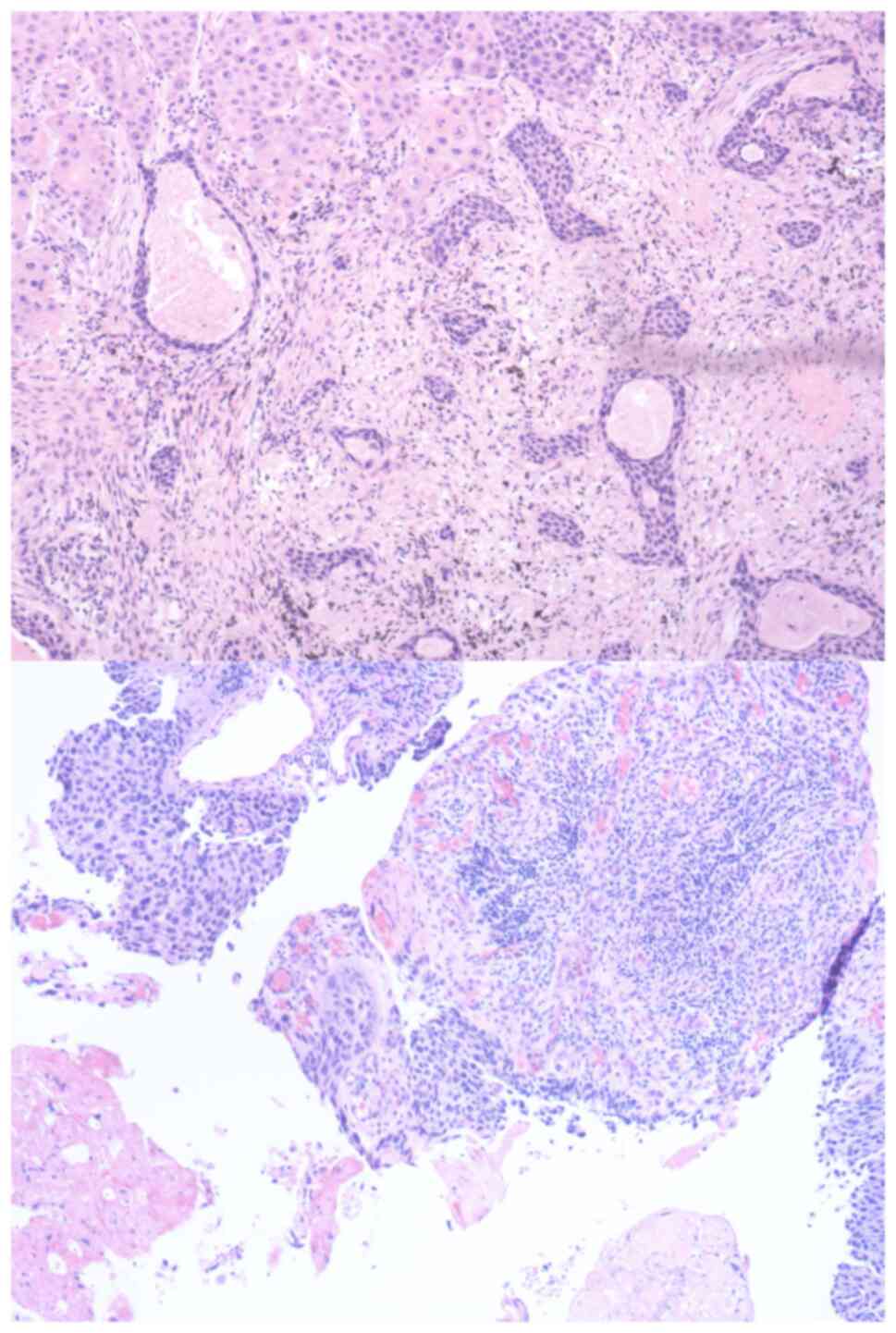
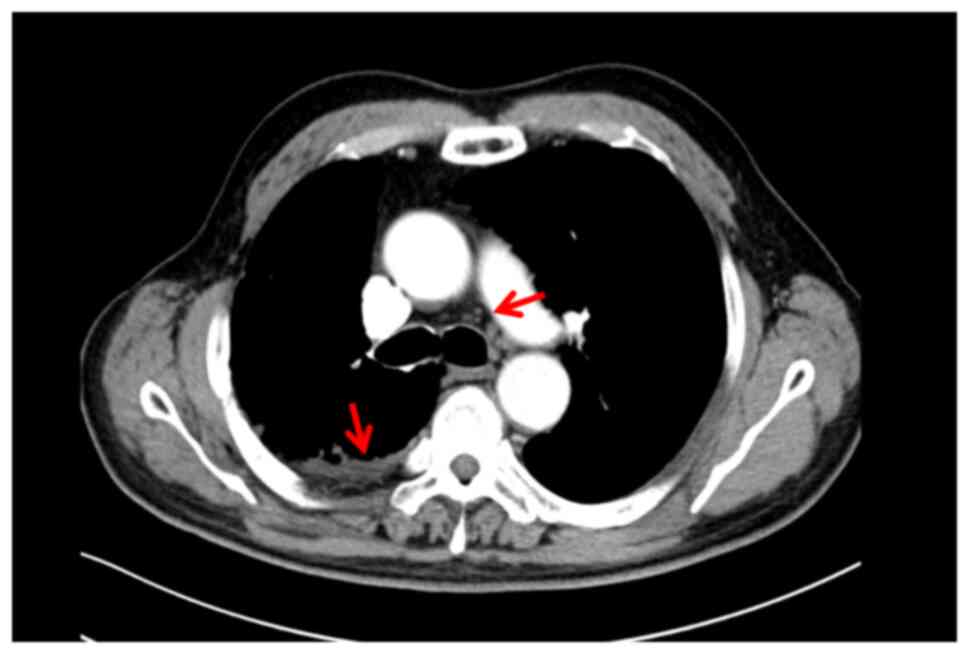
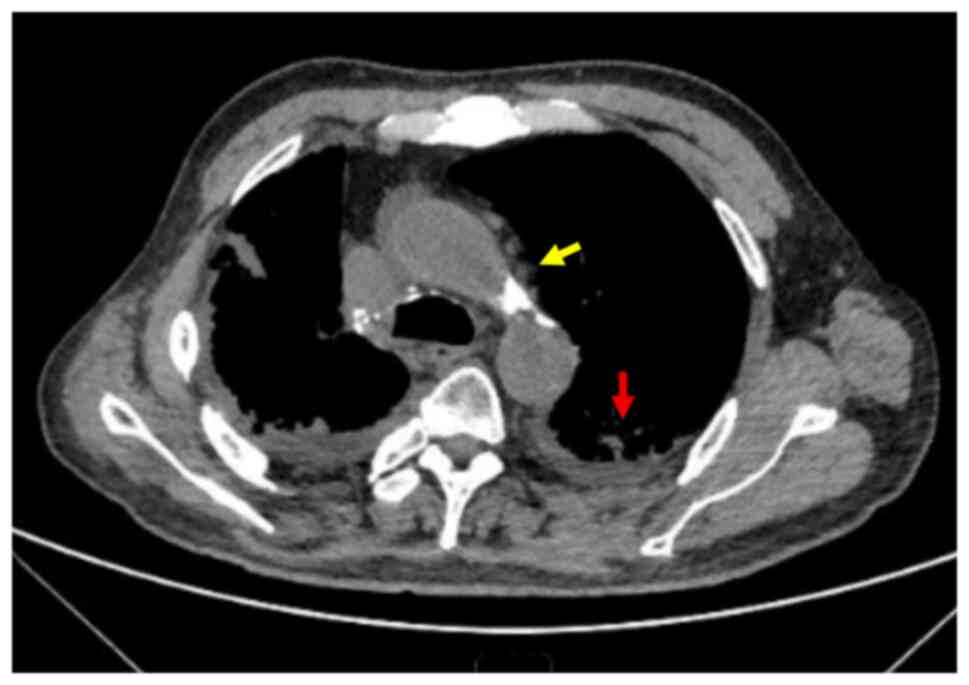
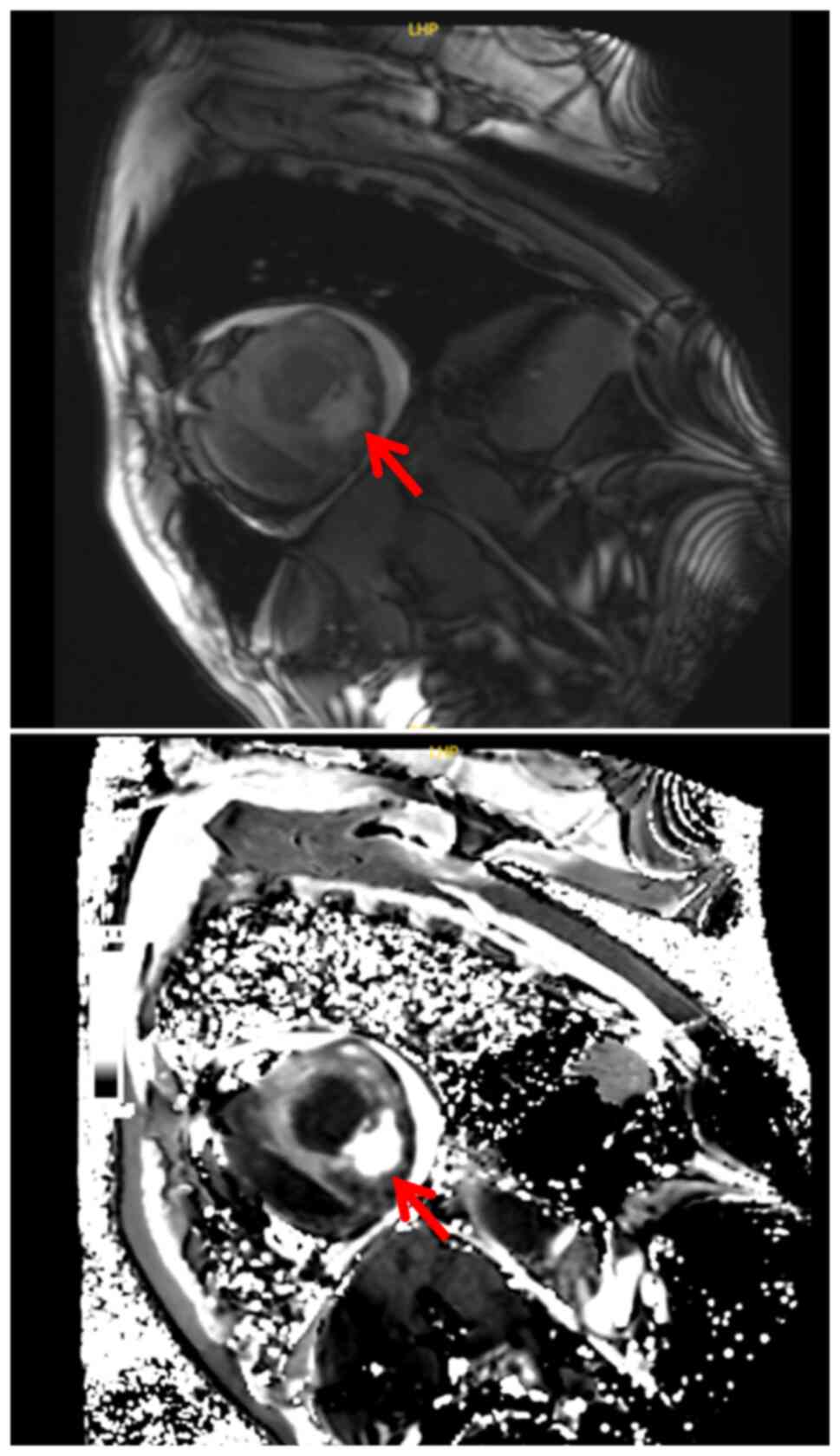
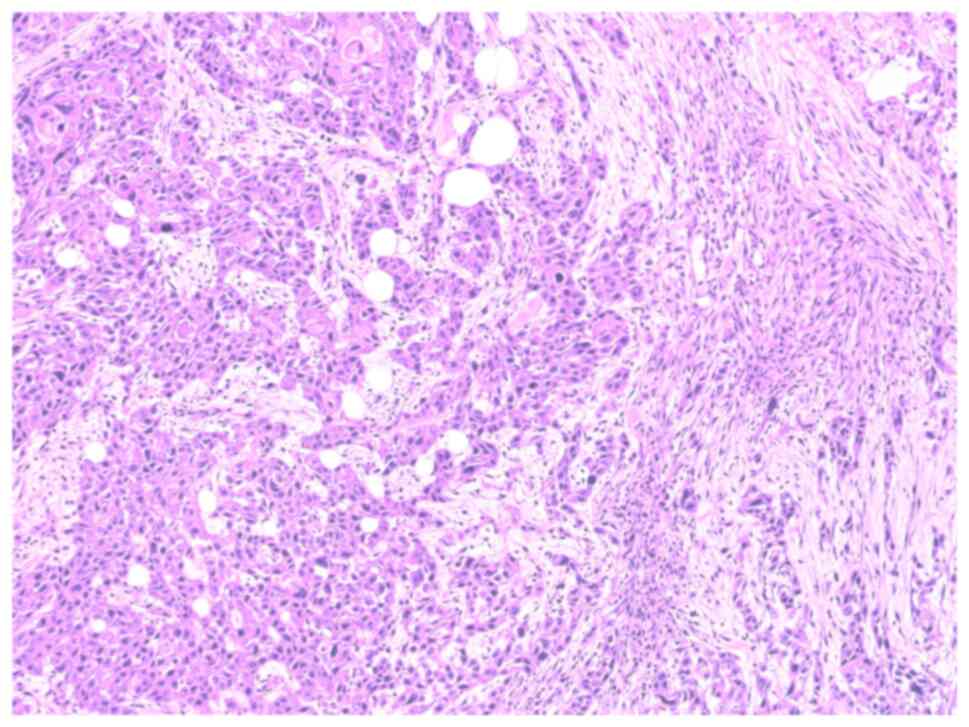
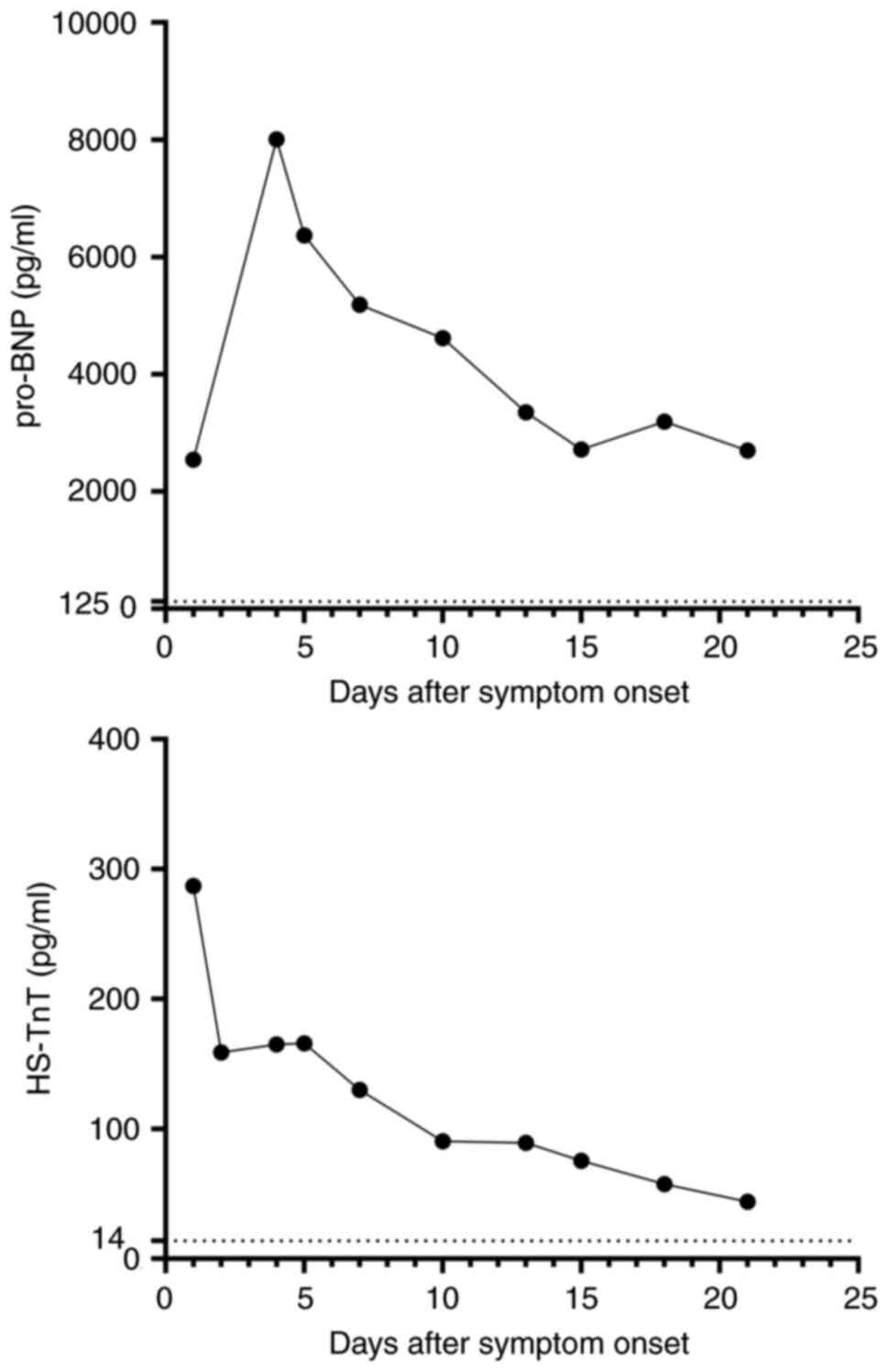
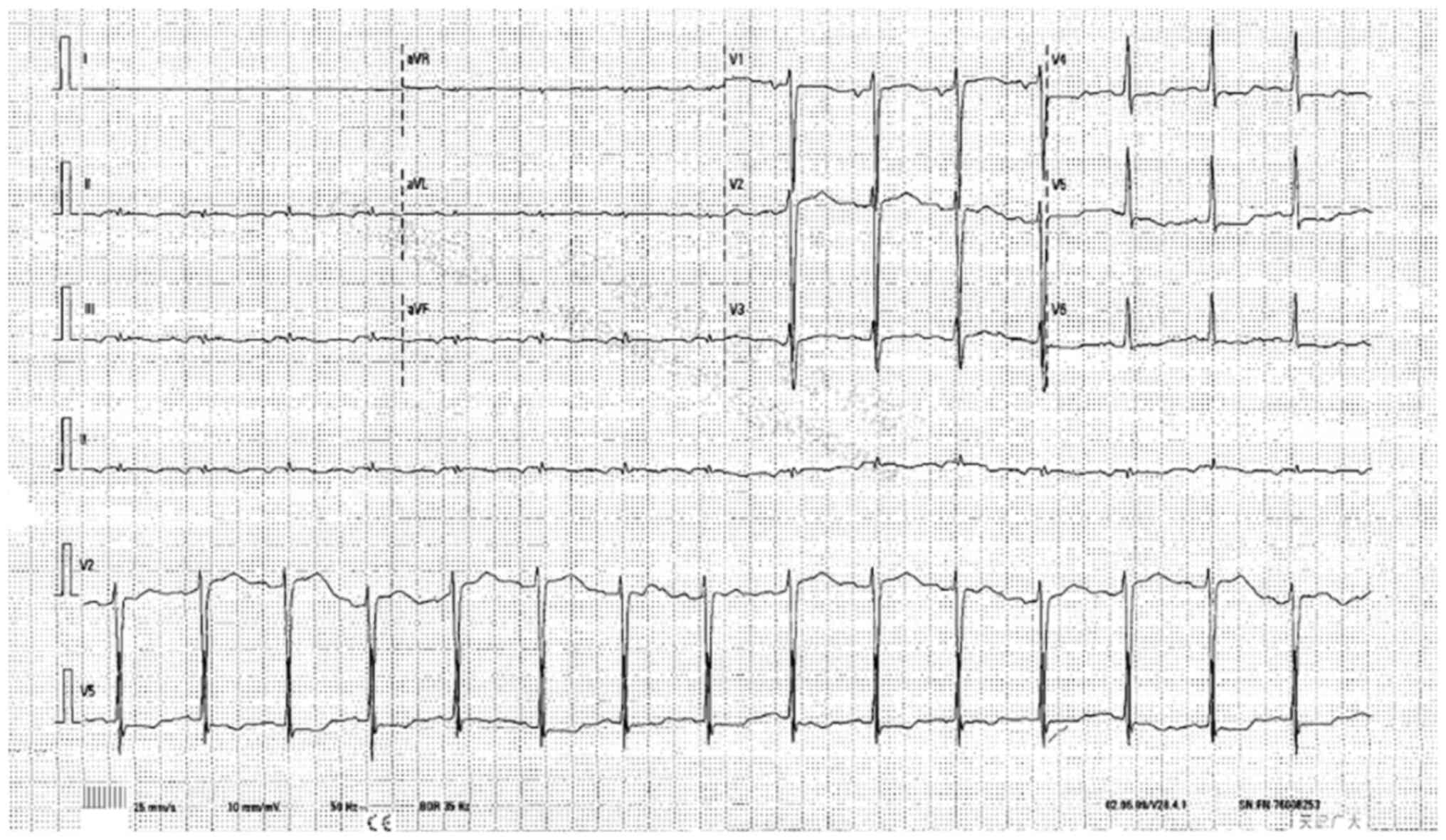
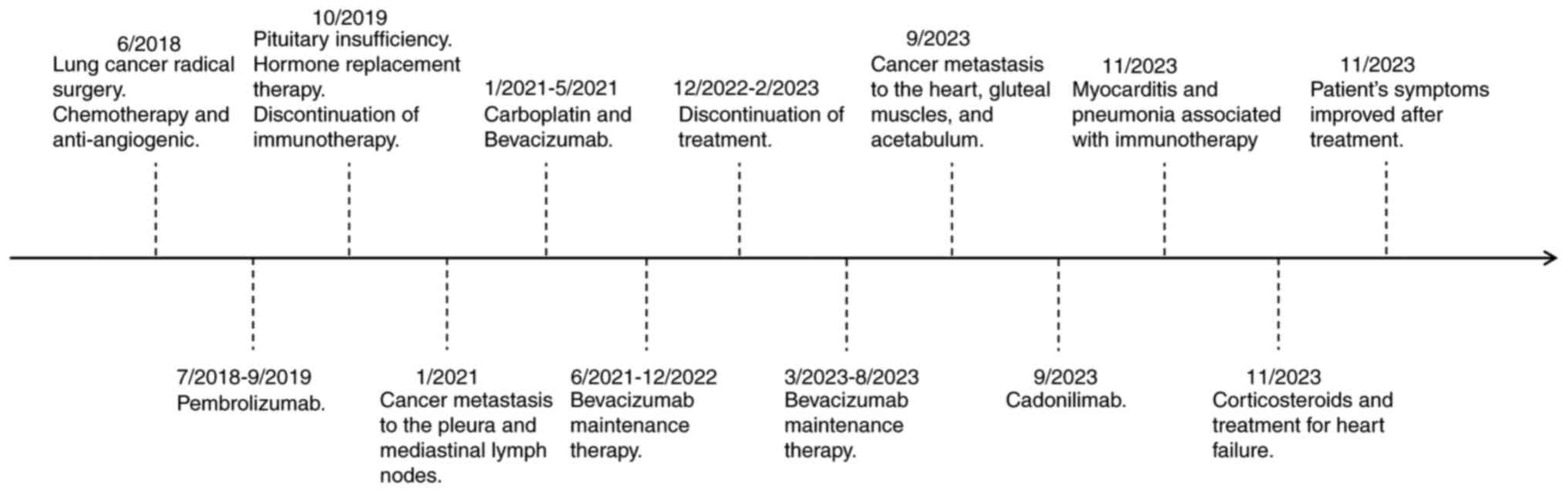
|
1
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao Y, Ma Y, Fan Y, Zhou J, Yang N, Yu Q,
Zhuang W, Song W, Wang ZM, Li B, et al: A multicenter, open-label
phase Ib/II study of cadonilimab (anti PD-1 and CTLA-4 bispecific
antibody) monotherapy in previously treated advanced non-small-cell
lung cancer (AK104-202 study). Lung Cancer. 184:1073552023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
