Introduction
Multiple primary malignant neoplasms (MPMNs) are
defined as the presence of two or more histologically distinct
primary cancer types arising in separate organs or anatomical sites
in the same patient (1–4). Although the reported incidence of
MPMNs in China remains low (ranging from 0.4 to 2.4%) it has
steadily increased in recent years owing to advances in imaging and
diagnostic methodologies (4–6). The
widely accepted diagnostic framework combines the original Warren
criteria (1) with the modifications
from Liu et al (2), which
stipulate that: i) Each neoplasm must be confirmed as malignant by
histopathology; ii) each neoplasm must display unique pathological
features; iii) tumors must develop in non-contiguous locations; and
iv) each tumor must demonstrate an independent pattern of
metastasis, thereby excluding secondary spread or recurrence.
Nevertheless, clinicians frequently encounter challenges in
differentiating multiple primary tumors from metastases due to
overlapping clinical presentations, limitations of conventional
imaging and difficulties in accurately determining tumor origin on
pathological examination (7). These
factors contribute to increased rates of both misdiagnosis and
underdiagnosis.
In the present case report, the case of a
79-year-old male patient diagnosed with multiple primary malignant
neoplasms is described. The coexistence of distinct malignancies
posed substantial diagnostic challenges, particularly in
differentiating primary lesions from potential metastatic disease.
The present study highlights the utility of
99mTc-labeled methylene diphosphonate
(99mTc-MDP) three-phase bone imaging in providing
integrated diagnostic information that facilitates accurate
evaluation and clinical decision-making.
Case report
Chief complaint
A 79-year-old male patient, who had a forearm lesion
present for >5 years with recurrence noted in the past 6 months,
was admitted to the Department of Joint Surgery at the Third
Affiliated Hospital of Guangzhou Medical University (Guangzhou,
China) in March 2024.
History of present illness
The patient first noticed a painless nodular lesion
on the left forearm in 2020 without any apparent cause. After local
excision, histopathology revealed nodular fasciitis (Fig. S1). In 2021 (1 years later) the
lesion recurred at the same site and grew to 6×4 cm. Despite the
notable increase in size, the patient remained asymptomatic and
underwent a skin tumor resection at the Department of Joint
Surgery, The Third Affiliated Hospital of Guangzhou Medical
University. The excised tissue was fixed in 4% neutral
phosphate-buffered formaldehyde solution at room temperature
(~25°C) for 24 h. The specimens were sectioned at a thickness of 4
µm and stained using hematoxylin and eosin (H&E) reagents
[Roche Diagnostics (Shanghai) Co., Ltd.] at 25°C for 8 h. The
stained slides were examined under a light microscope (Olympus
BX43; Olympus Corporation) at ×100 magnification, and
representative images were captured for histopathological
evaluation. Histopathological analysis revealed fibrous tissue
proliferation, spindle-shaped tumor cells with nuclear atypia and
inflammatory cell infiltration, accompanied by myxoid degeneration,
hemorrhage and frequent mitotic figures (4 per high-power field)
(Fig. S2). The differential
diagnoses included nodular fasciitis and malignant mesenchymal
tumor. At this time, the clinical diagnosis was a cutaneous tumor,
and no further specific treatment was administered following the
excision.
In December 2023 (onset time), the patient noticed
two new lesions at the original site, measuring 3×3×2 and 2×2×1 cm.
The overlying skin showed erythema and swelling, but no additional
symptoms were reported. In March 2024, the patient presented to the
Surgical Outpatient Clinic at the Third Affiliated Hospital of
Guangzhou Medical University for evaluation. Throughout the course
of the illness, the patient maintained normal bowel movements and
stable mental status, but experienced urinary frequency and
urgency, and occasional hematuria, without significant changes in
sleep, appetite or weight.
Past medical and family history
The patient had no history of trauma, fractures,
tumors or other relevant medical conditions. The patient had no
family history of malignancy, and no history of exposure to
carcinogenic chemicals, radiation or toxic substances. The patient
also reported no history of smoking, alcohol use or illicit drug
use.
Physical examination
On March 26, 2024, a physical examination of the
left forearm revealed a 6×4 cm subcutaneous lesion with
well-defined borders and an indurated, rubbery consistency. The
lesion was partially movable upon palpation and was without any
signs of inflammation. Palpation revealed two additional lesions in
the left forearm. The larger lesion measured 3×3×2 cm with
well-defined borders and the smaller measured 2×2×1 cm with poorly
defined borders. A longitudinal surgical scar was noted on the skin
surface of the left forearm.
Preoperative laboratory tests
The preoperative laboratory tests revealed the
following results: Red blood cell count, 2.88×1012/l
(reference range, 4.3-5.8×1012/l); and hemoglobin, 81
g/l (reference range, 130–175 g/l). The white blood cell count,
platelet count, coagulation profile and liver and kidney function
tests were all within the normal limits.
Preoperative imaging
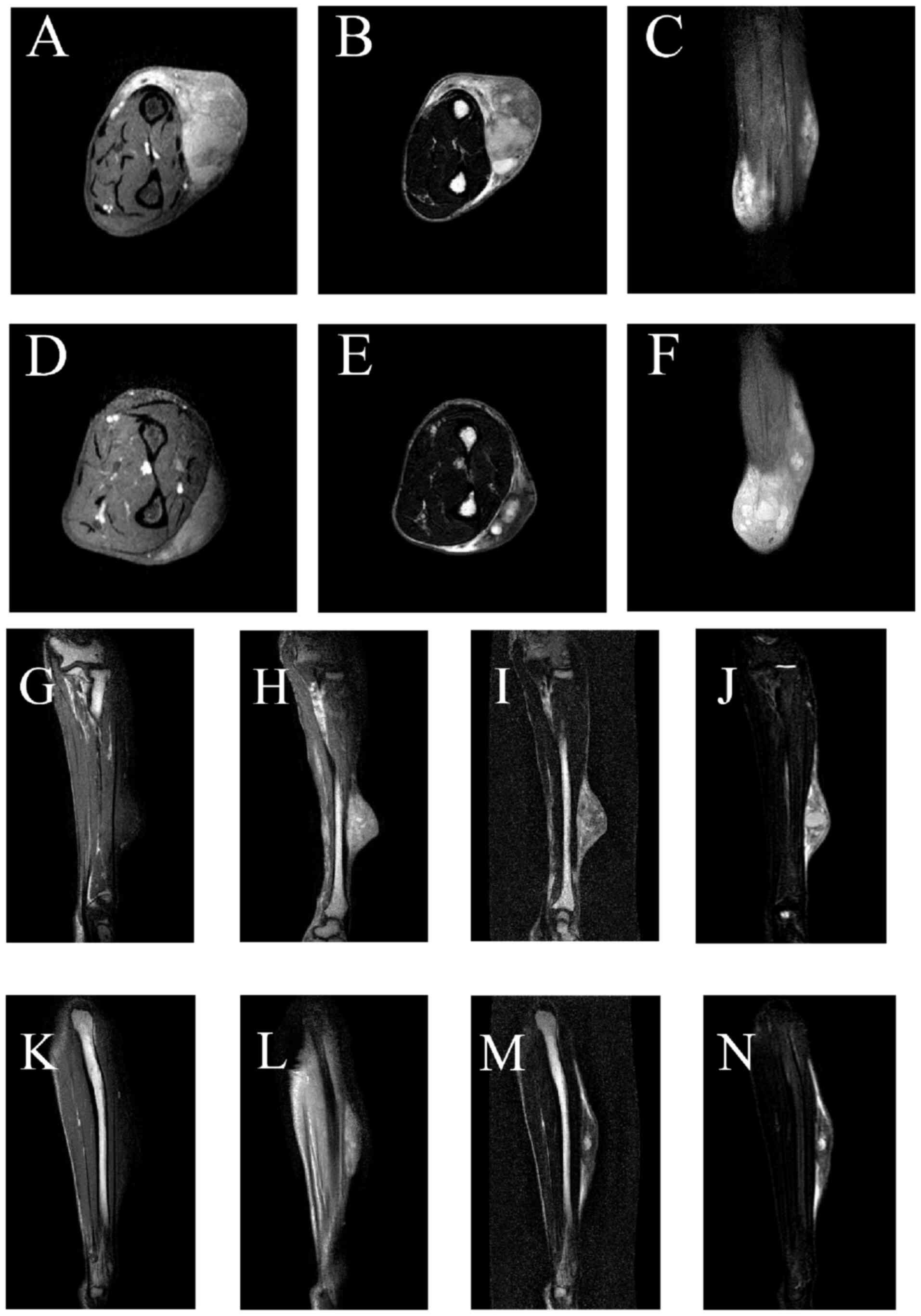
In April 2024, magnetic resonance imaging (MRI) of
the left forearm demonstrated multiple irregular subcutaneous
lesions on the dorsal aspect measuring 46×27×60 and 38×12×72 mm.
