Introduction
Inflammation constitutes a defensive mechanism of
the immune system in response to deleterious stimuli, including
pathogens, damaged cells or irritant agents (1). Moderate inflammation helps clear
infections and repair damage, serving a crucial role in maintaining
homeostasis of the body. However, previous studies have indicated
that chronic or excessive inflammation may serve a role in
oncogenesis (2,3), with >20% of cancers being
associated with inflammatory processes (4). Certain cytokines and growth factors
involved in the inflammatory response have the potential to enhance
cellular proliferation and induce mutations, consequently elevating
the risk of cancer development (5).
Globally, liver cancer is the sixth most common
cancer and the third leading cause of cancer-associated
mortalities. The World Health Organization's International Agency
for Research on Cancer reported that in 2022, there were ~870,000
new liver cancer cases and ~760,000 mortalities due to the disease
worldwide (6). Hepatocellular
carcinoma (HCC) represents 90% of primary liver cell carcinomas and
it is the most common type of liver cancer that usually develops in
individuals with chronic hepatitis (7).
Neutrophils are increasingly recognized as pivotal
contributors to inflammatory infiltration, exerting an inhibitory
influence on the cytolytic functions of immune cells. This includes
the suppression of natural killer cells, which serve a crucial role
in the eradication of tumor cells, as well as lymphocytes and
activated T cells (8). Previously,
the effect of neutrophils in tumor pathogenesis has garnered
notable attention. Emerging research highlights a cytotoxic
mechanism employed by neutrophils operating through the formation
of neutrophil extracellular traps (NETs) (9,10).
NETs are complex, web-like formations discharged by neutrophils
upon exposure to particular stimuli, such as pathogenic infections.
These structures consist of chromatin and DNA strands encased in
protease granules. Studies indicate that NETs are involved in the
initiation and progression of various inflammatory and autoimmune
diseases, such as hepatitis B virus (HBV) (11), COVID-19 (12) and rheumatoid arthritis (13). Moreover, NETs contribute to creating
a microenvironment that promotes tumor cell adhesion and
proliferation, thereby facilitating tumor cell migration and
invasion (14). Elevated levels of
NETs have been frequently detected in the bloodstream of patients
with lung cancer (15), pancreatic
cancer (16), bladder cancer
(17), HCC (18) and colorectal cancer (19).
Previous research has predominantly concentrated on
the roles and effects of NETs in hepatitis (20) and HCC (18). Nevertheless, emerging evidence
suggests that NETs are crucial in the progression from chronic
inflammation to cancer, especially within the field of hepatology.
As a result, the present review aimed to comprehensively
investigate the potential mechanisms through which NETs facilitate
the transformation from chronic liver inflammation to HCC and to
assess their clinical applicability as therapeutic targets for
liver cancers. A comprehensive literature review was performed
using PubMed and the Web of Science databases, encompassing
publications up to October 2025. The search strategy employed key
words such as ‘neutrophil extracellular traps’, ‘hepatocellular
carcinoma’, ‘hepatitis’, ‘liver cancer transformation’ and
‘inflammation’, ‘non-alcoholic steatohepatitis’, ‘non-alcoholic
fatty liver disease’ and ‘hepatic ischemia-reperfusion injury’,
with an expanded scope through various keyword combinations. The
inclusion criteria focused on original research articles and review
papers that investigated the role of NETs in the progression from
hepatitis to HCC, with a particular emphasis on molecular
mechanisms and potential therapeutic strategies. Studies were
excluded if they: i) Did not pertain to NETs or the
hepatitis-to-HCC transformation; ii) consisted of non-original
content such as conference abstracts, reviews or case reports; or
iii) lacked complete methodological data or had inaccessible full
texts. During the screening process, two reviewers (WYW and CYZ)
independently conducted preliminary screening of retrieved
literature titles and abstracts based on predefined
inclusion/exclusion criteria. Subsequently, full-texts of initially
qualified papers were obtained and independently evaluated by the
same two reviewers to determine final inclusion. If discrepancies
arose between reviewers regarding inclusion at any stage, they
would resolve them through mutual consultation. When consensus
could not be reached, a third senior researcher (CSG) would
arbitrate to ensure objective and fair decision-making. From the
ultimately included literature, the two reviewers independently
extracted the following information: First author, publication
year, study type (in vivo/in vitro/clinical),
research subjects, key findings and NETs-related molecular
mechanisms.
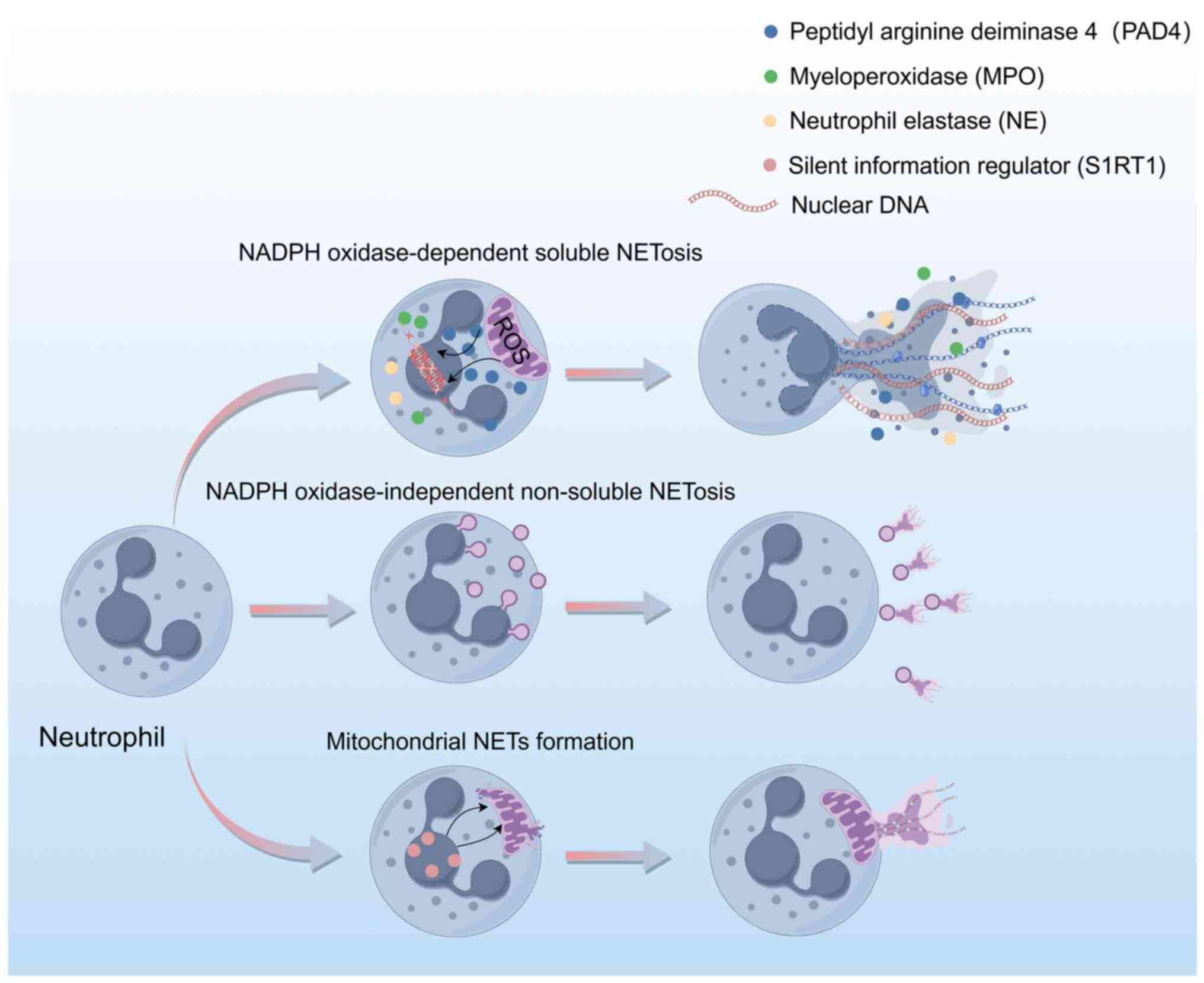
Formation of NETs
The formation of NETs can be elucidated from three
primary perspectives (Fig. 1). The
first perspective involves the NADPH-dependent NETosis pathway. In
this pathway, the activation of neutrophils by phorbol 12-myristate
13-acetate triggers the activation of NADPH oxidase, leading to the
generation of reactive oxygen species (ROS) within the cells
(21). ROS are pivotal in the
degradation of neutrophil granules and facilitate the release and
nuclear translocation of enzymes such as myeloperoxidase (MPO) and
neutrophil elastase (NE), which subsequently cleave histones
(22). Concurrently,
peptidylarginine deiminase 4 (PAD4) is activated, promoting the
citrullination of histone 3 (H3). This alteration causes chromatin
decondensation and nuclear membrane rupture by decreasing the
electrostatic interaction with DNA. The decondensed chromatin
translocates to the cytoplasm, where it associates with enzymes
such as MPO and NE, and is eventually released extracellularly to
form soluble NETs. Throughout this process, neutrophils undergo
apoptosis (23).
