Introduction
In the clinic, breast cancer (BC) has been commonly
characterized by the absence or presence of three key proteins:
estrogen receptor (ER), progesterone receptor) and the growth
factor receptor HER2 (1). This
information allows clinicians to choose specific treatment options
that target these receptors. BC that lacks the expression of all
three of these proteins is termed triple-negative breast cancer
(TNBC). In the case of TNBC, the drugs developed to target these
proteins are ineffective and standard chemotherapy is employed to
treat most patients. TNBC represents 15–20% of BC cases and it is
the most aggressive subtype of breast cancer with the lowest 5-year
survival rate (2). Gene expression
profiling provides an additional method to subtype BC into five
sub-groups according to their gene transcription patterns: Luminal
A, Luminal B, HER2, basal and normal-like (3). The basal subtype has significant
overlap with the TNBC subtype and its characteristics. TNBC will
frequently respond to chemotherapy initially but subsequently grow
back more aggressive and resistant to therapy. It is also more
likely to metastasize than other subtypes. Due to this re-growth
and metastasis after initial positive response to chemotherapy,
there is a need for safe and tolerable daily maintenance therapy
that can slow or prevent this re-growth.
Inflammatory breast cancer (IBC) is a rare type of
BC accounting for 2–6% of all breast cancer cases, however, it
accounts for 10% of all BC deaths (4,5). IBC
is primarily characterized by its clinical presentation that
includes diffuse skin thickening known as peasu d'orange
phenomenon, edema, erythema, nipple abnormalities and swelling of
the breast (6,7). These characteristics typically have a
rapid onset within a six-month period, may or may not include a
palpable lump and can be readily mistaken for infection or other
breast conditions. These distinctive visible hallmarks of IBC are
caused by tumor emboli blocking lymphatic ducts. IBC is an
extremely aggressive type of BC that has frequently metastasized by
the time of diagnosis. Although IBC clinically presents differently
than other BC subtypes, it is still evaluated and categorized into
the above subtypes (e.g., TNBC) and these biomarkers used to guide
treatment options. Thus, a case of IBC may also be subtyped as
triple-negative, referred to here as TN-IBC. TN-IBC has the lowest
survival rate within the IBC classification, compared to ER+ and
HER2+ subtypes (8). The standard of
care for IBC is neoadjuvant chemotherapy (traditional or targeted,
depending on subtype), radical mastectomy and then radiation
post-mastectomy (4,9).
While progress has been made in identifying drugs
for TNBC, e.g., PARP and immune checkpoint inhibitors, there is
still a lack of therapies specifically targeting triple-negative
forms of breast cancer. Drug repurposing is the identification of
new clinical applications for existing, Food and Drug
Administration (FDA)-approved drugs, thereby reducing the time and
financial cost associated with the traditional drug discovery
process (10). In addition, due to
already known clinical and safety data reducing the need for
extensive preclinical testing, the repurposed drug may be less
expensive for the patient and healthcare system. In the cancer
field, drug repurposing can entail either the repurposing of cancer
drugs approved for a different type of cancer or the use of
non-oncology drugs (NODs). NODs are FDA-approved (or other
agencies) drugs that were originally approved for indications other
than cancer. Many NODs have been discovered to have activity
against specific cancers in vitro and in vivo
(10). These NODs hold the
potential to be less toxic than standard chemotherapy. These unique
NODs may be administered simultaneously with chemotherapy to
enhance efficacy or reduce the dose of the more toxic
chemotherapies.
We propose additional use of NODs with anti-cancer
activity. New therapies are needed to reduce the risk of relapses
and slow cancer re-growth/metastasis in the interval after standard
treatments have completed-thus providing constant therapy to the
patient. We propose that tolerable, low toxicity NODs with
anticancer activity may serve as maintenance therapy that could be
taken on a regular basis for the interval between standard of care
treatments or after they are completed. This maintenance therapy is
envisioned to reduce the risk of recurrence or slow cancer
re-growth and metastasis, attenuating disease progression in the
interval between standard of care treatments. NODs are especially
well suited for this purpose due to their much better safety and
tolerability profiles that may allow daily dosing, unlike toxic
chemotherapy agents.
In this report, we sought to identify NODs with
activity against TN-IBC and TNBC. We employed the SUM-149 cell line
grown in spheroid culture as a model assay for TN-IBC. We used this
assay to screen a collection of NODs identified and curated from a
published database based on the screen of drugs against standard
TNBC cell lines (among others), but not IBC cell lines. In
parallel, we also tested a subset of these NODs against other TNBC
cell lines grown in two-dimension (2-D) culture. We identified 15
NODs with activity against SUM-149. Eight of these were also tested
against a panel of TNBC cell lines grown in spheroid culture to
assess breadth of activity. Several drugs, including eltrombopag
and papaverine, may have potential for repurposing for these
aggressive cancers.
Materials and methods
Materials
Plasticware and general reagents, including dimethyl
sulfoxide (DMSO), were obtained from Fisher Scientific. Cell
culture media and supplements were obtained from ThermoFisher and
Hyclone (GE Healthcare). CellTox Green stock solution and the
CellTiter Glo (CTG) 2.0 cell viability reagent were purchased from
Promega. Paclitaxel and cycloheximide were purchased from
Sigma-Aldrich; Merck KGaA. The drugs were purchased from
SelleckChem with a stated purity of ≥97%. D300 Digital Dispenser
and T4 and T8 cassettes were purchased from Tecan.
Cell culture
The cell lines MDA-MB-231, HCC-1806 and Hs578T were
obtained from American Type Tissue Collection (ATCC). MDA-MB-231
and Hs578T were grown in DMEM supplemented with 10% fetal bovine
serum (FBS) and Hs578T media was further supplemented with 0.01
mg/ml insulin. HCC-1806 were cultured in RPMI-1640 with 10% FBS.
SUM-149 and SUM-159 were obtained from BioIVT. These cell lines
were grown in DMEM/F12 supplemented with 10 mM HEPES, 5% FBS, 5
µg/ml insulin, 20 ng/ml EGF and 1 µg/ml hydrocortisone. The media
for all the cell lines were further supplemented with 2.5 mM
L-glutamine, 100 U/ml penicillin and 100 U/ml streptomycin. The
cells were grown in tissue-culture treated flasks in a humidified
incubator at 37°C and 5% CO2 and cells were harvested
for assays with trypsin-EDTA (Versene). Cell lines were tested
periodically for mycoplasma using the MycoAlert kit (Lonza, Basel,
Switzerland) and they tested negative for mycoplasma. The cell
lines were obtained directly from the vendors which authenticated
the cell lines. ATCC uses STR profiling to authenticate their cell
lines. All cell lines were amplified and stored frozen within 2–3
passages from original cells provided by the vendor. Experiments
were performed with cells that were less than twenty passages from
the vendor supplied cells.
Spheroid growth and cytotoxicity
assays
For the initial single concentration screening of
the 48 drugs with SUM-149, we used a combination of real time
imaging using a real-time microscopic imaging instrument (Incucyte
S3, Sartorius) to monitor spheroid area and fluorescence from the
CellTox Green cytotoxicity dye and subsequently, CTG was employed
to detect viable cells by ATP detection. The cell lines were
harvested, counted with a Vi-Cell XR Cell Viability Analyzer
(Beckman Coulter) and then plated in 96-well plates (Sbio, Catalog
# MS-9096UZ) at 5,000 cells/well in 100 µl/well complete growth
media. The plate was centrifuged at 200 × g for 10 min and then put
in the incubator for 72 h for spheroid formation. Subsequently, the
drugs and dye were added the same day. CellTox Green dye was added
to all wells at 1:1,000 final dilution (100 nl) of the stock
solution using the D300 Digital Dispenser with T8 cassettes.
Immediately after dye addition, the D300 was used to directly
deliver compounds dissolved in DMSO or DMSO only (for solvent
control wells) to create single point tests or 8-point half-log
serial dilutions (for follow up concentration response assays). The
D300 was employed to adjust DMSO in all wells to the same final
DMSO concentration of 0.1% with T8 or T4 cassettes following the
manufacturer's protocol for the instrument. Subsequently, the assay
plates were put into the imaging instrument and monitored every 4
or 6 h. After 96 h, 50 or 100 µl of CTG was added to the wells,
plate shaken 5 min and then incubated at room temp for 30 min
covered by aluminum foil to protect from light before luminescence
detected by Pherastar (BMG) or GloMax Explorer (Promega) plate
readers. The Incucyte Spheroid (Sartorius) software module was used
to determine spheroid area by phase contrast and total green
fluorescence of the well. The initial measurement for spheroid area
(0 h) was subtracted from the final measurement for each well (96
h) to determine change in area of the spheroid for each well. This
change in area reflects spheroid growth during the assay, while the
total fluorescence reflected a change in accessible DNA which
reflects cytotoxicity. Time 0 fluorescence values were used to
check for autofluorescence of the compound that may be the source
of higher fluorescence. Change in area of each well (spheroid) was
normalized to the mean of the change in area of the DMSO only wells
(used as 100% growth) to obtain percent of control which reflected
growth of spheroid. Total fluorescence was normalized to ‘no cells’
(media only) wells and DMSO wells. Thus, values >100%
represented cytotoxicity. Similarly, luminescence from the CTG part
of the assay was normalized using the mean of ‘no cells’ wells (0%
viability) and DMSO control wells (100% viability). Known cytotoxic
drug paclitaxel (0.1 µM) and cycloheximide (10 µM; primarily an
inhibitor of cell proliferation) were used as controls to confirm
detection of cell death and inhibition of cell proliferation,
respectively. The CTG single concentration data was assessed for
statistical significance using unpaired t-test to identify active
hits, defined as significantly (P<0.05) less than 100% of
control.
For subsequent assays to calculate the concentration
of drug that produced 50% growth inhibition (GI50), only
the CTG assay was performed as above. To establish the signal
representing no cell proliferation, a zero-day control plate was
used where it was plated along with other plates and at the time of
drug treatment, this plate was developed with CTG. The mean of this
zero-day control plate was subtracted from each well and then this
value normalized to the mean of no cells and DMSO control wells to
calculate cell proliferation as a percentage of control. The
GI50 values were calculated from the normalized cell
proliferation data (as a percentage of control) vs. drug
concentration graphs using a four-parameter non-linear regression
curve fit with the bottom of the curve fixed to 0% (representing no
proliferation) using Prism (Graphpad) graphing software. Values
below zero represented cytotoxicity.
2-D cell proliferation screening and
GI50 assays
For the initial screening of the drugs in 2-D, we
employed the Incucyte imager to monitor cell confluency in a
96-well format. The harvested cells were seeded at 5,000 cells/well
(90 µl) in a 96-well plate (Corning plate #3603) and incubated in
the biosafety cabinet for 45 min at room temperature before
transferring to an incubator at 37°C and 5% CO2
atmosphere. After 24 h, cells were treated manually with compounds
diluted into growth media (10 µl) in duplicate wells and incubated
in the Incucyte imager for 72 h. The final DMSO in compound treated
wells was 0.1% and DMSO was used at 0.1% final concentration for
maximum signal control wells. Paclitaxel (0.1 µM) and cycloheximide
(10 µM) were used as positive controls. The relative cell growth
(increase in confluency) for every well was calculated by first
subtracting the confluency data obtained from the first Incucyte
read (0 h, representing initial cell area) from the final 72 h read
(72 h read-0 h read) to get a net confluency change for every well.
This net confluency of every well was then divided by the mean net
confluency of the DMSO control well then multiplied by 100 to get
relative cell proliferation. This data was plotted using GraphPad
Prism and assessed for statistical significance using unpaired
t-test to identify active hits, defined as significantly
(P<0.05) less than 100% of control and having a mean of at least
20% inhibition. For subsequent GI50 and cytotoxicity
assays, we employed the CellTox Green dye to also measure
cytotoxicity along with proliferation in a 384-well format. Hs578T,
MDA-MB-231 and SUM-159 cells were seeded at 2,000, 1,500 and 600
cells/well (50 µl/well), respectively, in 384-well plates (Corning,
plate # 3764). The cells were incubated for 24 h at 37°C and 5%
CO2 and then treated with compound using a D300 digital
dispenser as triplicates for each compound concentration using an
8-point half-log or two-fold serial dilution scheme starting with
10 µM as the highest concentration. DMSO-only wells were used as a
solvent control representing maximal cell proliferation. All wells
were adjusted to a final concentration of 0.1% DMSO using the D300.
