|
1
|
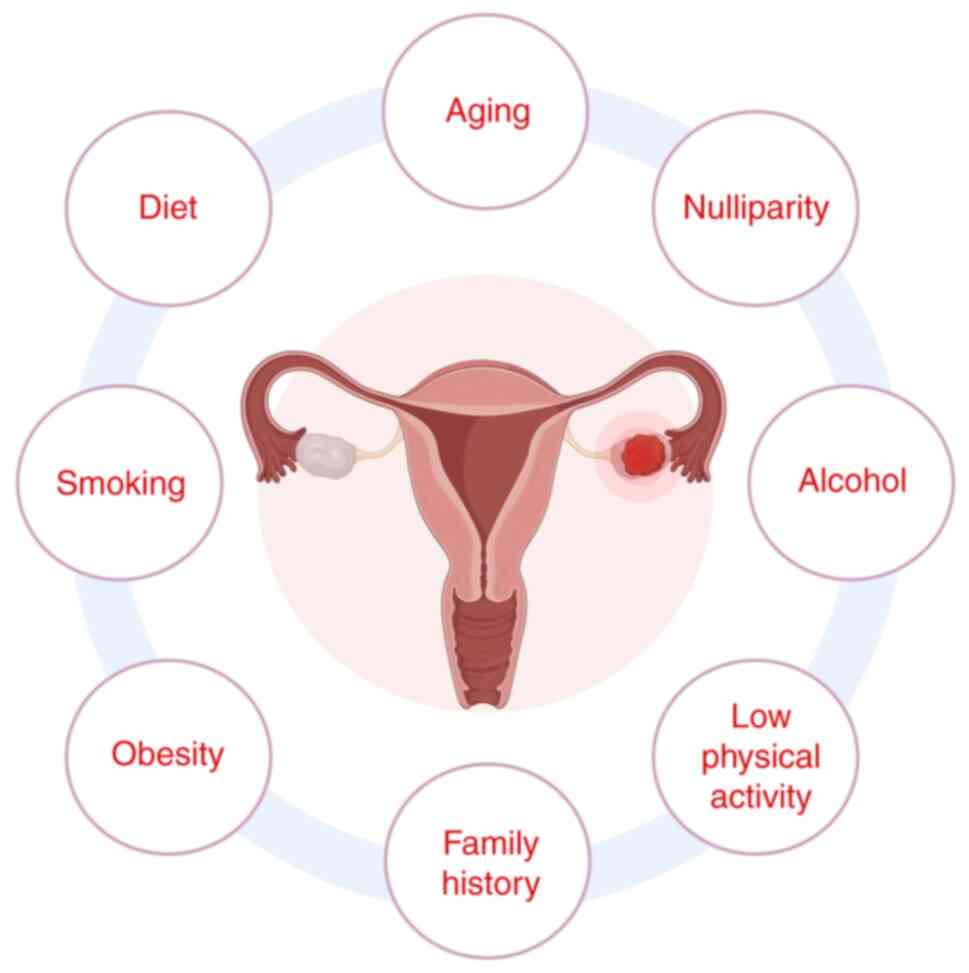
Ali A, Al-Ani O and Al-Ani F: Epidemiology
and risk factors for ovarian cancer. Prz Menopauzalny. 22:93–104.
