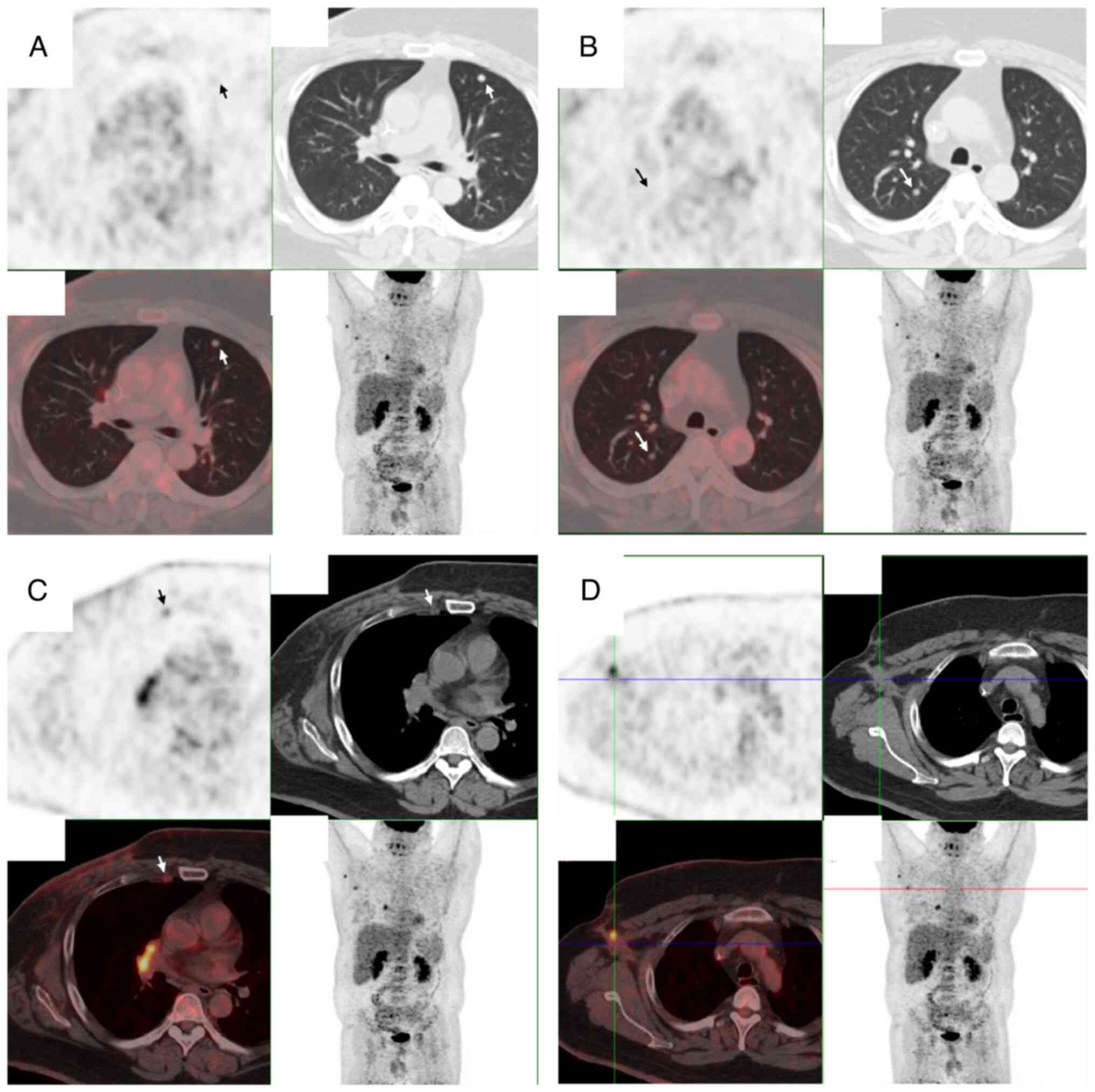
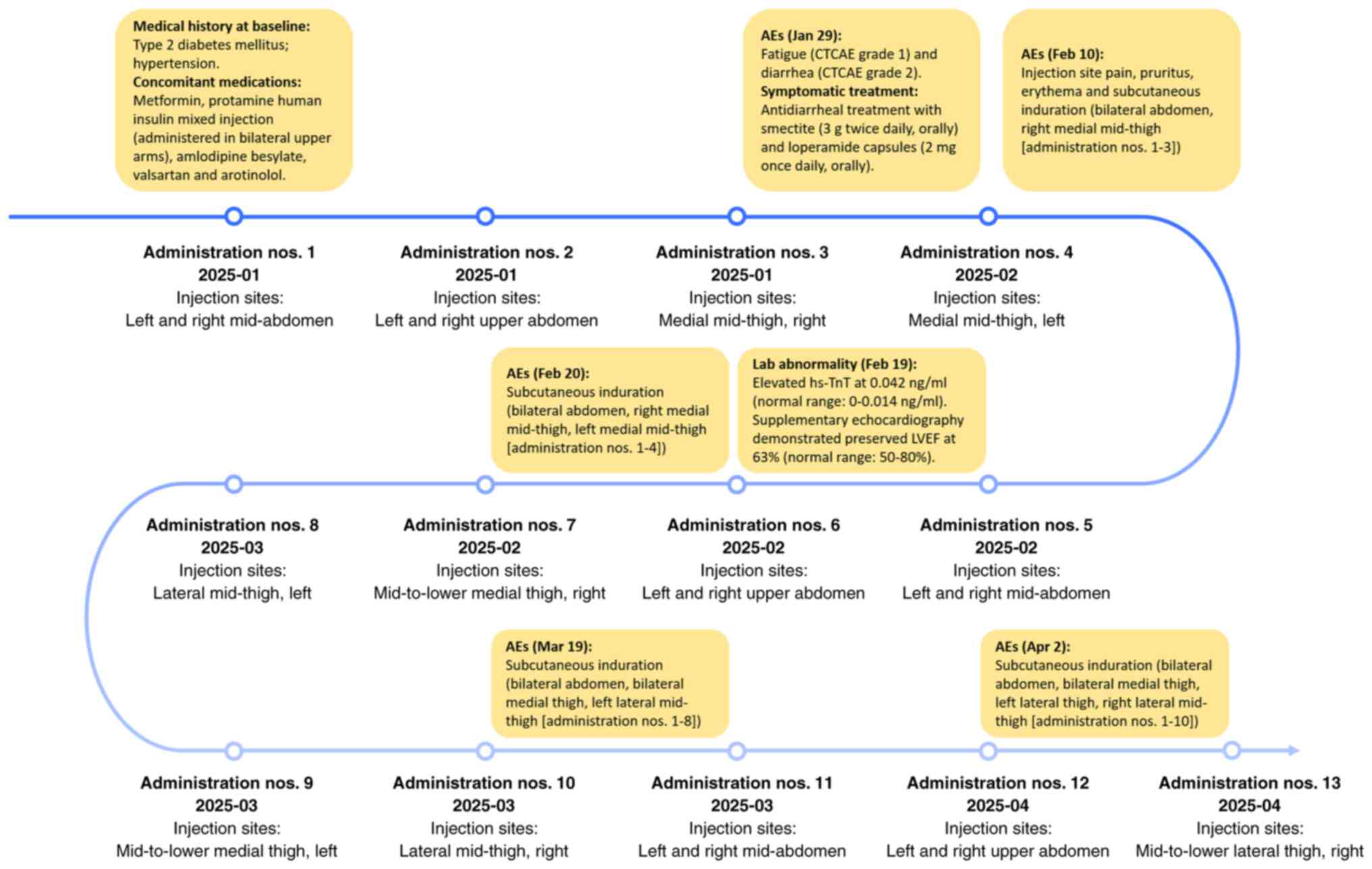
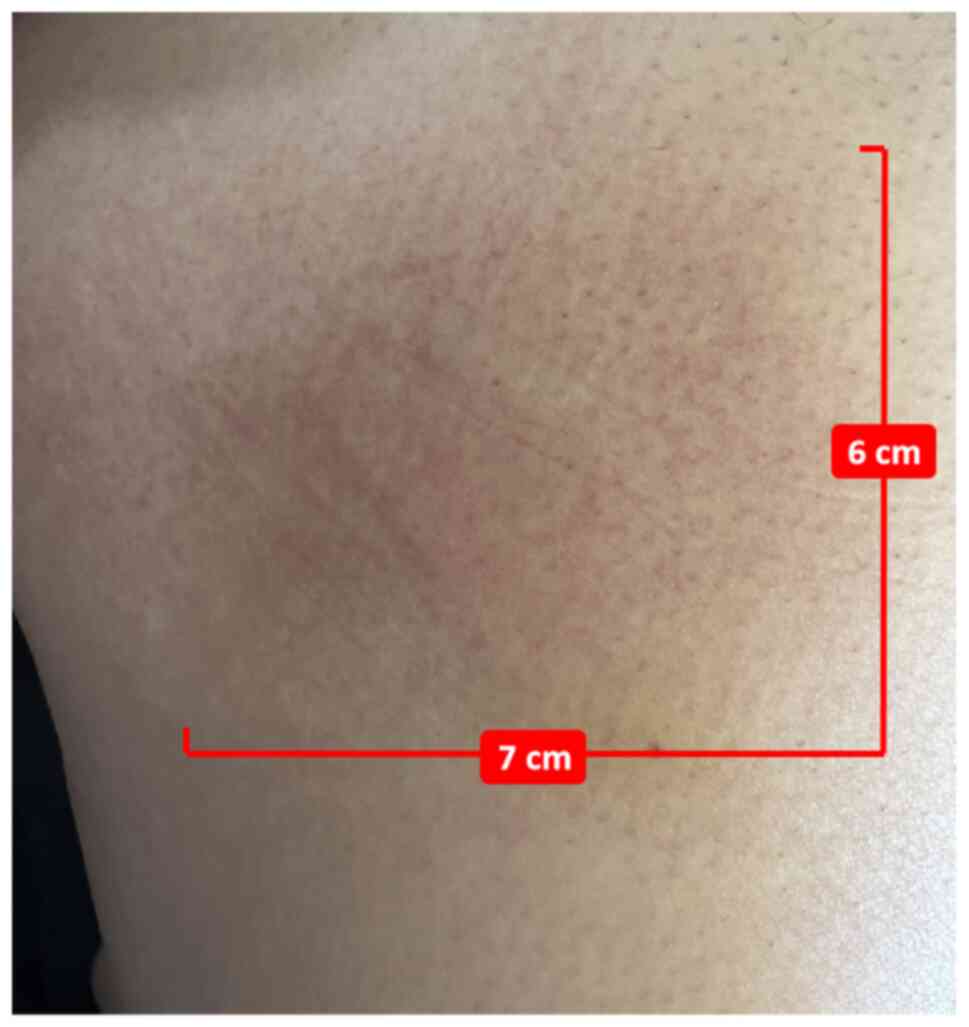
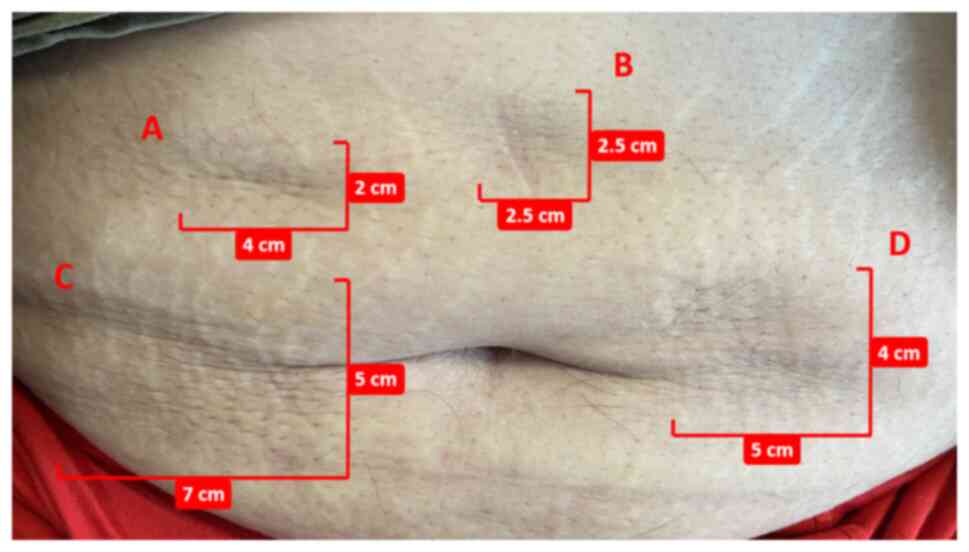
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
3
|
Zhao L, Cheng H, He D, Zhang Y, Chai Y,
Song A and Sun G: Decoding male breast cancer: Epidemiological
insights, cutting-edge treatments, and future perspectives. Discov
Oncol. 16:3602025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
An B, Che M, Liu Y, Yang X and Li Z:
Analysis and comparison of the burden of male breast cancer:
differences between the global, China, India, and the United
States. BMC Public Health. 25:22052025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ter-Zakarian A, Agelidis A and Jaloudi M:
Male breast cancer: Evaluating the current landscape of diagnosis
and treatment. Breast Cancer (Dove Med Press). 17:567–572.
