Introduction
Epstein-Barr virus (EBV)+ inflammatory
follicular dendritic cell (FDC) sarcoma (EBV+ IFDCS) is
an inert malignant tumor characterized by neoplastic FDC
hyperplasia, markedly reactive lymphoplasmacytic infiltration and
association with the EBV virus. EBV+ IFDCS most commonly
occurs in the liver and spleen, predominantly affecting females
(F:M=1.14:1), with an age range spanning 29 to 79 years (median age
of 62 years) (1). The tumor has a
large volume, clear boundaries and a gray-white cross-section.
Microscopically, the tumor cells are spindle-shaped, ovoid,
scattered or loosely bundled, accompanied by obvious
lymphoplasmacytic infiltration. Certain cases may have notable
granulomas, while a few cases may also have a large number of
eosinophils or plasma cell infiltration (2–5).
In 1986, Monda et al (6) described a non-lymphomatous primary
lymph node malignancy originating from FDC. In 1996, Shek et
al (7) first reported a case of
primary FDC tumor of the liver, which was revealed to be associated
with a clonal proliferation of EBV+ neoplastic FDC. In
2001, Cheuk et al (8)
proposed that inflammatory pseudotumor-like FDC tumors are a unique
variant of FDC tumors, morphologically mimicking inflammatory
pseudotumors, but with tumor cells expressing FDC markers and
positive in situ hybridization of RNA encoded by EBV. In
2023, the 5th edition of the World Health Organization
Classification of Haematolymphoid Tumours reclassified inflammatory
pseudotumor-like follicular/fibroblastic dendritic cell sarcoma as
EBV+ IFDCS and categorized it as mesenchymal dendritic
cell neoplasms (9). The present
study reports a rare case of EBV+ FDCS with clonal
immunoglobulin heavy chain (IGH) gene rearrangement, aiming to help
pathologists to better recognize the disease.
Case report
Case presentation
A 50-year-old man was admitted to Suining Central
Hospital (Suining, China) in July 2024 due to the identification of
a splenic space-occupying lesion during a physical examination,
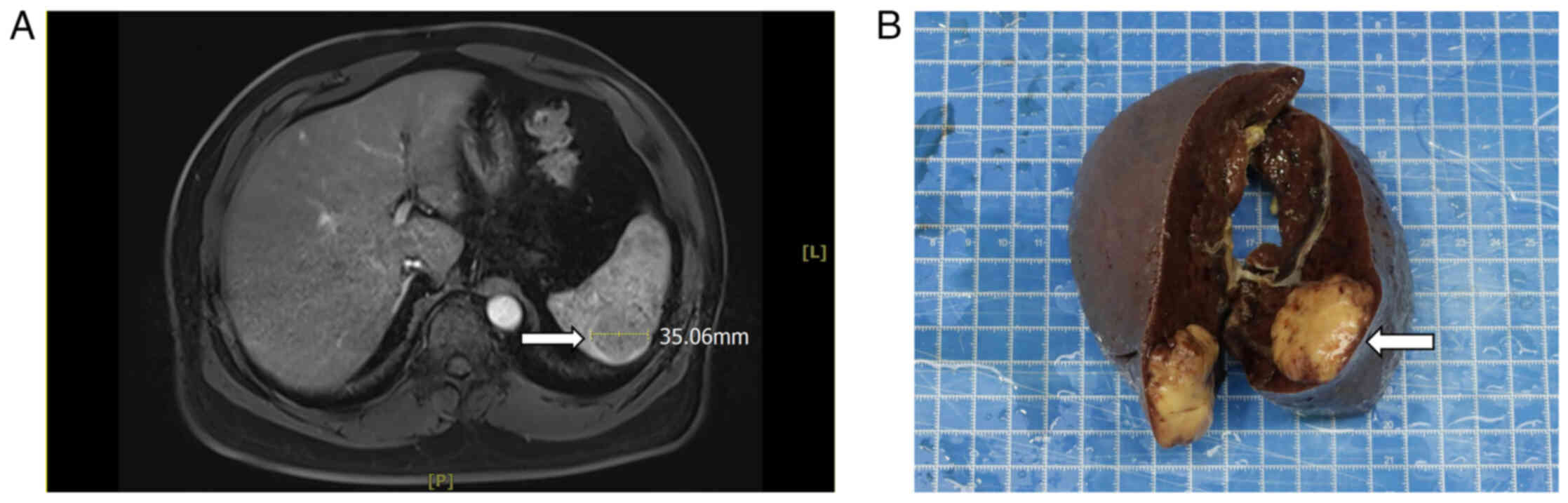
with no uncomfortable symptoms. MRI of the epigastric region
revealed a round-like abnormal signal in the spleen, ~35 mm, with a
slightly high signal in T1-weighted image (WI), a slightly high
signal in T2WI, a slightly high signal in diffusion-WI, a high
signal in apparent diffusion coefficient (Fig. S1) and the enhancement appeared to
be mildly intensified (Fig. 1A).
The CEA (6.43 ng/ml, normal range: <4.5 ng/ml) and CA19-9 (38.92
U/ml, normal range: <30 U/ml) of the patient were slightly
elevated. In July 2024, the patient underwent a total splenectomy.
