Introduction
Immune checkpoint inhibitors (ICIs) are antibodies
that block negative regulators of T-cell immune responses,
particularly programmed death 1 (PD-1) and cytotoxic T-lymphocyte
activator 4 (CTLA-4). Although ICIs are increasingly recommended as
standard therapies for the treatment of several types of cancer,
such as lung, esophageal and colorectal cancer, their
immune-enhancing effects can result in a wide range of
immune-related adverse events (irAEs), including cardiovascular
toxicity (1). Notably, it has been
reported that combination ICI therapy targeting both PD-1 and
CTLA-4 is considered as a major risk factor for the development of
ICI-related myocarditis. Cadonilimab, a bispecific tumor
immunotherapy drug, can simultaneously target PD-1 and CTLA-4
(2). Although cadonilimab exhibits
a more favorable safety profile compared with the conventional
PD-1/CTLA-4 combination therapy, myocarditis is still listed as a
potential adverse effect in its prescribing information.
Cadonilimab, as a novel dual ICI, has limited post-marketing
experience in the field of lung cancer. To the best of our
knowledge, the present study represents the first documented case
of immune-mediated myocarditis induced by this agent in patients
with malignant lung tumors, which renders this case particularly
distinctive. The present study aims to share these clinical
experiences to facilitate medical practice. Notably, this case also
exhibited concurrent cardiac metastasis, a convergence of multiple
rare events creating an exceptional clinical scenario. Whether
cardiac metastasis potentiates the risk of immune-mediated
myocarditis remains speculative and warrants further investigation
for validation.
Case report
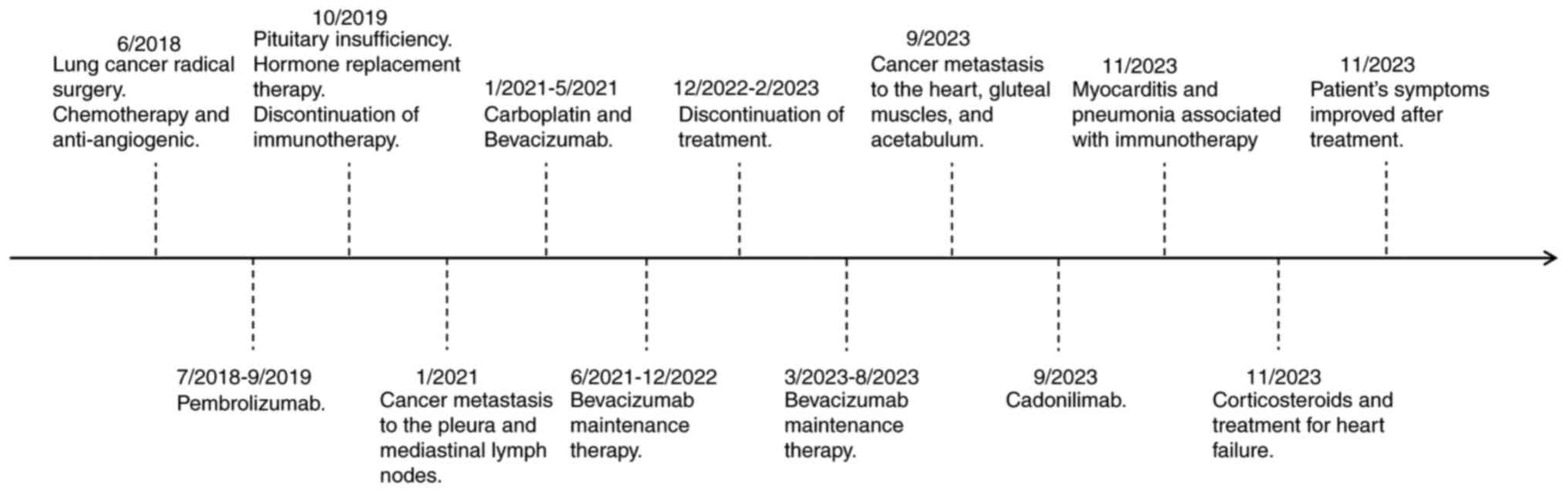
The present study reports the case of a 49-year-old
male previously diagnosed with TNM stage IIIA lung cancer (pT3N2M0)
(3), accompanied by multiple lymph
node metastases in the mediastinum and bilateral lungs. The
patient, who had undergone a radical lung cancer resection in June
2018, was admitted to Shandong Provincial Hospital (Shandong,
China) in November 2023 due to persistent dyspnea and paroxysmal
