Introduction
Epithelial ovarian cancer (EOC) is the primary cause
of mortality from gynecological malignancies and the sixth most
common cause of cancer-related fatalities overall in the Western
world. Women face a 1 in 70 to 80 a lifetime risk of developing EOC
and a chance of 1 in 90 to 100 of succumbing to this illness
(1). Currently, estimates indicate
that ~90% of these neoplasms originate from the ovary, while 5–7%
originate from the fallopian tubes. Non-epithelial ovarian tumors,
developed from stromal or germ cell neoplasms, make up the
remaining 3–5% (2). Ovarian cancer
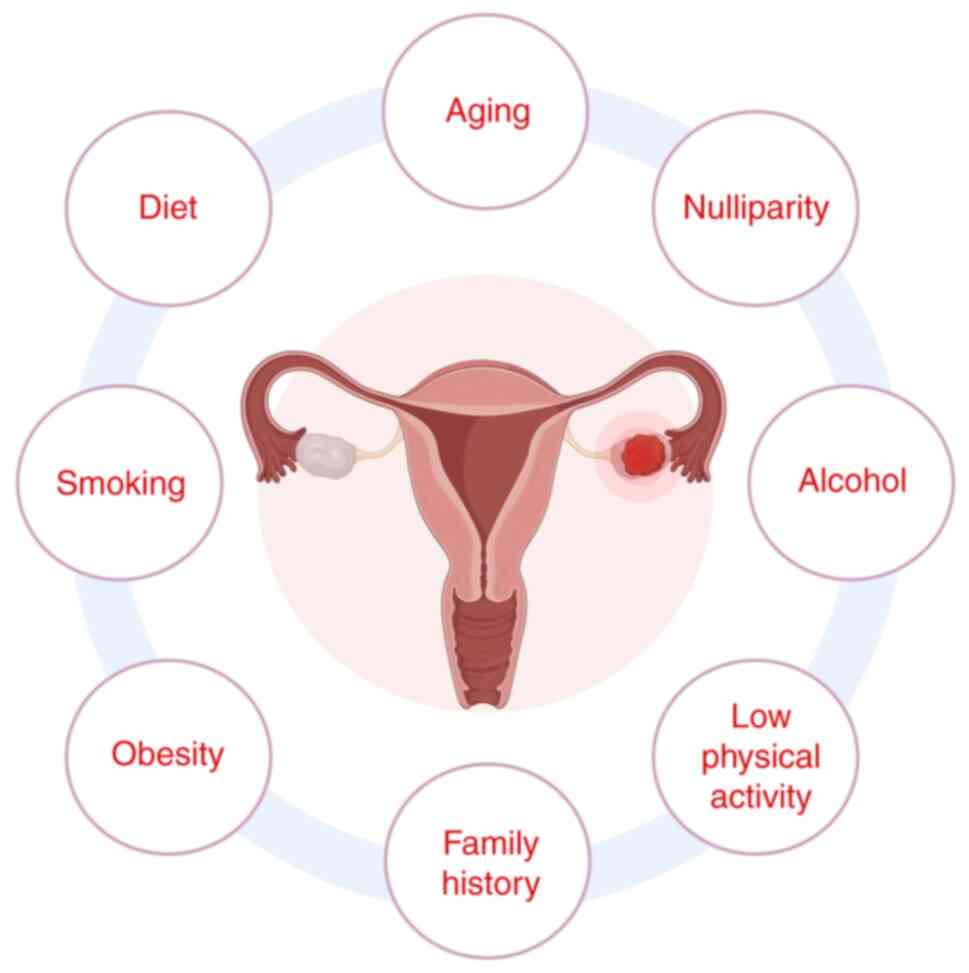
is a complex illness shaped by genetic, hormonal and environmental
influences. Principal risk factors encompass BRCA mutations,
nulliparity, early menarche, late menopause, hormone replacement
treatment, obesity and lifestyle choices (1) (Fig.
1). Although several studies indicated that dietary and
environmental exposures may serve a role in illness development,
causal associations remain ambiguous (3–8). The
clinical management of EOC presents considerable hurdles, as the
majority of cases are discovered at an advanced stage, hence
restricting therapy choices. Surgical intervention is fundamental
to therapy, with cytoreductive surgery markedly enhancing survival
rates. However, reconciling oncological results with minimally
invasive techniques (MITs) continues to be a topic of ongoing
research, especially in advanced-stage illness when optimum
cytoreduction is more challenging to attain (9). Innovations in surgical methodologies
seek to reduce patient stress while maintaining oncological safety;
nonetheless, the long-term advantages of minimally invasive surgery
(MIS) in EOC necessitate further research (10,11).
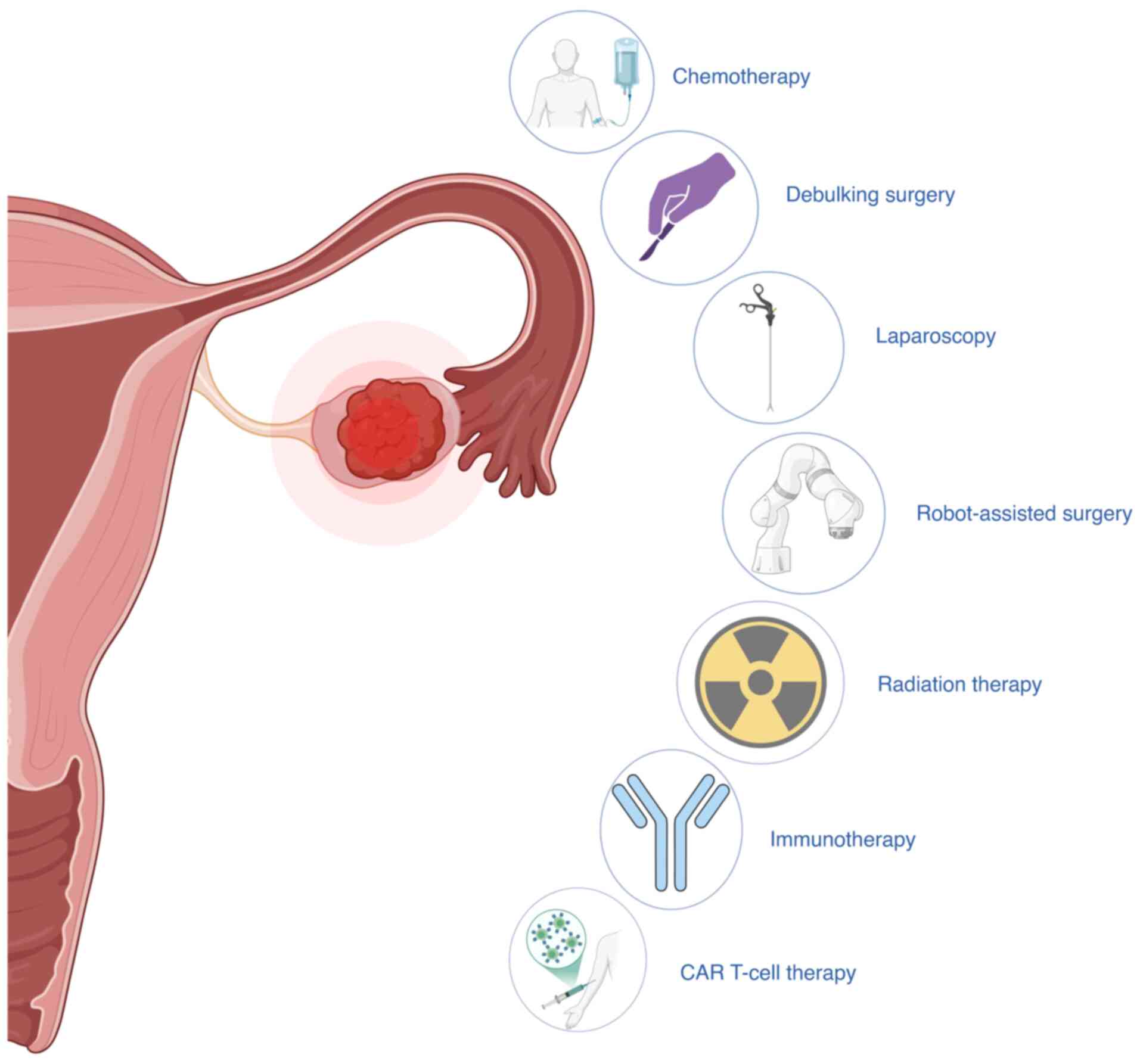
A multimodal approach is used to treat EOC, which
includes surgery and chemotherapy, with the specifics changing
based on the stage of the disease and the type of tissue involved.
The principal intervention is cytoreductive surgery, as achieving a
low residual tumor burden markedly associates with improved
progression-free and overall survival rates. In early-stage cancer,
surgery succeeded by platinum-based chemotherapy yields marked
results, whereas in advanced-stage disease, surgical cytoreduction
in conjunction with systemic treatment is the standard of care
(12,13).
Chemotherapy, primarily with platinum-taxane
regimens, continues to be the cornerstone of systemic treatment
(3,14). Novel targeted medicines, such as
poly(ADP-ribose) polymerase (PARP) inhibitors for BRCA-mutated
cancer types and angiogenesis inhibitors such as bevacizumab, have
broadened treatment alternatives; yet, therapeutic resistance
remains a notable concern (15,16).
Radiotherapy is infrequently employed in cases of EOC outside of
palliative care, given that its toxicity frequently surpasses its
advantages due to the low radiosensitivity of EOC. Current research
seeks to enhance therapy approaches by incorporating innovative
medicines and enhancing patient selection to increase treatment
effectiveness while reducing unwanted effects.
The present review was conducted as a narrative
review, with the aim of synthesizing recent advances in minimally
invasive surgical techniques and their integration with systemic
therapies in the management of EOC. A comprehensive literature
search was performed across the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Scopus (https://www.scopus.com/) and Web of Science
(https://www.webofscience.com/)
databases. The search focused on English-language peer-reviewed
publications from January 2009 to April 2024. The inclusion
criteria encompassed randomized controlled trials (RCTs),
prospective and retrospective clinical studies, large cohort
analyses, meta-analyses and relevant consensus guidelines
associated with surgical management, systemic therapies and their
integration in EOC. Studies focusing on non-surgical interventions,
preclinical or animal models or other types of gynecological cancer
were excluded. Titles and abstracts of 114 article were screened
and full texts of relevant articles were reviewed to ensure
alignment with the scope of the present review. The Scale for the
Assessment of Narrative Review Articles guidelines were followed to
enhance the methodological rigor and transparency of the present
narrative synthesis (17).
Surgical techniques in minimally invasive
management
The notion of MIS cancer cytoreduction was first
presented through the evaluation of two non-randomized studies that
indicated the non-inferiority of laparoscopy regarding
progression-free survival (PFS). A randomized phase III trial was
subsequently reported, which did not demonstrate non-inferiority in
overall survival for patients who had debulking by the minimally
invasive surgical strategy compared with open surgery (18,19).
Retrospective studies have reported an absence of drawbacks for the
MIS approach in terms of PFS and the finding of decreased
postoperative pain and tolerance to chemotherapy (20–22).
There are differences in study design and execution
between two major prospective randomized phase III trials that
investigated the MIS approach, such as operative duration and
center experience (19). The
different results have been attributed to various factors, such as
the longer duration and bleeding that come with laparoscopy and
that almost all of the participating centers have only performed a
few procedures each year, indicating less experience with the
procedure, which could negatively impact the outcomes. Regarding
the International Federation of Gynecology and Obstetrics (FIGO)
stage IA disease (23), the concept
of keeping the uterus stable during minimal surgical changes makes
the MIS approach more likely to be used. This could facilitate more
straightforward and standardized minimally invasive procedures for
complex surgeries. Further recommendations for patient selection
criteria, standardized surgical protocols and intraoperative safety
measures may emerge from analyses performed by skilled surgical
teams (24).
Laparoscopy
Imaging methods often cannot differentiate between
the different kinds of adnexal masses on their own, when it comes
to functional and benign variants. Accurately predicting the
presence of ovarian cancer and the need for surgery can both be
improved with laparoscopy. This method also helps in making a
correct diagnosis before surgery. Laparoscopy is an improved
technique as it helps reduce the need for individuals to undergo
unnecessary surgery to treat functional and benign ovarian cysts
(25,26). A histology examination of the tumor
and its implants typically offer additional diagnostic insights,
along with information regarding the stage of disease. Laparoscopy
facilitates the sampling of purulent peritoneal effusion for
culture testing, which aids in the identification of any
accompanying infection and in determining the appropriate surgical
approach (20).
