Introduction
Colorectal cancer (CRC) is one of the most prevalent
malignancies worldwide, accounting for a significant proportion of
cancer-related morbidity and mortality. According to global cancer
statistics, CRC is the third most commonly diagnosed cancer and
second leading cause of cancer-related deaths (1). Despite advancements in surgical
techniques and early detection methods, many patients present with
locally advanced or metastatic disease at diagnosis, necessitating
systemic chemotherapy to improve outcomes.
For metastatic CRC (mCRC), systemic chemotherapy has
been a cornerstone of treatment and is often combined with targeted
therapies. The introduction of monoclonal antibodies, including
bevacizumab, an anti-vascular endothelial growth factor (VEGF)
agent, and epidermal growth factor receptor (EGFR) inhibitors
(cetuximab and panitumumab), has significantly improved survival in
specific patient subgroups. The combination of fluorouracil,
leucovorin, oxaliplatin, and/or irinotecan (FOLFOX, FOLFIRI, or
FOLFOXIRI) with targeted agents has effected a median overall
survival (OS) >30 months in selected patients (2,3).
However, despite these advancements in CRC
treatment, resistance and progression remain major challenges for
later-line therapies. TAS-102, an effective treatment for
previously treated mCRC, exerts its cytotoxic effects through
trifluridine (FTD), which gets incorporated into DNA, thereby
disrupting its replication (4).
Additionally, preclinical CRC models have demonstrated that adding
bevacizumab enhances phosphorylated FTD levels, supporting the
rationale for this combination in mCRC (5).
In 2023, the results of the SUNLIGHT trial, a phase
III study, showed a significantly better OS for TAS-102 plus
bevacizumab compared with TAS-102 alone [10.8 vs. 7.5 months;
hazard ratio (HR)=0.61, P<0.001] (6).
Based on the results of the phase 1/2 C-TASK FORCE
trial (7) and after undergoing
Institutional Review Board approval, we have been actively
introducing and administering TAS-102 plus bevacizumab therapy as a
backward treatment since October 2016. The results of the SUNLIGHT
study were used as the basis for the present retrospective analysis
of the results and validity of TAS-102 plus bevacizumab
therapy.
Patients and methods
Patients
This retrospective observational study was approved
by the Ethics Committee of Gifu University Graduate School of
Medicine (Institutional Review Board approval no. 2024-144) and was
conducted in accordance with the national guidelines for human
research. The requirement for informed consent was waived by the
Ethics Committee due to the retrospective nature of the study.
This study analyzed data from electronic medical
records at our hospital. The study included patients with mCRC
resistant to at least one of the following: Fluoropyrimidine,
irinotecan, oxaliplatin, anti-VEGF therapy, and anti-EGFR therapy
(for KRAS wild-type tumors). These patients were administered
TAS-102 at our outpatient chemotherapy clinic between October 2016
and December 2023. The median age of the patients was 67 years
(range, 24–86 years).
KRAS/NRAS mutation status was analyzed at our
institution using a PCR-SSO (sequence-specific oligonucleotide)
method routinely performed in the clinical diagnostic laboratory,
covering exon 2 (codons 12, 13), exon 3 (codons 59, 61), and exon 4
(codons 117, 146). Primer sequences are proprietary and not
disclosed, but the assay is standardized and validated in our
institutional diagnostic setting.
Chemotherapy
Patients were administered TAS-102 (35
mg/m2 body surface area) orally twice daily on days 1–5
and 8–12 of a 28-day cycle, in combination with bevacizumab (5
mg/kg, infused intravenously over 30 min every 2 weeks).
For severe adverse events (grade 3–4 neutropenia), a
10-mg/day dose reduction was applied in subsequent cycles without
re-escalation. Treatment was suspended in cases of grade 3–4
neutropenia, thrombocytopenia, febrile neutropenia, elevated
bilirubin (>3.0 mg/dl), aspartate aminotransferase/alanine
aminotransferase level >150 U/l, creatinine level >1.5 mg/dl,
or grade 3–4 non-hematologic toxicities. After resolution,
treatment was resumed at a lower TAS-102 dose of 10 mg/day.
Statistical analysis
Statistical analyses were performed using JMP
Student Edition version 18 (SAS Institute Inc., Cary, NC, USA). The
significance level (α) was set at 0.05 (two-sided). Categorical
variables were compared using the χ2 test (for
frequencies), and continuous variables, such as age, were compared
using the Wilcoxon rank-sum test. Survival curves were analyzed
using both the log-rank test and the Gehan-Breslow-Wilcoxon test;
both were included because the log-rank test is more sensitive to
proportional hazards, whereas the Gehan-Breslow-Wilcoxon test
places more weight on early differences. Median survival times with
95% confidence intervals were calculated, and hazard ratios were
estimated using the Cox proportional hazards model. Both
univariable and multivariable analyses were performed.