cough following 2 cycles of cadonilimab (625 mg administered via
intravenous infusion every 3 weeks). The patient had no notable
family or social history, and no prior history of heart disease. A
right upper lobectomy was performed in June 2018, and the
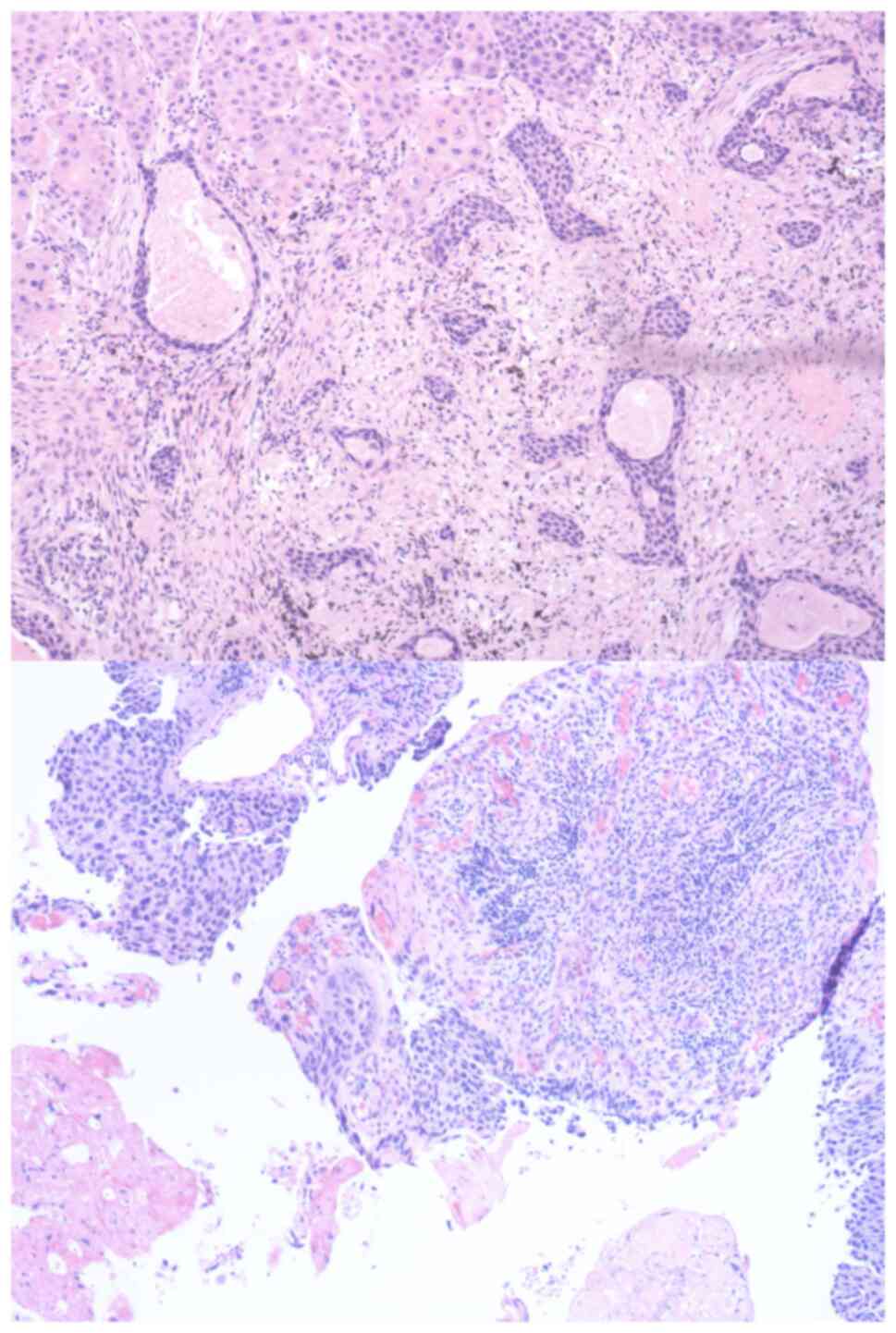
postoperative pathological examination confirmed non-small cell
lung cancer (NSCLC), while the immunohistochemical results revealed
programmed death ligand 1 (PD-L1) positivity (Fig. 1). The surgical intervention,
postoperative pathological diagnosis and subsequent
immunohistochemical analyses were all conducted at Shandong
Provincial Hospital. The patient was postoperatively treated with a
chemotherapy regimen that included pemetrexed and lobaplatin. The
patient underwent postoperative adjuvant chemotherapy consisting of
pemetrexed (800 mg) and lobaplatin (100 mg) administered via
intravenous infusion. The treatment regimen comprised 3 cycles,
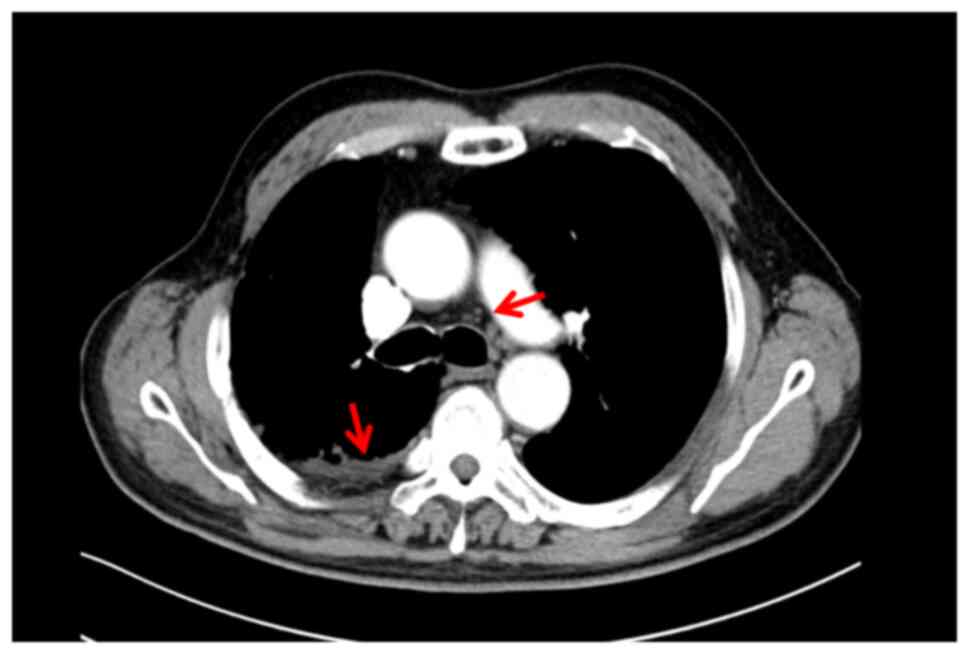
with each cycle repeated every 21 days. During treatment, a
follow-up chest computed tomography (CT) scan revealed a
right-sided pleural effusion (Fig.
2), thus prompting a change of the treatment protocol.
Therefore, the treatment was changed to pemetrexed and carboplatin
chemotherapy combined with pembrolizumab and Endostar. The
treatment protocol comprised one cycle of pemetrexed (800 mg) and
carboplatin (500 mg) chemotherapy combined with pembrolizumab (200
mg intravenously every 3 weeks for 7 cycles) plus recombinant human
endostatin (30 mg/day via continuous intravenous infusion pump for
7 days). In October 2019, the patient exhibited decreased cortisol
levels: 13.61 nmol/l at 8:00 am (reference range, >166 nmol/l)
and 11.83 nmol/l at 4:00 pm (reference range, >73.8 nmol/l).
Therefore, following consultation with the Department of
Endocrinology, immunotherapy was discontinued, and hydrocortisone
replacement therapy was initiated with hydrocortisone at a dosage
of 30 mg daily (20 mg at 8:00 am and 10 mg at 2:00 pm). Under the
guidance of the Department of Endocrinology, the patient was
transitioned to routine outpatient follow-up management at a
frequency of once per month.