2023.PubMed/NCBI
|
|
2
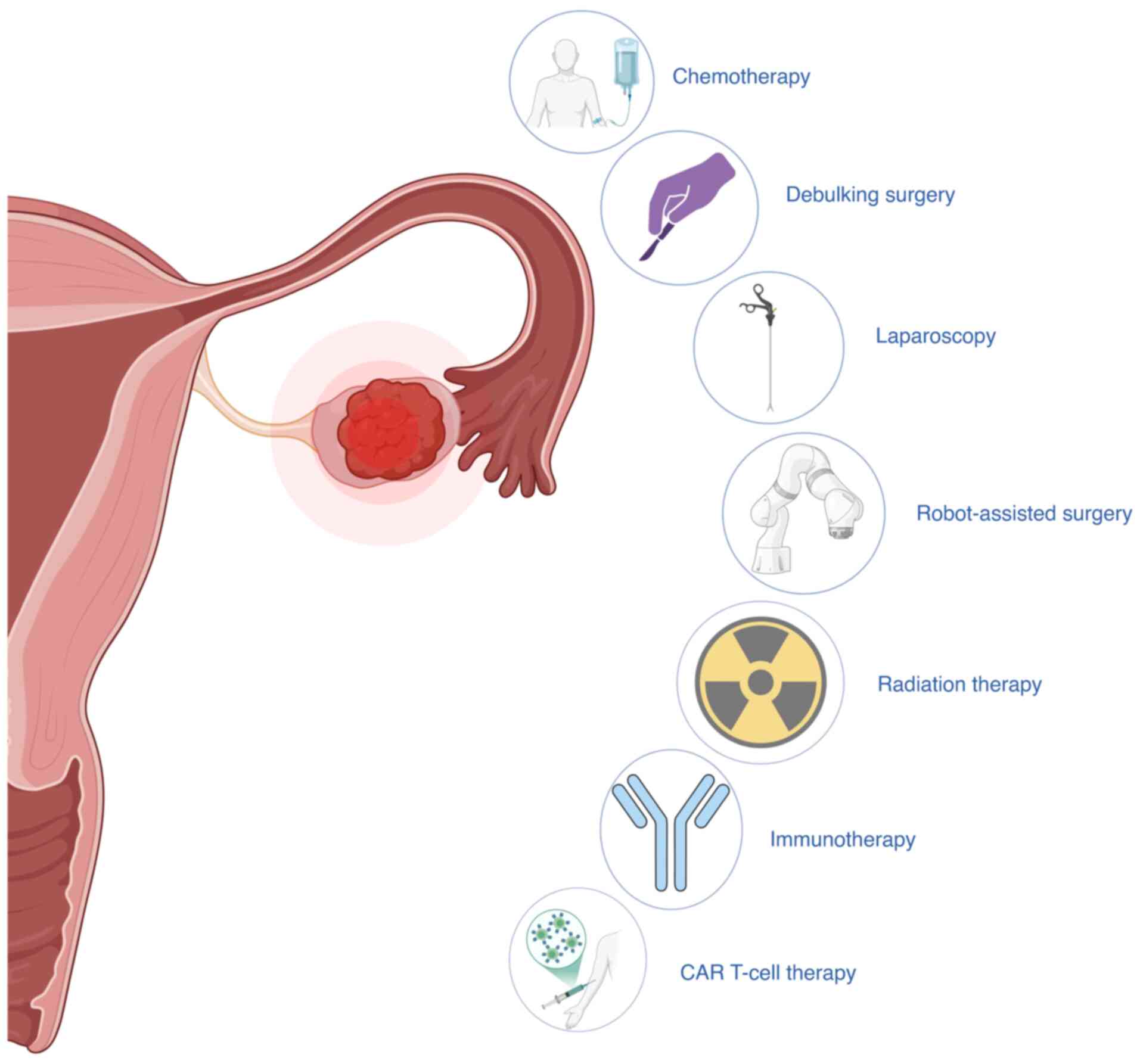
|
Silva EG: The origin of epithelial
neoplasms of the ovary: An alternative view. Adv Anat Pathol.
23:50–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crane TE, Khulpateea BR, Alberts DS,
Basen-Engquist K and Thomson CA: Dietary intake and ovarian cancer
risk: A systematic review. Cancer Epidemiol Biomarkers Prev.
23:255–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan A, Sun Q, Czernichow S, Kivimaki M,
Okereke OI, Lucas M, Manson JE, Ascherio A and Hu FB: Bidirectional
association between depression and obesity in middle-aged and older
women. Int J Obes (Lond). 36:595–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reid BM, Permuth JB and Sellers TA:
Epidemiology of ovarian cancer: A review. Cancer Biol Med. 14:9–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Risch HA: Hormonal etiology of epithelial
ovarian cancer, with a hypothesis concerning the role of androgens
and progesterone. J Natl Cancer Inst. 90:1774–1786. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Terry KL, Karageorgi S, Shvetsov YB,
Merritt MA, Lurie G, Thompson PJ, Carney ME, Weber RP, Akushevich
L, Lo-Ciganic WH, et al: Genital powder use and risk of ovarian
cancer: A pooled analysis of 8,525 cases and 9,859 controls. Cancer
Prev Res (Phila). 6:811–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faber MT, Kjær SK, Dehlendorff C,
Chang-Claude J, Andersen KK, Høgdall E, Webb PM, Jordan SJ;
Australian Cancer Study (Ovarian Cancer); Australian Ovarian Cancer
Study Group, ; et al: Cigarette smoking and risk of ovarian cancer:
A pooled analysis of 21 case-control studies. Cancer Causes
Control. 24:989–1004. 2013.PubMed/NCBI
|
|
9
|
Rauh-Hain JA, Melamed A, Pareja R, May T,
Sinno A, McNally L, Horowitz NS, De Iaco P, Michener CM, Van
Lonkhuijzen L, et al: Laparoscopic cytoreduction after neoadjuvant
chemotherapy in high-grade epithelial ovarian cancer: A LANCE
randomized clinical trial. JAMA Netw Open. 7:e2446325–e. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sambasivan S: Epithelial ovarian cancer.
