Introduction
Mandibular reconstruction following segmental
resection, often performed in the context of oral and maxillofacial
malignancies, is one of the most challenging procedures in head and
neck oncology. The accuracy of reconstruction critically affects
postoperative oral function, facial symmetry, and overall quality
of life. Due to both cosmetic and functional considerations,
including the maintenance of occlusion, mandibular reconstruction
requires precise alignment and preservation of the spatial
relationship between the divided bone fragments pre- and
postoperatively. After segmental mandibular resection, the bone may
be replaced with a graft, and various bone sources have been used,
including the fibula, iliac crest, scapula, and radius of the
forearm. When using bone replacements, sculpting them precisely to
fit the mandibular defect intraoperatively and aligning them
optimally to enhance bone approximation and promote bone healing is
crucial (1–4).
Traditionally, mandibular reconstruction has relied
on freehand techniques, which are highly dependent on the surgeon's
experience and intraoperative judgment. These methods often result
in variability in outcomes and prolonged surgical time.
Computer-assisted surgery (CAS) for mandibular reconstruction has
been increasingly adopted in clinical practice. CAS encompasses a
surgical approach and a set of methods that use computer technology
for surgical planning, guidance, or execution of interventions. CAS
in mandibular reconstruction was first implemented clinically by
determining the extent of mandibular resection, the shape of the
mandibular reconstruction metal plate, and the morphology and
position of the graft bone through preoperative computer
simulation, as described by Hirsch et al in 2009 (1). The resulting three-dimensional
(3D)-printed osteotomy guides and preshaped mandibular
reconstruction plates allow precisely planned surgery to be
executed with high accuracy and greater efficiency in the operating
room. These advancements reduce surgical duration and facilitate
technique optimization (2,5–15).
Currently, outsourced mandibular reconstruction CAS
systems, such as TruMatch® (DePuy Synthes, MA, USA), are
widely used (16). Introduced in
Japan in 2022, the TruMatch system has been applied in numerous
cases at our facility. However, these systems require long
preparation times, typically one month, and incur substantial
costs, which may limit their accessibility, especially in urgent
oncologic cases. Additionally, communication with external
engineers during planning may not fully capture the surgeon's
intent, and the hospital receives only the final product, with
limited control over the design process (17). To overcome these challenges, we
developed an in-house CAS system utilizing widely available 3D
printing and surgical simulation software. This system allows for
rapid planning, greater customization, and reduced costs, making it
a potentially viable alternative to commercial solutions. Despite
the growing interest in in-house CAS, few studies have directly
compared its accuracy with that of outsourced systems or
conventional methods.
Therefore, this study aimed to evaluate and compare
the accuracy of mandibular reconstruction using three approaches:
conventional surgery (non-CAS), outsourced CAS (TruMatch), and an
in-house CAS system. Given that the majority of cases involved
segmental mandibulectomy for malignant tumors, particularly oral
cancer, the study also sought to assess the feasibility and
precision of CAS-based reconstruction in oncologic settings.
Postoperative outcomes were analyzed using 3D model overlay
techniques to determine whether in-house CAS could provide accuracy
comparable to that of outsourced systems and support broader
clinical application in cancer-related mandibular
reconstruction.
Materials and methods
Patients
All patients who underwent mandibular reconstruction
surgery at our department (Center for Oral Cancer, The University
of Osaka Dental Hospital, Suita, Japan) between April 2018 and
January 2025 were included in this study. The cases were
categorized into three groups based on the surgical technique and
time period:
Non-CAS group: Cases treated before March 2021,
prior to the introduction of CAS at our institution. These cases
were managed using conventional freehand techniques with
miniplates. In cases where the mandibular segment would have been
too small following resection, segmental reconstruction was not
feasible, and a hemimandibulectomy was performed instead.
Outsourced CAS (TruMatch) group: Cases treated after
March 2021, where sufficient preparation time (≥1 month) and budget
permitted the use of outsourced CAS. TruMatch was not used in
urgent cases or when financial constraints precluded
outsourcing.
In-house CAS group: Cases treated after August 2022,
where outsourced CAS was not feasible due to time or cost
limitations. These cases underwent preoperative simulation and
guide design using Vincent and 3Shape software within our
institution. Standard reconstruction plates were manually contoured
to fit the simulated mandibular model and positioned
intraoperatively using custom-designed devices. The scapular graft
was adjusted and fixed in the same manner as in the TruMatch
group.
However, we excluded cases where one mandibular
condyle was completely resected and thus could not be analyzed,
cases where the reconstruction plate was temporarily positioned
directly on the mandible prior to resection during surgery, and
cases where pre- and postoperative computed tomography (CT) scans
were unavailable for comparison. All surgeries were performed by a
consistent surgical team specializing in oral and maxillofacial
oncology. The principal surgeons were board-certified oral and
maxillofacial surgeons with over 10 years of clinical experience in
oncologic head and neck surgery. The core members of this team
remained unchanged throughout the study period, thereby minimizing
variability related to surgeon experience. Of the 59 cases of
segmental mandibular resection performed during this period, 32
were included in this study. This retrospective study was approved
by the Institutional Review Board of The University of Osaka
Graduate School of Dentistry (approval number: R6-E35). Although
all patients were treated at The University of Osaka Dental
Hospital, ethics approval was obtained from the university-level
committee in accordance with institutional policy. At The
University of Osaka Graduate School of Dentistry, retrospective
clinical studies involving comparative analysis of multiple cases,
including those using opt-out consent, require formal review and
approval by the Graduate School of Dentistry Research Ethics
Committee to ensure consistent ethical oversight across affiliated
institutions. Patient data were collected from electronic medical
records and imaging archives maintained at The University of Osaka
Dental Hospital. In accordance with ethical guidelines, informed
consent was obtained from all patients using an opt-out method,
whereby information about the study was disclosed publicly and
patients were given the opportunity to decline participation. The
University of Osaka Dental Hospital is officially affiliated with
The University of Osaka Graduate School of Dentistry.
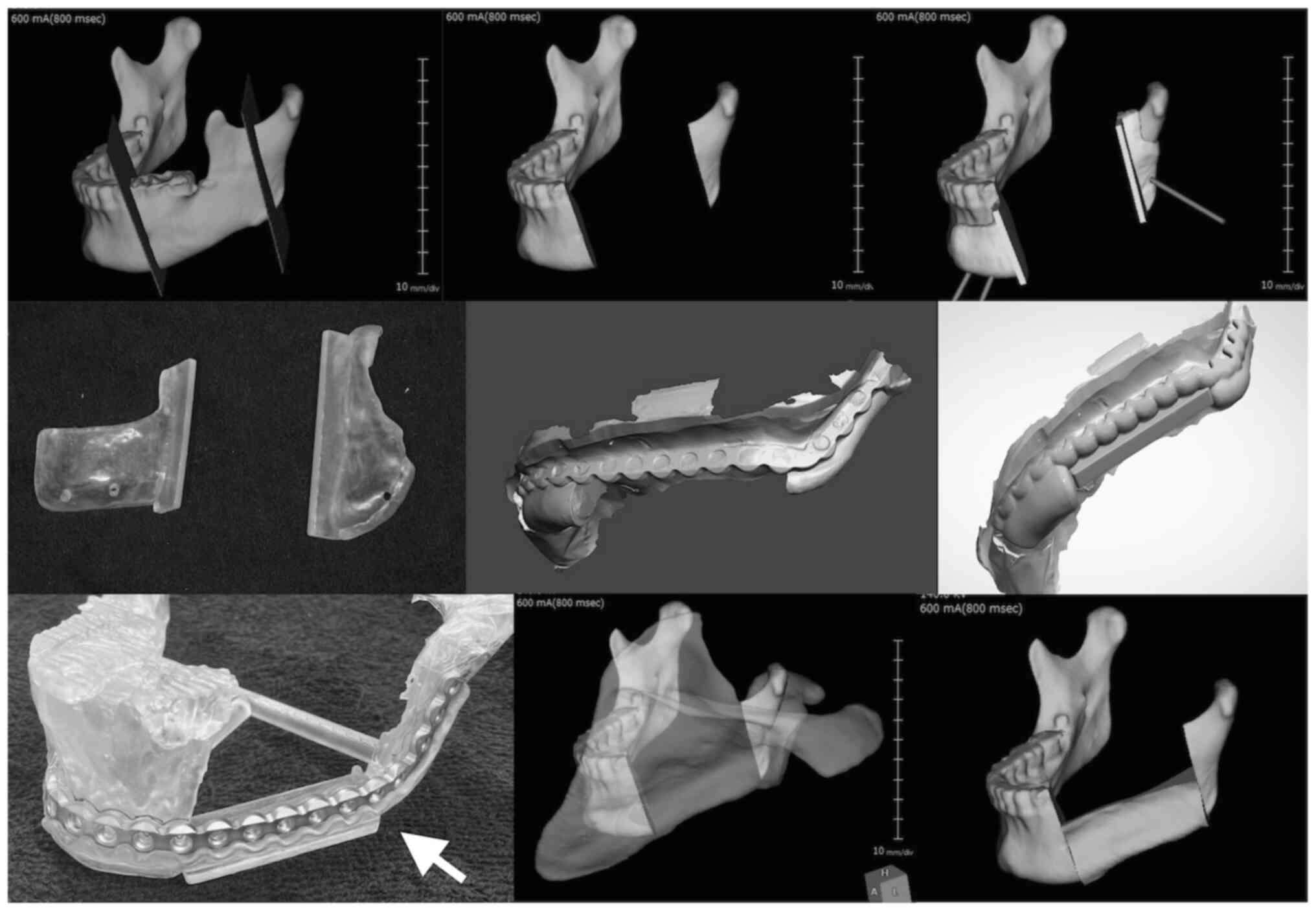
Virtual surgical planning and 3D
fabrication of surgical devices
All patients underwent CT scans of the mandible.