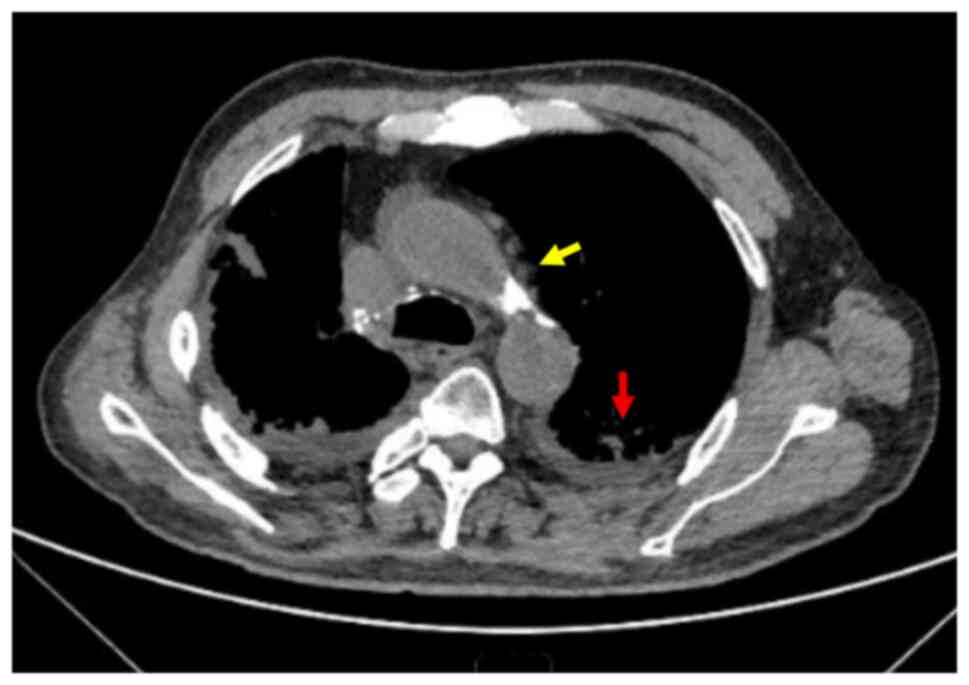
In January 2021, a CT scan showed nodular thickening
of the left interlobar pleura and partial enlargement of multiple
lymph nodes in the mediastinum and hilum of both lungs, suggestive
of metastatic disease (Fig. 3). Due
to the patient's prior development of grade III pituitary
dysfunction following immunotherapy, it was decided that
immunotherapy with ICIs should not be restarted. Between January
2021 and May 2021, the patient completed six treatment cycles
consisting of paclitaxel (400 mg), carboplatin (500 mg) and
bevacizumab (400 mg) administered intravenously every 3 weeks,
while maintaining the original hydrocortisone replacement regimen.
Following contraindication evaluation, three additional cycles were
administered in March, April and May 2023 using a modified protocol
of nab-paclitaxel (450 mg) and bevacizumab (500 mg) delivered via
intravenous infusion at 3-week intervals.
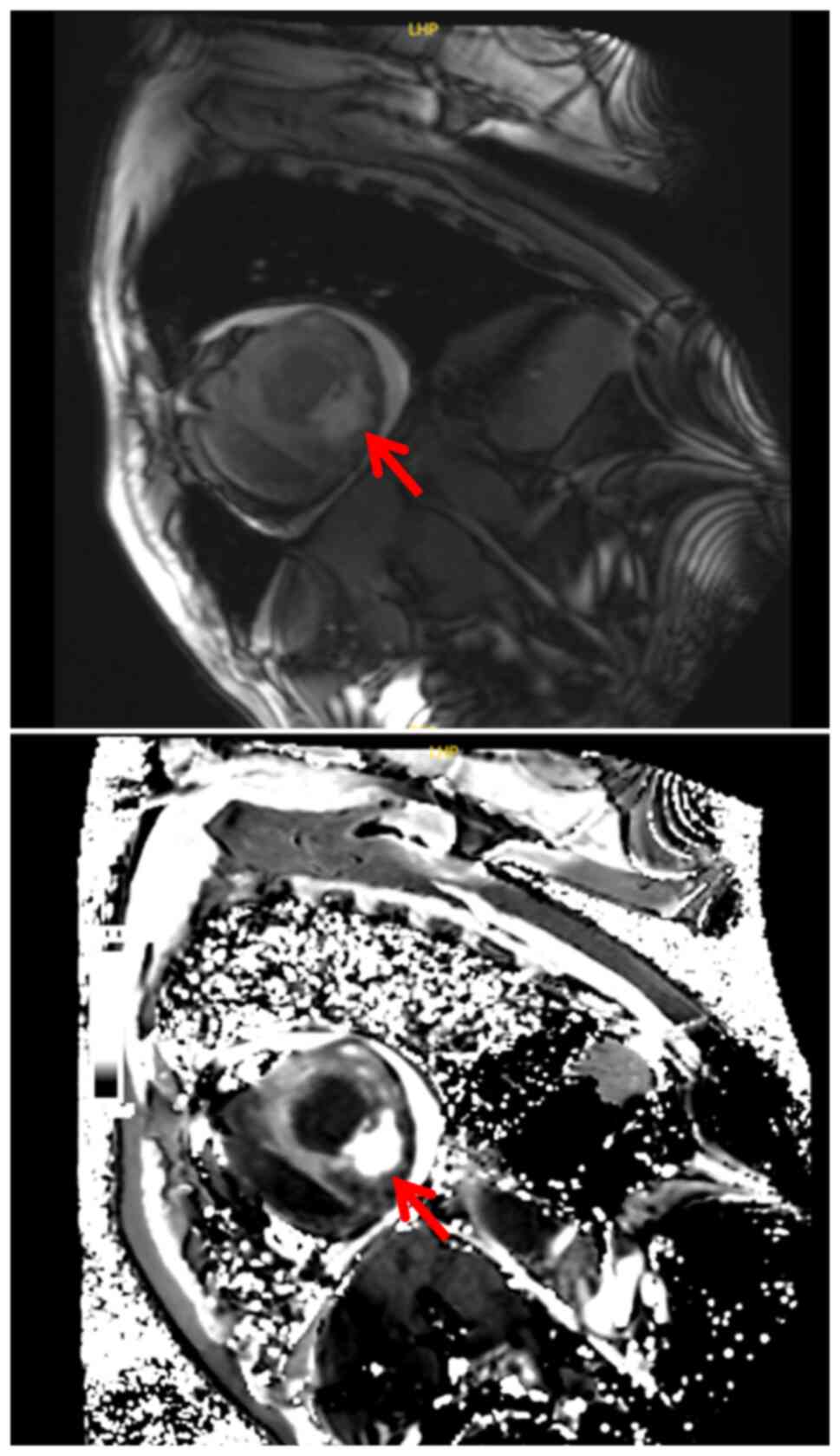
In September 2023, cardiac magnetic resonance
imaging (MRI) revealed localized thickening of the left and right
ventricular myocardium, with diffuse abnormal signal intensity and
contrast enhancement, accompanied by a significant amount of
pericardial effusion. These findings were suggestive of metastatic
disease (Fig. 4), and the patient
was considered to exhibit progressive disease. Due to the
considerable challenges in obtaining myocardial biopsy specimens
from the metastatic lesions, the diagnosis was primarily based on
imaging findings. Pelvic MRI performed at an external hospital
revealed signs of metastasis in the soft tissues of the gluteal