et al: Lung. AJCC cancer staging manual. 8th Edition. Springer
International Publishing; Cham, Switzerland: pp. 4472017
|
|
4
|
Garon EB, Hellmann MD, Rizvi NA, Carcereny
E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L,
et al: Five-year overall survival for patients with advanced
non-small-cell lung cancer treated with pembrolizumab: Results from
the phase I KEYNOTE-001 study. J Clin Oncol. 37:2518–2527. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrara R, Imbimbo M, Malouf R,
Paget-Bailly S, Calais F, Marchal C and Westeel V: Single or
combined immune checkpoint inhibitors compared to first-line
platinum-based chemotherapy with or without bevacizumab for people
with advanced non-small cell lung cancer. Cochrane Database Syst
Rev. 4:Cd0132572021.PubMed/NCBI
|
|
6
|
An E, Lu J and Chen L: Good response of
cadonilimab (PD-1/CTLA-4 bi-specific antibody) to patients with
advanced lung adenocarcinoma after immunotherapy resistance: A case
report. Asian J Surg. 47:3348–3349. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei SC, Meijers WC, Axelrod ML, Anang NAS,
Screever EM, Wescott EC, Johnson DB, Whitley E, Lehmann L, Courand
PY, et al: A genetic mouse model recapitulates immune checkpoint
inhibitor-associated myocarditis and supports a mechanism-based
therapeutic intervention. Cancer Discov. 11:614–625. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atallah-Yunes SA, Kadado AJ, Kaufman GP