In the context of malignant diseases, prioritizing
the complete removal of the primary tumor is key in ovarian cancer
as well. The primary objective of oncologists and gynecologists is
to ensure the safety of the patient; therefore, it is essential to
maintain a pathway for the potential conversion of laparoscopy to
laparotomy at any given time. Furthermore, depending on the
experience of the surgical oncologist, MITs such as laparoscopy can
provide patients with adnexal masses of unclear origin the same
diagnostic and therapeutic surgical staging benefits as laparotomy
(21). Furthermore, the prevention
of notable surgical procedures and complications can lead to a more
efficient recovery process for the patient. These factors enhance
the quality of life (QoL) and potentially the self-esteem of
patients while reducing their costs (21,27,28).
Robot-assisted surgery
In EOC surgery, the robotic system enhances
precision and dexterity of the surgeon, enabling fine dissection
and removal of primary tumors, targeted omentectomy and systematic
lymphadenectomy through small ports with three-dimensional
magnified visualization (29).
Robotic-assisted surgery is particularly crucial for patients with
cardiovascular, hepatic, renal or pulmonary comorbidities, as it
minimizes physiological stress by reducing intraoperative blood
loss, shortening operative time and maintaining stable
intra-abdominal pressures, thereby lowering cardiopulmonary
complication rates. In women aged 18–40 years who desire fertility
preservation, combining laparoscopy with robotic precision enables
accurate tumor resection, targeted omentectomy and unilateral
salpingo-oophorectomy while sparing the contralateral ovary
(29). Multiple retrospective
series have demonstrated that robotic cytoreduction reduces
hospital stays to an average of 2–3 days and decreases 90-day
morbidity compared with open surgery, driving its increasing
adoption among gynecological oncologists (21,28,30,31).
A 2-year retrospective observational study was
conducted, analyzing data on women diagnosed with ovarian cancer
and compared outcomes according to their surgical methods (29,30).
Robot-assisted surgery enables precise pelvic and para-aortic
lymphadenectomy, including dissection of nodal stations enriched
with hematopoietic progenitor cell niches, thereby improving
staging accuracy while minimizing collateral tissue trauma. This
allows the provision of gonadotoxic treatments to these patients,
which protects their ability to have children. The robot-assisted
surgical approach has been employed to perform systematic pelvic
and para-aortic lymphadenectomy, enabling accurate staging of
ovarian cancer and facilitating evaluation of the therapeutic
impact of varying lymph node harvests (31). Robotic surgery methods may help
identify high-risk patients who can have a full lymphadenectomy and
still receive fertility-preserving treatment. The growing adoption
of MITs in ovarian cancer surgery highlights various benefits,
including enhanced patient satisfaction and potential cost savings
associated with these approaches.
Molecular-based therapies in conjunction
with MITs
The clinical management of ovarian carcinoma poses
significant challenges for oncology professionals due to the
typically late-stage presentation of the disease, the high
propensity for peritoneal dissemination and the complexity in
achieving complete cytoreduction. According to the National
Comprehensive Cancer Network guidelines for ovarian cancer, optimal
management of advanced-stage EOC requires maximal cytoreductive
surgery, preferably achieving no macroscopic residual disease,
followed by platinum- and taxane-based chemotherapy, with
consideration of neoadjuvant chemotherapy (NACT) and interval
debulking in selected patients. Minimally invasive surgical
techniques in EOC allow optimal cytoreduction with outcomes
comparable to open surgery, while significantly reducing
perioperative morbidity and accelerating recovery. This faster
recovery enables earlier initiation of adjuvant therapy, so
patients can promptly receive novel systemic treatments that
improve disease control (27). This
faster recovery enables earlier initiation of adjuvant therapy, so
patients can promptly receive novel systemic treatments that
improve disease control (32).
Additionally, integrating immunotherapy (for example, checkpoint
inhibitors) is an emerging strategy, combination approaches, such
as a PARP inhibitor plus programmed cell death protein (PD)-1
blockade, have demonstrated higher response rates in refractory
ovarian cancer compared with either modality alone (33). In current practice, the combination
of MIS and these targeted therapies translates into improved
clinical outcomes: Patients experience shorter hospital stays,
improved treatment responses and extended remissions, which
demonstrate that surgical innovation complements systemic advances
without compromising oncological efficacy (32).
In EOC, combining MIS with novel systemic therapies
(PARP inhibitors or anti-angiogenic agents such as bevacizumab and
emerging immunotherapies) is shaping a more effective, multimodal
treatment paradigm. MIS (for example, laparoscopic or robotic
debulking) offers well-documented perioperative advantages over
open surgery, including shorter hospital stays, reduced blood loss
and fewer complications, which translate into faster recovery and
allows patients to proceed more swiftly to adjuvant systemic
therapy. This expedited post-surgery recovery means maintenance
treatments such as PARP inhibitor therapy or anti-angiogenic agents
can be initiated without undue delay, which maximizes their impact
(21,27,32,34,35).
By contrast, systemic therapies can shrink or control tumor burden
(for instance, NACT is often combined with bevacizumab in trials),
which thereby increases the likelihood that a subsequent
cytoreduction can be successfully performed via MIS. This
bidirectional synergy ensures each modality enhances the other:
Effective preoperative systemic therapy can render disease more
resectable for minimally invasive surgeons, while the lower
surgical morbidity of MIS preserves patient performance status for
the continuation of systemic treatments (27,36).
Clinical studies and outcomes underscore the value of this
integrated approach. The ongoing phase III Laparoscopic
Cytoreduction After Neoadjuvant Chemotherapy (LANCE) trial is
directly evaluating MIS vs. open laparotomy after NACT in advanced
EOC, reflecting the effort to confirm that a less invasive surgical
approach can achieve equivalent oncological outcomes (32,35)(Fig.
2).
The benefit of coupling surgery with targeted
post-operative therapy has also been reported, for example, in the
PAOLA-1 trial, patients who received standard surgery and
chemotherapy followed by maintenance olaparib (a PARP inhibitor)
plus bevacizumab had markedly prolonged PFS (22.1 months vs. 16.6
months with bevacizumab alone). This finding led to regulatory
approval of the olaparib-bevacizumab combination and illustrates
how surgery complemented by novel agents can improve patient
outcomes (35). Immunotherapy is
also being explored in combination with these modalities; although
checkpoint inhibitors alone have demonstrated limited efficacy in
EOC, integrating them with other treatments may unlock synergistic
effects. Preclinical evidence suggested that debulking surgery can
enhance the impact of immunotherapy by reducing immunosuppressive
tumor burden (for example, decreasing myeloid suppressor cells and
improving T-cell trafficking) (36)
and early-phase studies indicated that PARP inhibitors combined
with PD-1/PD-ligand 1 (L1) checkpoint blockade yield additive
antitumor activity in ovarian cancer (37). Clinical guidelines now advocate an
integrated, individualized approach to EOC management. The National
Comprehensive Cancer Network recommends offering minimally invasive
surgery for surgical staging in early-stage disease (FIGO I–IIA) or
for interval cytoreduction following neoadjuvant platinum-taxane
chemotherapy when key criteria are met: i) Disease confined to the
pelvis or superficial peritoneum without extensive diaphragmatic,
mesenteric, hepatic or splenic involvement; ii) at least a 50%
reduction in tumor volume on imaging to facilitate complete gross
resection via laparoscopy or robotics; iii) confirmation by
preoperative imaging or diagnostic laparoscopy that R0
cytoreduction is achievable without multiorgan resections; and iv)
patient performance status of ECOG 0–1 with well-controlled
comorbidities and anatomy suitable for minimally invasive access
(38,39). Concurrently, American Society of
Clinical Oncology/European Society for Medical Oncology consensus
guidelines endorse maintenance targeted therapy in patients with
advanced (FIGO III–IV) ovarian cancer who respond to first-line
platinum-taxane chemotherapy, specifying olaparib maintenance in
BRCA-mutated patients based on SOLO-1, niraparib maintenance in
BRCA wild-type or homologous recombination deficiency
(HRD)-positive patients per PRIMA, and the combination of olaparib
plus bevacizumab maintenance in HRD-positive cases according to
PAOLA-1 (27,35,40–42).
Together, these strategies create a cohesive treatment plan:
Maximal tumor reduction through surgery (increasingly via MIS to
minimize patient burden) followed by tailored systemic therapy to
eradicate microscopic disease and suppress recurrence. This
holistic approach has a compounding positive effect on patient
outcomes, which yields longer remissions and potentially improved
survival, while also improving QoL by reducing surgical trauma and
leveraging more precise, effective medications (27,32,35).
In summary, MIS and novel systemic therapies are not competing
alternatives but complementary approaches in the treatment of
ovarian cancer, as each reinforces the efficacy of the other, which
results in a synergistic improvement in clinical results for
patients with EOC.
Targeted therapies
Targeted therapies are fundamental in ovarian cancer
management as they are designed to interfere with specific
molecular pathways that cancer cells rely on for growth, survival
and proliferation, distinguishing them from traditional
chemotherapy agents such as carboplatin, paclitaxel and
doxorubicin, which broadly target all rapidly dividing cells.
Unlike conventional chemotherapy, which causes damage to both
malignant and normal cells leading to significant side effects such
as neutropenia, alopecia, mucositis and fatigue, targeted therapies
such as PARP inhibitors (including olaparib and rucaparib),
anti-angiogenic agents (including bevacizumab) and folate receptor
α inhibitors (including mirvetuximab), specifically attack pathways
activated only in cancer cells, resulting in fewer off-target
effects. These therapies work by inducing apoptosis through
disruption of cancer-specific signaling pathways, including
homologous recombination repair pathways and tumor angiogenesis
processes, which suppress tumor growth and promote cancer cell
death. Furthermore, predictive biomarkers such as BRCA mutations
and HRD status enable clinicians to identify patients who are most
likely to benefit from these targeted treatments, providing a
personalized approach that enhances efficacy (35,43–45).
Nonetheless, the emergence of drug resistance
presents a notable limitation. A potentially more effective
strategy may involve a combination therapy utilizing ≥2 targeted
agents or integrating chemotherapy with hormone therapy alongside a
targeted agent to enhance sensitivities (46). The foundation of these approaches
relies on current understanding of the molecular pathways that
serve a role in the development and progression of cancer. In the
past decade, research on EOC has highlighted mutations or
amplifications in specific genes that indicate the involvement of
pathways associated with DNA damage repair, apoptosis, cell cycle
control and anti-angiogenesis. The present review underscores the
necessity for innovative pharmaceuticals and the initiation of
clinical trials to explore therapeutic alternatives.
PARP inhibitors
In recent years, PARP inhibitors have emerged as a
prominent class of targeted therapies in EOC (32,33,47).
These agents act by disrupting the DNA repair process,
specifically, the repair of single-strand DNA breaks. When PARP is
inhibited, these breaks evolve into double-strand DNA damage, which
cannot be effectively repaired in cancer cells harboring BRCA1/2
mutations, which leads to cell death via synthetic lethality
(37,46,48).
Currently, three PARP inhibitors, olaparib, rucaparib and
niraparib, are approved by the Food and Drug Administration for
ovarian cancer treatment, based on a number of pivotal trials
(40,41,49).
For instance, the SOLO-1 trial demonstrated a
notable increase in PFS from 13.8 to 56 months in newly diagnosed
patients with BRCA-mutated EOC receiving olaparib as maintenance
therapy [Hazard ratio (HR), 0.30; P<0.001] (40). The PRIMA trial extended this benefit
to a broader population, which demonstrated improved PFS from 8.2
to 13.8 months with niraparib in both BRCA and non-BRCA advanced
cases (HR, 0.62; P<0.001) (41).