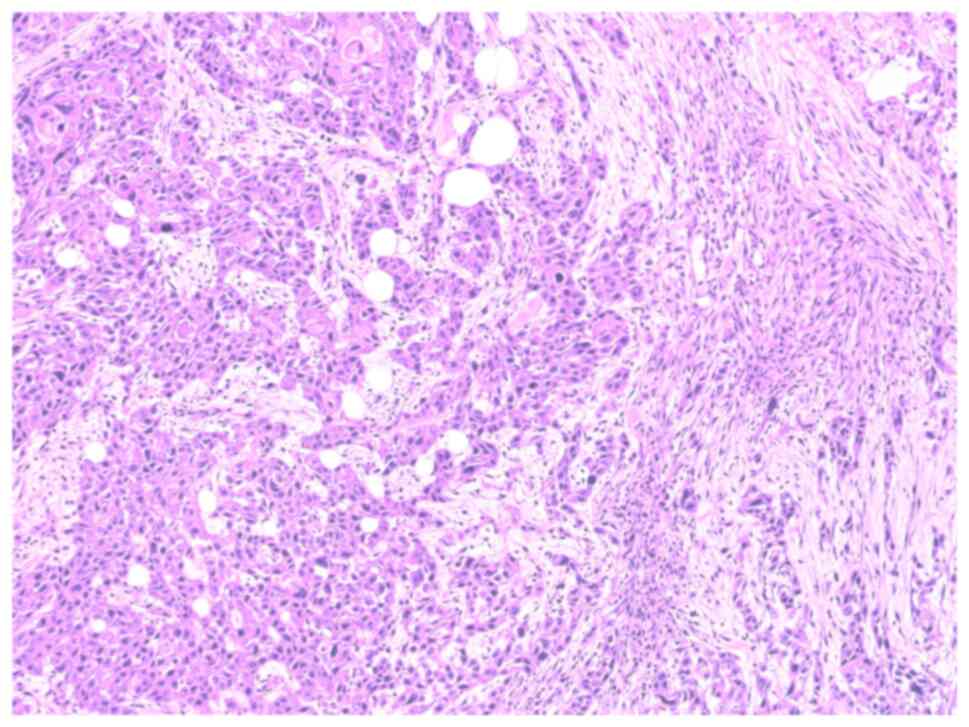
muscles and the right acetabulum. A biopsy of the gluteal
soft-tissue mass was subsequently performed at Shandong Provincial
Hospital, with pathological examination confirming squamous cell
carcinoma (Fig. 5). Given the
patient's prior resistance to single-agent ICI therapy and in
accordance with the patient's own preferences, cadonilimab was
chosen as the second-line treatment regimen. Following risk
assessment and according to the patient's wishes, treatment with
the PD-1/CTLA-4 bispecific antibody cadonilimab in combination with
single-agent chemotherapy and anti-angiogenic therapy was initiated
in September 2023. The patient was hospitalized for treatment
during the same month. The treatment protocol consisted of
gemcitabine (1.6 g) combined with anlotinib (8 mg) and cadonilimab
(625 mg) administered via intravenous infusion every 3 weeks.
At 2 months post-cadonilimab treatment initiation,
in November 2023, the patient experienced persistent dyspnea, a
paroxysmal cough and expectoration of small amounts of white mucoid
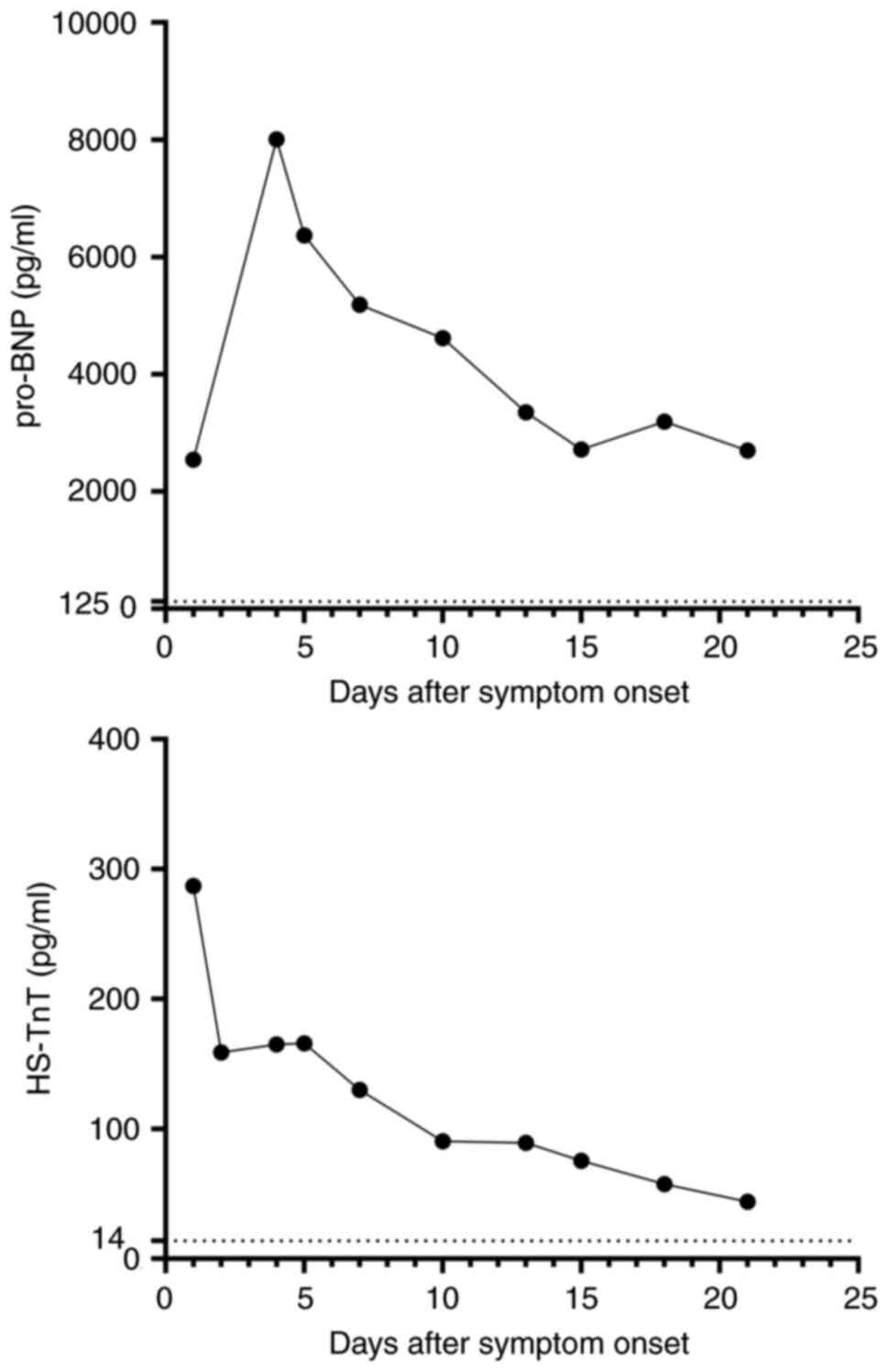
sputum. Laboratory tests showed elevated high-sensitivity cardiac
troponin T (HS-TnT) levels at 287.00 pg/ml (reference range, <14
pg/ml). The patient's pro-B-type natriuretic peptide (pro-BNP)
levels were elevated at 2,543.00 pg/ml (reference range, <125
pg/ml) (Fig. 6). Based on the
patient's treatment history with cadonilimab, reported irAEs and
imaging findings, immune-related pneumonia, potentially combined
with immune myocarditis, was suspected.
