Introduction
Undifferentiated pleomorphic sarcoma (UPS) is an
aggressive subtype of soft tissue sarcomas, characterized by a
limited response to therapy (1).
However, UPS shows higher immunogenicity compared to other sarcoma
subtypes, which supports the interest in immunotherapy for this
cancer (2). Immunotherapy has
changed oncological treatment in recent years, with several
patients now receiving it as a first-line therapy (3–6). A
number of these therapies, such as immune checkpoint inhibitors,
demonstrated notable outcomes for several patients (7). However, for numerous patients,
particularly those with solid tumors, immunotherapy still fails and
traditional therapeutic modalities, including cytotoxic
chemotherapy, need to be used in following therapy lines (8,9). In
addition, novel algorithms of oncological treatment may combine
multiple therapeutic approaches, such as chemotherapy and
immunotherapy or radiotherapy and immunotherapy (10,11).
However, despite the progress in the field, how therapeutic
combinations may affect each other and the overall therapeutic
efficacy remain to be elucidated. One of the key effector molecules
associated with immunotherapy is interferon γ (IFNγ) (12). This cytokine is primarily produced
by cytotoxic lymphocytes, including cytotoxic cluster of
differentiation(CD)8+ T cells, natural killer (NK) cells
and NK T cells, which serve a key role in eliminating cancer cells
(13,14). Thus, any treatment leading to the
effective removal of cancer cells by cytotoxic lymphocytes is
likely to be associated with the production of IFNγ and its
collateral impact not only on immune cells (15,16)
but also on other cells in the tumor microenvironment, including
cancer cells (17–19). Due to the impact of IFNγ on cancer
cells, it is difficult to determine how it can affect the efficacy
of individual therapeutic approaches or their combinations.
Previous studies have reported that IFNγ signatures, patterns of
genes, proteins or cellular changes that become activated in
response to this cytokine, are frequently associated with enhanced
responses to immunotherapy, and elevated IFNγ levels are typically
associated with immunotherapy efficacy (20,21).
Immunotherapy may sensitize tumors to subsequent chemotherapy
(22,23), yet it may also promote resistance to
chemotherapy (24,25). This duality implies that IFNγ
signatures may likewise exert dual, context-dependent roles across
these therapeutic modalities.
UPS is commonly linked to increased infiltration of
immune cells (26), which often
correlates with IFNγ signatures (27). However, the specific role of IFNγ in
UPS and its implications for immunotherapy and chemotherapy remain
unclear. To address this, the present study examined a recently
developed, primary tumor-derived, highly transformed, UPS cell line
termed JBT19 (28). The present
study further explored the mechanism by which prolonged exposure to
IFNγ influences the sensitivity of the cell line to cytotoxic
chemotherapy and antitumor immunity.
Materials and methods
Cell lines, cell culture and
IFNγ-mediated phenotype remodeling
The JBT19 cell line, which originated from a UPS,
high-grade soft tissue sarcoma, was established from the primary
tumor of a 65-year-old male patient, whose tumor was surgically
removed before any other therapeutic interventions and the cell
line was subsequently developed using tumor
fragmentation-dissociation techniques (28). After 30 passages and >30 months
in cell culture, the cell line already exhibited a high population
uniformity and was subsequently characterized (28). In the present study, the JBT19 cell
line and its green fluorescent protein (GFP)-transfected form
(JBT19-GFP) were used after 52 passages and after >35 months in
cell culture. The prostate carcinoma cell line PC-3 was used as an
epithelial-origin-derived tumor cell line model (29). Cells were adherently cultured (37°C;
5% CO2) in RPMI 1640-based FBS-containing complete
medium (KM+ medium). KM+ medium consisted of RPMI 1640 medium
(Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(non-heat-inactivated; HyClone™; Cytiva), 100 U/ml
penicillin-streptomycin, 2 mM GlutaMax, 1 mM sodium pyruvate
(Thermo Fisher Scientific, Inc.) and 1 mM non-essential amino acid
mix (Thermo Fisher Scientific, Inc.) in tissue culture flasks or
plates (TPP Techno Plastic Products AG or SARSTEDT AG & Co.
KG). The cells were passaged as previously described using
trypsin/EDTA solution (Thermo Fisher Scientific, Inc.) and KM+
medium (28). To reprogram the
phenotype of JBT19 cells, the adherent cultured JBT19 cells were
supplemented with 200 ng/ml IFNγ (PeproTech, Inc.; Thermo Fisher
Scientific, Inc.) for the indicated times. Every 2–4 days, the
cells were supplemented with fresh KM+ medium and IFNγ (200 ng/ml).
To reverse the phenotype of IFNγ-reprogrammed cells, the cells were
extensively rinsed (>3 times) with fresh KM+ medium and cultured
for the indicated times. The cells were supplemented with fresh KM+
medium every 2–4 days and/or passaged.
Phenotypic characterization
To evaluate the expression levels of cell surface
markers in JBT19 cells, the cells were rinsed with PBS and
harvested using trypsin/EDTA solution and KM+ medium. The harvested
cells were rinsed with PBS supplemented with 2 mM EDTA (PBS/E) and
stained on ice for 30–60 min with the following fluorophore-labeled
antibodies: Anti-CD44-Phycoerythrin (PE; clone MEM-263; cat. no.
1P-341-T100; EXBIO Praha, a.s.), anti-CD47-allophycocyanin (APC;
clone CC2C6; cat. no. 323124; BioLegend, Inc.), anti-CD95 (Fas)-APC
(clone DX2; cat.# 305611; BioLegend, Inc.), anti-fibroblast
activation protein (FAP)-α-PE (clone 427819; cat.# FAB3715P-100;
R&D Systems, Inc.), anti-CD274 (PD-L1)-APC (clone MIH3; cat.#
374514; BioLegend, Inc.), anti-human leukocyte antigen (HLA)-ABC
[major histocompatibility complex (MHC)]-(I)-PE (clone W6/32; cat.#
311406; BioLegend, Inc.) and HLA-DP, DQ, DR (MHC-II)-APC (clone
Tü39; cat.# 361714; BioLegend, Inc.). The stained cells were rinsed
with PBS/E and supplemented with 100 ng/ml DAPI (Thermo Fisher
Scientific, Inc.) shortly before cell analysis. To evaluate the
expression levels of intracellular cell markers, the harvested
cells were stained on wet ice using LIVE/DEAD Fixable Aqua Dead
Cell Stain (Thermo Fisher Scientific, Inc.), fixed and overnight
permeabilized as previously described (30). Cells were then stained on wet ice
with the following fluorophore-labeled antibodies: Anti-Ki-67-PE
(clone Ki-67; cat. no. 1P-155-T100; EXBIO Praha, a.s.),
anti-multidrug resistance (MDR)-1-PE (CD243, clone UIC2; cat.#
1P-764-T100; EXBIO Praha, a.s.), anti-B-cell lymphoma (Bcl)-2-PE
(clone Bcl-2/100; cat.# 556535; Becton, Dickinson and Company) and
anti-signal transducer and activator of transcription (STAT)1-Alexa
Fluor 647 (clone 1/STAT1; cat.# 558560; Becton, Dickinson and
Company). After staining, cells were rinsed with PBS/E, and the
surface of the cells or intracellularly stained cells were analyzed
with a FACSAria II or FACSFortesa flow cytometer (Becton, Dickinson
and Company). The acquired data were processed using FlowJo
software (version 10.10.0; FlowJo LLC; BD Biosciences). The gating
strategy used fluorescence minus one staining.
MTT assay, cell cycle assay and
annexin V analysis
The cytotoxic impact of treating the cell line with
docetaxel, doxorubicin (Selleck Chemicals) or mitomycin C
(MilliporeSigma; Merck KGaA), was determined using the MTT assay
(MilliporeSigma; Merck KGaA). Briefly, flat-bottom 96-well-cultured
adherent JBT19 cells (5,000–20,000 cells/well) were treated with
the indicated concentrations of docetaxel, doxorubicin or mitomycin
C for 3 days. The cell cultures were then supplemented with 0.5
mg/ml MTT substrate (MilliporeSigma; Merck KGaA) and cultured for 3
h. The supernatant was removed, and the insoluble formazan
dissolved with acidified isopropanol (MilliporeSigma; Merck KGaA).
The absorbance was acquired at 570 nm and referenced at 630 nm. The
acquired data were processed using GraphPad Prism (version 10.2.0;
Dotmatics) to determine the half-maximal inhibitory concentration
(IC50). Cell cycle analysis was performed as described
previously using PI staining (31),
with the exception that the data were analyzed using the
Dean/Jett/Fox algorithm
(docs.flowjo.com/flowjo/experiment-based-platforms/cell-cycle-univariate/)
with FlowJo software (version 10.10.0; FlowJo LLC; BD Biosciences).
Annexin V staining was performed as described previously (32) using annexin V-FITC (cat. no.
EXB0024; EXBIO Praha, a.s.).
Preparation of JBT19-reactive
lymphocytes
The source cell material were buffy coats from 4
healthy blood donor volunteers obtained from the Institute of
Hematology and Blood Transfusion (Prague, Czech Republic). This
material was used to isolate and cryopreserve peripheral blood
mononuclear cells (PBMCs) as previously described (30,33).
The volunteers provided a prior signed written informed consent for
the use of the biological material for research. The research
adhered to the ethics standards of the institutional research
committee and was approved by the Ethics Committee of the
University Hospital Motol in Prague (approval no. EK-602.4/22;
Prague, Czech Republic). The research was performed in compliance
with the 1964 Helsinki Declaration and its later amendments. The
JBT19-reactive lymphocytes were produced as described previously
(28) with minor modifications.
Briefly, the cryopreserved PBMCs were reconstituted overnight
(2–3×106 cells/ml) in RPMI 1640-based human
serum-containing complete medium [lympho medium (LM), consisting of
RPMI 1640 medium (Thermo Fisher Scientific, Inc.) containing 5%
human serum (One Lambda, Inc.; Thermo Fisher Scientific, Inc.) and
other non-serum supplements as in KM+ medium] supplemented with
human IL-2 (500 IU/ml; PeproTech, Inc.; Thermo Fisher Scientific,
Inc.). The reconstituted cells were harvested, pelleted and
reconstituted in fresh LM with 500 IU/ml IL-2 at 4×106
cells/ml. The cells were then combined at a 1:1 ratio with
trypsin/EDTA-harvested and UV-inactivated (312 nm; 2.55
J/cm2) JBT19 cells that were previously treated or not
with 200 ng/ml human IFNγ for 7 days (1×106 cells/ml)
[final ratio, 4:1 (PBMC/JBT19)]. The combined cells were cultured
in a flat-bottom 48-well plate well (Nalgene; Thermo Fisher
Scientific, Inc.). On days 3, 5 and 6, the cell cultures were
hemi-depleted and supplemented with fresh LM and 500 IU/ml IL-2. On
day 7, the cell cultures were transferred to a flat-bottom 12-well
plate well (Nalgene; Thermo Fisher Scientific, Inc.) and
supplemented with an equal volume of fresh LM and 500 IU/ml IL-2.
On days 10 and 11, the cell cultures were hemi-depleted and
supplemented with fresh LM and 500 IU/ml of IL-2. On day 12, the
cells were analyzed and/or cryopreserved.
Rapid expansion protocol (REP) for
JBT19-reactive lymphocytes
The 12-day cryopreserved cell cultures enriched with
lymphocytes reactive to JBT19 cells or IFNγ-treated JBT19 cells
were reconstituted as aforementioned in LM with 500 IU/ml IL-2. The
reconstituted cells were then large-scale expanded for 14 days
using an REP as previously described (34), with the exception that following the
initial IL-2 concentration of 4,000 IU/ml, the cells were next
cultured with 2,000 IU/ml IL-2. On day 14, the large-scale expanded
cells were analyzed and/or cryopreserved.
Analyses of JBT19-reactive
lymphocytes
The cultured lymphocytes (enriched or large-scale
expanded) were analyzed with small modifications using procedures
described previously (28).
Briefly, the cultured lymphocytes were harvested, pelleted and
resuspended in fresh LM with 500 IU/ml IL-2 at 4×106
cells/ml. The lymphocytes were then combined at a 1:1 ratio with
trypsin/EDTA-harvested JBT19 cells treated or not with 200 ng/ml
IFNγ for 7 days [1×106 cells/ml; final ratio, 4:1 of
lymphocytes (effector)/JBT19 (target)]. The combined cells were
then transferred to a U-bottom 96-well plate (Nalgene; Thermo
Fisher Scientific, Inc.) and cultured (stimulated) at 37°C in the
presence of 5% CO2. After 1 h, the cells were
supplemented with Brefeldin A (BioLegend, Inc.) to prevent cytokine
secretion from the cells and cultured for an additional 4 h. The
cells were transferred to a V-bottom 96-well plate (Nalgene; Thermo
Fisher Scientific, Inc.), pelleted, rinsed with PBS/E and stained
on wet ice with LIVE/DEAD Fixable Aqua Dead Cell Stain (Thermo
Fisher Scientific, Inc.). The stained cells were next fixed,
overnight permeabilized and stained on wet ice with
anti-CD3-peridinin-chlorophyll-protein-cyanine (Cy)5.5 (clone SK7;
cat. no. T9-173-T100), anti-CD4-PE-Cy7 (clone MEM-241; cat. no.
T7-359-T100), anti-CD8-Alexa Fluor 700 (clone MEM-31; cat. no.
A7-207-T100; EXBIO Praha, a.s.), anti-TNFα-APC (clone monoclonal
antibody 11; cat.# 562084) and anti-IFNγ-PE (clone B27; cat.#
559327; Becton, Dickinson and Company) antibodies. The stained
cells were pelleted, rinsed with PBS/E and analyzed by flow
cytometry as aforementioned. Reactive cell frequency was calculated
as the difference between the proportion of IFNγ- and/or
TNFα-producing cells in the JBT19 cell-stimulated sample and the
corresponding vehicle-stimulated control from the same sample. In
certain experiments, lymphocytes were pretreated for 30 min with 20
µg/ml anti-MHC-I (clone W6/32; cat. no. 311428; BioLegend, Inc.) or
anti-MHC-II (clone Tü39; cat.# 555556; Becton, Dickinson and
Company) blocking antibodies in serum-free RPMI 1640 medium (Thermo
Fisher Scientific, Inc.) and then stimulated in the presence or
absence of 10 µg/ml antibodies in LM. The flow cytometry was
performed as aforementioned.