Conversely, an alternative mechanism of NETosis
operates independently of NADPH (24). In this pathway, stimuli such as
bacteria or their derivatives, in conjunction with Toll-like
receptor (TLR)2, activate neutrophils, subsequently triggering PAD4
activation. This activation results in chromatin decondensation and
the translocation of NE to the nucleus. Notably, in this pathway,
chromatin, embellished with cytoplasmic and nuclear proteins, is
expelled through the formation of nuclear membrane blebs, thereby
preserving the nuclear membrane's integrity (25). As a result, neutrophils do not
undergo cell death and retain their phagocytic and chemotactic
capabilities (26).
In addition, under conditions where mitochondrial
oxidative respiration generates notable levels of ROS, neutrophils
possess the ability to release NETs that are comprised of
mitochondrial DNA rather than nuclear DNA (27). Research using a mouse model of lung
metastases from breast cancer has revealed that neutrophils can
activate sirtuin 1 through nicotinamide phosphoribosyl transferase
secreted by the primary tumor. This activation leads to the opening
of the mitochondrial permeability transition pore, thereby
facilitating the release of mitochondrial DNA and resulting in the
formation of mitochondrial-dependent NETs, which serve a crucial
role in this context (28).
NETs promote inflammatory-cancer
transformation
Previous studies have shown that NETs serve a
critical role in the pathogenesis of liver inflammatory diseases
and the progression of HCC (11,29–31).
The present review also aimed to elucidate the impact of NETs in
HBV, non-alcoholic steatohepatitis (NASH), hepatic
ischemia-reperfusion injury (HIRI) and HCC (Table I).
 | Table I.NETs in HBV, NASH, HIRI and HCC key
research summary. |
Table I.
NETs in HBV, NASH, HIRI and HCC key
research summary.
| Type of
disease | Research
models | Key findings | (Refs.) |
|---|
| HBV | i) FVH mouse model
(induced by MHV-3); ii) clinical patient samples and hydrodynamic
injection model; iii) clinical patient samples, human liver
chimeric mouse model of HBV infection, cell co-culture model. | i) FGL2 and MCOLN3
interact to promote autophagy and NETs formation; DNase1
degradation of NETs can improve liver function; ii) HBV core
protein and e protein promote chronic infection by inhibiting
autophagy and NETs release through mTOR; and iii) HBV activates
toxic natural killer cells, causing hepatocytes to die through
GSDMD/caspase-8 dependent apoptosis pathway, and neutrophils
subsequently form NETs, exacerbating HBV-ACLF. | (11,35,36) |
| NASH | i) STAM mouse model
(STZ + high fat diet at birth); ii) diet-induced LDLR−/−
mouse NASH model; vi) NE gene knockout mice and wild-type
mice. | i) The formation of
NETs is associated with neutrophil accumulation and elevated levels
of inflammatory factors, and affects the inflammatory environment
of NASH by attracting mononuclear macrophages; ii) the lack of MPO
markedly reduced activation of hepatic stellate cells, slowed the
development of fibrosis and reduced hepatocyte injury; and iii) the
levels of NE were elevated in the livers of wild-type mice with
NASH, while the NE-deficient NASH mice showed characteristics such
as weight loss, improved blood lipid indexes and reduced
inflammatory response. | (29,41,42) |
| HIRI | i) Mouse liver
ischemia-reperfusion model; ii) mouse liver ischemia-reperfusion
model and clinical patient samples; iii) machine learning to
analyze RNA-sequencing data; and iv) mouse liver ischemia
reperfusion model. | i) During hepatic
ischemia-reperfusion injury, activated neutrophils release a large
amount of ROS and proteases to promote the formation of NETs, thus
aggravating liver injury; ii) in the process of liver ischemia and
reperfusion, toxic aldehydes, such as acrolein, produced can
activate the signaling pathways of NOX2 and p38MAPK in neutrophils
to induce the formation of NETs, which further promote liver
injury; iii) after HIRI, interleukin-17A promotes neutrophil
infiltration and NET formation, which exacerbates liver injury; and
iv) HMGB1 induces chromatin decondensation through TLR4 to form
NETs and exert inflammatory effects. | (30,46–48) |
| HCC | i) Clinical patient
cohort models; ii) animal models of post-surgical metastasis in
simulated liver and PAD4 knockout mice; iii) mouse liver cancer
lung metastasis model, mouse in situ liver cancer model; iv)
in situ liver cancer model in mice and clinical patient
samples; and v) in situ liver cancer model in mice. | i) Preoperative NET
levels were associated with patient survival and could serve as a
novel biomarker for predicting recurrence-free survival and overall
survival in patients with primary liver cancer; ii) liver surgical
trauma releases HMGB1, which activates TLR2/4 receptor to recruit
neutrophils to form NETs, directly enhancing the ability of tumor
cells to migrate, invade and proliferate; iii) NETs release MPO to
produce hypochlorous acid, chlorinated layer adhesion protein α5 in
the extracellular matrix, activate the integrin β1/FAK signaling
pathway, promote the invasion and metastasis of liver cancer cells
and further promote the progression of liver cancer; iv) NETs
formed in neutrophils in patients with HCC (particularly metastatic
liver cancer) can activate the TLR4/9-COX2 signaling pathway in HCC
cells and promote metastasis of liver cancer; and v) intestinal
flora imbalance induces intrahepatic metastasis in mouse models by
enhancing neutrophil-mediated inflammatory response and inducing
excessive production of NETs. | (14,56,57,65,70) |
NETs promote the development of liver
inflammation
HBV
HBV is a critical human pathogen implicated in a
range of liver diseases. According to the ‘Global Hepatitis Report
2024’, ~254 million individuals were infected with HBV and 50
million with hepatitis C worldwide in 2022, with a daily mortality
rate of 3,500 attributed to these infections (32). Research performed by Li et al
(11) demonstrated that in the
fulminant viral hepatitis (FVH) mouse model, induced through
infection with mouse hepatitis virus strain-3, fibrinogen-like
protein 2 (FGL2) interacts with mucolipin-3 to modulate calcium ion
influx, thereby triggering autophagy and leading to the formation
of NETs. Reduced autophagy was associated with FGL2 deficit and
this resulted in a decrease in NET synthesis. Moreover, the
depletion of NETs using DNase1 improved liver function and survival
rates in FVH mice (Fig. 2).
Clinical data further indicate that patients with HBV-induced acute
liver injury or acute liver failure exhibit markedly elevated
levels of NETs in both plasma and liver tissue compared with
patients with chronic HBV and healthy controls. In individuals with
chronic HBV infection, there is a reduced release of NETs and an
inverse association has been identified between the levels of HBV
surface antigen, hepatitis B e antigen (HBeAg), and HBV core
antibody and NETs release (33).
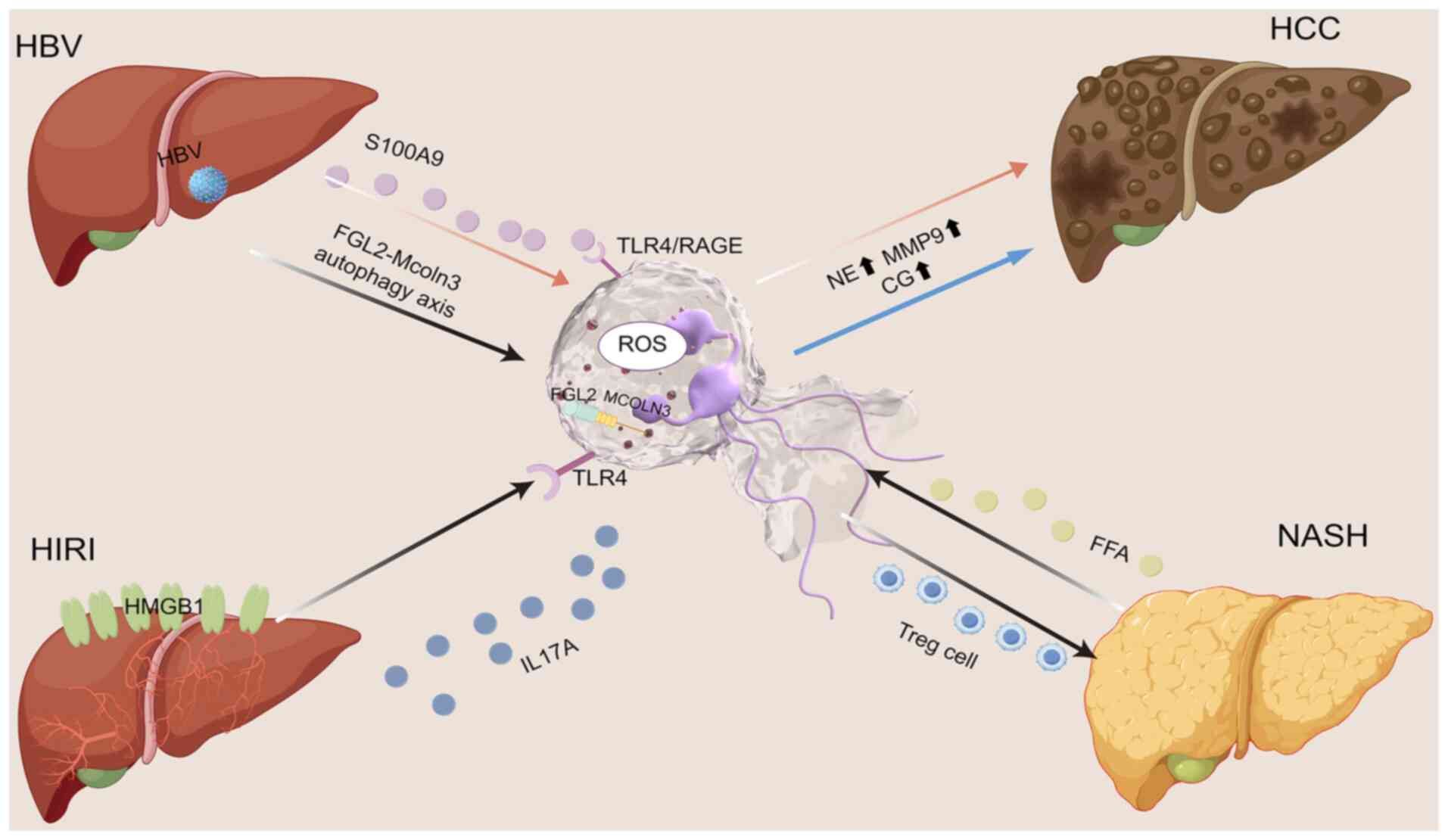 | Figure 2.Interplay between NETs and HBV, HIRI,
NASH and HCC. Hepatic inflammations such as HBV, HIRI and NASH can
promote the formation of NETs by neutrophils; this may therefore
promote the development of HCC. The figure was drawn using Figdraw
(https://www.figdraw.com/static/index.html#/). NETs,
neutrophil extracellular traps; HBV, hepatitis B virus; HIRI,
hepatic ischemia-reperfusion injury; HCC, hepatocellular carcinoma;
NASH, non-alcoholic steatohepatitis; IL, interleukin; ROS, reactive
oxygen species; TLR, Toll-like receptor; MMP9, matrix
metallopeptidase 9; NE, neutrophil elastase; Treg, regulatory T
cell; FFA, free fatty acids; CG, cholyglycine; MCOLN3, mucolipin
TRP cation channel 3; RAGE, receptor for advanced glycation end
products; FGL2, fibrinogen-like protein 2; HMGB1, high mobility
group box 1. |
Researchers have developed a scoring system based on
NETs to predict the 90-day mortality rate in patients with
HBV-related acute-on-chronic liver failure, demonstrating superior
predictive performance compared with traditional models (34). The research performed by Hu et
al (35) elucidates that
infection with the HBV induces the HBV core and HBeAg proteins to
enhance the activity of the mammalian target of rapamycin. This
enhancement results in a diminished autophagic activity in
neutrophils and suppresses the activation of ROS-dependent
extracellular signal-regulated kinase (ERK) and p38
mitogen-activated protein kinase (MAPK) pathways. Consequently,
this suppression inhibits the release of NETs, thereby impairing
both innate and adaptive immune responses against HBV and
ultimately facilitating the establishment of chronic liver
infection (28). Another previous
study found that HBV can activate cytotoxic natural killer cells,
triggering gasdermin/caspase-8-dependent apoptosis in hepatocytes.