On the following days, the patient's cardiac
biomarkers continued to fluctuate with an upward trend.
Transthoracic echocardiogram revealed a left ventricular ejection
fraction of 53%. Serological testing did not indicate the presence
of myocarditis-related viral infections. Considering the temporal
association with cadonilimab initiation, a multidisciplinary team
consultation supported a preliminary diagnosis of secondary immune
cadonilimab-induced myocarditis.
The patient continued to experience symptoms of
shortness of breath, paroxysmal cough and the production of small
amounts of white mucoid sputum. Given the rapid progression
commonly associated with irAEs, the patient received 160 mg
methylprednisolone daily. Subsequently, the patient showed
significant clinical improvement, with reduced coughing and
dyspnea, and only an occasional dry cough. Laboratory tests
revealed elevated C-reactive protein (CRP) and human serum amyloid
A levels at 128.30 mg/l (reference range, <10 mg/l) and 279.73
mg/l (reference range, <10 mg/l), respectively. Procalcitonin
and interleukin-6 (IL-6) levels were also measured, with the
results showing mildly elevated procalcitonin levels at 0.26 ng/ml
(reference range, <0.05 ng/ml). Considering the improvement in
the patient's symptoms and the decrease in HS-TnT levels to 287.00
pg/ml (reference range, <14 pg/ml), the preliminary diagnosis of
immune-related pneumonia and immune myocarditis was established.
Therefore, the dose of methylprednisolone was increased to 200
mg/day.
Another 2 days later, the patient experienced a
worsening cough, persistent chest tightness and dyspnea. Continuous
oxygen therapy was therefore administered via nasal cannula. A
respiratory rate of 22–25 breaths/min (reference range, 16–20
breaths/min), a heart rate of 86 beats/min (reference range, 60–100
beats/min) and elevated CRP levels at 24.45 mg/l (reference range,
<10 mg/l) were recorded. Despite improvement in infection
markers, pro-BNP levels were significantly increased at 8,010 pg/ml
(reference range, <125 pg/ml.), while the bilateral lung rales
were reduced. Considering the overall clinical picture, the
exacerbation of dyspnea was primarily attributed to newly developed
heart failure. Due to the severity of the patient's dyspnea,
continuous oxygen therapy was required. Additionally, diuretic
therapy with furosemide (20 mg daily) was initiated and maintained
for 3 days until clinical symptom resolution. A cardiology
consultation was obtained, and based on the consequent
recommendations, a low-dose diuretic regimen was initiated,
comprising hydrochlorothiazide (12.5 mg administered orally once
daily), spironolactone (10 mg administered orally once daily) and
coenzyme Q10 (10 mg administered orally three times daily).
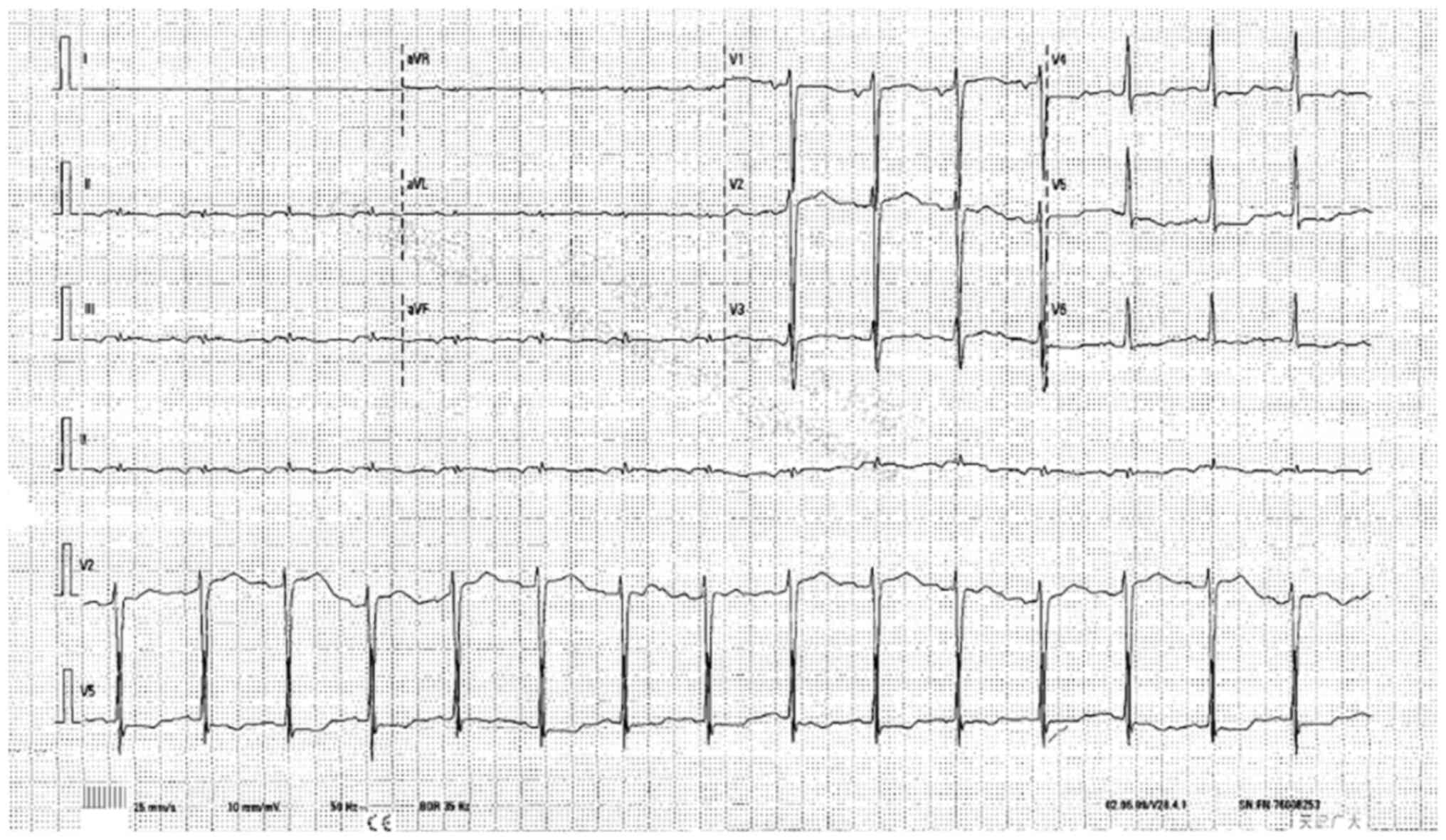
Electrocardiography revealed a possible atrial ectopic rhythm, with
ST-T abnormalities in the anterior wall, suggesting myocardial
ischemia (Fig. 7). A follow-up
chest CT scan showed significant improvement in the bilateral
pneumonia compared with the that in previous scans.