The surgical specimen revealed a mass of the spleen, size 3.5×3.3×3
cm, gray-white, solid, medium texture and a clear border with the
surrounding tissue (Fig. 1B).
Histopathological features
Tissue specimens were fixed in 10% neutral formalin
at room temperature for 24 h. The tumor tissues were cut into small
pieces, which were subjected to dehydration, clearing and wax
infiltration. Subsequently, the tissues were made into sections
with a thickness of 4 µm. Hematoxylin-eosin staining was performed
using a Leica automatic stainer (Leica Microsystems, Inc.). Under
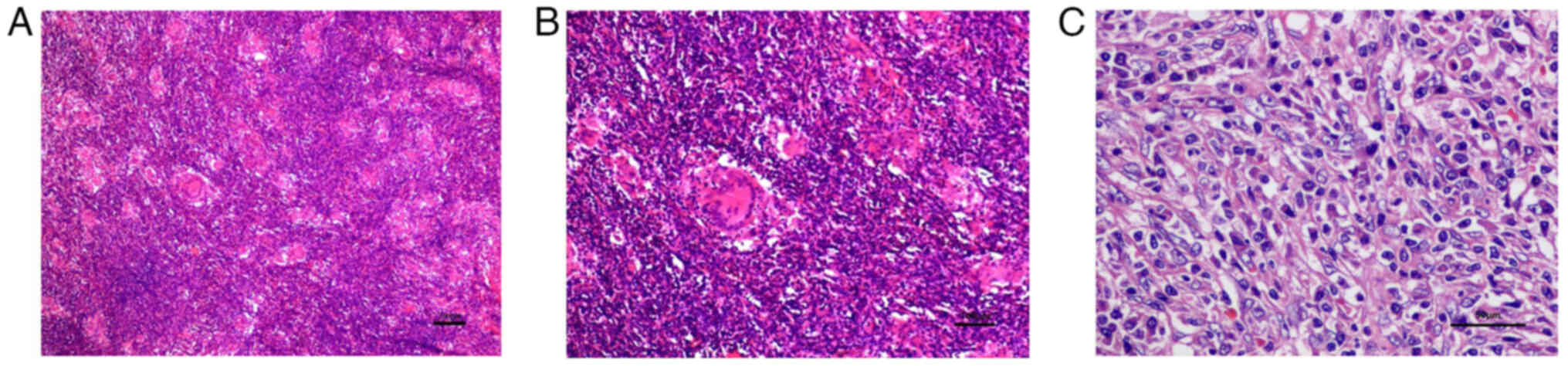
the microscope (BX43; Olympus Corporation), the normal structure of
the spleen appeared to be destroyed, and round, ovoid and
spindle-shaped cells were seen scattered in the background of a
large number of lymphocytes and plasma cells. The tumor cells were
arranged in bundles and cords, with unclear boundaries. The
cytoplasm is sparse and eosinophilic. The nuclei of the tumor cells
were rounded and ovoid. The tumor contains a large number of
non-caseous epithelioid granulomas (Fig. 2A).
Immunophenotypic findings
Using 4 µm tissue sections, after dewaxing and
rehydration, staining was performed using a fully automated
immunohistochemistry machine (Roche BenchMark ULTRA; Roche
Diagnostics). Primary antibodies employed included: Smooth muscle
actin (SMA; cat. no. UMAB237) and CD23 (cat. no. UMAB101) purchased
from Wuxi Origene Biotechnology Co., Ltd., CD3 (cat. no. MAB-0740),
CD5 (cat. no. MAB-0827), CD8 (cat. no. RMA-0514), CD20 (cat. no.
Kit-0001), CD21 (cat. no. RMA-0811), CD35 (cat. no. RMA-0768),
S-100 (cat. no. RMA-1075), podoplanin monoclonal antibody (D2-40;
cat. no. MAB-0567), C-X-C chemokine ligand 13 (CXCL13; cat. no.
GAB-0616) and anaplastic lymphoma kinase (ALK; cat. no. MAB-0848),
all procured from Fuzhou Maxin Biotechnology Development Co., Ltd.