These lesions showed hypointensity on T1-weighted imaging and
heterogeneous signal intensity on T2-weighted imaging and proton
density-weighted imaging, along with notable heterogeneous
enhancement and poorly defined borders on contrast-enhanced imaging
(Fig. 1). Despite the indistinct
margins, the lesions remained well-demarcated from the adjacent
muscles. The osseous structures appeared normal with an intact and
continuous cortex. No evidence of destructive bony changes, osseous
metastasis or abnormal signal intensity was observed. T1-weighted
contrast-enhanced imaging revealed no abnormal bone enhancement
(Fig. 1C, F, H and L). Although
X-ray, thin-section bone window computed tomography (CT) and 3D-CT
were not performed due to financial constraints and clinical
priorities, the absence of peri-tumoral bone destruction on MRI
definitively excluded direct osseous invasion, strongly suggesting
local recurrence of the soft-tissue sarcoma.
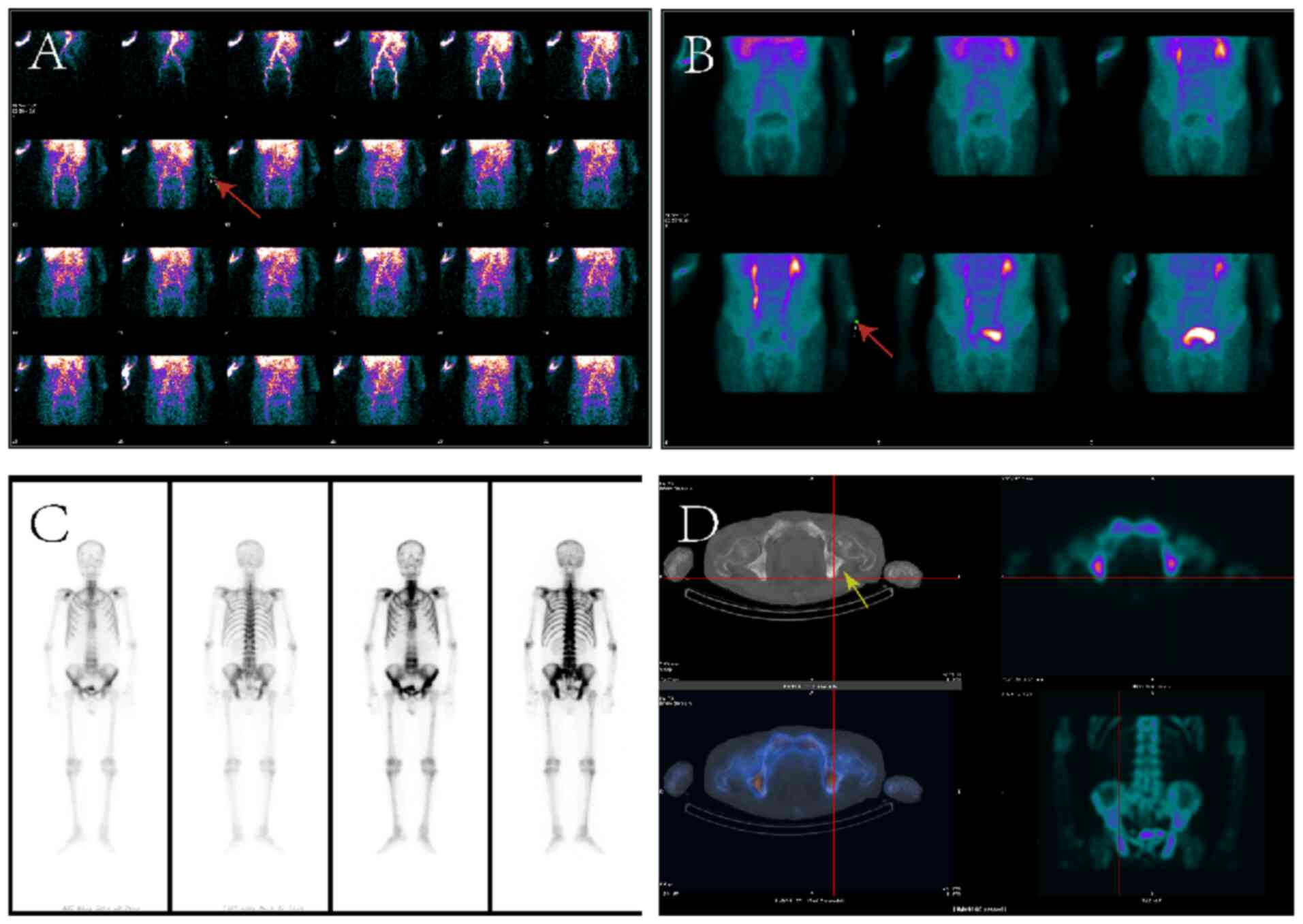
Concurrent 99mTc-MDP three-phase bone
imaging revealed markedly increased perfusion to the left forearm
following radiotracer injection, along with localized abnormal
radiotracer uptake during the blood pool phase, suggesting the
possibility of soft tissue pathology (Fig. 2A and B). Whole-body bone imaging
demonstrated diffusely increased skeletal radiotracer uptake, with
multiple irregularly shaped, heterogeneous foci exhibiting moderate
to high intensity in the cervical and thoracolumbar spine,
bilateral ribs, pelvis and proximal femurs (Fig. 2C and D). Such imaging results are
consistent with widespread bone metastasis, indicating extensive
involvement and heterogeneous uptake across the skeletal system.
The kidneys exhibited minimal tracer uptake, and the bladder showed
limited concentration, resulting in a characteristic ‘superscan’
pattern (Fig. 2C and D). Single
photon emission CT (SPECT)/CT fusion imaging confirmed multiple
osteoblastic changes in the thoracolumbar spine, ribs and pelvis,
along with soft-tissue lesions in the dorsal left forearm showing
reduced 99mTc-MDP uptake, without evidence of
significant bone involvement (Fig. 2C
and D). Due to the financial constraints and advanced age of
the patient, X-ray, thin-section bone window CT and 3D CT imaging
were not performed.