After 3 days of furosemide diuretic therapy, the
patient's respiratory-related symptoms were significantly improved.
The diagnosis of heart failure was ultimately confirmed based on
symptomatic relief and the corresponding reduction in NT-proBNP
levels to 6,371 pg/ml (reference range, <125 pg/ml) following
diuretic therapy. No significant changes in HS-TnT levels were
reported. According to the recommendations from the cardiology
consultation, the dose of methylprednisolone was increased to 280
mg, while the oral administration of furosemide and spironolactone
was continued. Ongoing monitoring of pro-BNP levels and cardiac
biomarkers was advised, along with regular assessment of albumin
and electrolyte levels. On the same day, routine cardiac ultrasound
revealed a solid myocardial mass, suggestive of tumor
metastasis.
After 5 days of furosemide diuretic therapy, the
patient reported resolution of chest tightness and dyspnea, with
only an occasional cough and minor production of white mucoid
sputum. Laboratory examinations demonstrated elevated pro-BNP
levels at 5,185.00 pg/ml (reference range, <25 pg/ml), and
HS-TnT levels at 130.00 pg/ml (reference range, <14 pg/ml). The
patient exhibited decreased serum calcium and potassium levels.
Despite the aforementioned abnormal laboratory results, the
clinical condition of the patient continued to improve, with
pro-BNP levels showing a downward trend, thus allowing the
continuation of diuretic therapy. However, since diuretic therapy
led to hypoalbuminemia, the patient received artificial albumin.
Given the persistently increased HS-TnT levels, the management plan
included continuation of 280 mg of methylprednisolone once daily
for 3 more days.
After 8 days of furosemide diuretic therapy, the
patient was clinically stable, with no fever, notable coughing,
sputum production or symptoms of chest tightness and dyspnea.
Potassium levels increased from 2.72 to 4.12 mmol/l (reference
range, 3.5–5.3 mmol/l) and albumin levels increased from 26.8 to
31.1 g/l (reference range, 40–55 g/l), and therefore the
intravenous administration of artificial albumin was discontinued,
while oral potassium citrate granules were continued for potassium
replacement. The serum levels of HS-TnT and pro-BNP continued to
decline, and the dose of methylprednisolone was reduced to 200 mg.
The other treatment regimens remained unchanged.
At 12 days after the initial onset of the persistent
dyspnea and paroxysmal cough, the patient's immune myocarditis and
pneumonia were under control, and the clinical condition was
stable. Following a multidisciplinary discussion with the
Department of Cardiology, it was decided that no further adjustment
to the corticosteroid therapy was necessitated. Anti-inflammatory
and diuretic treatment was continued.
At 14 days after the initial onset of the persistent
dyspnea and paroxysmal cough, the patient reported a recurrence of
significant dyspnea. The levels of HS-TnT had increased to 75.50
pg/ml. Further laboratory examinations revealed pro-BNP levels at
2,719.00 pg/ml (Fig. 6). Despite
the aforementioned symptoms, a follow-up examination indicated
improvement in cardiac function, and therefore the dose of
methylprednisolone was reduced to 160 mg once daily.
At 17 days after the initial onset of the persistent
dyspnea and paroxysmal cough, evaluation of the cardiac biomarkers
showed HS-TnT levels at 57.50 pg/ml (reference range, <14 pg/ml)
and pro-BNP levels at 3,191.00 pg/ml (reference range, <125
pg/ml) (Fig. 6). Due to persistent
hypoalbuminemia, hypocalcemia and hypokalemia, oral potassium and
calcium supplementation was continued, while the dose of
methylprednisolone was further reduced to 120 mg once daily.
20 days after the initial onset of persistent
dyspnea and paroxysmal cough, significant improvement in the levels
of the three cardiac biomarkers was observed, with HS-TnT and
pro-BNP levels at 43.90 pg/mland 2,701.00 pg/ml, respectively
(Fig. 6). Since the clinical
condition of the patient remained stable, with largely controlled
immune myocarditis and pneumonia, and no indications for further
antitumor therapy, the patient was discharged with instructions for
regular home monitoring (Fig. 8).
Regarding steroid therapy, the patient received 80 mg prednisone
once daily for 1 week, followed by a weekly reduction of 20 mg
until discontinuation after 4 weeks.