Results
Patient backgrounds
A total of 101 patients with CRC (65 men and 36
women; median age, 67 years) were administered TAS-102 plus
bevacizumab during the study period (Table I). Approximately 60% of the patients
had a primary rectal tumor, and 90% had ≤2 metastatic organs. The
metastatic organs included 48 livers, 47 lungs, 25 lymph nodes, 11
local, and 6 others. Approximately 60% and <20% of the patients
were administered the study treatment as third- and second-line
therapies, respectively. Wild-type RAS was present in approximately
half of the patients, and mutant BRAF and low microsatellite
instability (MSI) were observed in two patients each. The
chemotherapy history included fluoropyrimidine (101 patients,
100%), oxaliplatin (95 patients, 94.1%), irinotecan (82 patients,
81.2%), anti-VEGF antibody (92 patients, 91.1%), and anti-EGFR
antibody (41 patients, accounting for 87.2% of the RAS wild-type
subgroup). Subsequent therapy was administered in 66 patients
(65.3%), and regorafenib was used in 54 patients, accounting for
81.8% of those who received subsequent therapy. The median duration
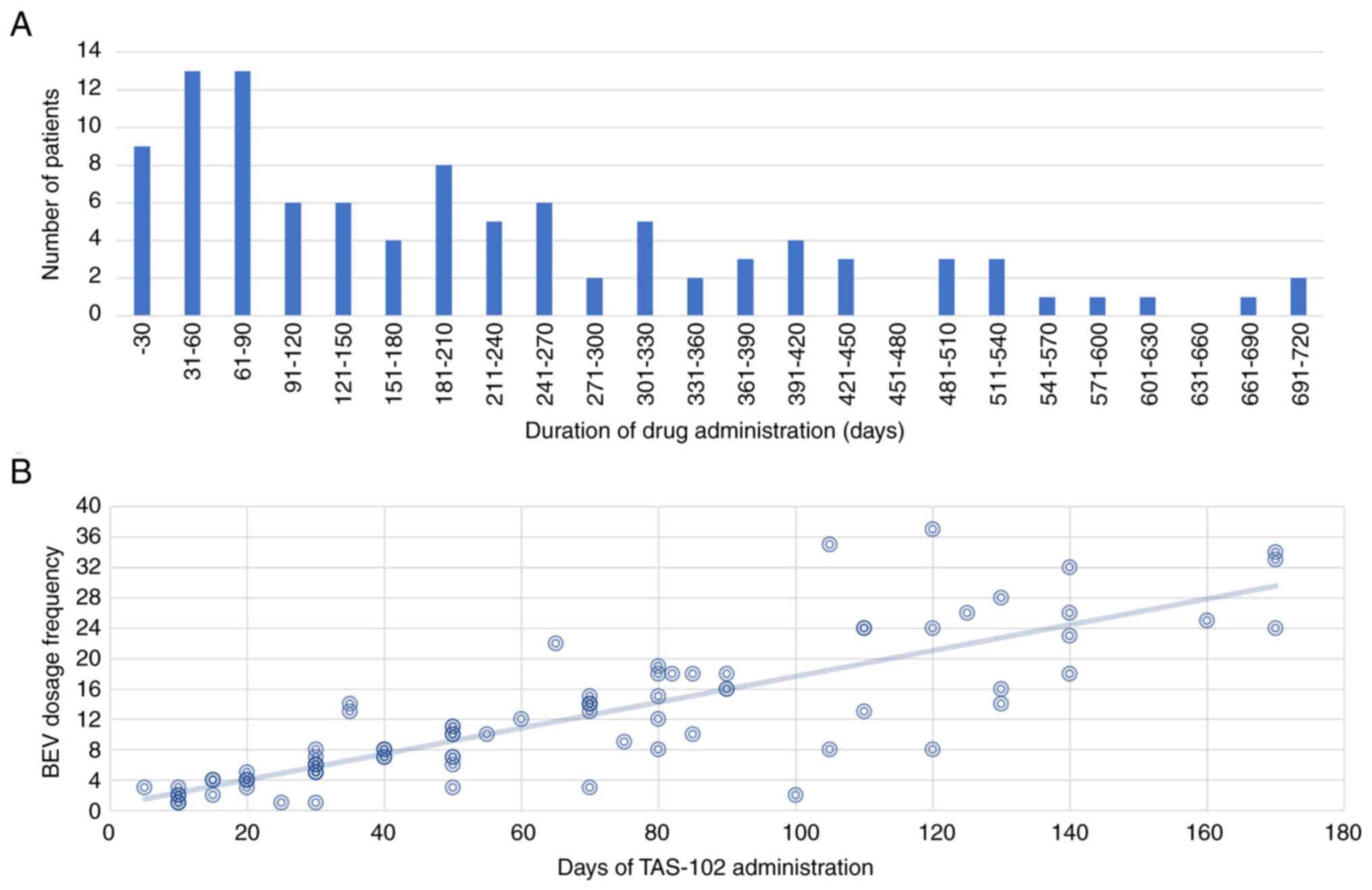
of TAS-102 plus bevacizumab treatment was 184 days (Fig. 1A). The longest treatment duration
was approximately 2 years, but most cases lasted for 9 months.
Although most patients were administered the same regimen, those
who skipped FTD/tipiracil (TPI) and received more bevacizumab and
those who skipped bevacizumab and received more FTD/TPI were
approximately the same (Fig. 1B).
The most frequent grade ≥3 adverse event was neutropenia [25
(24.8%) grade 3 and 18 (17.8%) grade 4], followed by leukopenia [25
(24.8%)], anemia [16 (15.8%)], and thrombocytopenia [6 (5.9%)].
Among non-hematological toxicities, grade 3 hypertension [12
(11.9%)], proteinuria [8 (7.9%)], and diarrhea [6 (5.9%)] were
observed. Notably, no grade 5 treatment-related adverse events
occurred (Table II).
 | Table I.Subject and patient background. |
Table I.