In recurrent BRCA-mutated EOC, the ARIEL3 trial confirmed the
efficacy of rucaparib, with PFS increasing from 5.4 to 10.8 months
(HR, 0.36; P<0.0001) (49). The
VELIA trial assessed veliparib added to first-line chemotherapy and
as maintenance, which reported an increase in PFS from 17.3 to 23.5
months (HR, 0.68; P<0.001), which supports the role of PARP
inhibitors in newly diagnosed EOC (50). Notably, the PAOLA-1 trial evaluated
the combination of olaparib with bevacizumab in HRD-positive
patients, which demonstrated improved PFS from 16.6 to 22.1 months
(HR, 0.59; P<0.001), which suggested potential synergy between
DNA repair inhibition and anti-angiogenic therapy (35). Together, these trials establish the
clinical efficacy of PARP inhibitors across different settings:
BRCA-mutated, HRD-positive and BRCA wild-type subpopulations. While
resistance mechanisms and optimal combinations remain areas of
active research, ongoing research continues to explore the role of
PARP inhibitors in frontline and maintenance therapy (Table I). These results have firmly
established PARP inhibitors, particularly olaparib and niraparib,
as standard of care in maintenance therapy for EOC.
 | Table I.Efficacy of targeted therapies in
improving PFS across patient populations with ovarian cancer. |
Table I.
Efficacy of targeted therapies in
improving PFS across patient populations with ovarian cancer.
| A, PARP
inhibitors |
|---|
|
|---|
| First author,
year | Clinical trial | Drug | NCT no. | Clinical trial
subject/design | Control to
treatment PFS improvement, months | HR (95% CI) | P-value | (Ref.) |
|---|
| Moore et al,
2018 | SOLO-1 | Olaparib | NCT01844986 | Newly diagnosed
BRCA-mutated EOC | 13.8–56 | 0.3 (0.2–0.4) | <0.001 | (40) |
| Psomiadou et
al, 2021 | PRIMA | Niraparib | NCT02655016 | Advanced ovarian
cancer (BRCA and non-BRCA) | 8.2–13.8 | 0.6 (0.5–0.7) | <0.001 | (29) |
| Abitbol et
al, 2019 | ARIEL3 | Rucaparib | NCT01968213 | Recurrent
BRCA-mutated EOC | 5.4–10.8 | 0.3 (0.3–0.4) | <0.0001 | (30) |
| Chen et al,
2023 | VELIA | Veliparib | NCT02470585 | Newly diagnosed
EOC | 17.3–23.5 | 0.6 (0.5–0.8) | <0.001 | (31) |
| Baum et al,
2022 | PAOLA-1 | Olaparib +
bevacizumab | NCT02477644 | HRD-positive
ovarian cancer | 16.6–22.1 | 0.6 (0.6–0.7) | <0.001 | (24) |
|
| B, Angiogenesis
inhibitors |
|
| First author,
year | Clinical
trial | Drug | NCT no. | Clinical trial
subject/design | Control to
treatment PFS improvement, months | HR (95%
CI) | P-value | (Ref.) |
|
| Rauh-Hain et
al, 2024 | GOG-0218 | Bevacizumab | NCT00262847 | Advanced ovarian
cancer (first-line) | 10.3–14.1 | 0.7 (0.6–0.8) | <0.001 | (9) |
| Ray-Coquard et
al, 2023 | ICON7 | Bevacizumab | NCT00483782 | Newly diagnosed
advanced ovarian cancer | 17.3–19 | 0.8 (0.7–0.9) | 0.004 | (32) |
| Zhao et al,
2024 | OCEANS | Bevacizumab | NCT00434642 | Platinum-sensitive
recurrent ovarian cancer | 8.4–12.4 | 0.5 (0.4–0.6) | <0.0001 | (33) |
| Nezhat et
al, 2024 | AGO-OVAR16 | Pazopanib | NCT00484442 | Advanced ovarian
cancer (maintenance) | 12.3–17.9 | 0.8 (0.6–0.9) | 0.0021 | (34) |
|
| C, Tyrosine
kinase inhibitors |
|
| First author,
year | Clinical
trial | Drug | NCT no. | Clinical trial
subject/design | Control to
treatment PFS improvement, months | HR (95%
CI) | P-value | (Ref.) |
|
| Ray-Coquard et
al, 2019 | ALTER-GO-010 | Anlotinib | NCT02773524 | Newly diagnosed
advanced ovarian cancer: A phase II, single-arm | 6 months, 100%; 9
months, 100% | 5.3 (3.7–8) | Final median PFS
not yet reached due to ongoing follow-up | (35) |
| Predina et
al, 2012 | MITO 11 | Pazopanib +
Paclitaxel | NCT01335226 |
Platinum-resistant/refractory advanced
ovarian cancer | 3.5–6.3 | 0.4 (0.2–0.7) | 0.0002 | (36) |
| Xiao et al,
2024 | NCT03367741 | Cabozantinib +
Nivolumab |
| Recurrent
endometrial cancer | 1.9–5.3 | 0.6 (90% CI,
0.3–1) | 0.09 | (37) |
Angiogenesis inhibitors
Angiogenesis serves a key role in ovarian cancer
progression, which supports tumor growth and metastasis by
establishing a dedicated blood supply. Targeting this pathway has
yielded promising therapeutic strategies, particularly with
bevacizumab, a monoclonal antibody against VEGF and pazopanib, a
multi-targeted angiogenesis inhibitor (16,51).
In the GOG-0218 trial, bevacizumab added to standard
first-line chemotherapy extended PFS from 10.3 to 14.1 months (HR,
0.717; P<0.001), which highlighted its benefit in newly
diagnosed advanced ovarian cancer (16). Similarly, the ICON7 trial
demonstrated a modest but notable improvement in PFS (17.3–19
months) in patients receiving bevacizumab with chemotherapy (HR,
0.81; P=0.004) (52). In the
recurrent setting, the OCEANS trial confirmed the benefit of
bevacizumab in platinum-sensitive relapse, with PFS rising from 8.4
to 12.4 months (HR, 0.484; P<0.0001) (53). For maintenance therapy, the
AGO-OVAR16 study evaluated pazopanib and observed a PFS extension
from 12.3 to 17.9 months in advanced ovarian cancer (HR, 0.77;
P=0.0021) (51).
In recurrent settings, the OCEANS trial demonstrated
that adding bevacizumab to chemotherapy improved PFS from 8.4 to
12.4 months (HR, 0.484; P<0.0001) in platinum-sensitive
relapses. Similarly, the AGO-OV/AR16 study demonstrated that
pazopanib maintenance extended PFS from 12.3 to 17.9 months in
advanced ovarian cancer (HR, 0.77; P=0.0021). Despite these
positive results, resistance to anti-angiogenic therapy remains a
challenge and future directions may involve biomarker-guided
patient selection or combination regimens with immunotherapy or
PARP inhibitors (Table I). While
bevacizumab is incorporated into standard regimens based on
GOG-0218 and ICON7, other agents such as pazopanib warrant further
investigation.
Tyrosine kinase inhibitors (TKIs)
TKIs represent a diverse class of targeted agents
designed to interfere with signaling pathways that mediate tumor
cell proliferation, survival, angiogenesis and invasion (54–56).
TKIs block multiple oncogenic signaling cascades that contribute to
ovarian cancer growth and spread. By inhibiting the VEGFR and
platelet-derived growth factor receptor, TKIs disrupt angiogenesis
and stromal support for tumor proliferation, while blockade of
fibroblast growth factor receptor signaling further impairs
neovascularization and cell survival. In addition, inhibition of
the hepatocyte growth factor/c-MET axis reduces tumor cell motility
and invasion, and targeting other kinases such as AXL and RET
overcome resistance mechanisms and attenuates survival pathways in
cancer cells (57–59). Several TKIs have been investigated
in ovarian and endometrial cancer types, with growing evidence
supporting their utility, especially in combination strategies. For
example, anlotinib, a multi-targeted TKI assessed in the
ALTER-GO-010 phase II trial for newly diagnosed advanced ovarian
cancer. This single-arm study reported a 100% PFS at both 6 and 9
months, with a median PFS of 5.36 months (95% CI, 3.68–7.98),
although final PFS was not yet reached due to ongoing follow-up
(54,60). The MITO 11 study, while primarily
assessing anti-angiogenesis, also demonstrated the efficacy of
pazopanib, which possesses TKI activity. Pazopanib combination with
paclitaxel yielded notable improvements in PFS in a
platinum-resistant/refractory cohort (55). A novel combination of a TKI and
immunotherapy was explored in trial NCT03367741, which evaluated
cabozantinib plus nivolumab in recurrent endometrial cancer.
Patients receiving the combination had a PFS of 5.3 vs. 1.9 months
with nivolumab alone (HR, 0.59; P=0.09) (56). Although these trials focused on
endometrial compared with ovarian cancer, these findings highlight
the expanding landscape of TKI-based combinations in gynecological
oncology.
Furthermore, in the NCT03367741 study, the
combination of cabozantinib and nivolumab was investigated in
recurrent endometrial cancer, which indicated a PFS improvement
from 1.9 to 5.3 months (HR, 0.59), which suggests potential synergy
between TKIs and immune checkpoint inhibitors, although these
findings remain preliminary and warrant further exploration in
ovarian cancer (56). Overall,
while TKIs alone have demonstrated modest efficacy, their
integration into combinatorial regimens, especially with immune
checkpoint inhibitors, may offer enhanced therapeutic outcomes.
Ongoing trials continue to assess the optimal positioning of TKIs
in ovarian cancer treatment, including in front-line, maintenance
and recurrent settings (Table I).
TKI-based combinations, such as those explored in ALTER-GO-010 and
NCT03367741, remain investigational and require validation through
larger trials.
Immunotherapeutic strategies in EOC
Vaccines
Several types of vaccines have been investigated in
EOC, most containing proteins or peptides that are associated with
ovarian cancer. Examples of tumor-associated antigens targeted in
ovarian cancer vaccines include MUC1, cancer-testis antigen
NY-ESO-1, WT1, folate receptor α and mesothelin (61). Numerous studies have seen T-cell
responses or longer disease stability in patients who are getting
treatment; however, not all of these studies demonstrated the same
benefits (62–64). The initial vaccine evaluated in EOC
and the only vaccine assessed in a randomized phase III trial was
canvaxin. Autologous tumor cell lysates were changed to add a novel
glycosylation site to the mucin 1 protein, which is a known antigen
located in ovarian tumors. The vaccine generated antibody responses
in 88% of vaccinated patients and seemed to improve tolerance
compared with the chemotherapy recommended by the physician in a
selected group of women. Eventually, this trial had to be
discontinued because the tumors were not under control (65–67).
However, canvaxin was still investigated in other studies as an
adjuvant, with and without chemotherapy, specifically in patients
with EOC (68,69). Several recent clinical trials have
evaluated novel antigen-targeted vaccines in EOC (61). The phase I study of folate
receptor-α peptide vaccine in patients with advanced ovarian cancer
demonstrated induction of FRα-specific CD8+ T-cell
responses in 90% of participants and disease stabilization in 40%
at 6 months. A multicenter phase II trial of the MUC1-liposomal
vaccine tecemotide added to neoadjuvant platinum-taxane
chemotherapy reported enhanced MUC1-specific CD4+ and
CD8+ T-cell responses in 75% of patients, with a
12-month PFS of 65% compared with 50% historical controls. These
treatments demonstrate the potential to prepare women for
subsequent therapy with immune checkpoint inhibitors (61,67).
Cell-based interventions
Adoptive transfer of tumor-infiltrating lymphocytes
and engineered CAR-T cells targeting mesothelin, HER2 or folate
receptor α have demonstrated potent antigen-specific cytotoxicity
and clinical activity in early-phase trials of EOC, especially for
antibody-coated T cells, chimeric antigen receptor T cells (CAR-T),
specifically chosen tumor-infiltrating lymphocytes or those that
have been engineered to express a tumor-specific T-cell receptor
(70–72). By using genetic engineering to
change the T cells of a patient, CAR-T therapies make it possible
for these cells to express specific receptors that help find and
kill cancer cells. CAR molecules have an antigen-binding domain
that is outside of cells, a transmembrane domain and an
intracellular signaling domain that activates more co-stimulatory
receptors after interacting with the target epitope (73,74).
Therefore, CAR-Ts demonstrate specificity for a certain antigen
while also being less dependent on HLA expression. CAR-T therapies
have evolved in recent years and gaining attention in the field.
The anti-CD19 therapy has demonstrated potential in treating
patients with B-cell malignancies (75). Concerning EOC, CAR-T-based
approaches have been created to target different antigens
associated with tumors, such as mesothelin, HER2 and folate
receptor α. These treatment approaches have demonstrated potential
in early-stage clinical trials (76–79).
