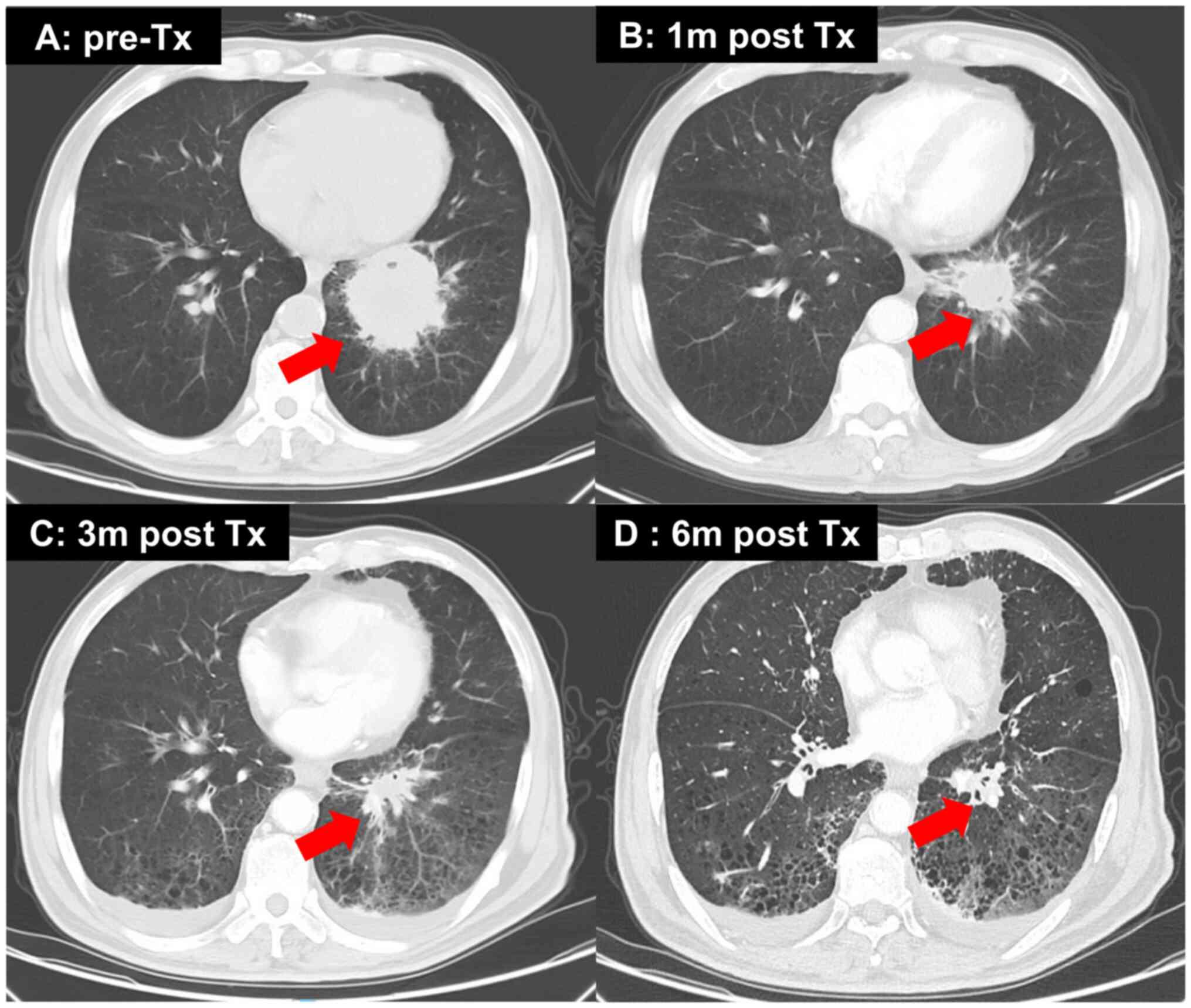
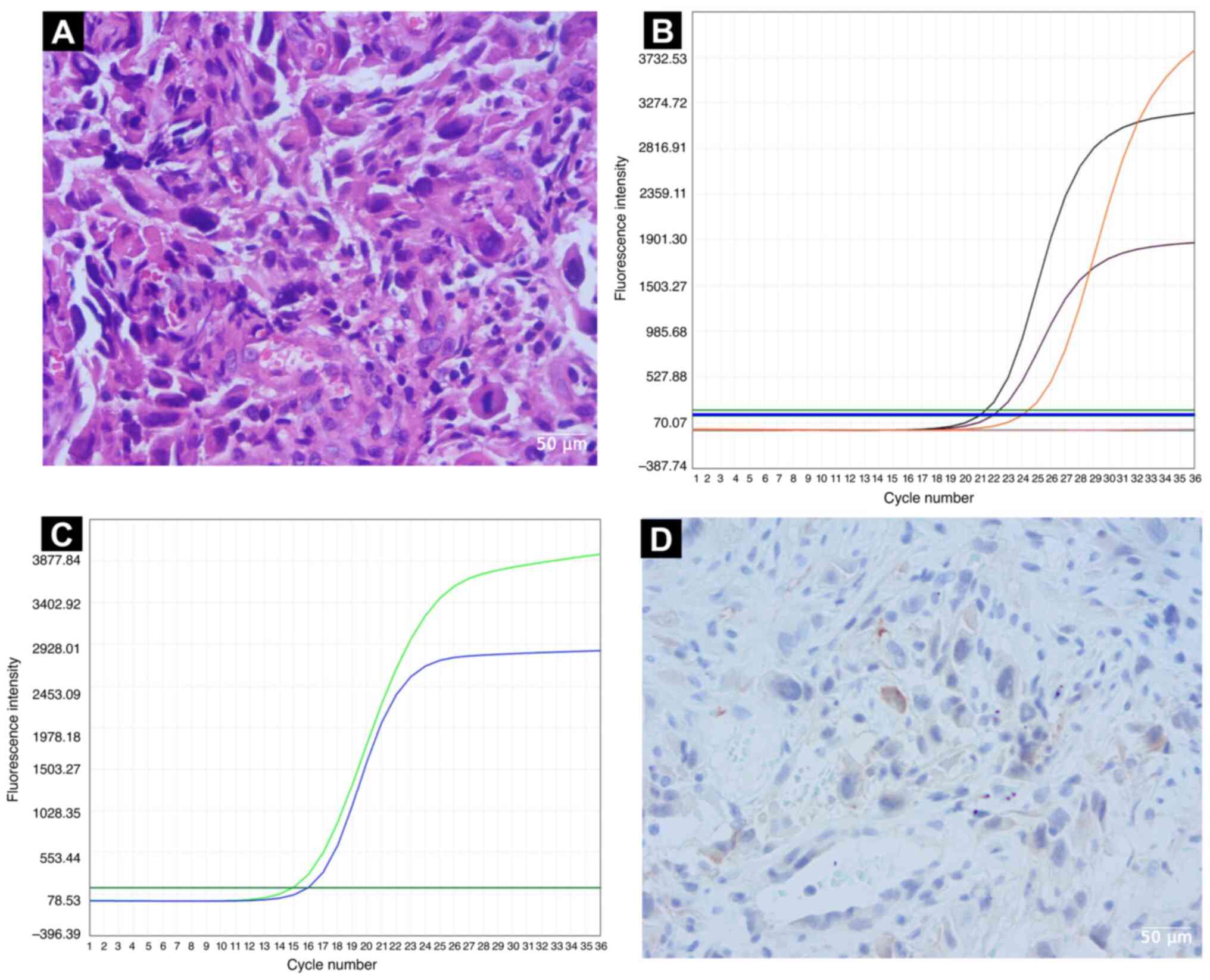
|
1
|
Kratzer TB, Bandi P, Freedman ND, Smith
RA, Travis WD, Jemal A and Siegel RL: Lung cancer statistics, 2023.