Analysis of the cell-mediated
cytotoxic impact on JBT19-GFP cells
JBT19-GFP cells were generated as previously
described (28). The cytotoxic
impact of lymphocytes (enriched or large-scale expanded) on
JBT19-GFP cells treated or not with 200 ng/ml IFNγ for 7 days was
assessed using a previously described protocol with a small
modification (28,35). Briefly, 0.1×106 JBT19-GFP
cells (target) were passaged into a flat-bottom 48-well plate well
and cultured for 1 day. Upon supernatant removal, the wells were
supplemented with 1.0 ×106 lymphocytes (effector) in 1
ml LM with IL-2 (500 IU/ml). After lymphocyte sedimentation for 10
min, the fluorescence of JBT19-GFP cells in the wells was acquired
with a fluorescence microscope and the mean fluorescence intensity
(MFI) at day 0 was calculated using image analysis with ImageJ
1.44p (National Institutes of Health) as previously described
(36). The cells were cocultured
for 3 days and MFIs were determined as on day 0.
Statistical analysis
Values were calculated from the n sample size
using GraphPad Prism 10.2.0 (GraphPad; Dotmatics). Data are
presented as the mean ± SEM of ≥3 independent experimental repeats.
Differences between groups were determined with the indicated
paired or unpaired two-tailed Student's t-test or with one-way
ANOVA followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference, unless
indicated otherwise.
Results
IFNγ abrogates in vitro proliferation
of JBT19 cells, increases their expression levels of CD44, CD47 and
CD95 (FAS) and induces docetaxel resistance
IFNγ is a cytokine produced by activated NK cells
and cytotoxic CD8+ T cells (13). These cells are key effector cells
responsible for cancer cell elimination (14,37)
and their presence in tumors is often associated with improved
prognosis of the disease (38,39).
However, once released in the tumor microenvironment, its chronic
impact can also modulate cancer cells. Therefore, the present study
used the recently established UPS sarcoma cell line JBT19 (28) and investigated the long-term
(chronic) impact of this cytokine on the proliferation and
phenotype of JBT19 cells. The rationale for using this cell line
was based on the observation that, although
two-dimensional-cultured JBT19 cells exhibited little-to-no surface
expression levels of MHC-II molecules, as determined by flow
cytometry, supplementation of the culture medium with IFNγ for 3
days induced robust surface expression levels of MHC-II (Fig. 1A). This response was concentration
dependent, reaching a plateau at concentrations >5 ng/ml
(Fig. 1B). Furthermore, IFNγ
supplementation reduced the proliferation of JBT19 cells. To
further investigate this effect, JBT19 cells were cultured in the
presence of 200 ng/ml IFNγ for 7 days, ensuring a robust and stable
response within the plateau range and cell proliferation was
subsequently evaluated in the presence or absence of the cytokine.
After the initial delay in the cell proliferation in the first 3
days, presumably due to a transient adverse impact of the passaging
procedure on cell proliferation, IFNγ markedly suppressed the
proliferation of JBT19 cells, increasing their doubling time
>3-fold, from ~2 to >6 days (Fig.
1C and D). This suppression led to a marked decrease in the
proliferation of the cell culture. Whereas the non-treated cell
culture exhibited a 150–200-fold increase in cell numbers in 12
days, cells treated with IFNγ expanded 3-4-fold.
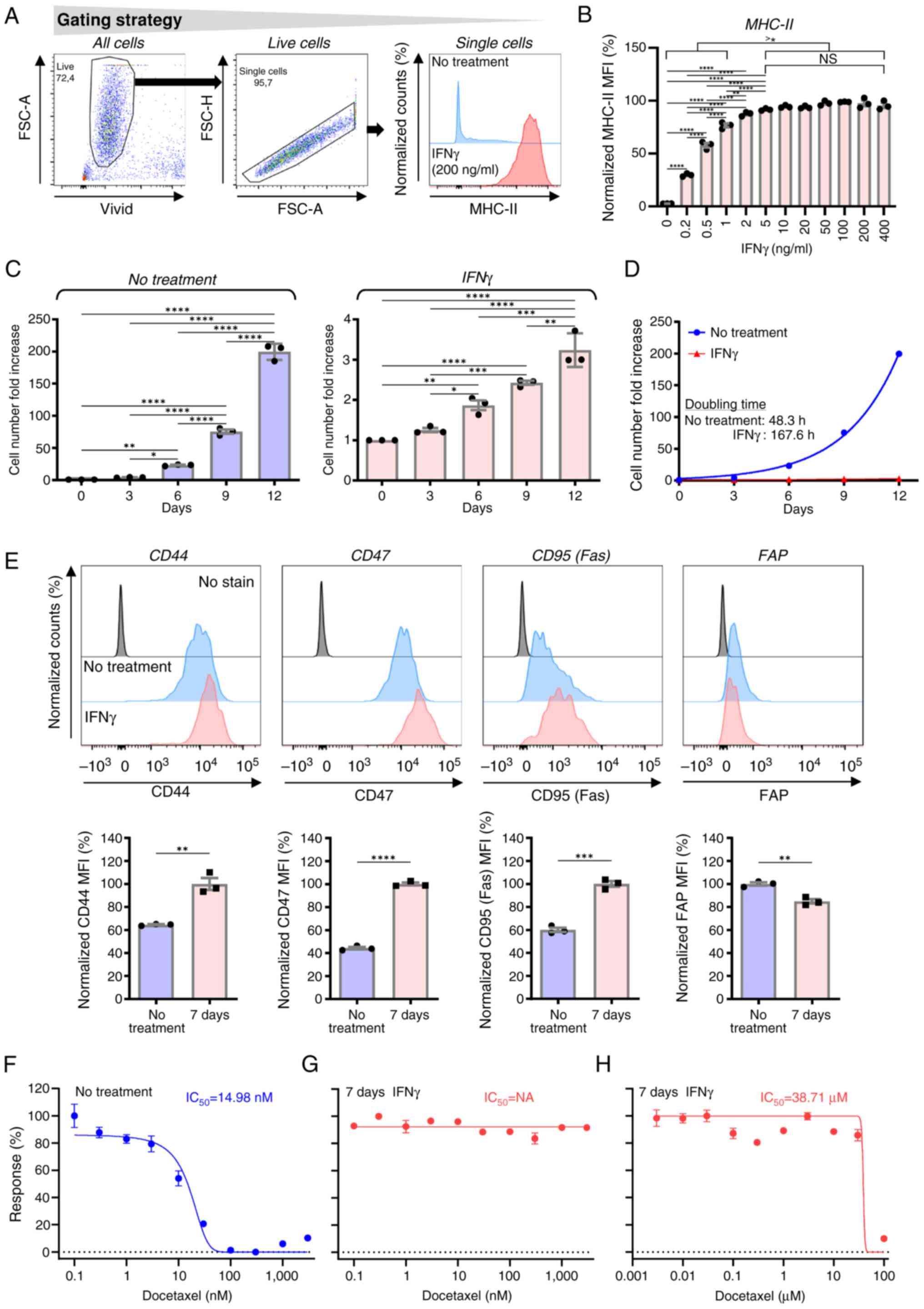 | Figure 1.Impact of IFNγ on the in vitro
proliferation of JBT19 cells and their expression levels of MHC-II,
CD44, CD47 and CD95 (Fas), and their sensitivity to docetaxel. (A)
Gating strategy of flow cytometry data. (B) Extracellular MHC-II
staining of 3-day treated JBT19 cells with the indicated
concentrations of IFNγ. (C) Cell number fold increase in
vehicle-treated JBT19 cells (No treatment, left panel) or 7-day
pre-treated and then cultured with IFNγ (200 ng/ml; IFNγ, right
panel). The evaluated are represented as means ± SEM. *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001; n=3 independent
experiments; one-way ANOVA with Tukey's post hoc test). (D)
Calculated proliferation curves and pertinent doubling times from
the data shown in panel C. (E) Extracellular staining of 7-day
vehicle-treated (No treatment) or 7-day IFNγ-treated (IFNγ, 200
ng/ml) JBT19 cells. The cells were stained with specific antibodies
against CD44, CD47, CD95 (Fas) or FAP. In the top panels,
representative histograms are shown and the control (No stain) for
individual fluorochromes refers to staining with vehicle alone. In
the bottom panels, the evaluated data presented as means ± SEM are
shown and statistically significant differences between the groups
are indicated. **P<0.01, ***P<0.001 and ****P<0.0001. MTT
assay of (F) vehicle-treated JBT19 cells (No treatment), or (G and
H) 7-day IFNγ-treated (IFNγ, 200 ng/ml) JBT19 cells (7 days IFNγ)
exposed to docetaxel at the indicated concentrations for 3 days.
The calculated IC50 from three independent experiments
is shown. MFI, mean fluorescence intensity; MHC, major
histocompatibility complex; nM, nanomolar; FAP, fibroblast
activation protein. |
Next, the present study investigated how this marked
decrease in JBT19 cell proliferation affected the expression levels
of cell surface markers, which are reported to be possibly
associated with modulated resistance of cancer cells to the immune
system and chemotherapeutics. Among these markers were CD44 (which
is associated with cancer cell stemness), CD47 (a receptor
inhibiting phagocytosis by macrophages and its expression in
multiple cancer types, such as in ovarian and gastric cancer, is
associated with chemoresistance and poor prognosis) (40–42)
and CD95 (Fas; a death receptor inducing apoptosis) (43). As shown in Fig. 1E, 7-day treatment of JBT19 cells
with IFNγ increased the surface expression levels of CD44, CD47 and
CD95 (Fas) in JBT19 cells. By contrast, the surface expression
levels of FAP were reduced. These results demonstrated that IFNγ
suppressed the proliferation of JBT19 cells but induced a phenotype
that could possibly affect JBT19 cell resistance to cytotoxic
chemotherapy and immunotherapy.
The IFNγ-mediated inhibition of JBT19 cell
proliferation suggested that these cells may develop resistance to
chemotherapeutic agents that primarily target dividing cells
(44). Therefore, the present study
next examined the cytotoxic effects of docetaxel on IFNγ-treated
JBT19 cells. Docetaxel is a commonly used chemotherapy drug
(45) that targets dividing cells
by inhibiting the depolymerization of microtubules, causing mitotic
arrest and subsequent apoptosis (46). JBT19 cells were sensitive to
docetaxel at nanomolar (nM) concentrations (IC50=14.98
nM) as determined by MTT assay (Fig.
1F). However, 7-day treatment of these cells with IFNγ made
these cells highly resistance to docetaxel (Fig. 1G), decreasing their sensitivity by
>2,000-fold (IC50=38.71 µM; Fig. 1H). These results demonstrated that,
albeit IFNγ nearly blocked the proliferation of JBT19 cells, this
was associated with the acquisition of chemoresistance to a widely
used drug such as docetaxel, to which JBT19 cells were otherwise
sensitive at nM concentrations.
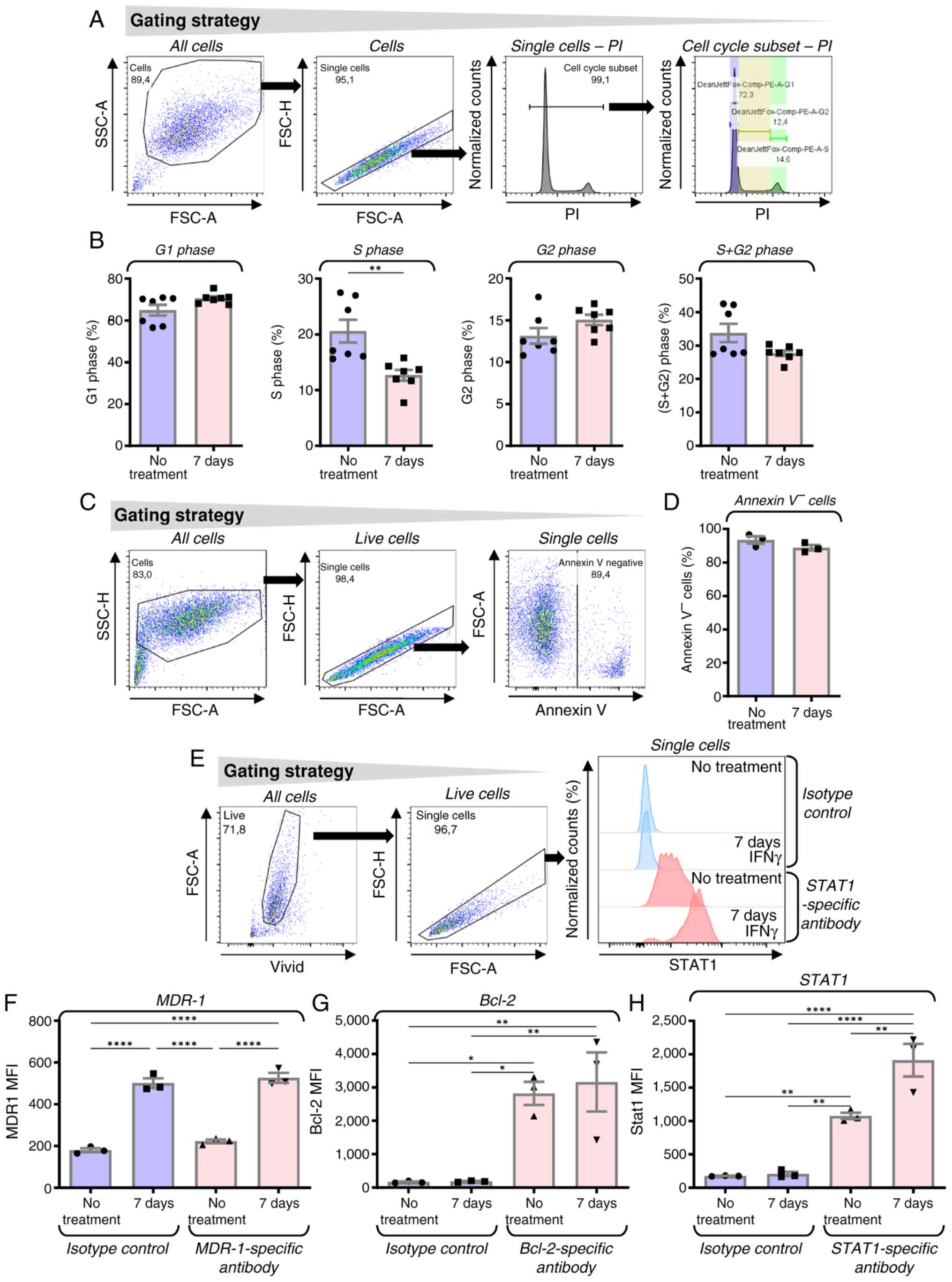
IFNγ changes the cell cycle, does not
induce apoptosis and increases STAT1 expression without inducing
the surface expression levels of multidrug resistance protein 1
(MDR-1) or enhancing the expression levels of Bcl-2 in JBT19
cells
IFNγ treatment is known to impact the cell cycle
(47); therefore, it was next
examined how the 7-day IFNγ treatment affected the cell cycle of
JBT19 cells. The results revealed a trend toward increased
frequencies of cells in G1 and G2 phases and
a decreased frequency in the combined G2 + S population.