Neutrophils selectively accumulate in the apoptotic liver, further
inducing NETs, thereby promoting HBV-associated acute-on-chronic
liver failure (36).
NASH
NASH represents a more severe form of non-alcoholic
fatty liver disease (NAFLD), distinguished by hepatic fat
accumulation alongside inflammation and hepatocellular injury
(37). Cirrhosis, liver fibrosis
and HCC can develop from this illness (38). Neutrophils have been consistently
identified within the inflammatory infiltrates associated with NASH
(39). In the context of NASH, free
fatty acids, such as non-esterified fatty acids, oleic acid and
linoleic acid, can activate neutrophils to generate NETs, which are
prevalent in NASH-affected livers (29) (Fig.
2). Research has indicated that the NETs hallmark proteins MPO
and NE are essential for the development of NASH (40). Furthermore, the levels of MPO-DNA in
the preoperative serum of patients with NASH are markedly elevated
compared with individuals with normal liver function, irrespective
of the presence of benign, primary malignant or metastatic hepatic
conditions (29) (Fig. 2).
In a STAM (NASH induced by neonatal streptozotocin
and high-fat diet) mouse model for NASH, initiated by administering
streptozotocin and a high-fat diet to newborn mice, the development
of NETs is associated with neutrophil accumulation and increased
levels of inflammatory cytokines. This mechanism influences the
inflammatory environment of NASH by attracting macrophages derived
from monocytes (29). Rensen et
al (41) showed that lacking
MPO markedly reduces the activation of hepatic stellate cells,
slows fibrosis development and decreases hepatocyte damage,
indicating that MPO is crucial in neuroendocrine tumors and
influences the pathogenesis and advancement of NASH. Furthermore,
Chen et al (42) established
NASH models in both NE gene knockout and wild-type mice. The study
revealed that NE levels were increased in the liver tissue of
wild-type mice with NASH, whereas NE-deficient NASH mice showed
weight reduction, improved lipid profiles and decreased
inflammatory responses. These findings imply that NE influences
ceramide metabolism in the liver, both in living organisms and in
laboratory settings, underscoring the crucial involvement of NETs
in driving inflammation linked to NASH. According to research, by
blocking the development of NETs connected to the production of MPO
and citrullinated H3, Tanshinone IIA may reduce inflammation and
liver cell death in mice with NASH (43). Some scholars have also found that
neutrophil-derived Notch-driven NETs promote the progression of
NAFLD to NASH by inducing cellular senescence (44).
HIRI
HIRI is a critical factor that notably impairs
postoperative recovery in patients undergoing hepatic surgery. The
onset of HIRI rapidly induces an acute inflammatory response,
resulting in substantial hepatocellular damage and liver
dysfunction, which may progress to multiple organ failure and
mortality (45). Research indicates
that during HIRI, activated neutrophils release substantial
quantities of ROS and proteases, facilitating the formation of NETs
and thereby exacerbating hepatic damage (46). At the molecular level, utilizing 101
machine learning combinations to analyze bulk and single-cell RNA
sequencing data, researchers identified the involvement of NETs in
the pathogenesis of HIRI and early allograft dysfunction. Notably,
the administration of C5AR1 antagonists was shown to reduce NETs
formation, thereby mitigating hepatic tissue inflammation and
enhancing liver function (47).
Furthermore, computational dynamic network analysis
has demonstrated that following HIRI, interleukin (IL)-17A
facilitates neutrophil infiltration and the formation of NETs,
thereby exacerbating liver damage (30) (Fig.
2). In a liver ischemia-reperfusion (I/R) injury model,
recombinant high mobility group box 1 protein induced chromatin
decondensation via TLR4 to form NETs, which exert pro-inflammatory
effects (48). Yazdani et al
(49) demonstrated that IL-33
released by liver sinusoidal endothelial cells promotes the
formation of NETs via the ST2 signaling pathway, thereby
exacerbating both sterile inflammation and systemic inflammation
within the liver. Additionally, hydroxychloroquine mitigated the
formation of NETs by inhibiting TLR9 and suppressing PAD4
expression, thereby alleviating liver I/R injury (46). In the histidine-rich glycoprotein
(HRG) mouse model, pretreatment with HRG reduced I/R injury in mice
by preventing the production of neutrophils and NETs (50). Additionally, the chemical compounds
benzoylphenylurea and Sivelestat sodium were shown to alleviate I/R
injury by inhibiting NETs formation (51). Subsequent studies demonstrated that
acrolein can trigger neutrophil chemotaxis and stimulate the
release of NETs in both in vitro and in vivo settings
(52,53). It was demonstrated that naringin and
F-apocynin suppress the production of inflammatory cytokines,
activation of the p38MAPK-ERK pathway and apoptotic signaling in
rat liver subjected to acrolein exposure and I/R. These compounds
effectively suppress acrolein-induced NETs release and mitigate the
associated liver I/R injury (54).