After adding the primary antibody, incubation was performed at 37°C
for 60 min. Subsequently, samples were incubated with the
UV-HRP-UNIV MULT and UV-DAB kits (Ventana Medical Systems)
separately at 37°C for 8 min each. Following color development,
counterstain with hematoxylin was performed. All reagents were
ready-to-use and required no dilution during the procedure. After
sealing with neutral gum, slides were observed and imaged under an
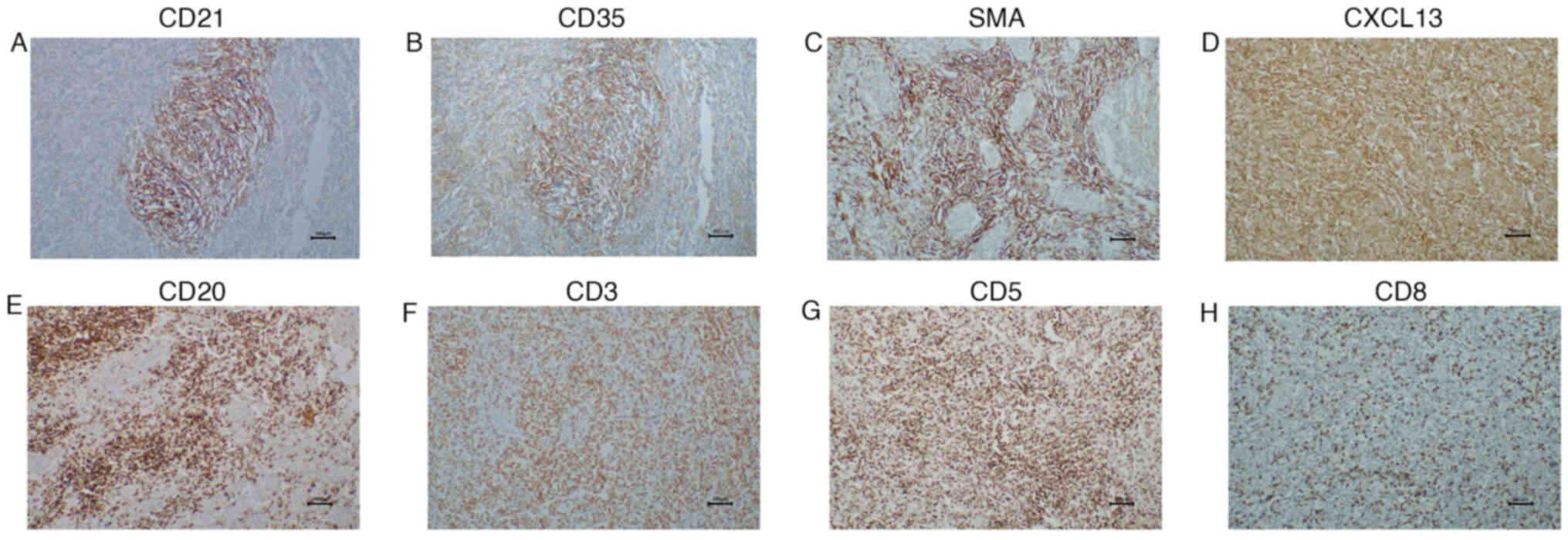
optical microscope. Immunohistochemical results revealed that tumor
cells expressed CD21, CD35, CXCL13 and SMA (Fig. 3A-D). Background lymphocytes were
partially positive for CD20, CD3, CD5 and CD8 (Fig. 3E-H). ALK, CD23, D2-40 and S-100
results were negative (data not shown).
In situ hybridization
Epstein-Barr encoding region (EBER) in situ
hybridization is the methodology of choice for the detection of the
EBV in tissue sections (10). All
operating procedures were performed in accordance with the
instructions of the EBV probe in situ hybridization kit
(cat. no. ISH-7001; OriGene Technologies, Inc.). After dewaxing
with xylene and anhydrous ethanol, the slices were air-dried for
5–10 min. Subsequently, 50–100 µl of gastric enzyme working
solution was added dropwise and incubated at 37°C for 30 min. After
discarding the gastric enzyme working solution, gradient ethanol
dehydration (75, 95 and 100% for 2 min each) was carried out and
samples were air-dried. Drops of 10 µl digoxigenin-labeled EBER
probe were added, coverslips were added and sealed with rubber
cement, and they were placed in an in situ hybridizer
(Thermobrite) and incubated at 37°C for 2 h. The coverslips were
removed and an appropriate amount of HRP-labeled digoxin antibody
was added dropwise and incubated at 37°C for 30 min. After washing
in PBS, DAB working solution was added, samples were counterstained
with hematoxylin staining solution, dehydrated, cleared and sealed.
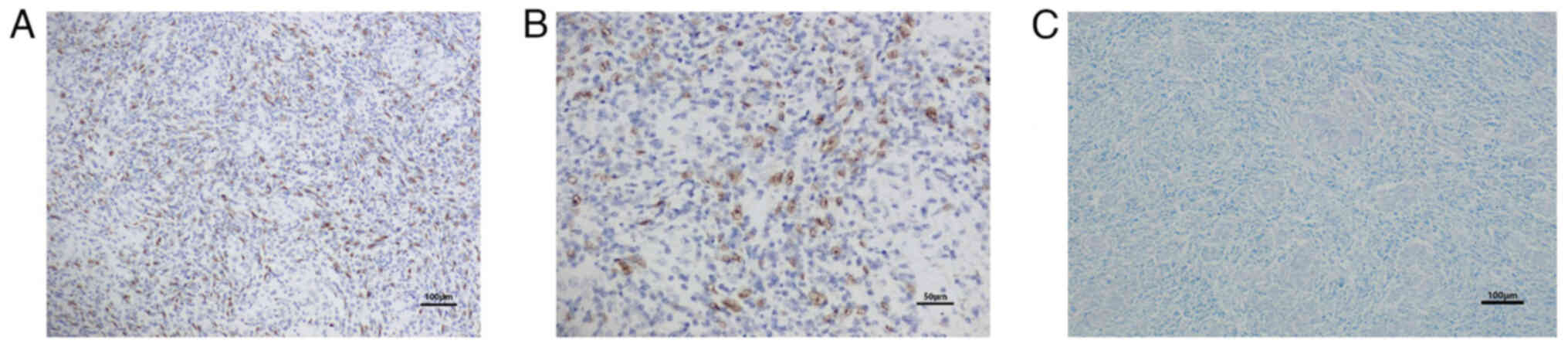
The results demonstrated that the tumor cell nuclei appeared
brownish yellow, indicating the presence of EBER (Fig. 4A and B).