and Hernandez-Montfort J: Immune checkpoint inhibitor therapy and
myocarditis: A systematic review of reported cases. J Cancer Res
Clin Oncol. 145:1527–1557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Axelrod ML, Meijers WC, Screever EM, Qin
J, Carroll MG, Sun X, Tannous E, Zhang Y, Sugiura A, Taylor BC, et
al: T cells specific for α-myosin drive immunotherapy-related
myocarditis. Nature. 611:818–826. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moslehi J, Lichtman AH, Sharpe AH,
Galluzzi L and Kitsis RN: Immune checkpoint inhibitor-associated
myocarditis: Manifestations and mechanisms. J Clin Invest.
131:e1451862021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palaskas N, Lopez-Mattei J, Durand JB,
Iliescu C and Deswal A: Immune checkpoint inhibitor myocarditis:
Pathophysiological characteristics, diagnosis, and treatment. J Am
Heart Assoc. 9:e0137572020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ali A, Caldwell R, Pina G, Beinart N,
Jensen G, Yusuf SW, Koutroumpakis E, Hamzeh I, Khalaf S, Iliescu C,
et al: Elevated IL-6 and tumor necrosis factor-α in immune
checkpoint inhibitor myocarditis. Diseases. 12:882024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Varricchi G, Galdiero MR and Tocchetti CG:
Cardiac toxicity of immune checkpoint inhibitors: Cardio-oncology
meets immunology. Circulation. 136:1989–1992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu H, Galdos FX, Lee D, Waliany S, Huang
