Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
prevalent subtype of non-Hodgkin lymphoma (NHL), characterized by
aggressive mature B-cell tumors with heterogeneous clinical
features and varying responses to treatment (1–3). Only
60% of patients achieve long-term remission, and approximately
one-third experience treatment resistance or relapse, despite
conventional immunochemotherapy with a rituximab, cyclophosphamide,
doxorubicin, vincristine and prednisone (R-CHOP) regimen (4).
The International Prognostic Index (IPI) (5) is the leading prognostic tool for
clinical decision-making, and incorporates age, stage, lactate
dehydrogenase (LDH) level, performance status and extranodal
involvement. Although gene expression profiling and molecular
markers (including MYD88, TP53 and KMT2D) provide useful prognostic
information (6,7), their high expense, technical
requirements and time-consuming procedures prevent widespread
clinical use. Therefore, there is an urgent need for accessible and
cost-effective prognostic biomarkers.
By inhibiting antitumor immunity, promoting
angiogenesis and providing trophic factors for malignant lymphocyte
survival, monocytes and tumor-associated macrophages play important
roles in the pathophysiology of lymphomas (8,9).
Absolute monocyte counts (AMCs) have demonstrated prognostic value
in lymphoma (10,11), but have important clinical
limitations. Patients with DLBCL frequently present with
significant hematological variations, including treatment-induced
leukopenia or leukocytosis, bone marrow involvement,
infection-related white blood cell (WBC) fluctuations (12) and corticosteroid effects (13). These factors can substantially alter
absolute counts while potentially masking the underlying immune
composition.
By contrast, monocyte percentage (MP) offers several
distinct advantages: First, MP reflects the relative immune cell
composition and the proportional balance between immunosuppressive
monocytes and other immune populations, which may more accurately
represent tumor-immune microenvironment dynamics given that
monocytes play pivotal roles in shaping immunosuppressive
environments through various mechanisms (14). Second, MP is less susceptible to the
confounding effects of total WBC variations, providing a more
stable measure across different clinical scenarios (15). Third, MP may eliminate variability
from laboratory-specific reference ranges, enhancing
standardization and applicability across diverse healthcare
settings. Despite these theoretical advantages and the established
role of monocytes in lymphoma pathogenesis, MP has been
underexplored as an independent prognostic marker in DLBCL.
Therefore, the present retrospective cohort study
was conducted to evaluate the prognostic value of MP in patients
with newly diagnosed DLBCL treated with R-CHOP, and to assess its
independent prognostic significance beyond established markers,
including analysis of IPI and AMC.
Patients and methods
Study design and participants
The present study included patients with newly
diagnosed DLBCL who received R-CHOP (rituximab, cyclophosphamide,
hydroxydaunorubicin, tumor protein, and prednisone) treatment and
evaluation at Ganzhou People's Hospital (Ganzhou, China) between
January 2015 and December 2023. This study was conducted in
accordance with the Declaration of Helsinki and was approved by the
Ganzhou People's Hospital Ethics Committee (Ganzhou, China;
approval no. PJB2025-210-01). The requirement for informed consent
was waived by the ethics committee due to the retrospective nature
of the study and the use of fully anonymized clinical data.
Exclusion criteria included autoimmune diseases, a human
immunodeficiency virus-positive status, inflammatory conditions,
other lymphomas (such as follicular lymphoma, mantle cell lymphoma)
or leukemias and previous chemoradiotherapy. The following clinical
baseline data were retrieved from medical records during diagnosis:
Sex, age, Eastern Cooperative Oncology Group Score (16), Ann Arbor tumor stage (17), molecular sub-type, extranodal
infiltration number, lactate dehydrogenase level, IPI score, bone
marrow involvement and pathology results, WBC count, MP, AMC,
absolute lymphocyte count, red blood cell (RBC) count, blood
platelet (PLT) count, hemoglobin level, fibrinogen level, LDH,
albumin level and immunophenotype. Clinicopathological criteria
from either an initial lymph node biopsy or an external biopsy of
the primary nodes were used to confirm the DLBCL diagnosis. The
molecular subtype of DLBCL was classified into germinal center
B-cell-like (GCB) and non-GCB subtypes using the Hans algorithm,
which is based on immunohistochemical staining for CD10, BCL6 and
MUM1 (6) When diagnosing DLBCL, an
automated complete blood count (CBC) is frequently used to measure
the MP in peripheral WBCs.
Grouping strategy
Patients were divided into two groups based on MP,
with the clinical laboratory standard upper limit threshold of 10%
used as the primary cutoff; however, receiver operating
characteristic (ROC) analysis using the Youden Index suggested
using an optimal threshold of 8.7%. The 10% threshold represents
the established upper limit for normal MP and defines monocytosis
in standard clinical practice. To evaluate how cutoff selection
affected prognostic performance, a sensitivity analysis was
conducted comparing the two thresholds. Participants were
classified based on a cut-off value of MP (≤10 and >10%) for the
primary analysis.
Follow-up and outcome definitions
Follow-up was conducted by reviewing medical
histories or through telephone conversations. Progression-free
survival (PFS) time was defined as the interval between the date of
diagnosis and disease progression, recurrence, death or the end of
follow-up. Overall survival (OS) time was defined as the interval
between diagnosis and death or the end of follow-up.
Statistical analysis
Categorical variables are presented as n (%), and
continuous variables that are normally distributed are presented as
mean ± standard deviation. Continuous variables with skewed
distribution are presented as median (interquartile range). All
comparisons were performed with appropriate statistical tests
(χ2/Fisher's exact test for categorical variables and
unpaired t-test/Mann-Whitney U-test for continuous variables) with
P-values reported.