2025.PubMed/NCBI
|
|
6
|
Hou J, Feng H, Zhang M, Wang D and Fan H:
Hotspots and future trends of male breast cancer: A global
perspective. Clin Transl Oncol. Aug 21–2025.(Epub ahead of print).
View Article : Google Scholar
|
|
7
|
Ross JS, Slodkowska EA, Symmans WF,
Pusztai L, Ravdin PM and Hortobagyi GN: The HER-2 receptor and
breast cancer: Ten years of targeted anti-HER-2 therapy and
personalized medicine. Oncologist. 14:320–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harder J, Ihorst G, Heinemann V, Hofheinz
R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S,
et al: Multicentre phase II trial of trastuzumab and capecitabine
in patients with HER2 overexpressing metastatic pancreatic cancer.
Br J Cancer. 106:1033–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshizawa A, Sumiyoshi S, Sonobe M,
Kobayashi M, Uehara T, Fujimoto M, Tsuruyama T, Date H and Haga H:
HER2 status in lung adenocarcinoma: A comparison of
immunohistochemistry, fluorescence in situ hybridization (FISH),
dual-ISH, and gene mutations. Lung Cancer. 85:373–378. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blok EJ, Kuppen PJ, van Leeuwen JE and
Sier CF: Cytoplasmic overexpression of HER2: A key factor in
colorectal cancer. Clin Med Insights Oncol. 7:41–51. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verri E, Guglielmini P, Puntoni M,
Perdelli L, Papadia A, Lorenzi P, Rubagotti A, Ragni N and Boccardo
F: HER2/neu oncoprotein overexpression in epithelial ovarian
cancer: Evaluation of its prevalence and prognostic significance.
Clinical study. Oncology. 68:154–161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei H, Zhang Y, Lu Y, Zou Y, Zhou L, Qin X
and Jiang Q: Is ADC a rising star in solid tumor? An umbrella
review of systematic reviews and meta-analyses. BMC Cancer.
25:3802025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wedam S, Fashoyin-Aje L, Gao X, Bloomquist
E, Tang S, Sridhara R, Goldberg KB, King-Kallimanis BL, Theoret MR,
Ibrahim A, et al: FDA approval summary: Ado-trastuzumab emtansine
for the adjuvant treatment of HER2-positive early breast cancer.
Clin Cancer Res. 26:4180–4185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dilawari A, Zhang H, Shah M, Gao X, Fiero
M, Bhatnagar V, Pierce W, Mixter B, Pazdur R and Amiri-Kordestani
L: US Food and Drug Administration approval summary: trastuzumab
deruxtecan for the treatment of adult patients with hormone
receptor-positive, unresectable or metastatic human epidermal
growth factor receptor 2-low or human epidermal growth factor
receptor 2-ultralow breast cancer. J Clin Oncol. 43:2942–2951.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deeks ED: Disitamab vedotin: First
approval. Drugs. 81:1929–1935. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lemech C, Wei J, O'Neill S, et al: 1496
JSKN033, an innovative subcutaneous-injected fixed-dose combination
(FDC) of biparatopic anti-HER2 antibody drug conjugate (ADC) and
PD-L1 inhibitor in advanced solid tumor. J Immunotherapy of Cancer.