NETs promote the development of
HCC
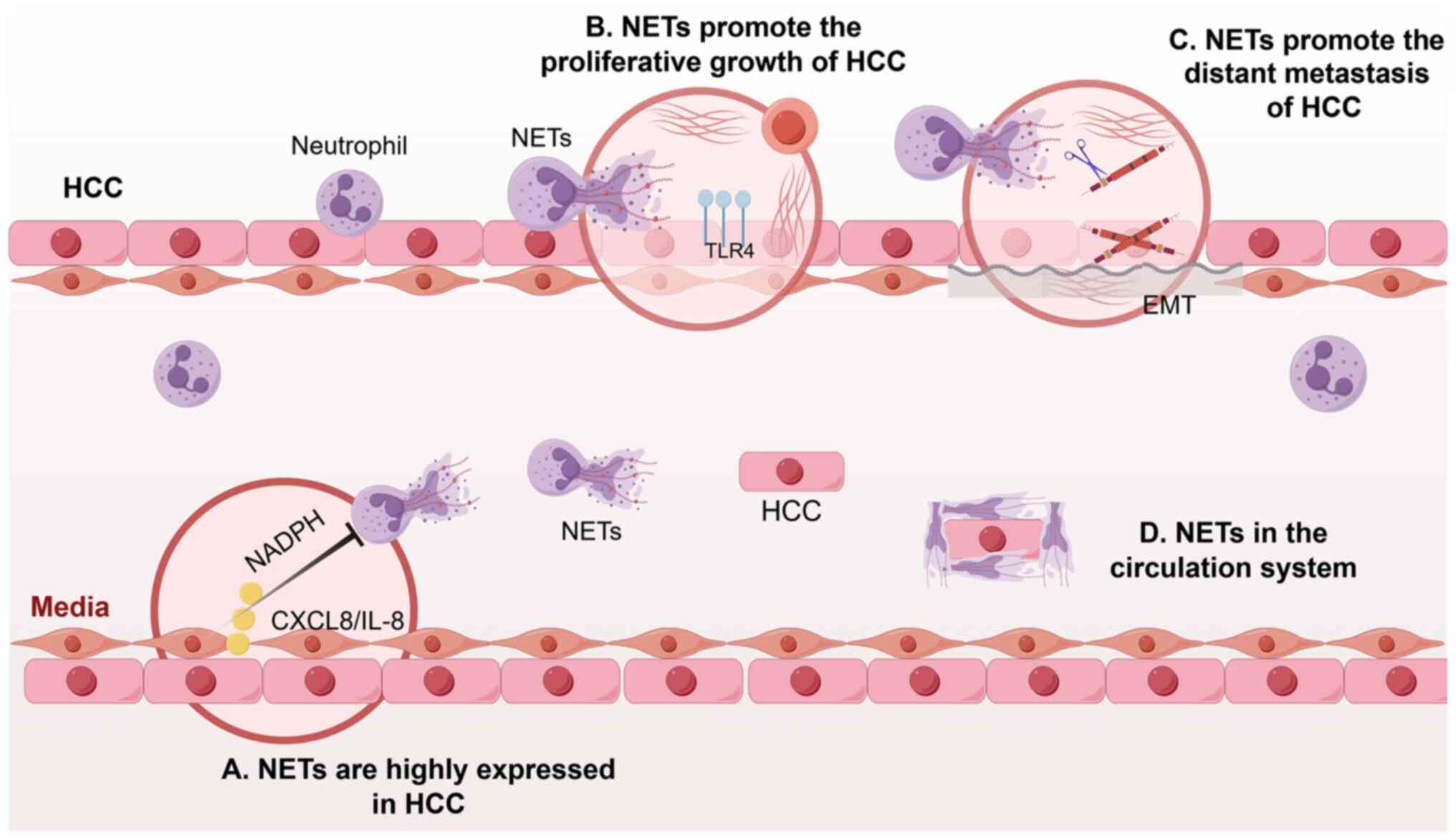
NETs are highly expressed in HCC
In HCC, higher NETs formation is associated with
raised biomarker levels, such as MPO-DNA in the bloodstream and H3
citrullination in tissue samples. These markers are extensively
utilized in clinical trials to identify and quantify NETs formation
(55). Kaltenmeier et al
(56), through a retrospective
analysis of serum and tissue samples from patients with liver
malignancies, demonstrated that preoperative NETs levels are
associated with patient survival and can serve as novel biomarkers
for predicting no recurrence and overall survival in individuals
with primary carcinoma of the liver. Guan et al (57) suggested that IL-8, derived from HCC
cells, stimulates the activation of tumor-associated neutrophils to
form NETs via the NADPH pathway (Fig.
3). Furthermore, a study assessing the levels of histone DNA,
double-stranded DNA and NE in the blood of patients with HCC
complicated by portal vein thrombosis (PVT) found that NETs markers
were positively associated with the severity of liver disease.
Disseminated tumor cells can induce NET formation and expedite the
development of PVT (58).
Furthermore, empirical evidence suggests that neutrophils producing
NETs are predominantly localized near blood vessels within HCC
tumor tissues (14). These
neutrophils enhance glycolysis by upregulating the pentose
phosphate pathway (59). The
investigations by Awasthi et al (60) demonstrated that glycolysis
facilitates the release of NETs via the NADPH oxidase-ROS pathway
and also that mitochondrial ROS (mitoROS) are implicated in various
aspects of energy metabolism and cellular homeostasis. A study has
revealed that neutrophils from patients with HCC exhibit elevated
levels of mitoROS and generate NETs enriched with oxidized
mitochondrial DNA in a mitoROS-dependent manner (61).
NETs promote the proliferation and
growth of HCC
Tumor growth is a multifaceted process influenced by
a variety of cellular and humoral environmental factors. Yazdani
et al (62). found that
neutrophil elastase (NE), produced by NETs, activates TLR4 in tumor
cells. This activation leads to increased mitochondrial metabolism,
which in turn enhances energy production and accelerates tumor cell
growth (62) (Fig. 3). Furthermore, by altering the
expression of the angiopoietin 2 (Ang-2) gene in endothelial cells,
NETs can promote the growth of tumors (63). Ang-2, belonging to the angiopoietin
family, is pivotal in altering tumor blood vessels across different
pathological states by variably influencing receptor signaling,
which in turn enhances tumor progression (64). NETs were found by Tohme et al
(14) to promote HCC growth and
metastasis. Inhibition of C-X-C motif chemokine ligand 2 can reduce
neutrophil recruitment to the tumor site and subsequent NETs
formation, thereby impeding the progression of HCC.
NETs promote the metastasis of
HCC
Research has demonstrated that NETs facilitate the
metastasis of liver cancer. Specifically, NETs contribute to the
degradation of the extracellular matrix through surface-bound
proteases, including matrix metallopeptidase 9 and NE. This process
results in the release of vascular endothelial growth factor, which
subsequently enhances tumor invasion and promotes angiogenesis
(31). On the other hand, research
has indicated that NETs serve a role in promoting the process of
epithelial-mesenchymal transition (EMT) within cancer cells.
Mechanistic investigations revealed that during NETs formation and
release, hypochlorous acid is generated, resulting in the
chlorination of tyrosine residues within the LKDYEDLR peptide
segment of laminin in the extracellular matrix. This modification
of laminin subunit γ-1 by hypochlorous acid can activate the
integrin/focal adhesion kinase signaling pathway, subsequently
modulating the expression of molecules associated with EMT. This
process enhances the migratory and invasive capabilities of tumor
cells, thereby advancing the progression of HCC (65) (Fig.
3).
Additionally, NETs can induce the downregulation of
VE-cadherin in endothelial cells, compromising the integrity of the
tumor vasculature, facilitating tumor infiltration and promoting
tumor cell metastasis (66). Within
the bloodstream, NETs can encapsulate circulating tumor cells
(CTCs) with platelets, creating a robust physical barrier that
impedes the interaction between immune cells and CTCs, thereby
facilitating the immune evasion of tumor cells (67) (Fig.
3). Additionally, NETs can augment the adhesive properties of
tumors, leading to the capture of CTCs by NETs, which subsequently
form stable micro-metastatic foci and progress to larger metastatic
sites (68).
In a previous study, it was reported that
co-culturing NETs with liver cancer cell lines, specifically Hep3B
and CSQT-2, enhances the migratory capacity of these cells.
Similarly, the study demonstrated that NETs facilitate inflammatory
cell infiltration in the livers of C57BL/6 mice and lead to
elevated Ki-67 protein levels in liver tissue following tail vein
injection of metastatic tumors (69). This implies that NETs aid in the
spread of liver cancer cells throughout the liver (55). Furthermore, research by Guan et
al (57) revealed a notable
increase in NET formation within the neutrophils of patients with
HCC, particularly those with metastatic HCC. These NETs possess the
ability to ensnare HCC cells, leading to increased resistance to
apoptosis and greater invasiveness, thereby boosting their
metastatic capabilities. This process is fueled by the
TLR4/9-cyclooxygenase-2 (COX2) signaling pathway being activated as
a result of HCC cells absorbing NETs. Blocking this pathway can
markedly reduce the metastatic potential that NETs confer.
Additionally, DNase 1 can directly break down NETs and, when used
alongside anti-inflammatory drugs such as aspirin and
hydroxychloroquine, it showed notable effectiveness in decreasing
HCC metastasis in mouse models (57). A recent study also demonstrated that
gut microbiota dysbiosis can promote intrahepatic metastasis in
mouse models by enhancing neutrophil-mediated inflammatory
responses and leading to the excessive formation of NETs. This
implies that the formation of NETs can be reduced through healthy
fecal microbiota transplantation, thereby inhibiting tumor
angiogenesis and tissue necrosis, to prevent and treat intrahepatic
metastasis of HCC (70).
NETs promote the transformation of
hepatitis into HCC
A previous study indicates that >20% of tumors
are attributable to chronic inflammation resulting from infections
(4). The inflammatory
microenvironment can impair the cytotoxic function of human immune
cells against tumor cells, enhance extracellular matrix deposition
and angiogenesis and ultimately initiate tumorigenesis. Research
has demonstrated that Helicobacter pylori infection can
induce chronic gastritis, with prolonged inflammatory responses
potentially facilitating the progression to gastric cancer
(71). Similarly, chronic
pancreatitis can result in sustained inflammation of pancreatic
tissue, thereby elevating the risk of pancreatic cancer (72). Chronic infection with HBV or HCV can
lead to persistent hepatitis, with long-term hepatic inflammation
potentially progressing to HCC (65). NET development is triggered by
inflammatory stimulation, which accelerates the progression of
inflammation to malignancy (73).
Research has indicated that compared to patients with HCC without
HBV infection, the levels of S100A9 protein are markedly elevated
in cancer tissues and serum of patients with HBV-associated HCC.
Activating neutrophils with S100A9 can induce NETs formation
through TLR4/receptor for advanced glycation end products receptors
and ROS (65) (Fig. 4).