Discussion
The present study reports the case of a patient with
lung cancer who developed irAEs, including both pneumonitis and
myocarditis induced by the bispecific ICI cadonilimab, concurrently
with cardiac metastasis, a relatively uncommon clinical
manifestation. The patient successfully recovered following
treatment with glucocorticoids and diuretics for heart failure,
combined with symptomatic management. Currently, to the best of our
knowledge, there are no other reports on cadonilimab-induced immune
myocarditis. In recent years, immunotherapy has fundamentally
changed the treatment strategy for NSCLC (4). The use of PD-1/PD-L1 inhibitors
combined with anti-CTLA-4 antibodies as first-line therapy has
significantly improved survival outcomes in patients with advanced
NSCLC compared with platinum-based chemotherapy alone (5). Cadonilimab is a PD-1/CTLA-4 bispecific
tumor immunotherapy drug that can simultaneously bind to PD-1 and
CTLA-4, thus inhibiting both immune checkpoint pathways. A clinical
trial indicated that in patients with metastatic NSCLC, cadonilimab
showed notable efficacy as a second-line monotherapy after the
failure of platinum-based double chemotherapy and initial
immunotherapy, with outcomes similar to those of other ICIs used
after first-line chemotherapy (2).
Furthermore, a reported case of immunotherapy-resistant advanced
lung adenocarcinoma demonstrated favorable therapeutic efficacy
with cadonilimab combined with chemotherapy (6).
However, in particular cases, the therapeutic
benefits of ICIs can be offset by the development of severe irAEs.
For instance, myocarditis is a rare but serious side effect of
ICIs, characterized by a high mortality rate of 39.7% (1). Compared with combination ICI therapy,
cadonilimab exhibits a more favorable safety profile. However, the
prescribing information of the drug still indicates a potential
risk of inducing immune myocarditis. ICIs targeting CTLA-4 or
PD-1/PD-L1 are associated with the development of several irAEs,
including immune-mediated pneumonia, immune-mediated colitis and
myocarditis. A previous study demonstrated that PD-1/CTLA-4
combination therapy-related mortality was typically attributed to
colitis (32/86 of reported cases; mortality rate, 37%) and
myocarditis (22/88 of reported cases; mortality rate, 25%), with
myocarditis showing the highest fatality rate (52/131 of reported
cases; mortality rate, 39.7%) (7).
The majority of cases of myocarditis and related mortality occur
early after the initiation of ICI therapy (8). The median time to onset of myocarditis
is ~27 days (range, 5–155 days) (1). Combination ICI therapy is a major risk
factor for ICI-related myocarditis, with a higher incidence of
myocarditis being reported in patients receiving combination
therapy compared with that in patients treated with CTLA-4 or
PD-1/PD-L1 monotherapy (8).
Cumulative evidence has demonstrated that combined CTLA-4 and PD-1
inhibition represents the primary risk factor for immune checkpoint
inhibitor-induced myocarditis. Both CTLA-4 and PD-1 can inhibit
T-cell activation. However, they act through different cellular and
molecular mechanisms (9).
Therefore, the increased incidence of myocarditis observed with
combination therapy could be attributed to the additive
immunomodulating effects of targeting both biological pathways or
from the functional interactions between CTLA-4 and PD-1 that
exacerbate the development of myocarditis (10). The management of ICI-induced
myocarditis typically involves high-dose corticosteroids, which
have been shown to reduce the risk of major adverse cardiac events
(11). In the current case,
high-dose methylprednisolone treatment was administered, resulting
in a favorable clinical recovery.
The patient exhibited elevated IL-6 levels 1 day
after the onset of myocarditis symptoms. A single-center
retrospective cohort study indicated that IL-6 and tumor necrosis
factor-α were the most commonly elevated cytokines in ICI-related
myocarditis, thus supporting the diagnostic potential of these
markers (12). Emerging evidence
has highlighted the presence of shared antigens or high-frequency
T-cell receptor sequences among the myocardium, skeletal muscle and
tumor tissues (13), thus
suggesting that cadonilimab could target myocardial cells while
exerting its antitumor effects. The loss of CTLA-4/PD-1 axis
function could also result in the development of autoimmune
myocarditis and dilated cardiomyopathy, indicating that these
molecules could serve a key role in preventing autoimmunity
(9). Furthermore, PD-L1 gene
deletion and anti-PD-L1 treatment could promote the progression of
transient myocarditis into a fatal form, thus implying that
PD-1/PD-L1 and CTLA-4 could play a crucial role in limiting T
cell-mediated autoimmune myocarditis (9,10).
In patients with ICI-related myocarditis, expansion
of cytotoxic CD8+ T effector cells, and more
particularly CD45RA cells (Temra CD8+ cells), has been
reported. Transcriptomic analyses of these Temra CD8+
clones demonstrated a highly activated and cytotoxic phenotype
(9). It was therefore speculated
that following treatment with ICIs, activated T cells could not
only recognize tumor antigens, but also shared myocardial antigens,
thereby triggering myocarditis (14). Furthermore, it has been also
reported that cadonilimab can effectively induce the secretion of
IL-2 and IFN-γ, which may contribute to myocardial damage. Notably,
high doses of IL-2 have been associated with severe cardiac
toxicity. In a study on IL-2-induced cardiac toxicity, among 57
patients receiving high-dose IL-2 therapy, 2 cases (3.5%) developed
IL-2-induced myocarditis (15). In
the present case, following multidisciplinary consultation and
considering the patient's condition, a high-dose corticosteroid
regimen was chosen, resulting in a favorable recovery. However, in
more severe cases of immune myocarditis, high-dose corticosteroids
alone may not effectively reverse disease deterioration, thus
requiring more effective therapeutic approaches. A study
establishing a murine ICI-induced myocarditis model demonstrated
that depletion of CD8+ T cells or macrophages, combined
with IFN-γ signaling blockade, significantly reduced cardiac
infiltration of C-X-C motif chemokine ligand-expressing
(CXCL9+ and CXCL10+) macrophages, thereby
attenuating myocarditis progression. This therapeutic approach may
confer translational implications for future myocarditis
management. ICI-related myocarditis is associated with the
expansion of a specific population of inflammatory IFN-γ-induced
macrophages, thus suggesting that IFN-γ blockade could be a
potential therapeutic approach for this condition (16). In a previous study, treatment with
CTLA4-immunoglobulin (CTLA4-Ig) successfully rescued mice with
lethal myocarditis developed in Ctla4+/−
Pdcd1−/− mice. The severity of myocarditis was found to
be gene dosage-dependent, thus suggesting that restoring CTLA4
and/or PD-1 signaling could be sufficient to prevent disease
progression. Abatacept, a recombinant CTLA4-Ig, could significantly
reduce mortality in Ctla4+/− Pdcd1−/− mice
(17). Therefore, CTLA4-Ig could
hold therapeutic potential in treating severe refractory
ICI-related myocarditis. Additionally, a case report on a patient
with corticosteroid-refractory ICI-related myocarditis showed that
treatment with abatacept resulted in favorable clinical outcomes
(18). In addition, in another case
of severe fulminant myocarditis induced by nivolumab, an ICI,
extracorporeal membrane oxygenation was also an effective
therapeutic option in life-threatening situations (19). The present case report serves as an
important reminder for clinicians. Particular vigilance is needed
when administering ICIs to patients with cardiac metastasis, since
they can be at enhanced risk for developing irAEs, such as
myocarditis.