Acid-fast staining
All procedures were performed according to the
instructions provided in the acid-fast staining kit (cat. no.
BA4090; Zhuhai Beiso Biotechnology Co., Ltd.). After
deparaffinization of the slices, without alcohol treatment, a
solution of stone carbonate was added to the slices and they were
stained at room temperature for 10–15 min. They were washed with
water and an acidic alcohol solution was added to decolorize for
1–2 min. Subsequently, they were washed again with water and
methylene blue solution was added to stain for 20–30 sec, and they
were finally washed with water and blown dry, and sealed with
neutral gum. The results indicated that Ziehl-Neelsen special stain
did not detect acid-fast bacilli (Fig.
4C).
Molecular assays for gene
rearrangements
Human genomic DNA was extracted from paraffin
samples according to the instructions for nucleic acid extraction
and purification reagents (cat. no. W006; Shanghai Yuanqi
Biotechnology Co., Ltd.). DNA purity and concentration were
measured using a NanoDrop 2000C microvolume spectrophotometer.
Patient T-cell receptor (TCR) and immunoglobulin (IG) gene
rearrangements were detected using the BIOMED-2 multiplex PCR
system (Thermo Fisher Scientific, Inc.). Multiplex PCR reactions
were performed as described previously (11). Detectable primers included three
VH-JH, two DH-JH, two Ig κ (IGK), one Ig λ, three TCR β, two TCR γ,
one TCR δ (12). A reaction system
of 20 µl was prepared. PCR amplification conditions on the PCR
amplifier (Hangzhou Bori Technology Co., Ltd.) were set as follows:
Pre-denaturation at 95°C for 7 min, followed by 40 cycles of
denaturation at 95°C for 45 sec, annealing at 60°C for 45 sec and
extension at 72°C for 90 sec. A final extension step was performed
at 72°C for 10 min. Following the manufacturer's instructions, the
PCR amplification products were denatured at 95°C for 3 min and
immediately placed on ice for 3 min. The products were analyzed by
capillary electrophoresis on a 3500DX Gene Analyzer (Thermo Fisher
Scientific, Inc.) and electrophoresis patterns were analyzed using
GeneMapper software (version 6.0; Thermo Fisher Scientific, Inc.).
All experiments included appropriate positive and negative
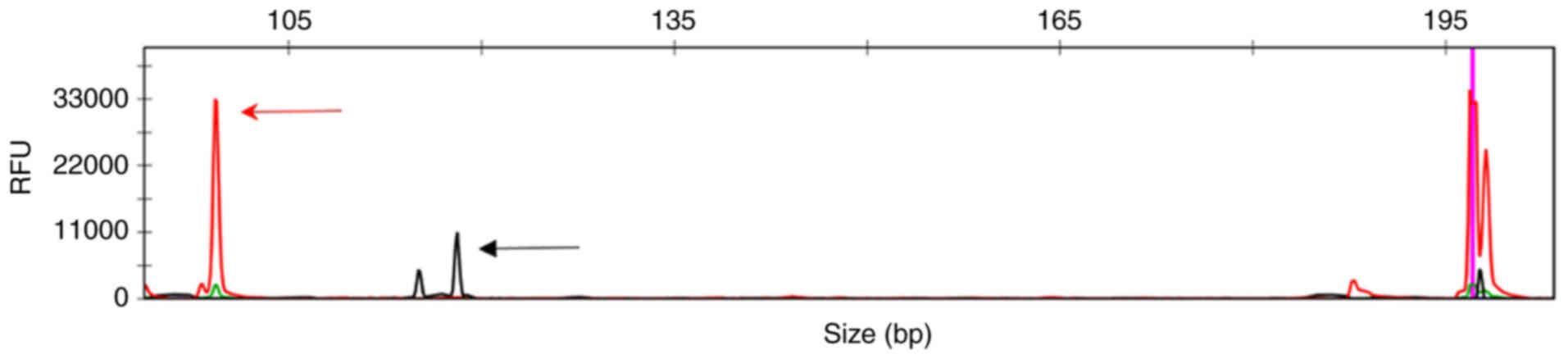
controls. The results revealed that an amplification peak appeared
in the DH7-JH region of IGH (Fig.
5).
Pathological diagnosis
According to the 5th edition of the World Health
Organization Classification, the patient was diagnosed with
EBV-positive inflammatory follicular dendritic cell sarcoma (August
2024).
Follow-up
The patient received anticoagulant therapy after
splenectomy surgery, taking aspirin enteric-coated tablets (0.1 g,
orally, once daily) and did not undergo radiotherapy or
chemotherapy postoperatively. Monthly telephone follow-ups were
conducted, and the patient is currently in good condition. As of
the date of this case submission (August 2025), the patient has
survived for one year.
