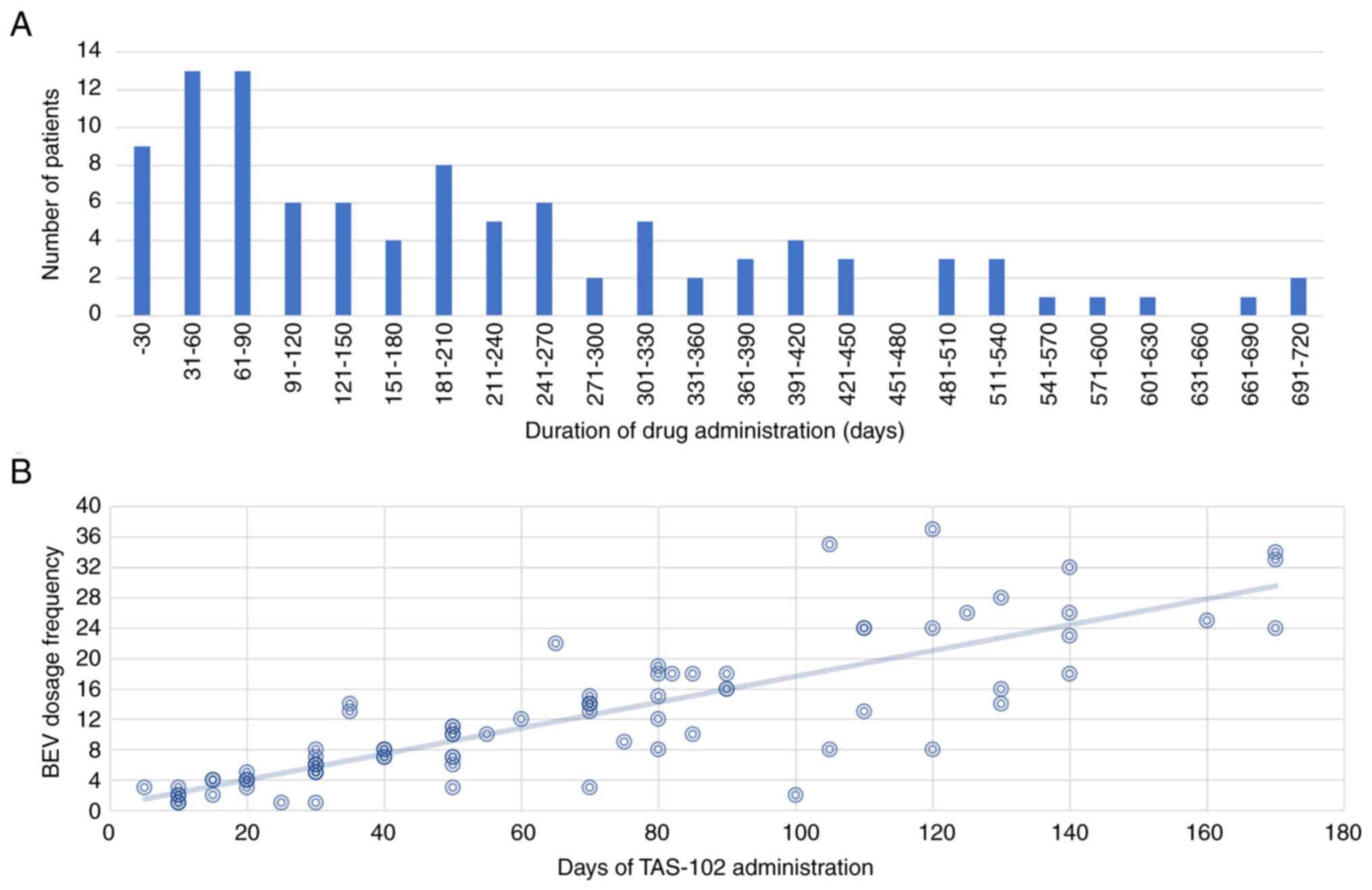
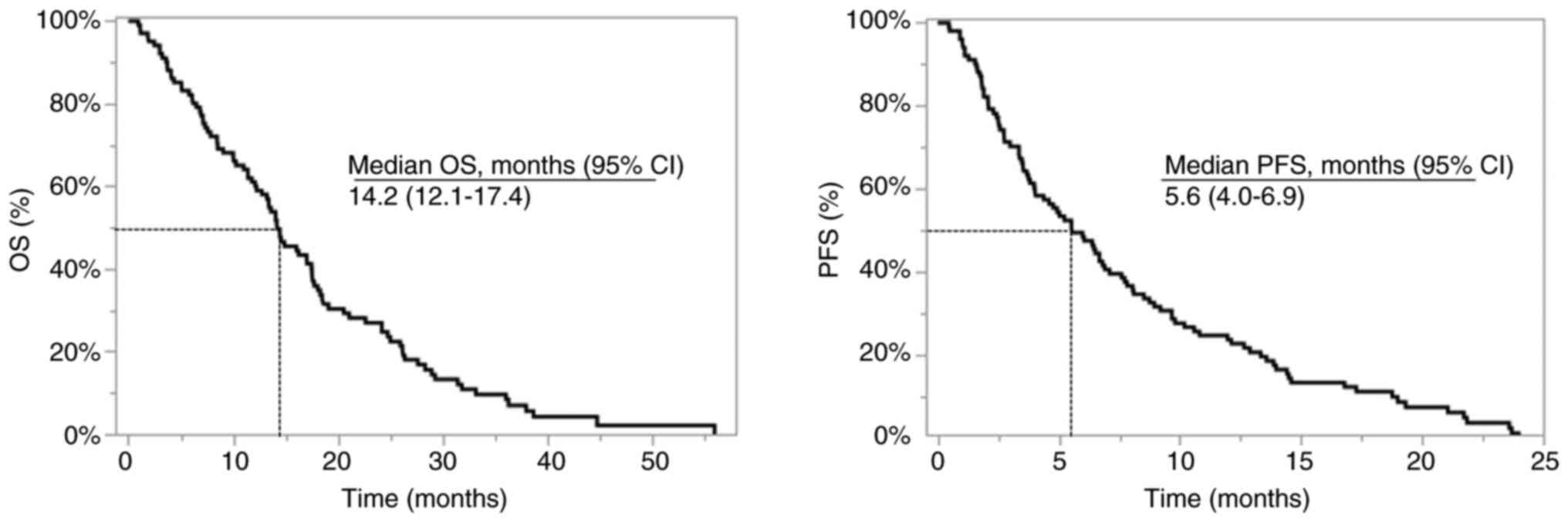
