Introduction
Male breast cancer (MBC) is a rare malignant tumor
that accounts for ~1% of all breast cancer cases (1). Globally, the incidence of MBC is
relatively low, with GLOBOCAN 2020 data reporting an annual rate of
0.5–1.0 cases per 100,000 individuals (2). The mortality rate of MBC in 2021 was
0.34 cases per 100,000 individuals [95% uncertainty interval (UI),
0.23–0.41], which was markedly lower compared with the rate of
female breast cancer (FBC) at 14.55 cases per 100,000 individuals
(95% UI, 13.45–15.56) (3). Due to
its rarity, MBC is underrepresented in breast cancer trials,
resulting in a lack of prospective or randomized data specific to
men. Therefore, treatment decisions are typically extrapolated from
data derived from female patients (4), whose adverse event (AE) profiles often
lack characteristics specific to men. Therefore, notable emphasis
should be placed on including male patients in clinical trials of
breast cancer and reporting AE data.
Primary treatment modalities for MBC include
surgery, radiotherapy, chemotherapy, endocrine therapy, targeted
therapy and immunotherapy (IO) (5,6).
Mastectomy has traditionally been considered the standard surgical
approach for early-stage MBC due to limited breast tissue in men
and the typical proximity of tumors to the nipple-areolar complex
(5). Since the majority of MBCs are
hormone receptor-positive, adjuvant endocrine therapy, primarily
tamoxifen, constitutes the cornerstone of treatment for hormone
receptor-positive disease. Radiotherapy is considered to provide
clinically notable benefits for male patients with early-stage and
locally advanced disease. Furthermore, the management of advanced
MBC typically aligns with established approaches used for female
patients, including chemotherapy, HER2-targeted agents, IO and
poly(ADP ribose) polymerase inhibitors (5,6).
HER2 is expressed in tumor tissues and cells of
various advanced malignant solid tumors, such as breast (7), gastric (8), pancreatic (9), lung (10), colorectal (11) and ovarian (12) cancer. In breast cancer, the
incidence of HER2 gene amplification and upregulation can reach
15–20% (13). The HER2 receptor is
recognized as an effective therapeutic target for tumors with HER2
amplification or upregulation. Antibody-drug conjugates (ADCs)
combine the high specificity of monoclonal antibodies with the
potent antitumor activity of cytotoxic payloads. The targeted
delivery mechanism of ADCs enhances safety profiles, making them a
prominent research focus in oncology therapeutics (14). Although several HER2-directed ADCs,
such as trastuzumab emtansine (T-DM1) (15), trastuzumab deruxtecan (16) and disitamab vedotin (17), have been approved for clinical use,
subcutaneous (SC) formulations of HER2-directed ADCs remain in the
emerging phase of clinical investigation, with limited reported
data on associated AEs.
In January 2025, the Phase I Unit of Fudan
University Shanghai Cancer Center (Shanghai, China) initiated a
phase I/II clinical trial evaluating the safety, tolerability,
pharmacokinetics/pharmacodynamics and antitumor activity of JSKN033
(18). JSKN033 is a fixed-dose
combination for SC injection that comprises JSKN003, a biparatopic
HER2-directed ADC, and envafolimab, a programmed death-ligand 1
(PD-L1) inhibitor approved by the National Medical Products
Administration of China (18). In
the dose-escalation phase, patients received SC JSKN033 across
three doses (5.6, 6.7 and 8.4 mg/kg, once weekly) following a
modified 3+3 design. Safety evaluations were based on
treatment-related AEs (TRAEs) and dose-limiting toxicities to
assess the primary endpoints of safety and tolerability. The
dose-limiting toxicity assessment period was 21 days. Investigators
evaluated tumor response every 6 weeks using the Response
Evaluation Criteria in Solid Tumors (version 1.1; RECIST) (19) and performed tumor imaging
assessments with computed tomography (CT) or magnetic resonance
imaging (MRI). Preliminary study data indicated that the most
common TRAE of JSKN033 was mild to moderate (grade 1 and 2)
injection site reactions (90.9%). This was followed by diarrhea
(54.5%), nausea (45.5%), increased aspartate aminotransferase
(27.3%), decreased appetite (27.3%), increased alanine
aminotransferase (18.2%) and maculo-papular rash (18.2%). No grade
3 or higher TRAEs or serious AEs were observed and no TRAEs led to
treatment discontinuation (18).
During the same month of JSKN033 initiation, a male patient with
breast cancer was enrolled and subsequently developed evident
systemic and injection-site adverse reactions after treatment,
including fatigue, diarrhea and localized injection-site
manifestations of pruritus, pain, cutaneous depression and SC
induration.
The development of SC formulations for anticancer
agents has gained increasing attention in oncological therapeutics
due to their notable convenience and improved treatment experience.
Notable examples include subcutaneous formulations of the
monoclonal antibodies trastuzumab (20), the fixed-dose combination of
pertuzumab and trastuzumab (21),
and the PD-L1 inhibitor envafolimab (22). JSKN033 is the first global SC
coformulation consisting of an ADC and an immune checkpoint
inhibitor, and to the best of our knowledge, research on the
underlying mechanisms of JSKN033-associated AEs and their clinical
management is limited (18,23). The present case report focuses on
JSKN033-associated AEs, which may contribute to the understanding
of the toxicity profiles of SC-administered ADCs and the
optimization of management strategies. Furthermore, as the present
case includes a male patient with breast cancer, it may provide
distinctive data and insights that are potentially valuable for
future research.