Discussion
EBV+ IFDCS is a rare disease that
exhibits morphological and immunophenotypic features of FDCs and
the exact etiology and pathogenesis remain to be fully elucidated
(13). FDCs are major members of
primary and secondary lymphoid follicles, which present antigens in
a spatially defined form to B cells and maintain humoral immune
responses. They are derived from the mesenchyme and express markers
of FDC differentiation (14).
EBV+ FDCS is prevalent in the young to
middle-aged population, with a predominance in women and most
reports from Asian countries. It often involves the liver and
spleen, with a few cases occurring in the colon and other sites
(4,15). Patients are usually asymptomatic;
certain patients present with abdominal pain, abdominal discomfort
and a few present with systemic symptoms such as fever and weight
loss (15). Based on morphological
features, they are classified into classic, lymphomatoid and
hemangiomatoid subtypes (13). The
classic type has distinct EBV+ tumor cells with a
fascicular or columnar growth pattern, variable lymphoplasmacytic
infiltration and vascularity. The lymphoma-like subtype has a
prominent lymphoplasmacytic infiltrate and monodisperse distinct
EBV+ tumor cells. The hemangioma-like subtype has
prominent blood vessels, accompanied by transparent and/or
fibrinoid degeneration, dispersed individual EBV+ tumor
cells and limited lymphoplasma cell infiltration (13).
Tumor cells specifically express FDC markers, of
which CD21, CD23 and CD35 are extensively used as preferred FDC
markers. Other markers such as SMA, fascin, β-3 tubulin, clusterin,
D2-40, γ-synuclein and CXCL13 may also be positive. Tumor cells do
not express ALK, desmin, CD31, CD34 and S100 (16–18).
EBV+ IFDCS is an entity with an extensive morphological
spectrum and immunophenotype and tumor cells typically lose at
least one conventional FDC marker. Using a set of FDC markers is
more advantageous for diagnosis than relying on a single marker
(16). In the present case, tumor
cells expressed FDC markers (CD21, CD35 and CXCL13) to varying
degrees. EBV+ IFDCS exhibited a fibroblast/myoid
immunophenotype with positive expression of SMA (14). Positive expression of CD3, CD5, CD8
and CD20 suggested that the background lymphocytes were a mixture
of T and B cells.
EBV is considered to serve a key role in the
occurrence of IFDCS (19). EBER
in situ hybridization is a key method for the accurate
diagnosis of EBV+ IFDCS, with a positive result
suggesting the diagnosis of EBV+ IFDCS. Chen et
al (20) reported a 30 bp
deletion of exon 3 of the latent membrane protein-1 (LMP-1) gene in
hepatic FDC tumors. It has been reported that EBV preferentially
infects B lymphocytes by binding to the CD21 receptor on the
surface of B cells via the envelope glycoprotein (gp)350 and
binding gp42 to human leukocyte antigen class II (21,22).
The pathogenesis of EBV infection in EBV+ IFDCS remains
to be elucidated. Abe et al (23) speculated that EBV initially infects
human B lymphocytes in lymphoid follicles. EBV lurks in B cells and
begins to infect FDCs. EBER or EBV-encoded LMP-1 is released from
EBV into FDCs, leading to inhibition of apoptosis and FDC
proliferation by amplifying the CD40 signaling pathway. EBV can
infect any resting B cells, driving them out of their resting state
and becoming activated proliferating lymphoblasts. EBV-encoded
LMP-1 (CD40 homolog of EBV) and LMP2A (B-cell receptor homolog of
EBV) are considered to provide signals independent of
antigen-driven interactions with helper T cells or FDCs and can
utilize the normal pathway of B cell differentiation to transform
EBV-infected B progenitor cells into resting memory cells (24,25).
LMP-1 is the main oncogene of EBV, which can activate various
cellular signaling pathways and upregulate anti-apoptotic proteins,
thereby inhibiting apoptosis and promoting tumor development
(25,26).
Previously, Lorenzi et al (27) investigated FDCS using whole-genome
and whole-exome sequencing, observing CDK inhibitor 2A deletion and
frequent mutations on retinoblastoma 1, BRCA2, Werner syndrome
RecQ-like helicase and tumor protein 53. The accumulation of
inactivating mutations on these genes was associated with poor
prognosis. Currently, there is limited molecular research on
EBV-associated IFDCS. Li et al (13) identified clonal TCR rearrangements
in three cases of lymphoma-like subtypes, one of which was
accompanied by clonal IG gene rearrangement. Xu et al
(28) also reported a case of
EBV+ IFDCS with clonal IGH gene rearrangement occurring
in the colon. The underlying mechanisms of these findings warrant
further investigation. The present study reported a case of
EBV+ IFDCS with clonal IGH gene rearrangement. There are
three main hypotheses regarding clonal receptor gene rearrangement
in histiocytic and dendritic cell tumors, including
dedifferentiation, common progenitor and transdifferentiation
(29). ‘De-differentiation’ occurs
by returning to the pluripotent progenitor stage and then
re-differentiating along the histiocytic/dendritic lineage. ‘Common
progenitor’ refers to the existence of a common premalignant
progenitor cell, particularly a pluripotent progenitor, that
differentiates at different times along the B cell and
histiocytic/dendritic lineages. ‘Transdifferentiation’ bypasses the
progenitor cell stage and directly differentiates from tumor B
cells into malignant histiocytes or dendritic cells (30–32).