Notably, IFNγ treatment induced a significant reduction in the
proportion of cells in S phase (Fig. 2A
and B). This pattern of IFNγ-mediated cell cycle changes was
consistent with observations in other cell types (48,49).
The treatment of JBT19 cells with IFNγ was not associated with
increased apoptosis, expression levels of MDR-1 receptor (50) or modulation of the expression levels
of the antiapoptotic signaling molecule Bcl-2 (51) (Fig.
2C-G). However, sustained exposure to IFNγ for 7 days enhanced
the expression levels of the STAT1 signaling molecule (Fig. 2H), a response previously reported in
multiple cell types (52–55).
IFNγ decreases the expression levels
of Ki-67 and increases the expression levels of PD-L1 and MHC-II in
JBT19 cells
The marked impact of IFNγ on the performance of
JBT19 cells towards chemoresistance was associated with long-term
(7-day) exposure of JBT19 cells to this cytokine. The present study
next evaluated a shorter exposition of JBT19 cells to this cytokine
and investigated a possible reversibility of its effect on their
proliferation. To do that, Ki-67 was used as a surrogate marker of
cell proliferation (56). Ki-67
expression was analyzed in JBT19 cells treated with IFNγ (200
ng/ml) for 7 days, 2 days or 2 days followed by its extensive
removal and additional 5-day cell culture. As shown in Fig. 3A-C, 2-day IFNγ treatment decreased
the levels of Ki-67 nearly to the levels observed in 7-day treated
cells. Furthermore, these levels were sustained even after the
following 5 days of cell culture in the absence of IFNγ. These
findings demonstrated that the anti-proliferative impact of IFNγ on
JBT19 cells was induced after a shorter time exposure but was not
reversed even 5 days later.
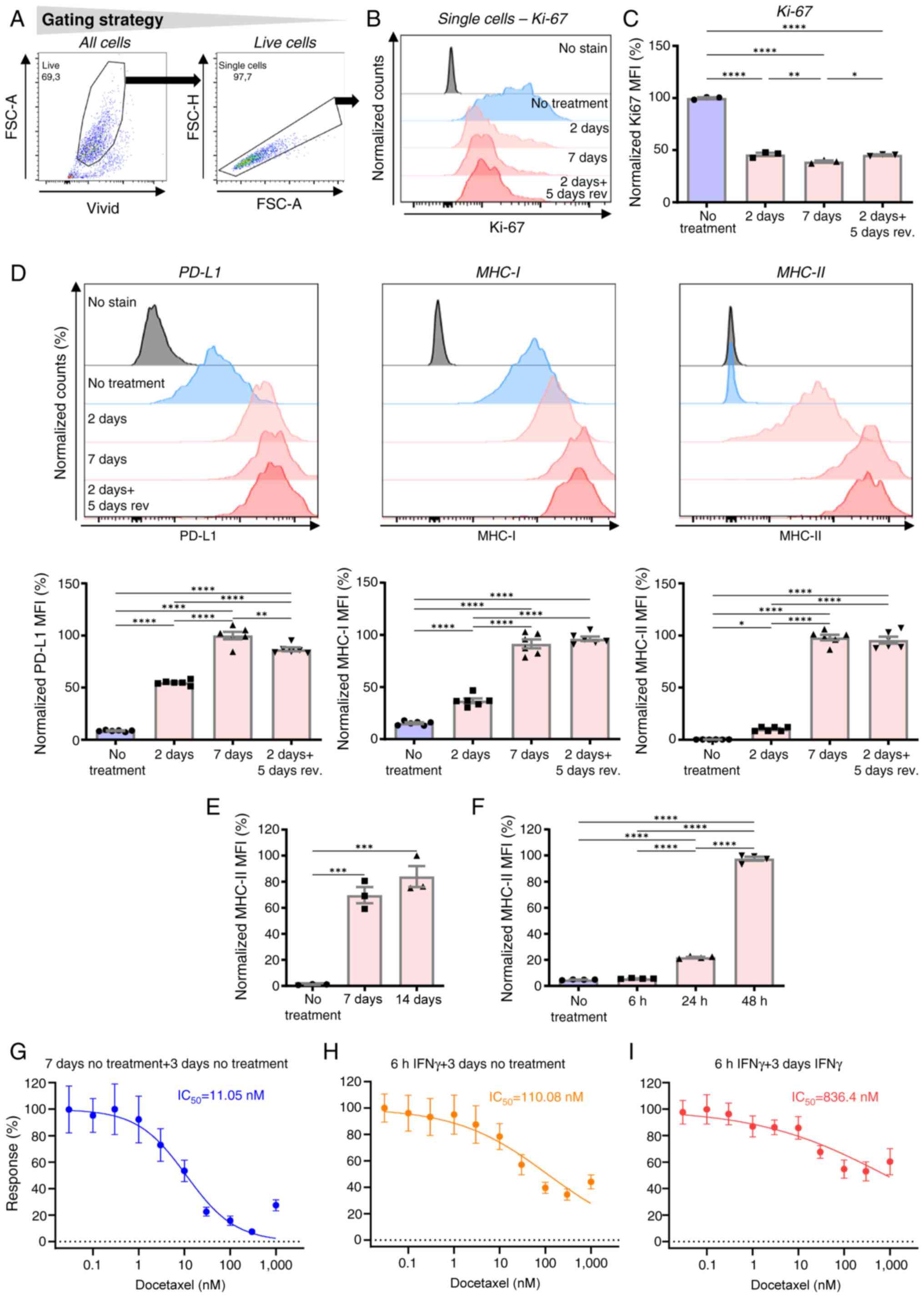 | Figure 3.In JBT19 cells, IFNγ decreases the
intracellular expression levels of Ki-67, increases the
extracellular expression levels of PD-L1 and MHC-I and enhances the
expression levels of MHC-II. (A) Gating strategy of flow cytometry
data. (B) Intracellular staining with anti-Ki-67-specific antibody
in vehicle-treated (No treatment), 2-day treated (2 d) or 7-day
treated (7 d) JBT19 cells with IFNγ (200 ng/ml). Alternatively, the
cells were treated for 2 days with IFNγ (200 ng/ml) and the
cytokine was then extensively removed, while the cells were
cultured in vehicle alone for additional 5 days (2d + 5d rev). As
control (No stain) for individual fluorochromes, staining with
vehicle alone was used. (C) Evaluation of the MFI staining in panel
B. (D) Histograms of extracellular staining with anti-PD-L1-,
MHC-I- or MHC-II-specific antibodies in samples treated as in panel
B. The control (No stain) for individual fluorochromes was staining
with vehicle alone. *P<0.05 and **P<0.01 and ****P<0.0001;
C, n=3 (Ki-67) independent experiments. D, n=6. (E) Extracellular
MHC-II staining of 14-day vehicle-treated (No treatment), 7-day or
14-day IFNγ-treated (IFNγ, 200 ng/ml) JBT19 cells. (F)
Extracellular MHC-II staining of 48-h vehicle-treated or 6-, 24- or
48-h IFNγ-treated (IFNγ, 200 ng/ml) JBT19 cells. *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001; E, n=3 independent
experiments. F, n=4 independent experiments. Panels E and F,
one-way ANOVA with Tukey's post hoc test. MTT assay of (G)
vehicle-treated JBT19 cells or (H and I) JBT19 cells treated for 6
h with IFNγ (200 ng/ml). Next, the treated JBT19 cells were rinsed
and exposed to docetaxel at the indicated concentrations for 3 days
in the (G and H) absence (no treatment + 3 d no treatment, 6 h
IFNγ+3 d no treatment) or (I) presence of IFNγ (200 ng/ml). The
calculated IC50 from n=3 independent experiments is
shown. IFNγ, interferon γ; MFI, mean fluorescence intensity; d,
days; PD-L1, programmed cell death-ligand 1; MHC, major
histocompatibility complex; nM, nanomolar. |
The sustained IFNγ-mediated inhibition of JBT19 cell
proliferation suggested that their phenotype could also be
maintained in the absence of IFNγ. However, since the IFNγ-induced
differences in the surface expression levels of the previously
investigated markers [CD44, CD47, CD95 (Fas) and FAP] were not
extensive, the investigation next focused on other possible markers
whose expression was previously reported to be sensitive to IFNγ
treatment, including PD-L1 (19),
MHC-I (57) and MHC-II (58). The first two markers were cell
surface-expressed in JBT19 cells and the expression levels of
MHC-II were found to be significantly expressed after IFNγ
treatment in the present study. In these experiments, JBT19 cells
were treated with IFNγ as for the Ki-67 analyses and the cell
surface expression levels of PD-L1, MHC-I and MHC-II was
determined. As shown, although JBT19 cells were previously reported
to be PD-L1+ (28), the
increase in PD-L1 expression after treatment with IFNγ was robust
(Fig. 3D). Similar to Ki-67, the
expression levels of PD-L1 was the highest after 7 days of
treatment. However, notable differences were observed compared with
the impact on Ki-67 expression. Specifically, 5 days after 2-day
IFNγ treatment, PD-L1 expression was considerably higher compared
with that in cells treated for 2 days and nearly reached the levels
observed in 7-day-treated cells (Fig.
3D). These results indicated that, after 2-day IFNγ treatment,
the removal of the cytokine did not prevent the cells from further
increasing the expression levels of PD-L1. These findings were also
observed in the expression levels of MHC-I (Fig. 3D). However, the most notable finding
was observed upon the evaluation of the surface expression levels
of MHC-II: JBT19 cells that were negative or weakly positive for
this molecule became positive after 2-day treatment with IFNγ and
the levels were largely enhanced after 7-day treatment with IFNγ
(Fig. 3D). Furthermore, the
expression levels of MHC-II followed the same pattern as PD-L1 and
MHC-I molecules; namely, the levels of MHC-II in cells cultured for
5 days after 2-day IFNγ treatment were markedly higher compared
with the levels in 2-day-treated cells and nearly reached the
levels of 7-day-treated cells. With an extended exposure to
14-day-treatment, the levels of MHC-II did not exhibit any further
notable increase, which indicated that the IFNγ-elicited phenotypic
changes were reaching their plateau within this length of treatment
(Fig. 3E).
Onset of IFNγ-induced phenotypic
remodeling and docetaxel resistance occurs shortly after exposure
to IFNγ
IFNγ induces a signaling pathway that results in
changes in the gene expression pattern within hours after the
stimulation (52,55). Using the expression levels of MHC-II
as a marker of the IFNγ-elicited phenotypic remodeling, it was
identified that MHC-II expression was significantly enhanced after
24-h treatment with IFNγ (Fig. 3F).
However, it was revealed that 6-h treatment with IFNγ enhanced
their resistance to docetaxel by ~10-fold when IFNγ was
subsequently removed during MTT assay (Fig. 3G and H). When IFNγ was present (not
removed) during the subsequent 3-day MTT assay, their resistance to
docetaxel increased ~100-fold (Fig. 3G
and I). These findings indicated that the onset of
JBT19-induced functionality changes (chemoresistance) occurred
within hours after their exposure to IFNγ and continued to be
enhanced with ongoing exposure.
IFNγ-induced phenotypic remodeling and
docetaxel resistance is sustained for extended time but is
reversible
The results in Fig.
3 indicated that the impact of IFNγ on JBT19 cells was
sustained and possibly irreversible, potentially causing permanent
changes in JBT19 cells. To investigate this, 7-day treated JBT19
cells were further cultured in the absence of IFNγ for 9 days
(Fig. 4A, Rev0-9) and their
proliferation rate was compared with that of cells cultured in the
presence of IFNγ (Fig. 4A, IFNγ).
Removal of IFNγ from the cells revealed a tendency to accelerate
cell proliferation (Fig. 4B); the
9-day cell number fold increase accelerated from ~2- to 4-fold
increase. Therefore, 9 days after cytokine removal, the cells were
again passaged and cultured for subsequent 15 days and their
proliferation rate (Fig. 4A,
Rev9-24) was compared with that of IFNγ non-treated JBT19 cells
(Fig. 4A, No treatment). The
proliferation rates of the treated (Rev9-24) and control cells (No
treatment) were comparable (Fig.
4C). To confirm that JBT19 cells regained the same
proliferation rate after 24 days of culture without IFNγ, doubling
times were determined for both treated (Fig. 4, Rev24) and control cells (Fig. 4, No treatment 2). The expansion of
the previously treated JBT19 cells was now similar to that of their
non-treated counterpart and both cell groups demonstrated
comparable doubling times (Fig.
4D). Thus, the impact of IFNγ on cell proliferation was
completely reversed 24 days after IFNγ removal.