Case report
A 38-year-old man underwent a right-sided simple
mastectomy at Changzhou Wujin People's Hospital (Changzhou, China)
in March 2023 after self-identifying a 7×7-cm mass in the right
breast. Postoperative pathology [based on histopathological slides
from Changzhou Wujin People's Hospital and subsequent consultation
with the Department of Pathology of Fudan University Shanghai
Cancer Center (Shanghai, China)] revealed grade 3 invasive ductal
carcinoma according to the Nottingham histological grading system
(24) [estrogen receptor (ER), 90%;
progesterone receptor, 90%; HER2, 2+; and Ki-67, 40%). The
immunohistochemistry and H&E images are not available as the
original histopathological slides were processed and diagnosed by
Changzhou Wujin People's Hospital (data not shown). In April 2023,
the patient received a modified radical mastectomy followed by
adjuvant chemotherapy consisting of 4 cycles of cyclophosphamide
[1.5 g intravenously (IV) every 3 weeks] plus epirubicin (180 mg IV
every 3 weeks) and 4 cycles of trastuzumab (1,120 mg IV every 3
weeks), pertuzumab (840 mg IV every 3 weeks) and docetaxel (202.4
mg IV every 3 weeks). The patient subsequently received adjuvant
radiotherapy in November 2023 (total dose of 4,256 cGy in 16
fractions at 266 cGy/fraction), followed by 2 cycles of T-DM1 (500
mg IV every 3 weeks), which was discontinued due to disease
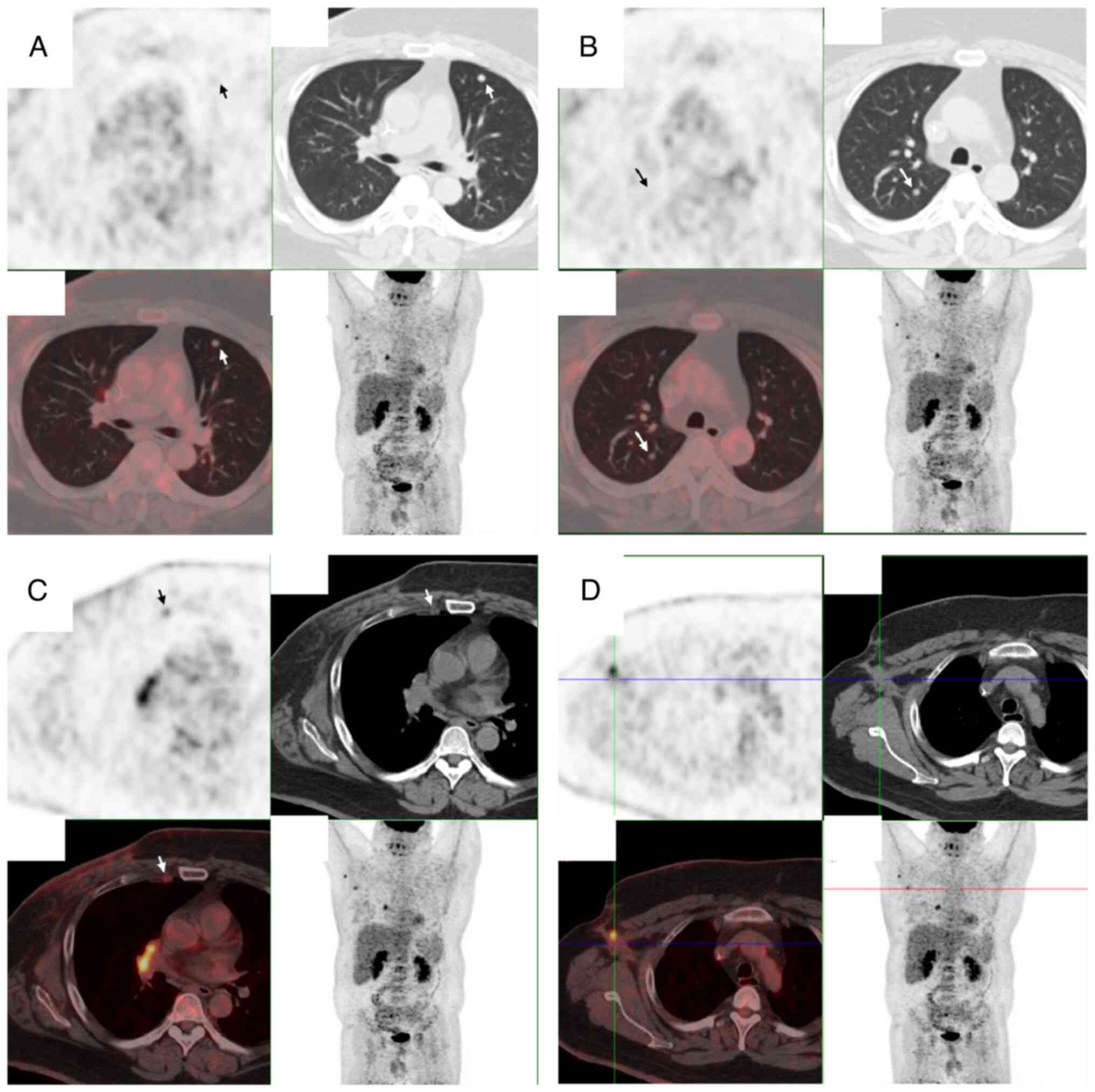
progression. In December 2023, positron emission tomography-CT
revealed disease recurrence in the chest wall with multiple
pulmonary and lymph node metastases (Fig. 1). The patient was subsequently
enrolled in two Phase 1 clinical trials [SMP-656 (CTR20233290) and
ASKG-915 (CTR20232767)] from January to June 2024 but was withdrawn
from both trials due to progressive disease.
The patient was enrolled in the JSKN033 phase I/II
clinical trial (CTR20244896) at the Phase I Clinical Trial Center
of Fudan University Shanghai Cancer Center in January 2025. The
patient had a medical history of type 2 diabetes mellitus and
hypertension, with concomitant medications, including metformin
(0.5 g twice daily, orally), protamine human insulin mixed
injection (30 IU once daily, administered subcutaneously in the
bilateral upper arms), amlodipine besylate (5 mg once daily,
orally), valsartan (100 mg three times daily, orally) and
arotinolol (10 mg three times daily, orally). No history of drug or
disinfectant allergies was reported. Physical examination revealed
no ecchymoses, erythema or SC nodules on the skin or mucous
membranes. Baseline target lesion selection was performed in
January 2025, identifying a left pulmonary nodule as the target
lesion, with a sum of the longest diameters measuring 17.3 mm.
JSKN033 [180 mg (1 ml)/vial] consisted of 80 mg of JSKN003 and 100
mg of envafolimab. The JSKN033 treatment regimen included weekly SC
administration. The dose administered to the patient was calculated
as 560 mg (3.1 ml) per administration on the basis of body weight,
which was divided into two SC injections (2+1.1 ml) due to protocol
recommendations (maximum 2 ml per injection) (25,26),
which meant that the patient received two SC injections per weekly
dose. The first dose of JSKN033 was administered in January 2025.