Treatment process and follow-up
pathology
Following the 99mTc-MDP whole-body
three-phase bone scan, the left forearm lesion was highly suspected
to be malignant, with augmented blood flow and increased bone
metabolic activity. Nevertheless, the extensive bone metastases,
indicative of multiple secondary lesions, are atypical for
fibrosarcoma. In April 2024, a prostate-specific antigen (PSA) test
revealed a markedly elevated total PSA level of 563.864 ng/ml
(reference range for age >80 years, 0–8 ng/ml), raising a strong
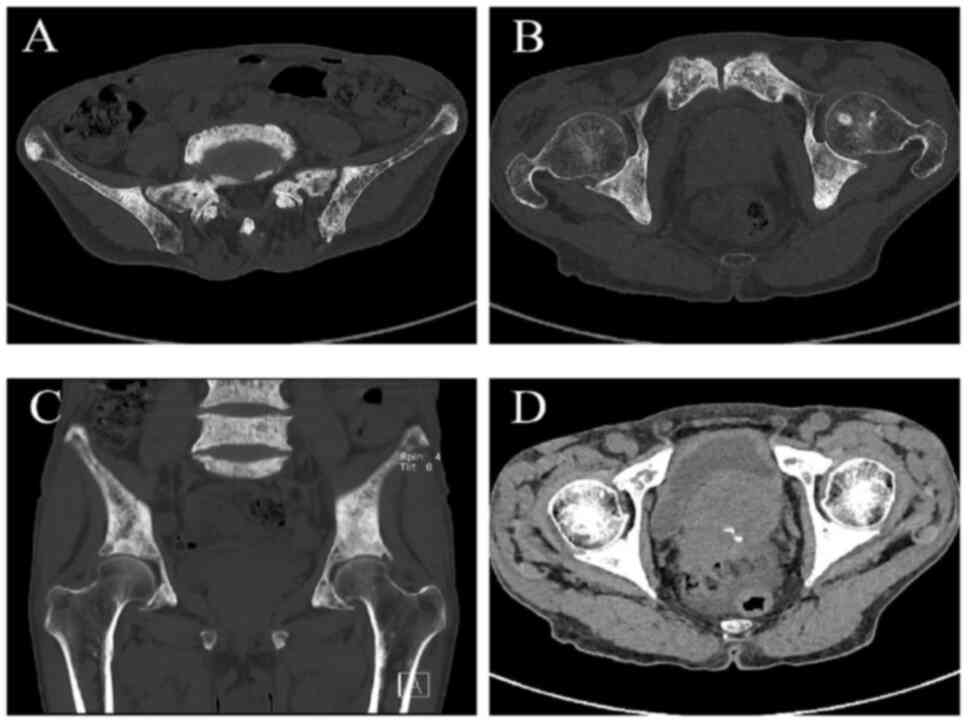
suspicion of an underlying malignancy. A subsequent CT scan
demonstrated heterogeneous bone density with multiple sclerotic and
lytic lesions in the hips and lumbar vertebrae, findings that are
highly indicative of metastatic bone disease (Fig. 3A-C). Additionally, significant
prostate enlargement was identified (Fig. 3D), which, in conjunction with the
elevated PSA levels and the extent of skeletal involvement,
provided compelling evidence supporting the diagnosis of advanced
prostate cancer. Pathological examination of the pubic bone biopsy
confirmed metastatic adenocarcinoma consistent with a prostatic
primary origin. The diagnosis of advanced prostate adenocarcinoma
had been established previously, as aforementioned.
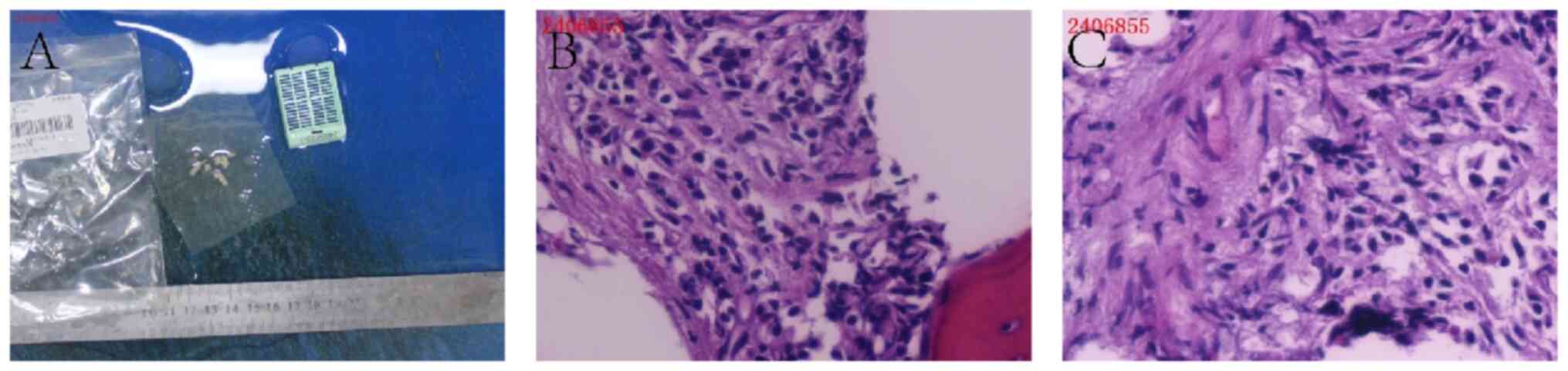
After ruling out surgical contraindications, the
patient underwent wide excision of the left forearm tumor with skin
grafting, vacuum sealing drainage closure and a pubic bone biopsy
in April 2024 (Fig. 4A). The
operation was successful without obvious postoperative acute
complications. The excised tissue was paraffin-embedded after
fixation in 4% neutral phosphate-buffered formaldehyde solution at
room temperature (~25°C) for 24 h. Sections were cut at a thickness
of 4 µm and stained with H&E using reagents supplied by Roche
Diagnostics (Shanghai) Co., Ltd. at 25°C for 8 h. The stained
slides were examined under a light microscope (Olympus BX43;
Olympus Corporation) and representative images were captured for
histopathological evaluation. Histopathological analysis of the
left forearm tumor demonstrated key features of a high-grade
malignant mesenchymal tumor, suggesting a diagnosis of high-grade
myxofibrosarcoma (MFS) (4). The
tumor was characterized by spindle cells with marked atypia,
frequent mitotic figures and multinucleated giant cells, along with
myxoid degeneration within the stroma and extensive inflammatory
cell infiltration, findings consistent with high-grade MFS
(Fig. 4B-D). Additionally, the
poorly defined margins, combined with notable cellular
pleomorphism, further supported the diagnosis of a highly
aggressive malignant mesenchymal tumor.
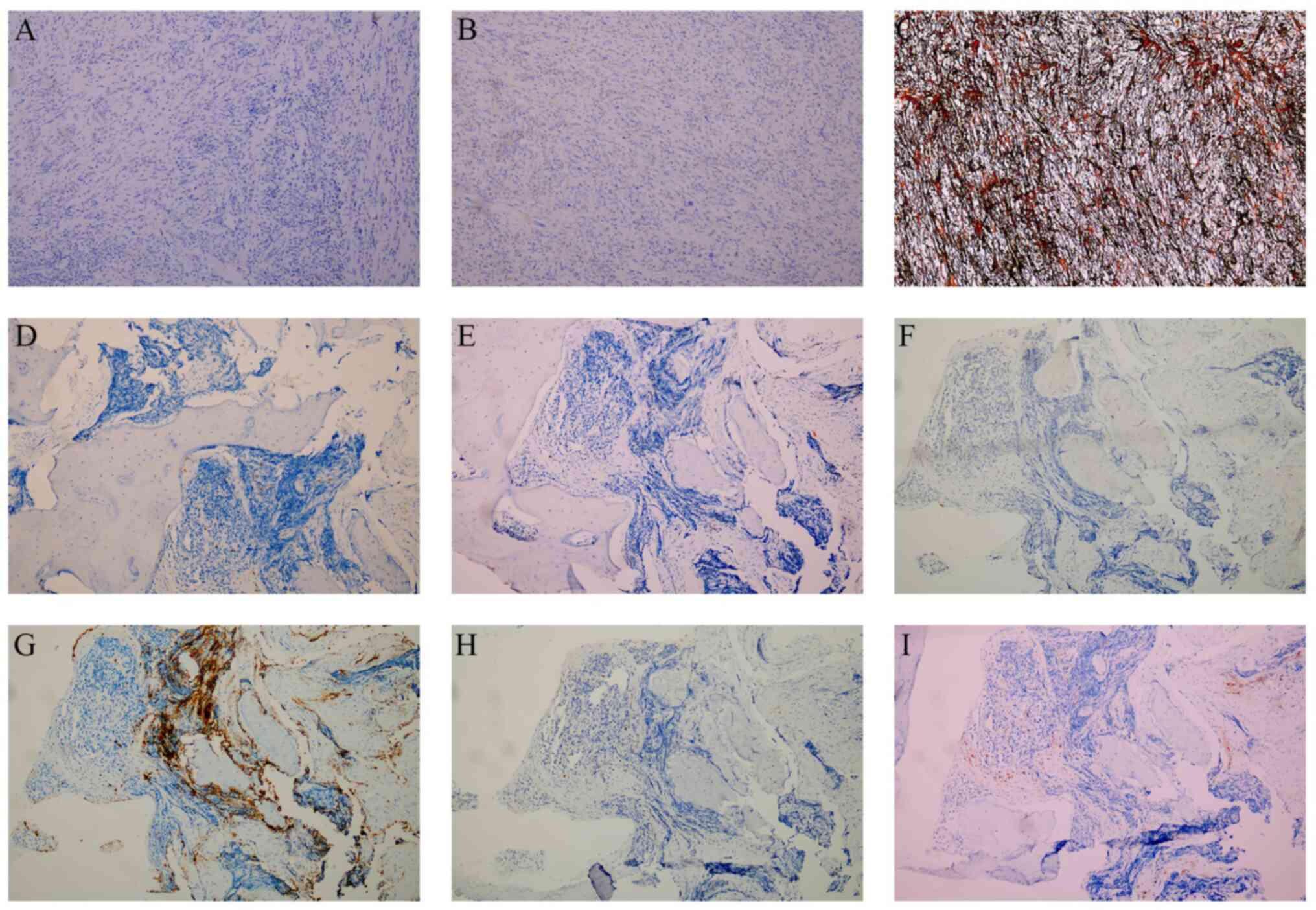
Immunohistochemical analysis of the left forearm
tumor revealed a high proliferative index (Ki-67, 60%) with focal
cytoplasmic desmin expression and scattered p16 immunoreactivity
(Fig. 5A-C). Tumor cells
demonstrated wild-type p53 nuclear expression pattern and weak MDM2
positivity (Fig. 5D and E).