To measure cytotoxic activity, 50 nl of the DNA-binding fluorescent
dye CellTox Green stock solution was added to each well immediately
after compound addition, resulting in a 1:1,000 dilution from the
stock solution provided by the manufacturer. Cells were incubated
and monitored for 96 h in the Incucyte as above. To obtain relative
cell growth, the confluency data was analyzed by subtracting the
initial confluency from the 72 h confluency (72-0 h reads) to get
net confluency for each well and then normalizing to the mean net
confluency of the DMSO control wells using the same method as
above. The GI50 values were calculated as above where
the bottom was fixed to zero (representing no change in
confluency/growth). Relative cytotoxicity values were separately
calculated using a published method (11) where the percent green fluorescence
confluency values (area of green fluorescence) were divided by the
total brightfield confluency (area of cell monolayer), multiplied
by 100, where these values were generated by the Incucyte
software.
Statistical analysis
In all cases where statistical analysis was
performed, an unpaired t-test was employed with n=4 or 6 data
points aggregated from replicate experiments where the comparison
is between drug treated wells and control wells. An alpha level of
0.05 was used. Error bars on all graphs and variability (±)
provided with values represent standard deviation (SD).
Results
NOD screening approach
We sought to identify NODs with the potential to be
repurposed for TN-IBC, and TNBC in general as maintenance therapy.
As a first step towards this goal, we utilized the high throughput
screening data from Corsello et al (12) that is publicly available in the
PRISM database (https://depmap.org/repurposing). This group screened a
large collection of compounds and drugs using 578 different cancer
cell lines by testing drugs against multiple pools of 25
DNA-encoded cell lines mixed in a well in a highly multiplexed cell
viability/proliferation assay. We queried this database to obtain
all the activity data for non-cancer drugs and approved drugs
(Fig. 1). Further selection was
applied to those NODs with measurable IC50 values (≤10
µM) and with activity in at least three TNBC cell lines, as there
were no IBC cell lines in the original screen. These criteria
resulted in 100 candidate compounds. From this list, we eliminated
actives that were not applicable for direct drug repurposing since
they would require steps similar to de novo drug discovery, were
redundant or otherwise not of practical use (Fig. 1). Thus, drugs were eliminated that
were approved for topical use only, not genuinely approved drugs,
highly similar to cancer drugs, prodrugs of other drugs on the
list, controlled access drugs and those approved only for animal
use. We also chose just one member of highly selective drug classes
such as HMG CoA reductase inhibitors (statins). After this further
selection process, 48 drugs (Fig.
2) remained on our list and were sourced from vendors for
testing in our assays.
We screened these 48 NODs for cytotoxic and
anti-proliferative activity against the TN-IBC cell line SUM-149
grown as spheroids. In parallel, we also queried PubMed with the 48
NODs to identify those that have been understudied for TNBC, i.e.,
≤1 publication using the terms TNBC and the drug name. The
resulting 33 drugs were then screened for activity using two TNBC
cell lines, MDA-MB-231 and SUM-159, grown in standard 2-D culture.
The 2-D assay was a more rapid screening method enabling the
initial screening using two different TNBC cell lines. Since the
resultant 2-D data cannot necessarily be directly compared to
spheroid data, the hits of highest interest in the 2-D assays were
re-tested in spheroid assays as well. Confirmed actives from the
SUM-149 and TNBC screens that were also identified as understudied
in IBC or TNBC were tested against a panel of TNBC cells grown as
spheroids to determine the breadth of activity. Representative
images of spheroids for each cell line at treatment time support
spheroid integrity (Fig. S1).
Prior to treatment, control wells were assessed for viability using
CTG and this value was also used to calculate cell proliferation as
a percentage of controls.
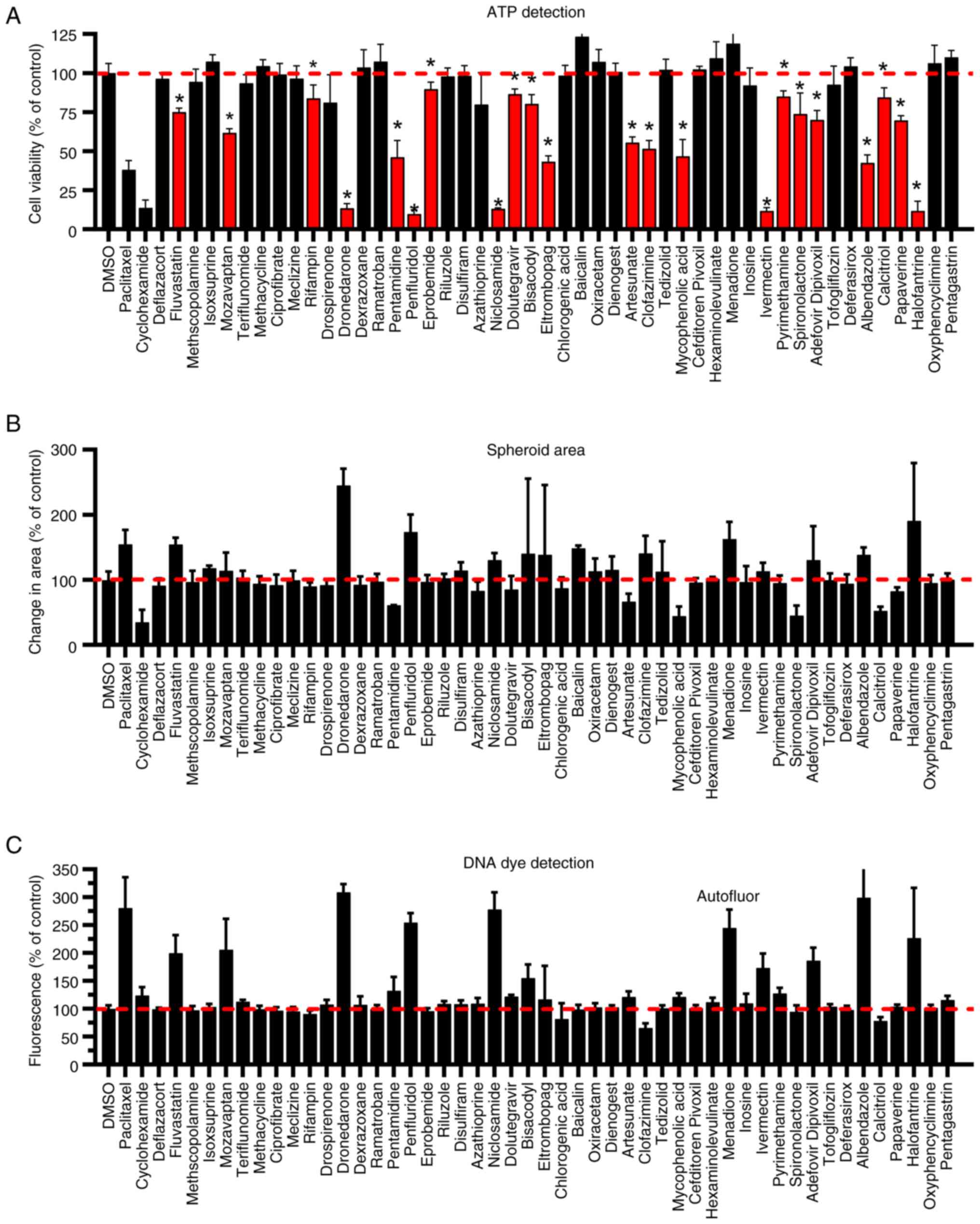
Initial screens for activity in NOD
collection
We screened the collection of 48 NODs at 10 µM drug
concentration using the SUM-149 spheroid growth assay in which we
measured three parameters as indicators of drug activity (Fig. 2). During the 96 h of drug exposure,
we monitored the spheroids with the Incucyte imaging system. After
the final reading in the Incucyte imager, we measured total cell
viability via ATP detection (CellTiter Glo) (Fig. 2A). We also determined the change in
area of the spheroid (Fig. 2B) and
cytotoxicity using CellTox Green dye (Fig. 2C) using the Incucyte software. This
dye is non-permeant to cells unless the membrane is compromised,
upon which the dye binds DNA and its fluorescence increases.
Therefore, higher fluorescence indicates cell death. The area of
the spheroid was decreased by some drugs and increased by other
drugs. An increase in size can be due to cytotoxicity resulting in
decreased cell-cell cohesion, reversing a tightly compacted
spheroid, resulting in increased total area of the spheroid
(13). This phenomenon was observed
with the known cytotoxic agent paclitaxel, used as a control in
this assay. Paclitaxel generated a mean of 155% change in area
compared to control, increased fluorescence and reduced signal in
the ATP assay, all consistent with a cytotoxic compound. A decrease
in size can be due to decreased cell proliferation during the 96 h
assay. For example, this was observed with cycloheximide, a
predominately anti-proliferative agent, which decreased mean area,
only slightly increased fluorescence and decreased ATP signal.
Based on the data, both types of spheroid area changes were
observed in this set of compounds. Of the 12 drugs that increased
the change in area by ≥125% of control, 9 also displayed an
increase in fluorescence in the CellTox Green assay, excluding
menadione which displayed autofluorescence and therefore unknown
cytotoxicity by this method. Baicalin and menadione, two of the
drugs that increased change in area but not fluorescence (baicalin)
or were autofluorescent (menadione), also increased the ATP content
over DMSO (solvent) control. Thus, these drugs appear to have
enhanced proliferation in this cell line. Clofazimine increased the
change in area relative to control, but showed no increase in
fluorescence, yet clearly inhibited cell proliferation/viability in
the ATP content assay. One drug, ivermectin, had no significant
effect on the spheroid area, but increased fluorescence and
dramatically decreased signal in the ATP assay. If area alone was
used in the screen, this drug would have been a false negative and
not detected. Thus, use of area alone may result in false positives
and false negatives when screening drugs for anti-cancer activity.
In another case, niclosamide increased the change in area by a mean
of 130%, while the signal in the ATP assay was inhibited by 87%,
the latter representing a more robust assay response. In the
cytotoxicity assay, 11 drugs (excluding menadione) produced
fluorescence >125% of control (DMSO). All these drugs were
present as a subset of the ATP assay hits. However, some drugs,
e.g., papaverine and spironolactone, were also detected with
activity in the ATP assay that showed no increase in fluorescence
suggesting they may be only anti-proliferative. Due to the lack of
specificity and sensitivity of the area measurement, the robustness
of the ATP assay and our interest in identifying both cytotoxic and
purely anti-proliferative drugs, the ATP detection assay was used
as the primary indicator of activity. Using the ATP assay, we
identified drugs that produced a signal significantly lower than
control (P<0.05) resulting in 22 drugs from this initial screen
(Fig. 2A) being selected for
confirmatory dose response studies.