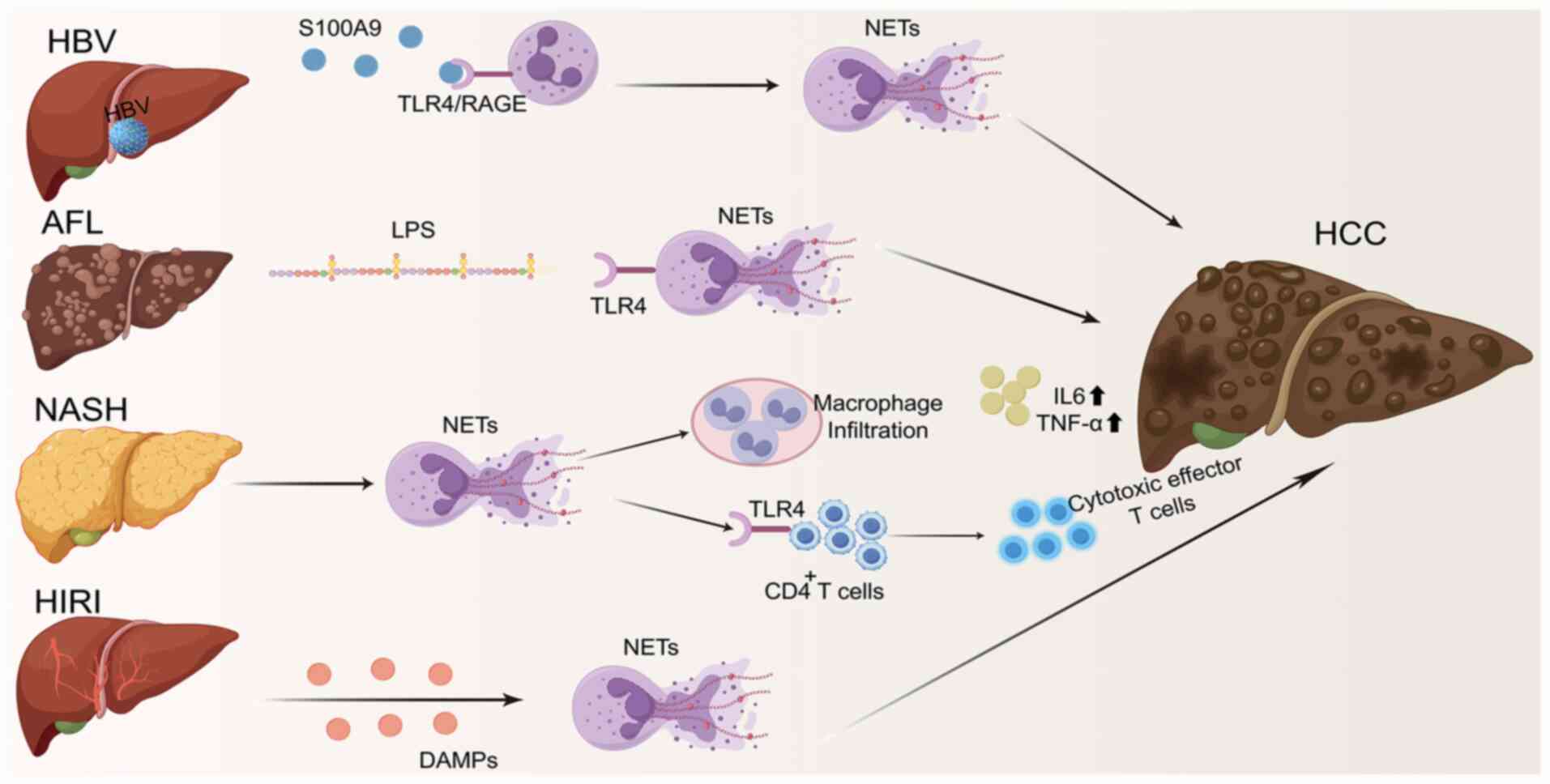 | Figure 4.NETs promote the transformation of
hepatitis into HCC. NETs can promote the transformation of HBV,
AFL, NASH and HIRI to HCC. The figure was drawn using Figdraw
(https://www.figdraw.com/static/index.html#/). HCC,
hepatocellular carcinoma; NETs, neutrophil extracellular traps;
TLR, Toll-like receptor; IL, interleukin; LPS, lipopolysaccharide;
HBV, hepatitis B virus; AFL, alcoholic fatty liver; NASH,
non-alcoholic steatohepatitis; HIRI, hepatic ischemia-reperfusion
injury; DAMPs, damage-associated molecular patterns; RAGE, receptor
for advanced glycation end products. |
Animal models have demonstrated that DNase I, ROS
scavengers (such as N-acetylcysteine) and S100A9 inhibitors (such
as paquinimod) effectively reduce NET formation while notably
inhibiting tumor growth and metastasis. This evidence confirms that
targeting the ‘HBV-S100A9-NETs’ axis represents a promising
therapeutic strategy (65).
Previous investigations uncovered a notable association between
serum levels of lipopolysaccharide (LPS) and MPO-DNA in individuals
suffering from alcoholic fatty liver disease and alcoholic HCC
(74). A previous study performed
on mouse models demonstrated that LPS originating from the gut
enhances the formation of NETs through the TLR4 signaling pathway,
exacerbating liver inflammation and advancing the transition from
alcoholic fatty liver disease to HCC (14) (Fig.
4).
In a study performed by van der Windt et al
(29), NETs generated in NASH mouse
models were shown to attract macrophage infiltration, elevate the
levels of inflammatory cytokines IL-6 and TNF-α, modify the
inflammatory milieu and consequently promote the development of
HCC. A study revealed that in NASH-associated liver disease,
neutrophils release NETs which, through the TLR4 signaling pathway,
alter the metabolic profile of CD4+ T cells. This
triggers their differentiation into immunosuppressive regulatory T
cells while suppressing the development of cytotoxic effector T
cells. The establishment of this immunosuppressive microenvironment
ultimately contributes to the progression of NASH-related HCC
(75). NETs serve a crucial role in
liver I/R injury. Following the initiation of hepatic I/R injury,
hepatocytes under stress release damage-associated molecular
patterns, which facilitate the infiltration of innate immune cells
and subsequently initiate an inflammatory cascade and cytokine
storm (76). Upon reperfusion,
neutrophils are among the earliest cells to infiltrate the hepatic
tissue. Within the liver, neutrophils may be pivotal in
exacerbating tissue damage and promoting tumor progression through
the formation of NETs, which contribute to the pro-metastatic
cascade (77) (Fig. 4).
Numerous studies have demonstrated that NETs
primarily serve a pro-tumor role (78–80).
Nonetheless, investigations within alternative cancer models
indicate a limited involvement of neutrophils during the initial
phases of tumorigenesis. Spiegel et al (81) demonstrated that the depletion of
neutrophils markedly reduced lung metastasis in models of
pancreatic and breast cancer, yet had no discernible effect on the
growth of primary tumors. These apparent inconsistencies do not
necessarily indicate fundamental contradictions but rather
underscore the intricate and dynamic nature of neutrophil biology
within the tumor microenvironment. Factors contributing to these
variations include distinct triggers of NETosis, such as IL-8 and
LPS, heterogeneity in NETs composition and the diverse stages of
HCC progression. Despite these complexities, NETs generally exhibit
pro-tumorigenic effects during the transformation of HCC. Future
research exploring the dual roles of NETs in both promoting and
inhibiting tumorigenesis will be essential for the development of
targeted therapeutic strategies.
Treatment strategies targeting NETs in
HCC
HCC, a malignant neoplasm characterized by high
global incidence and mortality rates, has attracted considerable
attention from the medical community in efforts to advance
treatment modalities. NETs are integral to the pathogenesis,
progression, invasion, metastasis and thrombus formation associated
with tumors, thereby representing a promising target for
oncological therapies (82). In
light of the relevance of NETs in HCC, researchers have
investigated various therapeutic strategies aimed at targeting NETs
to enhance treatment efficacy and improve patient quality of
life.
The release of NETs can be effectively inhibited
through the administration of pharmacological agents such as PAD4
inhibitors (83), NE inhibitors
(84) and gasdermin D pore
formation inhibitors (85). These
agents function by suppressing critical enzymes or molecules
integral to NETs formation, thereby diminishing their
production.
Enzymatic degradation of the DNA components of NETs
is achieved using DNase I, which disrupts the structural integrity
of the DNA scaffold. Furthermore, the synergistic application of
DNase I with anti-inflammatory agents, including aspirin and
hydroxychloroquine, demonstrated efficacy in markedly reducing HCC
metastasis in murine models (86).
NETs facilitate the capture of HCC cells via the TLR4/9-COX2
signaling pathway, thereby enhancing their invasive and metastatic
capabilities. Inhibition of this signaling pathway has the
potential to abrogate the metastatic propensity induced by NETs
(55).
However, currently, no phase III clinical trials
explicitly focus on NETs as a primary target in HCC, and
translating NET-targeted therapy from preclinical findings to
clinical application faces multiple challenges. Firstly, the
etiologies of HCC are diverse and its tumor microenvironment, as
well as the specific roles/mechanisms of NETs, exhibit notable
heterogeneity. Therefore, it is necessary to identify specific
predictive biomarkers for NET-targeted therapy response to help
understand treatment outcomes timely and accurately. Secondly, NETs
serve dual roles in combating infections and promoting tumor
metastasis. Indiscriminate suppression of NETs may increase
treatment risks. Determining the therapeutic time window and
precisely targeting pathogenic NETs (rather than all NETs) is
critical. Additionally, if NET-targeted therapy is applied
clinically, urgent issues such as optimizing combination regimens
with current treatments, managing overlapping toxicities and
screening appropriate indications will need to be addressed.
Limitations of current research
Although numerous preclinical findings support
targeting NETs for HCC treatment, current research in this field
still faces numerous limitations.