As of April 2025, the patient had received 13 weekly doses,
totaling 26 SC injections over the 13-week period. On the basis of
the protocol recommendation that the optimal injection sites are
the abdomen and thighs (25,26),
the injection sites were selected in the following order: i)
Left/right mid-abdomen, ii) left/right upper abdomen, iii)
left/right lower abdomen, iv) medial mid-to-lower left/right thigh;
and v) lateral mid-to-lower left/right thigh. New injection sites
were selected for each administration and no single site was
injected twice consecutively; when repeated injections in the same
anatomical region were necessary due to practical constraints,
subsequent injections were administered ≥2.5 cm from previous
sites. Please refer to Fig. 2 for a
detailed illustration of the injection site rotation strategy. With
the protocol recommended injection rate of ≤0.06 ml/sec, the
minimum injection time of each administration was calculated as 52
sec. Postinjection observation was required for 1 h before
discharge.
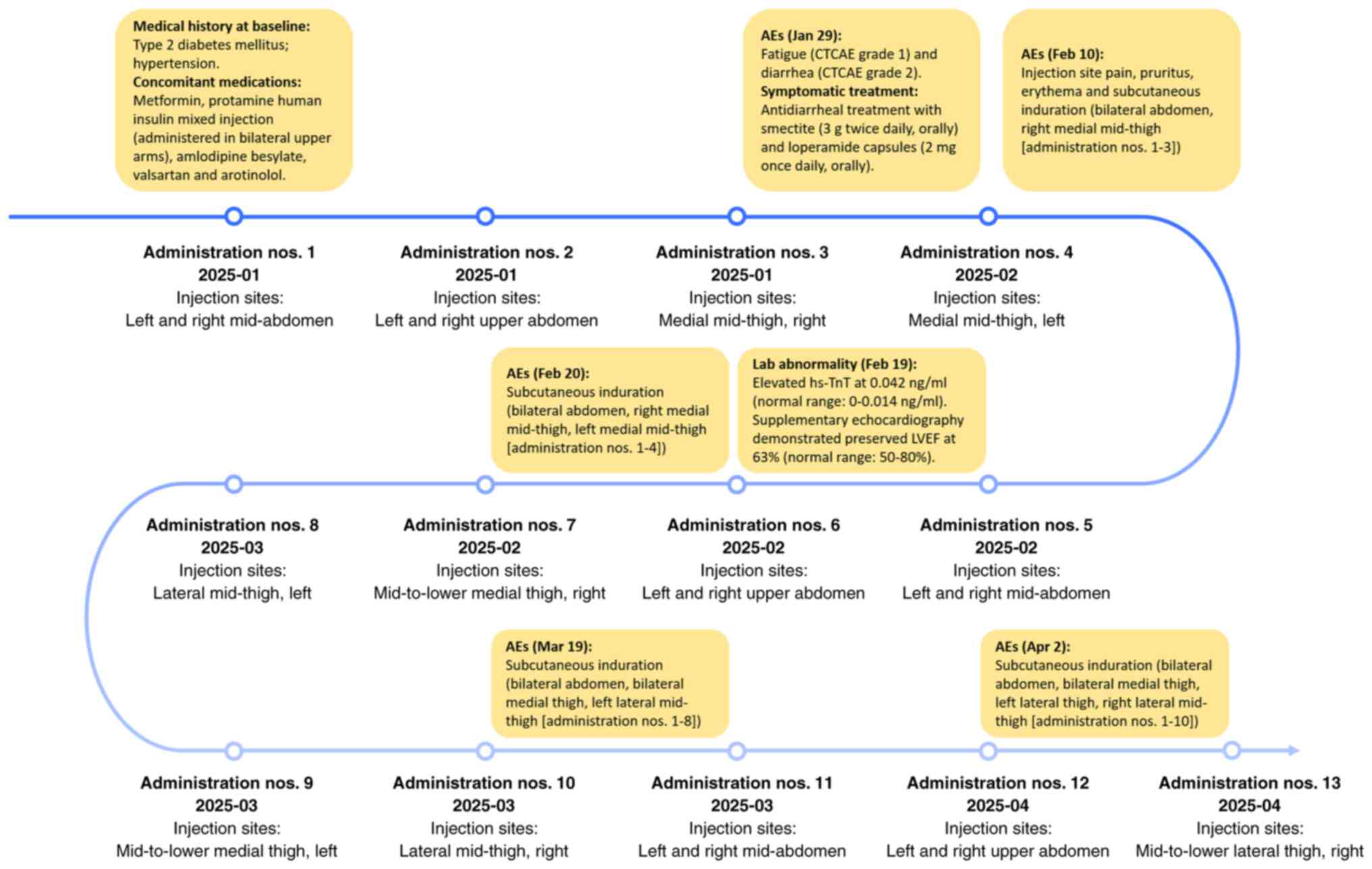 | Figure 2.Timeline of treatment interventions
and AEs. Each administration required two separate subcutaneous
injections (2+1.1 ml), with injection sites within the same
anatomical region spaced ≥2.5 cm apart (administration nos. 3, 4,
7, 8, 9, 10 and 13). CTCAE, Common Terminology Criteria for Adverse
Events; AEs, adverse events; LVEF, left ventricular ejection
fraction; hs-TnT, high-sensitivity troponin T. |
AEs were classified and graded according to the
Common Terminology Criteria for Adverse Events (CTCAE) version 5.0
(27). The patient developed
systemic reactions 2 weeks after administration (January 2025),
presenting with fatigue (CTCAE grade 1) and diarrhea (watery
stools, 4–5 episodes/day; CTCAE grade 2). The patient demonstrated
no evidence of anemia or thyroid dysfunction at baseline or during
treatment. Therefore, these factors were excluded as potential
causes of fatigue. The diarrhea improved to 1–2 episodes/day (CTCAE
grade 1) following antidiarrheal treatment with smectite (3 g twice
daily, orally) and loperamide capsules (2 mg once daily, orally).
Furthermore, 5 weeks after administration (February 2025), the
patient exhibited an elevated high-sensitivity troponin T
concentration of 0.042 ng/ml (normal range, 0–0.014 ng/ml).