In 2009, Chen et al (33)
reported that a high frequency of clonal IG receptor gene
rearrangements was detected in sporadic histiocyte/dendritic cell
(H/DC) sarcomas and 4 cases of IGH/IGK+ H/DC sarcomas
were positive for organic cation transporter 2, indicating that
these H/DC sarcomas may inherit the B cell genotype and may
originate from stereotyped B-cell progenitor cells. Huang et
al (34) detected not only
clonal IGH/IGK but also TCRβ/γ gene rearrangements in 33 cases of
H/DC sarcomas, which seems to support the idea that H/DC tumors
develop from lymphoid stereotyped progenitor cells. The two
different types of tumors may derive from a common precursor and
differentiate in two different directions under pathological
conditions. In 2004, Xie et al (35) reprogrammed B cells into macrophages
and reported that the reprogrammed macrophages exhibited heavy and
light chain IG rearrangements. Barone et al (36) reported a case of mantle cell
lymphoma with an apparent lineage transition to EBV+
T-cell lymphoma, indicating that a lineage transition from a mature
B-cell phenotype to a mature T-cell phenotype is possible.
Based on the aforementioned findings, we
hypothesized the following: i) EBV latent in B cells infects FDCS
and EBER or LMP-1 is released from EBV into FDCS, leading to
inhibition of apoptosis and FDC proliferation by amplifying the
CD40 signaling pathway; ii) EBV infects resting B cells and turns
them into activated proliferating lymphoblasts that then
differentiate along FDCs; and iii) EBV-infected B cells
differentiate directly into FDCs. The clonal IGH gene rearrangement
detected in the present case may also be associated with
differentiation of clonal EBV-infected B cells to FDC. The existing
data could not draw a clear conclusion and the mechanism of
occurrence still warrants further exploration in future
research.
The main differential diagnoses of EBV+
IFDCS include inflammatory myofibroblastoma (IMT) and classic
Hodgkin's lymphoma (CHL). IMT demonstrated elongated or polygonal
myofibroblasts with eosinophilic or biphasic cytoplasm, infiltrated
by plasma cells, lymphocytes and eosinophils. The histological
morphology is similar to that of EBV+ IFDCS, but IMT is
prevalent among children ranging from 2 months to 24 years of age
(median age, 9.5 years) and the majority of cases express ALK,
desmin and SMA but not FDC markers and are EBER- (37,38).
CHL is a lymphoblastic neoplasm with a small number of diagnostic
Reed-Sternberg cells and their variants scattered in a
characteristic reactive cellular background. CD30 and CD15 are
usually expressed (39).
Furthermore, the present case exhibits prominent granulomas,
necessitating differential diagnosis from granulomatous diseases
such as sarcoidosis and tuberculosis. Sarcoidosis typically
involves multiple systems, particularly the lungs, but FDC
immunomarkers and EBER are negative, while tuberculosis
demonstrates positive acid-fast staining (2).
Due to the rarity of this disease, there is no
standard treatment. Saygin et al (14) conducted a comprehensive analysis of
462 cases of dendritic cell sarcoma and reported that surgery was
the most effective treatment method. Adjuvant radiotherapy had no
significant impact on the overall survival of patients and the role
of chemotherapy in the treatment of advanced diseases is
controversial. By contrast, Pang et al (40) observed a significantly lower local
recurrence rate in patients receiving surgery plus radiotherapy
compared with those undergoing surgery alone when studying FDCS of
the head and neck. In clinical practice, chemotherapy is often used
for patients with metastatic FDCS, although there is no consensus
on the most effective regimen (41). At present, due to the lack of
consensus on the specific roles of chemotherapy and radiotherapy in
the management of FDCS, surgical resection remains the main
treatment method. In the future, large-scale multicenter
prospective studies are warranted to effectively evaluate adjuvant
therapy.
In conclusion, the present study described a rare
case of EBV+ IFDCS occurring in the spleen with clonal
IGH gene rearrangement. Two hypotheses were proposed: i) Clonal
EBV-infected B cells differentiation to FDC; and ii) IGH gene
rearrangement occurring in the background B cells. The key
principles behind the present case finding still warrants further
research in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures of this article.
Authors' contributions
WZ evaluated the samples cytologically and
histologically and clarified the diagnosis. JH completed the
relevant experiments. QY and JZ participated in the analysis and
interpretation of the results. QY, JZ, JH and WZ confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of anonymized data and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jin J, Zhu X, Wan Y and Shi Y:
Epstein-barr virus (EBV)-positive inflammatory pseudotumor-like
follicular dendritic cell sarcoma (IPT-like FDCS) presenting as
thrombocytopenia: A case report and literature review. Heliyon.