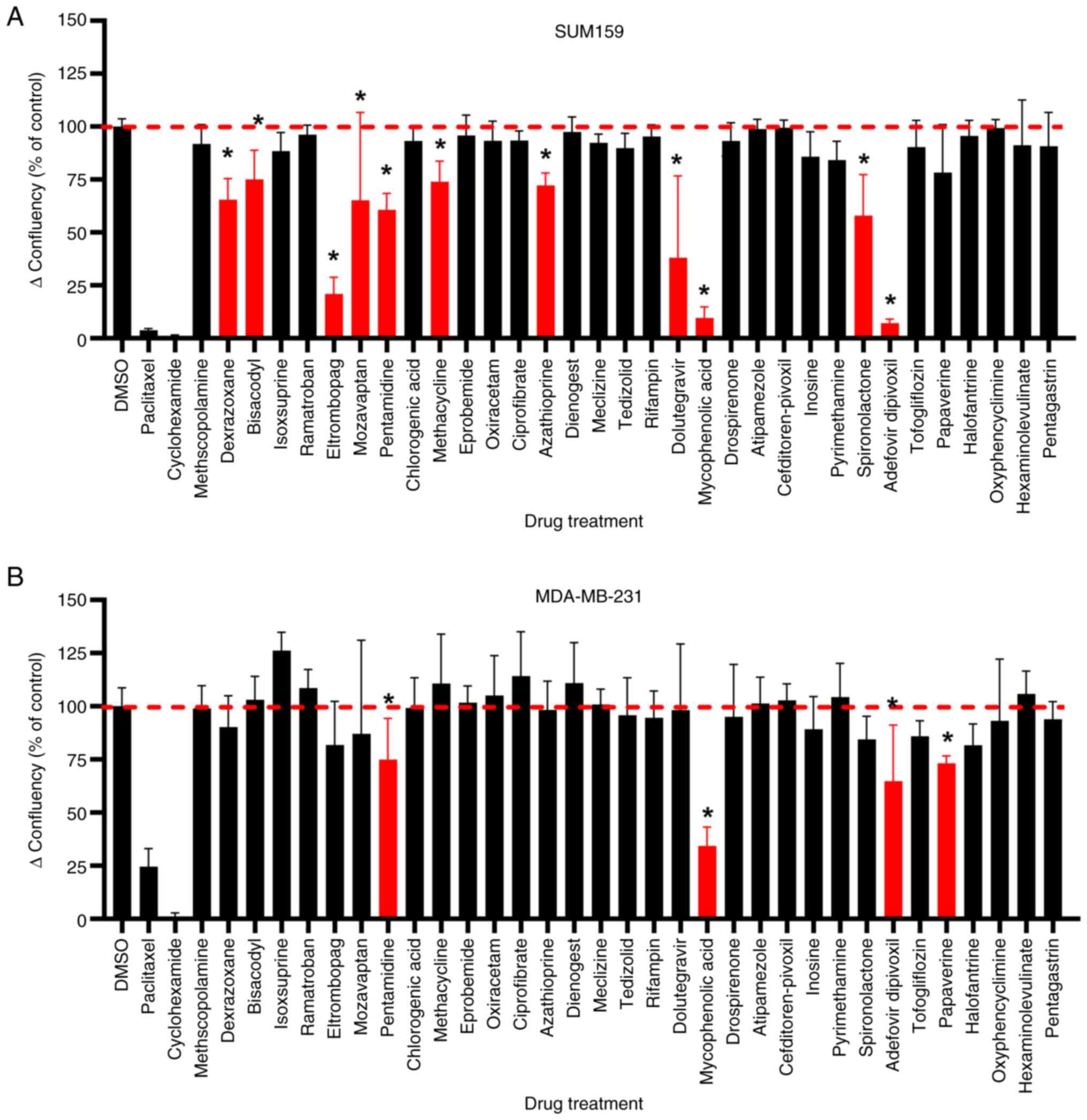
In a parallel study, we assessed a subset of 33
drugs (those from the set of 48 drugs with few literature
references) for their activity against non-IBC TNBC cell lines
SUM-159 and MDA-MB-231 (Fig. 3). A
standard 2-D cell culture model was employed, measuring change in
percent confluency as a measure of cell growth during the assay
using the Incucyte imaging system. The initial confluency at the
time of drug treatment (10 µM) was subtracted from the final
confluency to get an actual measure of cell proliferation
inhibition. Since there were too many drugs with p≤0.05 that had
minimal % inhibition (<10% inhibition) in the 2-D screen, we
added a percent inhibition cut-off to the 2-D screen hit criteria.
In this screen, 10 actives for SUM-159 and 4 actives for MDA-MB-231
were identified using a cut-off of ≥25% inhibition with P≤0.05
compared to DMSO control (Fig. 3).
One drug, mozavaptan, that showed unusually high variability in
activity against SUM-159, was also re-tested in potency assays. Due
to the inclusion of mozavaptan and significant overlap in actives,
the screen resulted in 12 unique drugs selected for confirmation
assays.
Confirmation concentration response
assays
The active drugs from the SUM-149 spheroid screen
were tested in concentration response assays using the same format,
except that only the ATP assay was used as a measure of cell
viability and proliferation. An initial measure of luminescence
(‘day 0’ signal) allowed for subtraction of the signal derived from
plated cells and therefore, enabled the plotting of spheroid growth
relative to controls to derive a GI50. Thus, this method
allowed detection of both cytostasis and cytotoxicity since the
normalized 0 signal represents no cell growth and negative values
indicate cytotoxicity. GI50 was determined, rather than
simple IC50, since our interest was to identify
potential maintenance therapy, including drugs that only slowed
re-growth of cancer cells after standard of care treatment. Since
NODs were not originally designed as cytotoxic chemotherapy agents,
the expectation was that at clinical doses, many of these NODs may
only inhibit cell growth. Thus, NODs that are purely
anti-proliferative would still be of interest and we wanted to
accurately quantify this anti-proliferative activity. 15 drugs had
calculable GI50 values (reached at least 50% inhibition)
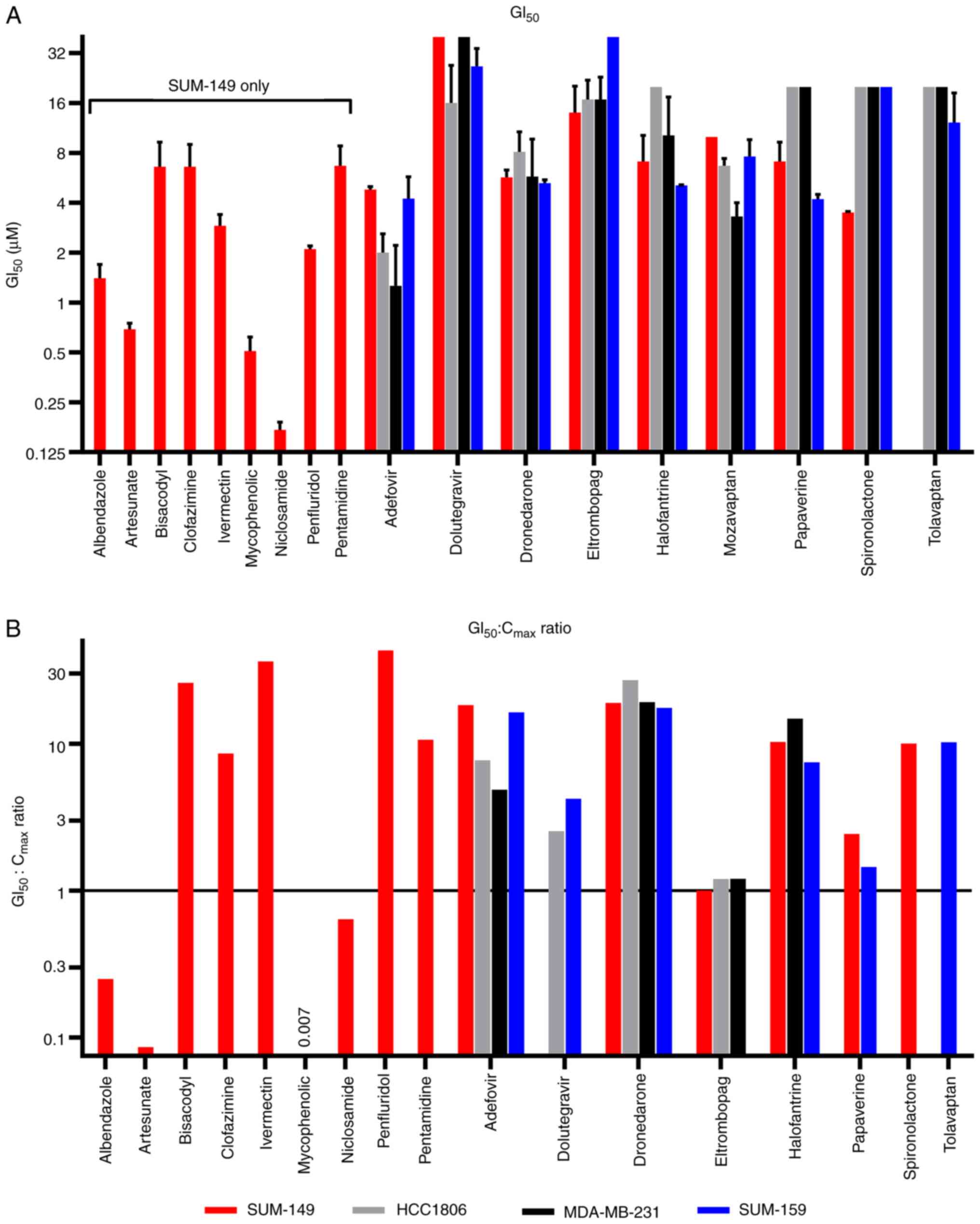
(Fig. 4 and Table I). The GI50 values ranged
from 0.17 to 14 µM and originated from various drug classes and
approved indications (Table I). At
the higher potency end of this collection of drugs (<2 µM
GI50), the anti-cancer activity of niclosamide
(GI50=0.17 µM), mycophenolic acid (GI50=0.51
µM), artesunate (GI50=0.69) and albendazole
(GI50=1.4 µM) have all been extensively described
(14). In addition to TNBC,
niclosamide has also been shown by others to have activity against
SUM-149 (15–17). While the activity of artesunate and
albendazole has been described for TNBC cell lines (18–21),
their activity against SUM-149 (or other IBC cell lines) does not
appear to have been previously reported.
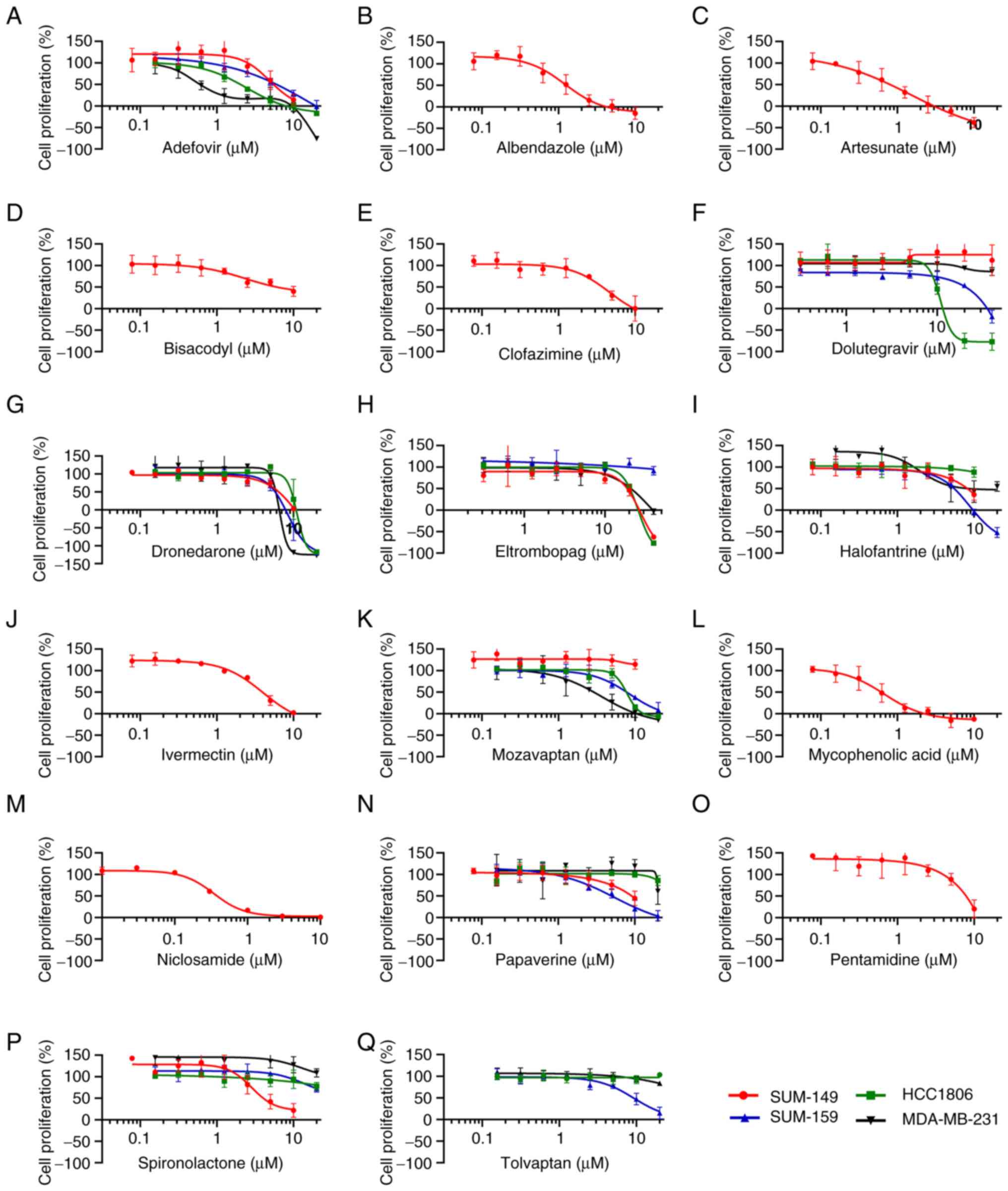 | Figure 4.GI50 determinations for
active drugs against SUM-149 and a panel of other TNBC cell lines
grown as spheroids. Relative cell proliferation (as a percentage of
controls) was determined in response to the indicated
concentrations of drugs tested against SUM-149. Selected compounds
were also profiled with an additional three TNBC cell lines,
SUM-159, HCC1806 and MDA-MB-231, as indicated. The concentration
response data was plotted for (A) adefovir, (B) albendazole, (C)
artesunate, (D) bisacodyl, (E) clofazimine, (F) dolutegravir, (G)
dronedarone, (H) eltrombopag, (I) halofantrine, (J) ivermectin, (K)
mozavaptan, (L) mycophenolic acid, (M) niclosamide, (N) papaverine,
(O) pentamidine, (P) spironolactone and (Q) tolvaptan. All cell
lines were grown in spheroid form for this assay with 96 h drug
treatment followed by viable cell number assessed by CellTiter Glo.