Supplementary echocardiography demonstrated a preserved left
ventricular ejection fraction of 63% (normal range, 50–80%). No
elevated liver enzymes or hematological toxicity was observed
during the treatment course. The patient self-reported
postinjection localized tension-type pain, quantified as 2 on a
0–10 numeric rating scale, where 0 indicates no pain and 10
represents the worst possible pain (28). Mild pruritus (CTCAE grade 1) at the
injection site was noted and worsened with thermal stimulation.
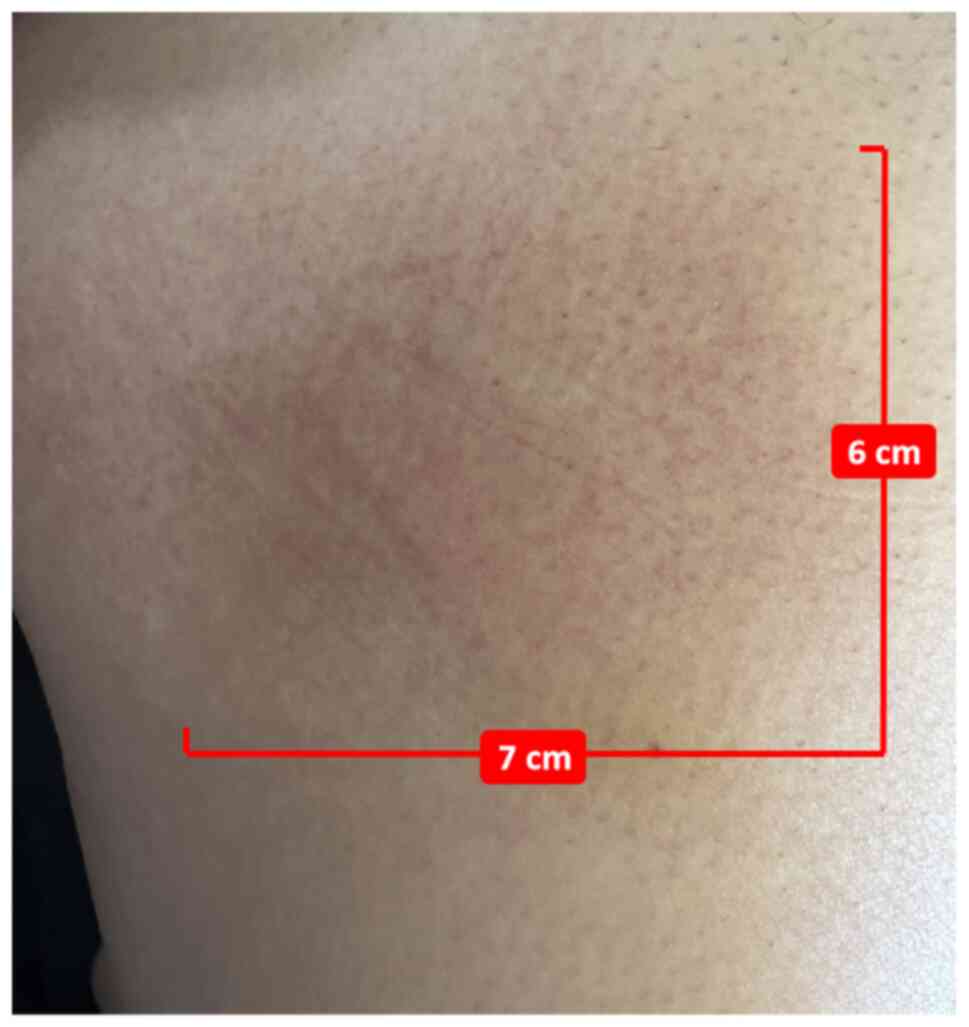
Furthermore, the patient experienced local erythema (Fig. 3) without swelling at ~1 week, which
typically resolved within 2–3 weeks, resulting in post-inflammatory
hyperpigmentation. Persistent cutaneous changes included
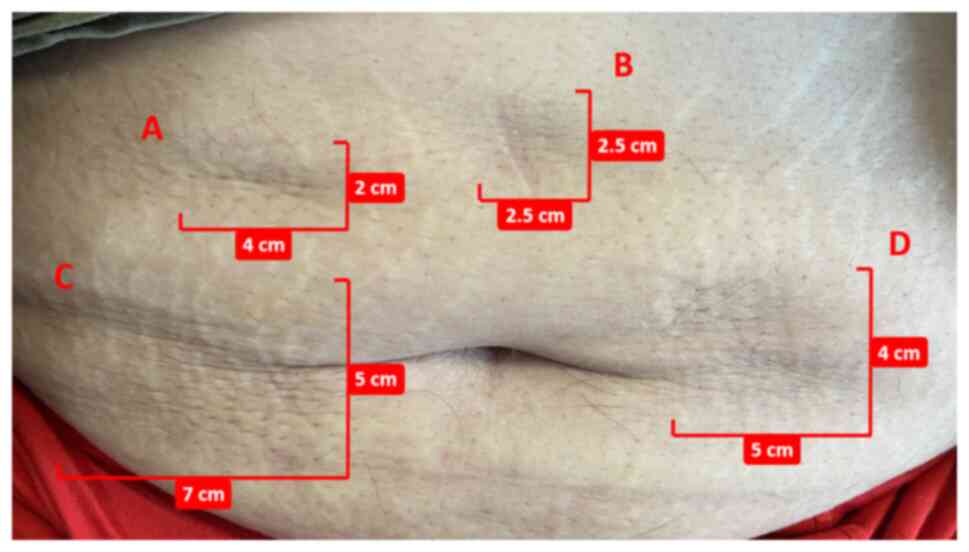
injection-site depression (Fig. 4),
enlarged pores, deepened skin folds and SC induration resistance to
resolution. All cutaneous abnormalities presented in Figs. 3 and 4 occurred following single injections at
each site. The overall treatment process and progression of AEs are
shown in Fig. 2.
The patient was followed up weekly, and tumor
assessments were performed using CT or MRI every 6 weeks (±7 days).