A multivariate Cox proportional hazard regression
model was used to adjust for potential confounders to estimate the
association of MP with OS and PFS in newly diagnosed DLBCL. The
lower group (≤10%) was the reference group. Model 1 had no
adjustments, whereas model 2 was adjusted for age and sex. Model 3
was further adjusted for Ann Arbor tumor stage, molecular subtype,
double expression, extranodal infiltration number, IPI score, bone
marrow involvement, Epstein-Barr virus infection, WBC count, RBC
count, PLT count, hemoglobin level, fibrinogen level and albumin
level. To determine the association of MP with OS and PFS, the
cumulative Kaplan-Meier curve was plotted. Eventually, by
calculating the area under the ROC curve (AUC) and 95% confidence
intervals (CIs) for outcomes, the predictive power of IPI scores
and their combination with the MP was evaluated. P<0.05 was used
to indicate a statistically significant difference.
All statistical analyses were conducted using the
software package R (http://www.R-project.org) and Empower Stats
(http://www.empowerstats.com; X&Y
Solutions, Inc.).
Results
Patient characteristics
A total of 169 newly diagnosed DLBCL patients were
included in the present study, with a median age of 59.0±13.0 years
(median, 61.0 years; IQR 53.0-68.0) and 53.8% male predominance.
Based on the optimal cutoff of 10% MP, 117 patients (69.2%) were
classified in the low MP group and 52 (30.8%) in the high MP group
(Table I).
 | Table I.Association between MP and clinical
characteristics in patients with newly diagnosed diffuse large
B-cell lymphoma. |
Table I.
Association between MP and clinical
characteristics in patients with newly diagnosed diffuse large
B-cell lymphoma.
| Variables | Total (n=169) | MP ≤10% (n=117) | MP >10%
(n=52) | P-value |
|---|
| Sex, n (%) |
|
|
| 0.738 |
|
Female | 78 (46.2) | 53 (45.3) | 25 (48.1) |
|
| Male | 91 (53.8) | 64 (54.7) | 27 (51.9) |
|
| Age, years |
|
|
| 0.757 |
| ≤60 | 75 (44.4) | 51 (43.6) | 24 (46.2) |
|
|
>60 | 94 (55.6) | 66 (56.4) | 28 (53.8) |
|
| Mean ± SD | 59.0±13.0 | 59.0±13.2 | 59.2±12.7 | 0.893 |
| Median (IQR) | 61.0 (53.0,
68.0) | 61.0 (53.0,
68.0) | 61.0 (53.0,
69.0) | 0.969 |
| Hans algorithm, n
(%) |
|
|
|
|
| GCB | 112 (66.3) | 70 (59.8) | 42 (80.8) |
|
|
Non-GCB | 57 (33.7) | 47 (40.2) | 10 (19.2) |
|
| Double expression, n
(%) |
|
|
| 0.149 |
| No | 126 (74.6) | 91 (77.8) | 35 (67.3) |
|
| Yes | 43 (25.4) | 26 (22.2) | 17 (32.7) |
|
| Ann Arbor clinical
stage, n (%) |
|
|
| 0.016 |
|
I–II | 27 (16.0) | 24 (20.5) | 3 (5.8) |
|
|
III–IV | 138 (81.7) | 89 (76.1) | 49 (94.2) |
|
| NA | 4 (2.4) | 4 (3.4) | 0 (0.0) |
|
| IPI score, n
(%) |
|
|
| 0.278 |
| 0 | 12 (7.1) | 10 (8.5) | 2 (3.8) |
|
| 1 | 34 (20.1) | 26 (22.2) | 8 (15.4) |
|
| 2 | 41 (24.3) | 27 (23.1) | 14 (26.9) |
|
| 3 | 43 (25.4) | 32 (27.4) | 11 (21.2) |
|
| 4 | 27 (16.0) | 14 (12.0) | 13 (25.0) |
|
| 5 | 12 (7.1) | 8 (6.8) | 4 (7.7) |
|
| Extranodal
involvement, n (%) |
|
|
| 0.062 |
|
<2 | 73 (43.2) | 45 (38.5) | 28 (53.8) |
|
| ≥2 | 96 (56.8) | 72 (61.5) | 24 (46.2) |
|
| Bone marrow
infiltration, n (%) |
|
|
| 0.769 |
| No | 145 (85.8) | 101 (86.3) | 44 (84.6) |
|
|
Yes | 24 (14.2) | 16 (13.7) | 8 (15.4) |
|
| Combined with
cancer, n (%) |
|
|
| 0.703 |
| No | 161 (95.3) | 112 (95.7) | 49 (94.2) |
|
|
Yes | 8 (4.7) | 5 (4.3) | 3 (5.8) |
|
| EBV DNA >500
copies/ml, n (%) |
|
|
| 0.253 |
| No | 136 (80.5) | 98 (83.8) | 38 (73.1) |
|
|
Yes | 24 (14.2) | 14 (12.0) | 10 (19.2) |
|
| NA | 9 (5.3) | 5 (4.3) | 4 (7.7) |
|
| Mean WBC ± SD,
×109/l | 6.4±3.1 | 6.9±3.3 | 5.1±2.4 | <0.001 |
| AMC,
×109/la | 0.5 (0.3-0.7) | 0.4 (0.3-0.6) | 0.7 (0.5-1.0) | <0.001 |
| ALC,
×109/la | 1.3 (0.9-1.7) | 1.4 (1.0-1.8) | 0.9 (0.5-1.4) | <0.001 |
| Mean RBC ± SD,
×1012/l | 4.2±0.8 | 4.3±0.7 | 3.8±0.9 | <0.001 |
| Mean Hb ± SD,
g/l | 122.9±25.5 | 127.5±23.2 | 112.3±27.3 | <0.001 |
| PLT,
×109/la | 219.0
(159.0-284.0) | 234.0
(188.0-308.0) | 172.5
(122.2-246.2) | <0.001 |
| Mean Fbg ± SD,
g/l | 3.8±1.3 | 3.8±1.2 | 3.8±1.4 | 0.980 |
| Mean Alb ± SD,
g/l | 40.7±5.7 | 41.6±5.4 | 38.6±6.0 | 0.001 |
| Ferritin,
ng/mla | 261.2
(148.9-464.9) | 244.7
(144.1-440.6) | 317.0
(168.2-748.0) | 0.045 |
| LDH,
U/la | 233.0
(182.5-448.5) | 208.0
(172.0-327.0) | 344.5
(209.2-550.8) | 0.001 |
| LDH >ULN, n
(%) | 69 (40.8) | 36 (30.8) | 33 (63.5) | <0.001 |
Baseline characteristics were generally balanced
between groups regarding age, sex, double expression, IPI score,
extranodal involvement, bone marrow infiltration, concurrent
malignancy, EBV DNA levels and fibrinogen levels (all P>0.05).