Similarly, patients planned for scapular grafting underwent
scapular CT imaging. The CT scans were saved as Digital Imaging and
Communications in Medicine files, segmented, and converted into a
virtual 3D model in Stereolithography (STL) format. For cases using
the TruMatch (DePuy Synthes, MA, USA) system, the order was placed
at least 1 month prior to surgery and discussed with the company
technician during preoperative planning. For the in-house CAS
system, a virtual mandibulectomy and reconstruction of the planned
mandibular defect were performed using Vincent software (Fujifilm,
Japan), which allowed for interactive 3D modeling of the mandible
and donor bone. Cases with severely deformed mandibles due to
tumors, fractures, or segmental defects were modeled based on the
contralateral, unaffected side using the mirror image principle
(8,12,18).
The cutting guide and bone/plate-positioning devices were designed
using Vincent software and the 3Shape Dental System (3Shape,
Denmark) (Fig. 1). The 3D models of
the mandible, bone/plate-positioning devices, and cutting-guide
devices were fabricated in photopolymer resin using a 3D printer
(Formlabs Inc., MA, USA). A 3D-printed model of the reconstructed
mandible was created. Preoperatively, a 2.6-mm locking titanium
mandibular reconstruction plate was manually contoured to fit the
3D-reconstructed mandibular model.
Vincent software, originally developed as a
diagnostic imaging viewer and analysis tool for radiology, is
primarily used to evaluate CT and magnetic resonance imaging data
and does not include surgical simulation functions by default. In
this study, we adapted the Vincent software for virtual
mandibulectomy and 3D modeling by utilizing its segmentation and
visualization capabilities. The 3Shape Dental System, primarily
intended for designing dental prostheses such as crowns, bridges,
custom impression trays, and complete or partial dentures, was
repurposed in our workflow. Specifically, we employed its CAD
modules to design bone and plate positioning devices for mandibular
reconstruction.
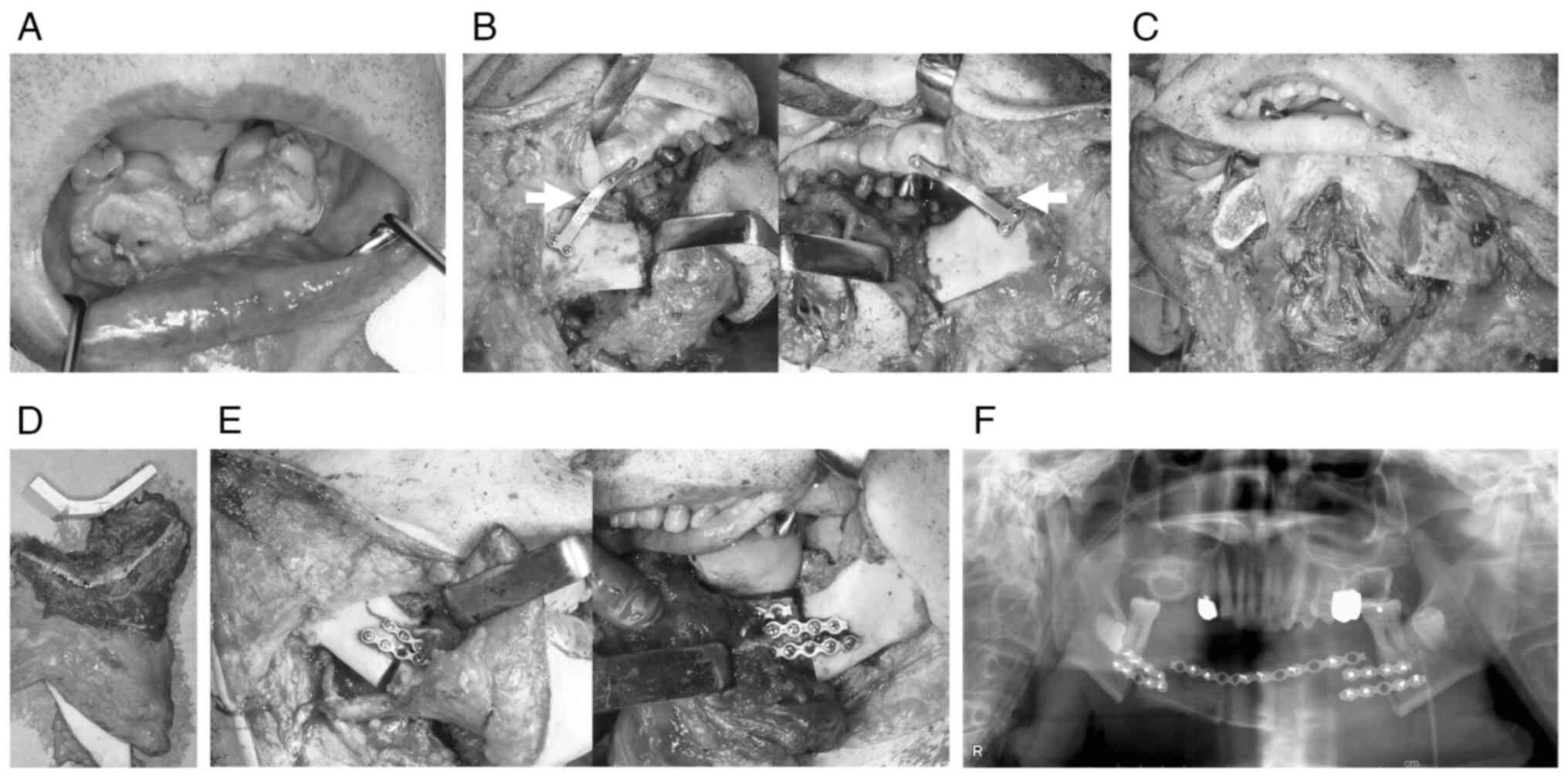
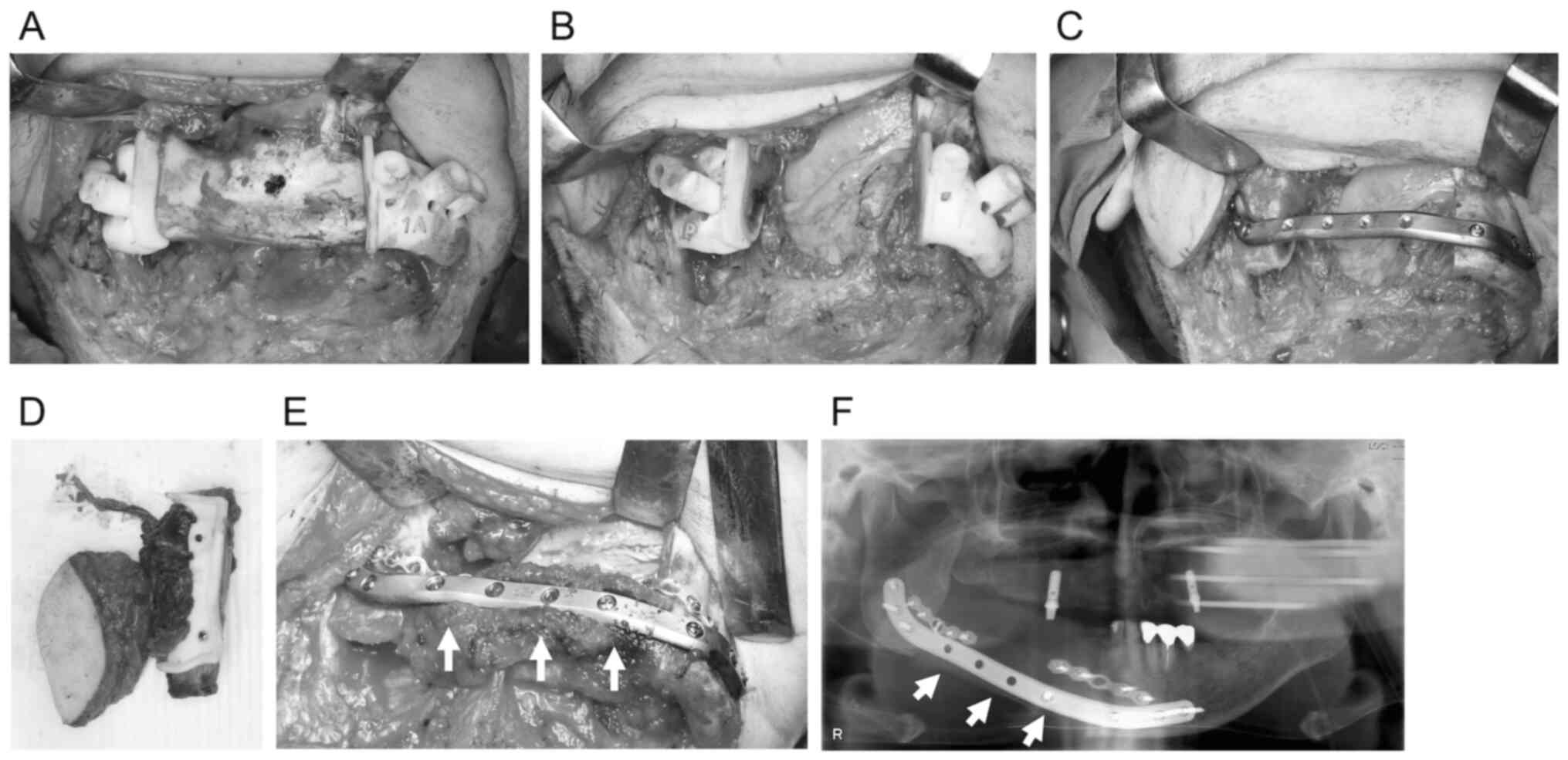
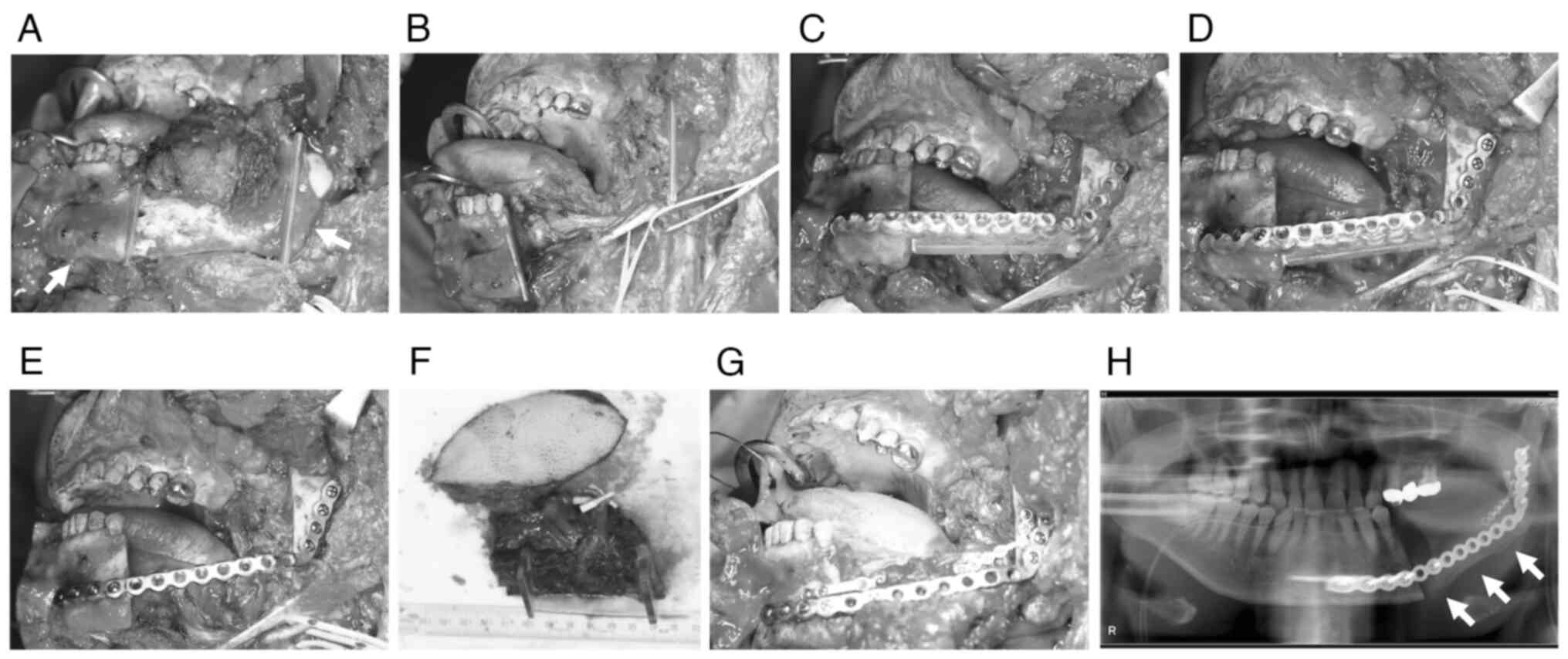
Surgical procedure
The surgeries were broadly divided into the
following three types: non-CAS, TruMatch, and in-house CAS
(Fig. 2, Fig. 3, Fig.
4). In non-CAS, intermaxillary fixation was used to maintain
the mandible's position after amputation, or a positioning plate
was used to fix it to the maxilla.