Relative cell proliferation (as opposed to total cells) was
calculated by subtracting mean 0-day plate values from all wells
and normalizing to controls such that a 0 value indicated no cell
proliferation during the course of the assay (same number of viable
cells as on day of treatment), while negative values indicated
cytotoxicity (fewer viable cells than on day of drug treatment).
Data shown are representative of n≥3 independent experiments and
the data points and error bars represent the mean ± standard
deviation of triplicate technical replicates. GI, growth
inhibition; TNBC, triple-negative breast cancer. |
 | Table I.GI50 values in the SUM-149
spheroid assay and pharmacological data for identified drugs. |
Table I.
GI50 values in the SUM-149
spheroid assay and pharmacological data for identified drugs.
| Drug | GI50
(µM)a | SD (µM) |
GI50:Cmax |
Cmaxb (µM) | Drug class or
MOA | Approved
indication |
|---|
|
Adefovir-dipivoxil | 4.8 | 0.2 | 18.5 | 0.26 | Nucleotide analog
reverse transcriptase inhibitor | Hepatitis B |
| Albendazole | 1.4 | 0.3 | 0.3 | 5.70 | Tubulin
inhibitor | Parasitic worm
infections |
| Artesunate | 0.7 | 0.1 | 0.1 | 8.00 | Generates free
radicals | Malaria |
| Bisacodyl | 6.6 | 2.7 | 26.4 | 0.25b | Stimulates
adenylate cyclase | Constipation |
| Clofazimine | 6.6 | 2.4 | 8.6 | 0.77 | Acts at bacterial
membranes | Leprosy |
| Dolutegravir | >40c | - | NA | 6.30 | Integrase
inhibitor | HIV-1 |
| Dronedarone | 5.7 | 0.6 | 19.0 | 0.30 | Multichannel
blocker | Atrial
fibrillation |
| Eltrombopag | 14.0d | 6.2 | 1.0 | 14.00 | Thrombopoietin
receptor agonist |
Thrombocytopenia |
| Halofantrine | 7.1 | 3.1 | 10.3 | 0.69 | Targets
ferritoporphyrin IX | Malaria |
| Ivermectin | 2.9 | 0.5 | 36.3 | 0.08 | Chloride ion
channels | Multiple
parasites |
| Mozavaptan | >10e | - | NA | Unk | Vasopressin
receptor antagonist |
Hyponatremiaf |
| Mycophenolic
acid | 0.5 | 0.1 | 0.01 | 78.00 | IMPDH
inhibitor |
Immunosuppressant |
| Niclosamide | 0.2 | 0.02 | 0.6 | 0.27 | Uncoupling of
electron transport | Tapeworm
infectionsg |
| Papaverine | 7.1 | 2.2 | 2.4 | 2.90 | Nonxanthine PDE
inhibitor | Myocardial
infarction/angina |
| Penfluridol | 2.1 | 0.1 | 42.0 | 0.05 | Dopamine receptor
blocker |
Antipsychotich |
| Pentamidine | 6.7 | 2.1 | 10.6 | 0.63 | Unknown | Pneumocystis
pneumonia |
| Spironolactone | 3.5 | 0.1 | 10.0 | 0.35 | MR antagonist | Hypertension, heart
failure |
The active drugs from the TNBC 2-D screen were
tested in concentration response assays using the same format as
the initial screen, except that CellTox Green dye was used as a
measure of cytotoxicity, while confluency was used to measure cell
proliferation. All the actives were tested against three TNBC cell
lines: SUM-159, MDA-MB-231 and Hs578T. Five drugs had confirmed
GI50 potencies of <10 µM for at least one cell line:
adefovir, eltrombopag, mozavaptan, mycophenolic acid and
pentamidine (Fig. 5 and Table II). Halofantrine was a sixth drug
that was also tested in this 2-D panel since it had borderline
activity against MDA-MB-231 and activity in the spheroid screen.
Mozavaptan was the only drug in this set that had activity against
all three cell lines with mean GI50 values of 2.2 to 4.6
µM. MDA-MB-231 appeared to be more drug resistant in general within
this panel than the other two cell lines since only two of these
drugs had calculatable GI50s against this cell line. The
most potent drugs were adefovir (mean GI50=1.2, 3.3 and
>10 µM) and mycophenolic acid (mean GI50=0.5, 3.1 and
>10 µM). Adefovir showed cytotoxicity only against SUM-159.
Mycophenolic acid has been extensively studied for its anti-cancer
activity, including against TNBC cell line MDA-MB-231 (22,23).
However, no studies to date have reported its activity against
SUM-149 or other IBC cell lines. A measure of cytotoxicity was
calculated by dividing the area of green fluorescence (from the
CellTox Green dye) by the area of confluency (from the brightfield
image) (Fig. 5). Mozavaptan was the
only drug that was cytotoxic against all three cell lines where it
generated cytotoxicity at concentrations above 1 µM. In contrast to
mozavaptan, the other drugs in this set that showed some
cytotoxicity only had cytotoxic activity against one cell line
(e.g. mycophenolic acid and adefovir) or weak cytotoxic activity
(only at 10 µM) against one or two cell lines (e.g. pentamidine and
halofantrine). Eltrombopag did not show cytotoxicity against any of
the cell lines and thus appeared to be purely anti-proliferative
under these 2-D conditions. Adefovir and mycophenolic acid were
only cytotoxic for SUM-159 at 3 µM and above.
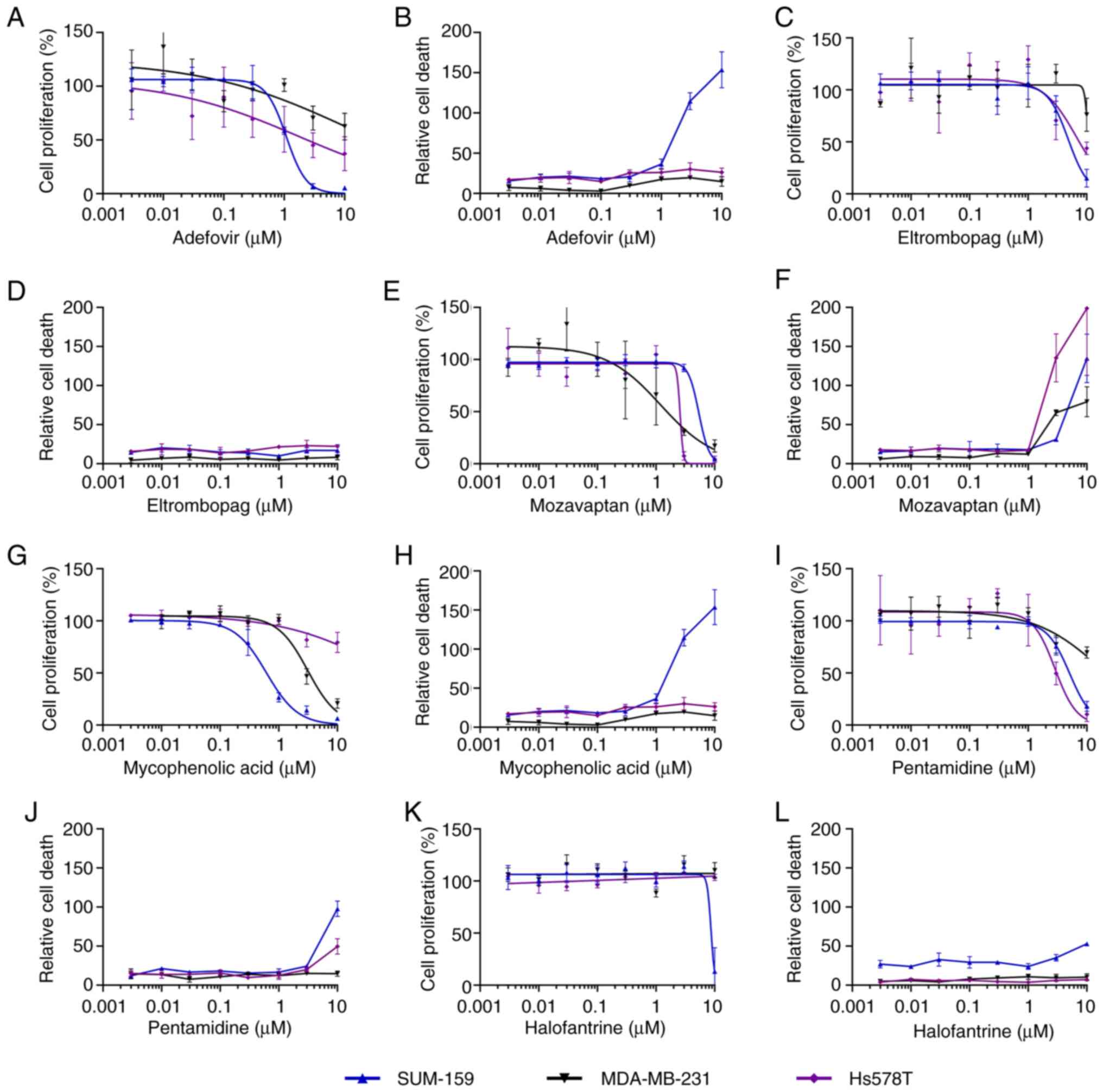 | Figure 5.GI50 determination of
active drugs from 2-D screen against a panel of three TNBC cell
lines. Relative cell proliferation (as a percentage of controls)
and cytotoxicity was determined in response to the indicated
concentrations of drugs tested against SUM-159, HCC1806 and
MDA-MB-231, as indicated. All cell lines were grown in 2-D.
Immediately after drug treatment, confluency and CellTox Green dye
fluorescence (cytotoxicity) was monitored for 72 h with the
Incucyte. Relative cell proliferation was calculated by subtracting
the initial confluency (based the first read) from the final
confluency measurement for each well and normalized to controls.
Therefore, a 0% value indicates no change in confluency compared to
the start of the assay. Relative cell death after 72 h was
calculated by dividing the area of green dye fluorescence by the
total confluency area (based on brightfield imaging) and
multiplying by 100. Separate plots are provided for cell
proliferation and cytotoxicity for (A and B, respectively)
adefovir, (C and D, respectively) eltrombopag, (E and F,
respectively) mozavaptan, (G and H, respectively) mycophenolic
acid, (I and J, respectively) pentamidine and (K and L,
respectively) halofantrine. Data shown are representative of n≥3
independent experiments and the data points and error bars
represent the mean ± standard deviation of triplicate technical
replicates. GI, growth inhibition; TNBC, triple-negative breast
cancer; 2-D, two-dimension. |
 | Table II.GI50 for drugs identified
in 2-D cell culture screen. |
Table II.