Nov 5–2024.(Epub ahead of print).
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ismael G, Hegg R, Muehlbauer S, Heinzmann
D, Lum B, Kim SB, Pienkowski T, Lichinitser M, Semiglazov V,
Melichar B and Jackisch C: Subcutaneous versus intravenous
administration of (neo)adjuvant trastuzumab in patients with
HER2-positive, clinical stage I–III breast cancer (HannaH study): A
phase 3, open-label, multicenter, randomized trial. Lancet Oncol.
13:869–878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan AR, Im SA, Mattar A, Colomer R,
Stroyakovskii D, Nowecki Z, De Laurentiis M, Pierga JY, Jung KH,
Schem C, et al: Fixed-dose combination of pertuzumab and
trastuzumab for subcutaneous injection plus chemotherapy in
HER2-positive early breast cancer (FeDeriCa): A randomized,
open-label, multicenter, non-inferiority, phase 3 study. Lancet
Oncol. 22:85–97. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Markham A: Envafolimab: First approval.
Drugs. 82:235–240. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang HP, Le HK and Shah DK:
Pharmacokinetics and pharmacodynamics of antibody-drug conjugates
administered via subcutaneous and intratumoral routes.
Pharmaceutics. 15:11322023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boudreau A: Practical considerations for
the integration of subcutaneous targeted therapies into the
oncology clinic. Can Oncol Nurs J. 29:267–270. 2019.PubMed/NCBI
|
|
26
|
Davis JD, Bravo Padros M, Conrado DJ,
Ganguly S, Guan X, Hassan HE, Hazra A, Irvin SC, Jayachandran P,
Kosloski MP, et al: Subcutaneous administration of monoclonal
antibodies: Pharmacology, delivery, immunogenicity, and learnings
from applications to clinical development. Clin Pharmacol Ther.
115:422–439. 2024. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
National Cancer Institute. Common
Terminology Criteria for Adverse Events (CTCAE) v5.0, . National
Cancer Institute website. Updated November 27 and 2017. May
26–2025Available from. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
|
|
28
|
Farrar JT, Young JP Jr, LaMoreaux L, Werth
JL and Poole MR: Clinical importance of changes in chronic pain
intensity measured on an 11-point numerical pain rating scale.
Pain. 94:149–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Q and Liu Y: Technical, preclinical,
and clinical developments of Fc-glycan-specific antibody-drug
conjugates. RSC Med Chem. 16:50–62. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joubert N, Beck A, Dumontet C and
Denevault-Sabourin C: Antibody-drug conjugates: The last decade.
Pharmaceuticals (Basel). 13:2452020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang Y, Zhang P, Li F, Lai H, Qi T and
Wang Y: Advances in the study of marketed antibody-drug conjugates
(ADCs) for the treatment of breast cancer. Front Pharmacol.
14:13325392024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hobson AD, Xu J, Marvin CC, McPherson MJ,
Hollmann M, Gattner M, Dzeyk K, Fettis MM, Bischoff AK, Wang L, et
al: Optimization of drug-linker to enable long-term storage of
antibody-drug conjugate for subcutaneous dosing. J Med Chem.
66:9161–9173. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ballestin P, López de Sá A, Diaz-Tejeiro
C, Paniagua-Herranz L, Sanvicente A, López-Cade I, Pérez-Segura P,
Alonso-Moreno C, Nieto-Jiménez C and Ocaña A: Understanding the
toxicity profile of approved ADCs. Pharmaceutics. 17:2582025.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan HN, Morcillo MA, Lopez J, Minchom A,
Sharp A, Paschalis A, Silva-Fortes G, Raobaikady B and Banerji U:
Treatment-related adverse events of antibody drug-conjugates in
clinical trials. J Hematol Oncol. 18:712025. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schettini F, Nucera S, Pascual T,
Martínez-Sáez O, Sánchez-Bayona R, Conte B, Buono G, Lambertini M,
Punie K, Cejalvo JM, et al: Efficacy and safety of antibody-drug
conjugates in pretreated HER2-low metastatic breast cancer: A
systematic review and network meta-analysis. Cancer Treat Rev.
