Introduction
Lung cancer remains the primary cause of
cancer-related mortality on a global scale (1). The discovery of driver mutations in
lung cancer has revolutionized treatment by enabling personalized
targeted therapies. As a result, the screening of patients with
lung cancer for oncogenic drivers and the subsequent administration
of tailored targeted treatments hold great importance (2). Tyrosine kinase inhibitors (TKIs) are
currently considered the primary initial treatment choice for
patients with advanced non-small cell lung cancer (NSCLC) who have
a known driver mutation (3).
Epidermal growth factor receptor (EGFR) mutations
are prevalent genetic alterations, constituting ~10–15% of NSCLC
incidences in individuals of European heritage and ~30% in those of
East Asian ancestry (4).
Osimertinib, a third generation, irreversible EGFR-TKI, is approved
as a first-line drug for the treatment of patients with metastatic
EGFR-mutated NSCLC based on the results of the FLAURA trial
(5).
The mesenchymal-epithelial transition (MET) exon 14
skipping mutation, a splice-site oncogenic mutation, is found in
2–3% of patients with NSCLC (6).
Patients with this condition exhibit a good response to MET-TKIs,
such as gumarontinib, which garnered approval from the Food and
Drug Administration of the P.R. China based on the findings of the
GLORY study (7).
Previous research has shown that EGFR mutation and
MET exon 14 skipping mutation were mutually exclusive (8). The simultaneous presence of these
mutations in a patient with adenocarcinoma represents a unique and
rare molecular subtype of NSCLC (8). The present study reported on the case