GI50 for drugs identified
in 2-D cell culture screen.
| Cell line | SUM-159 | MDA-MB-231 | Hs578T |
|---|
|
|
|
|
|---|
| Drug | GI50
(µM)a | SD | GI50
(µM)a | SD | GI50
(µM)a | SD |
|---|
| Adefovir
dipivoxil | 1.2 | 0.3 | >10 | - | 3.3b | 1.5 |
| Eltrombopag | 8.1 | 1.0 | >10 | - | 5.3b | 1.3 |
| Halofantrine | 7.6 | 1.1 | >10 | - | >10 | - |
| Mozavaptan | 4.6 | 0.5 | 2.2 | 0.7 | 2.7 | 0.7 |
| Mycophenolic
acid | 0.5 | 0.1 | 3.1 | 0.3 | >10 | - |
| Pentamidine | 7.4 | 1.8 | >10c | - | 2.0b | 0.9 |
Activity of understudied NODs in a
panel of TNBC cell lines
Since SUM-149 is the only commercially available
TN-IBC cell line, we were unable to test the active hits against
other TN-IBC cell lines. Therefore, we sought to test understudied
NODs from the SUM-149 screen against standard TNBC cell lines to
assess the breadth of activity. Of the 15 drugs that were confirmed
in the SUM-149 screen, 7 of them were assessed to be understudied
in TN-IBC/TNBC based on PubMed literature searches. Similarly, 5 of
the hits in the 2-D screen with TNBC cell lines had few studies
related to their activity in TNBC. We combined these sets of drugs,
resulting in 8 unique understudied drugs, due to high overlap in
the lists. Only mozavaptan was a novel hit in the 2-D screen
compared to the SUM-149 screen hits. Dolutegravir was understudied
in the literature but was highly variable in the SUM-149 potency
assays for unknown reasons, so we included it for testing against
this panel. We tested these eight drugs in concentration response
against TNBC cell lines SUM-159, HCC1806 and MDA-MB-231 grown in
the spheroid assay format with ATP detection as the measure of cell
viability and proliferation (Fig.
4, Table III and Fig. 6A). Since we performed a ‘Day 0’
plate to obtain the signal for the initial cell number at the time
of drug treatment, a negative cell proliferation value at the end
of drug treatment indicated fewer viable cells, i.e., cytotoxicity.
Hs578T failed to proliferate in the spheroid growth conditions
employed, so we used HCC1806 in its place. Eltrombopag and
dolutegravir were tested at higher max concentration (40 µM) due to
their higher Cmax values. Adefovir, an anti-hepatitis B
antiviral drug, and dronedarone, a multi-channel blocker for atrial
fibrillation, were the only drugs in this selected drug panel to
inhibit all four cell lines with a calculatable GI50
value. Adefovir produced mean GI50 values ranging from
1.3 to 4.8 µM and it appeared to be cytotoxic for MDA-MB-231 at 20
µM, but not for the other cell lines. Dronedarone produced
GI50 values in the range of 5.3 to 8.1 µM and was
cytotoxic at higher concentrations across the cell lines. Three of
the drugs had measurable GI50 values in three of the
four cell lines. These drugs and their GI50s for
SUM-149, HCC1806, SUM-159 and MDA-MB-231, respectively, included
eltrombopag (GI50=14, 16.8, >40 and 16.8 µM),
halofantrine (GI50=7.1, >20, 5.1, 10.2 µM) and
mozavaptan (GI50=>10, 6.7, 7.6, 3.3 µM). Since
mozavaptan is a vasopressin receptor antagonist, we sought to test
another member of this class, tolvaptan, for its activity (Table III, Fig. 4Q). Tolvaptan only had weak activity
against SUM-159 (GI50=12 µM) out of the three TNBC cell
lines tested. Eltrombopag was clearly cytotoxic only at 40 µM for
two (SUM149 and HCC1806) of the three sensitive cell lines.
Papaverine had activity against SUM-149 (GI50=7.1 µM)
and SUM-159 (4.2 µM), but minimal activity (GI50 >20
µM) against the other two cell lines. Dolutegravir exhibited
activity only against SUM-149 and HCC1806 and showed notable
cytotoxicity for HCC1806 at concentrations of 20 µM and above.
Spironolactone was the only drug in this subset of drugs that was
selective for SUM-149 (GI50=3.5 µM), with no activity
against the other three cell lines.
 | Table III.Anti-proliferative activity of
selected drugs in a panel of cell lines grown as spheroids. |
Table III.
Anti-proliferative activity of
selected drugs in a panel of cell lines grown as spheroids.
| Cell line | SUM-149 | HCC-1806 | SUM-159 | MDA-MB-231 |
|---|
|
|
|
|
|
|---|
| Drug | GI50
(µM)a | SD | GI50
(µM) | SD | GI50
(µM) | SD | GI50
(µM) | SD |
|---|
| Adefovir | 4.8 | 0.2 | 2.0 | 0.6 | 4.2 | 1.5 | 1.3 | 1.0 |
| Dolutegravir | >40b | - | 16.0 | 11.0 | 27 | 7.5 | >40 | - |
| Dronedarone | 5.7 | 0.6 | 8.1 | 2.6 | 5.3 | 0.3 | 5.8 | 3.9 |
| Eltrombopag | 14.0 | 6.2 | 16.8 | 5.2 | >40 | - | 16.8 | 6.1 |
| Halofantrine | 7.1 | 3.1 | >20c | - | 5.1 | 0.03 | 10.2 | 7.2 |
| Mozavaptan | >10d | - | 6.7 | 0.7 | 7.6 | 2.0 | 3.3 | 0.7 |
| Papaverine | 7.1 | 2.2 | >20c | - | 4.2 | 0.3 | >20c | - |
| Spironolactone | 3.5 | 0.05 | >20 | - | >20c | - | >20c | - |
| Tolvaptan | NT | - | >20 | - | 12.2 | 6.2 | >20 | - |
GI50 values are more informative when
viewed relative to the maximal concentration (Cmax)
achievable at clinical doses in humans. We obtained Cmax
values for the identified drugs using published Cmax
values from human clinical studies (Table I) (24–37).
By comparing the GI50 values from the SUM-149 data to
the Cmax values (Table I
and Fig. 6B), 9 drugs had
GI50 values that were 10-fold or more higher than the
Cmax (GI50:Cmax ratio of ≥10). One
drug, mozavaptan, had no published Cmax data. However,
there were 6 drugs that had GI50 values that ranged from
approximately twice their Cmax to 153-fold lower than
their Cmax. These drugs and their approximate
GI50:Cmax ratio included the following:
albendazole (GI50:Cmax=0.3), artesunate
(GI50:Cmax=0.09), mycophenolic acid
(GI50:Cmax=0.007), Niclosamide
(GI50:Cmax=0.6), eltrombopag
(GI50:Cmax=1) and papaverine
(GI50:Cmax=2). Eltrombopag and papaverine had
similar relative potencies (GI50:Cmax ratios)
for the other cell lines that were sensitive to them. Adefovir had
a Cmax of 0.2 µM and a GI50:Cmax of 18 for
SUM-149. However, in MDA-MB-231 cells, adefovir produced a
GI50 of 1.3 µM which is only 5-fold above
Cmax. Interestingly, this drug was inactive (>10 µM
GI50) in the 2-D potency assay with MDA-MB-231. This
contrasts with the results obtained with the same drug in SUM-159,
where the potency shifted almost 4-fold, indicating a less
sensitive response in the spheroid assay, suggesting a shift in
potency due to the spheroid culture conditions. It has been well
established that compound potency shifts can frequently happen when
comparing 2-D and 3-D cell culture, including many instances when
lower potency is observed in the 3-D culture (38,39).
In sum, we screened a collection of 48 NODs
identified through a published database for anti-proliferative
activity against the IBC cell line SUM-149 grown in spheroid
format. We identified 15 NODs with activity against SUM-149. A
subset of 33 NODs was also screened using two TNBC cell lines grown
in 2-D culture resulting in the identification of six drugs. There
was complete overlap in these sets of drugs, except for mozavaptan
which was only identified in the TNBC screen. In the SUM-149 assay,
the most potent drugs (<1 µM GI50) were artesunate,
mycophenolic acid, and niclosamide. Six of these drugs active
against SUM-149 (albendazole, artesunate, mycophenolic acid,
niclosamide, eltrombopag and papaverine) have potentially
clinically relevant GI50:Cmax ratios of ≤2.
Eight drugs were further tested against a panel of three non-IBC
TNBC cell lines grown in spheroid format. Selectivity varied from
two drugs (adefovir and dronedarone), which had activity against
all four cell lines, to one (spironolactone), which had activity
only against SUM-149. Eltrombopag and papaverine were identified as
having newly discovered activity against SUM-149, and we expanded
their activity profiling to multiple TNBC cell lines. In addition,
the eltrombopag and papaverine had GI50:Cmax
ratios of 1 and 2, respectively, indicating that they could reach
therapeutically relevant drug levels in vivo for anti-cancer
treatment.
Discussion
In this report, we screened a collection of NODs
based on a published database to identify NODs with activity
against TN-IBC and/or TNBC. Using the TN-IBC cell line SUM-149, we
identified 15 drugs with calculable GI50 values against
this cell line grown in spheroid form. In a parallel screening
approach, we tested a subset of these NODs for activity against two
other TNBC cell lines grown in 2-D. This second approach resulted
in a list of active drugs that overlapped with the SUM-149 hits,
except for mozavaptan which was uniquely active against SUM-149.
Rather than just using the absolute GI50 for assessing
their potential against TN-IBC, we also assessed these drugs in
terms of their potency (GI50) relative to their
Cmax reported in human studies. Many of these drugs
turned out to be well-known NODs that have many reports of their
anti-cancer activity. For example, the well-studied NODs,
albendazole (anti-parasitic), artesunate (anti-malarial),
niclosamide (for tapeworm infections) and mycophenolic acid
(immunosuppressant) have been extensively reported to have
anti-cancer activity against multiple types of cancers (10,14).
These drugs also have Cmax values in humans that are
above the GI50 reported here for SUM149 and hence may
have activity against IBC in patients. From the two screening
approaches, eight of these NODs were identified as being less
studied in TNBC, and so we tested these drugs against a panel of
other TNBC cell lines grown in spheroid format. Only two drugs
generated calculable GI50 values in all four cell lines:
adefovir (antiviral) and dronedarone (multichannel blocker for
atrial fibrillation). However, these drugs also had
GI50:Cmax ratios that were high, at
approximately 5- to 30-fold, and raises the question of how
effective they would be at clinical doses. The sensitivity to
adefovir varied between cell lines with MDA-MB-231 being the most
sensitive with a GI50 of 5-fold above Cmax.
Halofantrine (antimalarial) had activity in 3 of 4 cell lines, but
the GI50:Cmax values were all over 7-fold.
Dolutegravir (HIV drug) had activity against two cell lines (but
not SUM-149) with a GI50:Cmax of ~3-4-fold.
While this drug was reported to inhibit proliferation of BT-20, a
TNBC cell line, it was also found to increase metastasis in the 4T1
(mouse TNBC cell line) xenograft model (40). Two drugs, eltrombopag and papaverine
had GI50: Cmax ratios of 1 to 2 in sensitive
cell lines. These drugs have not been previously reported to have
activity in an IBC cell line.
A limitation of this study was the reliance on
SUM-149 as the sole TN-IBC model, since the observed drug activity
could be due to cell line-specific effects and not generalizable to
most TN-IBC. Hence, these drugs should be tested against other
TN-IBC cell lines. Suggestive evidence against unique SUM-149
effects is that most of the drugs active against SUM-149 were also
active against other TNBC cell lines. Another limitation of this
study was that the simple spheroid model does not fully
recapitulate IBC-specific in vivo mechanisms such as tumor
emboli formation and lymphangiogenesis. The use of Cmax in our data
analysis also has its limitations. Protein binding of drugs in
serum may alter the concentration of free drug in vivo
compared to 10% serum used in our in vitro studies. In
addition, the Cmax only reflects the concentration achieved in
blood, while the degree of tissue penetration may alter the
concentration of drug experienced by the tumor. In addition,
chronic dosing may increase the concentration of drug in the serum
and tumor. Thus, our use of Cmax in assessing the drugs is only a
rough guideline, but an improvement over ignoring the realities of
clinically achievable in vivo drug concentrations and how it
dramatically varies between drugs. The spheroids were not
characterized for properties described in the literature for
spheroid culture, including hypoxia, proliferation gradients and
necrotic centers. Therefore, validation of these properties in our
assays needs to be confirmed in future work.
Eltrombopag is a thrombopoietin receptor (TPOR)
agonist that is approved for treatment of thrombocytopenia. Despite
this originally designated target, eltrombopag has been
experimentally demonstrated to bind to multiple targets including
proteins such as BAK (41), TFEB
(42) and Syndecan-4 (43). Eltrombopag has also been reported to
directly inhibit BAX and prevent cell death (44). It has been reported to bind
double-stranded DNA (45), chelate
iron (46–48) and have anticancer activity in
multiple cell lines from many different tissue origins (49–52).
Despite low or undetectable TPOR in most tissues, eltrombopag has
been reported to inhibit cell growth in multiple different cancer
cell lines including breast cancer cell lines MCF-7, BT-474 and
TNBC cell line HCC1937 (50).