YV, Ryan J, Dang K, Neal JW, Wakelee HA, Reddy SA, et al:
Identification of pathogenic immune cell subsets associated with
checkpoint inhibitor-induced myocarditis. Circulation. 146:316–335.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisner RM, Husain A and Clark JI: Case
report and brief review: IL-2-induced myocarditis. Cancer Invest.
22:401–404. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma P, Liu J, Qin J, Lai L, Heo GS,
Luehmann H, Sultan D, Bredemeyer A, Bajapa G, Feng G, et al:
Expansion of pathogenic cardiac macrophages in immune checkpoint
inhibitor myocarditis. Circulation. 149:48–66. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frascaro F, Bianchi N, Sanguettoli F,
Marchini F, Meossi S, Zanarelli L, Tonet E, Serenelli M, Guardigli
G, Campo G, et al: Immune checkpoint inhibitors-associated
myocarditis: Diagnosis, treatment and current status on
rechallenge. J Clin Med. 12:77372023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salem JE, Allenbach Y, Vozy A, Brechot N,
Johnson DB, Moslehi JJ and Kerneis M: Abatacept for severe immune
checkpoint inhibitor-associated myocarditis. N Engl J Med.
380:2377–2379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang F, Liu Y, Xu W, Zhang C, Lv J and Ma
S: Fulminant myocarditis induced by immune checkpoint inhibitor
nivolumab: A case report and review of the literature. J Med Case
Rep. 15:3362021. View Article : Google Scholar : PubMed/NCBI
|