Subject and patient background.
| Characteristic | Value |
|---|
| Age,
yearsa | 67 (24–86) |
| Sex |
|
| Male | 65 (64.4%) |
|
Female | 36 (35.6%) |
| Primary
diagnosis |
|
|
Right-sided colon | 16 (15.8%) |
|
Left-sided colon | 23 (22.8%) |
|
Rectum | 62 (61.4%) |
| Time from diagnosis
of first metastasis to treatment |
|
| <18
months | 38 (37.6%) |
| ≥18
months | 63 (62.4%) |
| Number of metastatic
sites |
|
| 1 | 55 (54.5%) |
| 2 | 37 (36.6%) |
| 3 | 7 (6.9%) |
| 4 | 2 (2.0%) |
| RAS status |
|
| Wild
type | 47 (46.5%) |
|
Mutation | 52 (51.5%) |
|
Unknown | 2 (2.0%) |
| BRAF status |
|
| Wild
type | 66 (65.3%) |
|
Mutation | 2 (2.0%) |
|
Unknown | 33 (32.7%) |
| MMR and MSI
status |
|
| MMR
deficient and high MSI | 2 (2.0%) |
| MMR
proficient and stable or low MSI | 72 (71.3%) |
|
Unknown | 27 (26.7%) |
| Number of previous
treatment regimens |
|
| 1 | 17 (16.8%) |
| 2 | 63 (62.4%) |
| ≥3 | 21 (20.8%) |
| Subsequent
therapy |
|
| Yes | 66 (65.3%) |
|
Regorafenib | 54
(81.8%)b |
| No | 35 (34.7%) |
| Drugs used in the
previous treatment regimen |
|
|
Fluoropyrimidine | 101 (100.0%) |
|
Irinotecan | 82 (81.2%) |
|
Oxaliplatin | 95 (94.1%) |
|
Anti-VEGF monoclonal
antibody | 92 (91.1%) |
|
Anti-EGFR monoclonal
antibody | 41/47
(87.2%)c |
 | Table II.Treatment-related adverse events of
grade ≥3. |
Table II.
Treatment-related adverse events of
grade ≥3.
| Adverse event | Grade 3, n (%) | Grade 4, n (%) | Total, n (%) |
|---|
| Neutropenia | 25 (24.8) | 18 (17.8) | 43 (42.6) |
| Leukopenia | 25 (24.8) | 0 | 25 (24.8) |
| Anemia | 16 (15.8) | 0 | 16 (15.8) |
|
Thrombocytopenia | 6 (5.9) | 0 | 6 (5.9) |
| Hypertension | 12 (11.9) | 0 | 12 (11.9) |
| Proteinuria | 8 (7.9) | 0 | 8 (7.9) |
| Diarrhea | 6 (5.9) | 0 | 6 (5.9) |
OS and progression-free survival
(PFS)
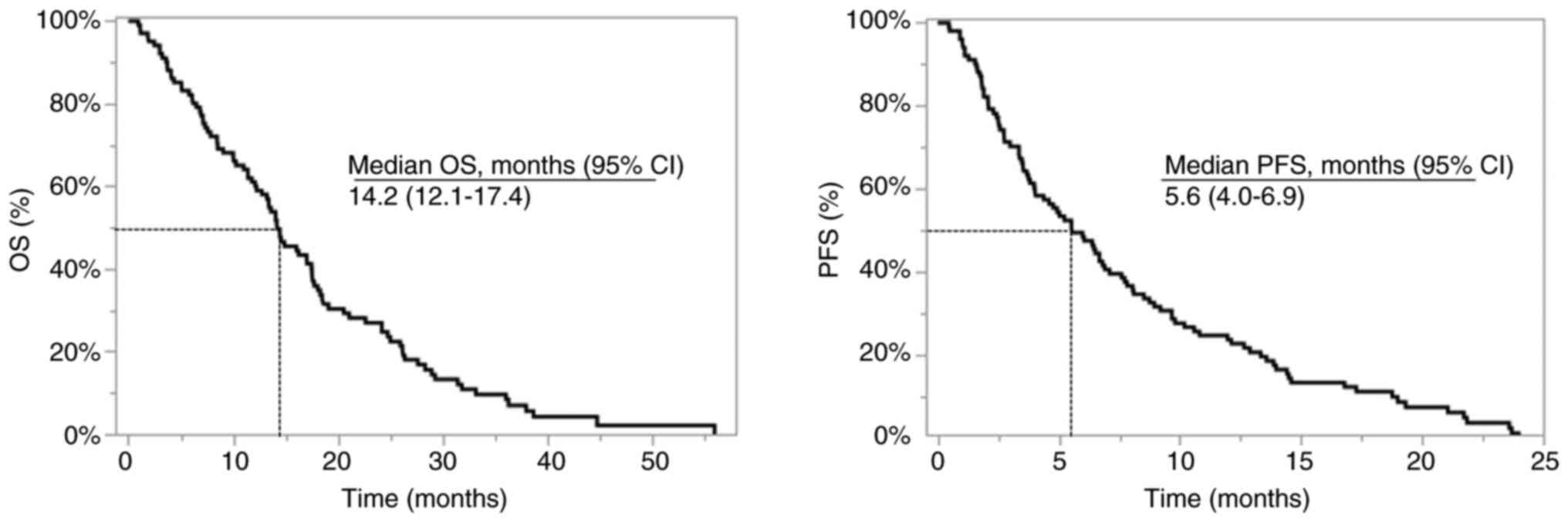
Overall, the median PFS and OS were 5.6 and 14.2
months, respectively (Fig. 2).
Regarding the primary tumor site, the median PFS was 4.0 months for
colon cancer and 6.9 months for rectal cancer, and the median OS
values were 11.0 and 17.6 months, respectively. Baseline
characteristics of patients with colon and rectal cancers are
summarized in Table III.