Eltrombopag has been reported as an inhibitor of the mRNA-binding
protein, HuR (human antigen R), which has been associated with poor
prognosis in multiple cancers (51,53,54).
In Zhu et al (53),
eltrombopag also inhibited cell proliferation in multiple cancer
cell lines, but no human TNBC cell lines were tested. In their
work, using the murine TNBC cell line 4T1, eltrombopag had an in
vitro IC50 of 13.5 µM, similar to our data with
human TN-IBC/TNBC cell lines. In a mouse allograft model using 4T1,
eltrombopag also had in vivo activity where it inhibited
tumor growth and metastasis and displayed anti-angiogenesis
activity (53,54). Our data adds to this body of
research since we demonstrated that eltrombopag had activity in 3
of 4 human TNBC cell lines grown in spheroid form, including a
TN-IBC cell line, with potencies similar to its Cmax. At
the highest concentration tested, 40 µM, eltrombopag was cytotoxic
in two of the four cell lines. The weak potency of eltrombopag
against SUM-159 (GI50 >40 µM) also suggests some TNBC
cancers may be resistant to this drug. According to RNAseq data
accessed through the Depmap portal (https://depmap.org), the expression level of the gene
for TPOR (MPL) is very low (close to undetectable) in all four cell
lines used in this study, which is consistent with undetectable
expression of MPL mRNA in 118 advanced or metastatic breast cancer
samples from patients (50).
However, TPOR expression in the cell lines used in this study needs
to be confirmed in future studies. Due to the many off-target
activities that have been ascribed to eltrombopag, it is not clear
which mechanism was producing the observed activity in our assays.
The Cmax of 14 µM cited herein was the result of a
single dose of 50 mg of eltrombopag in healthy adults (35). Another study dosed healthy adults
with up to 75 mg of eltrombopag per day for 10 days and concluded
that there were no significant adverse effects compared to placebo
(55). In this study, the 30-, 50-,
and 75-mg dose levels resulted in mean increases in platelet counts
of 24.1, 42.9, and 50.4%, respectively. The normal platelet count
in adults ranges from 150,000 to 450,000 platelets per microliter,
with counts exceeding 450,000 platelets per microliter defined as
thrombocytosis (56). Thus,
depending on an individual's baseline platelet count, even a 50%
increase in platelet count caused by eltrombopag treatment may
still be within normal range. A long-term study in patients with
chronic immune thrombocytopenia taking eltrombopag for up to 3
years (median daily dosing of 51.5 mg) found that the most common
adverse events were headache, nasopharyngitis, upper respiratory
tract infection, and fatigue, but these were mostly mild (57). Of note, 13% of patients experienced
≥1 adverse event, leading to study withdrawal. Importantly, no new
or increased incidence of safety issues were identified in this
study and the authors concluded that long-term treatment with
eltrombopag was generally safe and well tolerated. Overall, our
data indicated that eltrombopag may have potential for repurposing
for TN-IBC and TNBC in general.
Papaverine, a vasodilator traditionally used to
treat vascular spasms, has been reported to primarily target the
phosphodiesterase PDE10A and, less potently, other
phosphodiesterases, leading to increased intracellular
concentrations of cAMP (58). In
MDA-MB-231, cell growth has been reported to be inhibited by agents
that elevate intracellular cAMP levels, i.e., 8-bromo-cAMP, cholera
toxin, forskolin, and papaverine (59,60).
Papaverine has also been reported to inhibit mitochondrial complex
1, reducing oxidative phosphorylation and oxygen consumption in
cells (61). This latter mechanism
has been proposed to explain papaverine's ability to enhance
radiation sensitivity of cancer cells, since higher oxygen levels
lead to more radiation damage. Papaverine has also been reported to
inhibit cell proliferation and migration of non-small cell lung
cancer cell lines through allosteric binding directly to CDK5 and
inhibiting its activity (62). This
drug has also been reported to inhibit a panel of human liver cell
lines with IC50 values ranging from 1.7 to 52 µM
(63). In a different report,
papaverine was reported to selectively inhibit the growth of human
prostate cancer cell line PC-3 and induce apoptosis (64). Papaverine was identified in a screen
for small molecules that sensitize tumor cells to glucose
starvation and this effect was confirmed with four different cell
lines (65). In a xenograft mouse
model using the DLD1 colon cell line, modest in vivo
anti-tumor activity of papaverine was observed, but a strong
combination effect was observed when combined with bevacizumab, an
inhibitor of vascular endothelial growth factor (VEGF) (65). In another study, papaverine
demonstrated anti-proliferative activity for MCF-7 and MDA-MB-231
breast cancer cell lines (66).
Notably, we are the first to report papaverine activity against an
IBC cell line and human TNBC cell lines other than MDA-MB-231. In
contrast, our data for papaverine showed weak to no activity
against MDA-MB-231 (>12 to >20 µM GI50) which may
be due to differing culture conditions-we employed spheroid growth
instead of traditional 2-D culture. It is well known that spheroid
growth can shift the potency of compounds compared to 2-D growth.
In follow-up mechanism studies, it will be important to determine
if there is a correlation between cAMP elevation or mitochondrial
inhibition in papaverine treated cells and cell line sensitivity to
this drug. There is an oral form of papaverine available and one
study using chronic dosing of oral papaverine reported no adverse
events associated with the drug (67). However, hepatotoxicity in some
patients taking oral papaverine has been reported (68).
Mozavaptan is a vasopressin V2 receptor (V2R)
antagonist that is primarily used for hyponatremia. In our study,
mozavaptan inhibited proliferation of 3 out of 4 TNBC cell lines
grown in spheroid form and Hs578T grown in 2-D with a range of
GI50s of 2.7–7.6 µM. Mozavaptan has been approved in
Japan, but not by the FDA, so we also tested another member of
vasopressin receptor antagonist family that is
FDA-approved-tolvaptan. Tolvaptan did not show the same
activity/potency as mozavaptan. For example, mozavaptan inhibited
MDA-MB-231 cells with a GI50 of 3.3 µM, but tolvaptan
was >20 µM. Tolvaptan has a reported IC50 of 0.2 nM
for its target in one cell-based assay (69) and mozavaptan has a reported
IC50 of 19 nM in a CHO V2R expression system (70). Thus, given (1) the high potency of mozavaptan and
tolvaptan for V2R in contrast to our relatively high
GI50 values and (2)
inconsistent activity between the two drugs in our study, our data
is highly suggestive that the antiproliferative activity of both
drugs in our study were due to off-target activity. According to
The Human Protein Atlas database (https://www.proteinatlas.org), the RNA level of the
V2R gene (AVPR2) was 0.0 normalized transcript per million (nTPM)
for MDA-MB-231, SUM-159 and HCC1806, while RNA level for SUM-149
was 1.3 nTPM. Thus, this data indicated a lack of correlation
between V2R RNA expression and sensitivity to mozavaptan since we
observed mozavaptan activity in the cell lines not expressing V2R
RNA, but no activity in SUM-149 that may express some level of V2R
RNA (but very low). This database also reports undetectable V2R
protein levels in MDA-MB-231 and HCC1806, with no data on the other
cell lines. In contrast, one study showed V2R expression in
MDA-MB-231 (and MCF-7 and mouse F3II mammary cell line) by
immunohistochemistry (71). This
same study demonstrated that a synthetic peptide agonist for V2R,
desmopressin, was cytostatic in proliferation assays using
MDA-MB-231 and reduced tumor growth and angiogenesis in mouse
models using MDA-MB-231 and F3II. However, tolvaptan and mozavaptan
are both potent antagonists of V2R and thus V2R antagonism as a
mechanism would be inconsistent with this prior study. One
hypothesis to explain all this data is that V2R does not play a
role in the mechanisms for these drugs in our assays and the broad
activity observed for mozavaptan is off-target activity for a
target not shared by tolvaptan (or same off-target, but
differential potency) due to structural differences between the
drugs. It was not possible to determine the potency of mozavaptan
relative to the Cmax since this data was not readily
available in the literature. Thus, it is difficult to assess the
potential for in vivo activity of mozavaptan at
physiological doses. In terms of adverse events, mozavaptan has
been reported to have no serious adverse events, with the most
common being dry mouth (72).
Spironolactone is a mineralocorticoid receptor
antagonist along with being a nonselective antagonist of androgen
and progesterone receptors (73).
It functions as a potassium-sparing diuretic that is used to treat
high blood pressure, heart failure and other conditions.
Spironolactone only had activity against the TN-IBC cell line
suggesting some unique target or pathway dependency in this cell
line compared to the other TNBC cell lines. Since spironolactone is
a weak antagonist of the androgen receptor (AR), it is possible
that this was the target in SUM-149. However, it has been reported
that SUM-149 does not express AR, but SUM-159 does (74), which does not fit our activity
results. Spironolactone has also been reported to be a nucleotide
excision repair (NER) inhibitor (75). This mechanism involves
spironolactone inducing degradation of the XPB helicase that is
part of the TFIIH transcription/repair complex involved in NER.
This group also reported no inhibition with 10 µM spironolactone in
four- or five-day cell viability assays using HeLa, A2780 and
HCT-116 cell lines. These cell lines along with MDA-MB-231, HCC1806
and SUM-159 have wild type BRCA1 (76–80).
Unique in our panel, SUM-149 has a BRCA1 frameshift mutation and
allele loss that may make it more dependent on remaining DNA repair
functions (76). Notably, only 3
out of 41 human breast cancer cell lines had BRCA1 mutations (with
allele loss), with SUM-149 being one of them. This DNA repair
defect may make SUM-149 more sensitive to inhibition of NER by
spironolactone. Thus, the SUM-149-specific sensitivity to
spironolactone may be due to a synthetic lethal type response in
this cell line and this would explain the unusual sensitivity of
SUM-149 to spironolactone. The spironolactone GI50 of
3.5 µM against SUM-149 is 10-fold higher than the Cmax
of 0.35 µM. Therefore, it is unclear if it would have any
significant activity in humans. Spironolactone has been reported to
have no significant impact on the risk of breast cancer in a
meta-analysis (81). However, one
recent study of younger women taking spironolactone indicated a
slight beneficial effect in terms of BC risk (82). If there is a positive impact for the
relatively rare IBC, then it would probably not be detectable
within a population of mostly non-IBC patients. The common adverse
event associated with long-term spironolactone use is menstrual
irregularities, which are dose-dependent and can be countered with
oral contraceptives or intrauterine devices (83). Other infrequent adverse events
include increased urination, lightheadedness, headaches, nausea,
vomiting, breast discomfort, and breast enlargement. Since it is a
potassium-retaining diuretic, elevated potassium level
(hyperkalemia) is another possible side effect, especially in
patients with renal dysfunction or heart failure.
The adverse effects of the other identified drugs
also provide insight into their potential for re-purposing. For
adefovir, the most common adverse events were similar to placebo,
except that headache and abdominal pain occurred more frequently
with adefovir but did not lead to discontinuation (84). Adefovir is associated with
dose-dependent increased risk of renal toxicity and thus,
monitoring would be important. For dolutegravir, the most common
adverse events reported were diarrhea, fatigue, and headache
(85). However, the majority of
these adverse events were mild or moderate in severity. Based on a
recent analysis of the FDA Adverse Event Reporting System database,
dolutegravir treatment decision should also include restrictions on
contraindicated populations (e.g. pregnant, hepatobiliary
disorders) (86). In a study with
healthy volunteers, no serious adverse events occurred during a
7-day study of dronedarone (87).
However, dronedarone can induce prolonged RR and QT intervals as a
function of dose, without effect on circadian patterns, which
suggests potential proarrhythmic risk (88). For the anti-malarial drug
halofantrine, clinical studies are short term in nature. The main
serious adverse effect associated with halofantrine use is QT
interval prolongation which prevents its use for long term
treatment at normal doses (89).
In summary, we identified eltrombopag and papaverine
as having anti-proliferative activity against SUM-149 and other
TNBC cell lines. Furthermore, these drugs had
GI50:Cmax ratios of 1–2 for sensitive cell
lines, suggesting that they are potent enough to potentially have
activity in vivo. Therefore, these drugs should be further
tested against patient-derived organoids (PDO) and xenograft models
of TN-IBC and TNBC to assess their activity alone and in
combination with other drugs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The project described was partially supported by the National
Institutes of Health through the RCMI grant NIH/NIMHD (grant no.