In conclusion, ICIs have the potential to induce
severe irAEs. More particularly, with ICIs, such as cadonilimab,
early recognition and timely treatment are crucial for improving
clinical outcomes. Close monitoring of the patient's early response
to immunotherapy is essential, along with awareness of potential
risk factors, such as cardiac metastases. In terms of treatment,
high-dose corticosteroids are considered as the cornerstone of
treatment of several irAEs, including myocarditis. Based on our
clinical experience, we recommend that treatment plans should be
developed through multidisciplinary consultations to ensure prompt
management of irAEs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TL, ZY, QL and RZ were responsible for analyzing
patient data and treatment administration. TL, BZ, ZK and PZ
contributed to advising on patient treatment and obtaining medical
images. TL wrote the manuscript. All authors have read and approved
the final version of the manuscript. TL, ZY, QL, RZ, BZ, ZK and PZ
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ICI
|
immune checkpoint inhibitors
|
|
irAEs
|
immune-related adverse events
|
|
CTLA-4
|
cytotoxic T-lymphocyte activator 4
|
|
PD-1
|
programmed death 1
|
|
PD-L1
|
programmed death ligand 1
|
|
NSCLC
|
non-small cell lung cancer
|
References
|
1
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao Y, Ma Y, Fan Y, Zhou J, Yang N, Yu Q,
Zhuang W, Song W, Wang ZM, Li B, et al: A multicenter, open-label
phase Ib/II study of cadonilimab (anti PD-1 and CTLA-4 bispecific
antibody) monotherapy in previously treated advanced non-small-cell
lung cancer (AK104-202 study). Lung Cancer. 184:1073552023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
et al: Lung. AJCC cancer staging manual. 8th Edition. Springer
International Publishing; Cham, Switzerland: pp. 4472017
|
|
4
|
Garon EB, Hellmann MD, Rizvi NA, Carcereny
E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L,
et al: Five-year overall survival for patients with advanced
non-small-cell lung cancer treated with pembrolizumab: Results from
the phase I KEYNOTE-001 study. J Clin Oncol. 37:2518–2527. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrara R, Imbimbo M, Malouf R,
Paget-Bailly S, Calais F, Marchal C and Westeel V: Single or
combined immune checkpoint inhibitors compared to first-line
platinum-based chemotherapy with or without bevacizumab for people
with advanced non-small cell lung cancer. Cochrane Database Syst
Rev. 4:Cd0132572021.PubMed/NCBI
|
|
6
|
An E, Lu J and Chen L: Good response of
cadonilimab (PD-1/CTLA-4 bi-specific antibody) to patients with
advanced lung adenocarcinoma after immunotherapy resistance: A case
report. Asian J Surg. 47:3348–3349. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei SC, Meijers WC, Axelrod ML, Anang NAS,
Screever EM, Wescott EC, Johnson DB, Whitley E, Lehmann L, Courand
PY, et al: A genetic mouse model recapitulates immune checkpoint
inhibitor-associated myocarditis and supports a mechanism-based
therapeutic intervention. Cancer Discov. 11:614–625. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atallah-Yunes SA, Kadado AJ, Kaufman GP
and Hernandez-Montfort J: Immune checkpoint inhibitor therapy and
myocarditis: A systematic review of reported cases. J Cancer Res
Clin Oncol. 145:1527–1557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Axelrod ML, Meijers WC, Screever EM, Qin
J, Carroll MG, Sun X, Tannous E, Zhang Y, Sugiura A, Taylor BC, et
al: T cells specific for α-myosin drive immunotherapy-related
myocarditis. Nature. 611:818–826. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moslehi J, Lichtman AH, Sharpe AH,
Galluzzi L and Kitsis RN: Immune checkpoint inhibitor-associated
myocarditis: Manifestations and mechanisms. J Clin Invest.
131:e1451862021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palaskas N, Lopez-Mattei J, Durand JB,
Iliescu C and Deswal A: Immune checkpoint inhibitor myocarditis:
Pathophysiological characteristics, diagnosis, and treatment. J Am
Heart Assoc. 9:e0137572020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ali A, Caldwell R, Pina G, Beinart N,
Jensen G, Yusuf SW, Koutroumpakis E, Hamzeh I, Khalaf S, Iliescu C,
et al: Elevated IL-6 and tumor necrosis factor-α in immune
checkpoint inhibitor myocarditis. Diseases. 12:882024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Varricchi G, Galdiero MR and Tocchetti CG:
Cardiac toxicity of immune checkpoint inhibitors: Cardio-oncology
meets immunology. Circulation. 136:1989–1992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu H, Galdos FX, Lee D, Waliany S, Huang
YV, Ryan J, Dang K, Neal JW, Wakelee HA, Reddy SA, et al:
Identification of pathogenic immune cell subsets associated with
checkpoint inhibitor-induced myocarditis. Circulation. 146:316–335.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisner RM, Husain A and Clark JI: Case
report and brief review: IL-2-induced myocarditis. Cancer Invest.
22:401–404. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma P, Liu J, Qin J, Lai L, Heo GS,
Luehmann H, Sultan D, Bredemeyer A, Bajapa G, Feng G, et al:
Expansion of pathogenic cardiac macrophages in immune checkpoint
inhibitor myocarditis. Circulation. 149:48–66. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frascaro F, Bianchi N, Sanguettoli F,
Marchini F, Meossi S, Zanarelli L, Tonet E, Serenelli M, Guardigli
G, Campo G, et al: Immune checkpoint inhibitors-associated
myocarditis: Diagnosis, treatment and current status on
rechallenge. J Clin Med. 12:77372023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salem JE, Allenbach Y, Vozy A, Brechot N,
Johnson DB, Moslehi JJ and Kerneis M: Abatacept for severe immune
checkpoint inhibitor-associated myocarditis. N Engl J Med.
380:2377–2379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang F, Liu Y, Xu W, Zhang C, Lv J and Ma
S: Fulminant myocarditis induced by immune checkpoint inhibitor
nivolumab: A case report and review of the literature. J Med Case
Rep. 15:3362021. View Article : Google Scholar : PubMed/NCBI
|