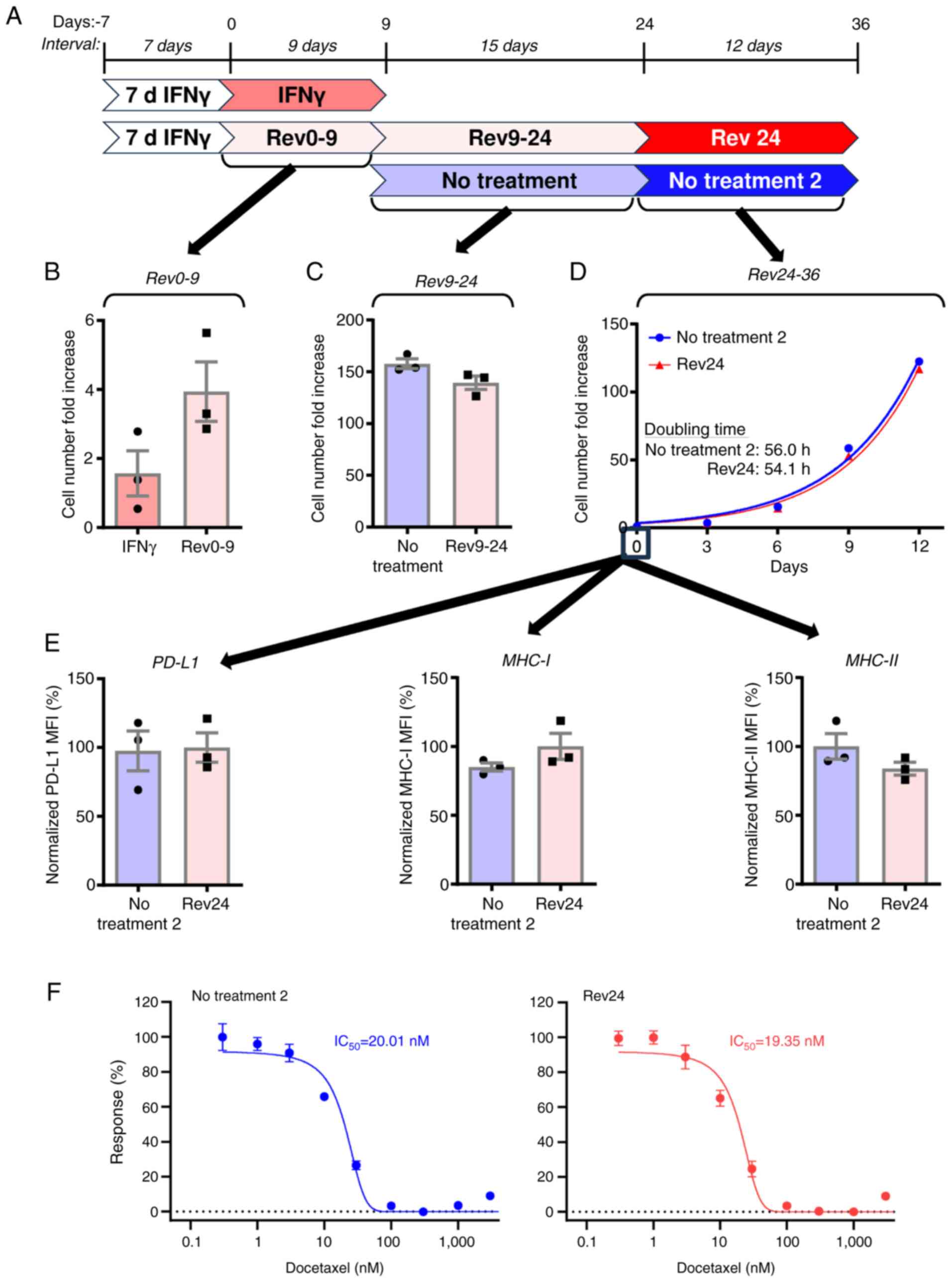 | Figure 4.IFNγ-induced phenotypic remodeling
and docetaxel resistance are sustained for an extended time but are
reversible. (A) Schematic representation of the times of the
experiment. (B) JBT19 cells were 7-day treated with IFNγ (200
ng/ml). The cells were then passaged and cultured for 9 days in the
presence (IFNγ; dark pink) or absence (Rev0-9; No treatment, light
pink) of IFNγ (200 ng/ml) and cell number fold increase was
determined. (C) The Rev0-9 sample of JBT19 cells from panel B
(Rev9-24, light pink) or JBT19 cells not treated with IFNγ (No
treatment, light blue) were passaged and cultured in the absence of
IFNγ for 15 days and the cell number fold increase was determined.
(D) Rev9-24 sample of JBT19 cells from C (Rev24, light pink) and
vehicle-treated sample of JBT19 cells from C (No treatment, light
blue) were passaged and cultured for 12 days. Next, the cell number
fold increase was determined at the indicated days and
proliferation curves and pertinent doubling times were calculated.
(E) Evaluation of the mean fluorescence intensities of
extracellular staining with anti-PD-L1-, MHC-I- or MHC-II-specific
antibodies in the Rev24 sample of JBT19 cells at day 0 from panel D
(Rev24, light pink) and vehicle-treated sample of JBT19 cells at
day 0 (No treatment 2, light blue). (F) Results of MTT assay of No
treatment 2 (blue) and Rev24 (red) samples at day 0 from panel D.
Cells were exposed to docetaxel at the indicated concentrations for
3 days. The calculated IC50 are shown (n=3 independent
experiments). MFI, mean fluorescence intensity (arbitrary units);
IFNγ, interferon γ; nM, nanomolar; PD-L1, programmed cell
death-ligand 1; MHC, major histocompatibility complex. |
Next, the present study investigated cell
proliferation recovery also translated into the reversion of the
cell surface markers PD-L1, MHC-I and MHC-II. As shown, the
expression levels of PD-L1, MHC-I and MHC-II returned to the levels
of the IFNγ non-treated cells (Fig.
4E).
To evaluate whether the extended cell
culture-elicited reversion also translated into resensitization of
the cells to docetaxel, the cells were analyzed by MTT assay. The
previously treated JBT19 cells regained the same sensitivity to
docetaxel as their non-treated counterparts (Fig. 4F). Collectively, these results
demonstrated that the changes elicited by IFNγ were sustained but
not permanent because the treated JBT19 cells could regain back the
phenotypic and functional properties of the non-treated cells.
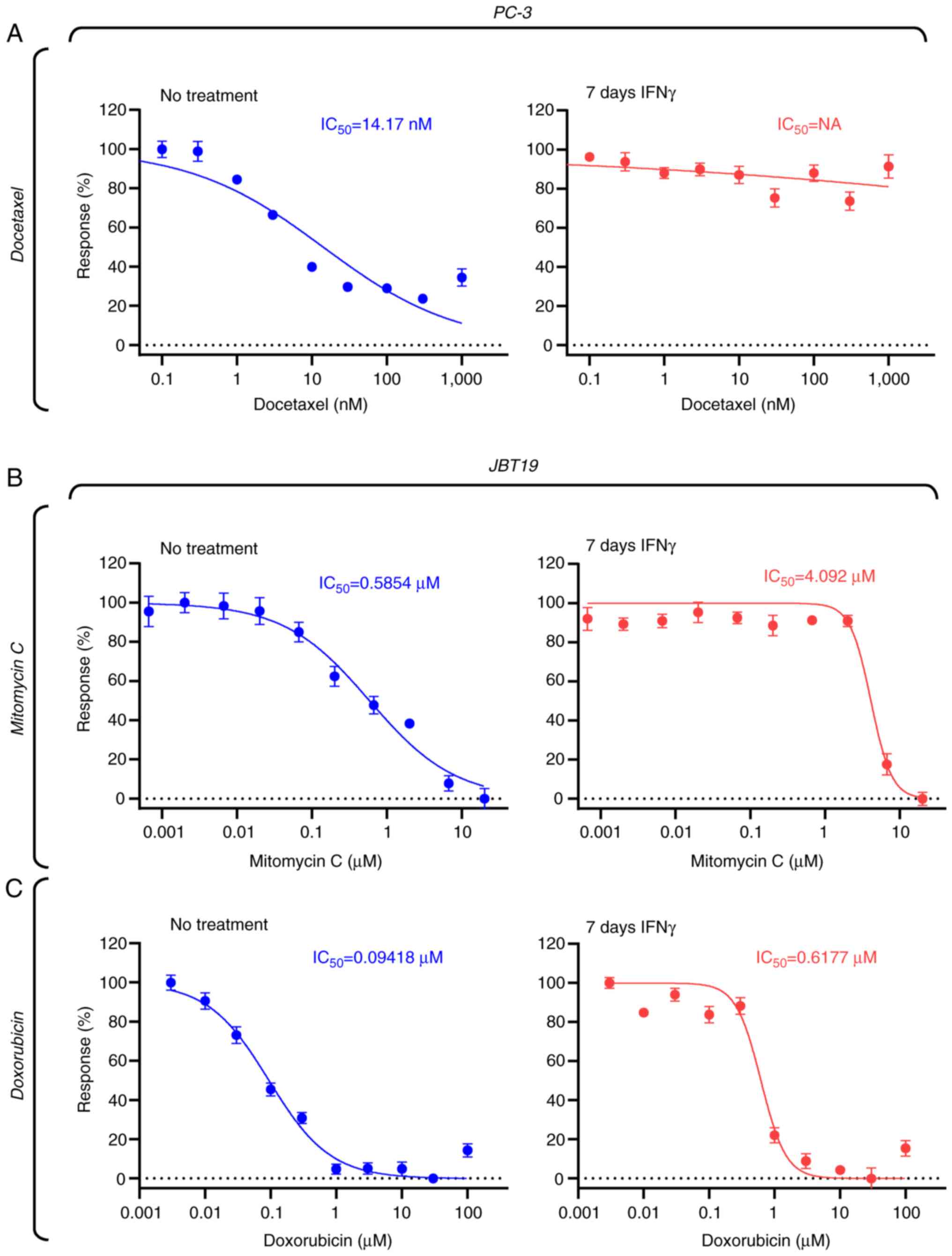
IFNγ-elicited chemoresistance is not
JBT19 cell-restricted and can be mitigated by mitomycin C or
doxorubicin in JBT19 cells
The JBT19 cell line was established from soft tissue
sarcoma (28), which is a tumor of
mesenchymal origin (59). To
determine whether IFNγ induced docetaxel resistance in
epithelial-derived tumors, the prostate carcinoma cell line PC-3
was utilized (29). Similar to
JBT19 cells, 7-day IFNγ treatment induced resistance to docetaxel
in PC-3 cells (Fig. 5A). indicating
that the mechanism observed in JBT19 cells also takes place in cell
lines of distinct histological origin.
Docetaxel is a chemotherapeutic agent that primarily
targets rapidly dividing cells (60,61).
The present study results indicated that IFNγ treatment markedly
reduced the proliferation rate of JBT19 cells, suggesting that this
decrease in proliferation could be the main mechanism underlying
JBT19 cell resistance to docetaxel. To determine whether
chemotherapeutics capable of targeting slowly dividing cells could
overcome IFNγ-induced resistance, mitomycin C (62,63)
and doxorubicin (64) were
evaluated. The results revealed that both agents effectively
targeted JBT19 cells at submicromolar concentrations (Fig. 5B and C) and that these agents were
still effective against IFNγ-treated JBT19 cells, albeit their
effective concentrations increased ~10-fold for mitomycin C and
6-fold for doxorubicin (Fig. 5B and
C). Nevertheless, their performance was markedly improved
compared with that of docetaxel, for which the effective
concentration increased >2,000-fold following IFNγ treatment, as
aforementioned. These results thus suggested that the IFNγ-elicited
decrease in the proliferation of JBT19 cells could be the
underlying mechanisms of the observed docetaxel resistance.
IFNγ treatment of JBT19 cells has no
impact on in vitro antitumor adaptive immune response
The IFNγ-induced chemoresistance of JBT19 cells may
limit the treatment options once similarly behaving cancer cells
are present in tumors of the patients. To investigate whether IFNγ
treatment could also limit the ability of the immune system to
elicit a specific adaptive immune response, JBT19-reactive
lymphocytes were in vitro produced as described previously
(28), using either non-treated
JBT19 cells (JBT19-primed, to produce JBT19-reactive lymphocytes)
or 7-day IFNγ-treated JBT19 cells (γJBT19-primed, to produce
γJBT19-reactive lymphocytes) to stimulate and enrich cell cultures
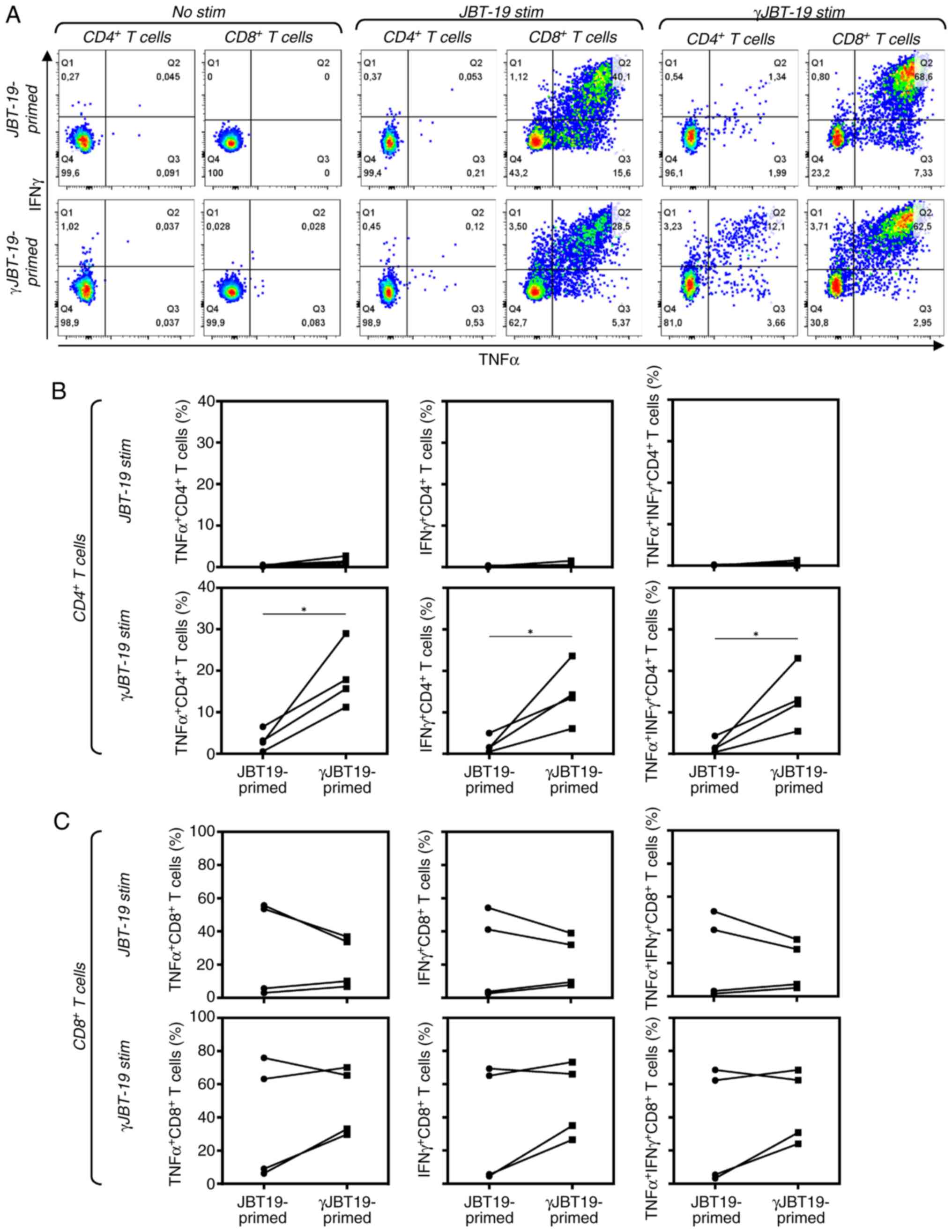
with reactive lymphocytes. As shown in Fig. S1A and B, the enriched cell cultures
were viable and the majority of cells were T cells exhibiting
tendencies to increased frequencies of CD4+ and
CD8+ populations in the γJBT19-primed cell cultures. The
produced JBT19- or γJBT19-reactive lymphocytes were then stimulated
with either JBT19 or γJBT19 cells and their reactivity was
evaluated through the expression levels of IFNγ and TNFα in the
treated lymphocytes, as determined by intracellular staining and
flow cytometry analyses. As shown in Fig. 6, CD8+ T cells of the
produced JBT19-primed or γJBT19-primed lymphocytes reacted with
both JBT19 or γJBT19 cells (Fig. 6A and
C). Although there were no differences in the reactivity of
CD8+ T cells between JBT19-primed and γJBT19-primed
cultures in high-responder donors, γJBT19-primed lymphocyte
cultures demonstrated enhanced enrichment, with reactive
CD8+ T cells in the low-responder donors, indicating
that IFNγ treatment of JBT19 cells promoted their potential to
increase lymphocyte reactivity and cell culture enrichment with
reactive CD8+ T cells in low-responder donors,
presumably through enhanced cell surface expression levels of MHC-I
(Fig. 6C).