β-catenin exhibited preserved membranous localization (Fig. 5F). Notably, pan-tropomyosin receptor
kinase (pan-TRK) staining showed weak focal cytoplasmic positivity
in 10% of tumor cells without nuclear expression (Fig. 5G). S-100 highlighted scattered
interstitial cells, whereas smooth muscle actin (SMA) and CD34
positivity confirmed vascular components (Fig. 5H-J). Finally, the tumor was negative
for anaplastic lymphoma kinase (ALK) and SRY-box transcription
factor 10 (SOX10), while the reticulin stain demonstrated delicate
fibers encircling individual tumor cells (Fig. 6A-C).
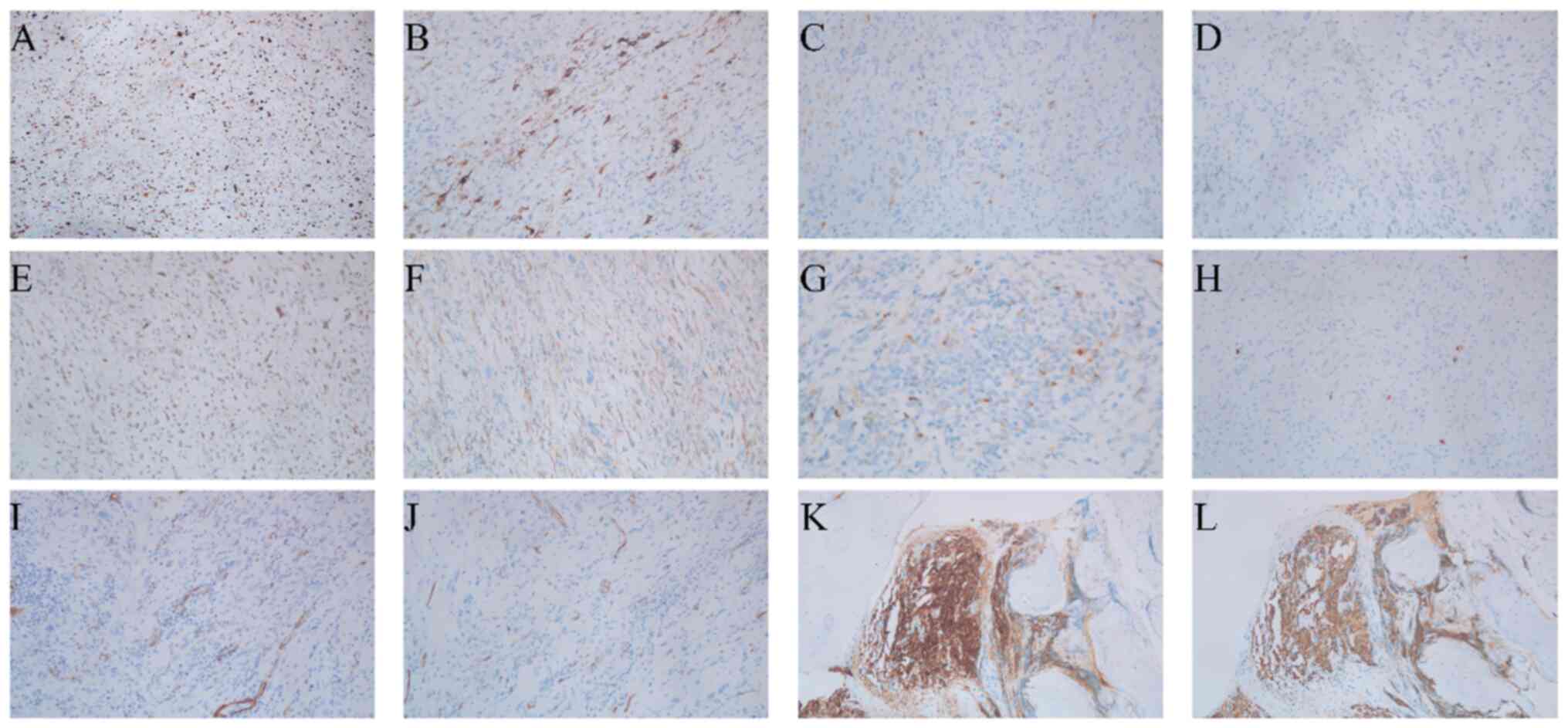 | Figure 5.Representative immunohistochemical
staining of myxofibrosarcoma and pubic bone metastasis. (A) Ki-67,
(B) desmin, (C) p16, (D) p53, (E) MDM2, (F) β-catenin, (G)
Pan-tropomyosin receptor kinase, (H) S-100, (I) smooth muscle
actin, (J) CD34, (K) prostate-specific antigen, (L) cytokeratin.
Magnification, ×100. |
The tissue processing and immunohistochemical
staining procedures were as follows: Tumor tissues were fixed in 4%
neutral phosphate-buffered formaldehyde for 24 h at room
temperature (25°C) and subsequently paraffin-embedded. Paraffin
blocks were sectioned into 4-µm slices and baked at 65°C for 60 min
or overnight at 58°C. Sections were then deparaffinized in three
xylene baths (5–10 min each) and rehydrated through a descending
alcohol series (100, 95, 85 and 75% each for 2 min). Antigen
retrieval and immunostaining were performed using a BenchMark ULTRA
automated immunostainer (Roche Diagnostics) at 99°C for 16 min with
an immunohistochemistry antigen retrieval buffer (cat. no.
24005424569188; Roche Diagnostics). Endogenous peroxidase activity
was blocked using a peroxidase inhibitor for 5 min at 25°C. Primary
antibodies (prediluted; Roche Diagnostics) were incubated with the
sections at 25°C for 16 min, followed by the OptiView DAB Detection
Kit (Roche Diagnostics) according to the manufacturer's protocol.
Sections were incubated with OptiView HQ Universal Linker
containing horseradish peroxidase (HRP) for 8 min at 25°C, and
subsequently with HRP Multimer for 8 min to visualize staining.
The detailed information on the primary antibodies
used in this study is as follows. Ki-67 (Xiamen Talent Biomedical
Technology Co., Ltd.; cat. no. AM0241; clone MIB1; 1:200), SMA
(Fuzhou Maixin Biotechnology Development Co., Ltd.; cat. no.
kit-0006; clone 1A4; 1:100), Desmin [GeneTech (Shanghai) Co., Ltd.;
cat. no. GT225229; clone GTM2; 1:300], p16 (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.; cat. no. ZM-0205; clone 1C1;
1:300), p53 (Quan Hui Trading International Co., Ltd.; cat. no.
AQ20025; clone DO-7; 1:100), MDM2 (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.; cat. no. ZM-0425; clone 0TI17B3;
ready-to-use), ALK (Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd.; cat. no. TA800712; clone 0TI1H7; ready-to-use), CD34
(Fuzhou Maixin Biotechnology Development Co., Ltd.; cat. no.
kit-0004; clone QBEND/10; 1:100), β-Catenin (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.; cat. no. ZM-0442; clone
UMAB15; 1:300), pan-TRK (VENTANA Roche; cat. no. 08494665001; clone
EPR17341-4; ready-to-use), S-100 (Fuzhou Maixin Biotechnology
Development Co., Ltd.; cat. no. kit-0007; clone 4C4.9; 1:100),
SOX10 (Xiamen Talent Biomedical Technology Co., Ltd.; cat. no.