Immune checkpoint inhibitors
Immune checkpoint inhibitors have transformed the
treatment landscape for multiple malignancies, including metastatic
melanoma, non-small cell lung cancer and renal cell carcinoma. By
blocking receptors such as PD-1 on T cells or ligands like PD-L1 on
tumor cells, these agents prevent inhibitory signaling that
normally dampens T-cell activation. Specifically, PD-1/PD-L1
blockade restores T-cell receptor signaling, leading to increased
proliferation of effector T cells, enhanced cytokine production and
sustained antitumor cytotoxicity. The goal of anti-PD-1/PD-L1
therapies was to revive lymphocytes that had been eliminated by
advanced tumors. Anti-PD-1/PD-L1 therapies demonstrated efficacy
for several types of cancer that exhibited HLA-antigen
presentation; therefore, the demonstrating potential for these
types of drugs to help treat EOC. The effectiveness of these drugs
could be increased by combining them with other treatments, such as
VEGF inhibitors, PARP inhibitors or platinum-based chemotherapy
(64,80). The efficacy of both anti-PD-1 and
anti-PD-L1 antibodies have been assessed in patients with EOC.
Anti-PD-1/PD-L1 monotherapy in EOC has produced modest clinical
benefits. In the phase II trial of nivolumab in platinum-resistant
ovarian cancer, the objective response rate was 15% and the disease
control rate (responses plus stable disease ≥6 months) was 45%,
with a median PFS of 3.5 months. Similarly, pembrolizumab in the
KEYNOTE-100 study achieved an ORR of 8% and a DCR of 34%, with a
median PFS of 2.1 months, indicating that while a subset of
patients experiences prolonged stabilization, the overall impact on
disease control remains limited (81,82).
As a stand-alone treatment, PD-L1 blockade has had response rates
of <15%. This suggests that blocking the PD-L1/PD-1 interaction
may not be enough to activate and grow T cells, unlike what has
been reported with anti-CTLA-4 therapies. Consequently, further
immunotherapy approaches are currently being investigated (64,80).
Combining targeted therapies with immunotherapy or
chemotherapy holds notable potential in improving outcomes for
patients with EOC. PARP inhibitors, when combined with immune
checkpoint inhibitors, may enhance antitumor immunity by increasing
the tumor neoantigen load and modulating the tumor microenvironment
(37,83). Similarly, combining anti-angiogenic
agents with immunotherapy can normalize tumor vasculature,
promoting enhanced immune cell infiltration. However, these
combinations present challenges, including increased risk of
overlapping toxicities, optimal treatment schedule, dosing
strategies and patient selection. Optimal sequencing refers to the
strategic order and timing of administering each therapy to
maximize synergistic efficacy and minimize cumulative toxicity. In
EOC combinations: For PARP inhibitor + ICI: Sequencing may involve
giving the PARP inhibitor first to induce DNA damage and neoantigen
release, then administering PD-1/PD-L1 blockade at the peak of
antigen presentation to reinvigorate T cells. For anti-angiogenic +
ICI: VEGF inhibition is often delivered prior to checkpoint
blockade to normalize abnormal tumor vasculature and improve
effector T-cell infiltration, followed by PD-1/PD-L1 antibodies to
sustain the immune response (37,84,85).
Furthermore, identifying reliable biomarkers to predict response
remains an unmet need (86).
Ongoing trials are actively exploring these strategies and future
research will be key to refining combination approaches and
determining their place in clinical practice (37).
Clinical outcomes and efficacy of MITs
Over the past few decades, MITs such as laparoscopy
and robotic-assisted surgery have increasingly been incorporated
into the surgical management of EOC. Originally introduced for
early-stage disease, these approaches have expanded into selected
cases of advanced-stage (FIGO III–IV) cancer (9,87).
Previous studies have demonstrated that, in appropriately selected
patients with EOC, MITs can reduce blood loss, shorten hospital
stays and accelerate postoperative recovery, all while maintaining
long-term oncological outcomes comparable to open laparotomy
(27,88). Furthermore, the enhanced
visualization of retroperitoneal spaces during MIS may support more
accurate staging and surgical precision.
However, outcomes associated with MIS depend heavily
on surgeon expertise, institutional experience and careful patient
selection. Integrating MIS into oncological practice requires
gynecological oncologists to master advanced laparoscopic
techniques, particularly when pursuing complete cytoreduction in
high-risk patients. Standardization of surgical protocols,
including safe tumor handling and use of containment systems,
remains essential to minimize the risk of intraoperative tumor
spread. The clinical efficacy of MIS in advanced EOC has been
summarized from cohort studies, meta-analyses and emerging
randomized trial data; the residual disease rates, survival
outcomes, recurrence patterns and real-world implementation across
high-volume centers were also assessed (Table II).
 | Table II.Summary of key comparative studies on
minimally invasive vs. open cytoreductive surgery in advanced-stage
EOC. |
Table II.
Summary of key comparative studies on
minimally invasive vs. open cytoreductive surgery in advanced-stage
EOC.
| First author,
year | Design | Country | Setting | No. of patients
(stage) | Intervention vs.
control | Key findings | (Ref.) |
|---|
| Jorgensen et
al, 2023 | NCDB retrospective
cohort (multicenter). | USA | Interval debulking
after NACT (2013–2018) | 7,897 (stage
III–IV). MIS, 2,021; Open, 5,876 (after matching) | MIS IDS vs. open
IDS (propensity-matched) | OS: Median 46.7
months MIS vs. 41.0 months open (HR, 0.86; 95% CI, 0.79–0.94; no
detriment). 5-year OS: ~38 vs. 35% (ns). Residual disease: R0
achieved in 76% MIS vs. 73% open (MIS had slightly lower residual
rates). Morbidity: 90-day mortality 1.4 vs. 2.5% (P<0.01);
shorter LOS (~3 vs. 5 days). MIS usage rose to ~29% by 2018; cases
had less extensive disease (fewer multi-organ resections). | (27) |
| Alletti et
al, 2016 | Single-institution
case-control | Italy | Interval debulking
(after NACT) | 46 (stage III).
MIS, 23; open, 23 (matched on chemotherapy response) | Laparoscopic IDS
vs. open IDS | OS/PFS: No
significant difference in 5-year OS or PFS between groups (matched
selection). One analysis demonstrated median PFS 18 MIS vs. 12
months open (P<0.05), attributed to selection. Residual disease:
100% R0 in both groups (all patients optimally debulked).
Perioperative: MIS had less blood loss and shorter hospital stay.
Demonstrated feasibility of complete laparoscopy in
responders. | (92) |
| Feuer et al,
2013 | Single-center
retrospective | Israel | Mixed PDS/IDS cases
(stage III–IV) | 89 (advanced EOC).
MIS, 63; open, 26 (open included conversions) | Robotic-assisted
MIS vs. open (mixed timing) | OS/PFS: No
differences in PFS or OS between robotic MIS and open laparotomy.
Residual disease: laparotomy. Residual disease: Comparable R0
rates. All cases were R0 by inclusion (complete gross resection
achieved). More patients in MIS group had received NACT (52 vs. 15%
in open), which indicates selection of responders for MIS.
Demonstrated safety of robotic debulking with similar
outcomes. | (21) |
| Magrina et
al, 2011 | Multi-center
retrospective | Spain and USA | Mixed PDS and IDS
(primary or secondary cytoreduction) | 171 (stage III–IV).
Robot, 25; lap, 27; open, 119 | Robotic MIS vs.
conventional laparoscopy vs. open | OS: No OS
differences among the three groups. Residual disease: R0 was
achieved more often in open surgery (data from one center
demonstrated higher complete resection in laparotomy), but all
groups had high optimal debulk rates (>90%). PFS: MIS groups had
longer PFS compared with open in this series (P<0.05)
Perioperative: MIS had longer OR time in robot group, but much
lower EBL and shorter LOS. Highlighted that extensive disease cases
went open, explaining R0 difference. | (22) |
| Pereira et
al, 2022 | Dual-center
retrospective | Spain and USA | Both PDS and IDS
analyzed (2012–2013) | 89 (stage IIIC-IV
unresectable at diagnosis). IDS, 59 after NACT; PDS, 30 upfront.
Subgroups, MIS vs. open | MIS vs. open at
IDS; also some MIS vs. open PDS | OS: At last
follow-up, survival favored MIS (47.5 MIS vs. 30% open alive in IDS
cohort), although groups differed. No statistically significant OS
disadvantage for MIS in either PDS or IDS. Recurrence: No
difference in recurrence rate between MIS and open. Findings: MIS
was viable for IDS in selected patients without compromising
survival. Significantly shorter hospital stays with MIS (2 days vs.
8 days) and fewer bowel resections at IDS. | (90) |
| Hayek, 2024 | NCDB analysis
subseta; multi-center | USA | Interval R0 cases
only | 2,412 (stage III–IV
IDS with complete gross resection). MIS, 25.9%; open, 74.1% | MIS vs. open
Interval R0 (real-world subset) | OS: No difference
in OS for MIS vs. open R0 debulking (median OS ~51 vs. 46 months;
HR, 1.10; 95% CI, 0.94–1.26; P=0.17). Achieving R0 negated route as
a factor in survival. Morbidity: MIS had shorter hospital stays.
30/90-day mortality and readmission were low and similar between
groups. Other: MIS use increased from ~12% in 2010 to 36.5% in
2019. Open surgery group had more ‘extensive’ procedures (53% vs.
41%; P<0.001). Real-world data from community centers mirrored
high-volume outcomes. | (93) |
Residual disease rates
Achieving minimal or no gross residual disease (R0)
is critical in advanced ovarian cancer. Recent studies indicate
that MIS can attain similar optimal cytoreduction rates as open
surgery in selected patients. A 2024 meta-analysis stratified by
surgery timing reported no notable difference in optimal debulking
rates between MIS and laparotomy for both primary debulking surgery
(PDS) and interval debulking surgery (IDS). In interval debulking
after NACT, the pooled risk ratio for achieving optimal
cytoreduction with MIS was ~1.03 relative to open surgery (95% CI,
0.96–1.11; P=0.05), essentially indicating equivalent success
rates. Similarly, the rate of complete gross resection (no
macroscopic disease) did not differ between MIS and open approaches
(risk ratio, ~1.01; 95% CI, 0.97–1.07) (34). These findings have been similarly
reported by other studies (34,42,89); a
2022 systematic review reported no notable disparity in R0
(complete debulking) or R1 (≤1 cm residual) rates between
laparoscopic/robotic IDS and open IDS (89).
Individual cohort studies reinforce that
well-selected MIS cases can achieve high R0 rates. In the
multi-center MISSION trial (2019), which evaluated 127 patients
undergoing MIS IDS, 96.1% of patients had no gross residual disease
at surgery. The conversion to laparotomy in that series was only
~3.9%, which implied that nearly all intended MIS procedures could
be completed with optimal resection (42). Certain single-center studies have
reported slightly lower complete resection rates with MIS, but
these differences appear to reflect case selection. For example,
Pereira et al (90) observed
a higher R0 rate in their laparotomy group compared with MIS
groups, potentially because surgeons attempted MIS only in cases
with smaller tumor burden. Across numerous reports, when MIS was
chosen for patients with limited disease distribution or marked
chemotherapy response, residual disease outcomes were comparable
with to open surgery (34,89).
Overall survival
Long-term survival outcomes (overall survival and
PFS) have been a central concern when comparing MIS to open
debulking in advanced ovarian cancer. Notably, no RCT has yet
reported survival comparisons, although the phase III LANCE trial
is currently evaluating MIS vs. open laparotomy in this setting
(9,27,91).
However, multiple large observational studies and meta-analyses in
the last 5 years consistently reported no OS disadvantage with MIS
and some even suggest non-inferiority or parity in survival
(34,88,89).
A comprehensive 2023 analysis of nearly 7,900
patients from the U.S. National Cancer Database (https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/)
demonstrated no notable difference in overall survival between MIS
and open surgery for interval debulking after NACT. After
propensity score matching, the median OS was ~46.7 months with MIS
vs. 41.0 months with laparotomy (HR, 0.86; 95% CI, 0.79–0.94);
5-year survival probabilities were essentially equivalent (38.3%
MIS vs. 34.8% open; P<0.01 in favor of MIS. This apparent
advantage may have been influenced by differences in patient
selection). There was no detriment to survival with MIS, if
anything, MIS reported a slight survival benefit in this weighted
analysis (27). This aligns with a
Japanese Gynecologic Oncology Committee meta-analysis (2024) which
reported no notable OS differences between MIS and open approaches
in advanced-stage disease, whether for PDS or IDS. For instance, in
advanced primary debulking cases, the pooled odds of survival were
similar (OR, ~0.66; 95% CI, 0.36–1.22; P>0.05), and likewise no
OS disadvantage was seen for MIS IDS (OR, ~0.93; CI 0.25–3.44)
(88).