of a patient with NSCLC harboring EGFR Del19 and MET exon 14
skipping, who achieved significant remission through the
administration of two corresponding TKIs. The case is presented in
accordance with the CARE reporting checklist (9).
Case report
In December 2023, a 70-year-old man was admitted to
Shandong Cancer Hospital and Institute (Jinan, Chian) with a
1-month history of chest pain. The patient had no previous history
of smoking or alcohol consumption and no family history of
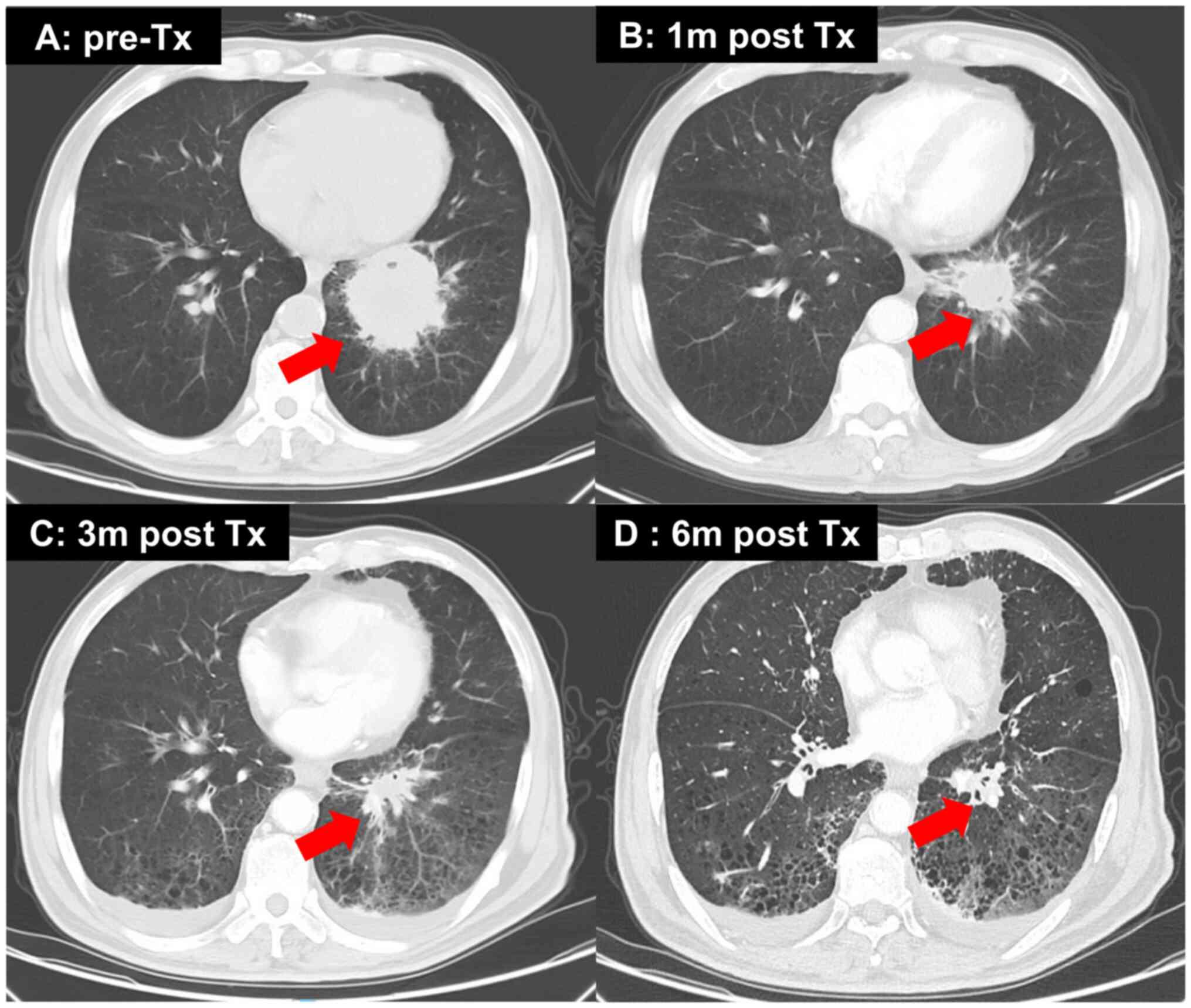
malignancy. A chest computed tomography (CT) scan revealed a left
lower lobe mass measuring ~5.8×5.6 cm, along with multiple enlarged
mediastinal and hilar lymph nodes, bilateral lung metastasis and
bone metastasis (Fig. 1A). Magnetic
resonance imaging of the brain showed no malignant metastases. A
biopsy specimen taken by endoscopic ultrasound-guided
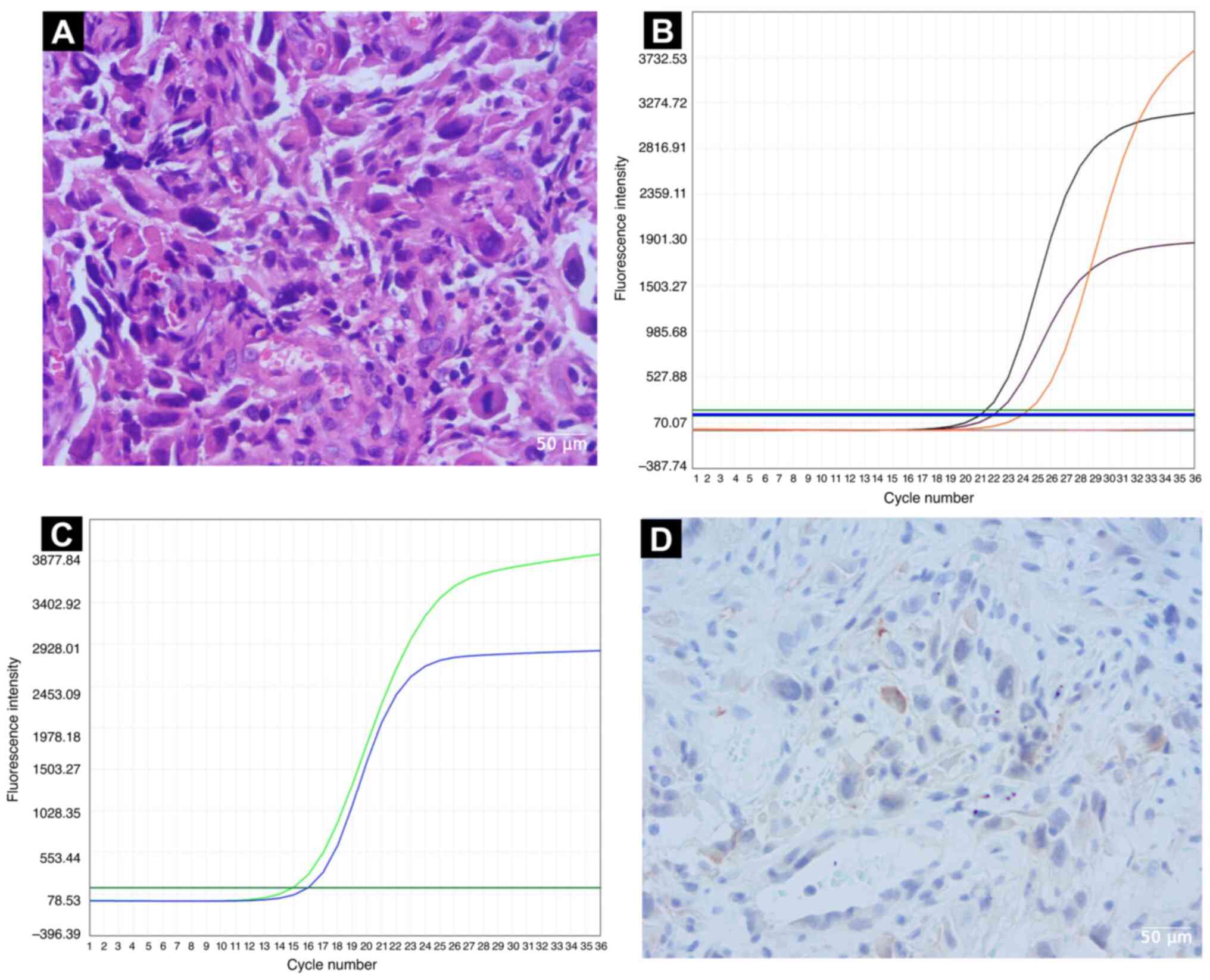
transbronchial needle aspiration showed lung adenocarcinoma
(Fig. 2A). The histology protocol
is provided in the Supplemental
methods. The patient was clinically diagnosed with lung
adenocarcinoma, with a clinical stage of cT3N2M1c (pulmonary and
bone metastases), cStage IVB according to the 8th Edition of TNM in
Lung Cancer (10) and Eastern
Cooperative Oncology Group Performance Status 1 (11). Amplification refractory mutation
system-polymerase chain reaction technology (ADx-ARMS kit; Amoy
Diagnostics, Co., Ltd.) examination performed according to the
manufacturer's instructions revealed the known EGFR Del 19 mutation
(Fig. 2B), but also a concomitant