U54MD012392). This project was also partially funded by internal
grants from BRITE at North Carolina Central University.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The dataset used in this
article to identify candidate non-oncology drugs has been
previously published (12) and is
publicly available in the PRISM database (https://depmap.org/repurposing).
Authors' contributions
SA performed spheroid-based cell
proliferation/cytotoxicity assays, analyzed the results and
interpreted the data. SK performed the 2-D cell
proliferation/cytotoxicity assays, analyzed the results and
interpreted the data. KPW contributed to the conception and design
and revision of the manuscript. JES contributed to the conception
and design, analysis of the results, interpretation of the data and
drafting of the manuscript. SA and SK confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Kevin P. Williams, ORCID: 0000000269304630; John E.
Scott, ORCID: 0000-0002-5987-9334.
Glossary
Abbreviations
Abbreviations:
|
BC
|
breast cancer
|
|
TNBC
|
triple-negative breast cancer
|
|
IBC
|
inflammatory breast cancer
|
|
TN-IBC
|
triple-negative inflammatory breast
cancer
|
|
DMSO
|
dimethyl sulfoxide
|
|
GI
|
growth inhibition
|
|
CTG
|
CellTiter Glo
|
|
FBS
|
fetal bovine serum
|
|
2-D
|
two-dimension
|
|
NOD
|
non-oncology drug
|
|
Cmax
|
maximal concentration
|
|
SD
|
standard deviation
|
|
ER
|
estrogen receptor
|
|
FDA
|
Food and Drug Administration
|
|
ATCC
|
American Type Tissue Collection
|
|
TPOR
|
thrombopoietin receptor
|
|
V2R
|
vasopressin V2 receptor
|
|
NER
|
nucleotide excision repair
|
|
AR
|
androgen receptor
|
References
|
1
|
Beňačka R, Szabóová D, Guľašová Z,
Hertelyová Z and Radoňák J: Classic and new markers in diagnostics
and classification of breast cancer. Cancers (Basel). 14:54442022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee J: Current treatment landscape for
early triple-negative breast cancer (TNBC). J Clin Med.
12:15242023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Russnes HG, Lingjærde OC, Børresen-Dale AL
and Caldas C: Breast cancer molecular stratification: From
intrinsic subtypes to integrative clusters. Am J Pathol.
187:2152–2162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devi GR, Hough H, Barrett N, Cristofanilli
M, Overmoyer B, Spector N, Ueno NT, Woodward W, Kirkpatrick J,
Vincent B, et al: Perspectives on inflammatory breast cancer (IBC)
research, clinical management and community engagement from the
duke IBC consortium. J Cancer. 10:3344–3351. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boussen H, Berrazaga Y, Sherif K, Manai M,
Berrada N, Mejri N, Siala I, Levine PH and Cristofanilli M:
Inflammatory breast cancer: Epidemiologic data and therapeutic
results. Int Rev Cell Mol Biol. 384:1–23. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matro JM, Li T, Cristofanilli M, Hughes
ME, Ottesen RA, Weeks JC and Wong YN: Inflammatory breast cancer
management in the national comprehensive cancer network: The
disease, recurrence pattern, and outcome. Clin Breast Cancer.
15:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hester RH, Hortobagyi GN and Lim B:
Inflammatory breast cancer: Early recognition and diagnosis is
critical. Am J Obstet Gynecol. 225:392–396. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biswas T, Efird JT, Prasad S, James SE,
Walker PR and Zagar TM: Inflammatory TNBC breast cancer: Demography
and clinical outcome in a large cohort of patients with TNBC. Clin
Breast Cancer. 16:212–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chainitikun S, Saleem S, Lim B, Valero V
and Ueno NT: Update on systemic treatment for newly diagnosed
inflammatory breast cancer. J Adv Res. 29:1–12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al Khzem AH, Gomaa MS, Alturki MS, Tawfeeq
N, Sarafroz M, Alonaizi SM, Al Faran A, Alrumaihi LA, Alansari FA
and Alghamdi AA: Drug repurposing for cancer treatment: A
comprehensive review. Int J Mol Sci. 25:124412024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szalai P and Engedal N: An image-based
assay for high-throughput analysis of cell proliferation and cell
death of adherent cells. Bio Protoc. 8:e28352018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Corsello SM, Nagari RT, Spangler RD,
Rossen J, Kocak M, Bryan JG, Humeidi R, Peck D, Wu X, Tang AA, et
al: Discovering the anti-cancer potential of non-oncology drugs by
systematic viability profiling. Nat Cancer. 1:235–248. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Culley J, Nagle PW, Dawson JC and
Carragher NO: Patient derived glioma stem cell spheroid reporter
assays for live cell high content analysis. SLAS Discov. 28:13–19.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pfab C, Schnobrich L, Eldnasoury S,
Gessner A and El-Najjar N: Repurposing of antimicrobial agents for
cancer therapy: What do we know? Cancers (Basel). 13:31932021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhattacharya U, Kamran M, Manai M,
Cristofanilli M and Ince TA: Cell-of-origin targeted drug
repurposing for triple-negative and inflammatory breast carcinoma
with HDAC and HSP90 inhibitors combined with niclosamide. Cancers
(Basel). 15:3322023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
da Silva Fernandes T, Gillard BM, Dai T,
Martin JC, Chaudhry KA, Dugas SM, Fisher AA, Sharma P, Wu R,
Attwood KM, et al: Inosine monophosphate dehydrogenase 2 (IMPDH2)
modulates response to therapy and chemo-resistance in triple
negative breast cancer. Sci Rep. 15:10612025. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JH, Park S, Jung E, Shin J, Kim YJ,
Kim JY, Sessler JL, Seo JH and Kim JS: A dual-action
niclosamide-based prodrug that targets cancer stem cells and
inhibits TNBC metastasis. Proc Natl Acad Sci USA.
120:e23040811202023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greenshields AL, Fernando W and Hoskin DW:
The anti-malarial drug artesunate causes cell cycle arrest and
apoptosis of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3
breast cancer cells. Exp Mol Pathol. 107:10–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zadeh T, Lucero M and Kandpal RP:
Artesunate-induced cellular effects are mediated by specific EPH
receptors and ephrin ligands in breast carcinoma cells. Cancer
Genomics Proteomics. 19:19–26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Sun H, Zhang B, Liu S, Deng S, Weng
Z, Zuo B, Yang J and He Y: 18F-FDG PET imaging for
monitoring the early anti-tumor effect of albendazole on
triple-negative breast cancer. Breast Cancer. 27:372–380. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Priotti J, Baglioni MV, Garcia A, Rico MJ,
Leonardi D, Lamas MC and Menacho Márquez M: Repositioning of
anti-parasitic drugs in cyclodextrin inclusion complexes for
treatment of triple-negative breast cancer. AAPS PharmSciTech.
19:3734–3741. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kieliszek AM, Mobilio D, Bassey-Archibong
BI, Johnson JW, Piotrowski ML, de Araujo ED, Sedighi A, Aghaei N,
Escudero L, Ang P, et al: De novo GTP synthesis is a metabolic
vulnerability for the interception of brain metastases. Cell Rep
Med. 5:1017552024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benjanuwattra J, Chaiyawat P, Pruksakorn D
and Koonrungsesomboon N: Therapeutic potential and molecular
mechanisms of mycophenolic acid as an anticancer agent. Eur J
Pharmacol. 887:1735802020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdelwahab MT, Wasserman S, Brust JCM,
Gandhi NR, Meintjes G, Everitt D, Diacon A, Dawson R, Wiesner L,
Svensson EM, et al: Clofazimine pharmacokinetics in patients with
TB: Dosing implications. J Antimicrob Chemother. 75:3269–3277.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bullingham RE, Nicholls AJ and Kamm BR:
Clinical pharmacokinetics of mycophenolate mofetil. Clin
Pharmacokinet. 34:429–455. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ceballos L, Alvarez L, Lifschitz A and
Lanusse C: Ivermectin systemic availability in adult volunteers
treated with different oral pharmaceutical formulations. Biomed
Pharmacother. 160:1143912023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friedrich C, Richter E, Trommeshauser D,
de Kruif S, van Iersel T, Mandel K and Gessner U: Absence of
excretion of the active moiety of bisacodyl and sodium picosulfate
into human breast milk: An open-label, parallel-group,
multiple-dose study in healthy lactating women. Drug Metab
Pharmacokinet. 26:458–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Girard PM, Clair B, Certain A, Bidault R,
Matheron S, Regnier B and Farinotti R: Comparison of plasma
concentrations of aerosolized pentamidine in nonventilated and
ventilated patients with pneumocystosis. Am Rev Respir Dis.
140:1607–1610. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kouakou YI, Tod M, Leboucher G, Lavoignat
A, Bonnot G, Bienvenu AL and Picot S: Systematic review of
artesunate pharmacokinetics: Implication for treatment of resistant
malaria. Int J Infect Dis. 89:30–44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Milton KA, Edwards G, Ward SA, Orme ML and
Breckenridge AM: Pharmacokinetics of halofantrine in man: Effects
of food and dose size. Br J Clin Pharmacol. 28:71–77. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naccarelli GV, Wolbrette DL, Levin V,
Samii S, Banchs JE, Penny-Peterson E and Gonzalez MD: Safety and
efficacy of dronedarone in the treatment of atrial
fibrillation/flutter. Clin Med Insights Cardiol. 5:103–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ross LL, Song IH, Arya N, Choukour M, Zong
J, Huang SP, Eley T, Wynne B and Buchanan AM: No clinically
significant pharmacokinetic interactions between dolutegravir and
daclatasvir in healthy adult subjects. BMC Infect Dis. 16:3472016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schulz M and Schmoldt A: Therapeutic and
toxic blood concentrations of more than 800 drugs and other
xenobiotics. Pharmazie. 58:447–474. 2003.PubMed/NCBI
|
|
34
|
Schweizer MT, Haugk K, McKiernan JS,
Gulati R, Cheng HH, Maes JL, Dumpit RF, Nelson PS, Montgomery B,
McCune JS, et al: A phase I study of niclosamide in combination
with enzalutamide in men with castration-resistant prostate cancer.
PLoS One. 13:e01983892018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Williams DD, Peng B, Bailey CK, Wire MB,
Deng Y, Park JW, Collins DA, Kapsi SG and Jenkins JM: Effects of
food and antacids on the pharmacokinetics of eltrombopag in healthy
adult subjects: Two single-dose, open-label, randomized-sequence,
crossover studies. Clin Ther. 31:764–776. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu FG, Zhang ZJ, Dong HJ, Tian Y, Liu Y
and Chen Y: Bioequivalence assessment of two formulations of
spironolactone in Chinese healthy male volunteers.
Arzneimittelforschung. 58:117–121. 2008.PubMed/NCBI
|
|
37
|
Zou J, Di B, Zhang J, Dai L, Ding L, Zhu
Y, Fan H and Xiao D: Determination of adefovir by LC-ESI-MS-MS and
its application to a pharmacokinetic study in healthy Chinese
volunteers. J Chromatogr Sci. 47:889–894. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abbas ZN, Al-Saffar AZ, Jasim SM and
Sulaiman GM: Comparative analysis between 2D and 3D colorectal
cancer culture models for insights into cellular morphological and
transcriptomic variations. Sci Rep. 13:183802023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Filipiak-Duliban A, Brodaczewska K,
Kajdasz A and Kieda C: Spheroid culture differentially affects
cancer cell sensitivity to drugs in melanoma and RCC models. Int J
Mol Sci. 23:11662022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Lin J, Lin JR, Farris M, Robbins L,
Andrada L, Grohol B, Nong S and Liu Y: Dolutegravir inhibits
proliferation and motility of BT-20 tumor cells through inhibition
of human endogenous retrovirus type K. Cureus.
14:e265252022.PubMed/NCBI
|
|
41
|
Chen M, Hu L, Bao X, Ye K, Li Y, Zhang Z,
Kaufmann SH, Xiao J and Dai H: Eltrombopag directly activates BAK
and induces apoptosis. Cell Death Dis. 14:3942023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin Y, Shi Q, Yang G, Shi F, Zhou Y, Wang
T, Xu P, Li P, Liu Z, Sun H, et al: A small-molecule drug inhibits
autophagy gene expression through the central regulator TFEB. Proc
Natl Acad Sci USA. 120:e22136701202023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cui C, Pan Y, Zhang C, Zhu D, Xuan Y, Hao
P, Ke X, Zhou X and Qu Y: Eltrombopag binds SDC4 directly and
enhances MAPK signaling and macropinocytosis in cancer cells. Am J
Cancer Res. 12:2697–2710. 2022.PubMed/NCBI
|
|
44
|
Spitz AZ, Zacharioudakis E, Reyna DE,
Garner TP and Gavathiotis E: Eltrombopag directly inhibits BAX and
prevents cell death. Nat Commun. 12:11342021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheraghi S, Senel P, Dogan Topal B, Agar
S, Majidian M, Yurtsever M, Bellur Atici E, Gölcü A and Ozkan SA:
Elucidation of DNA-eltrombopag binding: electrochemical,
spectroscopic and molecular docking techniques. Biosensors (Basel).