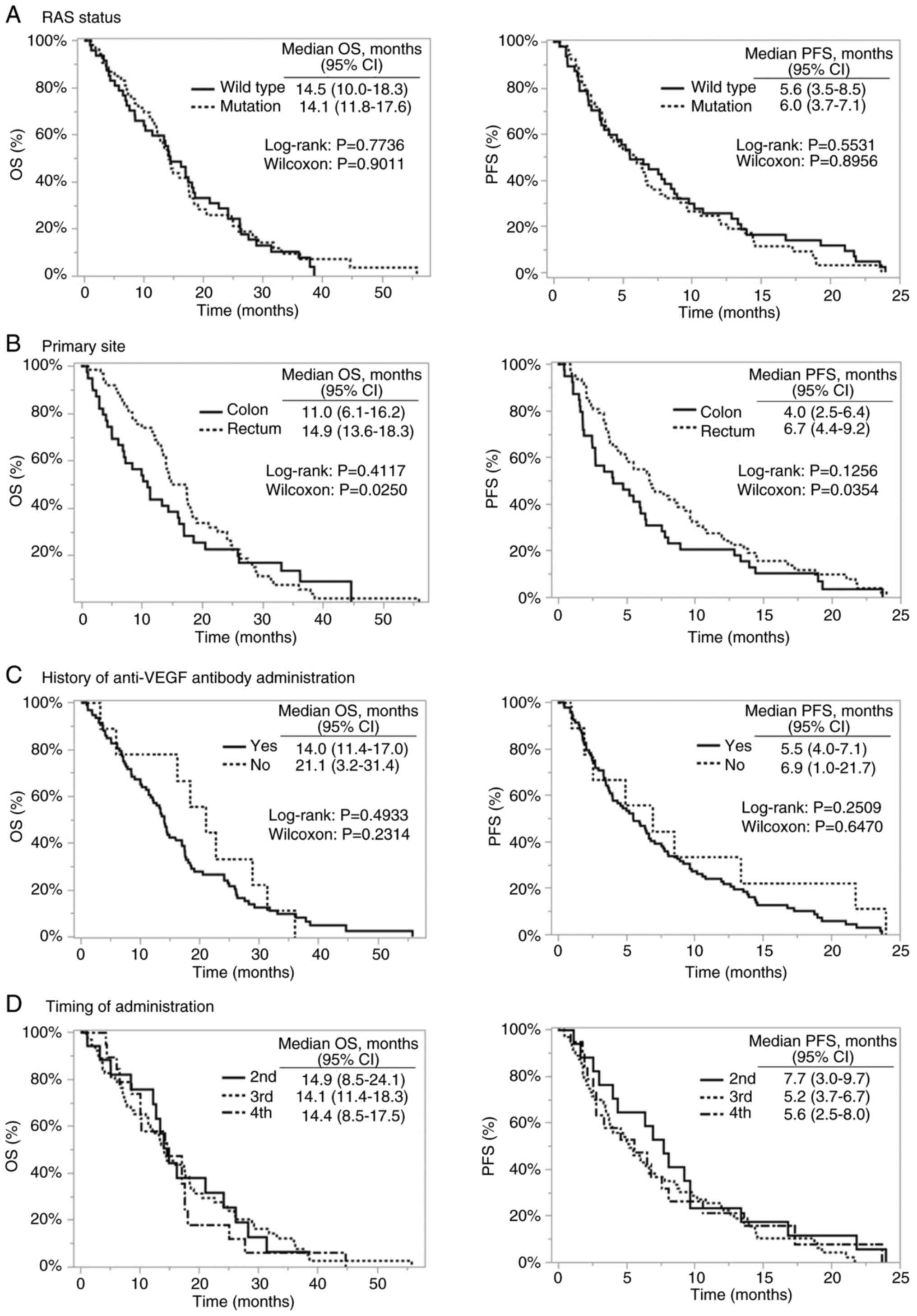
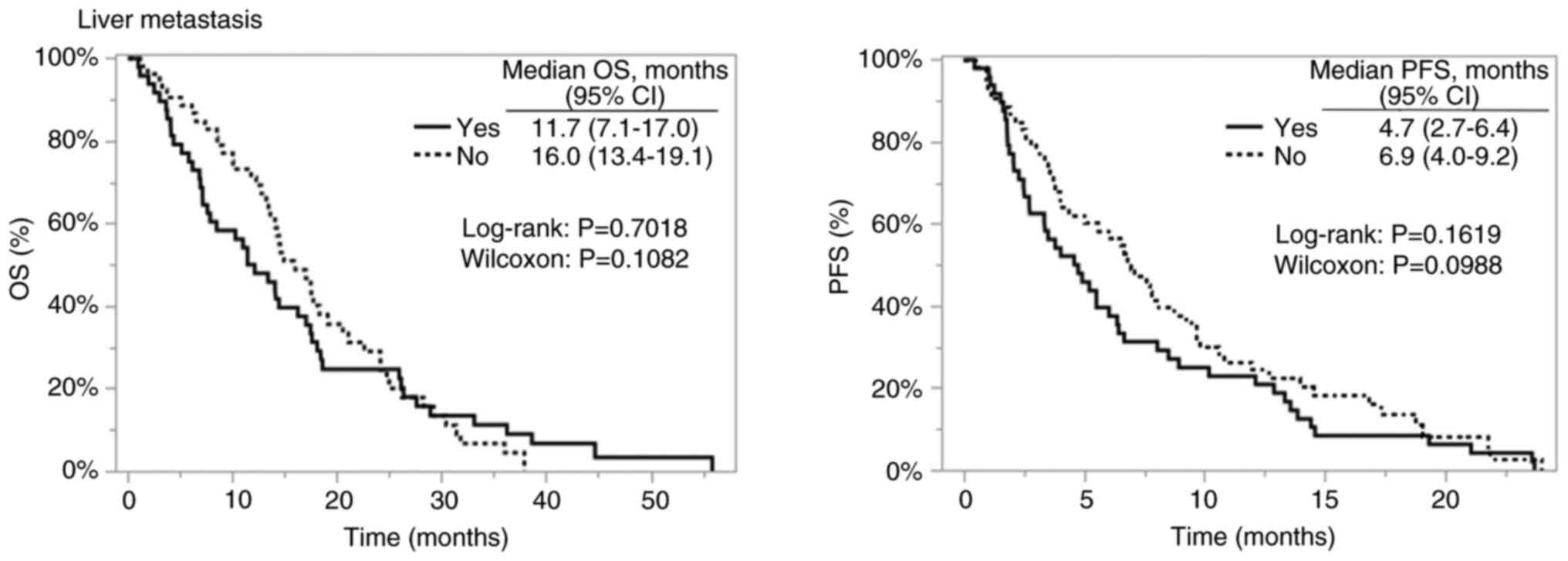
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48.