MET exon 14 skipping mutation (MET gene fusion between exon 13 and
exon 15) (Fig. 2C).
Immunohistochemical analysis of the tumor (protocol is provided in
the Supplemental methods) showed
that programmed cell death ligand 1 expression was negative
(Fig. 2D). Therefore, the patient
commenced a combination therapy regimen consisting of Osimertinib
(80 mg, oral, daily) and gumarontinib (300 mg, oral, twice daily)
(12). A significant reduction in
the primary mass and all lymph nodes was observed after 1 month,
and the efficacy was evaluated as a partial response based on the
Response Evaluation Criteria In Solid Tumors (13) (Fig.
1B-D). Continuous monitoring of chest CT scans revealed a
gradual reduction in tumor size. During subsequent treatment, the
patient developed a first-degree rash, which was managed with
topical corticosteroids and resolved within 7 days. Concurrently,
the patient was diagnosed with Grade 2 pneumonia based on clinical
symptoms and radiographic findings. This was managed with a course
of oral corticosteroids (prednisone 0.5 mg/kg/day), temporary
withholding of the anticancer therapy and supportive care. The
pneumonia and associated symptoms subsided completely within 7 days
and a follow-up chest X-ray confirmed resolution. The patient
passed away due to cerebral infarction.
Discussion
The present study reported a rare case of a patient
with lung adenocarcinoma with co-occurrence of EGFR mutations and
MET exon 14 skipping. The co-existing of EGFR Del 19 and MET exon
14 skipping is relatively rare (~0.2%) (14). To the best of our knowledge, this is
the first case that has been documented.