13:3002023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Argenziano M, Di Paola A, Tortora C, Di
Pinto D, Pota E, Di Martino M, Perrotta S, Rossi F and Punzo F:
Effects of iron chelation in osteosarcoma. Curr Cancer Drug
Targets. 21:443–455. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Argenziano M, Tortora C, Paola AD, Pota E,
Martino MD, Pinto DD, Leva CD and Rossi F: Eltrombopag and its iron
chelating properties in pediatric acute myeloid leukemia.
Oncotarget. 12:1377–1387. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Waters T, Goss KL, Koppenhafer SL, Terry
WW and Gordon DJ: Eltrombopag inhibits the proliferation of Ewing
sarcoma cells via iron chelation and impaired DNA replication. BMC
Cancer. 20:11712020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dogra N, Singh P and Kumar A: A multistep
in silico approach identifies potential glioblastoma drug
candidates via inclusive molecular targeting of glioblastoma stem
cells. Mol Neurobiol. 61:9253–9271. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Erickson-Miller CL, Pillarisetti K,
Kirchner J, Figueroa DJ, Ottesen L, Martin AM, Liu Y, Kamel YM and
Messam C: Low or undetectable TPO receptor expression in malignant
tissue and cell lines derived from breast, lung, and ovarian
tumors. BMC Cancer. 12:4052012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Idlin N, Krishnamoorthy S, Wolczyk M,
Fakhri M, Lechowski M, Stec N, Milek J, Mandal PK, Cendrowski J,
Spanos C, et al: Effects of genetic ablation and pharmacological
inhibition of HuR on gene expression, iron metabolism, and hormone
levels. BMC Biol. 23:242025. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Roth M, Will B, Simkin G, Narayanagari S,
Barreyro L, Bartholdy B, Tamari R, Mitsiades CS, Verma A and Steidl
U: Eltrombopag inhibits the proliferation of leukemia cells via
reduction of intracellular iron and induction of differentiation.
Blood. 120:386–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhu Y, Yang L, Xu J, Yang X, Luan P, Cui
Q, Zhang P, Wang F, Li R, Ding X, et al: Discovery of the
anti-angiogenesis effect of eltrombopag in breast cancer through
targeting of HuR protein. Acta Pharm Sin B. 10:1414–1425. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen Y, Zhang R, Yang L, Zhang P, Wang F,
Lin G, Zhang J and Zhu Y: Eltrombopag inhibits metastasis in breast
carcinoma by targeting HuR protein. Int J Mol Sci. 24:31642023.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jenkins JM, Williams D, Deng Y, Uhl J,
Kitchen V, Collins D and Erickson-Miller CL: Phase 1 clinical study
of eltrombopag, an oral, nonpeptide thrombopoietin receptor
agonist. Blood. 109:4739–4741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shen CL, Hsieh TC, Wang TF, Huang WH, Chu
SC and Wu YF: Designing a scoring system for differential diagnosis
from reactive thrombocytosis and essential thrombocytosis. Front
Med (Lausanne). 8:7361502021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Saleh MN, Bussel JB, Cheng G, Meyer O,
Bailey CK, Arning M and Brainsky A; EXTEND Study Group, : Safety
and efficacy of eltrombopag for treatment of chronic immune
thrombocytopenia: Results of the long-term, open-label EXTEND
study. Blood. 121:537–545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bhat A, Tan V, Heng B, Chow S, Basappa S,
Essa MM, Chidambaram SB and Guillemin GJ: Papaverine, a
phosphodiesterase 10A inhibitor, ameliorates quinolinic
acid-induced synaptotoxicity in human cortical neurons. Neurotox
Res. 39:1238–1250. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fontana JA, Miksis G, Miranda DM and
Durham JP: Inhibition of human mammary carcinoma cell proliferation
by retinoids and intracellular cAMP-elevating compounds. J Natl
Cancer Inst. 78:1107–1112. 1987.PubMed/NCBI
|
|
60
|
Gomes DA, Joubert AM and Visagie MH: In
vitro effects of papaverine on cell proliferation, reactive oxygen
species, and cell cycle progression in cancer cells. Molecules.
26:63882021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Benej M, Hong X, Vibhute S, Scott S, Wu J,
Graves E, Le QT, Koong AC, Giaccia AJ, Yu B, et al: Papaverine and
its derivatives radiosensitize solid tumors by inhibiting
mitochondrial metabolism. Proc Natl Acad Sci USA. 115:10756–10761.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Laure A, Royet C, Bihel F, Baratte B, Bach
S, Peyressatre M and Morris MC: Ethaverine and papaverine target
cyclin-dependent kinase 5 and inhibit lung cancer cell
proliferation and migration. ACS Pharmacol Transl Sci. 7:1377–1385.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sahin ID, Christodoulou MS, Guzelcan EA,
Koyas A, Karaca C, Passarella D and Cetin-Atalay R: A small library
of chalcones induce liver cancer cell death through Akt
phosphorylation inhibition. Sci Rep. 10:118142020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Huang H, Li LJ, Zhang HB and Wei AY:
Papaverine selectively inhibits human prostate cancer cell (PC-3)
growth by inducing mitochondrial mediated apoptosis, cell cycle
arrest and downregulation of NF-κB/PI3K/Akt signalling pathway. J
BUON. 22:112–118. 2017.PubMed/NCBI
|
|
65
|
Marciano R, Prasad M, Ievy T, Tzadok S,
Leprivier G, Elkabets M and Rotblat B: High-throughput screening
identified compounds sensitizing tumor cells to glucose starvation
in culture and VEGF inhibitors in vivo. Cancers (Basel).
11:1562019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sajadian S, Vatankhah M, Majdzadeh M,
Kouhsari SM, Ghahremani MH and Ostad SN: Cell cycle arrest and
apoptogenic properties of opium alkaloids noscapine and papaverine
on breast cancer stem cells. Toxicol Mech Methods. 25:388–395.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gardos G, Cole JO and Sniffin C: An
evaluation of papaverine in tardive dyskinesia. J Clin Pharmacol.
16:304–310. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Shupack J, Stiller M, Meola T Jr and
Orbuch P: Papaverine hydrochloride in the treatment of atopic
dermatitis: A double-blind, placebo-controlled crossover clinical
trial to reassess safety and efficacy. Dermatologica. 183:21–24.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Reif GA, Yamaguchi T, Nivens E, Fujiki H,
Pinto CS and Wallace DP: Tolvaptan inhibits ERK-dependent cell
proliferation, Cl− secretion, and in vitro cyst growth
of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal
Physiol. 301:F1005–F1013. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tahara A, Saito M, Sugimoto T, Tomura Y,
Wada K, Kusayama T, Tsukada J, Ishii N, Yatsu T, Uchida W and
Tanaka A: Pharmacological characterization of the human vasopressin
receptor subtypes stably expressed in Chinese hamster ovary cells.
Br J Pharmacol. 125:1463–1470. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Garona J, Pifano M, Orlando UD, Pastrian
MB, Iannucci NB, Ortega HH, Podesta EJ, Gomez DE, Ripoll GV and
Alonso DF: The novel desmopressin analogue [V4Q5]dDAVP inhibits
angiogenesis, tumour growth and metastases in vasopressin type 2
receptor-expressing breast cancer models. Int J Oncol.
46:2335–2345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ectopic ADH Syndrome Therapeutic Research
Group, . Yamaguchi K, Shijubo N, Kodama T, Mori K, Sugiura T,
Kuriyama T, Kawahara M, Shinkai T, Iguchi H and Sakurai M: Clinical
implication of the antidiuretic hormone (ADH) receptor antagonist
mozavaptan hydrochloride in patients with ectopic ADH syndrome. Jpn
J Clin Oncol. 41:148–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ferreira JP, Pitt B and Zannad F:
Mineralocorticoid receptor antagonists in heart failure: An update.
Circ Heart Fail. 17:e0116292024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ethier SP, Guest ST, Garrett-Mayer E,
Armeson K, Wilson RC, Duchinski K, Couch D, Gray JW and Kappler C:
Development and implementation of the SUM breast cancer cell line
functional genomics knowledge base. NPJ Breast Cancer. 6:302020.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Alekseev S, Ayadi M, Brino L, Egly JM,
Larsen AK and Coin F: A small molecule screen identifies an
inhibitor of DNA repair inducing the degradation of TFIIH and the
chemosensitization of tumor cells to platinum. Chem Biol.
21:398–407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Elstrodt F, Hollestelle A, Nagel JHA,
Gorin M, Wasielewski M, van den Ouweland A, Merajver SD, Ethier SP
and Schutte M: BRCA1 mutation analysis of 41 human breast cancer
cell lines reveals three new deleterious mutants. Cancer Res.
66:41–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Martín-Bejarano P, Sánchez-Tapia EM,
Jessica P, Martín-Gómez T, Tocino RV, González-Sarmiento R and
Herrero AB: Functional characterization of BRCA1 variants of
unknown significance using homologous recombination repair assays.
Breast Cancer Res. 27:1742025. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Baloch T, López-Ozuna VM, Wang Q, Matanis
E, Kessous R, Kogan L, Yasmeen A and Gotlieb WH: Sequential
therapeutic targeting of ovarian cancer harboring dysfunctional
BRCA1. BMC Cancer. 19:442019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mita P, Sun X, Fenyö D, Kahler DJ, Li D,
Agmon N, Wudzinska A, Keegan S, Bader JS, Yun C and Boeke JD: BRCA1
and S phase DNA repair pathways restrict LINE-1 retrotransposition
in human cells. Nat Struct Mol Biol. 27:179–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Keung MY, Wu Y, Badar F and Vadgama JV:
Response of breast cancer cells to PARP inhibitors is independent
of BRCA status. J Clin Med. 9:9402020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bommareddy K, Hamade H, Lopez-Olivo MA,
Wehner M, Tosh T and Barbieri JS: Association of spironolactone use
with risk of cancer: A systematic review and meta-analysis. JAMA
Dermatol. 158:275–282. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Garate D, Thang CJ, Golovko G, Wilkerson
MG and Barbieri JS: A matched cohort study evaluating whether
spironolactone or tetracycline-class antibiotic use among female
acne patients is associated with breast cancer development risk.
Arch Dermatol Res. 316:1962024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lin G, Chen R, Wen C, Li Z, Yan X and Li
L: Analyzing real-world adverse events of spironolactone with the
FAERS database. PLoS One. 20:e03306592025. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kayaaslan B and Guner R: Adverse effects
of oral antiviral therapy in chronic hepatitis B. World J Hepatol.
9:227–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Min S, Sloan L, DeJesus E, Hawkins T,
McCurdy L, Song I, Stroder R, Chen S, Underwood M, Fujiwara T, et
al: Antiviral activity, safety, and
pharmacokinetics/pharmacodynamics of dolutegravir as 10-day
monotherapy in HIV-1-infected adults. AIDS. 25:1737–1745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Su J, He L and Wang M: Post-marketing
safety concerns with dolutegravir: A pharmacovigilance study based
on the FDA adverse event reporting system database. Front
Pharmacol. 16:16256012025. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tschuppert Y, Buclin T, Rothuizen LE,
Decosterd LA, Galleyrand J, Gaud C and Biollaz J: Effect of
dronedarone on renal function in healthy subjects. Br J Clin
Pharmacol. 64:785–791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wadhani N, Sarma JS, Singh BN, Radzik D
and Gaud C: Dose-dependent effects of oral dronedarone on the
circadian variation of RR and QT intervals in healthy subjects:
Implications for antiarrhythmic actions. J Cardiovasc Pharmacol
Ther. 11:184–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Charbit B, Becquemont L, Lepère B,
Peytavin G and Funck-Brentano C: Pharmacokinetic and
pharmacodynamic interaction between grapefruit juice and
halofantrine. Clin Pharmacol Ther. 72:514–523. 2002. View Article : Google Scholar : PubMed/NCBI
|