However, significant differences emerged in several key features.
Patients with high MP more frequently presented with advanced stage
III–IV disease (94.2 vs. 76.1%; P=0.016) and GCB subtype (80.8 vs.
59.8%; P=0.008). Laboratory analyses revealed that high MP was
associated with elevated LDH (median, 344.5 vs. 208.0 U/l;
P=0.001), with 63.5% exceeding the upper limit of normal LDH level
compared with 30.8% in the low MP group (P<0.001). Despite lower
total WBC counts (5.1×109/l vs. 6.9×109/l;
P<0.001), the high MP group had a higher AMC
(0.7×109/l vs. 0.4×109/l; P<0.001) but
lower ALC (0.9×109/l vs. 1.4×109/l;
P<0.001), indicating a shift in immune cell composition.
Additionally, high MP was associated with lower hemoglobin (112.3
vs. 127.5 g/l; P<0.001), PLTs (172.5×109/l vs.
234.0×109/l; P<0.001) and albumin (38.6 vs. 41.6 g/l;
P=0.001) levels, and a higher ferritin level (317.0 vs. 244.7
ng/ml; P=0.045), suggesting greater disease burden.
Univariate analysis
Using univariate Cox regression analysis, various
prognostic factors that affect PFS and OS were analyzed (Table II). PFS was strongly correlated
with Ann Arbor stages III to IV (HR=8.30, 95% CI: 1.14-60.23;
P=0.036), bone marrow involvement (HR=3.05, 95% CI: 1.64-5.68;
P<0.001), IPI scores >3 (HR=4.64, 95% CI: 2.68-8.02;
P<0.001), RBC (HR=0.59, 95% CI: 0.42-0.83; P=0.002), hemoglobin
(HR=0.99, 95% CI: 0.98-1.00; P=0.016), PLT count (HR=1.00, 95% CI:
0.99-1.00; P=0.023) and albumin level (HR=0.95, 95% CI: 0.91-1.00;
P=0.048). Similarly, OS was strongly associated with Ann Arbor
stages III to IV (HR=7.45, 95% CI: 1.24-54.68; P=0.002), IPI scores
>3 (HR=5.12, 95% CI: 1.62-16.18; P=0.005) and WBC count
(HR=0.75, 95% CI: 0.58-0.97; P=0.028).
 | Table II.Univariate analysis of
clinicopathological parameters for OS and PFS time in patients with
newly diagnosed diffuse large B-cell lymphoma. |
Table II.
Univariate analysis of
clinicopathological parameters for OS and PFS time in patients with
newly diagnosed diffuse large B-cell lymphoma.
|
| PFS | OS |
|---|
|
|
|
|
|---|
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Female | 1.28
(0.74-2.21) | 0.383 | 1.12
(0.40-3.14) | 0.831 |
| Age >60
years | 1.31
(0.76-2.26) | 0.335 | 1.00
(0.96-1.04) | 0.840 |
| Ann Arbor stage
III–IV | 8.30
(1.14-60.23) | 0.036 | 7.45
(1.24-54.68) | 0.002 |
| Hans type | 1.54
(0.87-2.72) | 0.137 | 1.08
(0.27-4.26) | 0.917 |
| IPI scores | 4.64
(2.68-8.02) | <0.001 | 5.12
(1.62-16.18) | 0.005 |
| Extranodal sites
≥2 | 1.20
(0.69-2.10) | 0.516 | 0.20
(0.41-3.54) | 0.744 |
| Bone marrow
involvement | 3.05
(1.64-5.68) | <0.001 | 1.96
(0.61-6.29) | 0.260 |
| EBV DNA >500
copies | 1.17
(0.46-3.00) | 0.736 | 2.10
(0.25-17.61) | 0.493 |
| Combined with
tumor | 2.26
(0.81-6.32) | 0.119 | 1.81
(0.40-8.29) | 0.442 |
| WBC | 0.92
(0.82-1.02) | 0.120 | 0.75
(0.58-0.97) | 0.028 |
| RBC | 0.59
(0.42-0.83) | 0.002 | 0.63
(0.35-1.15) | 0.133 |
| Hb | 0.99
(0.98-1.00) | 0.016 | 0.98
(0.97-1.00) | 0.072 |
| PLT | 1.00
(0.99-1.00) | 0.023 | 1.00
(0.99-1.00) | 0.383 |
| Fbg | 1.02
(0.81-1.29) | 0.843 | 0.64
(0.37-1.10) | 0.106 |
| Alb | 0.95
(0.91-1.00) | 0.048 | 0.92
(0.84-1.00) | 0.064 |
| Ferritins | 1.00
(1.00-1.00) | 0.337 | 1.00
(1.00-1.00) | 0.492 |
Cutoff value determination
The following performance metrics were observed by
comparing the ROC-optimal (8.7%) and clinical (10%) cutoffs: The
8.7% cutoff demonstrated higher sensitivity (62.3 vs. 45.3%),
Youden Index (0.286 vs. 0.194) and negative predictive value (NPV)
(79.4 vs. 74.8%), whereas the 10% cutoff showed superior
specificity (74.1 vs. 66.4%). Between the 8.7 and 10% cutoffs,
positive predictive values were comparable (45.8 vs. 44.4%;
difference 1.4%). The 10% threshold was selected for its clinical
practicality as a round number and higher specificity, which
minimizes false-positive risk stratification despite slightly lower
sensitivity and NPV. Table SI
summarizes the performance comparison.