Resistance to EGFR TKI therapy in EGFR-mutant lung
adenocarcinoma often involves the modification of the MET signaling
pathway (15). MET is essential for
regulating cell growth, survival and migration. The presence of MET
alterations has been linked to a less favorable prognosis in
patients with EGFR-mutant lung adenocarcinoma, serving as a
potential biomarker for predicting resistance to EGFR-TKI therapy
(16). Research indicates that
individuals with EGFR-mutant lung adenocarcinoma and MET
alterations are less likely to respond to EGFR-TKI treatment and
experience shorter progression-free survival than those without MET
alterations (17).
Other research findings indicate that simultaneous
blocking of EGFR and MET is necessary for achieving tumor
regression (18). MET exon 14
skipping has been recognized by Kauffmann-Guerrero et al
(19) as a significant factor
contributing to the development of resistance to EGFR TKI among
patients with sensitizing EGFR mutations. Gumarontinib, an oral MET
inhibitor, is highly selective. It has shown a favorable safety
profile in preclinical and preliminary clinical investigations
(20,21). The National Medical Products
Administration of China has granted conditional approval for
Gumarontinib in the treatment of locally advanced or metastatic
NSCLC with MET exon 14 skipping mutation. Gumarontinib currently
serves as an available therapeutic agent in China specifically
tailored to address MET exon 14 skipping mutations (7).
The combination therapy of osimertinib and
gumarontinib in this patient resulted in a partial response,
demonstrating the potential benefits of targeting multiple pathways
simultaneously. The observed reduction in tumor size suggests that
this approach may be effective in overcoming resistance mechanisms
associated with the MET mutation. It also raises important
questions regarding the optimal sequencing and combination of
therapies in the context of advanced lung cancer. Despite the
initial success, the patient's eventual demise due to cerebral
infarction highlights a critical aspect of managing patients with
lung cancer: The intricate interplay between cancer treatment and
the risk of thromboembolic events.
Several reports and early-phase studies have
documented a clinical benefit from dual targeted therapy in
patients with concurrent EGFR mutations and MET alterations, most
often using osimertinib in combination with MET inhibitors such
asor capmatinib, leading to partial responses or disease
stabilization despite limited evidence from small series and case
reports (12,19,22,23).
However, the feasibility of such approaches varies considerably
across regions due to differences in regulatory approval,
reimbursement policies and clinical guideline recommendations
(24–26). In resource-limited settings or under
restrictive insurance systems, the high cost of targeted agents,
limited availability of molecular testing and lack of trial
infrastructure represent major barriers to the real-world
implementation of combination strategies, underscoring the need for
global efforts to bridge disparities in access to precision
oncology (27).
It is noteworthy that the patient succumbed to a
cerebral infarction approximately 11 months after commencing
treatment. Given the patient's advanced age (70 years), which is a
primary risk factor for cerebrovascular events, this occurrence was
carefully evaluated. While the patient was on a combination of
osimertinib and gumarontinib, there is no established direct
evidence linking either agent, or their combination, to a
significantly increased incidence of cerebral infarction.
Therefore, after comprehensive assessment, the cerebral infarction
may be considered to be most likely unrelated to the anticancer
therapy and attributable to the patient's underlying age-related
vascular risk.
In the present case, the concurrent presence of EGFR
and MET mutations was detected in the biopsy specimen. Should
osimertinib monotherapy be employed, its efficacy in suppressing
the growth of EGFR-positive neoplastic cells may be limited
(28). Consequently, it was chosen
to employ a combination therapy incorporating gumarontinib. The
choice of combining gumarontinib or other inhibitors is still under
exploration (29–31). The present study reported the first
detection of MET exon 14 skipping and EGFR Del19 in a patient, to
the best of our knowledge. This patient achieved remission with
osimertinib combined with gumarontinib treatment. The present
findings provide valuable evidence for the subsequent treatment of
such patients.
In conclusion, this case emphasizes the importance
of understanding the genetic landscape of lung adenocarcinoma and
the implications of concurrent mutations in treatment response and
patient outcomes. Future clinical strategies should include
thorough genetic testing and possibly a shift towards combination
therapies that address multiple pathways to enhance treatment
efficacy while managing adverse effects. Continued research is
essential to refine therapeutic approaches and improve the
prognosis for patients with complex lung cancer profiles.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 82473254), Wu Jieping Medical
Foundation (grant nos. 320.6750.2023–16-6 and 320.6750.2025–20-12)
and the China Zhongguancun Precision Medicine Science and
Technology Foundation (grant no. GXZDH72) and the Clinical Research
Pioneering Program of Shandong First Medical University &
Shandong Academy of Medical Sciences (grant no. 607D25022).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XJ and HZ conceptualized the study and wrote the
original manuscript. LL and SL searched the literature and obtained
case-related data. SL and XZ analyzed data and relevant literature.