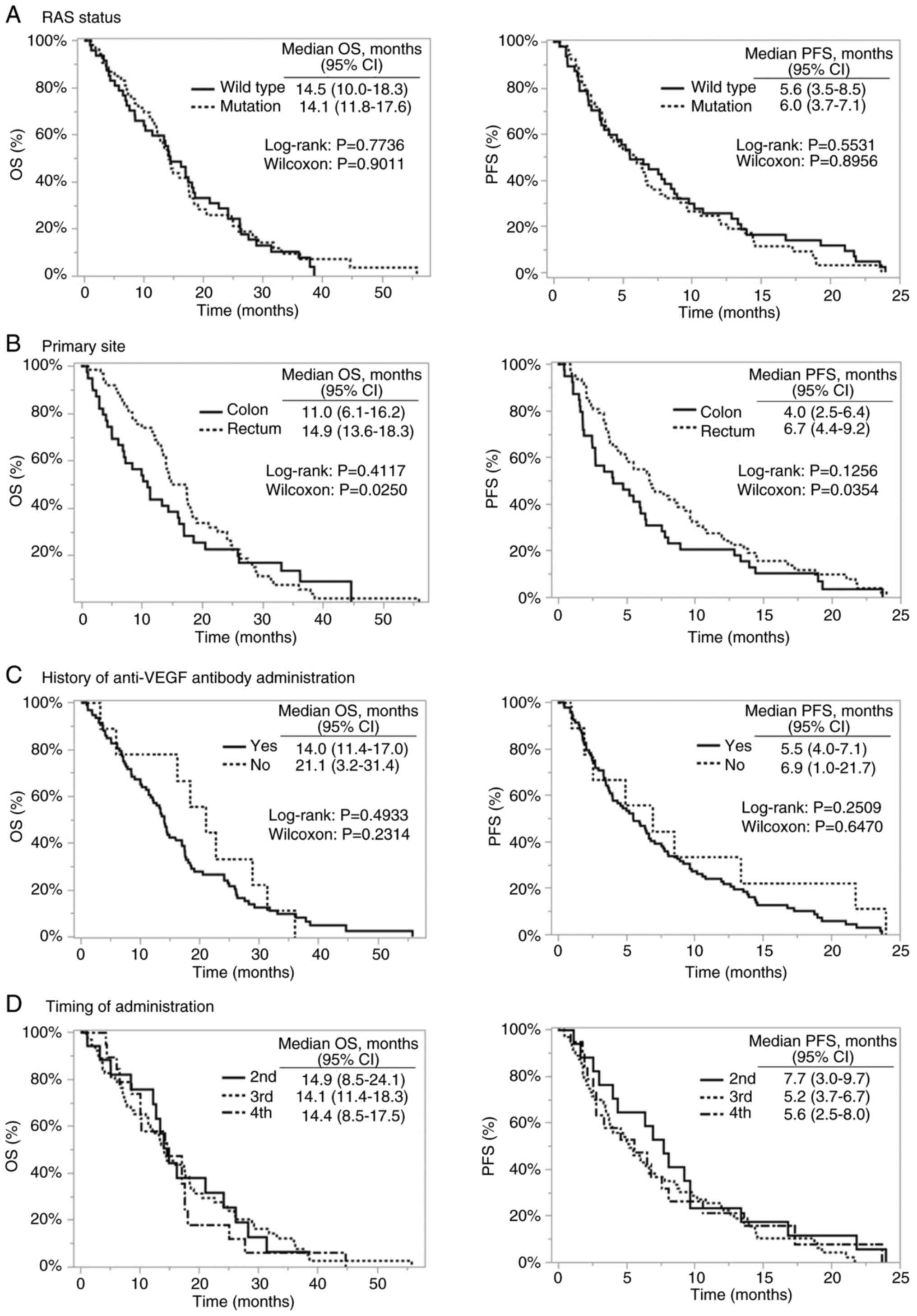
Concerning survival curve comparison, the Gehan-Breslow-Wilcoxon
test showed a statistically significant difference in favor of
rectal cancer (PFS: P=0.035; OS: P=0.025), whereas the log-rank
test did not show a statistically significant difference (PFS:
P=0.54; OS: P=0.65). In the multivariable Cox regression analysis
adjusting for RAS mutation, primary site, anti-VEGF antibody
administration, number of treatment lines, and liver metastasis,
primary site was not identified as an independent prognostic factor
for either PFS or OS (Table
IV).
 | Table III.Comparison of patient backgrounds in
the colon and rectum. |
Table III.
Comparison of patient backgrounds in
the colon and rectum.
| Characteristic | Colon (n=39) | Rectum (n=62) | P-value |
|---|
| Age,
yearsa | 64.7±13.8 | 65.6±10.7 | 0.7340 |
| Male sex | 22 (56.4%) | 43 (69.4%) | 0.1860 |
| RAS mutation | 20 (51.3%) | 33 (54.1%) | 0.8490 |
| BRAF mutation | 2 (8.0%) | 0 (0.0%) | 0.1463b |
| MSI high | 1 (3.6%) | 1 (2.2%) |
>0.999b |
| Number of previous
treatment regimens |
|
| 0.3718 |
| 1 | 4 | 13 |
|
| 2 | 26 | 37 |
|
| ≥3 | 9 | 12 |
|
| Subsequent
therapy |
|
| 0.2858c |
|
Yes | 23 | 43 |
|
|
Regorafenib | 19 | 35 |
|
| No | 16 | 19 |
|
| Number of
metastatic sites |
|
| 0.1662 |
| 1 | 21 | 34 |
|
| 2 | 12 | 25 |
|
| ≥3 | 6 | 3 |
|
| Sites of
metastasisd |
|
| 0.0103b |
|
Liver | 28 | 20 |
|
|
Lung | 13 | 34 |
|
| Lymph
node | 7 | 18 |
|
|
Local | 2 | 9 |
|
|
Bone | 0 | 1 |
|
|
Others | 2 | 3 |
|
 | Table IV.Multivariable Cox proportional
hazards analysis. |
Table IV.