In order to ascertain the predictive value of the MP
in peripheral blood, MP was measured from peripheral blood samples
collected at initial diagnosis. In the multivariate Cox regression
analysis, MP was analyzed both as a continuous variable and as a
dichotomized variable using a 10% cutoff.
Multivariate analysis
When MP was analyzed as a continuous variable, it
demonstrated consistent prognostic significance across all three
models. In model 1, each 1% increase in MP was associated with
worse PFS (HR, 1.10; 95% CI, 1.05-1.15; P<0.001) and OS (HR,
1.13; 95% CI, 1.04-1.22; P=0.003) (Table III). In model 2, MP remained
significantly associated with PFS (HR, 1.09; 95% CI, 1.03-1.15;
P=0.001) and OS (HR, 1.14; 95% CI, 1.05-1.24; P=0.002). In model 3,
MP continued to be an independent prognostic factor for both PFS
(HR, 1.14; 95% CI, 1.05-1.25; P=0.002) and OS (HR, 2.15; 95% CI,
1.15-4.03; P=0.017) (Table
III).
 | Table III.Multivariate Cox proportional
analysis of MP for the prediction of OS and PFS in patients with
DLBCL. |
Table III.
Multivariate Cox proportional
analysis of MP for the prediction of OS and PFS in patients with
DLBCL.
| A, Model 1 |
|---|
|
|---|
|
| Multivariate
analysis PFS | Multivariate
analysis OS |
|---|
|
|
|
|
|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| MP (per 1%
increase) | 1.10
(1.05-1.15) | <0.001 | 1.13
(1.04-1.22) | 0.003 |
| MP (>10 vs.
≤10%) | 1.94
(1.12-3.37) | 0.018 | 3.73
(1.01-13.77) | 0.049 |
|
| B, Model
2 |
|
|
| Multivariate
analysis PFS | Multivariate
analysis OS |
|
|
|
|
|
Parameter | HR (95%
CI) | P-value | HR (95%
CI) | P-value |
|
| MP (per 1%
increase) | 1.09
(1.03-1.15) | 0.001 | 1.14
(1.05-1.24) | 0.002 |
| MP (>10 vs.
≤10%) | 1.79
(1.02-3.13) | 0.042 | 3.92
(1.05-14.72) | 0.043 |
|
| C, Model
3 |
|
|
| Multivariate
analysis PFS | Multivariate
analysis OS |
|
|
|
|
|
Parameter | HR (95%
CI) | P-value | HR (95%
CI) | P-value |
|
| MP (per 1%
increase) | 1.14
(1.05-1.25) | 0.002 | 2.15
(1.14-4.03) | 0.017 |
| MP (>10 vs.
≤10%) | 2.54
(1.08-5.99) | 0.033 | 5.34
(0.57-50.14) | 0.143 |
When MP was analyzed as a dichotomized variable with
10% as the cutoff, patients with MP >10% showed significantly
worse outcomes compared with those with MP ≤10% (reference group).
In model 1, elevated MP (>10%) was associated with worse PFS
(HR, 1.94; 95% CI, 1.12-3.37; P=0.018) and OS (HR, 3.73; 95% CI,
1.01-13.77; P=0.049). In model 2, the associations remained
significant for both PFS (HR, 1.79; 95% CI, 1.02-3.13; P=0.042) and
OS (HR, 3.92; 95% CI, 1.05-14.72; P=0.043). In model 3, MP remained
an independent predictor of PFS, compared with the reference group
(HR, 2.54; 95% CI, 1.08-5.99; P=0.033). By contrast, no significant
difference was observed when comparing OS to the reference group
(HR, 5.34; 95% CI, 0.57-50.14; P=0.143) (Table III).