Cancer Treat Res Commun. 33:1006292022.PubMed/NCBI
|
|
11
|
Kuroki L and Guntupalli SR: Treatment of
epithelial ovarian cancer. BMJ. 371:m37732020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Delga B, Classe JM, Houvenaeghel G, Blache
G, Sabiani L, El Hajj H, Andrieux N and Lambaudie E: 30 years of
experience in the management of stage III and IV epithelial ovarian
cancer: Impact of surgical strategies on survival. Cancers (Basel).
12:7682020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurnit KC, Fleming GF and Lengyel E:
Updates and new options in advanced epithelial ovarian cancer
treatment. Obstet Gynecol. 137:108–1021. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bailly C, Thuru X and Quesnel B: Combined
cytotoxic chemotherapy and immunotherapy of cancer: Modern times.
NAR Cancer. 2:zcaa0022020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaitskell K, Rogozińska E, Platt S, Chen
Y, Abd El Aziz M, Tattersall A and Morrison J: Angiogenesis
inhibitors for the treatment of epithelial ovarian cancer. Cochrane
Database Syst Rev. 4:CD0079302023.PubMed/NCBI
|
|
16
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Incorporation of bevacizumab in the primary treatment of
ovarian cancer. N Engl J Med. 365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baethge C, Goldbeck-Wood S and Mertens S:
SANRA-a scale for the quality assessment of narrative review
articles. Res Integr Peer Rev. 4:52019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chi DS, Eisenhauer EL, Zivanovic O, Sonoda
Y, Abu-Rustum NR, Levine DA, Guile MW, Bristow RE, Aghajanian C and
Barakat RR: Improved progression-free and overall survival in
advanced ovarian cancer as a result of a change in surgical
paradigm. Gynecol Oncol. 114:26–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quénet F, Elias D, Roca L, Goéré D, Ghouti
L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, et al:
Cytoreductive surgery plus hyperthermic intraperitoneal
chemotherapy versus cytoreductive surgery alone for colorectal
peritoneal metastases (PRODIGE 7): A multicentre, randomised,
open-label, phase 3 trial. Lancet Oncol. 22:256–266. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nezhat F, Nezhat C, Welander CE and
Benigno B: Four ovarian cancers diagnosed during laparoscopic
management of 1011 women with adnexal masses. Am J Obstet Gynecol.
167:790–796. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feuer GA, Lakhi N, Barker J, Salmieri S
and Burrell M: Perioperative and clinical outcomes in the
management of epithelial ovarian cancer using a robotic or
abdominal approach. Gynecol Oncol. 131:520–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Magrina JF, Zanagnolo V, Noble BN, Kho RM
and Magtibay P: Robotic approach for ovarian cancer: Perioperative
and survival results and comparison with laparoscopy and
laparotomy. Gynecol Oncol. 121:100–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berek JS, Matias-Guiu X, Creutzberg C,
Fotopoulou C, Gaffney D, Kehoe S, Lindemann K, Mutch D and Concin
N; Endometrial Cancer Staging Subcommittee and FIGO Women's Cancer
Committee, : FIGO staging of endometrial cancer: 2023. Int J
Gynaecol Obstet. 162:383–394. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baum S, Alkatout I, Proppe L, Kotanidis C,
Rody A, Laganà AS, Sommer S and Gitas G: Surgical treatment of
endometrioid endometrial Carcinoma-laparotomy versus laparoscopy. J
Turk Ger Gynecol Assoc. 23:233–240. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Serur E, Emeney PL and Byrne DW:
Laparoscopic management of adnexal masses. JSLS. 5:143–151.