Multivariable Cox proportional
hazards analysis.
|
| OS | PFS |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Ras status (wild
type vs. mutation) | 0.62 | 0.34–1.65 | 0.38 | 0.56 | 0.31–1.29 | 0.37 |
| Primary site
(rectum vs. colon) | 1.20 | 0.65–1.78 | 0.65 | 1.18 | 0.73–1.82 | 0.54 |
| Anti-VEGF therapy
(yes vs. no) | 1.29 | 0.61–2.98 | 0.51 | 1.46 | 0.67–3.54 | 0.35 |
| Liver metastasis
(yes vs. no) | 1.08 | 0.71–2.08 | 0.75 | 1.36 | 0.87–2.12 | 0.18 |
| Treatment line
number | 1.02 | 0.64–1.47 | 0.95 | 0.92 | 0.67–1.37 | 0.82 |
Comparisons based on the presence or absence of RAS
mutations, history of anti-VEGF antibody administration, and
treatment line showed numerically longer OS in patients without
prior anti-VEGF antibody administration and a trend toward longer
PFS in the second-line treatment group; however, none of these
differences were statistically significant in
Gehan-Breslow-Wilcoxon, log-rank, or Cox analyses (Fig. 3; Table
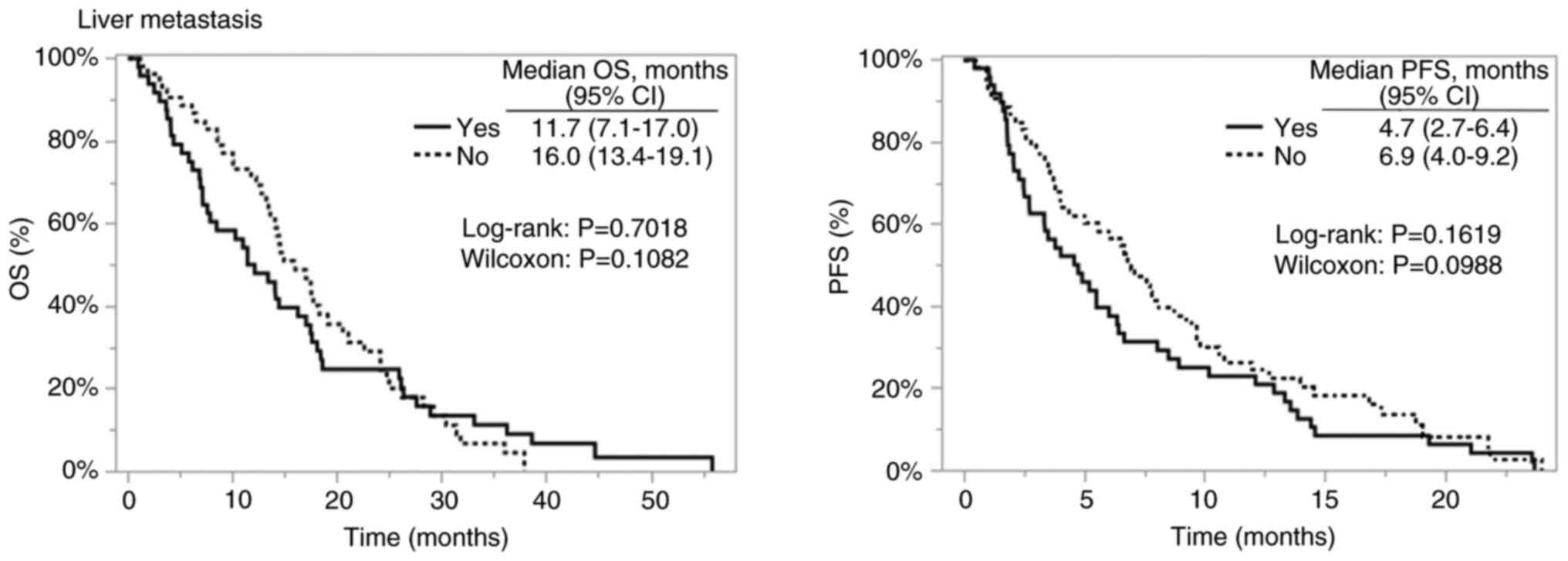
IV). In addition, comparisons according to the presence or
absence of liver metastasis are shown in Fig. 4, and the corresponding patient
characteristics and outcomes are summarized in Table V.
 | Table V.Comparison with and without liver
metastasis. |
Table V.
Comparison with and without liver
metastasis.
| Characteristic | With liver
metastasis (n=48) | Without liver
metastasis (n=53) | P-value |
|---|
| Age,
yearsa | 64.0±13.3 | 66.5±10.6 | 0.3097 |
| Male sex | 34 (70.8%) | 31 (58.5%) | 0.1959 |
| Primary
diagnosis |
|
| 0.0001 |
|
Colon | 28 (58.3%) | 11 (20.8%) |
|
|
Rectum | 20 (41.7%) | 42 (79.2%) |
|
| RAS mutation | 24 (50.0%) | 30 (56.6%) | 0.5063 |
| BRAF mutation | 2 (4.2%) | 0 (0.0%) | 0.2234b |
| MSI high | 2 (4.2%) | 0 (0.0%) | 0.2234b |
| Number of previous
treatment regimens |
|
| 0.0211 |
| 1 | 3 | 14 |
|
| 2 | 35 | 28 |
|
| ≥3 | 10 | 11 |
|
| Subsequent
therapy |
|
| 0.2701c |
|
Yes | 34 | 32 |
|
|
Regorafenib | 29 | 25 |
|
| No | 14 | 21 |
|
| Number of
metastatic sites |
|
| 0.1534b |
| 1 | 23 | 32 |
|
| 2 | 14 | 19 |
|
| ≥3 | 7 | 2 |
|
Discussion
Combination therapy with TAS-102 and bevacizumab has
demonstrated improved survival outcomes compared to TAS-102
monotherapy in patients with mCRC. Across multiple studies, the
median PFS for this combination ranged from 4.29 to 5.6 months,
whereas the median OS spanned 9.3 to 14.4 months (4,5,8–11)
(Table VI).