Accuracy analysis
Postoperative CT scans were taken approximately 1
month after surgery in all cases. These scans were used for
accuracy assessment through 3D model overlay analysis. The
postoperative CT scans were converted into 3D-STL models, which
were then superimposed on the preoperative 3D-STL models used for
virtual surgical planning, with the apex of the mandibular condyle
serving as the anchor point (software: Vincent, Fujifilm). The
discrepancy between the planned and actual mandibular
reconstruction was evaluated using two metrics: the intercondylar
distance (ICD, defined as the change in linear distance between the
apices of the bilateral mandibular condyles before and after
surgery, as described by Zhang et al) (19) and the 3D deviation distance (3DD,
defined as the spatial deviation of the apex of the affected
mandibular condyle between the planned and actual postoperative
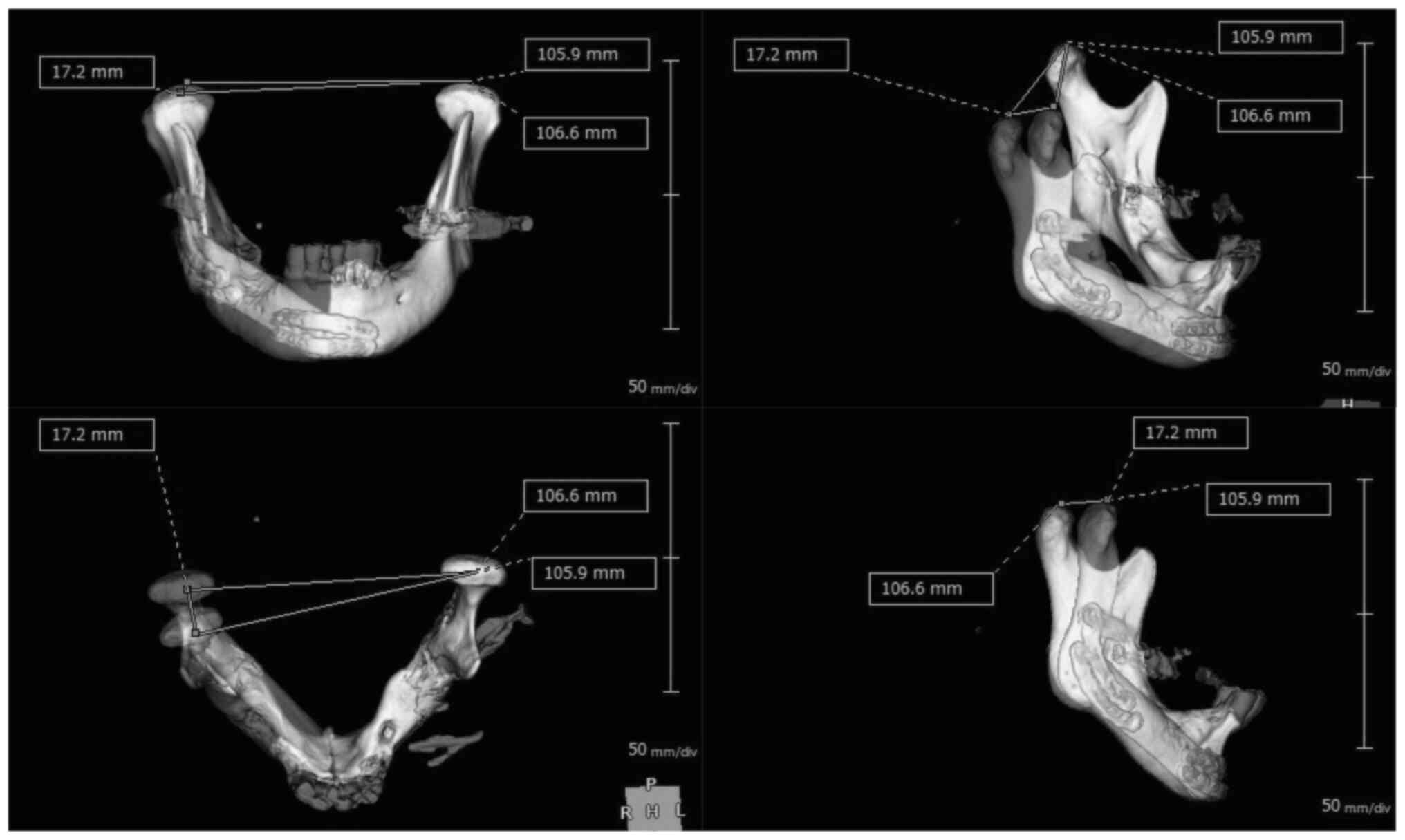
positions) (Fig. 5). The analysis
was performed using Vincent software (Fujifilm, Japan) as follows:
A single anatomical landmark, the apex of the mandibular condyle,
was defined as the most superior point of the condyle on the
preoperative CT scan. Using Vincent's overlay function, the
postoperative condyle was superimposed onto the preoperative
condyle for each side separately, and the same apex point was
defined at the postoperative condyles. Next, using the healthy side
of the mandible (including the mandibular body, angle, and ramus)
as a reference, the preoperative and postoperative models were
overlaid again using Vincent's overlay function. Based on this 3D
overlay, the ICD was calculated as the difference in ICD before and
after surgery, and the 3DD was calculated as the spatial deviation
of the affected condyle apex.
Statistical analysis
To determine the appropriate statistical tests, we
assessed the normality of the ICD and 3DD datasets using the
Shapiro-Wilk test. Based on the results and the limited sample
size, comparisons among the three groups were conducted using the
Kruskal-Wallis test, followed by Dunn's multiple comparisons test.
However, the comparison between two groups was analyzed using the
Mann-Whitney U test. Statistical analyses were performed using
GraphPad Prism version 10 (GraphPad Software, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Between April 2018 and January 2025, a total of 32
cases were included, comprising eight non-CAS cases, 13 TruMatch
cases, and 11 in-house CAS cases (Table
I, Table II, Table III). The overall mean age of
patients was 67.8 years, with 13 females and 19 males. Of the 32
cases, 23 involved malignant tumors, eight involved benign tumors,
and three were cases of osteomyelitis. Regarding mandibular
reconstruction, five cases involved plate-only reconstruction,
eight cases involved plate + pectoralis major myocutaneous flap, 16
cases involved plate + scapular flap, two cases involved plate +
fibula flap, and one case involved plate + rectus abdominis
musculocutaneous flap (Table IV).
Based on the classification of mandibular defects by Boyd et
al (HCL classification) (20),
nine cases (28.1%) were classified as lateral-condyle (LC) or
lateral-condyle-lateral (LCL) mandibular defects. Furthermore,
almost all in-house CAS cases involved malignant tumors, and half
of these cases were classified as LC or LCL. In both TruMatch and
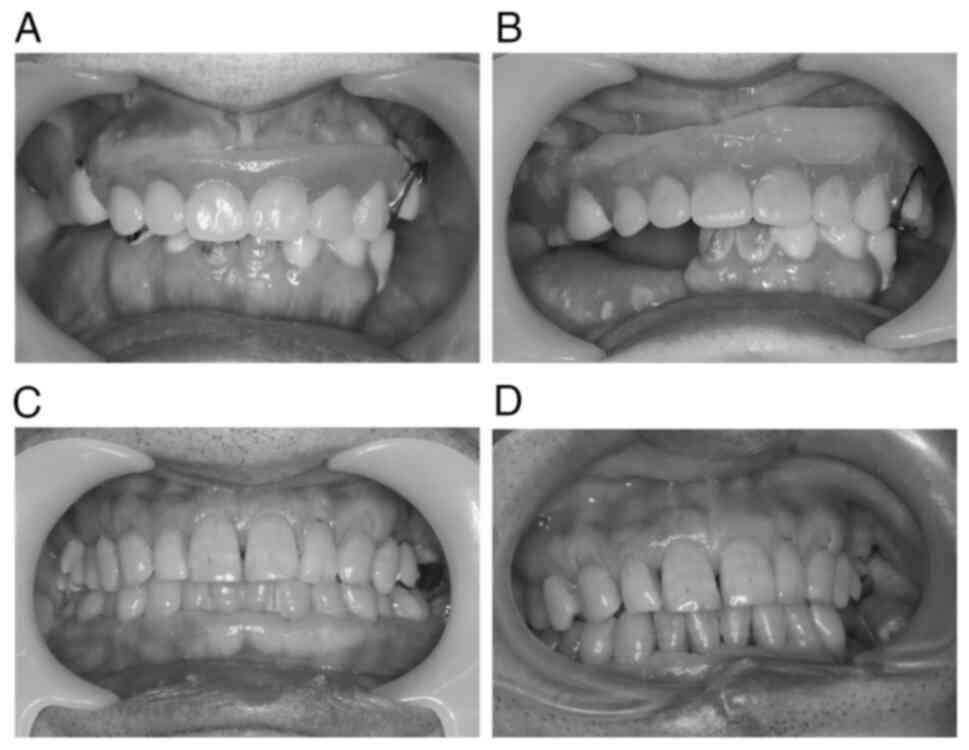
in-house CAS cases, the occlusal relationship was well maintained
pre- and postoperatively (Fig.