2001.PubMed/NCBI
|
|
26
|
Canis M, Mage G, Pouly JL, Wattiez A,
Manhes H and Bruhat MA: Laparoscopic diagnosis of adnexal cystic
masses: A 12-year experience with long-term follow-up. Obstet
Gynecol. 83:707–712. 1994.PubMed/NCBI
|
|
27
|
Jorgensen K, Melamed A, Wu CF, Nitecki R,
Pareja R, Fagotti A, Schorge JO, Ramirez PT and Rauh-Hain JA:
Minimally invasive interval debulking surgery for advanced ovarian
cancer after neoadjuvant chemotherapy. Gynecol Oncol. 172:130–137.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chamberlain E and Carlo BD: Ovarian Cancer
Update. Proceedings of UCLA Health. Vol 28:2024.
|
|
29
|
Psomiadou V, Prodromidou A, Fotiou A,
Lekka S and Iavazzo C: Robotic interval debulking surgery for
advanced epithelial ovarian cancer: Current challenge or future
direction? A systematic review. J Robot Surg. 15:155–163. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abitbol J, Gotlieb W, Zeng Z, Ramanakumar
A, Kessous R, Kogan L, Pare-Miron V, Rombaldi M, Salvador S,
Kucukyazici B, et al: Incorporating robotic surgery into the
management of ovarian cancer after neoadjuvant chemotherapy. Int J
Gynecol Cancer. 29:1341–1347. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Zheng Y and Yang F: Primary
debulking surgery for advanced epithelial ovarian cancer with
isolated enlarged para-aortic lymph node by robotic transumbilical
single port approach. Int J Gynecol Cancer. 33:1976–1977. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ray-Coquard I, Leary A, Pignata S, Cropet
C, González-Martín A, Marth C, Nagao S, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus bevacizumab First-line maintenance
in ovarian cancer: Final overall survival results from the
PAOLA-1/ENGOT-ov25 trial. Ann Oncol. 34:681–692. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao L, Zhai Y and Niu G: Research
progress of immune checkpoint inhibitors in ovarian cancer. Exp
Immunol. 4:853–870. 2024. View Article : Google Scholar
|
|
34
|
Nezhat F, Briskin C, Lakhi N, Fu R and
Pejovic T: Minimally invasive surgery for the management of ovarian
cancer: A systematic review and Meta-analysis. O G Open. 1:392024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ray-Coquard I, Pautier P, Pignata S, Pérol
D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus bevacizumab as first-line
maintenance in ovarian cancer. N Engl J Med. 381:2416–2428. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Predina JD, Kapoor V, Judy BF, Cheng G,
Fridlender ZG, Albelda SM and Singhal S: Cytoreduction surgery
reduces systemic myeloid suppressor cell populations and restores
intratumoral immunotherapy effectiveness. J Hematol Oncol.
5:342012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiao F, Wang Z, Qiao L, Zhang X, Wu N,
Wang J and Yu X: Application of PARP inhibitors combined with
immune checkpoint inhibitors in ovarian cancer. J Transl Med.
22:7782024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Armstrong DK, Alvarez RD, Bakkum-Gamez JN,
Barroilhet L, Behbakht K, Berchuck A, Chen LM, Cristea M, DeRosa M,
Eisenhauer EL, et al: Ovarian cancer, version 2.2020, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
19:191–226. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghirardi V, Fagotti A and Scambia G:
Laparoscopic selection for surgery in epithelial ovarian cancer. A
short review. Facts Views Vis Obgyn. 15:25–28. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
González-Martín A, Pothuri B, Vergote I,
DePont Christensen R, Graybill W, Mirza MR, McCormick C, Lorusso D,
Hoskins P, Freyer G, et al: Niraparib in patients with newly
diagnosed advanced ovarian cancer. N Engl J Med. 381:2391–2402.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fagotti A, Alletti SG, Corrado G, Cola E,
Vizza E, Vieira M, Andrade CE, Tsunoda A, Favero G, Zapardiel I, et
al: The INTERNATIONAL MISSION study: Minimally invasive surgery in
ovarian neoplasms after neoadjuvant chemotherapy. Int J Gynecol
Cancer. 29:5–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Monk BJ, Dalton H, Farley JH, Chase DM and
Benjamin I: Antiangiogenic agents as a maintenance strategy for
advanced epithelial ovarian cancer. Crit Rev Oncol Hematol.