 | Table VI.Treatment outcomes of TAS-102 plus
bevacizumab therapy. |
Table VI.
Treatment outcomes of TAS-102 plus
bevacizumab therapy.
| First author,
year | Median OS, months
(95% CI) | Median PFS, months
(95% CI) | (Refs.) |
|---|
| Satake et
al, 2020 | 10.86 | 4.29 | (5) |
| Chida et al,
2021 | 11.5
(9.9–13.9) | 4.4 (3.7–5.4) | (8) |
| Fujii et al,
2020 | 14.4 | 5.6 (TTF) | (9) |
| Joarder et
al, 2025 | 10.9
(8.9–12.8) | 4.4 (3.1–5.7) | (10) |
| Liu et al,
2021 | 10.41
(8.40–12.89) | 4.35
(3.05–6.20) | (11) |
| Martínez-Lago et
al, 2022 | 9.3 (6.6–12.1) | 4.3 (3.4–5.1) | (4) |
| Present study,
2025 | 14.2
(12.1–17.4) | 5.6 (4.0–6.9) | - |
Although TAS-102 plus bevacizumab therapy has shown
promising results in mCRC, to date, no study has specifically
analyzed or reported outcomes for patients with rectal cancer
separately from those for CRC as a whole. All existing studies have
combined data for CRC (including both colon and rectal cancers)
without a subgroup analysis for rectal cancer. This lack of
differentiation represents a significant gap in current research
and limits the ability to assess potential differences in treatment
efficacy or safety profiles between rectal and colon cancers.
Although both colon and rectal cancers are
classified as CRC, they differ in prognosis, recurrence patterns,
and treatment responses. Several studies have investigated the
survival rates and prognostic factors of these two malignancies,
revealing key differences in their outcomes.
Historically, colon cancer has demonstrated a mildly
better prognosis than rectal cancer, particularly in the early
stages. Population-based studies indicate that five-year survival
rates for colon cancer have improved from 51–57% to 62–66%, whereas
rectal cancer survival has increased from 44–51% to 59–65%
(12–14). These improvements are partly
attributable to advances in systemic therapy for colon cancer and
preoperative chemoradiotherapy and surgical techniques for rectal
cancer (14–16). Thus, the survival gap between colon
and rectal cancers has narrowed over time.
Additionally, survival rates differ with disease
recurrence. Colon cancer has a high propensity for liver
metastasis, whereas rectal cancer more frequently metastasizes to
the lungs, and local recurrence remains a major challenge (17–20).
These distinct metastatic patterns may partly explain the survival
differences observed in our cohort. Nevertheless, the effect of
primary tumor site on prognosis in patients with refractory mCRC
should be considered hypothesis-generating, and confirmation
through larger prospective datasets or pooled analyses is
warranted.
In our study, the comparison between rectal and
colon cancers revealed no significant differences in age, sex, RAS
or BRAF mutation status, MSI status, number of previous treatment
regimens, subsequent therapies, or the number of metastatic sites.
However, similar to the results of previous surveys, liver
metastases were less common in patients with rectal cancer than in
those with colon cancer, whereas lung metastases and local
recurrence were more common (Table
III).
Various studies have also reported prognostic
differences based on metastatic and recurrent sites in CRC. For
instance, some studies have suggested that solitary lung metastases
are associated with a better prognosis (21–23);
however, these analyses often included cases in which the
metastases were surgically resected. In our study, likely owing to
the small sample size, no significant prognostic differences were
observed among cases with solitary metastases based on the
metastatic site.
The present study is the first to evaluate the
differences in prognosis after salvage therapy. Contrary to
previous reports, most patients receiving the salvage line did not
have resectable distant metastases. In our analysis, patients with
liver metastases tended to have poorer OS and PFS compared with
those without metastases (Fig. 4).
Comparison of patient characteristics based on liver metastasis
status revealed that colon cancer was significantly more frequent
and the proportion of patients receiving second-line treatment was
significantly higher in patients with liver metastases. Although
the number of metastatic sites in three or more organs was
numerically higher in the liver metastasis group, the difference
was not statistically significant (Table VI). Notably, although the
proportion of patients receiving second-line treatment with a good
treatment response was higher in the liver metastasis group, the OS
and PFS still tended to be poorer in patients with liver
metastasis.
When comparing rectal and colon cancers, survival
curve analysis showed a significant difference in favor of rectal
cancer with the Gehan-Breslow-Wilcoxon test but no significant
difference with the log-rank test. Moreover, primary tumor site was
not identified as an independent prognostic factor in the
multivariable Cox regression model. This suggests that patients
with rectal cancer may experience better outcomes during the early
phase of treatment; however, these differences diminish over time
and may be confounded by other clinical factors.