6).
 | Table I.List of the non-computer-assisted
surgery system cases. |
Table I.
List of the non-computer-assisted
surgery system cases.
| No. | Age, years | Sex | Diagnosis | HCL
classification | Reconstruction |
|---|
| 1 | 69 | M | Carcinoma of
mandibular gingiva | L | Fibula flap,
mini-plate |
| 2 | 37 | F | Ossifying
fibroma | L | Scapula flap,
mini-plate |
| 3 | 77 | F | Carcinoma of
mandibular gingiva | LCL | Scapula flap,
mini-plate |
| 4 | 52 | F | Ameloblastoma | L | Scapula flap,
mini-plate |
| 5 | 78 | M | Carcinoma of floor of
mouth | LCL | Scapula flap,
mini-plate |
| 6 | 75 | M | Carcinoma of
mandibular gingiva | L | Fibula flap,
mini-plate |
| 7 | 65 | M | Carcinoma of
mandibular gingiva | L | Scapula flap,
mini-plate |
| 8 | 74 | M | Carcinoma of
mandibular gingiva | L | Scapula flap,
mini-plate |
 | Table II.List of the TruMatch system
cases. |
Table II.
List of the TruMatch system
cases.
| No. | Age, years | Sex | Diagnosis | HCL
classification | Reconstruction |
|---|
| 1 | 70 | M | Carcinoma of
mandibular gingiva | L | TruMatch plate,
scapula flap |
| 2 | 77 | M | Radiation
osteomyelitisa | L | TruMatch plate,
PMMC-flap |
| 3 | 49 | F | Ameloblastoma | L | TruMatch plate |
| 4 | 65 | M | Carcinoma of
mandibular gingiva | L | TruMatch plate,
scapula flap |
| 5 | 67 | M | Carcinoma of
mandibular gingiva | L | TruMatch plate,
PMMC-flap |
| 6 | 73 | M | Ameloblastoma | L | TruMatch plate |
| 7 | 71 | F | Ameloblastoma | L | TruMatch plate,
scapula flap |
| 8 | 56 | F | Carcinoma of
mandibular gingiva | L | TruMatch plate,
scapula flap |
| 9 | 65 | M | Carcinoma of
mandibular gingiva | LCL | TruMatch plate,
scapula flap |
| 10 | 29 | F |
Cementoblastoma | L | TruMatch plate,
scapula flap |
| 11 | 83 | F | Medication-Related
Osteonecrosis of the Jaw | LCL | TruMatch plate |
| 12 | 63 | M | Postoperative
mandibular gingival carcinoma | L | TruMatch plate,
scapula flap |
| 13 | 77 | M | Postoperative
mandibular gingival carcinoma | L | TruMatch plate |
 | Table III.List of the in-house
computer-assisted surgery system cases. |
Table III.
List of the in-house
computer-assisted surgery system cases.
| No. | Age, years | Sex | Diagnosis | HCL
classification | Reconstruction |
|---|
| 1 | 94 | F | Carcinoma of
mandibular gingiva | LC | In-house plate |
| 2 | 86 | F | Carcinoma of
mandibular gingiva | LCL | In-house plate,
PMMC-flap |
| 3 | 70 | M | Carcinoma of
mandibular gingiva | L | In-house plate,
scapula flap |
| 4 | 78 | M | Carcinoma of
mandibular gingiva | L | In-house plate,
PMMC-flap |
| 5 | 51 | M | Carcinoma of
mandibular gingiva | LCL | In-house plate,
PMMC-flap |
| 6 | 80 | F | Carcinoma of
mandibular gingiva | L | In-house plate,
PMMC-flap |
| 7 | 67 | F | Carcinoma of
mandibular gingiva | LCL | In-house plate,
PMMC-flap |
| 8 | 90 | F | Carcinoma of
mandibular gingiva | LCL | In-house plate,
PMMC-flap |
| 9 | 58 | M | Carcinoma of
mandibular gingiva | L | In-house plate,
scapula flap |
| 10 | 55 | M | Carcinoma of
mandibular gingiva | L | In-house plate,
scapula flap |
| 11 | 70 | M | Radiation
osteomyelitisa | L | In-house plate,
RA-flap |
 | Table IV.Patient characteristics. |
Table IV.
Patient characteristics.
| Characteristic | Total | Non-CAS | TruMatch | In-house |
|---|
| Mean age,
years | 67.8 | 65.9 | 65.0 | 72.6 |
| Cases | 32 | 8 | 13 | 11 |
| Sex |
|
|
|
|
|
Female | 13 | 3 | 5 | 5 |
|
Male | 19 | 5 | 8 | 6 |
| Reconstruction |
|
|
|
|
| Only
the plate | 5 | 0 | 4 | 1 |
| The
plate + PMMC-flap | 8 | 0 | 2 | 6 |
| The
plate + scapula flap | 16 | 6 | 7 | 3 |
| The
plate + fibula flap | 2 | 2 | 0 | 0 |
| The
plate + RA-flap | 1 | 0 | 0 | 1 |
| Primary
disease |
|
|
|
|
|
Malignant tumor | 23 | 6 | 7 | 10 |
| Benign
tumor | 6 | 2 | 4 | 0 |
|
Osteomyelitis | 3 | 0 | 2 | 1 |
| HCL
classification |
|
|
|
|
| L | 23 | 6 | 11 | 6 |
| LC or
LCL | 9 | 2 | 2 | 5 |
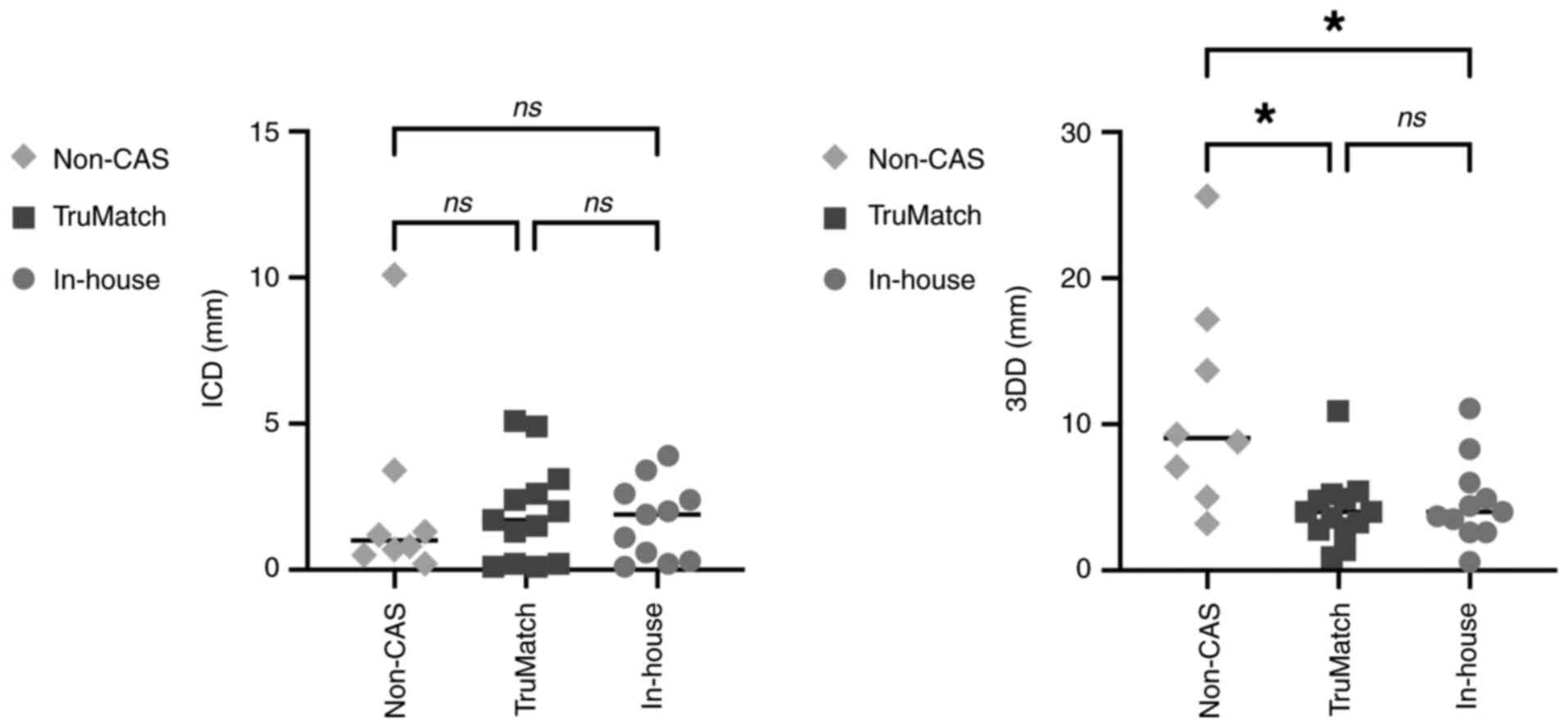
The results of the accuracy analysis using 3D model
overlay comparison are presented in Fig. 7. The ICD measurements were 2.3 mm on
average [standard deviation (SD): 3.3] in the non-CAS group, 1.9 mm
on average (SD: 1.7) in the TruMatch cases, and 1.8 mm on average
(SD: 1.3) in the in-house CAS group. No significant differences
were observed among the three groups. However, the 3DD results were
11.2 mm on average (SD: 7.4) in the non-CAS group, 4.2 mm on
average (SD: 2.4) in the TruMatch group, and 5.1 mm on average (SD:
2.7) in the in-house CAS group. The 3DD results indicated that
CAS-assisted cases were significantly more accurate, with no
significant difference between the TruMatch and in-house CAS
groups. However, significant differences were observed between the
TruMatch and non-CAS groups (adjusted P=0.0193) and between the
in-house CAS and non-CAS groups (adjusted P=0.0499).
Discussion
This study aimed to evaluate the accuracy of
mandibular reconstruction using three approaches: conventional
(non-CAS), outsourced CAS (TruMatch), and in-house CAS. The results
demonstrated that both CAS methods significantly improved
mandibular segment positioning compared with the conventional
method, with no significant difference in accuracy between
outsourced and in-house CAS.
In conventional mandibular reconstruction, plates
are typically pre-bent and temporarily fixed to the mandible before
resection to preserve the spatial relationship between bone
segments. However, this technique is feasible only when the bone
surface is clearly visible and unaffected by disease. In cases
involving tumor-related deformity or indistinct resection margins,
preoperative plate fixation is not possible, and attempting it may
result in plate protrusion and facial deformity.