A significant effect of JBT19 cell treatment with
IFNγ was identified for CD4+ T cells. As shown in
Fig. 6, CD4+ T cells
became stimulated only with γJBT19 cells and only significantly in
the γJBT19-primed lymphocytes, while only a negligible stimulation
was observed in JBT19-primed lymphocytes (Fig. 6A and B). These results demonstrated
that IFNγ treatment of JBT19 cells had no negative impact on the
in vitro stimulation of JBT19/γJBT19-reactive lymphocytes
and that the IFNγ-induced de novo expression levels of
MHC-II on the surface of JBT19 cells subsequently enriched the
reactive lymphocytes with γJBT19-reactive CD4+ T cells,
thus providing evidence of the immune functionality of IFNγ-induced
MHC-II on the surface of JBT19 cells.
IFNγ treatment of JBT19 cells does not
prevent in vitro amplification of antitumor-elicited adaptive
immune response
Effective antitumor immunity is dependent on its
amplification through a large-scale cell number expansion of
antitumor-reactive lymphocytes. To evaluate whether IFNγ treatment
of JBT19 cells used for stimulation and enrichment with
γJBT19-reactive lymphocytes could limit their later amplification,
the in vitro-enriched γJBT19-reactive lymphocytes were
large-scale expanded using a modified REP previously described for
the expansion of virus antigen-specific lymphocytes (34). Using this protocol, JBT19- or
γJBT19-primed cell cultures could be in vitro expanded with
enriched JBT19/γJBT19-reactive lymphocytes by ~2,000-fold (Fig. S1C). The large-scale expanded cell
cultures were viable and nearly exclusively contained T cells,
including their CD4+ and CD8+ populations
(Fig. S1D and E). Similar to the
enriched cell cultures, the CD8+ T cells of the expanded
cell cultures contained a significant population of JBT19- or
γJBT19-reactive cells (Fig. 7B). In
addition, within the limited number of donors, the expanded cell
cultures enriched through γJBT19 (γJBT19-primed) demonstrated a
notable tendency to enhanced frequencies of the γJBT19-reactive
population compared with the JBT19-primed cell cultures (Fig. 7B). By contrast, the frequencies of
JBT19-reactive CD8+ T cells varied between the donors,
revealing two high responders and two low responders (Fig. 7B). Regardless, the results
demonstrated that IFNγ treatment of JBT19 cells had no detrimental
effects on the ability of cells to induce and amplify a targeted
CD8+ T cell-based immune response against these cells or
their non-treated counterparts.
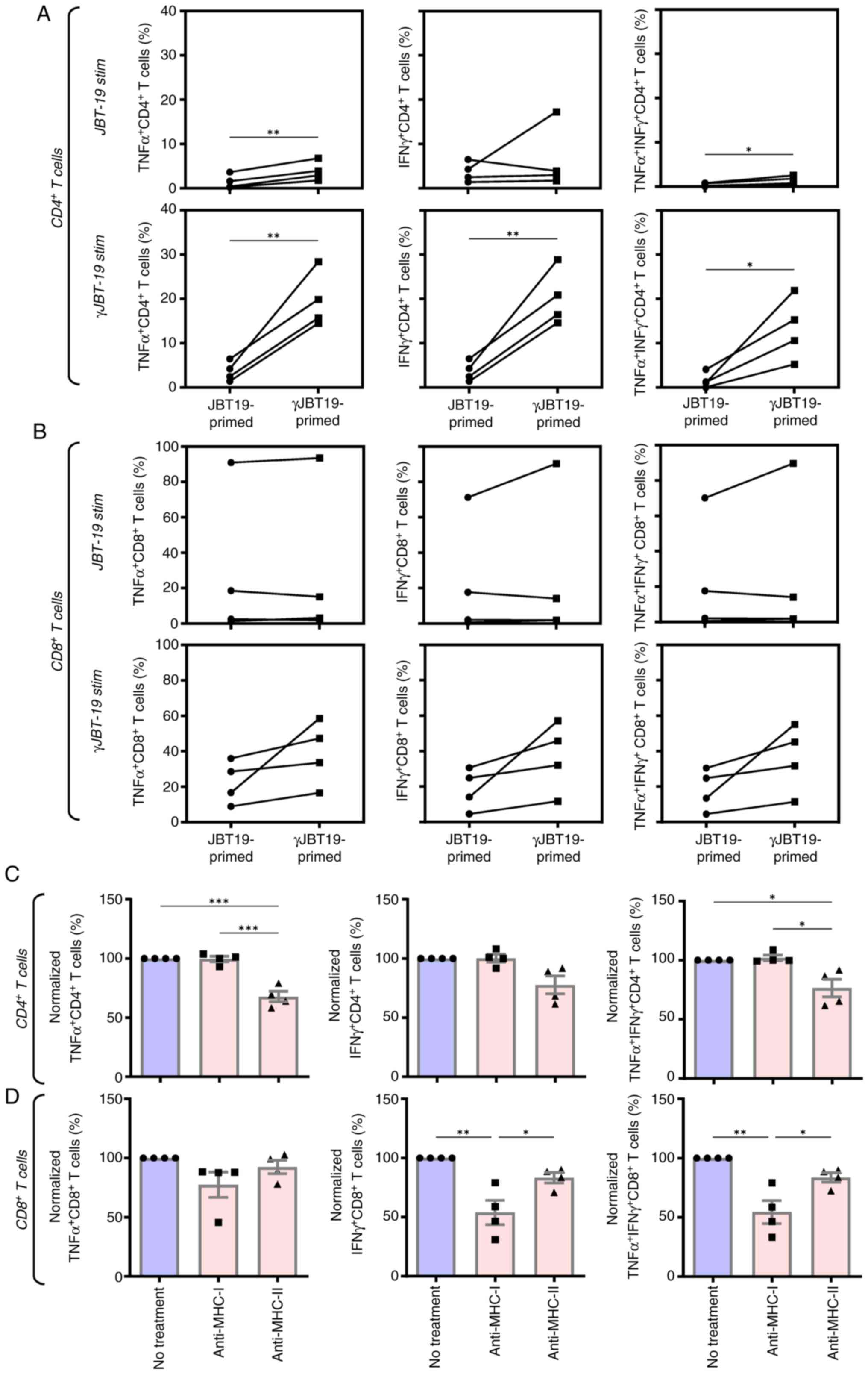
The reactivity pattern of CD4+ T cells
was similar to the pattern observed with the enriched cell
cultures. CD4+ T cells became stimulated only with
γJBT19 cells and only significantly in the γJBT19-primed and
expanded lymphocytes, while negligible stimulation was observed in
the JBT19-primed and expanded lymphocytes (Fig. 7A). Collectively, these results
indicated that treatment of JBT19 cells with IFNγ did not prevent
adaptive immunity to elicit and amplify a specific CD4+
and CD8+ T cell-based adaptive immune response against
treated JBT19 cells.
Next, the present study investigated whether MHC-T
cell receptor interactions on expanded lymphocytes contributed to
their activation. Using MHC-I and MHC-II blocking antibodies, a
significant reduction was observed in T cell response for TNFα- and
TNFα/IFNγ-producing CD4+ T cells (Fig. 7C) and for IFNγ- and
TNFα/IFNγ-producing CD8+ T cells (Fig. 7D). A notable fraction of reactive T
cells remained responsive despite the presence of blocking
antibodies, suggesting either incomplete blockade or the
involvement of bystander stimulation (65).
In vitro large-scale expanded
JBT19-reactive lymphocytes are efficient in eliminating
IFNγ-induced docetaxel-resistant JBT19 cells
The finding that IFNγ treatment of JBT19 cells did
not translate into impaired adaptive immune response suggested that
IFNγ-induced chemoresistance could be overcome by targeted adaptive
immunity. To investigate whether this targeted adaptive immunity
could also be effective in eliminating cancer cells, the present
study evaluated whether the JBT19- or γJBT19-primed and large-scale
expanded cell cultures with enriched JBT19/γJBT19-reactive
lymphocytes could eliminate cultured JBT19 or γJBT19 cells. For
this purpose, GFP-expressing JBT19 cells (JBT19-GFP) that the
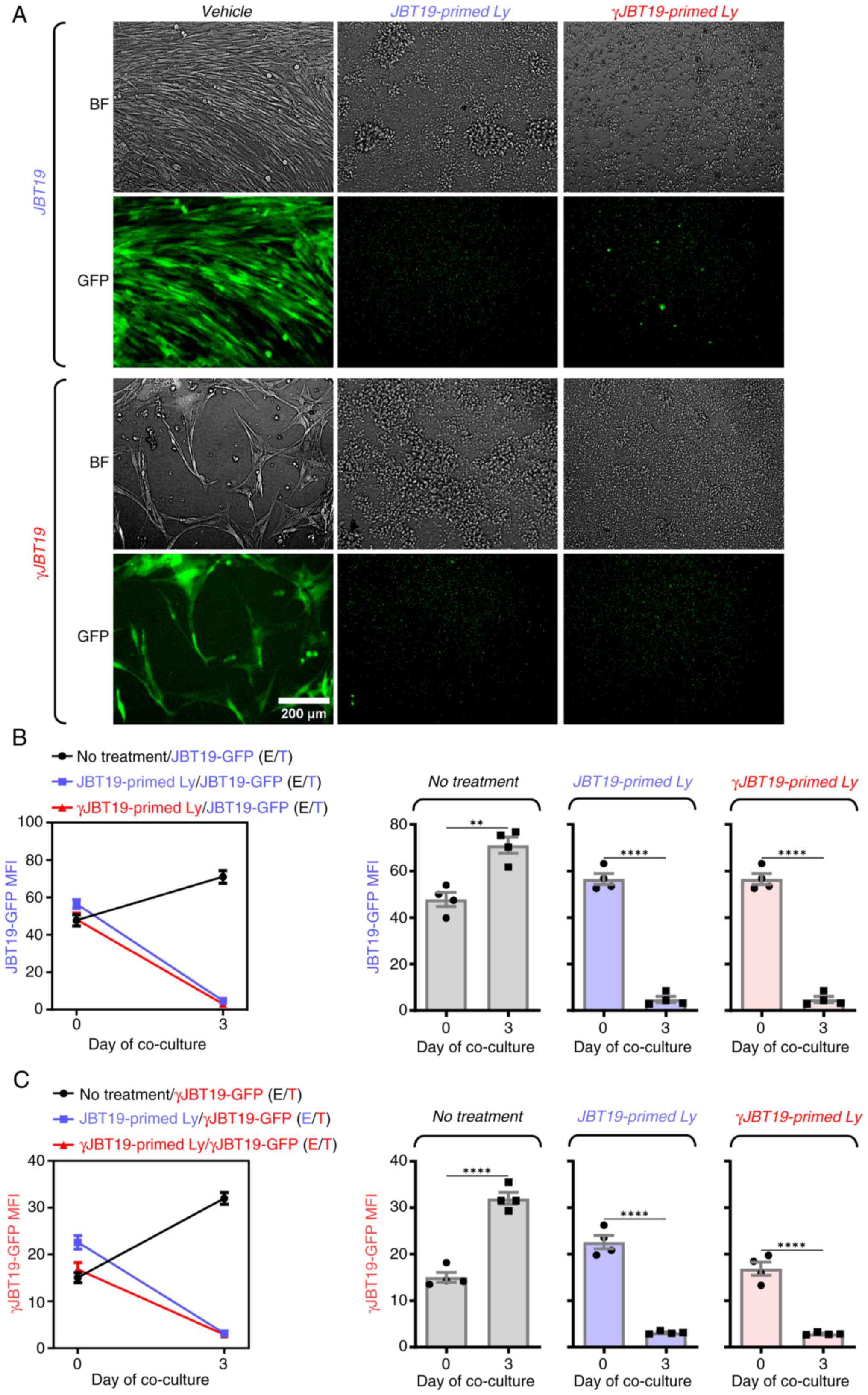
present study group had previously established were used (28). As shown in Fig. 8, regardless of whether JBT19- or
γJBT19-primed and large-scale expanded cell cultures were used,
both cell culture types were able to efficiently eliminate
IFNγ-treated (γJBT19-GFP) and non-treated (JBT19-GFP) JBT19-GPF
cells. Thus, these results revealed that, although IFNγ treatment
produced docetaxel-resistant JBT19 cells, these docetaxel-resistant
cells were not in vitro resistant to targeted adaptive
immune response.
Discussion
The present study demonstrated a dual impact of IFNγ
on the sensitivity of a UPS cell line, JBT19, to the widely used
chemotherapeutic docetaxel and the ability of this cell line to
in vitro elicit and amplify a targeted immune response.
IFNγ-elicited changes in JBT19 cells were identified to be
sustained but not permanent, since the cells were able to regain
their original phenotype and behavior prior to treatment with IFNγ.
However, reconstitution of this phenotype and behavior took >3
weeks, revealing that IFNγ markedly, yet still reversibly, change
the propensity of cancer cells for extended periods of time and
thus modulate their long-term behavior and resilience towards other
therapeutic interventions.
The tumor microenvironment serves a key role in
determining the behavior of the tumor and its resilience to
elimination by multiple therapeutic approaches, including
immunotherapy and chemotherapy (66,67).
The long-term (chronic) effects of this microenvironment on immune,
stromal and cancer cells can differ notably from short-term (acute)
effects, which may affect mast cell and T cell stimulation as well
as tumor cell metabolism (30,68–73).
Any intervention targeting the tumor microenvironment can alter
tumor behavior. Such changes may either make the tumor more
sensitive to therapy or cause it to develop resistance (74–76).
IFNγ is a cytokine that is a key component of the cell-mediated
response to cancer cells (13,15,77).