2023.PubMed/NCBI
|
|
2
|
Cremolini C, Loupakis F, Antoniotti C,
Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M,
Zaniboni A, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus
bevacizumab as first-line treatment of patients with metastatic
colorectal cancer: Updated overall survival and molecular subgroup
analyses of the open-label, phase 3 TRIBE study. Lancet Oncol.
16:1306–1315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shinozaki K, Yamada T, Nasu J, Matsumoto
T, Yuasa Y, Shiraishi T, Nagano H, Moriyama I, Fujiwara T, Miguchi
M, et al: A phase II study of folfoxiri plus bevacizumab as initial
chemotherapy for patients with untreated metastatic colorectal
cancer: TRICC1414 (BeTRI). Int J Clin Oncol. 26:399–408. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martínez-Lago N, Chucla TC, De Castro BA,
Ponte RV, Rendo CR, Rodriguez MIGR, Diaz SS, Suarez BG, Gomez JC,
Fernandez FB, et al: Efficacy, safety and prognostic factors in
patients with refractory metastatic colorectal cancer treated with
trifluridine/tipiracil plus bevacizumab in a real-world setting.
Sci Rep. 12:146122022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Satake H, Kato T, Oba K, Kotaka M, Kagawa
Y, Yasui H, Nakamura M, Watanabe T, Matsumoto T, Kii T, et al:
Phase Ib/II study of biweekly TAS-102 in combination with
bevacizumab for patients with metastatic colorectal cancer
refractory to standard therapies (BiTS Study). Oncologist.
25:e1855–e1863. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prager GW, Taieb J, Fakih M, Ciardiello F,
Van Cutsem E, Elez E, Cruz FM, Wyrwicz L, Stroyakovskiy D, Papai Z,
et al: Trifluridine-tipiracil and bevacizumab in refractory
metastatic colorectal cancer. N Engl J Med. 388:1657–1667. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuboki Y, Nishina T, Shinozaki E, Yamazaki
K, Shitara K, Okamoto W, Kajiwara T, Matsumoto T, Tsushima T,
Mochizuki N, et al: TAS-102 plus bevacizumab for patients with
metastatic colorectal cancer refractory to standard therapies
(C-TASK FORCE): An investigator-initiated, open-label, single-arm,
multicentre, phase 1/2 study. Lancet Oncol. 18:1172–1181. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chida K, Kotani D, Nakamura Y, Kawazoe A,
Kuboki Y, Kojima T, Taniguchi H, Watanabe J, Endo I and Yoshino T:
Efficacy and safety of trifluridine/tipiracil plus bevacizumab and
trifluridine/tipiracil or regorafenib monotherapy for
chemorefractory metastatic colorectal cancer: A retrospective
study. Ther Adv Med Oncol. April 20–2021.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujii H, Matsuhashi N, Kitahora M,
Takahashi T, Hirose C, Iihara H, Yamada Y, Watanabe D, Ishihara T,
Suzuki A and Yoshida K: Bevacizumab in combination with TAS-102
improves clinical outcomes in patients with refractory metastatic
colorectal cancer: A retrospective study. Oncologist. 25:e469–e476.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Joarder R, Patel D, Tiwari A, Choudhary J,
Vana P, Shenoy V, Mer N, Ramaswamy A, Bhargava P and Ostwal V:
TAS-102 plus bevacizumab as an effective and well tolerated regimen
in chemotherapy-refractory advanced colorectal cancers-A single
institution retrospective analysis. South Asian J Cancer. Jan
7–2025.(Epub ahead of print).