At the 12-week follow-up (April 2025), the therapeutic response was
assessed as a partial response according to RECIST (version 1.1),
with the target left pulmonary nodule demonstrating a sum of the
longest diameters of 9.8 mm (baseline, 17.3 mm). All observed AEs
were mild and clinically manageable. Treatment continuation was
approved, with ongoing monitoring.
Discussion
JSKN033 is a fixed-dose combination of JSKN003 and
envafolimab (18). JSKN003 is a
biparatopic HER2-directed ADC composed of a recombinant humanized
anti-HER2 bispecific antibody, a linker and a topoisomerase I
inhibitor payload. The JSKN003 mechanism of action includes
specific recognition and binding to HER2 on tumor cell surfaces,
followed by HER2-mediated internalization, intracellular release of
the topoisomerase I inhibitor payload, induction of DNA damage and
subsequent apoptosis, thereby exerting antitumor effects (29). The combination of ADCs with IO may
provide potential synergistic effects to enhance clinical benefits,
representing a promising direction for future ADC development
(30). The currently approved
HER2-targeted ADCs (for example, T-DM1, disitamab vedotin and
trastuzumab deruxtecan) (17,31)
are all administered via intravenous (IV) infusion. Furthermore,
both ADC-IO combinations and SC ADC formulations remain under
clinical investigation (18,32).
Based on current reports of HER2-targeted ADCs, the
commonly observed AEs include elevated liver enzymes, fatigue,
decreased left ventricular ejection fraction, interstitial lung
disease/non-infectious pneumonitis, loss of appetite, nausea,
vomiting, diarrhea, hematological toxicity and infusion-associated
reactions (14,33–35).
The toxicity profile of ADCs is primarily associated with target
selection and payload characteristics (33). In the present case, the systemic
reactions of the patient manifested mainly as diarrhea and fatigue,
which may be attributed to cross-reactivity of the HER2-targeted
ADC with gastrointestinal HER2 receptors during tumor antigen
binding (36) and the payload, a
topoisomerase I inhibitor, which can induce early-onset and
delayed-onset diarrhea (37). With
respect to non-infectious etiologies, mild diarrhea typically
responds to loperamide supplemented by fluid/electrolyte
replacement when necessary (38).
Overall, these systemic reactions remain mild and manageable
through symptomatic treatment and lifestyle modifications. From
both theoretical and clinical data, the systemic adverse reactions
associated with SC administration are generally consistent with
those associated with the IV route (in terms of classification
rather than specific incidence rates) (14,18,33–35).
Compared with IV administration, SC delivery avoids
infusion-associated reactions but may induce injection site
reactions (ISRs). In accordance with the preliminary clinical data
of JSKN033, 11 patients were enrolled in the dose-escalation phase
in Australia, 10 of whom (90.9%) experienced ISRs following
administration (18), all of which
were mild in severity, with no reported cases requiring dose
reduction or treatment discontinuation. To the best of our
knowledge, current research on the mechanisms underlying ISRs
associated with SC administration of HER2-targeted ADCs remains
limited. Potential contributing factors to these local reactions
may include the following: i) Drug-associated factors: The
cytotoxic payloads of ADCs are likely the primary causes of ISRs
(23). The slow absorption process
and prolonged local retention of large-molecule ADCs can lead to
sustained local drug exposure at the injection site. Furthermore,
enhanced immune cell uptake of the antibody-conjugated payload may
exacerbate immune system and skin inflammation (23). Furthermore, envafolimab in JSKN033
is the first approved global SC-administered PD-L1 inhibitor and is
marketed in China. Current data indicates that envafolimab induces
ISRs with no more than a 5% incidence rate, potentially associated
with immune system activation and inflammatory responses, possibly
contributing to the ISR presentation of the patient (39). ii) Injection technique factors: The
stability of the linker markedly influences payload release, where
premature release of the cytotoxic payload may result in off-target
toxicity (33). Improper handling
practices such as excessive heating or agitation before injection
could compromise linkers, thereby impairing therapeutic efficacy
and exacerbating AEs. Furthermore, injection parameters, including
volume, injection rate, technique and repeated dosing at the same
site, contribute to ISRs (40).
iii) Patient-specific factors: The present patient had a baseline
body weight of 120 kg, with a BMI >37 kg/m2. Previous
studies have indicated that pro-inflammatory immune cells and
adipocytes secrete inflammatory cytokines (TNF-α, IL-6, IL-1β and
leptin), creating a chronic low-grade inflammatory state in obesity
that likely exacerbates injection-site inflammatory responses and
induration formation (41–43). Furthermore, obesity affects skin
barrier integrity, which gets translated into clinical xerosis.
Obesity also causes altered collagen structure and impaired wound
healing due to decreased mechanical strength (44). These factors may have collectively
contributed to the development of ISRs in the present patient.
Furthermore, MBC is usually ER+. A large
multicenter retrospective cohort study demonstrated strong ER
expression in >90% of MBC cases (45). The gene encoding ERα acts as a key
regulator of several key hepatic drug-metabolizing enzymes,
including cytochrome P450 families, exerting broad effects on drug
metabolism (46). Therefore, the
high ER-positive profile characteristic of MBC may also represent
one of the factors influencing drug metabolism and the development
of AEs. It has been reported that certain sex-based differences
exist in the risk of AEs between male and female patients with
cancer receiving IO, targeted therapy or chemotherapy (47). Women are at a notably greater risk
of severe symptomatic AEs across multiple treatment domains,
including patients receiving immune checkpoint inhibitor therapy
and targeted therapies with kinase inhibitors (47). This disparity may be associated with
factors such as differences in sex hormone levels affecting drug
metabolism and sex variations in pharmacokinetics, pharmacogenomics
and treatment adherence (47).
JSKN033 is a combination of JSKN003 (a biparatopic HER2-directed
ADC) and envafolimab (a PD-L1 inhibitor) (18); therefore, its treatment-associated
AEs may also exhibit sex-specific variations. However, as JSKN033
is still in the clinical trial stage, robust statistical evidence
regarding sex differences in its toxicity profile remains
unavailable. Future research on sex-specific differences in
JSKN033-associated AEs would be valuable to inform optimized dosing
strategies for both male and female patients.