The present study confirmed its large production by the in
vitro-produced JBT19-reactive CD8+ T cells once
these cells were exposed to JBT19 cells. Therefore, under immune
attack, cancer cells are likely to be first exposed to this
cytokine before they become eliminated by reactive cytotoxic
lymphocytes. Using the cell line JBT19 as a model, the present
study demonstrated that this exposure could markedly change the
propensity of cancer cells under a presumable immune attack.
IFNγ-exposed JBT19 cells nearly stopped proliferating and started
to express multiple molecules that are associated with worse
disease prognosis, immunoresistance, metastatic behavior, cancer
cell stemness and cancer development [namely, CD44 (78), CD47 (41,79),
PD-L1 (80–82) and CD95 (Fas) (43,83,84)].
However, one of the expressed molecules triggered by IFNγ in JBT19
cells could have an ambiguous functional role towards antitumor
immunity: MHC-II. The expression levels of MHC-II are largely
associated with professional antigen-presenting cells, where it is
responsible for the stimulation of CD4+ T cells.
However, this molecule can also be expressed in other cell types
such as tumor-associated fibroblasts or cancer cells (85). The cancer cell expression levels of
MHC-II in murine tumor models is mostly associated with slower
tumor growth, improved tumor rejection and increased tumor
infiltration with immune cells (86,87).
Furthermore, previous studies reported that MHC-II expression in
tumor cells was associated with improved disease prognosis and
response to immunotherapy, including immune checkpoint inhibitors
(ICIs) (88–91). However, MHC-II expression in cancer
cells can also drive resistance to immunotherapy (92), which can promote cancer cell
metastasis (93). Using an
IFNγ-JBT19-based study system, the present study findings could
recapitulate and explain several of these contrasting findings
associated with the expression levels of MHC-II in cancer cells.
The expression levels of MHC-II in JBT19 cells were a consequence
of long-term exposure to IFNγ, which also caused a notable decrease
in the speed of proliferation of JBT19 cells. Furthermore, in
vitro-enriched and -expanded JBT19-reactive lymphocytes
produced IFNγ after their stimulation with JBT19 cells. Therefore,
a close-by antitumor activity in vivo upon which cytotoxic
lymphocytes attack cancer cells could be the source of IFNγ, which
may induce MHC-II expression in the not-yet attacked cancer cells.
As such, the expression levels of MHC-II in cancer cells could be
thus viewed as a marker sensing ongoing antitumor immune activity
in the vicinity of cancer cells. There, it could be considered that
the expression levels of MHC-II in cancer cells of tumors could
serve as a surrogate predictive marker for the efficacy of ICIs
(anti-PD-1, anti-PDL-1), as this efficacy is increased in inflamed
tumors (94). Similar conclusions
could be inferred from the expression levels of PD-L1 in
IFNγ-treated JBT19 cells, the expression levels of which are often
enhanced in inflamed tumors, where IFNγ could be present at high
levels in the tumor milieu, regulating, alongside other cytokines
(IL-27, IL-1α) (95), the
expression levels of PD-L1 in cancer cells (96). However, whether other cytokines in
the tumor milieu could promote PD-L1 or MHC-II expression in JBT19
cells, remains to be further investigated in follow-up in
vivo models using JBT19 cells and pertinent therapeutic
interventions in the future.
Antitumor immune activity is key to tumor
elimination. This activity is boosted or elicited by immunotherapy.
As immunotherapy has become the first-line treatment in numerous
oncological diagnoses, such as advanced non-small cell lung and
advanced head and neck cancer or metastatic melanoma (3–6),
antitumor immunity serves a key role in therapy-naïve tumors and
changes their behavior, which can cause cancer cell immune evasion
and resistance to immunotherapy (92,97,98).
Although the present study indicated that IFNγ-treated JBT19 cells
were in vitro efficiently eliminated by JBT19- or
γJBT19-reactive lymphocytes, this scenario may not hold in
vivo, where not all cancer cells often become readily and
entirely eliminated (99). These
cells may migrate to distant locations away from the tumor and
exhibit their IFNγ-induced characteristics. One possible
interaction could involve novel expression levels of MHC-II, which
in turn may lead to the activation of regulatory CD4+ T
cells in lymph nodes (93). The
present study demonstrated that γJBT19 cells in vitro
induced the enrichment and expansion of γJBT19-reactive
CD4+ T cells, the reactivity of which was presumably
dependent on the expression and functionality of MHC-II, since no
enrichment or expansion of γJBT19-reactive CD4+ T cells
was observed in IFNγ non-treated JBT19 cells. However, whether
these γJBT19-reactive CD4+ T cells hold or could later
acquire (92) a regulatory
potential remains to be further elucidated. Nevertheless, the
IFNγ-induced reversible but sustained reprogramming of cancer cells
not only indicates the cancer cell plasticity that can be
responsible for the disease resistance to immunotherapy and
possible promotion of metastatic behavior (100–102), but may also have notable
implications for other therapeutic modalities, including cytotoxic
chemotherapy.
The robustness of the novel IFNγ-JBT19-based system
helped demonstrate the marked impact of IFNγ on the proliferation
of JBT19 cells and their expansion in cell culture. IFNγ treatment
not only nearly stopped the expansion of JBT19 cells in the cell
culture, but also made them resistant to the chemotherapeutic agent
docetaxel, which is one of the widely used anticancer drugs
(45). Immunotherapy that elicits
or promotes an immune attack on tumors may indicate a therapeutic
impact leading to the elimination of cancer cells; by contrast, it
can also produce cancer cell immunoresistance. Therefore, although
IFNγ is often considered a marker of effective immunotherapy,
promoting both immune and cancer cells by increasing antigen
presentation and recruiting more cancer cell-targeting immune cells
to tumors, it can also exhibit the opposite role in the responses
of patients to immunotherapy by contributing to the development of
mechanisms associated with the acquisition of resistance to
immunotherapy, including resistance to ICIs (72,103).
To the best of our knowledge, the present study is, however, the
first to demonstrate that IFNγ can elicit resistance beyond
immunotherapy, impacting chemotherapy.
The present study indicated that chronic exposure of
JBT19 cells to IFNγ significantly slowed down their proliferation.
This suggested that the cell line could become resistant to
chemotherapeutics effective against dividing cells such as
docetaxel (46). The choice to
study docetaxel confirmed this expectation, as an increased
resistance to the drug was observed. However, the extent of this
acquired resistance was unexpected; a cell line that was initially
sensitive to nM concentrations of the drug became nearly resistant
to this drug after prolonged exposure to IFNγ. A similar
observation was made in PC-3 cells, suggesting that this mechanism
of resistance is not limited to sarcomas but may also be relevant
in carcinoma cell lines (59). This
finding contrasted with previous research, which demonstrated that
IFNγ sensitized cancer cells to chemotherapy in a model of
metastatic castration-resistant prostate cancer (104). However, this sensitization was
based on 40-fold lower concentrations of IFNγ, 5 ng/ml vs. 200
ng/ml in the present study and only 2-day treatment vs. 7-day
treatment. Furthermore, the cytotoxic effect was investigated in a
combination with recombinant human TNF-related apoptosis inducing
ligand (TRAIL), a molecule that serves a key role in the ability of
the immune system to selectively induce apoptosis (programmed cell
death) in cancer cells (105).
TRAIL receptor expression or functionality is enhanced by IFNγ,
which associates this modulation to both chemoresistance and
immunoresistance (106,107). Therefore, the conditions of the
present study were more robust concerning the IFNγ concentration
and exposure time, absence of the contributing TRAIL ligand/TRAIL
receptor-mediated cytotoxicity and presumably were more closely
mimicking in vivo conditions under which local
concentrations of IFNγ at sites of an ongoing and concentrated
attack on cancer cells by cytotoxic lymphocytes could be increasing
and the exposure time could be chronic.
Analysis of chemotherapeutics that target
non-dividing or slowly dividing cells via multiple mechanisms, such
as mitomycin C (108,109) and doxorubicin (110,111), further supports the notion that
downregulation of cell proliferation could be a key mechanism
underlying IFNγ-induced resistance. One potential driver of this
mechanism is the observed upregulation of STAT1 in IFNγ-treated
JBT19 cells. Upregulation of STAT1 has been associated not only
with chemoresistance, including resistance to docetaxel (112,113), but also to mechanisms that can be
mitigated by targeting the STAT signaling pathway (114,115). The detailed mechanism that
underpins this drug resistance acquisition warrants further
investigation, as well as its existence in other cell lines, its
performance in 3D-culture/co-culture models or in vivo
animal models and its potential interaction with other
chemotherapeutics, particularly those whose cytotoxic impact is not
as much cell proliferation-dependent, such as alkylating drugs,
proteasome inhibitors and autophagy inhibitors (116,117). Conducting these validation studies
will be key to determine the extent to which immune mechanisms
drive drug resistance. Regardless of the scope of this mechanism
that needs to be addressed in follow-up studies, the use of JBT19
cells in the present study enabled to describe a novel IFNγ-driven
mechanism that provides evidence of the interplay between
immunotherapy and cancer cell chemoresistance.
This novel mechanism could have notable
implications for immunotherapy and other treatment options
(targeted therapy) where IFNγ signatures can serve a notable role
(118). IFNγ signatures are
frequently associated with improved responses to immunotherapy
(20,21,72,119).
However, these responses are often insufficient to fully prevent
disease progression. After immunotherapy failure, chemotherapy
commonly remains a standard treatment option (120,121). In certain cases, the chemotherapy
outcome is greater than expected in the absence of prior
immunotherapy (22,23). In certain instances, chemotherapy
efficacy is not improved (122).
However, there are also studies indicating that chemotherapy can be
less effective following failed immunotherapy compared with
treatment-naïve conditions (24,25).
The present study revealed a novel mechanism that
may underline the immunotherapy-induced acquisition of transient
chemotherapy resistance, which could be associated with enhanced
IFNγ signatures often associated with immunotherapy (20,21,72,119).
The present study demonstrated that this IFNγ-mediated resistance
differentially impacted chemotherapeutic agents, rendering cancer
cells either completely resistant to certain drugs (such as
docetaxel) or partially resistant to others (such as mitomycin C
and doxorubicin). From a clinical perspective, these findings
suggested that therapeutic decision-making after immunotherapy
failure should account for this mechanism when selecting subsequent
chemotherapeutic regimens. Furthermore, similar considerations
should be applied during the design of next-generation
therapeutics, such as antibody-drug conjugates or nanoparticle-drug
conjugates, particularly when choosing the chemotherapeutic
payloads to maximize efficacy (123).
The present study reported a robust system based on
a novel UPS cell line, JBT19, through which a dual identity of IFNγ
towards chemoresistance and immunostimulation was demonstrated
in vitro. This dual identity materialized through sustained
but reversible phenotypical and functional changes in IFNγ-impacted
JBT19 cells, and produced chemoresistance on one side, and enhanced
immunostimulatory potential on the other. Although this dual
identity could be cell line- and condition-specific, its engagement
under specific disease conditions and therapy could markedly affect
the outcome of therapy. Therefore, these findings could have
potentially notable implications for combined therapies, namely for
the combinations of immunotherapy and chemotherapy in the
future.
In conclusion, to the best of our knowledge, the
present study revealed for the first time the ‘dual role’ in
chronic IFNγ exposure, leading to both chemoresistance and
immunosensitivity. This may have key implications for the interplay
between the effectiveness of cytotoxic chemotherapy and
immunotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ministry of Health, Czech
Republic (grant no. NU23-08-00071).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PT and DSt performed cell culture, viability and
flow cytometry analyses. DSt performed cytotoxic analyses. PT and
DSm conceived and designed the present study. DSm supervised the
present study. DSt contributed to the present study design. DSm
wrote the manuscript. PT and DSt wrote the manuscript and confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
ethics standards of the institutional, national research committee
(Ethics Committee of the University Hospital Motol in Prague;
approval no. EK-602.4/22; Prague, Czech Republic). All experiments
were performed in accordance with the 1964 Declaration of Helsinki
and its later amendments or comparable ethics standards. All
volunteers included in the present study signed an informed consent
form for the use of their blood-derived products in future
research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun H, Liu J, Hu F, Xu M, Leng A, Jiang F
and Chen K: Current research and management of undifferentiated
pleomorphic sarcoma/myofibrosarcoma. Front Genet. 14:11094912023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu JT, Nowak E, Imamura J, Leng J, Shepard
D, Campbell SR, Scott J, Nystrom L, Mesko N, Schwartz GK and Burke
ZDC: Immunotherapy in the treatment of undifferentiated pleomorphic
sarcoma and myxofibrosarcoma. Curr Treat Options Oncol. 26:891–909.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J and Wu L: First-line immunotherapy
for advanced non-small cell lung cancer: Current progress and
future prospects. Cancer Biol Med. 21:117–124. 2023.PubMed/NCBI
|
|
4
|
Reardon S: First cell therapy for solid
tumours heads to the clinic: What it means for cancer treatment.
Nature. Mar 11–2024.doi: 10.1038/d41586-024-00673-w (Epub ahead of
print).
|
|
5
|
Huang Y, Zhou H, Zhao G, Wang M, Luo J and
Liu J: Immune checkpoint inhibitors serve as the First-line
treatment for advanced head and neck cancer. Laryngoscope.
134:749–761. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lamba N, Ott PA and Iorgulescu JB: Use of
First-line immune checkpoint inhibitors and association with
overall survival among patients with metastatic melanoma in the
Anti-PD-1 Era. JAMA Netw Open. 5:e22254592022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yau T, Galle PR, Decaens T, Sangro B, Qin
S, da Fonseca LG, Karachiwala H, Blanc JF, Park JW, Gane E, et al:
Nivolumab plus ipilimumab versus lenvatinib or sorafenib as
first-line treatment for unresectable hepatocellular carcinoma
(CheckMate 9DW): An Open-label, randomised, phase 3 trial. Lancet.
405:1851–1864. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Diker O and Olgun P: Salvage chemotherapy
in patients with nonsmall cell lung cancer after prior
immunotherapy: Aa retrospective, real-life experience study.
Anticancer Drugs. 33:752–757. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Assi HI, Zerdan MB, Hodroj M, Khoury M,
Naji NS, Amhaz G, Zeidane RA and El Karak F: Value of chemotherapy
post immunotherapy in stage IV non-small cell lung cancer (NSCLC).