Standardization issues in NETs
detection and quantification
Current research lacks unified standards for the
biomarkers used to detect NETs (such as MPO-DNA complexes and H3
citrullination) and the technical methods employed (such as
immunofluorescence, ELISA and flow cytometry) (87). This makes it difficult to directly
compare results across different studies, thereby reference
intervals, medical decision levels and critical values for NETs
levels cannot be established. This hinders the application of NETs
as reliable biomarkers in clinical diagnosis and prognostic
evaluation.
Translational limitations of animal
models
Current research heavily relies on mouse models.
However, there are inherent differences between mice and humans in
terms of immune system function, liver physiology and the
mechanisms of NETs formation (88).
For example, mouse neutrophils exhibit different responsiveness to
certain stimuli compared with human neutrophils (8). Consequently, the efficacy and safety
of various targeted therapeutic strategies that demonstrate
promising results in animal models (such as DNase I and PAD4
inhibitors) still require validation in humans.
Relationship between NETs and HCC
treatment resistance
Previous studies have shown that levels of NETs are
elevated in patients with breast cancer and animal models with
developing drug resistance (89).
However, to the best of our knowledge, there are currently no
clinical studies on how NETs affect HCC resistance to targeted
therapies (such as sorafenib) and immune checkpoint inhibitors. The
main reason is that it is currently unclear which specific
components within NETs (such as NE, MPO or HMGB1 (High mobility
group box-1 protein) act as key effector molecules driving
resistance. Additionally, the precise signaling pathways that lead
to tumor cell desensitization to sorafenib or immune cell
unresponsiveness to programmed cell death protein 1 (PD-1)
inhibitors are yet to be elucidated. Due to the majority of current
studies being based on preclinical mouse models, it remains unclear
whether the levels of tumor-associated or circulating NETs in
patients with HCC undergoing treatment with sorafenib or immune
checkpoint inhibitors can serve as reliable biomarkers for
predicting treatment efficacy and survival outcomes. In conclusion,
further investigation is required to understand the mechanisms by
which NETs contribute to HCC resistance to targeted therapies, such
as sorafenib and immune checkpoint inhibitors, as well as the
potential for NET-targeted therapies to overcome such
resistance.
Summary and outlook
The present study systematically reviewed the
critical role of NETs in the inflammatory microenvironment of
chronic liver diseases and their transition to HCC. There is
growing evidence that NETs are not only a key component of the
liver's inflammatory response but also a critical mediator
promoting the ‘inflammation-to-cancer’ transition. In various liver
diseases, including HBV infection, NASH and HIRI, NETs notably
drive the initiation and progression of HCC by sustaining chronic
inflammation, disrupting immune surveillance and promoting tumor
cell proliferation, invasion and metastasis. Targeting NETs
formation (such as using PAD4 or NE inhibitors), degrading their
structure (such as with DNase I) or blocking related signaling
pathways (such as the TLR4/9-COX2 axis) has demonstrated promising
anti-HCC effects in preclinical studies, particularly in inhibiting
tumor metastasis and improving the tumor immune microenvironment.
Additionally, strategies to modulate the gut microbiota to reduce
NETs formation offer novel approaches for HCC prevention and
treatment.
In summary, the present research underscores the
notable role of the neutrophilic microenvironment, specifically
NETs, in the progression of HCC. At present, there is no research
on the dual application of NETs targeting agents and immunotherapy
in the literature. Due to the pivotal functions of NETs in
establishing immunosuppressive microenvironments and facilitating
therapeutic resistance, the integration of NET inhibitors such as
DNase I to degrade NETs, PAD4 inhibitors to prevent their formation
or specific antibodies to neutralize their components (90), with current PD-1/programmed death
ligand 1 inhibitors is poised to become a novel therapeutic
approach in the future. This strategy aims to dismantle the
physical and biochemical barriers imposed by NETs, thereby
enhancing T-cell infiltration and cytotoxicity within tumors and
reversing resistance to immune therapies. As this concept moves
toward clinical implementation, ensuring safety remains a critical
concern. Future research should prioritize assessing the long-term
effects of NET suppression on the body's anti-infective immunity
and developing strategies to achieve localized efficacy of NETs
within the tumor microenvironment. Identifying specific NET-related
biomarkers for patient selection will be essential in advancing
this precision combination therapy model.
Acknowledgements
Not applicable.
Funding
The present work was supported by the National Nature Science
Foundation of China (grant no. 31900569), Science and Technology
Research Project of Henan Province Science and Technology
Development Plan (grant no. 232102310196), Key Scientific Research
Projects of Henan Province Universities (grant no. 26A320010),
Henan Medical Science & Technology Research Plan
Co-construction Project (grant no. LHGJ20250488) and Postgraduate
Scientific and Technological Innovation Support Program of Xinxiang
Medical College (grant no. YJSCX202442Y).
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization was performed by CG and HL. YW and
CZ wrote the original draft. HW, YZ and YY reviewed and edited the
manuscript. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
The patient consents to publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Medzhitov R: Origin and physiological
roles of inflammation. Nature. 454:428–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Engblom C, Pfirschke C and Pittet MJ: The
role of myeloid cells in cancer therapies. Nat Rev Cancer.
16:447–462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Liu L, Zhou Y, Zhou P, Yan Q, Chen
X, Ding S and Zhu F: CKLF1 enhances inflammation-mediated
carcinogenesis and prevents doxorubicin-induced apoptosis via
IL6/STAT3 signaling in HCC. Clin Cancer Res. 25:4141–4154. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen Y, Zhu Y, Zhang C, Yang X, Gao Y, Li
M, Yang H, Liu T and Tang H: Chronic inflammation, cancer
development and immunotherapy. Front Pharmacol. 13:10401632022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miao WG, Zhou JY and Han RQ: Analysis of
global liver cancer statistics. Zhonghua Liu Xing Bing Xue Za Zhi.
45:865–869. 2024.(In Chinese). PubMed/NCBI
|
|
7
|
Nakagawa H and Maeda S: Inflammation- and
stress-related signaling pathways in hepatocarcinogenesis. World J
Gastroenterol. 18:4071–4081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liew PX and Kubes P: The neutrophil's role
during health and disease. Physiol Rev. 99:1223–1248. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brinkmann V, Reichard U, Goosmann C,
Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y and Zychlinsky A:
Neutrophil extracellular traps kill bacteria. Science.
303:1532–1535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hickey MJ and Kubes P: Intravascular
immunity: The host-pathogen encounter in blood vessels. Nat Rev
Immunol. 9:364–375. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Gao Q, Wu W, Hai S, Hu J, You J,
Huang D, Wang H, Wu D, Han M, et al: FGL2-MCOLN3-autophagy
axis-triggered neutrophil extracellular traps exacerbate liver
injury in fulminant viral hepatitis. Cell Mol Gastroenterol
Hepatol. 14:1077–1101. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ackermann M, Anders HJ, Bilyy R, Bowlin
GL, Daniel C, De Lorenzo R, Egeblad M, Henneck T, Hidalgo A,
Hoffmann M, et al: Patients with COVID-19: In the dark-NETs of
neutrophils. Cell Death Differ. 28:3125–3139. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corsiero E, Pratesi F, Prediletto E,
Bombardieri M and Migliorini P: NETosis as source of autoantigens
in rheumatoid arthritis. Front Immunol. 7:4852016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tohme S, Yazdani HO, Al-Khafaji AB, Chidi
AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H and Tsung A:
Neutrophil extracellular traps promote the development and
progression of liver metastases after surgical stress. Cancer Res.
76:1367–1380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Liu F, Chen L, Fang C, Li S, Yuan
S, Qian X, Yin Y, Yu B, Fu B, et al: Neutrophil extracellular traps
(NETs) promote non-small cell lung cancer metastasis by suppressing
lncRNA MIR503HG to activate the NF-κB/NLRP3 inflammasome pathway.
Front Immunol. 13:8675162022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu Y, Tao J, Gu Y, Liu Y, Qiu J, Su D,
Wang R, Luo W, Liu T, Zhang F, et al: Multiomics integration
reveals NETosis heterogeneity and TLR2 as a prognostic biomarker in
pancreatic cancer. NPJ Precis Onc. 8:1092024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herranz R, Oto J, Hueso M, Plana E, Cana
F, Castaño M, Cordón L, Ramos-Soler D, Bonanad S, Vera-Donoso CD,
et al: Bladder cancer patients have increased NETosis and impaired
DNaseI-mediated NET degradation that can be therapeutically
restored in vitro. Front Immunol. 14:11710652023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu W, Fan C, Dong S, Li X, Chen H and
Zhou W: Neutrophil extracellular traps regulating tumorimmunity in
hepatocellular carcinoma. Front Immunol. 14:12539642023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Wu S, Zhao Y, Dinh T, Jiang D,
Selfridge JE, Myers G, Wang Y, Zhao X, Tomchuck S, et al:
Neutrophil extracellular traps induced by chemotherapy inhibit
tumor growth in murine models of colorectal cancer. J Clin Invest.
134:e1750312024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Wu R, Zhan X, Wang XY, Xiang LW,
Duan YQ, You Y, Zhang JB, Wu R, Zhang YY and Duan L: Neutrophil
extracellular traps facilitate liver inflammation/fibrosis
progression by entering macrophages and triggering AIM2
inflammasome-dependent pyroptosis. Cell Commun Signal. 22:5562024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Varricchi G, Modestino L, Poto R,
Cristinziano L, Gentile L, Postiglione L, Spadaro G and Galdiero
MR: Neutrophil extracellular traps and neutrophil-derived mediators
as possible biomarkers in bronchial asthma. Clin Exp Med.