AM0417; clone SDM2; ready-to-use), PSA (Fuzhou Maixin Biotechnology
Development Co., Ltd.; cat. no. MAB-0146; clone ER-PR8; 1:50), CK
(Guangzhou Anbiping Pharmaceutical Technology Co., Ltd.; cat. no.
IM069; clone AE1&AE3; 1:150), CD3 (Quan Hui Trading
International Co., Ltd.; cat. no. NCL-L-CD3-565; clone LN10; 1:50),
CD20 (Fuzhou Maixin Biotechnology Development Co., Ltd.; cat. no.
kit-0001; clone L26; 1:1), CD61 (Agilent Technologies Singapore
International; cat. no. 8930248; clone VI-PL2; 1:100), CD235a
(Fuzhou Maixin Biotechnology Development Co., Ltd.; cat. no.
MAB-0603; clone JC159; ready-to-use), MPO (Quan Hui Trading
International Co., Ltd.; cat. no. AQ20285; polyclonal; 1:100) and
CD38 (Quan Hui Trading International Co., Ltd.; cat. no.
NCL-L-CD38-290; clone SPC32; 1:100).
Reticulin staining was performed on formalin-fixed
paraffin-embedded sections using standard protocols. Slides were
counterstained with hematoxylin for 8 min and eosin for 1 min at
room temperature and examined under a light microscope (Olympus
BX53) at ×100 magnification.
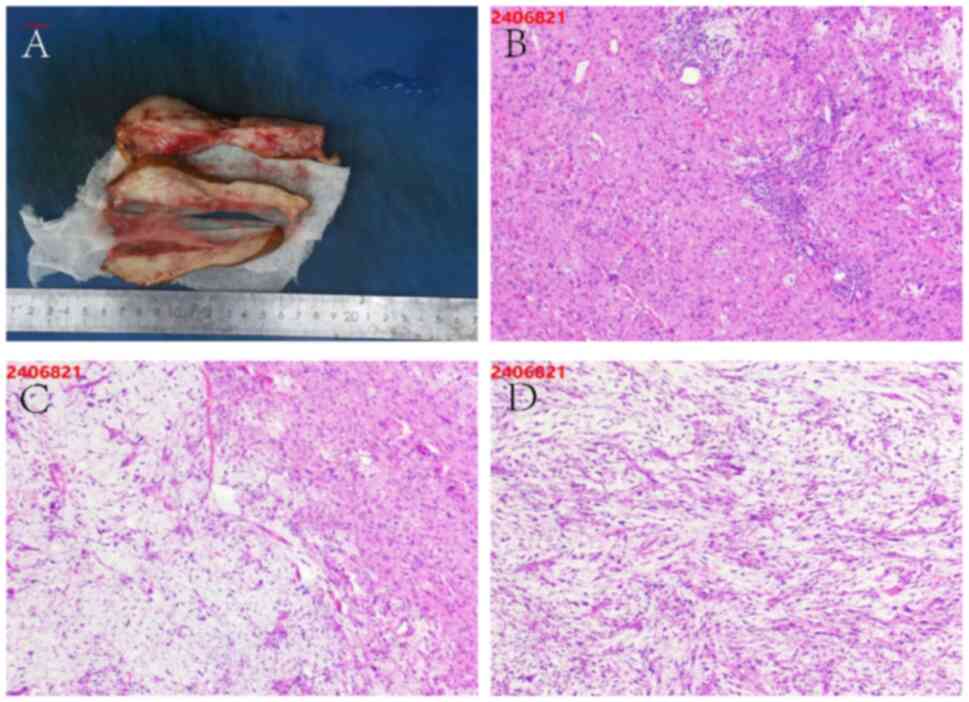
Subsequently, histopathological analysis of the
pubic bone biopsy confirmed metastatic prostate cancer. Gross
examination revealed multiple gray-white specimens from the pubic
bone (Fig. 7A). Microscopically,
the fibrous tissue surrounding the lesion exhibited marked
proliferation and infiltrative growth of epithelioid cells,
characterized by abundant cytoplasm, prominent nucleoli and
pleomorphic features, consistent with metastatic adenocarcinoma of
prostatic origin (Fig. 7B and C).
Immunohistochemistry further demonstrated PSA and cytokeratin
positivity (Fig. 5K and L), while
hematopoietic markers (CD3, CD20 and MPO), megakaryocytic/erythroid
markers (CD61 and CD235a) and the plasma cell marker (CD38) were
uniformly negative (Fig. 6D-I).
These findings, in conjunction with the notably elevated serum PSA
levels and the clinical profile, conclusively supported a diagnosis
of advanced metastatic prostate cancer. Given the unequivocal
histopathological confirmation of osseous metastasis in this
octogenarian patient, a prostate biopsy was deemed unwarranted per
multidisciplinary consensus guidelines.
Postoperatively, the patient was treated with
cefuroxime sodium (1.5 g twice daily, i.v.), papaverine
hydrochloride (30 mg i.v.), celecoxib (0.2 g twice daily, p.o.) and
rabeprazole sodium (10 mg p.o.) for anti-infection, circulation
improvement, analgesia, and gastric protection, respectively. The
patient demonstrated favorable recovery and remained in stable
condition, with no other notable concurrent interventions. The
patient exhibited good treatment compliance and reported no
significant discomfort. By August 2024, the patient had achieved a
marked recovery, maintained a stable appetite and regular sleep
patterns, adhered to scheduled follow-up visits and continued a
routine regimen of bone pain medication. As of September 2025, the
patient remained alive, in stable condition and under regular
follow-up. The final diagnosis of metachronous multiple primary
malignant neoplasms (MMPMNs; high-grade myxofibrosarcoma of the
left forearm and prostate adenocarcinoma with osseous metastasis)
was thus established.
Discussion
MFS, formerly classified as a subtype of malignant
fibrous histiocytoma, predominantly affects patients aged 40 to 80
years (8,9). In total, two-thirds of MFS cases arise
in superficial soft tissues, typically the deep dermis and subcutis
of the limbs, while the remainder occur in deeper subfascial or
intramuscular locations (9,10). Histologically, MFS is distinguished
by a myxoid extracellular matrix, pleomorphic spindle cells and
characteristic curvilinear blood vessels (11). Patients with MFS exhibit a notably
elevated risk of developing subsequent primary malignancies
(12). Following the analysis of a
retrospective cohort, Tateishi et al (13) reported 5- and 10-year cumulative
incidences of secondary cancers of 16.9%, significantly exceeding
rates seen in other sarcoma subtypes (hazard ratio, 2.34; 95%
confidence interval, 1.01-5.41; P=0.048). In that cohort, patients
with MFS who developed secondary malignancies exhibited a wide
spectrum of additional tumor types involving multiple organ
systems. The secondary cancers most frequently comprised
adenocarcinomas arising in the stomach, ovary, prostate, colon,
cecum, breast, kidney, lung and rectum. Other histological subtypes
included squamous cell carcinomas of the pharynx, tongue, gingiva
and esophagus, as well as transitional cell carcinoma of the
bladder and renal cell carcinoma of the kidney. These neoplasms
represented independent primary tumors coexisting with MFS rather
than metastatic lesions, with some diagnosed prior to and others
following the onset of sarcoma. This series also included a case of
metachronous prostate adenocarcinoma following MFS, mirroring the
observation of occult prostate cancer detected via superscan in the
present study.
Beyond the risk of additional primary tumors, MFS
poses its own clinical challenges due to aggressive behavior and
high local recurrence rates, particularly in high-grade tumors.
Recurrence rates for MFS rank among the highest of all soft-tissue
sarcomas, underscoring the necessity for rigorous surveillance
(14,15). Although distant metastases are less
frequent (occurring in 13–16% of cases), the lungs and brain are
the most common sites, with occasional spread to the small
intestine, pelvis, retroperitoneum, stomach, liver and adrenal
glands (16,17). In the present study, the paradoxical
finding of a superscan (indicating diffuse osteoblastic activity)
combined with intact cortical bone adjacent to the sarcoma and the
rarity of MFS metastasis to prostate or bone strongly supports the
diagnosis of multiple primary malignancies rather than metastatic
spread.