Survival analysis
Kaplan-Meier survival curves were used to compare
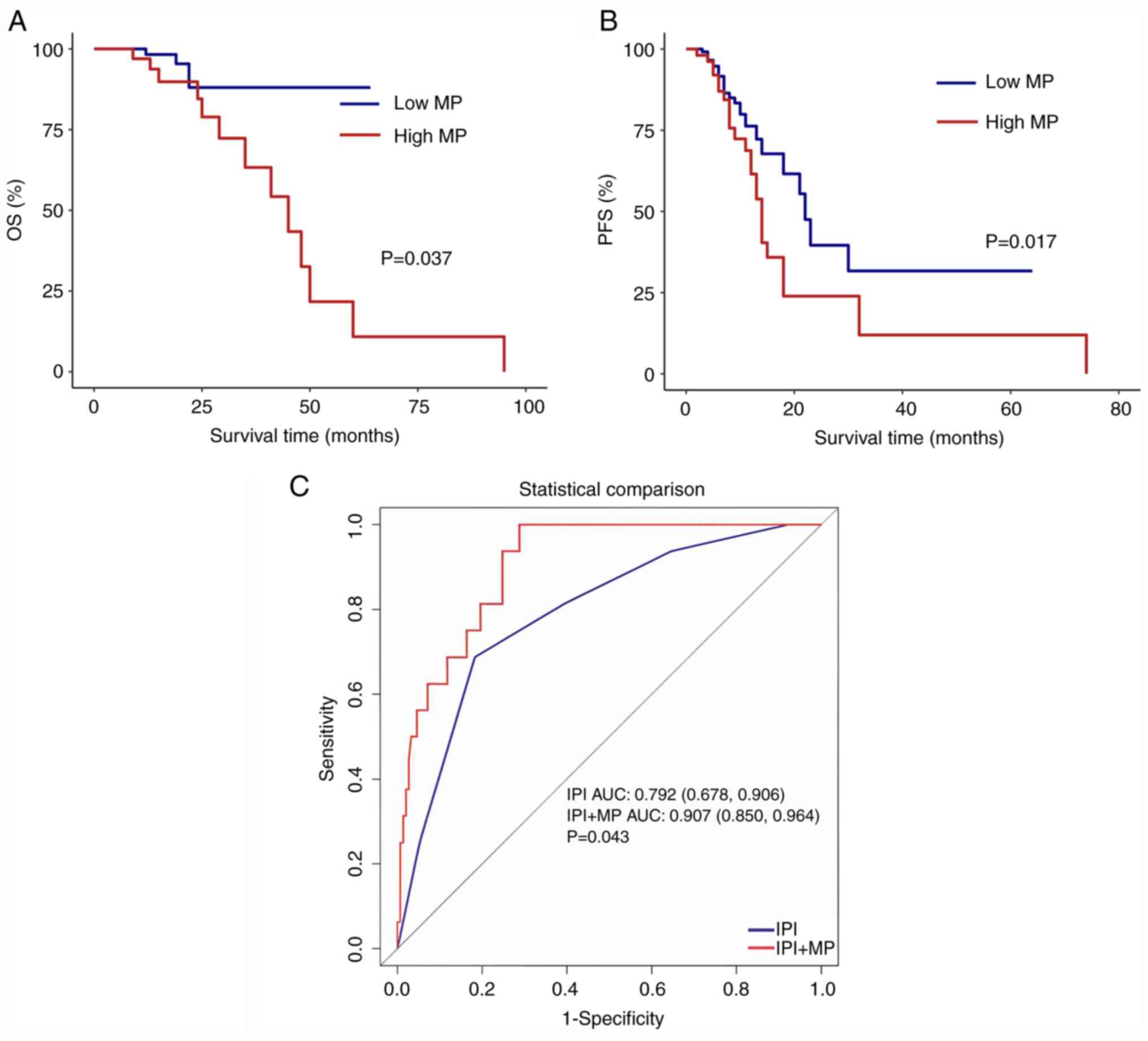
the two groups (≤10 and >10%). Both PFS (P=0.017) and OS
(P=0.037) were poor in the high MP group compared with those in the
low MP group (Fig. 1A and B).
To evaluate the predictive value of MP and assess
the impact of identifying multiple biomarkers on OS prediction, ROC
curves were plotted for patients with OS outcomes. Compared with
the international IPI score for assessing risk factors,
incorporating the MP increased the AUC (0.792 vs. 0.907; P=0.043)
(Fig. 1C).
Discussion
The results of the present study suggest that MP at
diagnosis may be utilized as a biomarker to predict survival in
patients with newly diagnosed DLBCL treated with R-CHOP. A high MP
was associated with poor PFS and OS. Additionally, MP provided
prognostic information independent of IPI score, and it may
increase the predictive effectiveness of outcomes when combined
with IPI score. Although the prognostic value of AMC in DLBCL has
been reported (10,11), the precise function of MP as a
readily available biomarker remains unknown. To the best of our
knowledge, the present study is the first to systematically
evaluate the value of MP as an independent prognostic indicator.
Compared with AMC, MP provides a standardized assessment method
that is unaffected by factors such as hemodilution or
hemoconcentration, offering better clinical applicability.
The tumor microenvironment and host immunity are
crucial factors in DLBCL outcomes (18,19).
Monocytes and monocytic myeloid-derived suppressor cells may
suppress host antitumor immunity through several mechanisms,
including secretion of immunosuppressive cytokines (e.g., IL-10,
TGF-β), depletion of amino acids via arginase-1 and inducible
nitric oxide synthase, production of reactive oxygen species, and
expression of immune checkpoint molecules such as PD-L1 (20). Additionally, myeloid cells have been
implicated in fostering tumor angiogenesis within the tumor
microenvironment (21).
Furthermore, immune-derived myeloid cells produced by tumors
directly promote tumor growth and vascularization by their
differentiation into endothelial cells (22). In human umbilical vein endothelial
cell cultures, monocytes actively promote endothelial cell
proliferation through C-X3-C motif chemokine receptor 1/fractalkine
interaction (23).
Beyond promoting tumor cell angiogenesis, monocytes
can suppress the host's antitumor immune response. T-cell
co-inhibitory ligand B7-homolog 1 (also known as programmed
death-ligand 1) is expressed in the tumor microenvironment by
peripheral blood monocytes and their progeny (24). Moreover, lymphoma B cells can
recruit monocytes via chemokine C-C motif ligand 5, which enhances
the survival and proliferation of neoplastic B lymphocytes while
suppressing the proliferation of healthy T cells (25). Finally, monocytes are an essential
source of soluble mediators, such as B lymphocyte stimulator, that
promote the growth and survival of healthy and malignant B cells
(26,27). It follows that monocytes promote the
growth of malignant lymphocytes in both T-cell and B-cell NHL
(8,28). The mechanism underlying the
association between monocytes and rituximab-mediated B-cell
lymphoma outcomes was elucidated by these cited earlier
investigations.
