy combined with
concurrent radiation and cisplatin, in newly diagnosed, I/II stage,
nasal type, extranodal natural killer/T-cell lymphoma. Oncotarget.
8:50155–50163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oh D, Ahn YC, Kim SJ, Kim WS and Ko YH:
Concurrent chemoradiation therapy followed by consolidation
chemotherapy for localized extranodal natural killer/t-cell
lymphoma, nasal type. Int J Radiat Oncol Biol Phys. 93:677–683.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niu S, Li Y, Shao H, Hu J, Wang J, Wang H
and Zhang Y: Phase 2 clinical trial of simultaneous boost intensity
modulated radiation therapy with 3 dose gradients in patients with
stage I–II nasal type natural killer/T-cell lymphoma: Long-term
outcomes of survival and quality of life. Int J Radiat Oncol Biol
Phys. 118:770–780. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu ZL, Bi XW, Liu PP, Lei DX, Jiang WQ
and Xia Y: The clinical utility of circulating epstein-barr virus
DNA concentrations in NK/T-cell lymphoma: A meta-analysis. Dis
Markers. 2018:19610582018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimura H and Kwong YL: EBV viral loads in
diagnosis, monitoring, and response assessment. Front Oncol.
9:622019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Porte J, Hennequin C, Krizch D, Vercellino
L, Guillerm S, Thieblemont C and Quéro L: Extranodal nasal-type
NK/T lymphoma treated with chemotherapy and radiotherapy: Case
series from a European tertiary referral center and review of the
literature. Strahlenther Onkol. 200:434–443. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Hu J, Dong M, Ding M, Zhu L, Wu J,
Sun Z, Li X, Zhang L, Li L, et al: DDGP vs. SMILE in
relapsed/refractory extranodal natural killer/T-cell lymphoma,
nasal type: A retrospective study of 54 patients. Clin Transl Sci.
14:405–411. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suzuki R, Yamaguchi M, Izutsu K, Yamamoto
G, Takada K, Harabuchi Y, Isobe Y, Gomyo H, Koike T, Okamoto M, et
al: Prospective measurement of Epstein-Barr virus-DNA in plasma and
peripheral blood mononuclear cells of extranodal NK/T-cell
lymphoma, nasal type. Blood. 118:6018–6022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhong H, Cheng S, Xiong J, Chen J, Mu R,
Yang H, Yi H, Song Q, Zhang H, Hu Y, et al: Dynamic change in
Epstein-Barr virus DNA predicts prognosis in early stage natural
killer/T-cell lymphoma with pegaspargase-based treatment: long-term
follow-up and biomarker analysis from the NHL-004 multicenter
randomized study. Haematologica. 2025.(Epub ahead of print).
View Article : Google Scholar
|
|
34
|
Damania B, Kenney SC and Raab-Traub N:
Epstein-Barr virus: Biology and clinical disease. Cell.
185:3652–3670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stelzl E, Kessler HH, Parulekar AD, Bier
C, Nörz D, Schneider T, Kumar S, Simon CO and Lütgehetmann M:
Comparison of four commercial EBV DNA quantitative tests to a new
test at an early stage of development. J Clin Virol.
161:1054002023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shannon-Lowe C, Rickinson AB and Bell AI:
Epstein-Barr virus-associated lymphomas. Philos Trans R Soc Lond B
Biol Sci. 372:201602712017. View Article : Google Scholar : PubMed/NCBI
|