86:161–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moore KN, Oza AM, Colombo N, Oaknin A,
Scambia G, Lorusso D, Konecny GE, Banerjee S, Murphy CG, Tanyi JL,
et al: Phase III, randomized trial of mirvetuximab soravtansine
versus chemotherapy in patients with platinum-resistant ovarian
cancer: Primary analysis of FORWARD I. Ann Oncol. 32:757–765. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Akter S, Rahman MA, Hasan MN, Akhter H,
Noor P, Islam R, Shin Y, Rahman MDH, Gazi MS, Huda MN, et al:
Recent advances in ovarian cancer: Therapeutic strategies,
potential biomarkers, and technological improvements. Cells.
11:6502022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang L, Wang Q, Xu Y, Cui M and Han L:
Advances in the treatment of ovarian cancer using PARP inhibitors
and the underlying mechanism of resistance. Curr Drug Targets.
21:167–178. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lord CJ and Ashworth A: PARP inhibitors:
Synthetic lethality in the clinic. Science. 355:1152–1158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Coleman RL, Oza AM, Lorusso D, Aghajanian
C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G,
et al: Rucaparib maintenance treatment for recurrent ovarian
carcinoma after response to platinum therapy (ARIEL3): A
randomised, Double-blind, placebo-controlled, phase 3 trial.
Lancet. 390:1949–1961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Coleman RL, Fleming GF, Brady MF, Swisher
EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat
Ben-Baruch N, Werner TL, et al: Veliparib with first-line
chemotherapy and as maintenance therapy in ovarian cancer. N Engl J
Med. 381:2403–2415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vergote I, Du Bois A, Floquet A, Rau J,
Kim JW, Del Campo J, Friedlander M, Pignata S, Fujiwara K, Colombo
N, et al: Overall survival results of AGO-OVAR16: A phase 3 study
of maintenance pazopanib versus placebo in women who have not
progressed after first-line chemotherapy for advanced ovarian
cancer. Gynecol Oncol. 155:186–191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, Kurzeder C, et al: A phase 3 trial of bevacizumab in
ovarian cancer. N Engl J Med. 365:2484–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aghajanian C, Blank SV, Goff BA, Judson
PL, Teneriello MG, Husain A, Sovak MA, Yi J and Nycum LR: OCEANS: A
randomized, double-blind, placebo-controlled phase III trial of
chemotherapy with or without bevacizumab in patients with
platinum-sensitive recurrent epithelial ovarian, primary
peritoneal, or fallopian tube cancer. J Clin Oncol. 30:2039–2045.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang Y, Gao Y, Zhou H, Cai Y, Yu J, Chen
Y, Xue J and Cheng W: Anlotinib combined with
carboplatin/paclitaxel and maintenance anlotinib as front-line
treatment for newly diagnosed advanced ovarian cancer: A phase II,
single-arm, multicenter study (ALTER-GO-010). Am Soc Clin Oncol.
41:55752023. View Article : Google Scholar
|
|
55
|
Pignata S, Lorusso D, Scambia G, Sambataro
D, Tamberi S, Cinieri S, Cinieri S, Mosconi AM, Orditura M, Brandes
AA, et al: Pazopanib plus weekly paclitaxel versus weekly
paclitaxel alone for platinum-resistant or platinum-refractory
advanced ovarian cancer (MITO 11): A randomised, open-label, phase
2 trial. Lancet Oncol. 16:561–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lheureux S, Matei DE, Konstantinopoulos
PA, Wang BX, Gadalla R, Block MS, Jewell A, Gaillard SL, McHale M,
McCourt C, et al: Translational randomized phase II trial of
cabozantinib in combination with nivolumab in advanced, recurrent,
or metastatic endometrial cancer. J Immunother Cancer.