Different molecular subtypes and clinical features
may affect the association between MP and DLBCL outcomes. Germinal
center B-cell-like (GCB) and non-GCB DLBCL subtypes exhibit
fundamentally different tumor microenvironments and inflammatory
profiles. In contrast to GCB subtypes, non-GCB subtypes may promote
more monocyte recruitment and activation owing to their
constitutive NF-κB activation and elevated inflammatory signaling
(29,30). By promoting angiogenesis and
suppressing the immune system, these activated monocytes can
differentiate into tumor-associated macrophages, which aid in tumor
growth (31).
Double expressor lymphomas
(MYC+/BCL2+) are a physiologically aggressive
subtype with distinct immune characteristics. MYC overexpression
has been associated with changes in immune cell recruitment and
function, which may affect the differentiation state of circulating
monocytes (32,33). By altering apoptotic pathways in
both tumor cells and immune effector cells, the concomitant
upregulation of BCL2 may further modulate the tumor-immune
interface (34). Similarly, more
aggressive disease biology and systemic inflammatory responses that
may influence MP and function are frequently reflected in
extranodal involvement.
Importantly, the multivariate analysis in the
present study demonstrated that MP maintained independent
prognostic value even after adjusting for the established molecular
markers (GCB subtype and double expressor status) and clinical
variables (extranodal involvement). The novelty of this study lies
in, to the best of our knowledge, the first demonstration that MP
retains independent prognostic value after adjusting for molecular
markers (GCB subtype and double expressor status) and clinical
variables. This suggests that MP may capture biological information
not reflected by existing molecular and clinical stratification
methods. Thus, MP may reflect the complex interplay between
systemic immune status and local tumor microenvironment dynamics,
capturing further biological information beyond the current
molecular and clinical stratification approaches.
In the present study, patients with DLBCL who had a
high MP at diagnosis tended to have poor PFS and OS. After
controlling for other key negative prognostic variables,
multivariate analysis retained the predictive value of MP. These
results support the adverse prognostic impact of elevated MP on
B-cell NHL and are in line with earlier research (19,35,36).
The majority of studies have suggested that the AMC may be
considered a prognostic indicator for DLBCL. However, the precise
association between a high MP acquired from a CBC and a poor
prognosis remains debatable. Notably, monocytes may contribute to
tumor immunity and the tumor microenvironment as vital components
of the immune system. Hemoglobin levels and PLT counts are
independent predictors of poor 5-year OS and disease-free survival
rates in patients with DLBCL (37).
Similarly, Li et al (38)
reported that decreased peripheral blood PLT count may be a factor
associated with a poor prognosis in newly diagnosed DLBCL. The
present results, which suggested that the PLT count was a
protective factor for PFS, are in line with this previous research.
Using univariate Cox regression analysis, the present study
demonstrated positive associations between hemoglobin levels and
PFS. However, the hemoglobin level did not affect OS. Unlike gene
expression profiling or immunohistochemistry, which require complex
testing, MP is a simple indicator readily obtainable from a routine
CBC, yet its prognostic value has been underrecognized. To the best
of our knowledge, the present study is the first to validate MP as
an independent prognostic biomarker and demonstrate its ability to
enhance the predictive power of the National Comprehensive Cancer
Network (NCCN)-IPI.
Despite increasing evidence emphasizing the
association between the tumor microenvironment and lymphoma, it is
challenging to incorporate tumor microenvironment-related
prognostic variables into standard clinical practice. This is
because gene expression profiles or immunohistochemical data often
form the basis for most research estimating such associations. By
contrast, the MP data achieved from a CBC may be widely used and
easily incorporated into clinical practice. The important
contribution of the present study is identifying MP as a unique
prognostic indicator from among numerous hematological parameters.
While previous studies have focused on AMC, MP as a relative
proportion indicator offers several advantages: i) It is unaffected
by individual blood volume variations; ii) it reflects the relative
balance of immune cell composition; and iii) it may more accurately
reflect tumor-associated systemic immune status changes.
Furthermore, MP, a novel prognostic metric derived from CBC that is
easily accessible in clinical data, presents information
independent of NCCN-IPI. Additionally, it may provide further
predictive value when combined with NCCN-IPI.
The present study has several limitations. First,
the limited number of OS events (n=16; 9.5%) resulted in large
confidence intervals and low statistical power for multivariate
analysis. Post-hoc power analysis suggested that ~50 OS events
would be required to detect the observed effect size with 80%
power. This explains why the hazard ratio remained high (HR, 5.34)
but had wide confidence intervals (95% CI, 0.57-50.14) in the
non-significant multivariate Cox model (P=0.143), compared with the
significant univariate Kaplan-Meier analysis (P=0.037). Second,
although the association between MP and DLBCL prognosis was
assessed using multivariate Cox regression analysis, the influence
of unaccounted-for confounding factors could not be excluded
entirely. Third, although the 10% MP cutoff was based on laboratory
reference ranges, it may not be optimal for prognostic
stratification and warrants further investigation in larger cohorts
using ROC-derived thresholds. Therefore, larger prospective
multi-center studies with longer follow-up periods are warranted to
confirm the predictive efficacy of MP, an easily available and
affordable parameter for determining patient prognosis in those
with DLBCL.
In conclusion, the present cohort study demonstrated
a significant association between elevated MP and poor survival
outcomes. Furthermore, combining MP with IPI may improve the
predictive efficiency of these results.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the talent project of
2025 ‘Technology + Medical’ Joint Plan Project/Ganzhou Science and
Technology Bureau (grant no. 2025YLCE0072).
Availability of data and materials
The data supporting this study's findings are
available online at DOI 10.6084/m9.figshare.25151393.