Cancer. 130:1330–1348. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu XD, Zhang Y and He HY: Targeted
next-generation sequencing of 491 lung cancers in clinical
practice: Implications for future detection strategy and targeted
therapy. Heliyon. 10:e275912024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fois SS, Paliogiannis P, Zinellu A, Fois
AG, Cossu A and Palmieri G: Molecular epidemiology of the main
druggable genetic alterations in non-small cell lung cancer. Int J
Mol Sci. 22:6122021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levantini E, Maroni G, Del Re M and Tenen
DG: EGFR signaling pathway as therapeutic target in human cancers.
Semin Cancer Biol. 85:253–275. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Michaels E and Bestvina CM: Meeting an
un-MET need: Targeting MET in non-small cell lung cancer. Front
Oncol. 12:10041982022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu Y, Zhou J, Li X, Goto K, Min X, Nishino
K, Cui J, Wu L, Sakakibara J, Shu Y, et al: Gumarontinib in
patients with non-small-cell lung cancer harbouring MET exon 14
skipping mutations: A multicentre, single-arm, open-label, phase
1b/2 trial. EClinicalMedicine. 59:1019522023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Skoulidis F and Heymach JV: Co-occurring
genomic alterations in non-small-cell lung cancer biology and
therapy. Nat Rev Cancer. 19:495–509. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gagnier JJ, Kienle G, Altman DG, Moher D,
Sox H and Riley D; CARE Group, : The CARE guidelines:
Consensus-based clinical case reporting guideline development. BMJ
Case Rep. 2013:bcr20132015542013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Marinis F, Kim TM, Bonanno L, Cheng S,
Kim SW, Tiseo M, Chu Q, Proto C, Sacher A, Luo YH, et al:
Savolitinib plus osimertinib in epidermal growth factor receptor
(EGFR)-mutated advanced non-small cell lung cancer with MET
overexpression and/or amplification following disease progression
on osimertinib: Primary results from the phase II SAVANNAH study.
Ann Oncol. 36:920–933. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Armato SG III and Nowak AK: Revised
modified response evaluation criteria in solid tumors for
assessment of response in malignant pleural mesothelioma (version
1.1). J Thorac Oncol. 13:1012–1021. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li WF, Kang J, Zhang XC, Jian S, Chen H,
Wang Z, Wang BC, Zhou Q, Tu HY, Wu YL and Yang JJ: Coexistence of
MET exon 14 mutations with EGFR mutations in non-small cell lung
cancer. J Clin Oncol. 35 (15 Suppl):e206362017. View Article : Google Scholar
|
|
15
|
Leonetti A, Sharma S, Minari R, Perego P,
Giovannetti E and Tiseo M: Resistance mechanisms to osimertinib in
EGFR-mutated non-small cell lung cancer. Br J Cancer. 121:725–737.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mazieres J, Vioix H, Pfeiffer BM, Campden
RI, Chen Z, Heeg B and Cortot AB: MET exon 14 skipping in NSCLC: A
systematic literature review of epidemiology, clinical
characteristics, and outcomes. Clin Lung Cancer. 24:483–497. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mi J, Huang Z, Zhang R, Zeng L, Xu Q, Yang
H, Lizaso A, Tong F, Dong X, Yang N and Zhang Y: Molecular
characterization and clinical outcomes in EGFR-mutant de novo
MET-overexpressed advanced non-small-cell lung cancer. ESMO Open.