10:e329972024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nie C, Xie X, Li H, Li Y, Chen Z, Li Y and
Li Z: Epstein-Barr virus-positive inflammatory follicular dendritic
cell sarcoma with significant granuloma: Case report and literature
review. Diagn Pathol. 19:342024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen HGD, Zhang Y and Leow WQ: Uncommon
granulomatous manifestation in Epstein-Barr virus-positive
follicular dendritic cell sarcoma: A case report. J Pathol Transl
Med. 59:133–138. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu J, Huang D, Xu C, Chen Y, Ma H and Shen
Z: Epstein-barr virus-positive inflammatory follicular dendritic
cell sarcoma presenting as a colonic polyp: Report of a case with a
literature review. Medicina (Kaunas). 59:13412023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen YY, Wang PM, Deng LZ, Li PH, Li CG,
He YH, Chen F and Yue JY: Imaging and pathological characteristics
of hepatosplenic EBV-positive inflammatory follicular dendritic
cell sarcoma. Front Oncol. 15:15521932025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monda L, Warnke R and Rosai J: A primary
lymph node malignancy with features suggestive of dendritic
reticulum cell differentiation. A report of 4 cases. Am J Pathol.
122:562–572. 1986.PubMed/NCBI
|
|
7
|
Shek TW, Ho FC, Ng IO, Chan AC, Ma L and
Srivastava G: Follicular dendritic cell tumor of the liver.
Evidence for an Epstein-Barr virus-related clonal proliferation of
follicular dendritic cells. Am J Surg Pathol. 20:313–324. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheuk W, Chan JK, Shek TW, Chang JH, Tsou
MH, Yuen NW, Ng WF, Chan AC and Prat J: Inflammatory
pseudotumor-like follicular dendritic cell tumor: A distinctive
low-grade malignant intra-abdominal neoplasm with consistent
Epstein-Barr virus association. Am J Surg Pathol. 25:721–731. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alaggio R, Amador C, Anagnostopoulos I,
Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D,
Calaminici M, et al: The 5th edition of the World health
organization classification of haematolymphoid tumours: Lymphoid
neoplasms. Leukemia. 36:1720–1748. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weiss LM and Chen YY: EBER in situ
hybridization for Epstein-Barr virus. Methods Mol Biol.
999:223–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sandberg Y, van Gastel-Mol EJ, Verhaaf B,
Lam KH, van Dongen JJ and Langerak AW: BIOMED-2 multiplex
immunoglobulin/T-cell receptor polymerase chain reaction protocols
can reliably replace Southern blot analysis in routine clonality
diagnostics. J Mol Diagn. 7:495–503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Dongen JJ, Langerak AW, Brüggemann M,
Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E,
García-Sanz R, et al: Design and standardization of PCR primers and
protocols for detection of clonal immunoglobulin and T-cell
receptor gene recombinations in suspect lymphoproliferations:
Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia.
17:2257–2317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Yang X, Tao L, Zeng W, Zuo M, Li S,
Wu L, Lin Y, Zhang Z, Yun J and Huang Y: Challenges in the
diagnosis of epstein-barr virus-positive inflammatory follicular
dendritic cell sarcoma: Extremely wide morphologic spectrum and
immunophenotype. Am J Surg Pathol. 47:476–489. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saygin C, Uzunaslan D, Ozguroglu M,
Senocak M and Tuzuner N: Dendritic cell sarcoma: A pooled analysis
including 462 cases with presentation of our case series. Crit Rev
Oncol Hematol. 88:253–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge R, Liu C, Yin X, Chen J, Zhou X, Huang
C, Yu W and Shen X: Clinicopathologic characteristics of
inflammatory pseudotumor-like follicular dendritic cell sarcoma.
Int J Clin Exp Pathol. 7:2421–2429. 2014.PubMed/NCBI
|
|
16
|
Choe JY, Go H, Jeon YK, Yun JY, Kim YA,
Kim HJ, Huh J, Lee H, Shin DH and Kim JE: Inflammatory
pseudotumor-like follicular dendritic cell sarcoma of the spleen: A
report of six cases with increased IgG4-positive plasma cells.
Pathol Int. 63:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen S, You Z, Chen X and Wang C:
Clinicopathological and molecular genetic insights into
EBV-positive inflammatory follicular dendritic cell sarcoma. Hum
Pathol. 153:1056682024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panigrahi C, Nayak HK, Patra S and Mitra
S: Hepatic follicular dendritic cell sarcoma with epithelioid
morphology: Histopathologist's perspective. J Clin Exp Hepatol.