10:e0042332022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Paik ES, Kim TH, Cho YJ, Ryu J, Choi JJ,
Lee YY, Kim TJ, Choi CH, Kim WY, Sa JK, et al: Preclinical
assessment of the VEGFR inhibitor axitinib as a therapeutic agent
for epithelial ovarian cancer. Sci Rep. 10:49042020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rafii SL: Narrative review of novel
chemotherapeutic agents in management of ovarian cancer. Gynecol
Pelvic Med. 42021.
|
|
59
|
Ansari MJ, Bokov D, Markov A, Jalil AT,
Shalaby MN, Suksatan W, Chupradit S, Al-Ghamdi HS, Shomali N,
Zamani A, et al: Cancer combination therapies by angiogenesis
inhibitors; a comprehensive review. Cell Commun Signal. 20:492022.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Monk BJ, Colombo N, Tewari KS, Dubot C,
Caceres MV, Hasegawa K, Shapira–Frommer R, Salman P, Yañez E, Gumus
M, et al: KEYNOTE-826: Final overall survival results from a
randomized, double-blind, phase 3 study of pembrolizumab+
chemotherapy vs. placebo+ chemotherapy for first-line treatment of
persistent, recurrent, or metastatic cervical cancer. J Clin Oncol.
41:55002023. View Article : Google Scholar
|
|
61
|
Tang M, Cai JH, Diao HY, Guo WM, Yang X
and Xing S: The progress of peptide vaccine clinical trials in
gynecologic oncology. Hum Vaccin Immunother. 18:20629822022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hamanishi J, Mandai M, Ikeda T, Minami M,
Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S,
et al: Safety and antitumor activity of anti-PD-1 antibody,
nivolumab, in patients with platinum-resistant ovarian cancer. J
Clin Oncol. 33:4015–4022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Disis ML, Taylor MH, Kelly K, Beck JT,
Gordon M, Moore KM, Patel MR, Chaves J, Park H, Mita AC, et al:
Efficacy and safety of avelumab for patients with recurrent or
refractory ovarian cancer: Phase 1b results from the JAVELIN solid
tumor trial. JAMA Oncol. 5:393–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gray HJ, Benigno B, Berek J, Chang J,
Mason J, Mileshkin L, Mitchell P, Moradi M, Recio FO, Michener CM,
et al: Progression-free and overall survival in ovarian cancer
patients treated with CVac, a mucin 1 dendritic cell therapy in a
randomized phase 2 trial. J Immunother Cancer. 4:342016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Martin Lluesma S, Wolfer A, Harari A and
Kandalaft LE: Cancer vaccines in ovarian cancer: How can we
improve? Biomedicines. 4:102016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chow S, Berek JS and Dorigo O: Development
of therapeutic vaccines for ovarian cancer. Vaccines (Basel).
8:6572020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lawrie TA, Winter-Roach BA, Heus P and
Kitchener HC: Adjuvant (post-surgery) chemotherapy for early stage
epithelial ovarian cancer. Cochrane Database Syst Rev.
12:CD0047062015.PubMed/NCBI
|
|
69
|
Coleridge S, Bryant A, Kehoe S and
Morrison J: Neoadjuvant chemotherapy before surgery versus surgery
followed by chemotherapy for initial treatment in advanced ovarian
epithelial cancer. Cochrane Database Syst Rev.
7:CD0053432021.PubMed/NCBI
|
|
70
|
Santoiemma PP and Powell DJ Jr: Tumor
infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther.
16:807–820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cutri-French C, Nasioudis D, George E and
Tanyi JL: CAR-T cell therapy in ovarian cancer: Where are we now?
Diagnostics (Basel). 14:8192024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kandalaft LE, Powell DJ and Coukos G: A
phase I clinical trial of adoptive transfer of folate
receptor-alpha redirected autologous T cells for recurrent ovarian
cancer. J Transl Med. 10:1572012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sarivalasis A, Morotti M, Mulvey A,
Imbimbo M and Coukos G: Cell therapies in ovarian cancer. Ther Adv
Med Oncol. 13:175883592110083992021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Xin Q, Chen Y, Sun X, Li R, Wu Y and Huang
X: CAR-T therapy for ovarian cancer: Recent advances and future
directions. Biochem Pharmacol. 226:1163492024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nguyen TT, Thanh Nhu N, Chen CL and Lin
CF: Effectiveness and safety of CD22 and CD19 dual-targeting
chimeric antigen receptor T-cell therapy in patients with relapsed
or refractory B-cell malignancies: A meta-analysis. Cancer Med.