Following intravenous administration of
99mTc-MDP, nuclear imaging techniques such as SPECT and
positron emission tomography (PET) assess the skeletal
biodistribution of the tracer, mediated by its affinity for
hydroxyapatite in bone tissue. While physiological uptake in soft
tissues may occasionally occur, abnormal extraosseous radiotracer
accumulation warrants further investigation. Research highlights a
strong correlation between radiotracer deposition and malignancies,
primarily driven by metastatic spread or primary soft-tissue
tumors, reflecting its critical diagnostic application in
oncological settings (18–20). Soft-tissue tumors in the limbs are
frequently characterized by increased blood volume within malignant
tissues, driven by tumor-induced angiogenesis. As a result, this
vascular proliferation leads to abnormal tracer accumulation as the
enhanced and permeable vasculature facilitates tracer retention in
non-target regions, complicating the accuracy of imaging
interpretation (21).
A superscan represents a distinctive pattern
observed in three-phase bone imaging, characterized by diffuse and
intense skeletal uptake, and accompanied by markedly diminished
soft tissue and renal activity. While, under normal conditions, the
tracer uptake ratio between the skeletal system and kidneys is
~2:3, in cases of a superscan, this ratio may shift to as high as
17:3 (22), indicating significant
deviations from typical tracer distribution. Given the inherent
complexities involved in achieving objective quantification, the
accurate diagnosis of a superscan is particularly challenging and
often necessitates the specialized expertise and interpretive
skills of experienced nuclear medicine physicians (23). Superscan is frequently associated
with conditions that promote diffuse reactive bone formation, such
as bone metastases from malignancies and certain metabolic bone
diseases (24,25). Notably, this phenomenon has been
documented in the literature as an unexpected indicator of occult
prostate cancer, underscoring the critical role of bone
scintigraphy as a diagnostic alert for asymptomatic secondary
malignancies (26). Data indicate
that superscan occurs in 15–23% of patients with prostate cancer,
establishing the disease as the most common underlying cause.
Although other malignancies, including gastric and breast cancer,
are also capable of inducing superscan, their incidence remains
significantly lower by comparison (25). Given the broad differential
diagnosis associated with superscan, visual interpretation alone is
insufficient. Laboratory tests (such as serum calcium and alkaline
phosphatase), tumor markers and additional imaging are critical for
an accurate diagnosis.
In the present study, X-ray, thin-section
bone-window CT and 3D-CT were not performed for the patient due to
financial constraints and comorbidities, which limits the
anatomical assessment and the exclusion of microscopic bone
invasion. Future similar cases should incorporate these imaging
modalities to enhance lesion characterization and diagnostic
precision.
Although the present case lacked molecular
profiling, available data indicate key genetic alterations in both
malignancies (MFS and prostate cancer) that may reveal shared
oncogenic pathways. MFS often harbors TP53 mutations and CDKN2A
deletions, leading to disrupted cell-cycle control and genomic
instability, while prostate adenocarcinoma frequently features
androgen receptor amplification and PTEN loss, driving tumor growth
and metastatic potential (27,28).
Furthermore, prostate-specific membrane antigen (PSMA; classically
upregulated in prostate cancer) is also detected in the
neovasculature of sarcomas (up to 35.3% in MFS), where it promotes
angiogenesis and may contribute to increased 99mTc-MDP
uptake in a superscan (29,30). Both tumor types express luteinizing
hormone-releasing hormone (LHRH) receptors, suggesting potential
benefit from LHRH-targeted therapies (31). Emerging evidence implicates
dysregulated p53 signaling, PI3K/AKT pathway activation and an
immunosuppressive tumor microenvironment characterized by
tumor-associated macrophage infiltration and T-cell exhaustion as
possible common mechanisms underlying multiple primary malignancies
(32–34). Future studies should employ
comprehensive genomic sequencing and immune profiling to identify
overlapping driver mutations, clarify the role of PSMA-mediated
angiogenesis and LHRH pathways, and evaluate whether targeted or
immunomodulatory treatments can mitigate the risk of metachronous
tumors.
MPMNs within the same individual are identified by
the presence of distinct pathological features in each tumor
(1–4). Traditionally, the diagnosis of MPMN is
based on the Warren criteria (35)
and revisions by Liu et al (36). However, the Surveillance,
Epidemiology and End Results (SEER) and International Agency for
Research on Cancer/International Association of Cancer Registries
(IARC/IACR) guidelines have become integral to modern diagnostics,
serving to enhance and extend the criteria used for diagnosing
MPMNs. SEER emphasizes tumor histology, anatomical location,
laterality and the time interval between diagnoses, thereby
facilitating the identification of multiple independent primary
tumors (37). By contrast, the
IARC/IACR criteria are more conservative, typically registering
only one tumor per organ or paired organs, emphasizing histological
consistency and adopting a cautious approach to multiple tumors in
the same anatomical site (38).
Synchronous MPMNs (SMPMNs) are defined as two or
more primary malignancies diagnosed within 6 months, while those
diagnosed after 6 months are termed metachronous MPMNs (MMPMNs).
MMPMNs have a lower incidence but similar survival rate compared
with SMPMNs (3,5). In the present case, the patient was
diagnosed with MMPMNs, comprising high-grade myxofibrosarcoma of
the left forearm and prostate adenocarcinoma with osseous
metastasis, as the two malignancies were identified more than 6
months apart. Tumor growth patterns, treatment approaches and
individual variability can affect the timing of diagnosis. Data
suggest a median interval of 3.45-6.13 years between the diagnoses
of the first and second primary tumors in MMPMN, but the prognostic
relevance of this interval remains uncertain (5,6,39).
Regardless, early detection, precise diagnosis and prompt treatment
are critical to improving outcomes in MPMNs. While imaging
techniques such as SPECT/CT and PET/CT enhance diagnostic accuracy,
pathological examination remains the gold standard when MPMN cannot
be ruled out.
In the evaluation of MFS,
18F-fluorodeoxyglucose (18F-FDG) PET/CT can
offer valuable metabolic information, while 99mTc-MDP
three-phase bone scintigraphy is traditionally used to detect
osteoblastic activity and assess potential bone metastases, often
demonstrating only minimal soft-tissue uptake (40). Although in the present study bone
scintigraphy revealed increased perfusion and focal
99mTc-MDP accumulation in the left forearm, suggestive
of soft-tissue malignancy, and produced a classic superscan pattern
indicative of diffuse skeletal involvement, it lacks the
specificity to distinguish primary sarcoma from metastatic disease.
By contrast, 18F-FDG PET/CT quantifies tumor glucose
metabolism, achieving sensitivity rates of >90% for soft-tissue
sarcomas compared with ~70% for planar bone scans, and can detect
microscopic cortical or marrow invasion that may be occult on bone
scintigraphy or MRI (41,42). In the present study,
18F-FDG PET/CT was not performed due to the advanced
age, frailty and financial constraints of the patient, which
constitutes a limitation of the diagnostic workup. While
99mTc-MDP SPECT/CT has high utility for detecting
widespread bone metastatic activity (superscan), its sensitivity
(~86.7%) and specificity (~98.8%) for prostate cancer bone
metastases are slightly inferior to those of
18Ffluorocholine PET/CT, which achieves a sensitivity of
up to 93.3% and a specificity of 100% in biochemical recurrence
settings (43,44). A prospective comparative study also
showed that NaF PET/CT attains a sensitivity of ~95% and a
specificity of ~93%, marginally outperforming SPECT/CT and MRI
(45). Moreover,
18F-DCFPyL PET/CT demonstrated superior diagnostic
accuracy vs. 99mTc-MDP SPECT/CT in a direct head-to-head
study (46). Together, these
findings indicate that PET/CT modalities, especially PSMA-targeted
or choline-based tracers and NaF PET, offer significantly higher
sensitivity and specificity compared with conventional bone
SPECT/CT in prostate cancer bone metastasis detection.
In the present study, preserved cortical bone
integrity was confirmed via histopathological microsectioning,
effectively ruling out local invasion despite the presence of a
superscan pattern. Given this discordance, PSA testing (563.864
ng/ml) and hip CT were pursued, which, combined with SPECT/CT
fusion imaging, identified prostate enlargement and mixed
sclerotic/lytic lesions consistent with metastatic prostate cancer.