7:1003472022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aubanel M, Swalduz A, Avrillon V, Doublet
L, Mastroianni B, Neidhardt-Bérard EM and Pérol M: Combining EGFR
and MET inhibition with crizotinib in EGFR-mutated lung
adenocarcinoma harboring MET amplification: A brief report. Clin
Lung Cancer. 21:e601–e606. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kauffmann-Guerrero D, Kahnert K, Kumbrink
J, Syunyaeva Z, Tufman A and Huber RM: Successful treatment of a
patient with NSCLC harboring an EGFR mutation and a concomitant met
exon 14 skipping mutation combining afatinib and crizotinib. Clin
Lung Cancer. 20:59–62. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ai J, Chen Y, Peng X, Ji Y, Xi Y, Shen Y,
Yang X, Su Y, Sun Y, Gao Y, et al: Preclinical evaluation of SCC244
(Glumetinib), a novel, potent, and highly selective inhibitor of
c-Met in MET-dependent cancer models. Mol Cancer Ther. 17:751–762.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J, Xu H, Li H, Ma L, Chen J, Yuan F,
Sheng L, Liu C, Chen W and Li X: Effect of food on the
pharmacokinetics and safety of a novel c-Met inhibitor SCC244: A
randomized phase I study in healthy subjects. Drug Des Devel Ther.
17:761–769. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lausoontornsiri W, Tan CK, Rajgor D and
Tang YC: Capmatinib treatment in a patient with
osimertinib-resistant NSCLC harboring two distinct MET alterations
revealed by tissue-based NGS testing. Cancer Pathog Ther.
3:357–360. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elghawy O, Barsouk A, Reed-Guy L, Stalker
M, Sussman J, Robinson K, Kosteva J, Singh A, Cohen RB, Langer C,
et al: Brief report: Osimertinib Plus capmatinib for patients with
MET-altered EGFR-mutant NSCLC following progression on front line
therapy. Clin Lung Cancer. 26:158–163.e2. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Passiglia F and Scagliotti GV: The
evolving paradigm of precision medicine in lung cancer. Curr Opin
Pulm Med. 27:249–254. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barrios C, de Lima Lopes G, Yusof MM,
Rubagumya F, Rutkowski P and Sengar M: Barriers in access to
oncology drugs-a global crisis. Nat Rev Clin Oncol. 20:7–15. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Febbraro M, Gheware A, Kennedy T, Jain D,
de Moraes FY and Juergens R: Barriers to access: Global variability
in implementing treatment advances in lung cancer. Am Soc Clin
Oncol Educ Book. 42:1–7. 2022.PubMed/NCBI
|
|
27
|
Fasola G, Barducci MC, Pelizzari G, Grossi
F, Pinto C, Daniele B, Giordano M, Ortega C, Silva RR, Tozzi VD, et
al: Implementation of precision oncology in clinical practice:
Results of a national survey for health care professionals.
Oncologist. 28:e324–e330. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Q, Yang S, Wang K and Sun SY: MET
inhibitors for targeted therapy of EGFR TKI-resistant lung cancer.
J Hematol Oncol. 12:632019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
A Multicenter, Randomized, Double-blind,
Phase III Clinical Study to Evaluate the Efficacy and Safety of
Savolitinib + Osimertinib Versus Placebo + Osimertinib as the First
Line Therapy for Patients With EGFRm+/MET+ NSCLC. 2021.https://clinicaltrials.gov/study/NCT05009836January
25–2025
|
|
30
|
A Prospective, Pilot Study of First-line
Osimertinib With or Without Savolitinib in de Novo MET Positive,
EGFR-mutant NSCLCs (FLOWERS). 2021.https://clinicaltrials.gov/study/NCT05163249January
25–2025
|
|
31
|
A Multi-centre Phase II, Double-Blind,
Randomised Study of Savolitinib in Combination With Osimertinib vs
Savolitinib in Combination With Placebo in Patients With EGFRm+ and
MET Amplified Locally Advanced or Metastatic Non-Small Cell Lung
Cancer Who Have Progressed Following Treatment With Osimertinib.
2020.https://clinicaltrials.gov/study/NCT04606771January
25–2025
|