12:677–685. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kiryu S, Takeuchi K, Shibahara J, Uozaki
H, Fukayama M, Tanaka H, Maeda E, Akahane M and Ohtomo K:
Epstein-Barr virus-positive inflammatory pseudotumour and
inflammatory pseudotumour-like follicular dendritic cell tumour. Br
J Radiol. 82:e67–e71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen TC, Kuo TT and Ng KF: Follicular
dendritic cell tumor of the liver: A clinicopathologic and
Epstein-Barr virus study of two cases. Mod Pathol. 14:354–360.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nemerow GR, Mold C, Schwend VK, Tollefson
V and Cooper NR: Identification of gp350 as the viral glycoprotein
mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d
receptor of B cells: Sequence homology of gp350 and C3 complement
fragment C3d. J Virol. 61:1416–1420. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borza CM and Hutt-Fletcher LM: Alternate
replication in B cells and epithelial cells switches tropism of
Epstein-Barr virus. Nat Med. 8:594–599. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abe K, Kitago M, Matsuda S, Shinoda M,
Yagi H, Abe Y, Oshima G, Hori S, Endo Y, Yokose T, et al:
Epstein-Barr virus-associated inflammatory pseudotumor variant of
follicular dendritic cell sarcoma of the liver: A case report and
review of the literature. Surg Case Rep. 8:2202022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thorley-Lawson DA: Epstein-Barr virus:
Exploiting the immune system. Nat Rev Immunol. 1:75–82. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hatton O, Martinez OM and Esquivel CO:
Emerging therapeutic strategies for Epstein-Barr virus+
post-transplant lymphoproliferative disorder. Pediatr Transplant.
16:220–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grimm T, Schneider S, Naschberger E, Huber
J, Guenzi E, Kieser A, Reitmeir P, Schulz TF, Morris CA and Stürzl
M: EBV latent membrane protein-1 protects B cells from apoptosis by
inhibition of BAX. Blood. 105:3263–3269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lorenzi L, Haferlach T, Mori L, Simbeni M,
Walter W, Balzarini P, Meggendorfer M, Döring C, Lonardi S, Bugatti
M, et al: Massive parallel sequencing unveils homologous
recombination deficiency in follicular dendritic cell sarcoma.
Haematologica. 109:1815–1824. 2024.PubMed/NCBI
|
|
28
|
Xu X, Li X, Deng Q, Yu K and Li J:
EBV-positive inflammatory follicular dendritic cell sarcoma of the
colon with clonal immunoglobulin gene rearrangement: A case report
and literature review. Heliyon. 10:e319472024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stoecker MM and Wang E:
Histiocytic/dendritic cell transformation of B-cell neoplasms:
Pathologic evidence of lineage conversion in differentiated
hematolymphoid malignancies. Arch Pathol Lab Med. 137:865–870.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feldman AL, Arber DA, Pittaluga S,
Martinez A, Burke JS, Raffeld M, Camos M, Warnke R and Jaffe ES:
Clonally related follicular lymphomas and histiocytic/dendritic
cell sarcomas: Evidence for transdifferentiation of the follicular
lymphoma clone. Blood. 111:5433–5439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feldman AL, Minniti C, Santi M, Downing
JR, Raffeld M and Jaffe ES: Histiocytic sarcoma after acute
lymphoblastic leukaemia: A common clonal origin. Lancet Oncol.
5:248–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie H and Orkin SH: Immunology: Changed
destiny. Nature. 449:410–411. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen W, Lau SK, Fong D, Wang J, Wang E,
Arber DA, Weiss LM and Huang Q: High frequency of clonal
immunoglobulin receptor gene rearrangements in sporadic
histiocytic/dendritic cell sarcomas. Am J Surg Pathol. 33:863–873.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang W, Qiu T, Zeng L, Zheng B, Ying J
and Feng X: High frequency of clonal IG and T-cell receptor gene
rearrangements in histiocytic and dendritic cell neoplasms.
Oncotarget. 7:78355–78362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie H, Ye M, Feng R and Graf T: Stepwise
reprogramming of B cells into macrophages. Cell. 117:663–676. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barone PD, Tam W, Geyer JT, Leonard JP,
Phillips A and Ouseph MM: Nodal T-cell lymphoma transdifferentiated
from mantle cell lymphoma with epstein-barr virus infection.
Pathobiology. 92:109–120. 2025.PubMed/NCBI
|
|
37
|
Mahajan P, Casanova M, Ferrari A, Fordham
A, Trahair T and Venkatramani R: Inflammatory myofibroblastic
tumor: Molecular landscape, targeted therapeutics, and remaining
challenges. Curr Probl Cancer. 45:1007682021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Casanova M, Brennan B, Alaggio R, Kelsey
A, Orbach D, van Noesel MM, Corradini N, Minard-Colin V, Zanetti I,
Bisogno G, et al: Inflammatory myofibroblastic tumor: The
experience of the European pediatric Soft Tissue Sarcoma Study
Group (EpSSG). Eur J Cancer. 127:123–129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang HW, Balakrishna JP, Pittaluga S and
Jaffe ES: Diagnosis of Hodgkin lymphoma in the modern era. Br J
Haematol. 184:45–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pang J, Mydlarz WK, Gooi Z, Waters KM,
Bishop J, Sciubba JJ, Kim YJ and Fakhry C: Follicular dendritic
cell sarcoma of the head and neck: Case report, literature review,
and pooled analysis of 97 cases. Head Neck. 38 (Suppl
1):E2241–E2249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Ren M, Bi F, Chen Y and Li Z:
Favorable response to PD-1 inhibitor plus chemotherapy as
first-line treatment for metastatic follicular dendritic cell
sarcoma of the spleen: a case report. Front Immunol.
14:12286532023. View Article : Google Scholar : PubMed/NCBI
|