22:285–300. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vorobjeva NV and Chernyak BV: NETosis:
Molecular mechanisms, role in physiology and pathology.
Biochemistry (Mosc). 85:1178–1190. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eghbalzadeh K, Georgi L, Louis T, Zhao H,
Keser U, Weber C, Mollenhauer M, Conforti A, Wahlers T and
Paunel-Görgülü A: Compromised anti-inflammatory action of
neutrophil extracellular traps in PAD4-deficient mice contributes
to aggravated acute inflammation after myocardial infarction. Front
Immunol. 10:23132019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sofoluwe A, Bacchetta M, Badaoui M, Kwak
BR and Chanson M: ATP amplifies NADPH-dependent and -independent
neutrophil extracellular trap formation. Sci Rep. 9:165562019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leshner M, Wang S, Lewis C, Zheng H, Chen
XA, Santy L and Wang Y: PAD4 mediated histone hypercitrullination
induces heterochromatin decondensation and chromatin unfolding to
form neutrophil extracellular trap-like structures. Front Immun.
3:3072012. View Article : Google Scholar
|
|
26
|
Tokuhiro T, Ishikawa A, Sato H, Takita S,
Yoshikawa A, Anzai R, Sato S, Aoyagi R, Arita M, Shibuya T, et al:
Oxidized phospholipids and neutrophil elastase coordinately play
critical roles in NET formation. Front Cell Dev Biol. 9:7185862021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yousefi S, Mihalache C, Kozlowski E,
Schmid I and Simon HU: Viable neutrophils release mitochondrial DNA
to form neutrophil extracellular traps. Cell Death Differ.
16:1438–1444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang C, Wang Z, Li L, Zhang Z, Jin X, Wu
P, Sun S, Pan J, Su K, Jia F, et al: Aged neutrophils form
mitochondria-dependent vital NETs to promote breast cancer lung
metastasis. J Immunother Cancer. 9:e0028752021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Der Windt DJ, Sud V, Zhang H, Varley
PR, Goswami J, Yazdani HO, Tohme S, Loughran P, O'Doherty RM,
Minervini MI, et al: Neutrophil extracellular traps promote
inflammation and development of hepatocellular carcinoma in
nonalcoholic steatohepatitis. Hepatology. 68:1347–1360. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tohme S, Yazdani HO, Sud V, Loughran P,
Huang H, Zamora R, Simmons RL, Vodovotz Y and Tsung A:
Computational analysis supports IL-17A as a central driver of
neutrophil extracellular trap-mediated injury in liver ischemia
reperfusion. J Immunol. 202:268–277. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Q, Zhang L, Li X and Zhuo W:
Neutrophil extracellular traps in tumor metastasis: Pathological
functions and clinical applications. Cancers (Basel). 13:28322021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burki T: WHO's 2024 global hepatitis
report. Lancet Infect Dis. 24:e362–e363. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu W, Sun S, Wang Y, Zhao R, Ren H, Li Z,
Zhao H, Zhang Y, Sheng J, Chen Z and Shi Y: Circulating neutrophil
dysfunction in HBV-related acute-on-chronic liver failure. Front
Immunol. 12:6203652021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Shi K, Zhu B, Feng Y, Liu Y and
Wang X: Neutrophil extracellular trap scores predict 90-day
mortality in hepatitis B-related acute-on-chronic liver failure.
Biomedicines. 12:20482024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu S, Liu X, Gao Y, Zhou R, Wei M, Dong J,
Yan H and Zhao Y: Hepatitis B virus inhibits neutrophil
extracellular trap release by modulating reactive oxygen species
production and autophagy. J Immunol. 202:805–815. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao Q, Chen DP, Chen HD, Wang YZ, Shi W,
Lu YT, Ren YZ, Wu YK, Pang YH, Deng H, et al: NK-cell-elicited
gasdermin-D-dependent hepatocyte pyroptosis induces neutrophil
extracellular traps that facilitate HBV-related acute-on-chronic
liver failure. Hepatology. 81:917–931. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chalasani N, Younossi Z, Lavine JE,
Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM and Sanyal AJ:
The diagnosis and management of nonalcoholic fatty liver disease:
Practice guidance from the American association for the study of
liver diseases. Hepatology. 67:328–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kanda T, Goto T, Hirotsu Y, Masuzaki R,
Moriyama M and Omata M: Molecular mechanisms: Connections between
nonalcoholic fatty liver disease, steatohepatitis and
hepatocellular carcinoma. Int J Mol Sci. 21:15252020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu L, Gao X, Guo Q, Li J, Yao J, Yan K, Xu
Y, Jiang X, Ye D and Guo J: The role of neutrophils in innate
immunity-driven nonalcoholic steatohepatitis: Lessons learned and
future promise. Hepatol Int. 14:652–666. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hwang S, Yun H, Moon S, Cho YE and Gao B:
Role of neutrophils in the pathogenesis of nonalcoholic
steatohepatitis. Front Endocrinol (Lausanne). 12:7518022021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rensen SS, Bieghs V, Xanthoulea S,
Arfianti E, Bakker JA, Shiri-Sverdlov R, Hofker MH, Greve JW and
Buurman WA: Neutrophil-derived myeloperoxidase aggravates
non-alcoholic steatohepatitis in low-density lipoprotein
receptor-deficient mice. PLoS One. 7:e524112012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen J, Liang B, Bian D, Luo Y, Yang J, Li
Z, Zhuang Z, Zang S and Shi J: Knockout of neutrophil elastase
protects against western diet induced nonalcoholic steatohepatitis
in mice by regulating hepatic ceramides metabolism. Biochem Biophys
Res Commun. 518:691–697. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu L, Liu X, Jia T, Sun Y, Du Y, Wei S,
Wang W, Zhang Y, Chen W and Zhang S: Tanshinone IIA ameliorates
nonalcoholic steatohepatitis in mice by modulating neutrophil
extracellular traps and hepatocyte apoptosis. Evid Based Complement
Alternat Med. 2022:57693502022.PubMed/NCBI
|
|
44
|
Xu M, Xu H, Ling YW, Liu JJ, Song P, Fang
ZQ, Yue ZS, Duan JL, He F and Wang L: Neutrophil extracellular
traps-triggered hepatocellular senescence exacerbates lipotoxicity
in non-alcoholic steatohepatitis. J Adv Res. Mar 9–2025.(Epub ahead
of print). View Article : Google Scholar
|
|
45
|
Jiménez-Castro MB, Cornide-Petronio ME,
Gracia-Sancho J and Peralta C: Inflammasome-mediated inflammation
in liver ischemia-reperfusion injury. Cells. 8:11312019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang S, Zhang Q, Wang F, Guo X, Liu T,
Zhao Y, Gu B, Chen H and Li Y: Hydroxychloroquine inhibiting
neutrophil extracellular trap formation alleviates hepatic
ischemia/reperfusion injury by blocking TLR9 in mice. Clin Immunol.
216:1084612020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Arumugam S, Girish Subbiah K, Kemparaju K
and Thirunavukkarasu C: Neutrophil extracellular traps in acrolein
promoted hepatic ischemia reperfusion injury: Therapeutic potential
of NOX2 and p38MAPK inhibitors. J Cell Physiol. 233:3244–3261.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu X, Yang Z, Wang H, Zhao Y, Gao X and
Zang B: High-mobility group box protein-1 induces acute
pancreatitis through activation of neutrophil extracellular trap
and subsequent production of IL-1β. Life Sci. 286:1192312021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yazdani HO, Chen HW, Tohme S, Tai S, van
der Windt DJ, Loughran P, Rosborough BR, Sud V, Beer-Stolz D,
Turnquist HR, et al: IL-33 exacerbates liver sterile inflammation
by amplifying neutrophil extracellular trap formation. J Hepatol.
68:130–139. 2018. View Article : Google Scholar
|
|
50
|
Huang H, Tohme S, Al-Khafaji AB, Tai S,
Loughran P, Chen L, Wang S, Kim J, Billiar T, Wang Y and Tsung A:
Damage-associated molecular pattern-activated neutrophil
extracellular trap exacerbates sterile inflammatory liver injury.
Hepatology. 62:600–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang CL, Wang Y, Jiang QL, Zeng Y, Yao QP,
Liu X, Li T and Jiang J: DNase I and sivelestat ameliorate
experimental hindlimb ischemia-reperfusion injury by eliminating
neutrophil extracellular traps. J Inflamm Res. 16:707–721. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang HT, Tong ZJ, Lin YR, Wei KC, Huang
CY, Chen PY, Chen KT, Lin YJ and Tsai HC: Acrolein-induced PKM2
modification drives NETosis and glioma progression. Free Radic Biol
Med. 241:567–581. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Burcham PC: Acrolein and human disease:
Untangling the knotty exposure scenarios accompanying several
diverse disorders. Chem Res Toxicol. 30:145–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xie M, He Z, Bin B, Wen N, Wu J, Cai X and
Sun X: Bulk and single-cell RNA sequencing analysis with 101
machine learning combinations reveal neutrophil extracellular trap
involvement in hepatic ischemia-reperfusion injury and early
allograft dysfunction. Int Immunopharmacol. 131:1118742024.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei
R, Lin ZF, Wang XY, Wang CQ, Lu M, et al: Increased neutrophil
extracellular traps promote metastasis potential of hepatocellular
carcinoma via provoking tumorous inflammatory response. J Hematol
Oncol. 13:32020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kaltenmeier CT, Yazdani H, Van der Windt
D, Molinari M, Geller D, Tsung A and Tohme S: Neutrophil
extracellular traps as a novel biomarker to predict recurrence-free
and overall survival in patients with primary hepatic malignancies.