Oncotarget. 14:517–525. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sordo-Bahamonde C, Lorenzo-Herrero S,
Gonzalez-Rodriguez AP, Martínez-Pérez A, Rodrigo JP, García-Pedrero
JM and Gonzalez S: Chemo-immunotherapy: A new trend in cancer
treatment. Cancers (Basel). 15:29122023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Liu X, Chen D and Yu J:
Radiotherapy combined with immunotherapy: The dawn of cancer
treatment. Signal Transduct Target Ther. 7:2582022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ni L and Lu J: Interferon gamma in cancer
immunotherapy. Cancer Med. 7:4509–4516. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Castro F, Cardoso AP, Goncalves RM, Serre
K and Oliveira MJ: Interferon-gamma at the crossroads of tumor
immune surveillance or evasion. Front Immunol. 9:8472018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martinez-Lostao L, Anel A and Pardo J: How
do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res.
21:5047–5056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhat P, Leggatt G, Waterhouse N and Frazer
IH: Interferon-γ derived from cytotoxic lymphocytes directly
enhances their motility and cytotoxicity. Cell Death Dis.
8:e28362017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mazet JM, Mahale JN, Tong O, Watson RA,
Lechuga-Vieco AV, Pirgova G, Lau VWC, Attar M, Koneva LA, Sansom
SN, et al: IFNgamma signaling in cytotoxic T cells restricts
anti-tumor responses by inhibiting the maintenance and diversity of
intra-tumoral stem-like T cells. Nat Commun. 14:3212023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jorgovanovic D, Song M, Wang L and Zhang
Y: Roles of IFN-γ in tumor progression and regression: A review.
Biomark Res. 8:492020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jing ZL, Liu GL, Zhou N, Xu DY, Feng N,
Lei Y, Ma LL, Tang MS, Tong GH, Tang N and Deng YJ: Interferon-γ in
the tumor microenvironment promotes the expression of B7H4 in
colorectal cancer cells, thereby inhibiting cytotoxic T cells. Sci
Rep. 14:60532024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abiko K, Matsumura N, Hamanishi J,
Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I
and Mandai M: IFN-γ from lymphocytes induces PD-L1 expression and
promotes progression of ovarian cancer. Br J Cancer. 112:1501–1509.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong CW, Huang YY and Hurlstone A: The
role of IFN-γ-signalling in response to immune checkpoint blockade
therapy. Essays Biochem. 67:991–1002. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reijers ILM, Rao D, Versluis JM, Menzies
AM, Dimitriadis P, Wouters MW, Spillane AJ, Klop WMC, Broeks A,
Bosch LJW, et al: IFN-γ signature enables selection of neoadjuvant
treatment in patients with stage III melanoma. J Exp Med.
220:e202219522023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Casadei B, Argnani L, Morigi A, Lolli G,
Broccoli A, Pellegrini C, Nanni L, Stefoni V, Coppola PE, Carella
M, et al: Effectiveness of chemotherapy after anti-PD-1 blockade
failure for relapsed and refractory Hodgkin lymphoma. Cancer Med.
9:7830–7836. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saleh K, Daste A, Martin N, Pons-Tostivint
E, Auperin A, Herrera-Gomez RG, Baste-Rotllan N, Bidault F, Guigay
J, Le Tourneau C, et al: Response to salvage chemotherapy after
progression on immune checkpoint inhibitors in patients with
recurrent and/or metastatic squamous cell carcinoma of the head and
neck. Eur J Cancer. 121:123–129. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goldinger SM, Buder-Bakhaya K, Lo SN,
Forschner A, McKean M, Zimmer L, Khoo C, Dummer R, Eroglu Z,
Buchbinder EI, et al: Chemotherapy after immune checkpoint
inhibitor failure in metastatic melanoma: A retrospective
multicentre analysis. Eur J Cancer. 162:22–33. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Black M, Barsoum IB, Truesdell P,
Cotechini T, Macdonald-Goodfellow SK, Petroff M, Siemens DR, Koti
M, Craig AW and Graham CH: Activation of the PD-1/PD-L1 immune
checkpoint confers tumor cell chemoresistance associated with
increased metastasis. Oncotarget. 7:10557–10567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lazcano R, Barreto CM, Salazar R, Carapeto
F, Traweek RS, Leung CH, Gite S, Mehta J, Ingram DR, Wani KM, et
al: The immune landscape of undifferentiated pleomorphic sarcoma.
Front Oncol. 12:10084842022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei X, Ruan H, Zhang Y, Qin T, Zhang Y,
Qin Y and Li W: Pan-cancer analysis of IFN-gamma with possible
immunotherapeutic significance: A verification of single-cell
sequencing and bulk omics research. Front Immunol. 14:12021502023.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taborska P, Lukac P, Stakheev D,
Rajsiglova L, Kalkusova K, Strnadova K, Lacina L, Dvorankova B,
Novotny J, Kolar M, et al: Novel PD-L1- and collagen-expressing
patient-derived cell line of undifferentiated pleomorphic sarcoma
(JBT19) as a model for cancer immunotherapy. Sci Rep. 13:190792023.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaighn ME, Narayan KS, Ohnuki Y, Lechner
JF and Jones LW: Establishment and characterization of a human
prostatic carcinoma cell line (PC-3). Invest Urol. 17:16–23.
1979.PubMed/NCBI
|
|
30
|
Taborska P, Stakheev D, Svobodova H,
Strizova Z, Bartunkova J and Smrz D: Acute conditioning of
Antigen-expanded CD8+ T cells via the GSK3β-mTORC axis
differentially dictates their immediate and distal responses after
antigen rechallenge. Cancers (Basel). 12:37662020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smrž D, Kim MS, Zhang S, Mock BA, Smrzová
S, DuBois W, Simakova O, Maric I, Wilson TM, Metcalfe DD and
Gilfillan AM: mTORC1 and mTORC2 differentially regulate homeostasis
of neoplastic and non-neoplastic human mast cells. Blood.
118:6803–6813. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smrž D, Dráberová L and Dráber P:
Non-apoptotic phosphatidylserine externalization induced by
engagement of glycosylphosphatidylinositol-anchored proteins. J
Biol Chem. 282:10487–10497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taborska P, Bartunkova J and Smrz D:
Simultaneous in vitro generation of human CD34+-derived dendritic
cells and mast cells from non-mobilized peripheral blood
mononuclear cells. J Immunol Methods. 458:63–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taborska P, Lastovicka J, Stakheev D,
Strizova Z, Bartunkova J and Smrz D: SARS-CoV-2 spike
glycoprotein-reactive T cells can be readily expanded from COVID-19
vaccinated donors. Immun Inflamm Dis. 9:1452–1467. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stakheev D, Taborska P, Kalkusova K,
Bartunkova J and Smrz D: LL-37 as a powerful molecular tool for
boosting the performance of ex vivo-Produced human dendritic cells
for cancer immunotherapy. Pharmaceutics. 14:27472022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stakheev D, Taborska P, Strizova Z,
Podrazil M, Bartunkova J and Smrz D: The WNT/β-catenin signaling
inhibitor XAV939 enhances the elimination of LNCaP and PC-3
prostate cancer cells by prostate cancer patient lymphocytes in
vitro. Sci Rep. 9:47612019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Molgora M, Cortez VS and Colonna M:
Killing the invaders: NK cell impact in tumors and Anti-tumor
therapy. Cancers (Basel). 13:5952021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang S, Liu W, Hu B, Wang P, Lv X, Chen S
and Shao Z: Prognostic significance of Tumor-infiltrating natural
killer cells in solid tumors: A systematic review and
Meta-analysis. Front Immunol. 11:12422020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun YP, Ke YL and Li X: Prognostic value
of CD8+ tumor-infiltrating T cells in patients with breast cancer:
A systematic review and meta-analysis. Oncol Lett. 25:392023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yaghobi Z, Movassaghpour A, Talebi M,
Abdoli Shadbad M, Hajiasgharzadeh K, Pourvahdani S and Baradaran B:
The role of CD44 in cancer chemoresistance: A concise review. Eur J
Pharmacol. 903:1741472021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang H, Tan M, Zhang S, Li X, Gao J, Zhang
D, Hao Y, Gao S, Liu J and Lin B: Expression and significance of
CD44, CD47 and c-met in ovarian clear cell carcinoma. Int J Mol
Sci. 16:3391–3404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yoshida K, Tsujimoto H, Matsumura K,
Kinoshita M, Takahata R, Matsumoto Y, Hiraki S, Ono S, Seki S,
Yamamoto J and Hase K: CD47 is an adverse prognostic factor and a
therapeutic target in gastric cancer. Cancer Med. 4:1322–1333.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peter ME, Hadji A, Murmann AE, Brockway S,
Putzbach W, Pattanayak A and Ceppi P: The role of CD95 and CD95
ligand in cancer. Cell Death Differ. 22:549–559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tilsed CM, Fisher SA, Nowak AK, Lake RA
and Lesterhuis WJ: Cancer chemotherapy: Insights into cellular and
tumor microenvironmental mechanisms of action. Front Oncol.
12:9603172022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Montero A, Fossella F, Hortobagyi G and
Valero V: Docetaxel for treatment of solid tumours: A systematic
review of clinical data. Lancet Oncol. 6:229–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Imran M, Saleem S, Chaudhuri A, Ali J and
Baboota S: Docetaxel: An update on its molecular mechanisms,
therapeutic trajectory and nanotechnology in the treatment of
breast, lung and prostate cancer. J Drug Delivery Sci Technol.
602020.
|
|
47
|
Sangfelt O, Erickson S and Grander D:
Mechanisms of interferon-induced cell cycle arrest. Front Biosci.
5:D479–D487. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kulkarni A, Scully TJ and O'Donnell LA:
The antiviral cytokine interferon-gamma restricts neural
stem/progenitor cell proliferation through activation of STAT1 and
modulation of retinoblastoma protein phosphorylation. J Neurosci
Res. 95:1582–1601. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xaus J, Cardo M, Valledor AF, Soler C,
Lloberas J and Celada A: Interferon gamma induces the expression of
p21waf-1 and arrests macrophage cell cycle, preventing induction of
apoptosis. Immunity. 11:103–113. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bossennec M, Di Roio A, Caux C and
Menetrier-Caux C: MDR1 in immunity: Friend or foe? Oncoimmunology.
7:e14993882018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cao ZH, Zheng QY, Li GQ, Hu XB, Feng SL,
Xu GL and Zhang KQ: STAT1-mediated down-regulation of Bcl-2
expression is involved in IFN-γ/TNF-α-induced apoptosis in NIT-1
cells. PLoS One. 10:e01209212015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cheon H and Stark GR: Unphosphorylated
STAT1 prolongs the expression of Interferon-induced immune
regulatory genes. Proc Natl Acad Sci USA. 106:9373–9378. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Morrow AN, Schmeisser H, Tsuno T and Zoon
KC: A novel role for IFN-stimulated gene factor 3II in IFN-γ
signaling and induction of antiviral activity in human cells. J
Immunol. 186:1685–1693. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Clark DN, O'Neil SM, Xu L, Steppe JT,
Savage JT, Raghunathan K and Filiano AJ: Prolonged STAT1 activation
in neurons drives a pathological transcriptional response. J
Neuroimmunol. 382:5781682023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yuasa K, Masubuchi A, Okada T, Shinya M,
Inomata Y, Kida H, Shyouji S, Ichikawa H, Takahashi T, Muroi M and
Hijikata T: Interferon-dependent expression of the human STAT1 gene
requires a distal regulatory region located approximately 6 kb
upstream for its autoregulatory system. Genes Cells. 30:e131882025.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lastovicka J, Rataj M and Bartunkova J:
Assessment of lymphocyte proliferation for diagnostic purpose:
Comparison of CFSE staining, Ki-67 expression and 3H-thymidine
incorporation. Hum Immunol. 77:1215–1222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou F: Molecular mechanisms of IFN-gamma
to up-regulate MHC class I antigen processing and presentation. Int
Rev Immunol. 28:239–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Steimle V, Siegrist CA, Mottet A,
Lisowska-Grospierre B and Mach B: Regulation of MHC class II
expression by interferon-gamma mediated by the transactivator gene
CIITA. Science. 265:106–109. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Benesova I, Kalkusova K, Kwon YS, Taborska
P, Stakheev D, Krausova K, Smetanova J, Ozaniak A, Bartunkova J,
Smrž D and Strizova ZO: Cancer-associated fibroblasts in human
malignancies, with a particular emphasis on sarcomas (review). Int
J Oncol. 67:792025. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hennequin C, Giocanti N and Favaudon V:
S-phase specificity of cell killing by docetaxel (Taxotere) in
synchronised HeLa cells. Br J Cancer. 71:1194–1198. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mosca L, Ilari A, Fazi F, Assaraf YG and
Colotti G: Taxanes in cancer treatment: Activity, chemoresistance
and its overcoming. Drug Resist Updat. 54:1007422021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tomasz M: Mitomycin C: Small, fast and
deadly (but very selective). Chem Biol. 2:575–579. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Paz MM, Zhang X, Lu J and Holmgren A: A
new mechanism of action for the anticancer drug mitomycin C:
Mechanism-based inhibition of thioredoxin reductase. Chem Res
Toxicol. 25:1502–1511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sritharan S and Sivalingam N: A
comprehensive review on Time-tested anticancer drug doxorubicin.
Life Sci. 278:1195272021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yosri M, Dokhan M, Aboagye E, Al Moussawy
M and Abdelsamed HA: Mechanisms governing bystander activation of T
cells. Front Immunol. 15:14658892024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wilczynski B, Dabrowska A, Kulbacka J and
Baczynska D: Chemoresistance and the tumor microenvironment: The
critical role of cell-cell communication. Cell Commun Signal.
22:4862024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Alsaafeen BH, Ali BR and Elkord E:
Resistance mechanisms to immune checkpoint inhibitors: Updated
insights. Mol Cancer. 24:202025. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ito T, Smrž D, Jung MY, Bandara G, Desai
A, Smržová Š, Kuehn HS, Beaven MA, Metcalfe DD and Gilfillan AM:
Stem cell factor programs the mast cell activation phenotype. J
Immunol. 188:5428–5437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jung MY, Smrž D, Desai A, Bandara G, Ito
T, Iwaki S, Kang JH, Andrade MV, Hilderbrand SC, Brown JM, et al:
IL-33 induces a hyporesponsive phenotype in human and mouse mast
cells. J Immunol. 190:531–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Desai A, Jung MY, Olivera A, Gilfillan AM,
Prussin C, Kirshenbaum AS, Beaven MA and Metcalfe DD: IL-6 promotes
an increase in human mast cell numbers and reactivity through
suppression of suppressor of cytokine signaling 3. J Allergy Clin
Immunol. 137:1863–1871. e18662016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chang TH and Ho PC: Interferon-driven
metabolic reprogramming and tumor microenvironment remodeling.