12:18767–1885. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fang J, Ding N, Guo X, Sun Y, Zhang Z, Xie
B, Li Z, Wang H, Mao W, Lin Z, et al: αPD-1-mesoCAR-T cells
partially inhibit the growth of advanced/refractory ovarian cancer
in a patient along with daily apatinib. J Immunother Cancer.
9:e0011622021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Liang Z, Dong J, Yang N, Li SD, Yang ZY,
Huang R, Li FJ, Wang WT, Ren JK, Lei J, et al: Tandem CAR-T cells
targeting FOLR1 and MSLN enhance the antitumor effects in ovarian
cancer. Int J Biol Sci. 17:4365–4376. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Banville AC, Wouters MC, Oberg AL, Goergen
KM, Maurer MJ, Milne K, Ashkani J, Field E, Ghesquiere C, Jones
SJM, et al: Co-expression patterns of chimeric antigen receptor
(CAR)-T cell target antigens in primary and recurrent ovarian
cancer. Gynecol Oncol. 160:520–529. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mai J, Wu L, Yang L, Sun T, Liu X, Yin R,
Jiang Y, Li J and Li Q: Therapeutic strategies targeting folate
receptor α for ovarian cancer. Front Immunol. 14:12545322023.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for cancer immunotherapy: Mechanism, combinations, and
clinical outcome. Front Pharmacol. 8:5612017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Matulonis U, Shapira-Frommer R, Santin A,
Lisyanskaya A, Pignata S, Vergote I, Raspagliesi F, Sonke GS,
Birrer M, Provencher DM, et al: Antitumor activity and safety of
pembrolizumab in patients with advanced recurrent ovarian cancer:
Results from the phase II KEYNOTE-100 study. Ann Oncol.
30:1080–1087. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Matulonis UA, Shapira R, Santin A,
Lisyanskaya AS, Pignata S, Vergote I, Raspagliesi F, Sonke GS,
Birrer M, Sehouli J, et al: Final results from the KEYNOTE-100
trial of pembrolizumab in patients with advanced recurrent ovarian
cancer. J Clin Oncol. 38:60052020. View Article : Google Scholar
|
|
83
|
Musacchio L, Cicala CM, Camarda F,
Ghizzoni V, Giudice E, Carbone MV, Ricci C, Perri MT, Tronconi F,
Gentile M, et al: Combining PARP inhibition and immune checkpoint
blockade in ovarian cancer patients: A new perspective on the
horizon? ESMO Open. 7:1005362022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
An D, Banerjee S and Lee JM: Recent
advancements of antiangiogenic combination therapies in ovarian
cancer. Cancer Treat Rev. 98:1022242021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lee WS, Yang H, Chon HJ and Kim C:
Combination of anti-angiogenic therapy and immune checkpoint
blockade normalizes vascular-immune crosstalk to potentiate cancer
immunity. Exp Mol Med. 52:1475–1485. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Färkkilä A, Gulhan DC, Casado J, Jacobson
CA, Nguyen H, Kochupurakkal B, Maliga Z, Yapp C, Chen YA, Schapiro
D, et al: Immunogenomic profiling determines responses to combined
PARP and PD-1 inhibition in ovarian cancer. Nat Commun.
11:14592020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bouter E, Lok C and Trum H: Robot-assisted
laparoscopic staging compared to conventional laparoscopic staging
and laparotomic staging in clinical early stage ovarian carcinoma.
Curr Opin Oncol. 34:490–496. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yokoi A, Machida H, Shimada M, Matsuo K,
Shigeta S, Furukawa S, Nishikawa N, Nomura H, Hori K, Tokunaga H,
et al: Efficacy and safety of minimally invasive surgery versus
open laparotomy for epithelial ovarian cancer: A systematic review
and meta-analysis. Gynecol Oncol. 190:42–52. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zeng S, Yu Y, Cui Y, Liu B, Jin X, Li Z
and Liu L: Efficacy and safety of minimally invasive surgery versus
open laparotomy for interval debulking surgery of advanced ovarian
cancer after neoadjuvant chemotherapy: A systematic review and a
meta-analysis. Front Oncol. 12:9002562022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pereira A, Magrina JF, Magtibay PM, Neto
JS, Siufi DFS, Chang YH and Perez-Medina T: Does MIS play a role in
the treatment of advanced ovarian cancer? Cancers (Basel).