PFS has also been reported to be equivalent between
minimally invasive and open surgery approaches. Numerous studies
found no notable PFS difference between MIS and open surgery groups
(20). In a meta-analysis of 6
studies (3,528 patients) focusing on IDS after NACT, MIS was
associated with a trend toward improved PFS but no OS difference
(89). Specifically, patients in
the MIS group had slightly improved PFS in some cohorts, possibly
due to selection of improved responders, but on the whole PFS is
equivalent when accounting for confounders. For example, Alletti
et al (92) reported median
PFS of 18 months with laparoscopy vs. 12 months with open in a
single-center IDS series, but no difference in 5-year OS. In the
pooled analysis of six studies (3,528 patients) after NACT, MIS was
associated with a median PFS of 15.8–18.0 months vs. 12.0–14.2
months with open surgery, translating into an absolute PFS gain of
1.6–3.8 months. From meta-analysis data, the pooled hazard ratio
for progression was 0.85 (95% CI, 0.73–0.99; P=0.04), indicating a
15% lower risk of progression with MIS, even though overall
survival did not differ between the groups (89).
Several individual center studies and registry
analyses further support OS parity. A study by Pereira et al
(90) reported that 5-year survival
rates were comparable with MIS. In their analysis, 47.5% of
patients with MIS IDS were alive at 5 years vs. 30% of patients
with open IDS (41 vs. 28% for MIS vs. open PDS). While such
differences reflect selection factors, MIS did not compromise
long-term survival in appropriate candidates. Additionally, a
recent conference report including >2,400 interval debulking
cases with R0 resection reported median OS ~51 months MIS vs. 46
months open, a difference that was not statistically significant
(HR, ~1.1; P=0.17) (92). Taken
together, current evidence indicated that overall survival with MIS
in advanced ovarian cancer is comparable to open surgery, provided
patients are selected for achievable complete cytoreduction
(88,93).
Recurrence patterns
Beyond survival duration, the pattern and timing of
disease recurrence are important considerations. Thus far, studies
have not identified any unique or worse recurrence patterns
associated with minimally invasive debulking. Recurrence rates
appear similar between MIS and open approaches in advanced-stage
patients. For patients with advanced-stage (FIGO III–IV) EOC
undergoing interval debulking after NACT, Zeng et al
(89) performed a meta-analysis of
six studies encompassing 3,528 patients and found no significant
difference in recurrence rates between minimally invasive surgery
and open laparotomy groups. In a dual-center retrospective cohort
of 89 patients with stage IIIC-IV EOC (59 MIS vs. 30 open IDS),
Pereira et al (90) reported
equivalent recurrence frequencies in both approaches. Similarly,
Alletti et al (92)
evaluated 46 patients with stage III EOC (23 MIS vs. 23 open IDS)
and observed no increase in early or atypical recurrences with
minimally invasive techniques. A single-center comparison of
robotic vs. open cytoreduction reported that the recurrence-free
survival was longer in the MIS cohort (potentially reflecting
case-specific factors such as patient selection bias, tumor stage
distribution, preoperative disease burden, performance status or
differences in adjuvant therapy protocols), but recurrence
frequency was similar across groups. No study to date has
demonstrated a higher risk of recurrence solely due to the
minimally invasive technique when surgical completeness is
equivalent (34).
A specific concern with MIS is the risk of port-site
metastases (tumor seeding at trocar sites). Port-site metastases
have been documented in advanced ovarian cancer after diagnostic
laparoscopy, with rates of 5–17% reported when no precautions were
taken. However, these metastases are usually detected at the time
of subsequent surgery or during chemotherapy and are resected or
sterilized by treatment. Furthermore, long-term follow-up has
indicated no adverse impact on prognosis from port-site implants if
patients receive standard therapy (94). In a novel published series of MIS
IDS (from 2015 onwards), port-site recurrences were rare (>1–2%
of cases) and have not been reported as a notable pattern of
failure compared to the overall recurrence rates in ovarian cancer
(89,95). The majority of recurrences after
either MIS or open debulking occur in typical intra-abdominal
locations (peritoneum and retroperitoneum account for 75–80% of
recurrence sites), with remaining recurrences distributed among
distant sites (liver, brain and lungs), reflecting the biology of
ovarian cancer compared with the surgical route (42,94).
Overall, current evidence suggests no difference in recurrence
patterns between MIS and open surgery; the key determinant is
whether complete cytoreduction is achieved, not the incision type
(89). Notably, no increase in
distant or early recurrence has been observed with MIS, unlike
early-stage cervical cancer MIS trials (95). Ongoing studies will continue to
monitor for any subtle differences in recurrence dynamics with
MIS.
Patient selection criteria
Patient selection is a key factor in the success of
minimally invasive cytoreductive surgery. Several previous studies
emphasize that MIS in advanced ovarian cancer should be reserved
for patients with a limited disease burden that can be addressed
with standard cytoreductive techniques not requiring extensive
multiorgan resections. Common selection criteria include: Notable
response to NACT (for interval cases) with substantial tumor
shrinkage; disease distribution confined to areas amenable to
laparoscopic resection (for example, superficial peritoneal
implants, easily accessible pelvic/abdominal disease, omental
caking that can be removed en bloc); absence of extensive
diaphragmatic or mesenteric disease requiring large incisions; and
patient factors such as body habitus and comorbidities that favor a
minimally invasive approach (42,94).
In practice, surgeons often perform a thorough preoperative imaging
assessment and may even use diagnostic laparoscopy to confirm that
complete resection may be feasible via MIS ports (89).
Previous studies demonstrated that when these
selection principles are applied, patients with MIS tend to have
lower initial tumor load. For example, in the National Cancer
Database (NCDB; http://www.facs.org/quality-programs/cancer/ncdb/)
study, MIS cases had significantly fewer ‘additional cytoreductive
procedures’ (for example, bowel or spleen resections) compared with
open cases (59.3 vs. 70.8%; P<0.01). This suggests surgeons
selected MIS for patients with less disseminated disease, hence
needing fewer organ resections. In the same dataset, the rate of
any gross residual disease was slightly lower in patients with MIS
(23.9 vs. 26.7% in open; corresponding to higher complete resection
rates) (27), which suggested that
surgeons attempted MIS primarily in cases where they anticipated
achieving R0. Another analysis noted patients with MIS were less
likely to require ‘extensive’ surgery compared with open patients
(41 vs. 53%; P<0.001). All of this reflects a selection bias:
Candidates for MIS are typically those with resectable disease of
low complexity (42,93). A multi-center consensus concluded
that MIS is appropriate when surgery can be limited to standard
cytoreductive procedures without major complexity (42).
Beyond tumor factors, patient factors serve a role;
a previous study reported that patients with MIS were on average
older compared with those undergoing open surgery (93), possibly because surgeons chose MIS
to minimize morbidity in older patients (≥65 years) who had
adequate response to chemotherapy. Other selection considerations
include performance status and surgeon experience. High-volume
centers with advanced MIS skills are more likely to attempt
laparoscopy/robotics on borderline cases, whereas less experienced
centers might opt for laparotomy for the same patient. In summary,
ideal candidates for MIS PDS/IDS are those with regionally confined
disease, notable response to therapy and no need for large en
bloc resections, as determined by imaging or laparoscopic
assessment. These selection criteria underlie the similar outcomes
seen, and differences in the characteristics of the patient groups,
such as age, comorbidities, response to chemotherapy and extent of
disease, rather than the surgical approach itself, may account for
any modest benefits observed in the MIS cohorts (27,89).
Data limitations and ongoing
studies
Although outcomes thus far are encouraging, it is
important to acknowledge the limitations of existing data.
Selection bias is the foremost limitation, as all comparative
studies to date are non-randomized and patients with MIS inevitably
had more favorable disease characteristics. Propensity score
matching and multivariable adjustments, as performed in the NCDB
analyses (27), help mitigate but
cannot eliminate this bias. Thus, it remains possible that
equivalent patients with extensive disease would fare differently
with MIS compared with open surgery. High-level evidence from
randomized trials to confirm oncological safety.
To date, no RCT has published definitive results
comparing MIS vs. open debulking in advanced ovarian cancer
(34). However, the first such
trial is presently underway. The international LANCE trial is a
phase III multicenter RCT designed to evaluate non-inferiority of
MIS vs. laparotomy for IDS in advanced-stage ovarian cancer after
3–4 cycles of NACT (95). The pilot
phase of the trial demonstrated feasibility and acceptable
conversion rates, enabling full accrual. The outcome of the trial
(disease-free survival as primary endpoint) will provide level I
evidence on the oncological efficacy of MIS in this setting
(9,95). Therefore, until the LANCE trial
results are reported, the present review relied on observational
data.
Another limitation is that most studies have been
conducted in specialized centers or databases from developed
healthcare systems, which may not reflect all practice settings
globally. There is a paucity of data on MIS outcomes in
resource-limited regions or low-volume centers. Additionally,
follow-up duration in previous MIS series was still short, as
several studies reported a median follow-up of 3–4 years, so
long-term survival equivalence and patterns of late recurrence
require continued investigation (42). Data on recurrence patterns
specifically (for example whether MIS might lead to different
metastatic spread) are limited. To date, no concerning safety
issues or unexpected complications have been reported. Numerous
analyses (for example, NCDB) lack specific details such as exact
residual tumor size or QoL outcomes. QoL and recovery metrics may
favor MIS (due to faster recovery), but robust prospective data on
patient-reported outcomes are needed.
In summary, although current evidence supports MIS
as an acceptable approach in advanced ovarian cancer, it is still
largely retrospective. Results must be interpreted with caution
given the non-randomized nature of published studies and potential
confounders (34). The ongoing
LANCE RCT and other prospective studies will be key to conclusively
establish whether MIS is comparable with open surgery in all
oncological outcomes or if differences may emerge when selection
bias is removed. To date, the National Comprehensive Cancer Network
(NCCN) and the European Society of Gynecological Oncology (96) guidelines emphasize that MIS for
advanced ovarian cancer should be offered only in highly selected
patients by experienced surgeons, preferably in the context of
clinical trials or protocols (38,89).
Furthermore, current evidence is largely derived from retrospective
studies with inherent selection biases. While several cohort
analyses indicate non-inferior outcomes for MIT compared with open
surgery, the lack of level I evidence from completed RCTs mandates
cautious interpretation. The ongoing LANCE trial is expected to
address this gap.
Real-world outcomes in high-volume
centers
Real-world data from high-volume gynecological
oncology centers have consistently reported that in appropriately
selected patients, MIS can safely replicate the oncological
outcomes of open cytoreduction. For instance, the NCDB analyses
demonstrated that between 2013 and 2019, MIS usage in IDS increased
significantly in the USA, with comparable overall survival (OS) and
even lower perioperative mortality compared with open surgery
(90-day mortality; 1.4 vs. 2.5%; P<0.01) (27). Similar findings were reported by the
MISSION study in Europe and South America (42) and by multi-center registries from
including a 2022 meta-analysis (89). These studies confirmed equivalent
5-year OS rates and high R0 rates with MIS when performed by
experienced teams (90,92). MIS has also been associated with
faster recovery, shorter hospital stays and earlier initiation of
postoperative chemotherapy in some cohorts (93). Collectively, these real-world data
suggest the feasibility and safety of MIS for advanced ovarian
cancer and support its broader implementation when patient
selection and surgical expertise are appropriate.
However, comprehensive data specifically addressing
QoL in advanced-stage EOC are limited. While some retrospective
analyses and small prospective series suggest potential QoL
benefits, large, well-powered studies are needed to establish
definitive conclusions. Furthermore, while some studies have
utilized validated patient-reported outcome instruments such as the
European Organization for Research and Treatment of Cancer Quality
of Life Questionnaire (QLQ)-Core 30 and QLQ-Ovarian Cancer Module
28 to assess postoperative QoL in patients with EOC, robust PRO
data specific to advanced-stage disease remain sparse and warrant
further prospective investigation (97).