132:1028652025. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Al-Dasooqi N, Bowen JM, Gibson RJ,
Sullivan T, Lees J and Keefe DM: Trastuzumab induces
gastrointestinal side effects in HER2-overexpressing breast cancer
patients. Invest New Drugs. 27:173–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Paulik A, Grim J and Filip S: Predictors
of irinotecan toxicity and efficacy in treatment of metastatic
colorectal cancer. Acta Medica (Hradec Kralove). 55:153–159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manna M, Brabant M, Greene R, Chamberlain
MD, Kumar A, Alimohamed N and Brezden-Masley C: Canadian expert
recommendations on safety overview and toxicity management
strategies for sacituzumab govitecan based on use in metastatic
triple-negative breast cancer. Curr Oncol. 31:5694–5708. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cui C, Wang J, Wang C, Xu T, Qin L, Xiao
S, Gong J, Song L and Liu D: Model-informed drug development of
envafolimab, a subcutaneously injectable PD-L1 antibody, in
patients with advanced solid tumors. Oncologist. 29:e1189–e1200.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
St Clair-Jones A, Prignano F, Goncalves J,
Paul M and Sewerin P: Understanding and minimising injection-site
pain following subcutaneous administration of biologics: A
narrative review. Rheumatol Ther. 7:741–757. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chin SH, Huang WL, Akter S and Binks M:
Obesity and pain: A systematic review. Int J Obes (Lond).
44:969–979. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hildebrandt X, Ibrahim M and Peltzer N:
Cell death and inflammation during obesity: ‘Know my methods,
WAT(son)’. Cell Death Differ. 30:279–292. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Erstad BL and Barletta JF: Implications of
obesity for drug administration and absorption from subcutaneous
and intramuscular injections: A primer. Am J Health Syst Pharm.
79:1236–1244. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hirt PA, Castillo DE, Yosipovitch G and
Keri JE: Skin changes in the obese patient. J Am Acad Dermatol.
81:1037–1057. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cardoso F, Bartlett J, Slaets L, van
Deurzen CHM, van Leeuwen-Stok E, Porter P, Linderholm B, Hedenfalk
I, Schröder C, Martens J, et al: Characterization of male breast
cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG international
male breast cancer program. Ann Oncol. 29:405–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang D, Lu R, Rempala G and Sadee W:
Ligand-free estrogen receptor α (ESR1) as master regulator for the
expression of CYP3A4 and other cytochrome P450 enzymes in the human
liver. Mol Pharmacol. 96:430–440. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Unger JM, Vaidya R, Albain KS, LeBlanc M,
Minasian LM, Gotay CC, Henry NL, Fisch MJ, Lee SM, Blanke CD and
Hershman DL: Sex differences in risk of severe adverse events in
patients receiving immunotherapy, targeted therapy, or chemotherapy
in cancer clinical trials. J Clin Oncol. 40:1474–1486. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Otoya I, Valdiviezo N, Morante Z, Calle C,
Ferreyra Y, Huarcaya-Chombo N, Polo-Mendoza G, Castañeda C,
Vidaurre T, Neciosup SP, et al: Subcutaneous trastuzumab: an
observational study of safety and tolerability in patients with
early HER2-positive breast cancer. Int J Breast Cancer.
2024:95517102024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhi L, Liu D and Shameem M: Injection site
reactions of biologics and mitigation strategies. AAPS Open.
11:52025. View Article : Google Scholar
|
|
50
|
Wang LY, Qin HY and Lu YH: Expert
consensus on breast cancer targeted therapy with subcutaneous
injection. Chin J Nurs. 60:43–47. 2025.
|
|
51
|
Kim PJ, Lansang RP and Vender R: A
systematic review and meta-analysis of injection site reactions in
randomized-controlled trials of biologic injections. J Cutan Med
Surg. 27:358–367. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cancer Clinical Pharmacy Committee of
China Anti-cancer Association, Case Management Committee of China
Anti-cancer Association, . Expert consensus on the standardized
construction of ‘Convenient Injection Centers’ for anti-tumor
subcutaneous preparations. Chin J Mod Nurs. 30:1121–1130. 2024.
|