Taken together, these findings suggest that liver
metastasis is associated with a poor prognosis in salvage therapy,
and the lower frequency of liver metastases in rectal cancer may
partially explain the relatively favorable outcomes in this
subgroup. However, these results should be interpreted with
caution. The observed survival advantage in rectal cancer is
hypothesis-generating, and thus confirmation is required via larger
prospective datasets or pooled analyses.
The proportion of elderly patients in the present
study was higher than that reported in the SUNLIGHT study. As a
result, irinotecan was administered less frequently, with a trend
towards the use of the less invasive TAS-102 plus bevacizumab
regimen as second-line therapy. Similarly, the rate of anti-EGFR
antibody administration was low, with a trend towards a higher rate
of bevacizumab combination therapy (Table VII). In addition, the high
proportion of patients with rectal cancer may have resulted in a
relatively good prognosis. Although the patient backgrounds
differed from those in the SUNLIGHT study and other previous
reports, similar results were obtained in the present study,
indicating the efficacy of TAS-102 plus bevacizumab therapy across
different patient populations.
 | Table VII.Comparison of patient backgrounds in
the SUNLIGHT trial and this study. |
Table VII.
Comparison of patient backgrounds in
the SUNLIGHT trial and this study.
| Characteristic | SUNLIGHT trial
(n=246) | Our cases
(n=101) |
|---|
| Age,
yearsa | 62 (20–84) | 67 (24–86) |
| Male sex | 122 (49.6%) | 65 (64.4%) |
| Primary
diagnosis |
|
|
|
Colon | 180 (73.2%) | 39 (38.6%) |
|
Rectum | 66 (26.8%) | 62 (61.4%) |
| Number of
metastatic sites |
|
|
| 1 or
2 | 152 (61.8%) | 92 (91.1%) |
| ≥3 | 94 (38.2%) | 9 (8.9%) |
| RAS status |
|
|
| Wild
type | 75 (30.5%) | 47 (46.5%) |
|
Mutation | 171 (69.5%) | 52 (51.5%) |
|
Unknown | 0 (0.0%) | 2 (2.0%) |
| Number of previous
treatment regimens |
|
|
| 1 | 11 (4.5%) | 17 (16.8%) |
| 2 | 229 (93.1%) | 63 (62.4%) |
| ≥3 | 6 (2.4%) | 21 (20.8%) |
| Drugs used in the
previous treatment regimen |
|
|
|
Fluoropyrimidine | 246 (100.0%) | 101(100.0%) |
|
Irinotecan | 246 (100.0%) | 82 (81.2%) |
|
Oxaliplatin | 241 (98.0%) | 95 (94.1%) |
|
Anti-VEGF monoclonal
antibody | 178 (72.4%) | 92 (91.1%) |
|
Anti-EGFR monoclonal
antibodyb | 67/71 (94.4%) | 41/47 (87.2%) |
In conclusion, in the present study, TAS-102
combined with bevacizumab in subsequent treatments showed greater
efficacy against rectal cancer than against colon cancer. This may
be because rectal cancer is associated with fewer liver metastases
than colon cancer. However, these results should be interpreted
with caution.
The limitations of this study include its
retrospective single-center design, potential selection bias,
relatively small sample size, and insufficient statistical power
for subgroup analyses. In particular, the lack of significant
differences in many subgroup comparisons is likely due to the
limited number of patients in each subgroup. Therefore, the
observed survival advantage for rectal cancer should be considered
hypothesis-generating, and further investigations using larger
prospective or pooled datasets are required to validate these
findings.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KMa and NM conceptualized the study, performed data
analysis, wrote the original draft and reviewed and edited the
manuscript. RY, CM, RA, JYT, ME, YH, TH, MF, IY, YS, YT and KMu
contributed to data acquisition, clinical supervision, and
interpretation of the data. KMa and NM confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethics approval and waiver of informed consent were
obtained from the Ethics Committee of Gifu University Graduate
School of Medicine (approval no. 2024-144).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the AI tools as necessary, taking full responsibility for the
ultimate content of the present manuscript.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
EGFR
|
epidermal growth factor receptor
|
|
FTD
|
trifluridine
|
|
HR
|
hazard ratio
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
TPI
|
tipiracil
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48.
2023.PubMed/NCBI
|
|
2
|
Cremolini C, Loupakis F, Antoniotti C,
Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M,
Zaniboni A, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus
bevacizumab as first-line treatment of patients with metastatic
colorectal cancer: Updated overall survival and molecular subgroup
analyses of the open-label, phase 3 TRIBE study. Lancet Oncol.
16:1306–1315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shinozaki K, Yamada T, Nasu J, Matsumoto
T, Yuasa Y, Shiraishi T, Nagano H, Moriyama I, Fujiwara T, Miguchi
M, et al: A phase II study of folfoxiri plus bevacizumab as initial
chemotherapy for patients with untreated metastatic colorectal
cancer: TRICC1414 (BeTRI). Int J Clin Oncol. 26:399–408. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martínez-Lago N, Chucla TC, De Castro BA,
Ponte RV, Rendo CR, Rodriguez MIGR, Diaz SS, Suarez BG, Gomez JC,
Fernandez FB, et al: Efficacy, safety and prognostic factors in
patients with refractory metastatic colorectal cancer treated with
trifluridine/tipiracil plus bevacizumab in a real-world setting.
Sci Rep. 12:146122022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Satake H, Kato T, Oba K, Kotaka M, Kagawa
Y, Yasui H, Nakamura M, Watanabe T, Matsumoto T, Kii T, et al:
Phase Ib/II study of biweekly TAS-102 in combination with
bevacizumab for patients with metastatic colorectal cancer
refractory to standard therapies (BiTS Study). Oncologist.