Challenges and limitations of MITs
MIS has the potential to improve the outcomes of EOC
surgery. However, the implementation of MIS must be accompanied by
high adherence to oncological principles, which will consequently
push the envelope in technically challenging cases. These are the
cases in which the use of conventional laparoscopy or
robotic-assisted laparoscopy can be limited, which leads to an
increased incompletion of surgery and residual disease. In
low-volume centers, adnexal masses may be misdiagnosed and thereby
given a less conservative approach, which can lead to stage
upstaging and incomplete surgery (42,87,98).
Therefore, preoperative and intraoperative protocols and criteria,
surgical expertise and the correct allocation of resources, that
is, ensuring that surgical expertise is available at several
tertiary reference centers as possible.
Previous studies have highlighted that
hepatobiliary involvement in ovarian cancer is associated with
increased tumor burden, necessitating more complex surgical
approaches and specialized expertise (42,87,98).
Findings by Di Donato et al (98) further confirmed that achieving
complete cytoreduction in such cases significantly impacted patient
survival outcomes. In the future, the use of novel imaging
techniques that can further assess tumor spread and the molecular
characteristics of cancer will contribute to patient selection,
allowing, whenever possible, an optimal MIS approach to be selected
for EOC surgery.
While MITs offer several advantages, they are not
free of risks; a key concern is the increased likelihood of
intraoperative complications, particularly in advanced-stage
ovarian cancer cases where optimal cytoreduction is technically
challenging. Additionally, suboptimal staging may lead to higher
recurrence rates due to the incomplete assessment of peritoneal
spread and lymph node involvement (34). Further studies are needed to
determine standardized protocols that minimize these risks and
ensure oncological safety.
Patient selection
Currently, there is a lack of consensus in the
field of gynecological oncology on the definition of ‘early stage’
ovarian cancer and its management. Typically, gynecological
oncologists identify most EOC types at an advanced stage and the
standard surgical intervention involves primary cytoreduction,
followed by discrimination (12,13).
However, two distinct cohorts of patients with apparently
early-stage disease (those with Stage I disease on imaging who are
ultimately found to have advanced disease at surgery and those with
localized Stage I disease) may potentially gain from varying
management approaches. In the early stages of the disease, surgery
could lead to the diagnosis of Stage IIIB serous carcinoma, which
may cause the disease stage to progress (12,13).
The NCCN guidelines currently advocate conducting
the standard staging of surgical procedures for tumors classified
as small stage I diseases (99). An
alternative biological perspective suggested evaluating an early
suspicious pelvic and/or para-aortic stage from a clinical
standpoint. It is possible for these rare conditions to be caused
by nodal endometriosis or dermoids or these conditions may be
associated with a slow progression of hematogenous
lymphangiomatosis, which usually occurs when endometriosis stays
the same (38,57,92,100).
Currently, research on the origins of these small, less aggressive
atypical nodular forms of cancer is being conducted. The surgical
techniques should vary when executed in a gynecology unit focused
on these distinct procedures. Only certain patients qualify as
suitable candidates for MIS in cases of advanced stage disease: i)
Patients with documented major response to NACT, defined by imaging
or laparoscopic assessment showing >80% reduction in tumor
volume and confinement of residual disease to easily accessible
pelvic and omental sites; ii) individuals whose disease
distribution is limited to superficial peritoneal implants, minor
omental caking that can be removed en bloc and absence of
extensive diaphragmatic or mesenteric involvement; iii) cases
without bulky upper abdominal disease or large bowel, splenic or
hepatic surface metastases, such that no multiorgan resections are
anticipated; iv) patients with Charlson comorbidity index <2 and
adequate performance status (ECOG 0–1), minimizing anesthesia risk
and facilitating rapid postoperative recovery; v) selected older
patients (aged ≥65 years) with limited tumor burden who stand to
benefit most from reduced perioperative morbidity and shorter
hospital stays; and vi) selected older patients (aged ≥65 years)
with limited tumor burden who stand to benefit most from reduced
perioperative morbidity and shorter hospital stays.
The feasibility and safety of MIS in selected
patients has been demonstrated in the MISSION study, where 96%
achieved no gross residual disease via MIS after NACT and
conversion to laparotomy occurred in only 3.9% of cases (57,92).
It could be suggested that patients should have: i) Imaging after
clinical evaluation by the multidisciplinary team with radiological
diagnosis; ii) FIGO stage IIIC or less; iii) Charlson comorbidity
index <2 (100); iv)
pauci-symptomatic small disease; v) imaging with pre-operative
diagnostic laparoscopy; and vi) no notable protein anemia. As for
the patients with carcinoma in situ (CIS), strong
de-swelling surgical procedures should be avoided; albumin and
hemoglobin must be normal; albumin and creatinine must be normal;
fitness status and adequate psycho-physiological support; no
overfeeding of the pathology or performance status; and ovarian
clear cell carcinoma and CIS, against serous papillary histotype
required.
Proper patient selection is key to achieve
favorable outcomes in MIS. While early-stage disease, lower
histological aggressiveness and a lower Charlson comorbidity index
favor the feasibility of this approach, further research is
required to validate these selection criteria across diverse
patient populations and advanced-stage cases.
Training and expertise
The role of MIS in the management of ovarian cancer
has been well documented in the form of low invasive techniques
such as laparoscopic, robotic or single port surgery. Consequently,
the approach has been associated with favorable reports in terms of
blood loss, time, length of stay, wound complications and recovery
time. However, despite favorable outcomes for recurrence, mortality
and overall survival for patients receiving MIS, favorable surgical
results are only associated with centers that have extensive
experience in MIS.
The viability of hepatobiliary cytoreduction is
markedly influenced by the skill level of the surgeon and the
available resources at the institution. Previous studies have
demonstrated that high-volume centers tend to achieve superior
oncological outcomes compared with lower-volume centers (42,89,98).
Di Donato et al (98)
demonstrated that complete cytoreduction can be achieved in
patients with hepatobiliary metastases when conducted in
specialized centers with a multidisciplinary approach. In this
situation, procedures such as sentinel node mapping and the removal
of lymph nodes using minimally invasive methods have been
recognized as important (42,89,98).
However, these procedures should only be performed by surgeons with
documented expertise in minimally invasive debulking surgery,
following established institutional guidelines and quality
standards to ensure patient safety and adequate disease
management.
Future directions
Novel technology brings novel challenges in
training and implementing these technologies in clinical practice.
The implementation of robotic surgery, for instance, is associated
with a steep learning curve. The effectiveness of different
training models remains to be elucidated. Furthermore, not all
departments can afford to start a robotics program directly.
Specifically, in resource-limited healthcare systems, a
multimillion investment could endanger other goals. In the
development of both robotic and laparoscopic surgical techniques,
training in simulations is key. Since there are several types and
complexities of intraoperative complications, in vivo
training combined with mentored procedures before leading a
procedure might be beneficial.
It will be important to choose patients who are in
medically fit before surgery, have few comorbidities (such as
hypertension without end-organ damage, diet-controlled diabetes,
mild COPD with preserved lung function or stable thyroid disease),
have early-stage EOC and have a low risk of complications, as not
every patient with stage I–IV EOC will gain advantages from a
minimally invasive approach. Prospective trials and retrospective
cohort studies may combine to create and assess these algorithms.
Furthermore, the formulation of these guidelines is essential,
grounded in real data compared with relying on automated training
databases. Among promising innovations in EOC management, liquid
biopsy and advanced imaging techniques are poised to serve
transformative roles, yet several hurdles must be addressed before
widespread clinical adoption. Liquid biopsy approaches, such as
circulating tumor DNA, circulating tumor cells and exosomal RNA
analyses, offer non-invasive means for early detection, monitoring
of minimal residual disease and treatment stratification (101,102). However, the sensitivity and
specificity of these assays remain variable and standardization
across platforms is still evolving. Regulatory approvals are
currently limited and liquid biopsy is notably viewed as a
complementary adjunct compared with a replacement for traditional
imaging and tissue biopsy (101,102). Similarly, advanced imaging
modalities, including diffusion-weighted MRI, PET/CT with novel
tracers and AI-enhanced imaging, hold potential to improve the
detection of small-volume peritoneal disease and informing surgical
planning (103,104). Nevertheless, variability in
imaging sensitivity, the need for specialized infrastructure and
the lack of established cost-effectiveness data pose notable
barriers to routine clinical integration. As ongoing prospective
studies refine the performance and clinical utility of these
technologies, their eventual incorporation into multimodal EOC
management strategies will require careful consideration of
regulatory, logistical and economic factors.
Conclusion
MITs and molecular-based therapies have reshaped
the management of EOC, particularly in selected cases of
advanced-stage disease. The current evidence, including large
cohort studies and recent meta-analyses, demonstrated that in
appropriately chosen patients, MIS can achieve comparable
oncological outcomes to open surgery, with the added benefits of
reduced perioperative morbidity, faster recovery and earlier
initiation of adjuvant treatment. These surgical innovations, when
aligned with novel systemic therapies such as PARP inhibitors,
anti-angiogenic agents and immune checkpoint inhibitors, offer an
integrated approach that enhances survival and maintains QoL.
For practicing gynecological oncologists, these
findings underscore the importance of individualized treatment
planning based on disease burden, response to neoadjuvant therapy
and institutional expertise. However, the adoption of these
strategies must be guided by rigorous criteria for patient
selection, as well as multidisciplinary coordination. Despite these
advances, several gaps remain. Long-term survival data from RCTs
comparing MIS with laparotomy in advanced EOC are still pending.
Additionally, real-world outcomes in low-volume or resource-limited
settings require further validation. QoL metrics and
cost-effectiveness analyses also need to be integrated into future
research to further inform clinical decision-making.
Currently, several studies support the use of MITs
for staging and interval debulking following NACT in carefully
selected patients with advanced-stage EOC, when performed by
experienced surgeons (27,42,90).
For systemic therapy, PARP inhibitors have become standard of care
for maintenance treatment in BRCA-mutated and HRD-positive EOC,
based on high-level evidence from pivotal trials such as SOLO-1 and
PRIMA. By contrast, immunotherapy remains investigational in this
setting, with ongoing trials evaluating optimal combinations. Key
gaps include the need for level I data validating the long-term
oncological safety of MITs in advanced-stage EOC, improved
strategies for the integration of systemic therapies and robust
patient-reported outcome data to guide treatment decisions.
Addressing these questions will be key for the translation of
evolving innovations into clinical practice and to optimize the
personalized management of EOC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RY, JG, YC, CL and JL conceptualized and designed
the present review. RY and JG prepared the original draft. YC and
CL wrote, edited and reviewed the manuscript. JL provided
supervision, conducted the analyses and revised the manuscript. All
authors commented on previous versions of the manuscript. All
authors read and approved the final manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
JL, ORCID no. 0009-0009-6755-2882.
References
|
1
|
Ali A, Al-Ani O and Al-Ani F: Epidemiology
and risk factors for ovarian cancer. Prz Menopauzalny. 22:93–104.
2023.PubMed/NCBI
|
|
2
|
Silva EG: The origin of epithelial
neoplasms of the ovary: An alternative view. Adv Anat Pathol.
23:50–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crane TE, Khulpateea BR, Alberts DS,
Basen-Engquist K and Thomson CA: Dietary intake and ovarian cancer
risk: A systematic review. Cancer Epidemiol Biomarkers Prev.
23:255–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan A, Sun Q, Czernichow S, Kivimaki M,
Okereke OI, Lucas M, Manson JE, Ascherio A and Hu FB: Bidirectional
association between depression and obesity in middle-aged and older
women. Int J Obes (Lond). 36:595–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reid BM, Permuth JB and Sellers TA:
Epidemiology of ovarian cancer: A review. Cancer Biol Med. 14:9–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Risch HA: Hormonal etiology of epithelial
ovarian cancer, with a hypothesis concerning the role of androgens
and progesterone. J Natl Cancer Inst. 90:1774–1786. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Terry KL, Karageorgi S, Shvetsov YB,
Merritt MA, Lurie G, Thompson PJ, Carney ME, Weber RP, Akushevich
L, Lo-Ciganic WH, et al: Genital powder use and risk of ovarian
cancer: A pooled analysis of 8,525 cases and 9,859 controls. Cancer
Prev Res (Phila). 6:811–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faber MT, Kjær SK, Dehlendorff C,
Chang-Claude J, Andersen KK, Høgdall E, Webb PM, Jordan SJ;
Australian Cancer Study (Ovarian Cancer); Australian Ovarian Cancer
Study Group, ; et al: Cigarette smoking and risk of ovarian cancer:
A pooled analysis of 21 case-control studies. Cancer Causes
Control. 24:989–1004. 2013.PubMed/NCBI
|
|
9
|
Rauh-Hain JA, Melamed A, Pareja R, May T,
Sinno A, McNally L, Horowitz NS, De Iaco P, Michener CM, Van
Lonkhuijzen L, et al: Laparoscopic cytoreduction after neoadjuvant
chemotherapy in high-grade epithelial ovarian cancer: A LANCE
randomized clinical trial. JAMA Netw Open. 7:e2446325–e. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sambasivan S: Epithelial ovarian cancer.