14:35792022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mori K, Hoppenot C, Helenowski I, Berry E,
Lurain J and Neubauer N: Minimally invasive surgery versus
laparotomy for interval cytoreduction after neoadjuvant
chemotherapy for ovarian cancer. Gynecol Oncol. 137:128–130. 2015.
View Article : Google Scholar
|
|
92
|
Alletti SG, Petrillo M, Vizzielli G,
Bottoni C, Nardelli F, Costantini B, Quagliozzi L, Gallotta V,
Scambia G and Fagotti A: Minimally invasive versus standard
laparotomic interval debulking surgery in ovarian neoplasm: A
single-institution retrospective case-control study. Gynecol Oncol.
143:516–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hayek J: Dr Hayek on Laparoscopic vs. Open
Surgery in Advanced Ovarian Cancer. SGO Annual Meeting; San Diego,
CA: 2024
|
|
94
|
Vergote I, Marquette S, Amant F, Berteloot
P and Neven P: Port-site metastases after open laparoscopy: A study
in 173 patients with advanced ovarian carcinoma. Int J Gynecol
Cancer. 15:776–779. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Nitecki R, Rauh-Hain JA, Melamed A,
Scambia G, Pareja R, Coleman RL, Ramirez PT and Fagotti A:
Laparoscopic cytoreduction after neoadjuvant ChEmotherapy (LANCE).
Int J Gynecol Cancer. 30:1450–1454. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ledermann JA, Matias-Guiu X, Amant F,
Concin N, Davidson B, Fotopoulou C, González-Martin A, Gourley C,
Leary A, Lorusso D, et al: ESGO-ESMO-ESP consensus conference
recommendations on ovarian cancer: Pathology and molecular biology
and early, advanced and recurrent disease. Ann Oncol. 35:248–266.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Colombo N, Gadducci A, Sehouli J, Rulli E,
Mäenpää J, Sessa C, Montes A, Ottevanger NB, Berger R, Vergote I,
et al: INOVATYON/ENGOT-ov5 study: Randomized phase III
international study comparing trabectedin/pegylated liposomal
doxorubicin (PLD) followed by platinum at progression vs.
carboplatin/PLD in patients with recurrent ovarian cancer
progressing within 6–12 months after last platinum line. Br J
Cancer. 128:1503–1513. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Di Donato V, Giannini A, D'Oria O, Schiavi
MC, Di Pinto A, Fischetti M, Lecce F, Perniola G, Battaglia F,
Berloco P, et al: Hepatobiliary disease resection in patients with
advanced epithelial ovarian cancer: Prognostic role and optimal
cytoreduction. Ann Surg Oncol. 28:222–230. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu J, Berchuck A, Backes FJ, Cohen J,
Grisham R, Leath CA, Martin L, Matei D, Miller DS, Robertson S, et
al: NCCN Guidelines® Insights: Ovarian Cancer/Fallopian
tube cancer/primary peritoneal cancer, version 3.2024. J Natl Compr
Canc Netw. 22:512–519. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Asante DB, Calapre L, Ziman M, Meniawy TM
and Gray ES: Liquid biopsy in ovarian cancer using circulating
tumor DNA and cells: Ready for prime time? Cancer Lett. 468:59–71.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shiao MS, Chang JM, Lertkhachonsuk AA,
Rermluk N and Jinawath N: Circulating exosomal miRNAs as biomarkers
in epithelial ovarian cancer. Biomedicines. 9:14332021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rivera-Piza A, Lee SH, Lee HH, Lee S, Shin
SJ, Kim J, Park JH, Yu JE, Lee SW, Park G, et al: Real-Time,
AI-Guided photodynamic laparoscopy enhances detection in a rabbit
model of peritoneal cancer metastasis. Cancer Sci. 116:966–975.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Fu C, Zhang B, Guo T and Li J: Imaging
evaluation of peritoneal metastasis: Current and promising
techniques. Korean J Radiol. 25:86–102. 2024. View Article : Google Scholar : PubMed/NCBI
|