SC administration improves patient experience,
treatment adherence and quality of life by markedly reducing
administration time, increasing treatment convenience, decreasing
hospitalization frequency, lowering both temporal and economic
health care burdens, and eliminating complications associated with
venipuncture (48). Therefore, the
development of SC formulations for anticancer agents has gained
increasing attention in oncological therapeutics. However, compared
with IV administration, the localized reactions associated with SC
injection may pose a limiting factor for its widespread adoption.
Therefore, proper injection techniques and skin protection measures
should be implemented when SC ADCs are administered to minimize or
prevent these localized reactions.
Excessive injection rates, and improper injection
angles or needle gauges may exacerbate ISRs (40,49).
Therefore, standardized SC administration is essential for
prevention. Since current research on the SC administration of ADCs
is limited, the present case report offers recommendations for
combining the pharmacological mechanisms of ADCs with those of
established SC injection techniques for biologics such as
monoclonal antibodies (40,49,50).
i) Medication preparation: After removal from 2–8°C refrigeration,
allow 30 min to let the medication reach room temperature prior to
injection, while avoiding improper heating or vigorous agitation to
maintain pharmaceutical stability. ii) Needle: SC injections
typically employ short (4–8 mm) and thin-wall needles with small
gauges (25–27 G) and sharp tips to minimize pain. iii) Injection
volume: The amount of drug injected should not be >2.0 ml per
injection site to prevent injection pain, leakage and tissue
distortion. iv) Injection site: Adipose-rich areas such as the
abdomen and thighs should be chosen for injection, ensuring that
the injection site exhibits no erythema, damage, ecchymosis,
scarring, induration or hyperpigmentation. v) Rotation techniques:
Injection sites should be rotated systematically to reduce
irritation and ISRs, with subsequent injection administered ≥2.5 cm
from previous sites. vi) Injection angle/technique: The
non-dominant hand should be used to elevate a skin fold and the
dominant hand should be used to insert the needle at a 30–40°
angle, which can be adjusted on the basis of SC fat thickness to
achieve optimal deposition. vii) Injection rate: The injection rate
should not exceed 0.06 ml/sec to ensure patient comfort and proper
drug dispersion. viii) Post-injection monitoring: A minimum of 1 h
of observation is needed after injection to confirm patient safety
before discharge.
Current clinical data indicates that ISRs are the
most frequently reported AEs associated with SC-administered
JSKN033, primarily manifesting as localized erythema, swelling,
pain and pruritus (18). Most ISRs
are mild in severity and typically resolve without intervention or
can be effectively managed with physician-directed antihistamine
therapy when necessary. For localized skin temperature elevation or
erythema, cold compresses or topical corticosteroids may be applied
(50). Analgesics should be
considered for patients with notable pain. Warm compresses and
physiotherapy may help alleviate induration. For severe cutaneous
reactions such as ulceration or necrosis, immediate treatment
discontinuation or regimen adjustment is warranted.
Although ISRs are usually mild and rarely classified
as severe AEs, they may have a notable effect on patient
satisfaction with treatment and even contribute to treatment
discontinuation (51). Adequate
patient education on the following aspects can help improve
treatment experience. Before injection, patients should be informed
about the procedure, precautions, common AEs and corresponding
management measures. During the injection, any discomfort, such as
pain at the injection site or systemic/local allergic reactions
(for example, fever, chills, rash, dizziness or chest tightness),
should be reported to medical staff immediately. After the
injection, patients should be instructed to apply proper pressure
to the injection site to minimize bleeding. The patients should
also be educated on self-identifying AEs and promptly reporting
them to medical staff, keeping the injection site clean and dry,
avoiding water exposure for ≥24 h and bathing only after the
injection site has fully healed. Furthermore, at 24 h
post-injection, patients should not apply heat, undergo
physiotherapy or vigorously massage the area in order to prevent
capillary rupture and bleeding (52).
Due to study limitations and the personal preference
of the patient, the present case report was unable to obtain
additional imaging data (for example, ultrasound and MRI scans) or
histological data regarding the AEs of the patient. The results
described in the study are based on pathology reports rather than
retrievable image files. Therefore, there is a lack of
histopathological or biopsy evidence to further support diagnostic
evaluation. Furthermore, during the current therapeutic response
assessment, HER2 fluorescence in situ hybridization analysis
was not performed, which represents a limitation of the present
case report. JSKN033 remains in the emerging phase of clinical
investigation. The AE mechanisms of JSKN033 and their management
are still being investigated, with little existing research
reported, to the best of our knowledge. Based on the present case
report and the pharmacological mechanisms of JSKN033, potential AE
mechanisms and management strategies were analyzed. However, these
analyses had limitations. Notably, the small sample size inherent
in a case report design limits the generalizability of the study
findings and precludes definitive statistical conclusions.
Furthermore, as the present case had only completed 13 weeks of
administration at the time of manuscript preparation, longer-term
follow-up outcomes were not yet available.
ADCs are currently a key research focus in oncology
therapeutics. SC administration has demonstrated notable potential
to improve the treatment experience and quality of life of patients
due to its enhanced convenience. However, SC ADC formulations
remain in the investigational stage and further study is warranted
to fully characterize their AE profiles, and the mechanisms,
prevention and management strategies of these AEs. The present
study reported a case of systemic and localized AEs associated with
SC ADC administration, aiming to provide data and potential
insights for associated research in this field. JSKN033 remains in
the emerging phase of clinical investigation, including the
underlying mechanisms and management of its associated AEs, with,
to the best of our knowledge, limited existing research. Based on
the present case report and the underlying pharmacological
mechanisms of JSKN033, the potential underlying mechanisms and
management strategies of JSKN033-associated AEs were analyzed.
However, these analyses had limitations. Future studies may collect
more comprehensive and long-term evidence to potentially improve
the mechanistic understanding and clinical management of AEs
associated with SC-administered ADCs to guide targeted management
strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
RY wrote the original draft, devised the
methodology, conducted the investigation and conceptualized the
present case report. JZ advised on patient treatment, analyzed
patient data, and critically reviewed and revised the manuscript.
YW reviewed and edited the manuscript, devised the methodology and
conceptualized the present case report. RY and YW confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present case report was approved by the Ethics
Committee of Fudan University Shanghai Cancer Center (Shanghai,
China; approval no. 2409305-22).