All CAS cases in this study were selected because
preoperative plate fixation could not be performed. By contrast,
the non-CAS group included patients in whom reconstruction plates
were not applicable, and miniplates with bone grafts were used
instead. This selection strategy ensured that all groups faced
comparable surgical challenges regarding mandibular segment
positioning. Importantly, the presence or absence of bone grafting
does not influence the accuracy of segment alignment, which was the
primary focus of this study. This statement is supported by
internal data comparing CAS cases with and without bone grafting,
which showed no significant difference in either ICD or 3DD
(Fig. S1). Therefore, we believe
that bone grafting does not affect the spatial positioning of
mandibular segments, and the comparison across groups remains
valid.
The advantages of using CAS in mandibular
reconstruction are as follows: i) the ability to define the
resection range precisely by simulating surgery on CT images; ii)
the cutting-guide device for the mandible enables accurate and
efficient bone resection; iii) the grafting-bone cutting-guide
reduces the preparation time required for bone adjustment; iv) the
occlusal relationship can be maintained almost identically to the
preoperative condition; and v) custom-made plates eliminate the
need for bending, minimizing the risk of plate fracture due to
metal fatigue (16,21). However, outsourced custom-made CAS
systems also have some limitations. First, they require a
preparation period of at least 1 month, making them challenging to
use in malignant tumor cases. Due to outsourcing, the high costs
make it difficult for hospitals to profit, and cancellations can
result in significant financial losses. Additionally, since the
meetings are held online, it can be difficult for engineers to
fully understand the surgeon's specific needs (17). The outsourcing retains all data
during the creation process, meaning the hospital only receives the
final product. To address these issues, we initiated mandibular
reconstruction using our in-house CAS system in 2022. In terms of
cost, the outsourced TruMatch system, including the custom plate,
screws, surgical guides, and 3D models, typically costs more than
¥800,000 per case in Japan. By contrast, our in-house CAS system
costs approximately ¥150,000 per case. This substantial cost
reduction is primarily attributed to our institution's ownership of
3D printing equipment and the availability of skilled dental
technicians proficient in the software and fabrication process.
Although these institutional advantages may not be universally
applicable, they demonstrate the potential for cost-effective CAS
implementation in facilities with comparable resources.
The reported improvement in efficiency from
shortening the operation time is between 80 and 88 min (6,22), and
the reduction in free-flap ischemia time is reported to be 36–50
min (19,22). One of the greatest advantages of
reconstruction using surgical devices with CAS is that the surgical
plan can be easily and rapidly reproduced in the operating room.
The learning curve for freehand graft bone sculpting and plate
bending without CAS is long, and outcomes vary between surgeons
depending on their experience and technical skills (23). CAS, through the use of surgical
guides, reduces the learning curve of mandibular reconstruction and
significantly enhances proficiency and precision (13,22).
Surgical results using surgical guides are consistent and
reproducible, even by surgeons with varying levels of experience,
which benefits less experienced surgeons (14). In our study, all surgeries were
performed by a consistent surgical team specializing in oral and
maxillofacial oncology. The core members of this team remained
unchanged across the three groups (non-CAS, outsourced CAS, and
in-house CAS), minimizing variability related to individual surgeon
experience. However, we acknowledge that other potential
confounding factors, such as differences in tumor size, defect
classification, and the presence or absence of bone grafting, may
have influenced the outcomes. To mitigate this, cases were selected
based on a common criterion: the inability to perform preoperative
plate fixation due to tumor-related bone deformity or unclear
resection margins. Furthermore, internal data comparing CAS cases
with and without bone grafting showed no significant difference in
ICD or 3DD, suggesting that grafting itself does not affect segment
positioning accuracy. These factors support the validity of our
comparative analysis.
Several classification systems for mandibular
defects have been proposed, with the HCL classification being the
most widely used due to its simplicity (20,24).
However, it may lack detail in distinguishing functional and
reconstructive differences within the same category. In Japan, the
CAT classification offers a more detailed framework by using six
anatomical landmarks, allowing for better prediction of
reconstruction strategies and outcomes (25). Although more complex, it provides
clearer guidance for clinical decision-making. In this study, the
HCL classification was used for consistency with international
standards. Generally, mandibular reconstruction may be more
difficult in LC and LCL cases due to mandibular defects in the
anterior region compared to cases classified as L. However, this
trend was not observed in this study. Although it might be
difficult to detect a trend due to the small number of cases, it is
possible that when CAS is used, accuracy is not affected even in
cases with mandibular defects in the anterior region.