Cancer Treat Res Commun. 33:1006292022.PubMed/NCBI
|
|
11
|
Kuroki L and Guntupalli SR: Treatment of
epithelial ovarian cancer. BMJ. 371:m37732020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Delga B, Classe JM, Houvenaeghel G, Blache
G, Sabiani L, El Hajj H, Andrieux N and Lambaudie E: 30 years of
experience in the management of stage III and IV epithelial ovarian
cancer: Impact of surgical strategies on survival. Cancers (Basel).
12:7682020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurnit KC, Fleming GF and Lengyel E:
Updates and new options in advanced epithelial ovarian cancer
treatment. Obstet Gynecol. 137:108–1021. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bailly C, Thuru X and Quesnel B: Combined
cytotoxic chemotherapy and immunotherapy of cancer: Modern times.
NAR Cancer. 2:zcaa0022020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaitskell K, Rogozińska E, Platt S, Chen
Y, Abd El Aziz M, Tattersall A and Morrison J: Angiogenesis
inhibitors for the treatment of epithelial ovarian cancer. Cochrane
Database Syst Rev. 4:CD0079302023.PubMed/NCBI
|
|
16
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Incorporation of bevacizumab in the primary treatment of
ovarian cancer. N Engl J Med. 365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baethge C, Goldbeck-Wood S and Mertens S:
SANRA-a scale for the quality assessment of narrative review
articles. Res Integr Peer Rev. 4:52019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chi DS, Eisenhauer EL, Zivanovic O, Sonoda
Y, Abu-Rustum NR, Levine DA, Guile MW, Bristow RE, Aghajanian C and
Barakat RR: Improved progression-free and overall survival in
advanced ovarian cancer as a result of a change in surgical
paradigm. Gynecol Oncol. 114:26–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quénet F, Elias D, Roca L, Goéré D, Ghouti
L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, et al:
Cytoreductive surgery plus hyperthermic intraperitoneal
chemotherapy versus cytoreductive surgery alone for colorectal
peritoneal metastases (PRODIGE 7): A multicentre, randomised,
open-label, phase 3 trial. Lancet Oncol. 22:256–266. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nezhat F, Nezhat C, Welander CE and
Benigno B: Four ovarian cancers diagnosed during laparoscopic
management of 1011 women with adnexal masses. Am J Obstet Gynecol.
167:790–796. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feuer GA, Lakhi N, Barker J, Salmieri S
and Burrell M: Perioperative and clinical outcomes in the
management of epithelial ovarian cancer using a robotic or
abdominal approach. Gynecol Oncol. 131:520–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Magrina JF, Zanagnolo V, Noble BN, Kho RM
and Magtibay P: Robotic approach for ovarian cancer: Perioperative
and survival results and comparison with laparoscopy and
laparotomy. Gynecol Oncol. 121:100–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berek JS, Matias-Guiu X, Creutzberg C,
Fotopoulou C, Gaffney D, Kehoe S, Lindemann K, Mutch D and Concin
N; Endometrial Cancer Staging Subcommittee and FIGO Women's Cancer
Committee, : FIGO staging of endometrial cancer: 2023. Int J
Gynaecol Obstet. 162:383–394. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baum S, Alkatout I, Proppe L, Kotanidis C,
Rody A, Laganà AS, Sommer S and Gitas G: Surgical treatment of
endometrioid endometrial Carcinoma-laparotomy versus laparoscopy. J
Turk Ger Gynecol Assoc. 23:233–240. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Serur E, Emeney PL and Byrne DW:
Laparoscopic management of adnexal masses. JSLS. 5:143–151.
2001.PubMed/NCBI
|
|
26
|
Canis M, Mage G, Pouly JL, Wattiez A,
Manhes H and Bruhat MA: Laparoscopic diagnosis of adnexal cystic
masses: A 12-year experience with long-term follow-up. Obstet
Gynecol. 83:707–712. 1994.PubMed/NCBI
|
|
27
|
Jorgensen K, Melamed A, Wu CF, Nitecki R,
Pareja R, Fagotti A, Schorge JO, Ramirez PT and Rauh-Hain JA:
Minimally invasive interval debulking surgery for advanced ovarian
cancer after neoadjuvant chemotherapy. Gynecol Oncol. 172:130–137.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chamberlain E and Carlo BD: Ovarian Cancer
Update. Proceedings of UCLA Health. Vol 28:2024.
|
|
29
|
Psomiadou V, Prodromidou A, Fotiou A,
Lekka S and Iavazzo C: Robotic interval debulking surgery for
advanced epithelial ovarian cancer: Current challenge or future
direction? A systematic review. J Robot Surg. 15:155–163. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abitbol J, Gotlieb W, Zeng Z, Ramanakumar
A, Kessous R, Kogan L, Pare-Miron V, Rombaldi M, Salvador S,
Kucukyazici B, et al: Incorporating robotic surgery into the
management of ovarian cancer after neoadjuvant chemotherapy. Int J
Gynecol Cancer. 29:1341–1347. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Zheng Y and Yang F: Primary
debulking surgery for advanced epithelial ovarian cancer with
isolated enlarged para-aortic lymph node by robotic transumbilical
single port approach. Int J Gynecol Cancer. 33:1976–1977. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ray-Coquard I, Leary A, Pignata S, Cropet
C, González-Martín A, Marth C, Nagao S, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus bevacizumab First-line maintenance
in ovarian cancer: Final overall survival results from the
PAOLA-1/ENGOT-ov25 trial. Ann Oncol. 34:681–692. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao L, Zhai Y and Niu G: Research
progress of immune checkpoint inhibitors in ovarian cancer. Exp
Immunol. 4:853–870. 2024. View Article : Google Scholar
|
|
34
|
Nezhat F, Briskin C, Lakhi N, Fu R and
Pejovic T: Minimally invasive surgery for the management of ovarian
cancer: A systematic review and Meta-analysis. O G Open. 1:392024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ray-Coquard I, Pautier P, Pignata S, Pérol
D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus bevacizumab as first-line
maintenance in ovarian cancer. N Engl J Med. 381:2416–2428. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Predina JD, Kapoor V, Judy BF, Cheng G,
Fridlender ZG, Albelda SM and Singhal S: Cytoreduction surgery
reduces systemic myeloid suppressor cell populations and restores
intratumoral immunotherapy effectiveness. J Hematol Oncol.
5:342012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiao F, Wang Z, Qiao L, Zhang X, Wu N,
Wang J and Yu X: Application of PARP inhibitors combined with
immune checkpoint inhibitors in ovarian cancer. J Transl Med.
22:7782024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Armstrong DK, Alvarez RD, Bakkum-Gamez JN,
Barroilhet L, Behbakht K, Berchuck A, Chen LM, Cristea M, DeRosa M,
Eisenhauer EL, et al: Ovarian cancer, version 2.2020, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
19:191–226. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghirardi V, Fagotti A and Scambia G:
Laparoscopic selection for surgery in epithelial ovarian cancer. A
short review. Facts Views Vis Obgyn. 15:25–28. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
González-Martín A, Pothuri B, Vergote I,
DePont Christensen R, Graybill W, Mirza MR, McCormick C, Lorusso D,
Hoskins P, Freyer G, et al: Niraparib in patients with newly
diagnosed advanced ovarian cancer. N Engl J Med. 381:2391–2402.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fagotti A, Alletti SG, Corrado G, Cola E,
Vizza E, Vieira M, Andrade CE, Tsunoda A, Favero G, Zapardiel I, et
al: The INTERNATIONAL MISSION study: Minimally invasive surgery in
ovarian neoplasms after neoadjuvant chemotherapy. Int J Gynecol
Cancer. 29:5–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Monk BJ, Dalton H, Farley JH, Chase DM and
Benjamin I: Antiangiogenic agents as a maintenance strategy for
advanced epithelial ovarian cancer. Crit Rev Oncol Hematol.
86:161–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moore KN, Oza AM, Colombo N, Oaknin A,
Scambia G, Lorusso D, Konecny GE, Banerjee S, Murphy CG, Tanyi JL,
et al: Phase III, randomized trial of mirvetuximab soravtansine
versus chemotherapy in patients with platinum-resistant ovarian
cancer: Primary analysis of FORWARD I. Ann Oncol. 32:757–765. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Akter S, Rahman MA, Hasan MN, Akhter H,
Noor P, Islam R, Shin Y, Rahman MDH, Gazi MS, Huda MN, et al:
Recent advances in ovarian cancer: Therapeutic strategies,
potential biomarkers, and technological improvements. Cells.
11:6502022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang L, Wang Q, Xu Y, Cui M and Han L:
Advances in the treatment of ovarian cancer using PARP inhibitors
and the underlying mechanism of resistance. Curr Drug Targets.
21:167–178. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lord CJ and Ashworth A: PARP inhibitors:
Synthetic lethality in the clinic. Science. 355:1152–1158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Coleman RL, Oza AM, Lorusso D, Aghajanian
C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G,
et al: Rucaparib maintenance treatment for recurrent ovarian
carcinoma after response to platinum therapy (ARIEL3): A
randomised, Double-blind, placebo-controlled, phase 3 trial.
Lancet. 390:1949–1961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Coleman RL, Fleming GF, Brady MF, Swisher
EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat
Ben-Baruch N, Werner TL, et al: Veliparib with first-line
chemotherapy and as maintenance therapy in ovarian cancer. N Engl J
Med. 381:2403–2415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vergote I, Du Bois A, Floquet A, Rau J,
Kim JW, Del Campo J, Friedlander M, Pignata S, Fujiwara K, Colombo
N, et al: Overall survival results of AGO-OVAR16: A phase 3 study
of maintenance pazopanib versus placebo in women who have not
progressed after first-line chemotherapy for advanced ovarian
cancer. Gynecol Oncol. 155:186–191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, Kurzeder C, et al: A phase 3 trial of bevacizumab in
ovarian cancer. N Engl J Med. 365:2484–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aghajanian C, Blank SV, Goff BA, Judson
PL, Teneriello MG, Husain A, Sovak MA, Yi J and Nycum LR: OCEANS: A
randomized, double-blind, placebo-controlled phase III trial of
chemotherapy with or without bevacizumab in patients with
platinum-sensitive recurrent epithelial ovarian, primary
peritoneal, or fallopian tube cancer. J Clin Oncol. 30:2039–2045.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang Y, Gao Y, Zhou H, Cai Y, Yu J, Chen
Y, Xue J and Cheng W: Anlotinib combined with
carboplatin/paclitaxel and maintenance anlotinib as front-line
treatment for newly diagnosed advanced ovarian cancer: A phase II,
single-arm, multicenter study (ALTER-GO-010). Am Soc Clin Oncol.
41:55752023. View Article : Google Scholar
|
|
55
|
Pignata S, Lorusso D, Scambia G, Sambataro
D, Tamberi S, Cinieri S, Cinieri S, Mosconi AM, Orditura M, Brandes
AA, et al: Pazopanib plus weekly paclitaxel versus weekly
paclitaxel alone for platinum-resistant or platinum-refractory
advanced ovarian cancer (MITO 11): A randomised, open-label, phase
2 trial. Lancet Oncol. 16:561–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lheureux S, Matei DE, Konstantinopoulos
PA, Wang BX, Gadalla R, Block MS, Jewell A, Gaillard SL, McHale M,
McCourt C, et al: Translational randomized phase II trial of
cabozantinib in combination with nivolumab in advanced, recurrent,
or metastatic endometrial cancer. J Immunother Cancer.