|
|
11
|
Liu CJ, Hu T, Shao P, Chu WY, Cao Y and
Zhang F: TAS-102 monotherapy and combination therapy with
bevacizumab for metastatic colorectal cancer. Gastroenterol Res
Pract. 2021:40146012021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brouwer NPM, Bos ACRK, Lemmens VEPP, Tanis
PJ, Hugen N, Nagtegaal ID, de Wilt JHW and Verhoeven RHA: An
overview of 25 years of incidence, treatment and outcome of
colorectal cancer patients. Int J Cancer. 143:2758–2766. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lemmens V, van Steenbergen L,
Janssen-Heijnen M, Martijn H, Rutten H and Coebergh JW: Trends in
colorectal cancer in the south of the Netherlands 1975–2007: Rectal
cancer survival levels with colon cancer survival. Acta Oncol.
49:784–796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Renouf DJ, Woods R, Speers C, Hay J, Phang
PT, Fitzgerald C and Kennecke H: Improvements in 5-year outcomes of
stage II/III rectal cancer relative to colon cancer. Am J Clin
Oncol. 36:558–564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fernandez-Martos C, Garcia-Albeniz X,
Pericay C, Maurel J, Aparicio J, Montagut C, Safont MJ, Salud A,
Vera R, Massuti B, et al: Chemoradiation, surgery and adjuvant
chemotherapy versus induction chemotherapy followed by
chemoradiation and surgery: Long-term results of the Spanish GCR-3
phase II randomized trial. Ann Oncol. 26:1722–1728. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yaffee P, Osipov A, Tan C, Tuli R and
Hendifar A: Review of systemic therapies for locally advanced and
metastatic rectal cancer. J Gastrointest Oncol. 6:185–200.
2015.PubMed/NCBI
|
|
17
|
Riihimäki M, Hemminki A, Sundquist J and
Hemminki K: Patterns of metastasis in colon and rectal cancer. Sci
Rep. 6:297652016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holch JW, Demmer M, Lamersdorf C, Michl M,
Schulz C, von Einem JC, Modest DP and Heinemann V: Pattern and
dynamics of distant metastases in metastatic colorectal cancer.
Visc Med. 33:70–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shinto E, Ike H, Ito M, Takahashi K, Ohue
M, Kanemitsu Y, Suto T, Kinugasa T, Watanabe J, Hida JI, et al:
Lateral node metastasis in low rectal cancer as a hallmark to
predict recurrence patterns. Int J Clin Oncol. 29:1896–1907. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi A, Sugito M, Ito M and Saito N:
Predictors of successful salvage surgery for local pelvic
recurrence of rectosigmoid colon and rectal cancers. Surg Today.
37:853–859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khattak MA, Martin HL, Beeke C, Price T,
Carruthers S, Kim S, Padbury R and Karapetis CS: Survival
differences in patients with metastatic colorectal cancer and with
single site metastatic disease at initial presentation: Results
from South Australian clinical registry for advanced colorectal
cancer. Clin Colorectal Cancer. 11:247–254. 2012.PubMed/NCBI
|
|
22
|
Pu H, Chen Y, Shen R, Zhang Y, Yang D, Liu
L, Dong X and Yang G: Influence of the initial recurrence site on
prognosis after radical surgery for colorectal cancer: A
retrospective cohort study. World J Surg Oncol. 21:1372023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawaguchi K, Uehara K, Nakayama G, Fukui
T, Fukumoto K, Nakamura S and Yokoi K: Growth rate of
chemotherapy-naïve lung metastasis from colorectal cancer could be
a predictor of early relapse after lung resection. Int J Clin
Oncol. 21:329–334. 2016. View Article : Google Scholar : PubMed/NCBI
|