This single-center retrospective study compared the
accuracy of reconstruction using CAS vs. non-CAS after mandibular
resection in cases where the reconstruction plate could not be
placed directly on the mandible before mandibular resection
intraoperatively. Among the cases using CAS, we also compared
outsourced CAS using TruMatch with in-house CAS. In these
comparisons, the 3DD values, which represent the displacement of
the mandibular condyle, showed that CAS-based reconstruction
methods were significantly more accurate. Furthermore, our in-house
CAS was comparable to the outsourced CAS, with almost identical
accuracy. There have been previous reports on the application of
CAS in mandibular reconstruction. For these methods to be
implemented and established as superior techniques, evaluating
their accuracy is essential. Measurements used to determine
accuracy include ICD, intergonial distance, anterior-posterior
distance, and gonial angle (26).
However, in many cases of mandibular segmental resection, the
mandibular angle and anterior teeth are also resected, and in
practice, only the ICD could be measured in almost all our cases.
Since the change in ICD may not reflect slight changes before and
after surgery, we chose to superimpose the healthy side using pre-
and postoperative 3D images and measure the displacement of the
affected mandibular condyle as 3DD. Therefore, in our cases, we did
not find a significant difference in ICD between non-CAS and CAS,
but we did find a significant difference in 3DD.
Several studies have reported on mandibular
reconstruction using either outsourced CAS or unique in-house CAS,
but few have directly compared the two (14). In our study, no significant
difference in accuracy was observed between in-house CAS and
outsourced CAS. Both approaches improved operation time and
occlusal relationships, and the fit between the reconstruction
plate and the mandible was enhanced. However, TruMatch employs a
custom plate, which may contribute to slightly higher accuracy.
In-house CAS requires the use of a standard reconstruction plate,
which is weaker than the custom-made outsourced plate, and this
limitation needs improvement in the future. However, because custom
plates take time to manufacture, the TruMatch system requires a
minimum of one month for preparation, limiting its usefulness in
malignant tumor surgeries. On the other hand, in-house CAS can be
used in as little as one week of preparation, making it suitable
for emergency surgeries. This explains why our results show a
higher proportion of malignant tumor patients in the in-house CAS
cases compared to TruMatch cases. Although occlusal stability
appeared visually consistent in CAS cases, a more detailed and
objective analysis is warranted. Future studies should address this
aspect to more accurately quantify functional outcomes. In
contrast, occlusal abnormalities were observed in several cases
within the non-CAS group, including malalignment and asymmetry of
the dental arches, as noted during routine postoperative follow-up
and radiographic evaluation. These findings suggest that even minor
deviations in mandibular positioning can lead to functional
impairment, particularly when preoperative plate fixation is not
feasible. While no severe mastication disorders or
temporomandibular joint dysfunction were reported within one month
postoperatively, the presence of occlusal discrepancies underscores
the importance of incorporating functional assessments into future
studies. Quantitative evaluation of occlusal force, mandibular
mobility, and long-term functional outcomes will be essential to
fully understand the clinical impact of CAS-based
reconstruction.
Directions for further improving mandibular
reconstruction using CAS include improving preoperative surgical
planning and revising and upgrading the equipment used during
surgery. For example, when determining the extent of resection,
which is a crucial factor in mandibular reconstruction for
malignant tumor surgeries, machine learning may be able to
automatically determine the appropriate resection extent by
superimposing not only CT scans but also magnetic resonance imaging
and positron emission tomography-CT images. Furthermore,
mathematical modeling and machine learning may reduce the reliance
on the capabilities of surgeons and clinical engineers to select
the optimal reconstruction solution and suggest the ideal shape of
the surgical device (27).
Additionally, 3D printed mandibular reconstruction plates are now
available and are preferred over pre-bent plates because they offer
more accurate results and are less likely to break. However,
attention should be given to disadvantages, such as plate exposure,
especially if the design becomes too complex (22,28).
To minimize the risk of breakage due to bending of ready-to-use
reconstruction plates, this can be addressed by developing
efficient bending techniques.
Although our in-house CAS system currently relies on
commercially available software such as Vincent (Fujifilm, Japan)
and 3Shape Dental System (3Shape, Denmark), it is used in a
customized manner not originally intended for clinical mandibular
reconstruction. To address concerns regarding generalizability and
reproducibility, we are actively developing a dedicated software
platform that integrates virtual surgical planning, guide design,
and plate fitting. This new system aims to standardize the workflow
and facilitate broader adoption of in-house CAS techniques in
diverse clinical settings.
Prior to implementing the in-house CAS system, we
gained experience with several cases using the outsourced TruMatch
system. The design of our in-house surgical guides and positioning
devices was informed by the structure and functionality of those
used in TruMatch, allowing us to adopt and adapt effective design
elements. This experience contributed to the reproducibility and
reliability of our in-house workflow, despite the continued use of
standard reconstruction plates.
In terms of plate fitting accuracy, the TruMatch
system clearly outperforms the in-house CAS approach. This
limitation arises from manual plate bending, which inevitably
introduces gaps between the plate and the bone surface. Such gaps
can result in misalignment during fixation, as the plate may be
secured in a position deviating from that planned on the 3D model.
To mitigate this issue, our in-house CAS workflow incorporates a
custom-designed device that enables the precise placement of
manually bent plates at the intended location. While this does not
eliminate the inherent limitations of manual bending, it ensures
that the plate is fixed exactly where planned, thereby improving
reproducibility and reducing positional errors.
As a future direction, we aim to develop techniques
or tools that further minimize the gap between manually bent plates
and the bone surface, enhancing the accuracy and reliability of
in-house CAS-based mandibular reconstruction. However, in this
study, the fit of the plate was not assessed objectively, as no
standardized imaging or intraoperative measurement protocols were
implemented. Future studies should incorporate objective methods to
evaluate plate fitting accuracy, such as quantitative measurement
of the plate-to-bone gap using high-resolution postoperative CT
imaging and assessment of the contact surface area with 3D modeling
software.
Finally, we acknowledge that this study evaluated
surgical accuracy only at approximately 1 month postoperatively.
Long-term functional outcomes, including occlusal stability, mouth
opening, aesthetic appearance, and postoperative complications such
as non-union or infection, were not assessed. These factors are
critical for determining the true clinical value of CAS-based
mandibular reconstruction. Future prospective studies with extended
follow-up periods are warranted to comprehensively evaluate both
functional and aesthetic outcomes over time.
In addition to technical improvements, future
research should focus on study design enhancements. Given the
retrospective nature of the present study, randomization and
stratification by surgeon experience were not feasible. Prospective
studies with randomized designs and clearly defined surgeon
qualifications and experience levels would help reduce potential
confounding factors and strengthen the evidence supporting
CAS-based mandibular reconstruction.
In conclusion, the use of CAS systems in mandibular
reconstruction surgery may be particularly advantageous in cases
involving mandibular resection where postoperative occlusal
reconstruction is challenging. In-house CAS systems provide
comparable and favorable outcomes to outsourced CAS, making them a
potentially cost-effective option for precise reconstructive
surgery and enabling a greater number of patients to benefit from
CAS technology in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported by the OU Master Plan Implementation
Project from The University of Osaka.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ShK, YM, TM and NU conceptualized the study and
designed the overall research approach. ShK, RK, KK, ATN and EO
performed experiments and ensured their completeness and integrity.
ShK and YM confirm the authenticity of the all the raw data. ShK,
YM, RK, TM, KK, ATN and EO performed formal statistical analyses.
YM and NU acquired funding for the project. ShK, RK, YU, SaK, YMM,
AT and KM conducted experimental and clinical investigations. ShK,
YM, RK, TM and KK developed and optimized the study methodology,
including experimental protocols and analysis pipelines. ShK, YM,
RK, TM, KK, AN, EO, YU, SaK, AT, KM and NU provided essential
resources, including access to clinical samples, instrumentation
and institutional facilities. ShK, YM, RK and TM developed and
maintained the software tools used for image processing and data
analysis. ShK, YM and NU supervised and validated the study steps
and results. ShK produced the visual materials (figures and
schematic diagrams) and, together with YM, prepared the initial
draft of the manuscript. ShK, SaK, YM, YMM, AT, KM and NU
critically revised the manuscript for important intellectual
content and approved the final version. All authors read and
approved the final manuscript, and agreed to be accountable for all
aspects of the work.