Immune Netw. 25:e82025. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wawrzyniak P and Hartman ML: Dual role of
interferon-gamma in the response of melanoma patients to
immunotherapy with immune checkpoint inhibitors. Mol Cancer.
24:892025. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nigam M, Mishra AP, Deb VK, Dimri DB,
Tiwari V, Bungau SG, Bungau AF and Radu AF: Evaluation of the
association of chronic inflammation and cancer: Insights and
implications. Biomed Pharmacother. 164:1150152023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zemek RM, Chin WL, Nowak AK, Millward MJ,
Lake RA and Lesterhuis WJ: Sensitizing the tumor microenvironment
to immune checkpoint therapy. Front Immunol. 11:2232020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gillet JP, Efferth T and Remacle J:
Chemotherapy-induced resistance by ATP-binding cassette transporter
genes. Biochim Biophys Acta. 1775:237–262. 2007.PubMed/NCBI
|
|
76
|
Wang X, Long M, Dong K, Lin F, Weng Y,
Ouyang Y, Liu L, Wei J, Chen X, He T and Zhang HZ: Chemotherapy
agents-induced immunoresistance in lung cancer cells could be
reversed by trop-2 inhibition in vitro and in vivo by interaction
with MAPK signaling pathway. Cancer Biol Ther. 14:1123–1132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Alizadeh D, Wong RA, Gholamin S, Maker M,
Aftabizadeh M, Yang X, Pecoraro JR, Jeppson JD, Wang D, Aguilar B,
et al: IFNγ is critical for CAR T Cell-mediated myeloid activation
and induction of endogenous immunity. Cancer Discov. 11:2248–2265.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xu H, Niu M, Yuan X, Wu K and Liu A: CD44
as a tumor biomarker and therapeutic target. Exp Hematol Oncol.
9:362020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Qu S, Jiao Z, Lu G, Xu J, Yao B, Wang T,
Wang J, Yao Y, Yan X, Wang T, et al: Human lung adenocarcinoma CD47
is upregulated by interferon-γ and promotes tumor metastasis. Mol
Ther Oncol. 25:276–287. 2022. View Article : Google Scholar
|
|
80
|
Zhao Y, Shi F, Zhou Q, Li Y, Wu J, Wang R
and Song Q: Prognostic significance of PD-L1 in advanced non-small
cell lung carcinoma. Medicine (Baltimore). 99:e231722020.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lin YM, Sung WW, Hsieh MJ, Tsai SC, Lai
HW, Yang SM, Shen KH, Chen MK, Lee H, Yeh KT and Chen CJ: High
PD-L1 expression correlates with metastasis and poor prognosis in
oral squamous cell carcinoma. PLoS One. 10:e01426562015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Klement JD, Redd PS, Lu C, Merting AD,
Poschel DB, Yang D, Savage NM, Zhou G, Munn DH, Fallon PG and Liu
K: Tumor PD-L1 engages myeloid PD-1 to suppress type I interferon
to impair cytotoxic T lymphocyte recruitment. Cancer Cell.
41:620–636.e9. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Qadir AS, Ceppi P, Brockway S, Law C, Mu
L, Khodarev NN, Kim J, Zhao JC, Putzbach W, Murmann AE, et al:
CD95/Fas increases stemness in cancer cells by inducing a
STAT1-dependent type I interferon response. Cell Rep. 18:2373–2386.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Risso V, Lafont E and Le Gallo M:
Therapeutic approaches targeting CD95L/CD95 signaling in cancer and
autoimmune diseases. Cell Death Dis. 13:2482022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dart A: Presenting fibroblasts. Nat Rev
Cancer. 22:1932022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mortara L, Castellani P, Meazza R, Tosi G,
De Lerma Barbaro A, Procopio FA, Comes A, Zardi L, Ferrini S and
Accolla RS: CIITA-induced MHC class II expression in mammary
adenocarcinoma leads to a Th1 polarization of the tumor
microenvironment, tumor rejection, and specific antitumor memory.
Clin Cancer Res. 12:3435–3443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Panelli MC, Wang E, Shen S, Schluter SF,
Bernstein RM, Hersh EM, Stopeck A, Gangavalli R, Barber J, Jolly D
and Akporiaye ET: Interferon gamma (IFNgamma) gene transfer of an
EMT6 tumor that is poorly responsive to IFNgamma stimulation:
Increase in tumor immunogenicity is accompanied by induction of a
mouse class II transactivator and class II MHC. Cancer Immunol
Immunother. 42:99–107. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Forero A, Li Y, Chen D, Grizzle WE, Updike
KL, Merz ND, Downs-Kelly E, Burwell TC, Vaklavas C, Buchsbaum DJ,
et al: Expression of the MHC Class II pathway in Triple-negative
breast cancer tumor cells is associated with a good prognosis and
infiltrating lymphocytes. Cancer Immunol Res. 4:390–399. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Roemer MGM, Redd RA, Cader FZ, Pak CJ,
Abdelrahman S, Ouyang J, Sasse S, Younes A, Fanale M, Santoro A, et
al: Major histocompatibility complex class ii and programmed death
ligand 1 expression predict outcome after programmed death 1
blockade in classic hodgkin lymphoma. J Clin Oncol. 36:942–950.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Axelrod ML, Cook RS, Johnson DB and Balko
JM: Biological consequences of MHC-II Expression by tumor cells in
cancer. Clin Cancer Res. 25:2392–2402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Macy AM, Herrmann LM, Adams AC and
Hastings KT: Major histocompatibility complex class II in the tumor
microenvironment: Functions of nonprofessional antigen-presenting
cells. Curr Opin Immunol. 83:1023302023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Johnson DB, Nixon MJ, Wang Y, Wang DY,
Castellanos E, Estrada MV, Ericsson-Gonzalez PI, Cote CH, Salgado
R, Sanchez V, et al: Tumor-specific MHC-II expression drives a
unique pattern of resistance to immunotherapy via LAG-3/FCRL6
engagement. JCI Insight. 3:e1203602018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lei PJ, Pereira ER, Andersson P, Amoozgar
Z, Van Wijnbergen JW, O'Melia MJ, Zhou H, Chatterjee S, Ho WW,
Posada JM, et al: Cancer cell plasticity and MHC-II-mediated immune
tolerance promote breast cancer metastasis to lymph nodes. J Exp
Med. 220:e202218472023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Shen J, Choi YL, Lee T, Kim H, Chae YK,
Dulken BW, Bogdan S, Huang M, Fisher GA, Park S, et al: Inflamed
immune phenotype predicts favorable clinical outcomes of immune
checkpoint inhibitor therapy across multiple cancer types. J
Immunother Cancer. 12:e0083392024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen S, Crabill GA, Pritchard TS, McMiller
TL, Wei P, Pardoll DM, Pan F and Topalian SL: Mechanisms regulating
PD-L1 expression on tumor and immune cells. J Immunother Cancer.
7:3052019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Mimura K, Teh JL, Okayama H, Shiraishi K,
Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, et al:
PD-L1 expression is mainly regulated by interferon gamma associated
with JAK-STAT pathway in gastric cancer. Cancer Sci. 109:43–53.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Landsberg J, Kohlmeyer J, Renn M, Bald T,
Rogava M, Cron M, Fatho M, Lennerz V, Wölfel T, Hölzel M and Tüting
T: Melanomas resist T-cell therapy through Inflammation-induced
reversible dedifferentiation. Nature. 490:412–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang B, Han Y, Zhang Y, Zhao Q, Wang H,
Wei J, Meng L, Xin Y and Jiang X: Overcoming acquired resistance to
cancer immune checkpoint therapy: Potential strategies based on
molecular mechanisms. Cell Biosci. 13:1202023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Goddard ET, Linde MH, Srivastava S, Klug
G, Shabaneh TB, Iannone S, Grzelak CA, Marsh S, Riggio AI, Shor RE,
et al: Immune evasion of dormant disseminated tumor cells is due to
their scarcity and can be overcome by T cell immunotherapies.
Cancer Cell. 42:119–134.e12. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yu M, Peng Z, Qin M, Liu Y, Wang J, Zhang
C, Lin J, Dong T, Wang L, Li S, et al: Interferon-gamma induces
tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase
separation. Mol Cell. 81:1216–1230.e9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mandai M, Hamanishi J, Abiko K, Matsumura
N, Baba T and Konishi I: Dual faces of IFNγ in cancer progression:
A role of PD-L1 induction in the determination of Pro- and
antitumor immunity. Clin Cancer Res. 22:2329–2334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Beziaud L, Young CM, Alonso AM, Norkin M,
Minafra AR and Huelsken J: IFNγ-induced stem-like state of cancer
cells as a driver of metastatic progression following
immunotherapy. Cell Stem Cell. 30:818–831.e6. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gocher AM, Workman CJ and Vignali DAA:
Interferon-gamma: Teammate or opponent in the tumour
microenvironment? Nat Rev Immunol. 22:158–172. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Korentzelos D, Wells A and Clark AM:
Interferon-γ increases sensitivity to chemotherapy and provides
immunotherapy targets in models of metastatic Castration-resistant
prostate cancer. Sci Rep. 12:66572022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gupta J, Abed HS, Uthirapathy S, Kyada A,
Rab SO, Shit D, Janney B, Nathiya D, Kadhim AJ and Mustafa YF:
Beyond TRAIL resistance: Novel strategies for potentiating
TRAIL-induced apoptosis in cancer. Exp Cell Res. 450:1146192025.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Merchant MS, Yang X, Melchionda F, Romero
M, Klein R, Thiele CJ, Tsokos M, Kontny HU and Mackall CL:
Interferon gamma enhances the effectiveness of tumor necrosis
factor-related apoptosis-inducing ligand receptor agonists in a
xenograft model of Ewing's sarcoma. Cancer Res. 64:8349–8356. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Johnsen JI, Pettersen I, Ponthan F,
Sveinbjornsson B, Flaegstad T and Kogner P: Synergistic induction
of apoptosis in neuroblastoma cells using a combination of
cytostatic drugs with interferon-gamma and TRAIL. Int J Oncol.
25:1849–1857. 2004.PubMed/NCBI
|
|
108
|
Gawrylak A, Brodaczewska K,
Iwanicka-Nowicka R, Koblowska M, Synowiec A, Bodnar L, Szczylik C,
Lesyng B, Stec R and Kieda C: Hypoxia alters the response of
ovarian cancer cells to the mitomycin C drug. Front Cell Dev Biol.
13:15751342025. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Strese S, Fryknas M, Larsson R and Gullbo
J: Effects of hypoxia on human cancer cell line chemosensitivity.
BMC Cancer. 13:3312013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Hultman I, Haeggblom L, Rognmo I, Jansson
Edqvist J, Blomberg E, Ali R, Phillips L, Sandstedt B, Kogner P,
Shirazi Fard S and Ährlund-Richter L: Doxorubicin-provoked increase
of mitotic activity and concomitant drain of G0-pool in
therapy-resistant BE(2)-C neuroblastoma. PLoS One. 13:e01909702018.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lyu YL, Kerrigan JE, Lin CP, Azarova AM,
Tsai YC, Ban Y and Liu LF: Topoisomerase IIbeta mediated DNA
double-strand breaks: Implications in doxorubicin cardiotoxicity
and prevention by dexrazoxane. Cancer Res. 67:8839–8846. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Stronach EA, Alfraidi A, Rama N, Datler C,
Studd JB, Agarwal R, Guney TG, Gourley C, Hennessy BT, Mills GB, et
al: HDAC4-regulated STAT1 activation mediates platinum resistance
in ovarian cancer. Cancer Res. 71:4412–4422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Patterson SG, Wei S, Chen X, Sallman DA,
Gilvary DL, Zhong B, Pow-Sang J, Yeatman T and Djeu JY: Novel role
of Stat1 in the development of docetaxel resistance in prostate
tumor cells. Oncogene. 25:6113–6122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhu H, Wang Z, Xu Q, Zhang Y, Zhai Y, Bai
J, Liu M, Hui Z and Xu N: Inhibition of STAT1 sensitizes renal cell
carcinoma cells to radiotherapy and chemotherapy. Cancer Biol Ther.
13:401–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Suzuki K, Yokoi A, Yoshida K, Suzuki H,
Kitagawa M, Asano-Inami E, Matsuo S, Yoshihara M, Tamauchi S,
Yoshikawa N, et al: Overcoming platinum-resistant ovarian cancer
targeting the activated JAK-STAT pathways via extracellular
vesicles. Commun Biol. 8:13052025. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Damen MPF, van Rheenen J and Scheele C:
Targeting dormant tumor cells to prevent cancer recurrence. FEBS J.
288:6286–6303. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
DeMichele A, Clark AS, Shea E, Bayne LJ,
Sterner CJ, Rohn K, Dwyer S, Pan TC, Nivar I, Chen Y, et al:
Targeting dormant tumor cells to prevent recurrent breast cancer: A
randomized phase 2 trial. Nat Med. 31:3464–3474. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li X, Lu F, Zhou J, Li X, Li Y, Ye W, Li
J, Yang L, Tang S, Zhou Y, et al: IFNγ augments TKI efficacy by
alleviating protein unfolding stress to promote GSDME-mediated
pyroptosis in hepatocellular carcinoma. Cell Death Dis. 16:5122025.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cui C, Xu C, Yang W, Chi Z, Sheng X, Si L,
Xie Y, Yu J, Wang S, Yu R, et al: Ratio of the interferon-γ
signature to the immunosuppression signature predicts anti-PD-1
therapy response in melanoma. NPJ Genom Med. 6:72021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Llop S, Plana M, Tous S, Ferrando-Díez A,
Brenes J, Juarez M, Vidales Z, Vilajosana E, Linares I, Arribas L,
et al: Salvage chemotherapy after progression on immunotherapy in
recurrent/metastatic squamous cell head and neck carcinoma. Front
Oncol. 14:14584792024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Reverdy T, Varnier R, de Talhouet S,
Duplomb S and Bruyas A: Analysis of the benefit of salvage
chemotherapy after progression on nivolumab in patients with
squamous cell carcinoma of the head and neck. Oral Oncol.
145:1065332023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Gaughan EM and Horton BJ: Outcomes from
cytotoxic chemotherapy following progression on immunotherapy in
metastatic melanoma: An institutional Case-series. Front Oncol.
12:8557822022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Abdelhamid MS, Wadan AS, Saad HA,
El-Dakroury WA, Hageen AW, Mohammed DH, Mourad S, Mohammed OA,
Abdel-Reheim MA and Doghish AS: Nanoparticle innovations in
targeted cancer therapy: Advancements in Antibody-drug conjugates.
Naunyn Schmiedebergs Arch Pharmacol. 398:6369–6389. 2025.
View Article : Google Scholar : PubMed/NCBI
|