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the case report, including any
potentially identifiable images or data.
Competing interests
The present case report has been published with the
written permission of the sponsor of JSKN033 (Jiangsu Alphamab
Biopharmaceuticals Co., Ltd.) from Clinical Trials ID no.
NCT06226766 and Chinese Clinical Trial Registry ID no. CTR20244896.
The authors declare that the research was conducted in the absence
of any commercial or financial relationships that could be
construed as a potential competing interest.
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
3
|
Zhao L, Cheng H, He D, Zhang Y, Chai Y,
Song A and Sun G: Decoding male breast cancer: Epidemiological
insights, cutting-edge treatments, and future perspectives. Discov
Oncol. 16:3602025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
An B, Che M, Liu Y, Yang X and Li Z:
Analysis and comparison of the burden of male breast cancer:
differences between the global, China, India, and the United
States. BMC Public Health. 25:22052025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ter-Zakarian A, Agelidis A and Jaloudi M:
Male breast cancer: Evaluating the current landscape of diagnosis
and treatment. Breast Cancer (Dove Med Press). 17:567–572.
2025.PubMed/NCBI
|
|
6
|
Hou J, Feng H, Zhang M, Wang D and Fan H:
Hotspots and future trends of male breast cancer: A global
perspective. Clin Transl Oncol. Aug 21–2025.(Epub ahead of print).
View Article : Google Scholar
|
|
7
|
Ross JS, Slodkowska EA, Symmans WF,
Pusztai L, Ravdin PM and Hortobagyi GN: The HER-2 receptor and
breast cancer: Ten years of targeted anti-HER-2 therapy and
personalized medicine. Oncologist. 14:320–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harder J, Ihorst G, Heinemann V, Hofheinz
R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S,
et al: Multicentre phase II trial of trastuzumab and capecitabine
in patients with HER2 overexpressing metastatic pancreatic cancer.
Br J Cancer. 106:1033–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshizawa A, Sumiyoshi S, Sonobe M,
Kobayashi M, Uehara T, Fujimoto M, Tsuruyama T, Date H and Haga H:
HER2 status in lung adenocarcinoma: A comparison of
immunohistochemistry, fluorescence in situ hybridization (FISH),
dual-ISH, and gene mutations. Lung Cancer. 85:373–378. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blok EJ, Kuppen PJ, van Leeuwen JE and
Sier CF: Cytoplasmic overexpression of HER2: A key factor in
colorectal cancer. Clin Med Insights Oncol. 7:41–51. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verri E, Guglielmini P, Puntoni M,
Perdelli L, Papadia A, Lorenzi P, Rubagotti A, Ragni N and Boccardo
F: HER2/neu oncoprotein overexpression in epithelial ovarian
cancer: Evaluation of its prevalence and prognostic significance.
Clinical study. Oncology. 68:154–161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei H, Zhang Y, Lu Y, Zou Y, Zhou L, Qin X
and Jiang Q: Is ADC a rising star in solid tumor? An umbrella
review of systematic reviews and meta-analyses. BMC Cancer.
25:3802025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wedam S, Fashoyin-Aje L, Gao X, Bloomquist
E, Tang S, Sridhara R, Goldberg KB, King-Kallimanis BL, Theoret MR,
Ibrahim A, et al: FDA approval summary: Ado-trastuzumab emtansine
for the adjuvant treatment of HER2-positive early breast cancer.
Clin Cancer Res. 26:4180–4185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dilawari A, Zhang H, Shah M, Gao X, Fiero
M, Bhatnagar V, Pierce W, Mixter B, Pazdur R and Amiri-Kordestani
L: US Food and Drug Administration approval summary: trastuzumab
deruxtecan for the treatment of adult patients with hormone
receptor-positive, unresectable or metastatic human epidermal
growth factor receptor 2-low or human epidermal growth factor
receptor 2-ultralow breast cancer. J Clin Oncol. 43:2942–2951.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deeks ED: Disitamab vedotin: First
approval. Drugs. 81:1929–1935. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lemech C, Wei J, O'Neill S, et al: 1496
JSKN033, an innovative subcutaneous-injected fixed-dose combination
(FDC) of biparatopic anti-HER2 antibody drug conjugate (ADC) and
PD-L1 inhibitor in advanced solid tumor. J Immunotherapy of Cancer.
Nov 5–2024.(Epub ahead of print).
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ismael G, Hegg R, Muehlbauer S, Heinzmann
D, Lum B, Kim SB, Pienkowski T, Lichinitser M, Semiglazov V,
Melichar B and Jackisch C: Subcutaneous versus intravenous
administration of (neo)adjuvant trastuzumab in patients with
HER2-positive, clinical stage I–III breast cancer (HannaH study): A
phase 3, open-label, multicenter, randomized trial. Lancet Oncol.
13:869–878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan AR, Im SA, Mattar A, Colomer R,
Stroyakovskii D, Nowecki Z, De Laurentiis M, Pierga JY, Jung KH,
Schem C, et al: Fixed-dose combination of pertuzumab and
trastuzumab for subcutaneous injection plus chemotherapy in
HER2-positive early breast cancer (FeDeriCa): A randomized,
open-label, multicenter, non-inferiority, phase 3 study. Lancet
Oncol. 22:85–97. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Markham A: Envafolimab: First approval.
Drugs. 82:235–240. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang HP, Le HK and Shah DK:
Pharmacokinetics and pharmacodynamics of antibody-drug conjugates
administered via subcutaneous and intratumoral routes.
Pharmaceutics. 15:11322023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boudreau A: Practical considerations for
the integration of subcutaneous targeted therapies into the
oncology clinic. Can Oncol Nurs J. 29:267–270. 2019.PubMed/NCBI
|
|
26
|
Davis JD, Bravo Padros M, Conrado DJ,
Ganguly S, Guan X, Hassan HE, Hazra A, Irvin SC, Jayachandran P,
Kosloski MP, et al: Subcutaneous administration of monoclonal
antibodies: Pharmacology, delivery, immunogenicity, and learnings
from applications to clinical development. Clin Pharmacol Ther.