Ethics approval and consent for
participation
The study was approved by The University of Osaka
Graduate School of Dentistry Research Ethics Committee (approval
number: R6-E35). All methods were performed in accordance with the
relevant guidelines and regulations of the Basel Declaration.
Informed consent was obtained from all patients using an opt-out
method.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hirsch DL, Garfein ES, Christensen AM,
Weimer KA, Saddeh PB and Levine JP: Use of computer-aided design
and computer-aided manufacturing to produce orthognathically ideal
surgical outcomes: A paradigm shift in head and neck
reconstruction. J Oral Maxillofac Surg. 67:2115–2122. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang EI, Jenkins MP, Patel SA and Topham
NS: Long-term operative outcomes of preoperative computed
tomography-guided virtual surgical planning for osteocutaneous free
flap mandible reconstruction. Plast Reconstr Surg. 137:619–623.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antony AK, Chen WF, Kolokythas A, Weimer
KA and Cohen MN: Use of virtual surgery and
stereolithography-guided osteotomy for mandibular reconstruction
with the free fibula. Plast Reconstr Surg. 128:1080–1084. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Largo RD and Garvey PB: Updates in head
and neck reconstruction. Plast Reconstr Surg. 141:271e–285e. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren W, Gao L, Li S, Chen C, Li F, Wang Q,
Zhi Y, Song J, Dou Z, Xue L and Zhi K: Virtual Planning and 3D
printing modeling for mandibular reconstruction with fibula free
flap. Med Oral Patol Oral Cir Bucal. 23:e359–e366. 2018.PubMed/NCBI
|
|
6
|
Wang YY, Zhang HQ, Fan S, Zhang DM, Huang
ZQ, Chen WL, Ye JT and Li JS: Mandibular reconstruction with the
vascularized fibula flap: Comparison of virtual planning surgery
and conventional surgery. Int J Oral Maxillofac Surg. 45:1400–1405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toto JM, Chang EI, Agag R, Devarajan K,
Patel SA and Topham NS: Improved operative efficiency of free
fibula flap mandible reconstruction with patient-specific,
computer-guided preoperative planning. Head Neck. 37:1660–1664.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumta S, Kumta M, Jain L, Purohit S and
Ummul R: A novel 3D template for mandible and maxilla
reconstruction: Rapid prototyping using stereolithography. Indian J
Plast Surg. 48:263–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Avraham T, Franco P, Brecht LE, Ceradini
DJ, Saadeh PB, Hirsch DL and Levine JP: Functional outcomes of
virtually planned free fibula flap reconstruction of the mandible.
Plast Reconstr Surg. 134:628e–634e. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu YF, Xu LW, Zhu HY and Liu SSY:
Technical procedures for template-guided surgery for mandibular
reconstruction based on digital design and manufacturing. Biomed
Eng Online. 13:632014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mazzoni S, Marchetti C, Sgarzani R,
Cipriani R, Scotti R and Ciocca L: Prosthetically guided
maxillofacial surgery: Evaluation of the accuracy of a surgical
guide and custom-made bone plate in oncology patients after
mandibular reconstruction. Plast Reconstr Surg. 131:1376–1385.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanasono MM and Skoracki RJ:
Computer-assisted design and rapid prototype modeling in
microvascular mandible reconstruction. Laryngoscope. 123:597–604.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Foley BD, Thayer WP, Honeybrook A, McKenna
S and Press S: Mandibular reconstruction using computer-aided
design and computer-aided manufacturing: An analysis of surgical
results. J Oral Maxillofac Surg. 71:e111–e119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pucci R, Weyh A, Smotherman C, Valentini
V, Bunnell A and Fernandes R: Accuracy of virtual planned surgery
versus conventional free-hand surgery for reconstruction of the
mandible with osteocutaneous free flaps. Int J Oral Maxillofac
Surg. 49:1153–1161. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodby KA, Turin S, Jacobs RJ, Cruz JF,
Hassid VJ, Kolokythas A and Antony AK: Advances in oncologic head
and neck reconstruction: Systematic review and future
considerations of virtual surgical planning and computer aided
design/computer aided modeling. J Plast Reconstr Aesthet Surg.
67:1171–1185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong QN, Karino M, Osako R, Ishizuka S,
Toda E, Kanayama J, Sato S, Okuma S, Okui T and Kanno T:
Computer-assisted fabrication of a cutting guide for marginal
mandibulectomy and a patient-specific mandibular reconstruction
plate: A case report. J Oral Maxillofac Surg Med Pathol.
33:505–512. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohyama Y, Hasegawa K, Uzawa N, Yamashiro
M, Michi Y, Inaba Y, Kubota M, Kanemaru T, Iwasaki T and Yoda T:
Vascularized bony reconstruction after mandibular resection-Report
and discussion of experience with 3 types of plates. Jpn J Oral
Maxillofac Surg. 69:493–498. 2023. View Article : Google Scholar
|
|
18
|
Metzler P, Geiger EJ, Alcon A, Ma X and
Steinbacher DM: Three-dimensional virtual surgery accuracy for free
fibula mandibular reconstruction: planned versus actual results. J
Oral Maxillofac Surg. 72:2601–2612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Liu Z, Li B, Yu H, Shen SG and
Wang X: Evaluation of computer-assisted mandibular reconstruction
with vascularized fibular flap compared to conventional surgery.
Oral Surg Oral Med Oral Pathol Oral Radiol. 121:139–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boyd JB, Gullane PJ, Rotstein LE, Brown DH
and Irish JC: Classification of mandibular defects. Plast Reconstr
Surg. 92:1266–1275. 1993.PubMed/NCBI
|
|
21
|
Koyachi M, Sugahara K, Tachizawa K,
Nishiyama A, Odaka K, Matsunaga S, Sugimoto M and Katakura A:
Mixed-reality and computer-aided design/computer-aided
manufacturing technology for mandibular reconstruction: A case
description. Quant Imaging Med Surg. 13:4050–4056. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blanc J, Fuchsmann C, Nistiriuc-Muntean V,
Jacquenot P, Philouze P and Ceruse P: Evaluation of virtual
surgical planning systems and customized devices in fibula free
flap mandibular reconstruction. Eur Arch Otorhinolaryngol.
276:3477–3486. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bartier S, Mazzaschi O, Benichou L and
Sauvaget E: Computer-assisted versus traditional technique in
fibular free-flap mandibular reconstruction: A CT symmetry study.
Eur Ann Otorhinolaryngol Head Neck Dis. 138:23–27. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jagtiani K, Gurav S, Singh G and Dholam K:
A review on the classification of mandibulectomy defects and
suggested criteria for a universal description. J Prosthet Dent.
132:270–277. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hashikawa K, Yokoo S and Tahara S: Novel
classification system for oncological mandibular defect: CAT
classification. Jpn J Head Neck Cancer. 34:412–418. 2008.
|
|
26
|
Annino DJ Jr, Sethi RK, Hansen EE, Horne
S, Dey T, Rettig EM, Uppaluri R, Kass JI and Goguen LA: Virtual
planning and 3D-printed guides for mandibular reconstruction:
Factors impacting accuracy. Laryngoscope Investig Otolaryngol.
7:1798–1807. 2022. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang E, Durham JS, Anderson DW and Prisman
E: Clinical evaluation of an automated virtual surgical planning
platform for mandibular reconstruction. Head Neck. 42:3506–3514.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang WF, Choi WS, Wong MCM, Powcharoen W,
Zhu WY, Tsoi JK, Chow M, Kwok KW and Su YX: Three-dimensionally
printed patient-specific surgical plates increase accuracy of
oncologic head and neck reconstruction versus conventional surgical
plates: A comparative study. Ann Surg Oncol. 28:363–375. 2021.
View Article : Google Scholar : PubMed/NCBI
|