The final diagnosis of metachronous MPMN (high-grade MFS and
prostate adenocarcinoma) was thus established. Nonetheless, the
absence of PET/CT imaging and molecular profiling remains a
limitation, and we recommend employing PET/CT or CT bone-window
imaging in similar future cases to enhance diagnostic accuracy and
to better distinguish primary soft-tissue tumors from skeletal
metastases.
In conclusion, the present study describes a rare
case involving dual primary malignancies (subcutaneous MFS and
prostate cancer with extensive bone metastases) diagnosed through
99mTc-MDP three-phase bone scintigraphy. The unique
imaging characteristics, specifically the extensive metastases on
superscan bone imaging and its established association with occult
prostate cancer, together with the patterns of recurrence and
metastasis of the primary tumor, were pivotal in informing and
shaping the subsequent therapeutic approach. Given the scarce
reports of MPMNs identified through 99mTc-MDP
scintigraphy, the present case highlights the need for heightened
clinical vigilance. For patients with recurrent soft-tissue sarcoma
who demonstrate a superscan on bone scintigraphy, routine screening
for occult prostate carcinoma should be undertaken. For any patient
newly diagnosed with a malignancy, clinicians should integrate
detailed clinical evaluation with three-phase bone imaging findings
to eliminate suspicion for additional primary tumors. Such a
strategy enhances diagnostic precision, reduces the likelihood of
missed or incorrect diagnoses and facilitates individualized
treatment planning that may improve overall patient outcomes.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JYL, JLL, HYZ, SXY and JSZ confirm the authenticity
of all the raw data. JYL, JLL, HYZ and JSZ contributed to the
conception of the study. JYL, JLL and SXY were responsible for
writing the original draft and reviewing and editing the
manuscript. SXY was responsible for the acquisition of clinical
data. JSZ was responsible for critical revision of the manuscript
and the analysis and interpretation of the data. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study passed ethical review by the Ethics
Committee of the Third Affiliated Hospital of Guangzhou Medical
University (Guangzhou, China). No information that could
potentially compromise patient privacy or personal identity was
included in this article. All the data used in this study were
collected from the Third Affiliated Hospital of Guangzhou Medical
University with both written and verbal informed consent from the
patient.
Patient consent for publication
All the test results, images and permission for
their publication were obtained with written consent from the
patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
18F-FDG
|
18F-fluorodeoxyglucose
|
|
IARC/IACR
|
International Agency for Research on
Cancer/International Association of Cancer Registries
|
|
LHRH
|
luteinizing hormone releasing
hormone
|
|
MFS
|
myxofibrosarcoma
|
|
MMPMN
|
metachronous multiple primary
malignant neoplasm
|
|
MPMN
|
multiple primary malignant
neoplasm
|
|
MRI
|
magnetic resonance imaging
|
|
PET
|
positron emission tomography
|
|
PSA
|
prostate-specific antigen
|
|
PSMA
|
prostate-specific membrane antigen
|
|
SEER
|
Surveillance Epidemiology and End
Results
|
|
SMPMN
|
synchronous multiple primary malignant
neoplasm
|
|
SPECT
|
single photon emission computed
tomography
|
|
99mTc-MDP
|
99mTc-labeled methylene
diphosphonate
|
References
|
1
|
Warren S and Gates O: Multiple primary
malignant tumors. A survey of the literature and stasistical study.
Am J Cancer. 16:1358–1414. 1932.
|
|
2
|
Liu F, Qin D and Wang Q: Clinical
pathological analysis of 172 cases with multiple primary carcinoma.
Chin J Oncol. 1:113–119. 1979.
|
|
3
|
Moertel CG, Dockerty MB and Baggenstoss
AH: Multiple primary malignant neoplasms. I. Introduction and
presentation of data. Cancer. 14:221–230. 1961. View Article : Google Scholar
|
|
4
|
Shi L, Zhou S, Jiang Y, Wan Y, Ma J, Fu S
and Cheng W: Gynecological malignant tumor related multiple primary
malignant neoplasms: Clinical analysis of 30 cases. Zhonghua Fu
Chan Ke Za Zhi. 49:199–203. 2014.
|
|
5
|
Xu LL and Gu KS: Clinical retrospective
analysis of cases with multiple primary malignant neoplasms. Genet
Mol Res. 13:9271–9284. 2014. View Article : Google Scholar
|
|
6
|
Wang Y, Jiao F, Yao J, Zhou X, Zhang X and
Wang L: Clinical features of multiple primary malignant tumors: A
retrospective clinical analysis of 213 Chinese patients at two
centers. Discov Med. 32:65–78. 2021.
|
|
7
|
Vogt A, Schmid S, Heinimann K, Frick H,
Herrmann C, Cerny T and Omlin A: Multiple primary tumours:
Challenges and approaches, a review. ESMO Open. 2:e0001722017.
View Article : Google Scholar
|
|
8
|
Pauli C, De Boni L, Pauwels JE, Chen Y,
Planas-Paz L, Shaw R, Emerling BM, Grandori C, Hopkins BD and Rubin
MA: A functional precision oncology approach to identify treatment
strategies for myxofibrosarcoma patients. Mol Cancer Res.
20:244–252. 2022. View Article : Google Scholar
|
|
9
|
Rachdi I, Daoud F, Khanchel F, Arbaoui I,
Somai M, Zoubeidi H, Aydi Z, Ben Dhaou B, Debbiche A and Boussema
F: Myxofibrosarcoma of the leg: A diagnostic challenge. Clin Case
Rep. 8:3332–3335. 2020. View Article : Google Scholar
|
|
10
|
Zhang P, Ruan SF, Huang J, Gong T and Ji
C: Multiple cutaneous myxofibrosarcoma on the right arm:
Clinicopathological features and differential diagnosis. Authorea.
2023.
|
|
11
|
Vanni S, De Vita A, Gurrieri L, Fausti V,
Miserocchi G, Spadazzi C, Liverani C, Cocchi C, Calabrese C,
Bongiovanni A, et al: Myxofibrosarcoma landscape: Diagnostic
pitfalls, clinical management and future perspectives. Ther Adv Med
Oncol. 14:175883592210939732022. View Article : Google Scholar
|
|
12
|
Fernebro J, Bladström A, Rydholm A,
Gustafson P, Olsson H, Engellau J and Nilbert M: Increased risk of
malignancies in a population-based study of 818 soft-tissue sarcoma
patients. Br J Cancer. 95:986–990. 2006. View Article : Google Scholar
|
|
13
|
Tateishi U, Hasegawa T, Yamamoto S,
Yamaguchi U, Yokoyama R, Kawamoto H, Satake M and Arai Y: Incidence
of multiple primary malignancies in a cohort of adult patients with
soft tissue sarcoma. Jpn J Clin Oncol. 35:444–452. 2005. View Article : Google Scholar
|
|
14
|
Sanfilippo R, Miceli R, Grosso F, Fiore M,
Puma E, Pennacchioli E, Barisella M, Sangalli C, Mariani L, Casali
PG and Gronchi A: Myxofibrosarcoma: Prognostic factors and survival
in a series of patients treated at a single institution. Ann Surg
Oncol. 18:720–725. 2011. View Article : Google Scholar
|
|
15
|
Mentzel T, Calonje E, Wadden C, Camplejohn
RS, Beham A, Smith MA and Fletcher CD: Myxofibrosarcoma.
Clinicopathologic analysis of 75 cases with emphasis on the
low-grade variant. Am J Surg Pathol. 20:391–405. 1996. View Article : Google Scholar
|
|
16
|
Neagu TP, Sinescu RD, Enache V, Achim SC,
Ţigliş M and Mirea LE: Metastatic high-grade myxofibrosarcoma:
Review of a clinical case. Rom J Morphol Embryol. 58:603–609.
2017.
|
|
17
|
Look Hong NJ, Hornicek FJ, Raskin KA, Yoon
SS, Szymonifka J, Yeap B, Chen YL, DeLaney TF, Nielsen GP and
Mullen JT: Prognostic factors and outcomes of patients with
myxofibrosarcoma. Ann Surg Oncol. 20:80–86. 2013. View Article : Google Scholar
|
|
18
|
Castaigne C, Martin P and Blocklet D:
Lung, gastric, and soft tissue uptake of Tc-99m MDP and Ga-67
citrate associated with hypercalcemia. Clin Nucl Med. 28:467–471.