115:422–439. 2024. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
National Cancer Institute. Common
Terminology Criteria for Adverse Events (CTCAE) v5.0, . National
Cancer Institute website. Updated November 27 and 2017. May
26–2025Available from. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
|
|
28
|
Farrar JT, Young JP Jr, LaMoreaux L, Werth
JL and Poole MR: Clinical importance of changes in chronic pain
intensity measured on an 11-point numerical pain rating scale.
Pain. 94:149–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Q and Liu Y: Technical, preclinical,
and clinical developments of Fc-glycan-specific antibody-drug
conjugates. RSC Med Chem. 16:50–62. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joubert N, Beck A, Dumontet C and
Denevault-Sabourin C: Antibody-drug conjugates: The last decade.
Pharmaceuticals (Basel). 13:2452020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang Y, Zhang P, Li F, Lai H, Qi T and
Wang Y: Advances in the study of marketed antibody-drug conjugates
(ADCs) for the treatment of breast cancer. Front Pharmacol.
14:13325392024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hobson AD, Xu J, Marvin CC, McPherson MJ,
Hollmann M, Gattner M, Dzeyk K, Fettis MM, Bischoff AK, Wang L, et
al: Optimization of drug-linker to enable long-term storage of
antibody-drug conjugate for subcutaneous dosing. J Med Chem.
66:9161–9173. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ballestin P, López de Sá A, Diaz-Tejeiro
C, Paniagua-Herranz L, Sanvicente A, López-Cade I, Pérez-Segura P,
Alonso-Moreno C, Nieto-Jiménez C and Ocaña A: Understanding the
toxicity profile of approved ADCs. Pharmaceutics. 17:2582025.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan HN, Morcillo MA, Lopez J, Minchom A,
Sharp A, Paschalis A, Silva-Fortes G, Raobaikady B and Banerji U:
Treatment-related adverse events of antibody drug-conjugates in
clinical trials. J Hematol Oncol. 18:712025. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schettini F, Nucera S, Pascual T,
Martínez-Sáez O, Sánchez-Bayona R, Conte B, Buono G, Lambertini M,
Punie K, Cejalvo JM, et al: Efficacy and safety of antibody-drug
conjugates in pretreated HER2-low metastatic breast cancer: A
systematic review and network meta-analysis. Cancer Treat Rev.
132:1028652025. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Al-Dasooqi N, Bowen JM, Gibson RJ,
Sullivan T, Lees J and Keefe DM: Trastuzumab induces
gastrointestinal side effects in HER2-overexpressing breast cancer
patients. Invest New Drugs. 27:173–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Paulik A, Grim J and Filip S: Predictors
of irinotecan toxicity and efficacy in treatment of metastatic
colorectal cancer. Acta Medica (Hradec Kralove). 55:153–159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manna M, Brabant M, Greene R, Chamberlain
MD, Kumar A, Alimohamed N and Brezden-Masley C: Canadian expert
recommendations on safety overview and toxicity management
strategies for sacituzumab govitecan based on use in metastatic
triple-negative breast cancer. Curr Oncol. 31:5694–5708. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cui C, Wang J, Wang C, Xu T, Qin L, Xiao
S, Gong J, Song L and Liu D: Model-informed drug development of
envafolimab, a subcutaneously injectable PD-L1 antibody, in
patients with advanced solid tumors. Oncologist. 29:e1189–e1200.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
St Clair-Jones A, Prignano F, Goncalves J,
Paul M and Sewerin P: Understanding and minimising injection-site
pain following subcutaneous administration of biologics: A
narrative review. Rheumatol Ther. 7:741–757. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chin SH, Huang WL, Akter S and Binks M:
Obesity and pain: A systematic review. Int J Obes (Lond).
44:969–979. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hildebrandt X, Ibrahim M and Peltzer N:
Cell death and inflammation during obesity: ‘Know my methods,
WAT(son)’. Cell Death Differ. 30:279–292. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Erstad BL and Barletta JF: Implications of
obesity for drug administration and absorption from subcutaneous
and intramuscular injections: A primer. Am J Health Syst Pharm.
79:1236–1244. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hirt PA, Castillo DE, Yosipovitch G and
Keri JE: Skin changes in the obese patient. J Am Acad Dermatol.
81:1037–1057. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cardoso F, Bartlett J, Slaets L, van
Deurzen CHM, van Leeuwen-Stok E, Porter P, Linderholm B, Hedenfalk
I, Schröder C, Martens J, et al: Characterization of male breast
cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG international
male breast cancer program. Ann Oncol. 29:405–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang D, Lu R, Rempala G and Sadee W:
Ligand-free estrogen receptor α (ESR1) as master regulator for the
expression of CYP3A4 and other cytochrome P450 enzymes in the human
liver. Mol Pharmacol. 96:430–440. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Unger JM, Vaidya R, Albain KS, LeBlanc M,
Minasian LM, Gotay CC, Henry NL, Fisch MJ, Lee SM, Blanke CD and
Hershman DL: Sex differences in risk of severe adverse events in
patients receiving immunotherapy, targeted therapy, or chemotherapy
in cancer clinical trials. J Clin Oncol. 40:1474–1486. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Otoya I, Valdiviezo N, Morante Z, Calle C,
Ferreyra Y, Huarcaya-Chombo N, Polo-Mendoza G, Castañeda C,
Vidaurre T, Neciosup SP, et al: Subcutaneous trastuzumab: an
observational study of safety and tolerability in patients with
early HER2-positive breast cancer. Int J Breast Cancer.
2024:95517102024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhi L, Liu D and Shameem M: Injection site
reactions of biologics and mitigation strategies. AAPS Open.
11:52025. View Article : Google Scholar
|
|
50
|
Wang LY, Qin HY and Lu YH: Expert
consensus on breast cancer targeted therapy with subcutaneous
injection. Chin J Nurs. 60:43–47. 2025.
|
|
51
|
Kim PJ, Lansang RP and Vender R: A
systematic review and meta-analysis of injection site reactions in
randomized-controlled trials of biologic injections. J Cutan Med
Surg. 27:358–367. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cancer Clinical Pharmacy Committee of
China Anti-cancer Association, Case Management Committee of China
Anti-cancer Association, . Expert consensus on the standardized
construction of ‘Convenient Injection Centers’ for anti-tumor
subcutaneous preparations. Chin J Mod Nurs. 30:1121–1130. 2024.
|