25:e1855–e1863. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prager GW, Taieb J, Fakih M, Ciardiello F,
Van Cutsem E, Elez E, Cruz FM, Wyrwicz L, Stroyakovskiy D, Papai Z,
et al: Trifluridine-tipiracil and bevacizumab in refractory
metastatic colorectal cancer. N Engl J Med. 388:1657–1667. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuboki Y, Nishina T, Shinozaki E, Yamazaki
K, Shitara K, Okamoto W, Kajiwara T, Matsumoto T, Tsushima T,
Mochizuki N, et al: TAS-102 plus bevacizumab for patients with
metastatic colorectal cancer refractory to standard therapies
(C-TASK FORCE): An investigator-initiated, open-label, single-arm,
multicentre, phase 1/2 study. Lancet Oncol. 18:1172–1181. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chida K, Kotani D, Nakamura Y, Kawazoe A,
Kuboki Y, Kojima T, Taniguchi H, Watanabe J, Endo I and Yoshino T:
Efficacy and safety of trifluridine/tipiracil plus bevacizumab and
trifluridine/tipiracil or regorafenib monotherapy for
chemorefractory metastatic colorectal cancer: A retrospective
study. Ther Adv Med Oncol. April 20–2021.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujii H, Matsuhashi N, Kitahora M,
Takahashi T, Hirose C, Iihara H, Yamada Y, Watanabe D, Ishihara T,
Suzuki A and Yoshida K: Bevacizumab in combination with TAS-102
improves clinical outcomes in patients with refractory metastatic
colorectal cancer: A retrospective study. Oncologist. 25:e469–e476.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Joarder R, Patel D, Tiwari A, Choudhary J,
Vana P, Shenoy V, Mer N, Ramaswamy A, Bhargava P and Ostwal V:
TAS-102 plus bevacizumab as an effective and well tolerated regimen
in chemotherapy-refractory advanced colorectal cancers-A single
institution retrospective analysis. South Asian J Cancer. Jan
7–2025.(Epub ahead of print).
|
|
11
|
Liu CJ, Hu T, Shao P, Chu WY, Cao Y and
Zhang F: TAS-102 monotherapy and combination therapy with
bevacizumab for metastatic colorectal cancer. Gastroenterol Res
Pract. 2021:40146012021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brouwer NPM, Bos ACRK, Lemmens VEPP, Tanis
PJ, Hugen N, Nagtegaal ID, de Wilt JHW and Verhoeven RHA: An
overview of 25 years of incidence, treatment and outcome of
colorectal cancer patients. Int J Cancer. 143:2758–2766. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lemmens V, van Steenbergen L,
Janssen-Heijnen M, Martijn H, Rutten H and Coebergh JW: Trends in
colorectal cancer in the south of the Netherlands 1975–2007: Rectal
cancer survival levels with colon cancer survival. Acta Oncol.
49:784–796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Renouf DJ, Woods R, Speers C, Hay J, Phang
PT, Fitzgerald C and Kennecke H: Improvements in 5-year outcomes of
stage II/III rectal cancer relative to colon cancer. Am J Clin
Oncol. 36:558–564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fernandez-Martos C, Garcia-Albeniz X,
Pericay C, Maurel J, Aparicio J, Montagut C, Safont MJ, Salud A,
Vera R, Massuti B, et al: Chemoradiation, surgery and adjuvant
chemotherapy versus induction chemotherapy followed by
chemoradiation and surgery: Long-term results of the Spanish GCR-3
phase II randomized trial. Ann Oncol. 26:1722–1728. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yaffee P, Osipov A, Tan C, Tuli R and
Hendifar A: Review of systemic therapies for locally advanced and
metastatic rectal cancer. J Gastrointest Oncol. 6:185–200.
2015.PubMed/NCBI
|
|
17
|
Riihimäki M, Hemminki A, Sundquist J and
Hemminki K: Patterns of metastasis in colon and rectal cancer. Sci
Rep. 6:297652016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holch JW, Demmer M, Lamersdorf C, Michl M,
Schulz C, von Einem JC, Modest DP and Heinemann V: Pattern and
dynamics of distant metastases in metastatic colorectal cancer.
Visc Med. 33:70–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shinto E, Ike H, Ito M, Takahashi K, Ohue
M, Kanemitsu Y, Suto T, Kinugasa T, Watanabe J, Hida JI, et al:
Lateral node metastasis in low rectal cancer as a hallmark to
predict recurrence patterns. Int J Clin Oncol. 29:1896–1907. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi A, Sugito M, Ito M and Saito N:
Predictors of successful salvage surgery for local pelvic
recurrence of rectosigmoid colon and rectal cancers. Surg Today.
37:853–859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khattak MA, Martin HL, Beeke C, Price T,
Carruthers S, Kim S, Padbury R and Karapetis CS: Survival
differences in patients with metastatic colorectal cancer and with
single site metastatic disease at initial presentation: Results
from South Australian clinical registry for advanced colorectal
cancer. Clin Colorectal Cancer. 11:247–254. 2012.PubMed/NCBI
|
|
22
|
Pu H, Chen Y, Shen R, Zhang Y, Yang D, Liu
L, Dong X and Yang G: Influence of the initial recurrence site on
prognosis after radical surgery for colorectal cancer: A
retrospective cohort study. World J Surg Oncol. 21:1372023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawaguchi K, Uehara K, Nakayama G, Fukui
T, Fukumoto K, Nakamura S and Yokoi K: Growth rate of
chemotherapy-naïve lung metastasis from colorectal cancer could be
a predictor of early relapse after lung resection. Int J Clin
Oncol. 21:329–334. 2016. View Article : Google Scholar : PubMed/NCBI
|