HZ reviewed and edited the final draft, and was responsible for
project administration and funding acquisition. XJ and HZ confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
This study was reviewed and approved by the Ethics
Committee of the Shandong Cancer Hospital and Institute (Jinan,
China; approval no. SDTHEC2024001026).
Patient consent for publication
Written informed consent for publication was
obtained from the patient and his family prior to the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kratzer TB, Bandi P, Freedman ND, Smith
RA, Travis WD, Jemal A and Siegel RL: Lung cancer statistics, 2023.
Cancer. 130:1330–1348. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu XD, Zhang Y and He HY: Targeted
next-generation sequencing of 491 lung cancers in clinical
practice: Implications for future detection strategy and targeted
therapy. Heliyon. 10:e275912024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fois SS, Paliogiannis P, Zinellu A, Fois
AG, Cossu A and Palmieri G: Molecular epidemiology of the main
druggable genetic alterations in non-small cell lung cancer. Int J
Mol Sci. 22:6122021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levantini E, Maroni G, Del Re M and Tenen
DG: EGFR signaling pathway as therapeutic target in human cancers.
Semin Cancer Biol. 85:253–275. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Michaels E and Bestvina CM: Meeting an
un-MET need: Targeting MET in non-small cell lung cancer. Front
Oncol. 12:10041982022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu Y, Zhou J, Li X, Goto K, Min X, Nishino
K, Cui J, Wu L, Sakakibara J, Shu Y, et al: Gumarontinib in
patients with non-small-cell lung cancer harbouring MET exon 14
skipping mutations: A multicentre, single-arm, open-label, phase
1b/2 trial. EClinicalMedicine. 59:1019522023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Skoulidis F and Heymach JV: Co-occurring
genomic alterations in non-small-cell lung cancer biology and
therapy. Nat Rev Cancer. 19:495–509. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gagnier JJ, Kienle G, Altman DG, Moher D,
Sox H and Riley D; CARE Group, : The CARE guidelines:
Consensus-based clinical case reporting guideline development. BMJ
Case Rep. 2013:bcr20132015542013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Marinis F, Kim TM, Bonanno L, Cheng S,
Kim SW, Tiseo M, Chu Q, Proto C, Sacher A, Luo YH, et al:
Savolitinib plus osimertinib in epidermal growth factor receptor
(EGFR)-mutated advanced non-small cell lung cancer with MET
overexpression and/or amplification following disease progression
on osimertinib: Primary results from the phase II SAVANNAH study.
Ann Oncol. 36:920–933. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Armato SG III and Nowak AK: Revised
modified response evaluation criteria in solid tumors for
assessment of response in malignant pleural mesothelioma (version
1.1). J Thorac Oncol. 13:1012–1021. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li WF, Kang J, Zhang XC, Jian S, Chen H,
Wang Z, Wang BC, Zhou Q, Tu HY, Wu YL and Yang JJ: Coexistence of
MET exon 14 mutations with EGFR mutations in non-small cell lung
cancer. J Clin Oncol. 35 (15 Suppl):e206362017. View Article : Google Scholar
|
|
15
|
Leonetti A, Sharma S, Minari R, Perego P,
Giovannetti E and Tiseo M: Resistance mechanisms to osimertinib in
EGFR-mutated non-small cell lung cancer. Br J Cancer. 121:725–737.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mazieres J, Vioix H, Pfeiffer BM, Campden
RI, Chen Z, Heeg B and Cortot AB: MET exon 14 skipping in NSCLC: A
systematic literature review of epidemiology, clinical
characteristics, and outcomes. Clin Lung Cancer. 24:483–497. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mi J, Huang Z, Zhang R, Zeng L, Xu Q, Yang
H, Lizaso A, Tong F, Dong X, Yang N and Zhang Y: Molecular
characterization and clinical outcomes in EGFR-mutant de novo
MET-overexpressed advanced non-small-cell lung cancer. ESMO Open.