HPB (Oxford). 23:309–320. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guan X, Lu Y, Zhu H, Yu S, Zhao W, Chi X,
Xie C and Yin Z: The crosstalk between cancer cells and neutrophils
enhances hepatocellular carcinoma metastasis via neutrophil
extracellular traps-associated cathepsin G component: A potential
therapeutic target. J Hepatocell Carcinoma. 8:451–465. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang L, Liu Q, Zhang X, Liu X, Zhou B,
Chen J, Huang D, Li J, Li H, Chen F, et al: DNA of neutrophil
extracellular traps promotes cancer metastasis via CCDC25. Nature.
583:133–138. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang L, Liu Y, Dai Y, Tang X, Yin T, Wang
C, Wang T, Dong L, Shi M, Qin J, et al: Single-cell RNA-seq
analysis reveals BHLHE40-driven pro-tumour neutrophils with
hyperactivated glycolysis in pancreatic tumour microenvironment.
Gut. 72:958–971. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Awasthi D, Nagarkoti S, Sadaf S, Chandra
T, Kumar S and Dikshit M: Glycolysis dependent lactate formation in
neutrophils: A metabolic link between NOX-dependent and independent
NETosis. Biochim Biophys Acta Mol Basis Dis. 1865:1655422019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang LY, Shen XT, Sun HT, Zhu WW, Zhang JB
and Lu L: Neutrophil extracellular traps in hepatocellular
carcinoma are enriched in oxidized mitochondrial DNA which is
highly pro-inflammatory and pro-metastatic. J Cancer. 13:1261–1271.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yazdani HO, Roy E, Comerci AJ, van der
Windt DJ, Zhang H, Huang H, Loughran P, Shiva S, Geller DA,
Bartlett DL, et al: Neutrophil extracellular traps drive
mitochondrial homeostasis in tumors to augment growth. Cancer Res.
79:5626–5639. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Srirajaskanthan R, Dancey G, Hackshaw A,
Luong T, Caplin ME and Meyer T: Circulating angiopoietin-2 is
elevated in patients with neuroendocrine tumours and correlates
with disease burden and prognosis. Endocr Relat Cancer. 16:967–976.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen Y, Hu H, Tan S, Dong Q, Fan X, Wang
Y, Zhang H and He J: The role of neutrophil extracellular traps in
cancer progression, metastasis and therapy. Exp Hematol Oncol.
11:992022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhan X, Wu R, Kong X, You Y, He K, Sun XY,
Huang Y, Chen WX and Duan L: Elevated neutrophil extracellular
traps by HBV-mediated S100A9-TLR4/RAGE-ROS cascade facilitate the
growth and metastasis of hepatocellular carcinoma. Cancer Commun
(Lond). 43:225–245. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jiang ZZ, Peng ZP, Liu XC, Guo HF, Zhou
MM, Jiang D, Ning WR, Huang YF, Zheng L and Wu Y: Neutrophil
extracellular traps induce tumor metastasis through dual effects on
cancer and endothelial cells. OncoImmunology. 11:20524182022.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ren J, He J, Zhang H, Xia Y, Hu Z,
Loughran P, Billiar T, Huang H and Tsung A: Platelet TLR4-ERK5 axis
facilitates NET-mediated capturing of circulating tumor cells and
distant metastasis after surgical stress. Cancer Res. 81:2373–2385.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cools-Lartigue J, Spicer J, McDonald B,
Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P and Ferri L:
Neutrophil extracellular traps sequester circulating tumor cells
and promote metastasis. J Clin Invest. 123:3446–3458. 2013.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang JL, Ma JB and Wang WX: Role and
mechanisms of neutrophil extracellular traps in hepatocellular
carcinoma metastasis. CJCB. 46:502–514. 2024.
|
|
70
|
Deng Z, Mei S, Ouyang Z, Wang R, Wang L,
Zou B, Dai J, Mao K, Li Q, Guo Q, et al: Dysregulation of gut
microbiota stimulates NETs-driven HCC intrahepatic metastasis:
Therapeutic implications of healthy faecal microbiota
transplantation. Gut Microbes. 17:24765612025. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Holleczek B, Schöttker B and Brenner H:
Helicobacter pylori infection, chronic atrophic gastritis
and risk of stomach and esophagus cancer: Results from the
prospective population-based ESTHER cohort study. Int J Cancer.
146:2773–2783. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gandhi S, de la Fuente J, Murad MH and
Majumder S: Chronic pancreatitis is a risk factor for pancreatic
cancer, and incidence increases with duration of disease: A
systematic review and meta-analysis. Clin Transl Gastroenterol.
13:e004632022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Castanheira FVS and Kubes P: Neutrophils
and NETs in modulating acute and chronic inflammation. Blood.
133:2178–2185. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li C, Li M and Wang Z: There is a linear
negative correlation between lipoprotein(a) and non-alcoholic fatty
liver disease. Sci Rep. 15:85382025. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang H, Zhang H, Wang Y, Brown ZJ, Xia Y,
Huang Z, Shen C, Hu Z, Beane J, Ansa-Addo EA, et al: Regulatory
T-cell and neutrophil extracellular trap interaction contributes to
carcinogenesis in non-alcoholic steatohepatitis. J Hepatol.
75:1271–1283. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Roh JS and Sohn DH: Damage-associated
molecular patterns in inflammatory diseases. Immune Netw.
18:e272018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kaltenmeier C, Yazdani HO, Handu S, Popp
B, Geller D and Tohme S: The role of neutrophils as a driver in
hepatic ischemia-reperfusion injury and cancer growth. Front
Immunol. 13:8875652022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang Y, Wang Z, Lu Y, Sanchez DJ, Li J,
Wang L, Meng X, Chen J, Kien TT, Zhong M, et al: Region-specific
CD16+ neutrophils promote colorectal cancer progression
by inhibiting natural killer cells. Adv Sci (Weinh).
11:24034142024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gao J, Liu J, Lu J, Zhang X, Zhang W, Li
Q, Cai J, Li M, Gan Y, Tang Y and Wu S: SKAP1 expression in cancer
cells enhances colon tumor growth and impairs cytotoxic immunity by
promoting neutrophil extracellular trap formation via the
NFATc1/CXCL8 axis. Adv Sci (Weinh). 11:e24034302024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Poto R, Cristinziano L, Modestino L, de
Paulis A, Marone G, Loffredo S, Galdiero MR and Varricchi G:
Neutrophil extracellular traps, angiogenesis and cancer.
Biomedicines. 10:4312022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Spiegel A, Brooks MW, Houshyar S,
Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J,
Zervantonakis IK, et al: Neutrophils suppress intraluminal NK
Cell-mediated tumor cell clearance and enhance extravasation of
disseminated carcinoma cells. Cancer Discov. 6:630–649. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Masucci MT, Minopoli M, Del Vecchio S and
Carriero MV: The emerging role of neutrophil extracellular traps
(NETs) in tumor progression and metastasis. Front Immunol.
11:17492020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu X, Arfman T, Wichapong K,
Reutelingsperger CPM, Voorberg J and Nicolaes GAF: PAD4 takes
charge during neutrophil activation: Impact of PAD4 mediated NET
formation on immune-mediated disease. J Thromb Haemost.
19:1607–1617. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Okeke EB, Louttit C, Fry C, Najafabadi AH,
Han K, Nemzek J and Moon JJ: Inhibition of neutrophil elastase
prevents neutrophil extracellular trap formation and rescues mice
from endotoxic shock. Biomaterials. 238:1198362020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Han F, Chen H, Chen L, Yuan C, Shen Q, Lu
G, Chen W, Gong W, Ding Y, Gu A and Tao L: Inhibition of gasdermin
D blocks the formation of NETs and protects acute pancreatitis in
mice. Biochem Biophys Res Commun. 654:26–33. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
He XY, Gao Y, Ng D, Michalopoulou E,
George S, Adrover JM, Sun L, Albrengues J, Daßler-Plenker J, Han X,
et al: Chronic stress increases metastasis via neutrophil-mediated
changes to the microenvironment. Cancer Cell. 42:474–486.e12. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Papayannopoulos V: Neutrophil
extracellular traps in immunity and disease. Nat Rev Immunol.
18:134–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mestas J and Hughes CCW: Of mice and not
men: Differences between mouse and human immunology. J Immunol.
172:2731–2738. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mousset A, Lecorgne E, Bourget I, Lopez P,
Jenovai K, Cherfils-Vicini J, Dominici C, Rios G, Girard-Riboulleau
C, Liu B, et al: Neutrophil extracellular traps formed during
chemotherapy confer treatment resistance via TGF-β activation.
Cancer Cell. 41:757–775.e10. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Canè S, Barouni RM, Fabbi M, Cuozzo J,
Fracasso G, Adamo A, Ugel S, Trovato R, De Sanctis F, Giacca M, et
al: Neutralization of NET-associated human ARG1 enhances cancer
immunotherapy. Sci Transl Med. 15:eabq62212023. View Article : Google Scholar : PubMed/NCBI
|