Authors' contributions
XX and CZ contributed to the study design and data
collection. XX performed the statistical analysis and data
analysis. CZ was involved in the writing and revision of the
manuscript. XX and CZ confirm the authenticity of all the raw data.
Both authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The studies involving human participants were
reviewed and approved by the Ganzhou People's Hospital Ethics
Committee (Ganzhou, China; approval no. PJB2025-210-01). The
requirement for informed consent was waived by the ethics committee
due to the retrospective nature of the study and the use of fully
anonymized clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu X, Zeng L, Liu J, Huang Y, Yao H,
Zhong J, Tan J, Gao X, Xiong D and Liu L: Artesunate induces
ferroptosis in diffuse large B-cell lymphoma cells by targeting
PRDX1 and PRDX2. Cell Death Dis. 16:5132025. View Article : Google Scholar
|
|
2
|
Barraclough A, Hawkes E, Sehn LH and Smith
SM: Diffuse large B-cell lymphoma. Hematol Oncol. 42:e32022024.
View Article : Google Scholar
|
|
3
|
Liang XJ, Song XY, Wu JL, Liu D, Lin BY,
Zhou HS and Wang L: Advances in multi-omics study of prognostic
biomarkers of diffuse large B-cell lymphoma. Int J Biol Sci.
18:1313–1327. 2022. View Article : Google Scholar
|
|
4
|
Poletto S, Novo M, Paruzzo L, Frascione
PMM and Vitolo U: Treatment strategies for patients with diffuse
large B-cell lymphoma. Cancer Treat Rev. 110:1024432022. View Article : Google Scholar
|
|
5
|
International Non-Hodgkin's Lymphoma
Prognostic Factors Project: A predictive model for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 329:987–994. 1993. View Article : Google Scholar
|
|
6
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar
|
|
7
|
Shen R, Fu D, Dong L, Zhang MC, Shi Q, Shi
ZY, Cheng S, Wang L, Xu PP and Zhao WL: Simplified algorithm for
genetic subtyping in diffuse large B-cell lymphoma. Signal
Transduct Target Ther. 8:1452023. View Article : Google Scholar
|
|
8
|
Wilcox RA, Wada DA, Ziesmer SC, Elsawa SF,
Comfere NI, Dietz AB, Novak AJ, Witzig TE, Feldman AL, Pittelkow MR
and Ansell SM: Monocytes promote tumor cell survival in T-cell
lymphoproliferative disorders and are impaired in their ability to
differentiate into mature dendritic cells. Blood. 114:2936–2944.
2009. View Article : Google Scholar
|
|
9
|
Shivakumar L and Ansell S: Targeting
B-lymphocyte stimulator/B-cell activating factor and a
proliferation-inducing ligand in hematologic malignancies. Clin
Lymphoma Myeloma. 7:106–108. 2006. View Article : Google Scholar
|
|
10
|
10.Shang CY, Wu JZ, Ren YM, Liang JH, Yin
H, Xia Y, Wang L, Li JY, Li Y and Xu W: Prognostic significance of
absolute monocyte count and lymphocyte to monocyte ratio in
mucosa-associated lymphoid tissue (MALT) lymphoma. Ann Hematol.
102:359–367. 2023. View Article : Google Scholar
|
|
11
|
Mohsen A, Taalab M, Abousamra N and Mabed
M: Prognostic significance of absolute lymphocyte count, absolute
monocyte count, and absolute lymphocyte count to absolute monocyte
count ratio in follicular non-hodgkin lymphoma. Clin Lymphoma
Myeloma Leuk. 20:e606–e615. 2020. View Article : Google Scholar
|
|
12
|
Conlan MG, Armitage JO, Bast M and
Weisenburger DD: Clinical significance of hematologic parameters in
non-Hodgkin's lymphoma at diagnosis. Cancer. 67:1389–1395. 1991.
View Article : Google Scholar
|
|
13
|
Jia WY and Zhang JJ: Effects of
glucocorticoids on leukocytes: Genomic and non-genomic mechanisms.
World J Clin Cases. 10:7187–7194. 2022. View Article : Google Scholar
|
|
14
|
Ugel S, Canè S, De Sanctis F and Bronte V:
Monocytes in the tumor microenvironment. Annu Rev Pathol.
16:93–122. 2021. View Article : Google Scholar
|
|
15
|
Arneth B: Complete blood count: Absolute
or relative values? J Hematol. 5:49–53. 2016. View Article : Google Scholar
|
|
16
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar
|
|
17
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian
Leukaemia; Lymphoma Group and Eastern Cooperative Oncology Group, ;
et al: Recommendations for initial evaluation, staging, and
response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano
classification. J Clin Oncol. 32:3059–3068. 2014. View Article : Google Scholar
|
|
18
|
Zeng J, Zhang X, Jia L, Wu Y, Tian Y and
Zhang Y: Pretreatment lymphocyte-to-monocyte ratios predict
AIDS-related diffuse large B-cell lymphoma overall survival. J Med
Virol. 93:3907–3914. 2021. View Article : Google Scholar
|
|
19
|
Kharroubi DM, Nsouli G and Haroun Z:
Potential prognostic and predictive role of monocyte and lymphocyte
counts on presentation in patients diagnosed with diffuse large
B-cell lymphoma. Cureus. 15:e356542023.
|
|
20
|
Condamine T and Gabrilovich DI: Molecular
mechanisms regulating myeloid-derived suppressor cell
differentiation and function. Trends Immunol. 32:19–25. 2011.