10:e0042332022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Paik ES, Kim TH, Cho YJ, Ryu J, Choi JJ,
Lee YY, Kim TJ, Choi CH, Kim WY, Sa JK, et al: Preclinical
assessment of the VEGFR inhibitor axitinib as a therapeutic agent
for epithelial ovarian cancer. Sci Rep. 10:49042020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rafii SL: Narrative review of novel
chemotherapeutic agents in management of ovarian cancer. Gynecol
Pelvic Med. 42021.
|
|
59
|
Ansari MJ, Bokov D, Markov A, Jalil AT,
Shalaby MN, Suksatan W, Chupradit S, Al-Ghamdi HS, Shomali N,
Zamani A, et al: Cancer combination therapies by angiogenesis
inhibitors; a comprehensive review. Cell Commun Signal. 20:492022.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Monk BJ, Colombo N, Tewari KS, Dubot C,
Caceres MV, Hasegawa K, Shapira–Frommer R, Salman P, Yañez E, Gumus
M, et al: KEYNOTE-826: Final overall survival results from a
randomized, double-blind, phase 3 study of pembrolizumab+
chemotherapy vs. placebo+ chemotherapy for first-line treatment of
persistent, recurrent, or metastatic cervical cancer. J Clin Oncol.
41:55002023. View Article : Google Scholar
|
|
61
|
Tang M, Cai JH, Diao HY, Guo WM, Yang X
and Xing S: The progress of peptide vaccine clinical trials in
gynecologic oncology. Hum Vaccin Immunother. 18:20629822022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hamanishi J, Mandai M, Ikeda T, Minami M,
Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S,
et al: Safety and antitumor activity of anti-PD-1 antibody,
nivolumab, in patients with platinum-resistant ovarian cancer. J
Clin Oncol. 33:4015–4022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Disis ML, Taylor MH, Kelly K, Beck JT,
Gordon M, Moore KM, Patel MR, Chaves J, Park H, Mita AC, et al:
Efficacy and safety of avelumab for patients with recurrent or
refractory ovarian cancer: Phase 1b results from the JAVELIN solid
tumor trial. JAMA Oncol. 5:393–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gray HJ, Benigno B, Berek J, Chang J,
Mason J, Mileshkin L, Mitchell P, Moradi M, Recio FO, Michener CM,
et al: Progression-free and overall survival in ovarian cancer
patients treated with CVac, a mucin 1 dendritic cell therapy in a
randomized phase 2 trial. J Immunother Cancer. 4:342016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Martin Lluesma S, Wolfer A, Harari A and
Kandalaft LE: Cancer vaccines in ovarian cancer: How can we
improve? Biomedicines. 4:102016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chow S, Berek JS and Dorigo O: Development
of therapeutic vaccines for ovarian cancer. Vaccines (Basel).
8:6572020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lawrie TA, Winter-Roach BA, Heus P and
Kitchener HC: Adjuvant (post-surgery) chemotherapy for early stage
epithelial ovarian cancer. Cochrane Database Syst Rev.
12:CD0047062015.PubMed/NCBI
|
|
69
|
Coleridge S, Bryant A, Kehoe S and
Morrison J: Neoadjuvant chemotherapy before surgery versus surgery
followed by chemotherapy for initial treatment in advanced ovarian
epithelial cancer. Cochrane Database Syst Rev.
7:CD0053432021.PubMed/NCBI
|
|
70
|
Santoiemma PP and Powell DJ Jr: Tumor
infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther.
16:807–820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cutri-French C, Nasioudis D, George E and
Tanyi JL: CAR-T cell therapy in ovarian cancer: Where are we now?
Diagnostics (Basel). 14:8192024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kandalaft LE, Powell DJ and Coukos G: A
phase I clinical trial of adoptive transfer of folate
receptor-alpha redirected autologous T cells for recurrent ovarian
cancer. J Transl Med. 10:1572012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sarivalasis A, Morotti M, Mulvey A,
Imbimbo M and Coukos G: Cell therapies in ovarian cancer. Ther Adv
Med Oncol. 13:175883592110083992021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Xin Q, Chen Y, Sun X, Li R, Wu Y and Huang
X: CAR-T therapy for ovarian cancer: Recent advances and future
directions. Biochem Pharmacol. 226:1163492024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nguyen TT, Thanh Nhu N, Chen CL and Lin
CF: Effectiveness and safety of CD22 and CD19 dual-targeting
chimeric antigen receptor T-cell therapy in patients with relapsed
or refractory B-cell malignancies: A meta-analysis. Cancer Med.
12:18767–1885. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fang J, Ding N, Guo X, Sun Y, Zhang Z, Xie
B, Li Z, Wang H, Mao W, Lin Z, et al: αPD-1-mesoCAR-T cells
partially inhibit the growth of advanced/refractory ovarian cancer
in a patient along with daily apatinib. J Immunother Cancer.
9:e0011622021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Liang Z, Dong J, Yang N, Li SD, Yang ZY,
Huang R, Li FJ, Wang WT, Ren JK, Lei J, et al: Tandem CAR-T cells
targeting FOLR1 and MSLN enhance the antitumor effects in ovarian
cancer. Int J Biol Sci. 17:4365–4376. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Banville AC, Wouters MC, Oberg AL, Goergen
KM, Maurer MJ, Milne K, Ashkani J, Field E, Ghesquiere C, Jones
SJM, et al: Co-expression patterns of chimeric antigen receptor
(CAR)-T cell target antigens in primary and recurrent ovarian
cancer. Gynecol Oncol. 160:520–529. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mai J, Wu L, Yang L, Sun T, Liu X, Yin R,
Jiang Y, Li J and Li Q: Therapeutic strategies targeting folate
receptor α for ovarian cancer. Front Immunol. 14:12545322023.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for cancer immunotherapy: Mechanism, combinations, and
clinical outcome. Front Pharmacol. 8:5612017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Matulonis U, Shapira-Frommer R, Santin A,
Lisyanskaya A, Pignata S, Vergote I, Raspagliesi F, Sonke GS,
Birrer M, Provencher DM, et al: Antitumor activity and safety of
pembrolizumab in patients with advanced recurrent ovarian cancer:
Results from the phase II KEYNOTE-100 study. Ann Oncol.
30:1080–1087. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Matulonis UA, Shapira R, Santin A,
Lisyanskaya AS, Pignata S, Vergote I, Raspagliesi F, Sonke GS,
Birrer M, Sehouli J, et al: Final results from the KEYNOTE-100
trial of pembrolizumab in patients with advanced recurrent ovarian
cancer. J Clin Oncol. 38:60052020. View Article : Google Scholar
|
|
83
|
Musacchio L, Cicala CM, Camarda F,
Ghizzoni V, Giudice E, Carbone MV, Ricci C, Perri MT, Tronconi F,
Gentile M, et al: Combining PARP inhibition and immune checkpoint
blockade in ovarian cancer patients: A new perspective on the
horizon? ESMO Open. 7:1005362022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
An D, Banerjee S and Lee JM: Recent
advancements of antiangiogenic combination therapies in ovarian
cancer. Cancer Treat Rev. 98:1022242021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lee WS, Yang H, Chon HJ and Kim C:
Combination of anti-angiogenic therapy and immune checkpoint
blockade normalizes vascular-immune crosstalk to potentiate cancer
immunity. Exp Mol Med. 52:1475–1485. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Färkkilä A, Gulhan DC, Casado J, Jacobson
CA, Nguyen H, Kochupurakkal B, Maliga Z, Yapp C, Chen YA, Schapiro
D, et al: Immunogenomic profiling determines responses to combined
PARP and PD-1 inhibition in ovarian cancer. Nat Commun.
11:14592020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bouter E, Lok C and Trum H: Robot-assisted
laparoscopic staging compared to conventional laparoscopic staging
and laparotomic staging in clinical early stage ovarian carcinoma.
Curr Opin Oncol. 34:490–496. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yokoi A, Machida H, Shimada M, Matsuo K,
Shigeta S, Furukawa S, Nishikawa N, Nomura H, Hori K, Tokunaga H,
et al: Efficacy and safety of minimally invasive surgery versus
open laparotomy for epithelial ovarian cancer: A systematic review
and meta-analysis. Gynecol Oncol. 190:42–52. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zeng S, Yu Y, Cui Y, Liu B, Jin X, Li Z
and Liu L: Efficacy and safety of minimally invasive surgery versus
open laparotomy for interval debulking surgery of advanced ovarian
cancer after neoadjuvant chemotherapy: A systematic review and a
meta-analysis. Front Oncol. 12:9002562022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pereira A, Magrina JF, Magtibay PM, Neto
JS, Siufi DFS, Chang YH and Perez-Medina T: Does MIS play a role in
the treatment of advanced ovarian cancer? Cancers (Basel).
14:35792022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mori K, Hoppenot C, Helenowski I, Berry E,
Lurain J and Neubauer N: Minimally invasive surgery versus
laparotomy for interval cytoreduction after neoadjuvant
chemotherapy for ovarian cancer. Gynecol Oncol. 137:128–130. 2015.
View Article : Google Scholar
|
|
92
|
Alletti SG, Petrillo M, Vizzielli G,
Bottoni C, Nardelli F, Costantini B, Quagliozzi L, Gallotta V,
Scambia G and Fagotti A: Minimally invasive versus standard
laparotomic interval debulking surgery in ovarian neoplasm: A
single-institution retrospective case-control study. Gynecol Oncol.
143:516–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hayek J: Dr Hayek on Laparoscopic vs. Open
Surgery in Advanced Ovarian Cancer. SGO Annual Meeting; San Diego,
CA: 2024
|
|
94
|
Vergote I, Marquette S, Amant F, Berteloot
P and Neven P: Port-site metastases after open laparoscopy: A study
in 173 patients with advanced ovarian carcinoma. Int J Gynecol
Cancer. 15:776–779. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Nitecki R, Rauh-Hain JA, Melamed A,
Scambia G, Pareja R, Coleman RL, Ramirez PT and Fagotti A:
Laparoscopic cytoreduction after neoadjuvant ChEmotherapy (LANCE).
Int J Gynecol Cancer. 30:1450–1454. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ledermann JA, Matias-Guiu X, Amant F,
Concin N, Davidson B, Fotopoulou C, González-Martin A, Gourley C,
Leary A, Lorusso D, et al: ESGO-ESMO-ESP consensus conference
recommendations on ovarian cancer: Pathology and molecular biology
and early, advanced and recurrent disease. Ann Oncol. 35:248–266.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Colombo N, Gadducci A, Sehouli J, Rulli E,
Mäenpää J, Sessa C, Montes A, Ottevanger NB, Berger R, Vergote I,
et al: INOVATYON/ENGOT-ov5 study: Randomized phase III
international study comparing trabectedin/pegylated liposomal
doxorubicin (PLD) followed by platinum at progression vs.
carboplatin/PLD in patients with recurrent ovarian cancer
progressing within 6–12 months after last platinum line. Br J
Cancer. 128:1503–1513. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Di Donato V, Giannini A, D'Oria O, Schiavi
MC, Di Pinto A, Fischetti M, Lecce F, Perniola G, Battaglia F,
Berloco P, et al: Hepatobiliary disease resection in patients with
advanced epithelial ovarian cancer: Prognostic role and optimal
cytoreduction. Ann Surg Oncol. 28:222–230. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu J, Berchuck A, Backes FJ, Cohen J,
Grisham R, Leath CA, Martin L, Matei D, Miller DS, Robertson S, et
al: NCCN Guidelines® Insights: Ovarian Cancer/Fallopian
tube cancer/primary peritoneal cancer, version 3.2024. J Natl Compr
Canc Netw. 22:512–519. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Asante DB, Calapre L, Ziman M, Meniawy TM
and Gray ES: Liquid biopsy in ovarian cancer using circulating
tumor DNA and cells: Ready for prime time? Cancer Lett. 468:59–71.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shiao MS, Chang JM, Lertkhachonsuk AA,
Rermluk N and Jinawath N: Circulating exosomal miRNAs as biomarkers
in epithelial ovarian cancer. Biomedicines. 9:14332021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rivera-Piza A, Lee SH, Lee HH, Lee S, Shin
SJ, Kim J, Park JH, Yu JE, Lee SW, Park G, et al: Real-Time,
AI-Guided photodynamic laparoscopy enhances detection in a rabbit
model of peritoneal cancer metastasis. Cancer Sci. 116:966–975.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Fu C, Zhang B, Guo T and Li J: Imaging
evaluation of peritoneal metastasis: Current and promising
techniques. Korean J Radiol. 25:86–102. 2024. View Article : Google Scholar : PubMed/NCBI
|