2003. View Article : Google Scholar
|
|
19
|
Flores LGI, Nagamachi S, Jinnouchi S,
Ohnishi T, Futami S, Nakahara H and Tamura S: Relationship between
extraosseous accumulation in bone scintigraphy with 99Tcm-HMDP and
histopathology. Nucl Med Commun. 19:347–354. 1998. View Article : Google Scholar
|
|
20
|
Zeng D, Chen Y, Cai L and Zhou F: Analysis
of abnormal uptake of extraskeletal soft tissue in
99mTc-MDP whole bone scan. Chongqing Med J.
45:2073–2077. 2016.(In Chinese).
|
|
21
|
Ostergaard L, Tietze A, Nielsen T, Drasbek
KR, Mouridsen K, Jespersen SN and Horsman MR: The relationship
between tumor blood flow, angiogenesis, tumor hypoxia, and aerobic
glycolysis. Cancer Res. 73:5618–5624. 2013. View Article : Google Scholar
|
|
22
|
Buckley O, O'Keeffe S, Geoghegan T, Lyburn
ID, Munk PL, Worsley D and Torreggiani WC: 99mTc bone scintigraphy
superscans: A review. Nucl Med Commun. 28:521–527. 2007. View Article : Google Scholar
|
|
23
|
Ren JYL, Li Y and Luo Y: Differentiation
of superscan in 99TCm-MDP bone scintigraphy:
A case report. Chin J Nucl Med Mol Imaging. 39:39–40. 2019.(In
Chinese).
|
|
24
|
Askari E, Shakeri S, Roustaei H, Fotouhi
M, Sadeghi R, Harsini S and Vali R: Superscan pattern on bone
scintigraphy: A comprehensive review. Diagnostics (Basel).
14:22292024. View Article : Google Scholar
|
|
25
|
Kovacsne A, Kozon I, Bentestuen M and
Zacho HD: Frequency of superscan on bone scintigraphy: A systematic
review. Clin Physiol Funct Imaging. 43:297–304. 2023. View Article : Google Scholar
|
|
26
|
Prakash R: Incidental detection of
skeletal metastases on technetium-99m DTPA renal scintigraphy.
Australas Radiol. 39:182–184. 1995. View Article : Google Scholar
|
|
27
|
Ogura K, Hosoda F, Arai Y, Nakamura H,
Hama N, Totoki Y, Yoshida A, Nagai M, Kato M, Arakawa E, et al:
Integrated genetic and epigenetic analysis of myxofibrosarcoma. Nat
Commun. 9:27652018. View Article : Google Scholar
|
|
28
|
Bui NQ, Przybyl J, Trabucco SE, Frampton
G, Hastie T, van de Rijn M and Ganjoo KN: A clinico-genomic
analysis of soft tissue sarcoma patients reveals CDKN2A deletion as
a biomarker for poor prognosis. Clin Sarcoma Res. 9:122019.
View Article : Google Scholar
|
|
29
|
Kleiburg F, van der Hulle T, Gelderblom H,
Slingerland M, Speetjens FM, Hawinkels LJAC, Dibbets-Schneider P,
van Velden FHP, Pool M, Lam SW, et al: PSMA expression and PSMA
PET/CT imaging in metastatic soft tissue sarcoma patients, results
of a prospective study. Eur J Nucl Med Mol Imaging. 52:3690–3699.
2025. View Article : Google Scholar
|
|
30
|
Spiridon IA, Ong SLM, Soukup J,
Topirceanu-Andreoiu OM, de Geus-Oei LF, Gelderblom H, Lam SW, de
Bruijn IHB, Akker BEWMVD, Hijmen L, et al: Neovascular prostate
specific membrane antigen (PSMA) expression in bone and soft tissue
sarcoma: A systematic analysis. Virchows Arch. Apr 9–2025.(Epub
ahead of print). View Article : Google Scholar
|
|
31
|
Deivaraju C, Temple HT, Block N, Robinson
P and Schally AV: LHRH receptor expression in sarcomas of bone and
soft tissue. Horm Mol Biol Clin Investig. 28:105–111. 2016.
View Article : Google Scholar
|
|
32
|
Wu H, Li J, Yao R, Liu J, Su L and You W:
Focusing on the interplay between tumor-associated macrophages and
tumor microenvironment: from mechanism to intervention.
Theranostics. 15:7378–7408. 2025. View Article : Google Scholar
|
|
33
|
Zhang Z and Wu Y: Research progress on
mechanisms of tumor immune microenvironment and gastrointestinal
resistance to immunotherapy: Mini review. Front Immunol.
16:16415182025. View Article : Google Scholar
|
|
34
|
Nair R, Somasundaram V, Kuriakose A,
Krishn SR, Raben D, Salazar R and Nair P: Deciphering T-cell
exhaustion in the tumor microenvironment: Paving the way for
innovative solid tumor therapies. Front Immunol. 16:15482342025.
View Article : Google Scholar
|
|
35
|
Swaroop VS, Winawer SJ, Kurtz RC and
Lipkin M: Multiple primary malignant tumors. Gastroenterology.
93:779–783. 1987. View Article : Google Scholar
|
|
36
|
Liu F, Qin D and Wang Q: Clinical and
pathological analysis of 172 cases of multiple primary cancers.
Chin J Oncol. 1:113–119. 1979.(In Chinese).
|
|
37
|
Adamo M, Johnson C, Ruhl J and Dicki L:
SEER program coding and staging manual. NIH publication number
12-5581. National Cancer Institute; Bethesda, MD: 2012
|
|
38
|
Working Group Report: International rules
for multiple primary cancers (ICD-0 third edition). Eur J Cancer
Prev. 14:307–308. 2005. View Article : Google Scholar
|
|
39
|
Tichansky DS, Cagir B, Borrazzo E, Topham
A, Palazzo J, Weaver EJ, Lange A and Fry RD: Risk of second cancers
in patients with colorectal carcinoids. Dis Colon Rectum. 45:91–97.
2002. View Article : Google Scholar
|
|
40
|
Ito K, Masuda-Miyata Y, Wada S, Morooka M,
Yamasawa K, Hashimoto M and Kubota K: F-18 FDG PET/CT imaging of
bulky myxofibrosarcoma in chest wall. Clin Nucl Med. 36:212–213.
2011. View Article : Google Scholar
|
|
41
|
Younis MH, Abu-Hijleh HA, Aldahamsheh OO,
Abualruz A and Thalib L: Meta-analysis of the diagnostic accuracy
of primary bone and soft tissue sarcomas by 18F-FDG-PET. Med Princ
Pract. 29:465–472. 2020. View Article : Google Scholar
|
|
42
|
Kassem TW, Abdelaziz O and Emad-Eldin S:
Diagnostic value of 18F-FDG-PET/CT for the follow-up and
restaging of soft tissue sarcomas in adults. Diagn Interv Imaging.
98:693–698. 2017. View Article : Google Scholar
|
|
43
|
Shen G, Deng H, Hu S and Jia Z: Comparison
of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the
diagnosis of bone metastases in patients with prostate cancer: A
meta-analysis. Skeletal Radiol. 43:1503–1513. 2014. View Article : Google Scholar
|
|
44
|
de Leiris N, Leenhardt J, Boussat B,
Montemagno C, Seiller A, Phan Sy O, Roux J, Laramas M, Verry C,
Iriart C, et al: Does whole-body bone SPECT/CT provide additional
diagnostic information over [18F]-FCH PET/CT for the detection of
bone metastases in the setting of prostate cancer biochemical
recurrence? Cancer Imaging. 20:582020. View Article : Google Scholar
|
|
45
|
Zamani-Siahkali N, Mirshahvalad SA, Farbod
A, Divband G, Pirich C, Veit-Haibach P, Cook G and Beheshti M:
SPECT/CT, PET/CT, and PET/MRI for response assessment of bone
metastases. Semin Nucl Med. 54:356–370. 2024. View Article : Google Scholar
|
|
46
|
Hu X, Cao Y, Ji B, Zhao M, Wen Q and Chen
B: Comparative study of 18F-DCFPyL PET/CT and
99mTc-MDP SPECT/CT bone imaging for the detection of
bone metastases in prostate cancer. Front Med (Lausanne).
10:12019772023. View Article : Google Scholar
|