View Article : Google Scholar
|
|
21
|
Yang F, Lee G and Fan Y: Navigating tumor
angiogenesis: Therapeutic perspectives and myeloid cell regulation
mechanism. Angiogenesis. 27:333–349. 2024. View Article : Google Scholar
|
|
22
|
Zha C, Yang X, Yang J, Zhang Y and Huang
R: Immunosuppressive microenvironment in acute myeloid leukemia:
Overview, therapeutic targets and corresponding strategies. Ann
Hematol. 103:4883–4899. 2024. View Article : Google Scholar
|
|
23
|
Kim JA, Kwak JY, Eunjung Y, Lee J, Park Y
and Broxmeyer HE: Fractalkine/CX3CR1 signaling promotes angiogenic
potentials in CX3CR1 expressing monocytes. Blood. 128:25072016.
View Article : Google Scholar
|
|
24
|
Song H, Chen L, Pan X, Shen Y, Ye M, Wang
G, Cui C, Zhou Q, Tseng Y, Gong Z, et al: Targeting tumor
monocyte-intrinsic PD-L1 by rewiring STING signaling and enhancing
STING agonist therapy. Cancer Cell. 43:503–518.e10. 2025.
View Article : Google Scholar
|
|
25
|
Le Gallou S, Lhomme F, Irish JM, Mingam A,
Pangault C, Monvoisin C, Ferrant J, Azzaoui I, Rossille D,
Bouabdallah K, et al: Nonclassical monocytes are prone to migrate
into tumor in diffuse large B-cell lymphoma. Front Immunol.
12:7556232021. View Article : Google Scholar
|
|
26
|
He B, Chadburn A, Jou E, Schattner EJ,
Knowles DM and Cerutti A: Lymphoma B cells evade apoptosis through
the TNF family members BAFF/BLyS and APRIL. J Immunol.
172:3268–3279. 2004. View Article : Google Scholar
|
|
27
|
Novak AJ, Grote DM, Stenson M, Ziesmer SC,
Witzig TE, Habermann TM, Harder B, Ristow KM, Bram RJ, Jelinek DF,
et al: Expression of BLyS and its receptors in B-cell non-Hodgkin
lymphoma: Correlation with disease activity and patient outcome.
Blood. 104:2247–2253. 2004. View Article : Google Scholar
|
|
28
|
Seiffert M, Schulz A, Ohl S, Döhner H,
Stilgenbauer S and Lichter P: Soluble CD14 is a novel
monocyte-derived survival factor for chronic lymphocytic leukemia
cells, which is induced by CLL cells in vitro and present at
abnormally high levels in vivo. Blood. 116:4223–4230. 2010.
View Article : Google Scholar
|
|
29
|
Davis RE, Ngo VN, Lenz G, Tolar P, Young
RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, et al:
Chronic active B-cell-receptor signalling in diffuse large B-cell
lymphoma. Nature. 463:88–92. 2010. View Article : Google Scholar
|
|
30
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:170232017. View Article : Google Scholar
|
|
31
|
Steidl C, Lee T, Shah SP, Farinha P, Han
G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al:
Tumor-associated macrophages and survival in classic Hodgkin's
lymphoma. N Engl J Med. 362:875–885. 2010. View Article : Google Scholar
|
|
32
|
Casey SC, Tong L, Li Y, Do R, Walz S,
Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M and Felsher
DW: MYC regulates the antitumor immune response through CD47 and
PD-L1. Science. 352:227–231. 2016. View Article : Google Scholar
|
|
33
|
Kortlever RM, Sodir NM, Wilson CH,
Burkhart DL, Pellegrinet L, Brown Swigart L, Littlewood TD and Evan
GI: Myc cooperates with Ras by programming inflammation and immune
suppression. Cell. 171:1301–1315.e14. 2017. View Article : Google Scholar
|
|
34
|
Xu-Monette ZY, Wei L, Fang X, Au Q, Nunns
H, Nagy M, Tzankov A, Zhu F, Visco C, Bhagat G, et al: Genetic
subtyping and phenotypic characterization of the immune
microenvironment and MYC/BCL2 double expression reveal
heterogeneity in diffuse large B-cell lymphoma. Clin Cancer Res.
28:972–983. 2022. View Article : Google Scholar
|
|
35
|
von Hohenstaufen KA, Conconi A, de Campos
CP, Franceschetti S, Bertoni F, Margiotta CG, Stathis A, Ghielmini
M, Stussi G, Cavalli F, et al: Prognostic impact of monocyte count
at presentation in mantle cell lymphoma. Br J Haematol.
162:465–473. 2013. View Article : Google Scholar
|
|
36
|
Tadmor T, Bari A, Sacchi S, Marcheselli L,
Liardo EV, Avivi I, Benyamini N, Attias D, Pozzi S, Cox MC, et al:
Monocyte count at diagnosis is a prognostic parameter in diffuse
large B-cell lymphoma: Results from a large multicenter study
involving 1191 patients in the pre- and post-rituximab era.
Haematologica. 99:125–130. 2014. View Article : Google Scholar
|
|
37
|
Troppan KT, Melchardt T, Deutsch A,
Schlick K, Stojakovic T, Bullock MD, Reitz D, Beham-Schmid C, Weiss
L, Neureiter D, et al: The significance of pretreatment anemia in
the era of R-IPI and NCCN-IPI prognostic risk assessment tools: A
dual-center study in diffuse large B-cell lymphoma patients. Eur J
Haematol. 95:538–544. 2015. View Article : Google Scholar
|
|
38
|
Li M, Xia H, Zheng H, Li Y, Liu J, Hu L,
Li J, Ding Y, Pu L, Gui Q, et al: Red blood cell distribution width
and platelet counts are independent prognostic factors and improve
the predictive ability of IPI score in diffuse large B-cell
lymphoma patients. BMC Cancer. 19:10842019. View Article : Google Scholar
|