7:1003472022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aubanel M, Swalduz A, Avrillon V, Doublet
L, Mastroianni B, Neidhardt-Bérard EM and Pérol M: Combining EGFR
and MET inhibition with crizotinib in EGFR-mutated lung
adenocarcinoma harboring MET amplification: A brief report. Clin
Lung Cancer. 21:e601–e606. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kauffmann-Guerrero D, Kahnert K, Kumbrink
J, Syunyaeva Z, Tufman A and Huber RM: Successful treatment of a
patient with NSCLC harboring an EGFR mutation and a concomitant met
exon 14 skipping mutation combining afatinib and crizotinib. Clin
Lung Cancer. 20:59–62. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ai J, Chen Y, Peng X, Ji Y, Xi Y, Shen Y,
Yang X, Su Y, Sun Y, Gao Y, et al: Preclinical evaluation of SCC244
(Glumetinib), a novel, potent, and highly selective inhibitor of
c-Met in MET-dependent cancer models. Mol Cancer Ther. 17:751–762.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J, Xu H, Li H, Ma L, Chen J, Yuan F,
Sheng L, Liu C, Chen W and Li X: Effect of food on the
pharmacokinetics and safety of a novel c-Met inhibitor SCC244: A
randomized phase I study in healthy subjects. Drug Des Devel Ther.
17:761–769. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lausoontornsiri W, Tan CK, Rajgor D and
Tang YC: Capmatinib treatment in a patient with
osimertinib-resistant NSCLC harboring two distinct MET alterations
revealed by tissue-based NGS testing. Cancer Pathog Ther.
3:357–360. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elghawy O, Barsouk A, Reed-Guy L, Stalker
M, Sussman J, Robinson K, Kosteva J, Singh A, Cohen RB, Langer C,
et al: Brief report: Osimertinib Plus capmatinib for patients with
MET-altered EGFR-mutant NSCLC following progression on front line
therapy. Clin Lung Cancer. 26:158–163.e2. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Passiglia F and Scagliotti GV: The
evolving paradigm of precision medicine in lung cancer. Curr Opin
Pulm Med. 27:249–254. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barrios C, de Lima Lopes G, Yusof MM,
Rubagumya F, Rutkowski P and Sengar M: Barriers in access to
oncology drugs-a global crisis. Nat Rev Clin Oncol. 20:7–15. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Febbraro M, Gheware A, Kennedy T, Jain D,
de Moraes FY and Juergens R: Barriers to access: Global variability
in implementing treatment advances in lung cancer. Am Soc Clin
Oncol Educ Book. 42:1–7. 2022.PubMed/NCBI
|
|
27
|
Fasola G, Barducci MC, Pelizzari G, Grossi
F, Pinto C, Daniele B, Giordano M, Ortega C, Silva RR, Tozzi VD, et
al: Implementation of precision oncology in clinical practice:
Results of a national survey for health care professionals.
Oncologist. 28:e324–e330. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Q, Yang S, Wang K and Sun SY: MET
inhibitors for targeted therapy of EGFR TKI-resistant lung cancer.
J Hematol Oncol. 12:632019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
A Multicenter, Randomized, Double-blind,
Phase III Clinical Study to Evaluate the Efficacy and Safety of
Savolitinib + Osimertinib Versus Placebo + Osimertinib as the First
Line Therapy for Patients With EGFRm+/MET+ NSCLC. 2021.https://clinicaltrials.gov/study/NCT05009836January
25–2025
|
|
30
|
A Prospective, Pilot Study of First-line
Osimertinib With or Without Savolitinib in de Novo MET Positive,
EGFR-mutant NSCLCs (FLOWERS). 2021.https://clinicaltrials.gov/study/NCT05163249January
25–2025
|
|
31
|
A Multi-centre Phase II, Double-Blind,
Randomised Study of Savolitinib in Combination With Osimertinib vs
Savolitinib in Combination With Placebo in Patients With EGFRm+ and
MET Amplified Locally Advanced or Metastatic Non-Small Cell Lung
Cancer Who Have Progressed Following Treatment With Osimertinib.
2020.https://clinicaltrials.gov/study/NCT04606771January
25–2025
|